Multi-Wavelength Excitable Multicolor Upconversion and Ratiometric Luminescence Thermometry of Yb3+/Er3+ Co-Doped NaYGeO4 Microcrystals
Abstract
1. Introduction
2. Results and Discussion
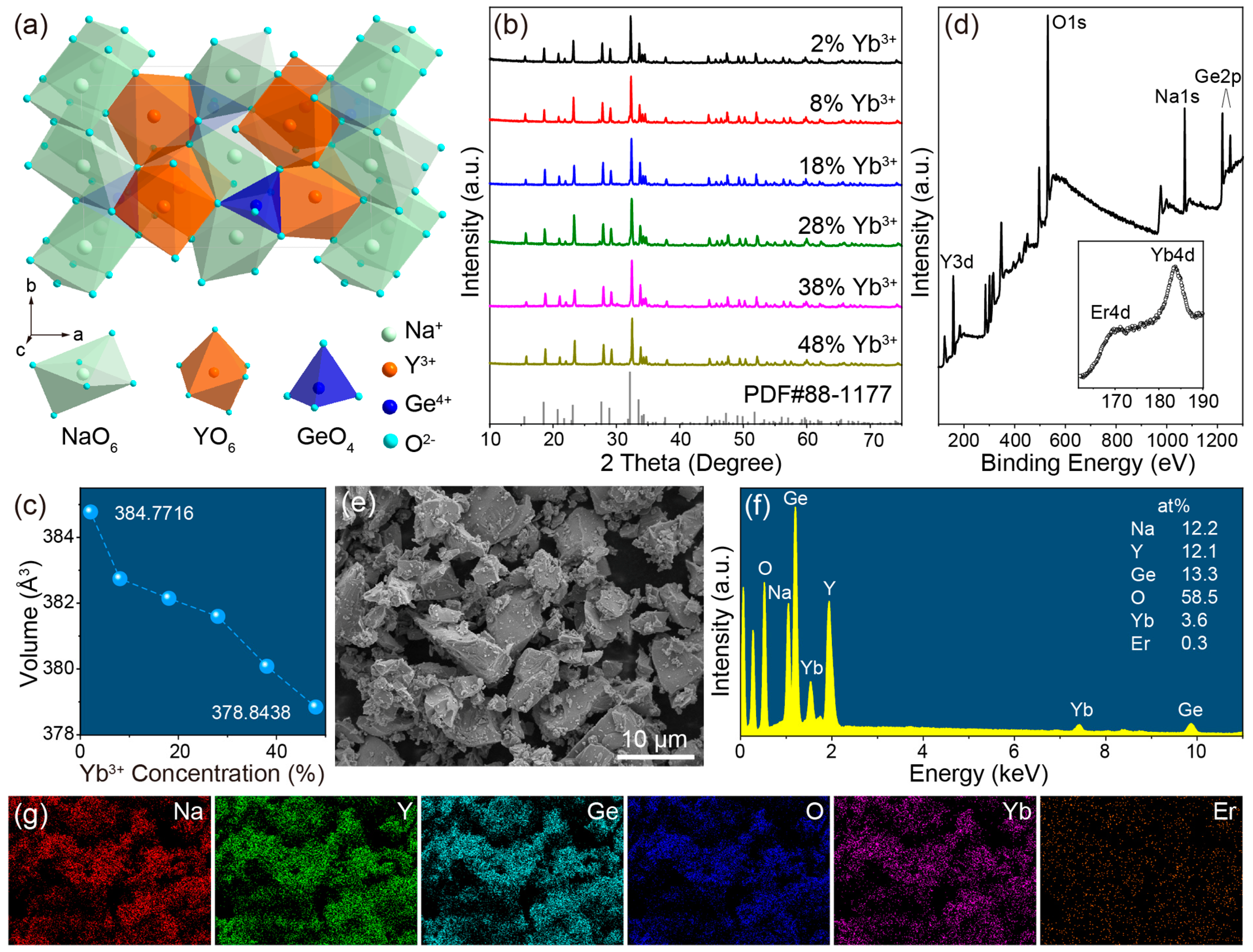
2.1. Structure, Composition and Morphology
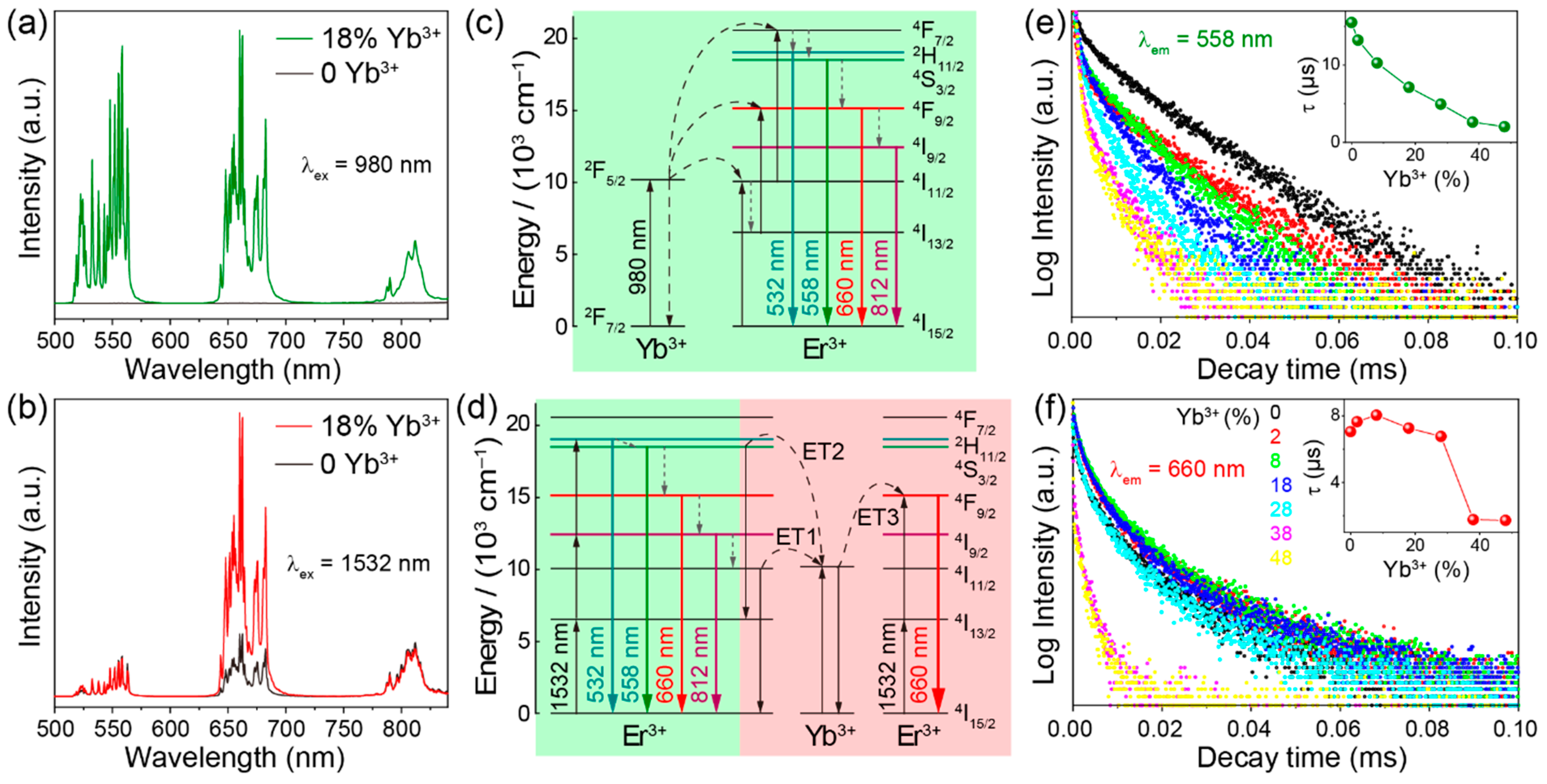
2.2. Upconversion Properties
2.3. Ratiometric Temperature Sensing Using Thermally Coupled Levels
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, P.; Wei, Y.; Liang, Y.; An, R.; Liu, S.; Lei, P.; Zhang, H. Near-Infrared-Responsive Rare Earth Nanoparticles for Optical Imaging and Wireless Phototherapy. Adv. Sci. 2023, 11, 2305308. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Hrovat, D.; Kumar, B.; Qu, G.; Houten, J.V.; Ahmed, R.; Piunno, P.A.E.; Gunning, P.T.; Krull, U.J. Lanthanide-Doped Upconversion Nanoparticles: Exploring A Treasure Trove of NIR-Mediated Emerging Applications. ACS Appl. Mater. Interfaces 2023, 15, 2499–2528. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.; Liu, X. Rare-Earth Doping in Nanostructured Inorganic Materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef]
- Huang, J.; Yan, L.; Liu, S.; Tao, L.; Zhou, B. Expanding the toolbox of photon upconversion for emerging frontier applications. Mater. Horiz. 2022, 9, 1167–1195. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Song, S.; Fan, D.; Zhang, H.; Liu, X. Remote manipulation of upconversion luminescence. Chem. Soc. Rev. 2018, 47, 6473–6485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Gao, J.; Zhang, K.; Gao, C.; Xie, X.; Huang, L. Physical Manipulation of Lanthanide-Activated Photoluminescence. Ann. Phys. 2019, 531, 1900026. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, J.; Yue, J.; Wei, Y.; Gao, C.; Xie, X.; Huang, L. Recent Development in Sensitizers for Lanthanide-Doped Upconversion Luminescence. Chem. Rev. 2022, 122, 15998–16050. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y. Orthogonal Emissive Upconversion Nanoparticles: Material Design and Applications. Small 2021, 17, 2004552. [Google Scholar] [CrossRef]
- Wen, H.; Zhu, H.; Chen, X.; Hung, T.F.; Wang, B.; Zhu, G.; Yu, S.F.; Wang, F. Upconverting near-infrared light through energy management in core-shell-shell nanoparticles. Angew. Chem. Int. Ed. 2013, 52, 13419–13423. [Google Scholar] [CrossRef]
- Li, X.; Guo, Z.; Zhao, T.; Lu, Y.; Zhou, L.; Zhao, D.; Zhang, F. Filtration Shell Mediated Power Density Independent Orthogonal Excitations-Emissions Upconversion Luminescence. Angew. Chem. Int. Ed. 2016, 55, 2464–2469. [Google Scholar] [CrossRef]
- Zhang, Z.; Jayakumar, M.K.G.; Zheng, X.; Shikha, S.; Zhang, Y.; Bansal, A.; Poon, D.J.J.; Chu, P.L.; Yeo, E.L.L.; Chua, M.L.K.; et al. Upconversion superballs for programmable photoactivation of therapeutics. Nat. Commun. 2019, 10, 4586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jayakumar, M.K.G.; Shikha, S.; Zhang, Y.; Zheng, X.; Zhang, Y. Modularly Assembled Upconversion Nanoparticles for Orthogonally Controlled Cell Imaging and Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 12549–12556. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Ling, X.; Mei, Q.; Fu, S.; Zhang, J.; Zhang, Y. An Excitation Navigating Energy Migration of Lanthanide Ions in Upconversion Nanoparticles. Adv. Mater. 2020, 32, 1906225. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Bansal, A.; Jayakumar, M.K.G.; Zhang, Z.; Zhang, J.; Huang, H.; Yu, D.; Ramachandra, C.J.A.; Hausenloy, D.J.; Soong, T.W.; et al. Manipulating energy migration within single lanthanide activator for switchable upconversion emissions towards bidirectional photoactivation. Nat. Commun. 2019, 10, 4416. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.J.; He, S.; Diao, S.; Chan, E.M.; Dai, H.; Almutairi, A. Direct Evidence for Coupled Surface and Concentration Quenching Dynamics in Lanthanide-Doped Nanocrystals. J. Am. Chem. Soc. 2017, 139, 3275–3282. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, Y.; Yuan, Z.; Wang, X.; Su, W.; Yin, L.; Xie, X.; Huang, L. Er3+ Sensitized Photon Upconversion Nanocrystals. Adv. Funct. Mater. 2018, 28, 1800208. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, X.; Huang, B.; Liang, L.; Han, S.; Yi, Z.; Wang, Y.; Li, Y.; Fan, D.; Huang, L.; et al. Confining Excitation Energy in Er3+-Sensitized Upconversion Nanocrystals through Tm3+ Mediated Transient Energy Trapping. Angew. Chem. Int. Ed. 2017, 56, 7605–7609. [Google Scholar] [CrossRef]
- Cheng, X.; Ge, H.; Wei, Y.; Zhang, K.; Su, W.; Zhou, J.; Yin, L.; Zhan, Q.; Jing, S.; Huang, L. Design for Brighter Photon Upconversion Emissions via Energy Level Overlap of Lanthanide Ions. ACS Nano 2018, 12, 10992–10999. [Google Scholar] [CrossRef]
- Zhao, J.; Chang, J.; Wang, N.; Zhao, P.; Zhu, M.; Liu, Y.; Guo, D.; Wang, Y.; Li, P.; Wang, Z.; et al. Thermal Enhancement of Er3+ NIR-II Luminescence by Ho3+-Mediated Energy-Trapping in Negative Thermal Expansion Nanocrystals. Laser Photonics Rev. 2024, 2400151, online version of record. [Google Scholar] [CrossRef]
- Liu, X.; Liu, T.; Tu, L.; Zuo, J.; Li, J.; Feng, Y.; Yao, C.J. Enhancing NIR-II Upconversion Monochromatic Emission for Temperature Sensing. Small 2024, 20, 2308748. [Google Scholar] [CrossRef]
- Bi, S.; Deng, Z.; Huang, J.; Wen, X.; Zeng, S. NIR-II Responsive Upconversion Nanoprobe with Simultaneously Enhanced Single-Band Red Luminescence and Phase/Size Control for Bioimaging and Photodynamic Therapy. Adv. Mater. 2023, 35, 2207038. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, L.; Li, J.; Wang, Z.; Zhang, J.; Zhang, L.; Luo, Y.; Wang, X. Selectively enhanced red upconversion luminescence and phase/size manipulation via Fe3+ doping in NaYF4:Yb,Er nanocrystals. Nanoscale 2015, 7, 14752–14759. [Google Scholar] [CrossRef]
- Lyu, T.; Dorenbos, P. Vacuum-Referred Binding Energies of Bismuth and Lanthanide Levels in ARE(Si,Ge)O4 (A = Li, Na; RE = Y, Lu): Toward Designing Charge-Carrier-Trapping Processes for Energy Storage. Chem. Mater. 2020, 32, 1192–1209. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, X.; Fan, B. Novel color tunable phosphors NaYGeO4:Tm3+, Tb3+, Eu3+ for ultraviolet excited white LEDs with good thermal stability. J. Mater. Sci. Mater. Electron. 2020, 31, 14434–14442. [Google Scholar] [CrossRef]
- Lyu, T.; Dorenbos, P.; Li, C.; Li, S.; Xu, J.; Wei, Z. Unraveling electron liberation from Bi2+ for designing Bi3+-based afterglow phosphor for anti-counterfeiting and flexible X-ray imaging. Chem. Eng. J. 2022, 435, 135038. [Google Scholar] [CrossRef]
- Wang, E.; Feng, K.; Li, J.; Zhou, X.; Sun, X. Luminescence characteristics of NaYGeO4:Bi3+/Tb3+/Eu3+ phosphors. J. Lumin. 2022, 250, 119108. [Google Scholar] [CrossRef]
- Melentsova, A.A.; Lipina, O.A.; Melkozerova, M.A.; Enyashin, A.N.; Chufarov, A.Y.; Tyutyunnik, A.P.; Zubkov, V.G. Infrared luminescence properties and energy transfer mechanism in NaYGeO4:Tm3+ powders. Ceram. Int. 2024, 50, 18681–18688. [Google Scholar] [CrossRef]
- Fan, B.; Zhou, W.; Qi, S.; Zhao, W. Eu3+-doped NaYGeO4: A novel red-emitting phosphors for ultraviolet or blue chips excited white LEDs. J. Solid State Chem. 2020, 283, 121158. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Hu, W.; Wang, W.; Zhang, J.; Yang, J.; Wang, Y. A terbium activated multicolour photoluminescent phosphor for luminescent anticounterfeiting. J. Rare Earths 2020, 38, 1039–1043. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Dai, Y.; Han, B. Synthesis and luminescence properties of novel host-sensitized germanate phosphors NaYGeO4:Ln (Ln = Eu3+, Sm3+, Dy3+). Optik 2020, 203, 163944. [Google Scholar] [CrossRef]
- SHANNON, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Tu, L.; Liu, X.; Wu, F.; Zhang, H. Excitation energy migration dynamics in upconversion nanomaterials. Chem. Soc. Rev. 2015, 44, 1331–1345. [Google Scholar] [CrossRef]
- Liu, X.; Deng, R.; Zhang, Y.; Wang, Y.; Chang, H.; Huang, L.; Liu, X. Probing the nature of upconversion nanocrystals: Instrumentation matters. Chem. Soc. Rev. 2015, 44, 1479–1508. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Han, S.; Zeng, X.; Wu, Y.; Song, S.; Zhang, H.; Liu, X. Rewritable Optical Memory Through High-Registry Orthogonal Upconversion. Adv. Mater. 2018, 30, 1801726. [Google Scholar] [CrossRef]
- Ryszczyńska, S.; Trejgis, K.; Marciniak, Ł.; Grzyb, T. Upconverting SrF2:Er3+ Nanoparticles for Optical Temperature Sensors. ACS Appl. Nano Mater. 2021, 4, 10438–10448. [Google Scholar] [CrossRef]
- Zhou, B.; Yan, L.; Huang, J.; Liu, X.; Tao, L.; Zhang, Q. NIR II-responsive photon upconversion through energy migration in an ytterbium sublattice. Nat. Photonics 2020, 14, 760–766. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Pang, T.; Chen, B.; Xin, F.; Xing, M.; Tian, M.; Fu, Y.; Luo, X.; Tian, Y. Engineering Er3+-sensitized nanocrystals to enhance NIR II-responsive upconversion luminescence. Nanoscale 2022, 14, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Sun, L.D.; Wang, Y.F.; Ke, J.; Si, R.; Xiao, J.W.; Lyu, G.M.; Shi, S.; Yan, C.H. Efficient Tailoring of Upconversion Selectivity by Engineering Local Structure of Lanthanides in NaxREF3+x Nanocrystals. J. Am. Chem. Soc. 2015, 137, 6569–6576. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Ma, H.; Huang, L. Colour modulation and enhancement of upconversion emissions in K2NaScF6:Yb/Ln (Ln = Er, Ho, Tm) nanocrystals. J. Rare Earths 2021, 39, 1477–1483. [Google Scholar] [CrossRef]
- Xiao, Q.; Dong, X.; Yin, X.; Wang, H.; Zhong, H.; Dong, B.; Luo, X. Dual-color up-conversion luminescence and temperature sensing of novel Na3Y(VO4)2: Yb3+, Er3+ phosphor under multi-wavelength excitation. Mater. Res. Bull. 2021, 141, 111326. [Google Scholar] [CrossRef]
- Ramos, T.J.S.; Longo, R.L.; Brites, C.D.S.; Ferreira, R.A.S.; Malta, O.L.; Carlos, L.D. Exploring the intra-4f and the bright white light upconversion emissions of Gd2O3:Yb3+,Er3+-based materials for thermometry. Nanoscale 2023, 15, 9993–10003. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.D.S.; Balabhadra, S.; Carlos, L.D. Lanthanide-Based Thermometers: At the Cutting-Edge of Luminescence Thermometry. Adv. Opt. Mater. 2019, 7, 1801239. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, T.; Li, H.; Xiang, Y.; Song, R.; Zhang, H.; Wang, B. Improving the up/down-conversion luminescence via cationic substitution and dual-functional temperature sensing properties of Er3+ doped double perovskites. Chem. Eng. J. 2023, 471, 144550. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Ma, Y.; Sun, X.; Zhang, Y.; Li, H. Simultaneous evolutions in composition, structure, morphology, and upconversion luminescence of BiOxFy:Yb/Er microcrystals and their application for ratiometric temperature sensing. J. Alloys Compd. 2024, 992, 174596. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Marin, R.; Suta, M.; Carneiro Neto, A.N.; Ximendes, E.; Jaque, D.; Carlos, L.D. Spotlight on Luminescence Thermometry: Basics, Challenges, and Cutting-Edge Applications. Adv. Mater. 2023, 35, 2302749. [Google Scholar] [CrossRef]
- Wang, C.; Jin, Y.; Zhang, R.; Yao, Q.; Hu, Y. A review and outlook of ratiometric optical thermometer based on thermally coupled levels and non-thermally coupled levels. J. Alloys Compd. 2022, 894, 162494. [Google Scholar] [CrossRef]
- Jia, M.; Sun, Z.; Zhang, M.; Xu, H.; Fu, Z. What determines the performance of lanthanide-based ratiometric nanothermometers? Nanoscale 2020, 12, 20776–20785. [Google Scholar] [CrossRef]
- Savchuk, O.A.; Carvajal, J.J.; Brites, C.D.S.; Carlos, L.D.; Aguilo, M.; Diaz, F. Upconversion thermometry: A new tool to measure the thermal resistance of nanoparticles. Nanoscale 2018, 10, 6602–6610. [Google Scholar] [CrossRef]



| Excitation Wavelength (nm) | LIR Used | Temperature Range (K) | Sr (% K−1) | δT (K) |
|---|---|---|---|---|
| 980 | I532/I558 (TCLs) | 295–823 | 0.15–1.17 | 0.20–1.59 |
| 1532 | I532/I558 (TCLs) | 295–823 | 0.15–1.18 | 0.14–1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Wang, Y.; Zhang, X.; Bu, X.; Liu, Z.; Li, H. Multi-Wavelength Excitable Multicolor Upconversion and Ratiometric Luminescence Thermometry of Yb3+/Er3+ Co-Doped NaYGeO4 Microcrystals. Molecules 2024, 29, 4887. https://doi.org/10.3390/molecules29204887
Zeng H, Wang Y, Zhang X, Bu X, Liu Z, Li H. Multi-Wavelength Excitable Multicolor Upconversion and Ratiometric Luminescence Thermometry of Yb3+/Er3+ Co-Doped NaYGeO4 Microcrystals. Molecules. 2024; 29(20):4887. https://doi.org/10.3390/molecules29204887
Chicago/Turabian StyleZeng, Hui, Yangbo Wang, Xiaoyi Zhang, Xiangbing Bu, Zongyi Liu, and Huaiyong Li. 2024. "Multi-Wavelength Excitable Multicolor Upconversion and Ratiometric Luminescence Thermometry of Yb3+/Er3+ Co-Doped NaYGeO4 Microcrystals" Molecules 29, no. 20: 4887. https://doi.org/10.3390/molecules29204887
APA StyleZeng, H., Wang, Y., Zhang, X., Bu, X., Liu, Z., & Li, H. (2024). Multi-Wavelength Excitable Multicolor Upconversion and Ratiometric Luminescence Thermometry of Yb3+/Er3+ Co-Doped NaYGeO4 Microcrystals. Molecules, 29(20), 4887. https://doi.org/10.3390/molecules29204887








