Investigation of the Sensing Properties of Lanthanoid Metal–Organic Frameworks (Ln-MOFs) with Terephthalic Acid
Abstract
1. Introduction
2. Results and Discussion
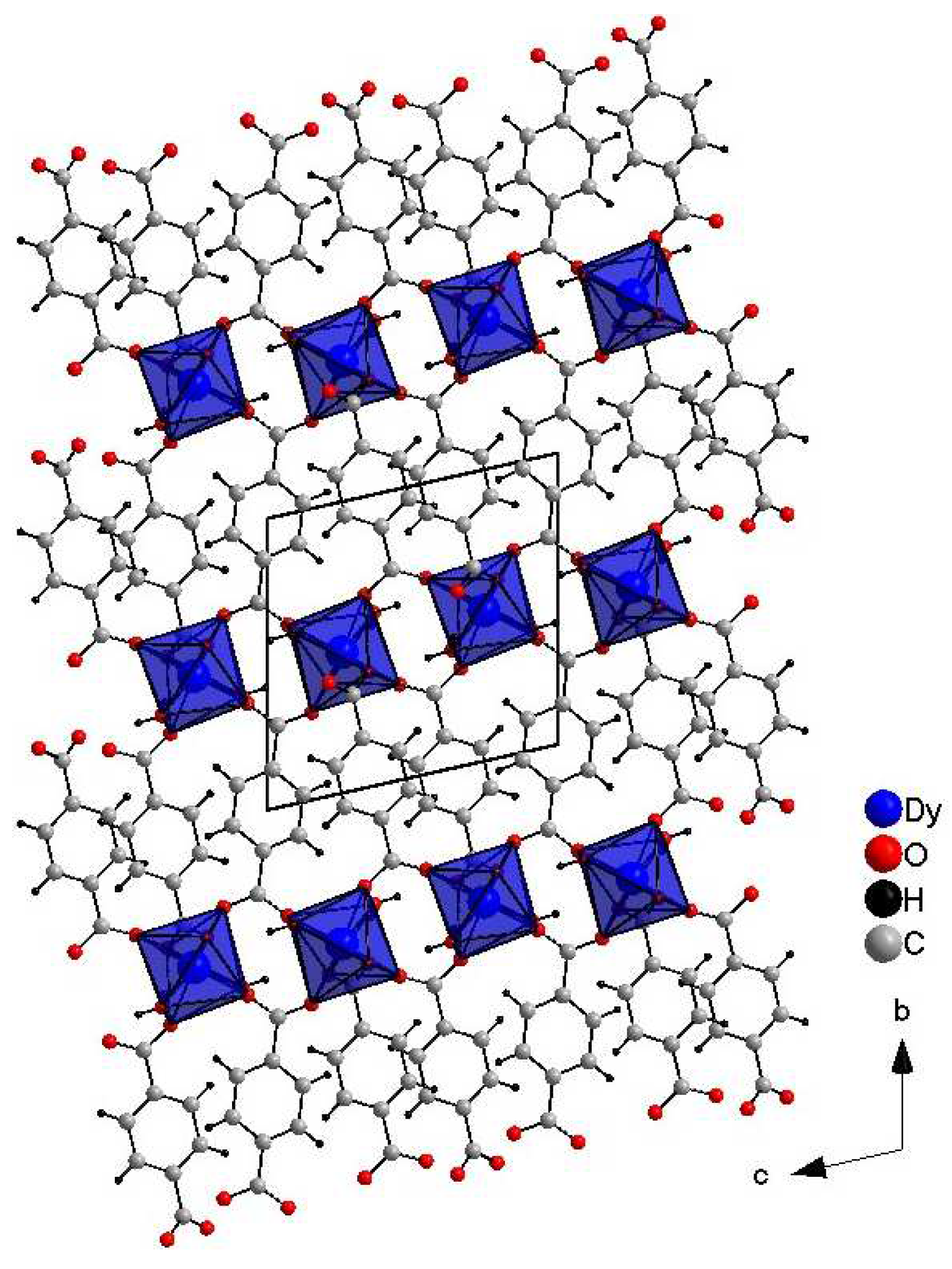
2.1. Crystal Structure of DyBDC–Single Crystal (SC)
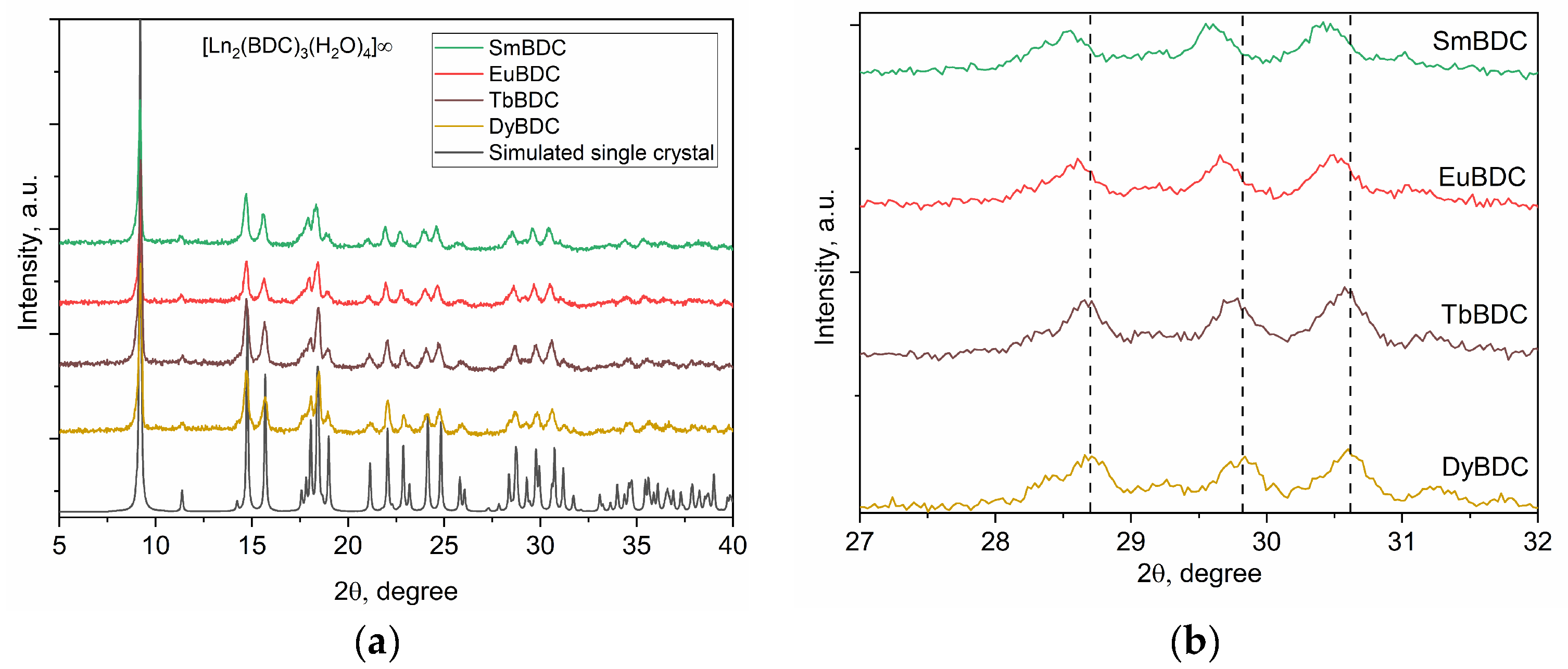
2.2. Powder X-ray Diffraction
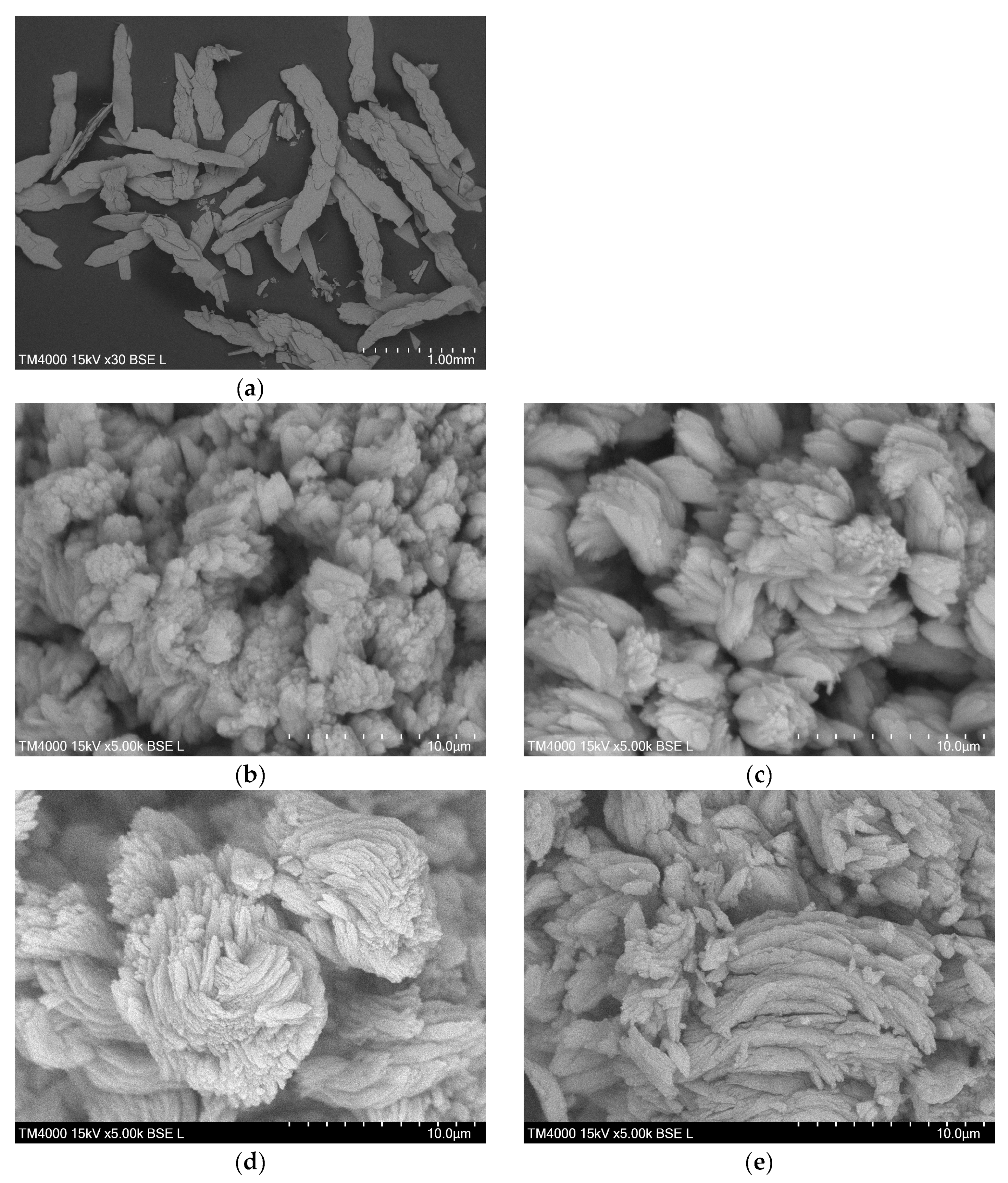
2.3. Morphology
2.4. Optical Properties
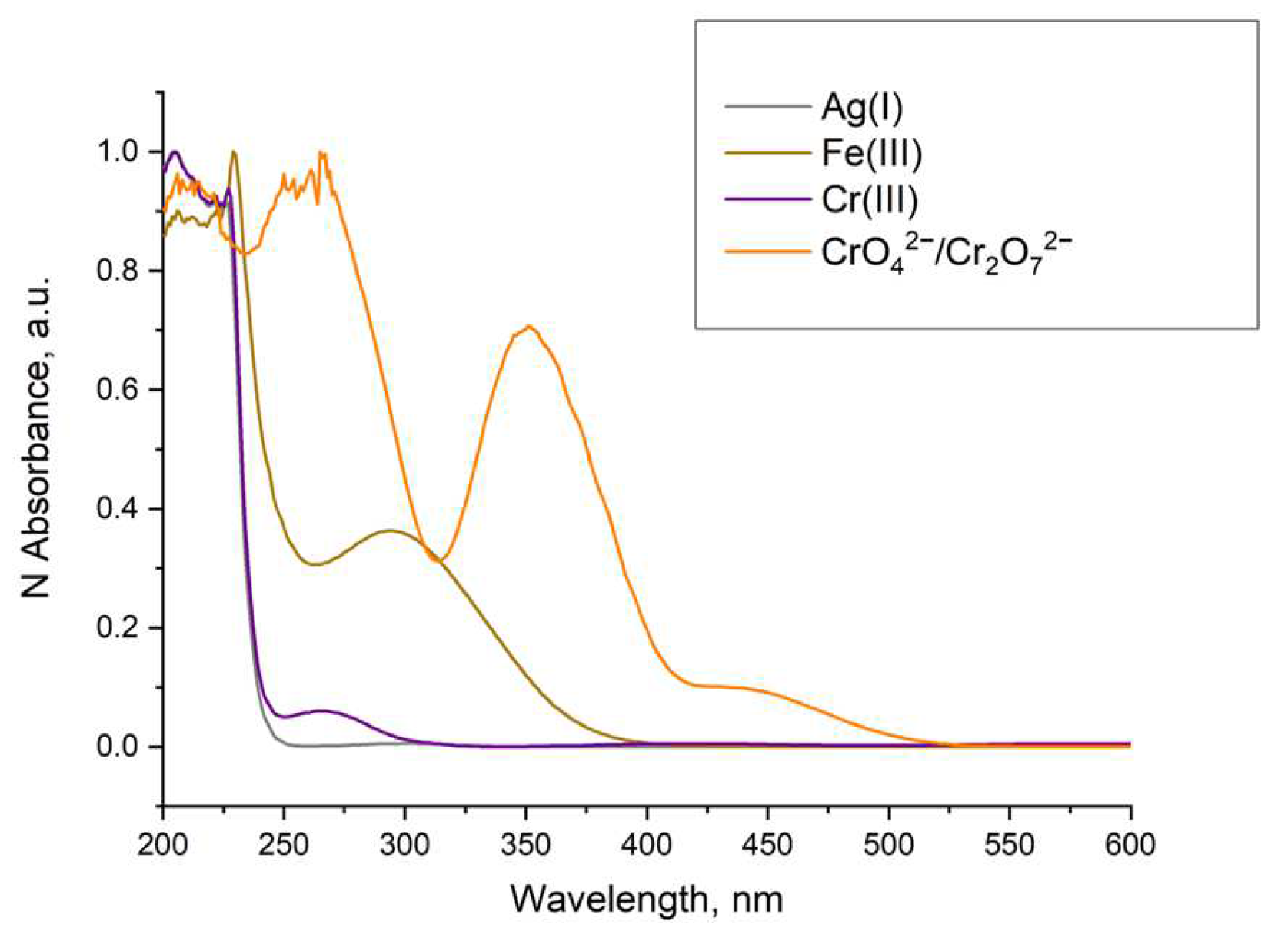
2.5. Sensor Properties
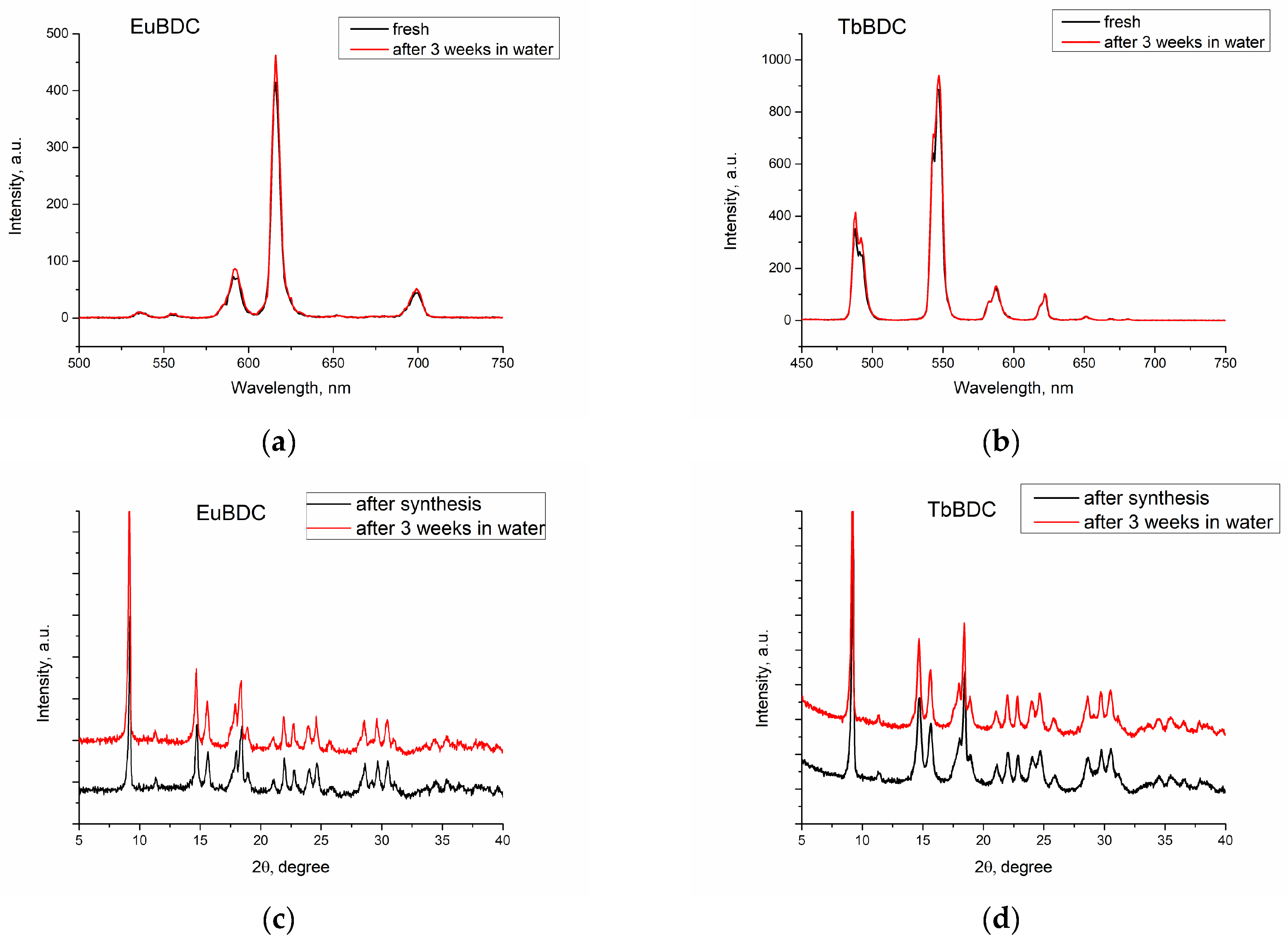
2.5.1. Stability of the Water Suspensions of the Samples
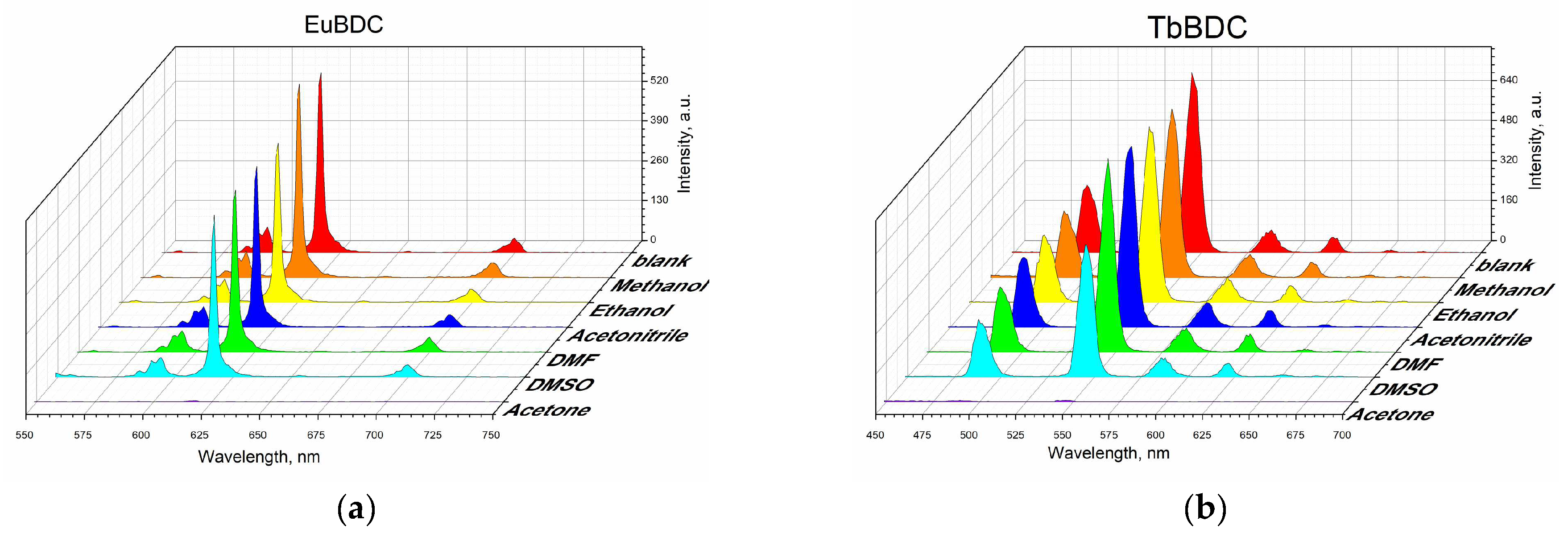
2.5.2. Behavior in Different Solvents
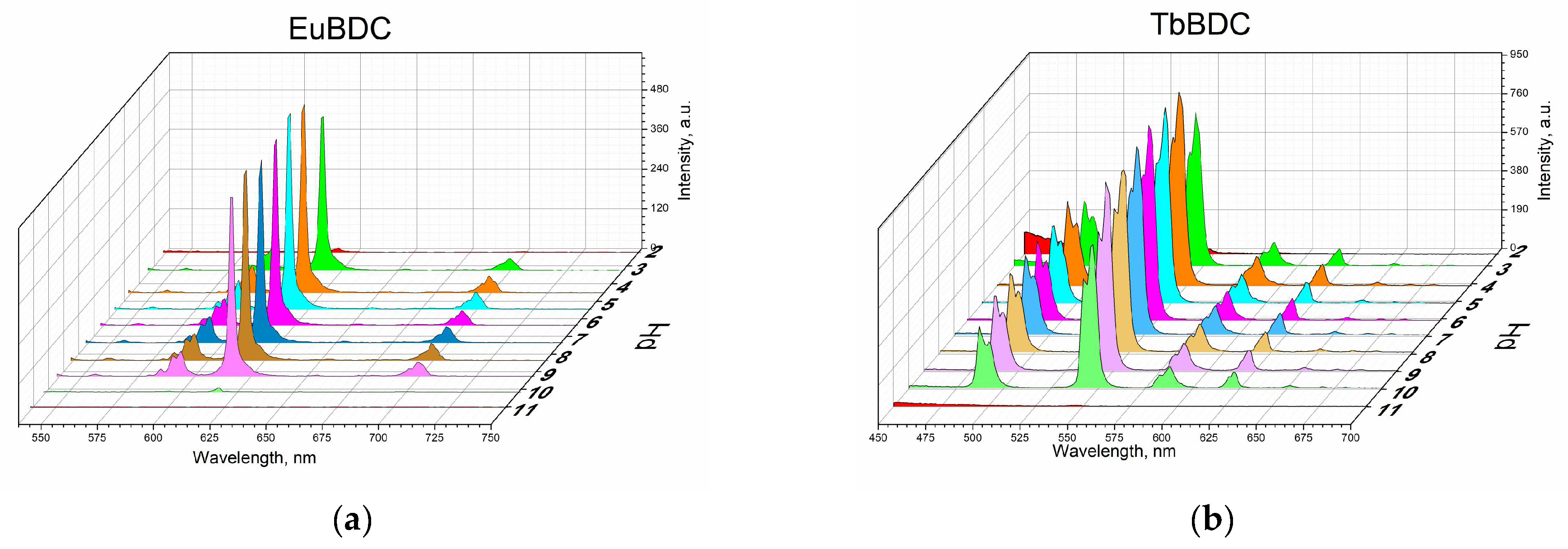
2.5.3. Behavior in Media with Different Acidity Levels
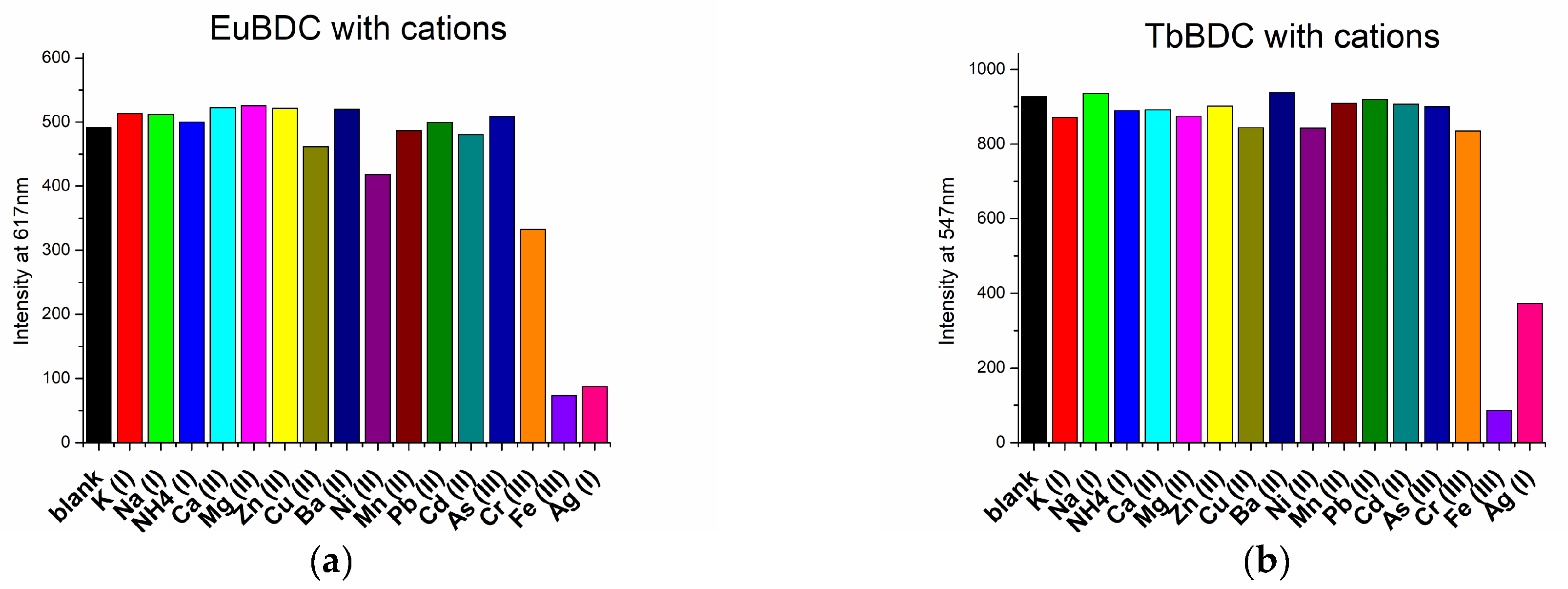
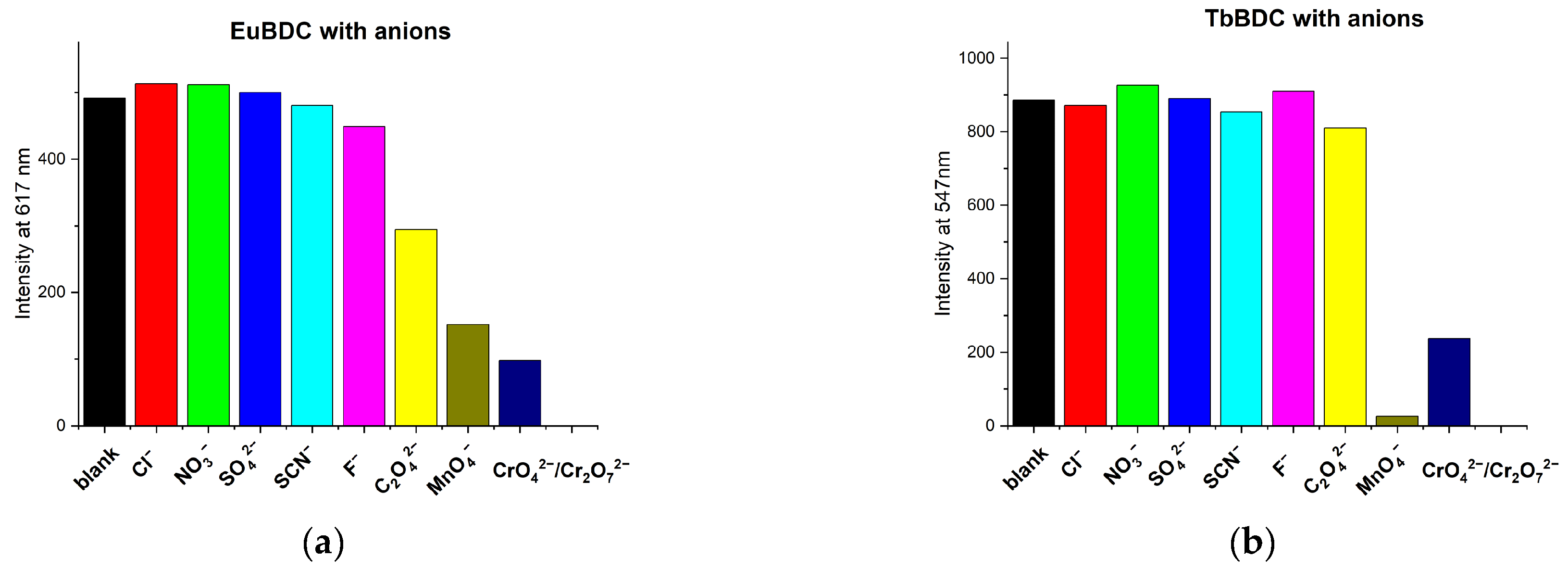
2.5.4. Behavior in the Presence of Different Cations and Anions
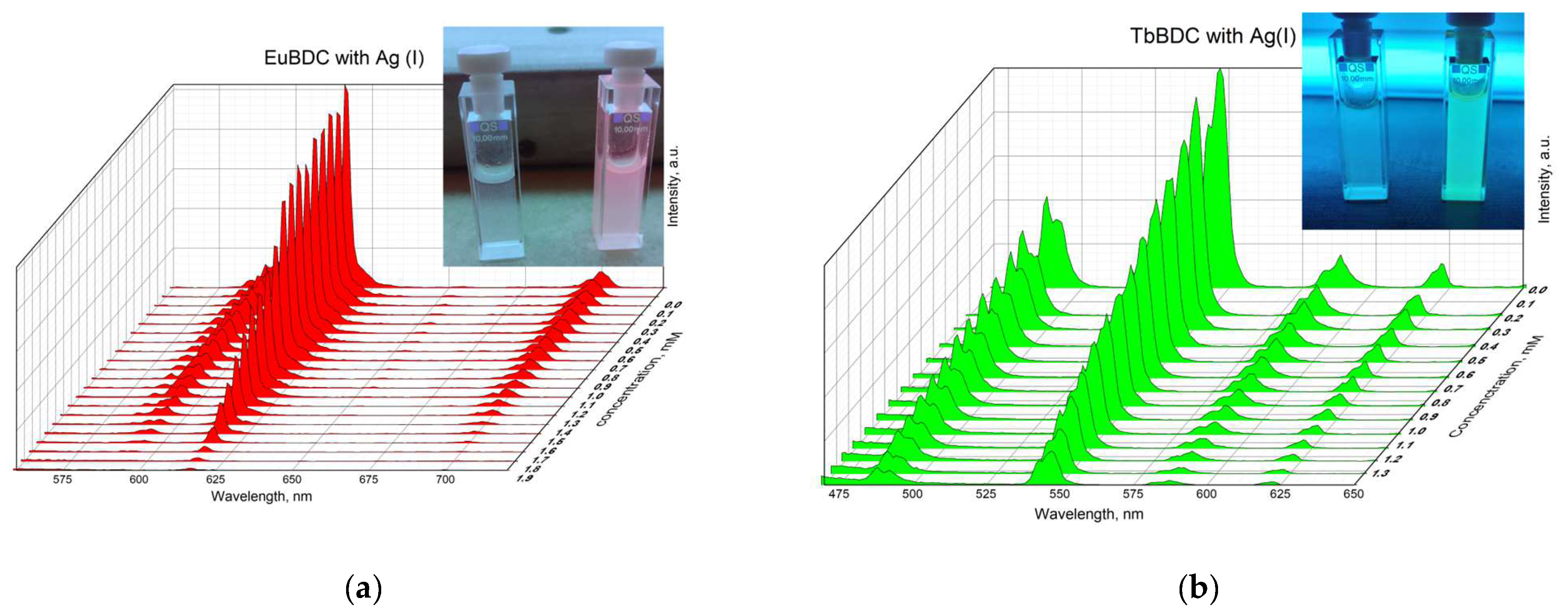
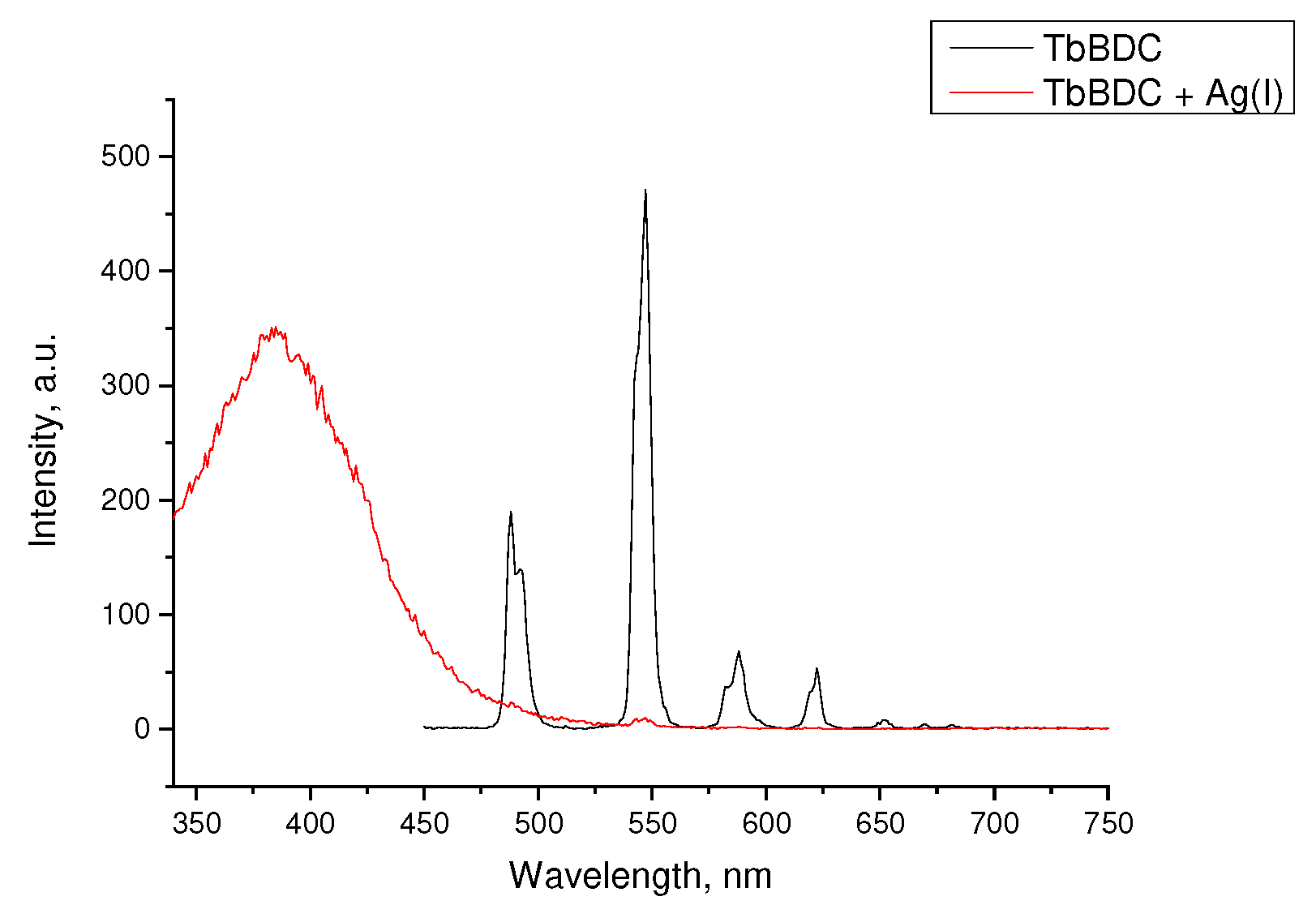
2.5.5. Silver(I) Sensitivity of EuBDC and of TbBDC
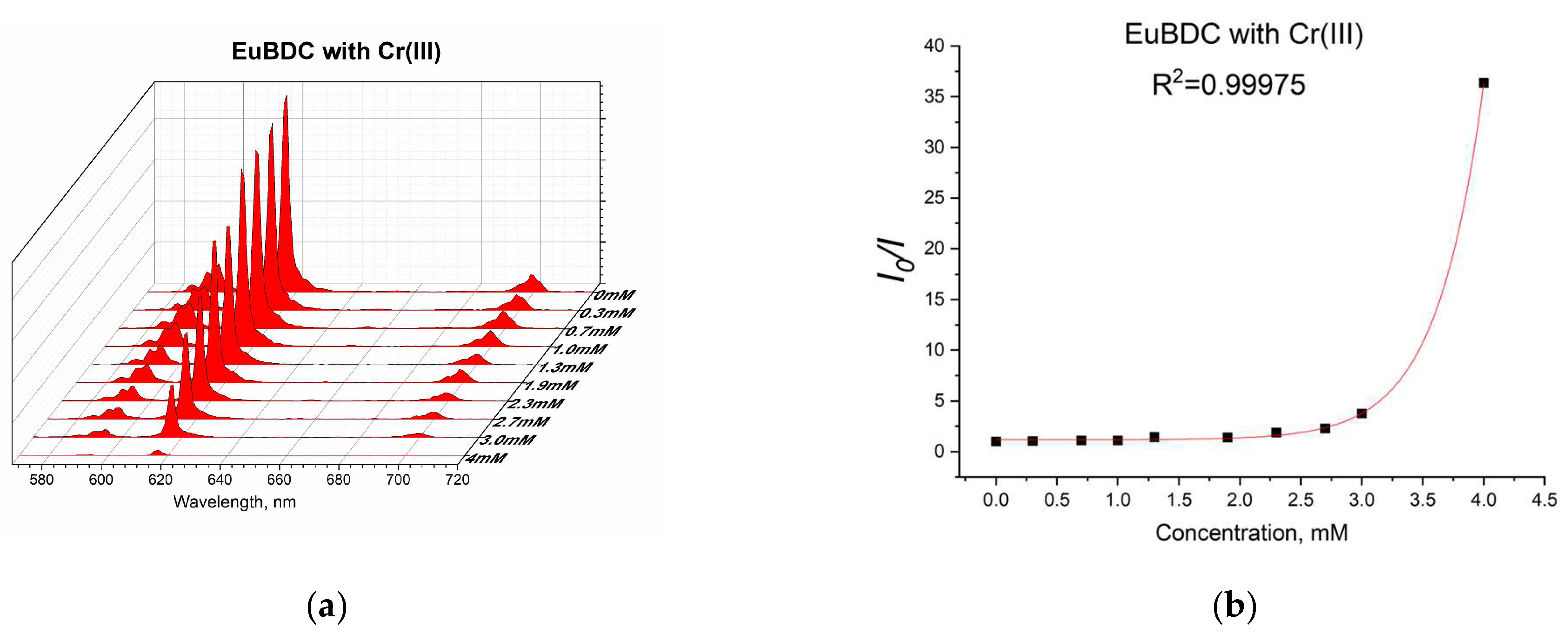
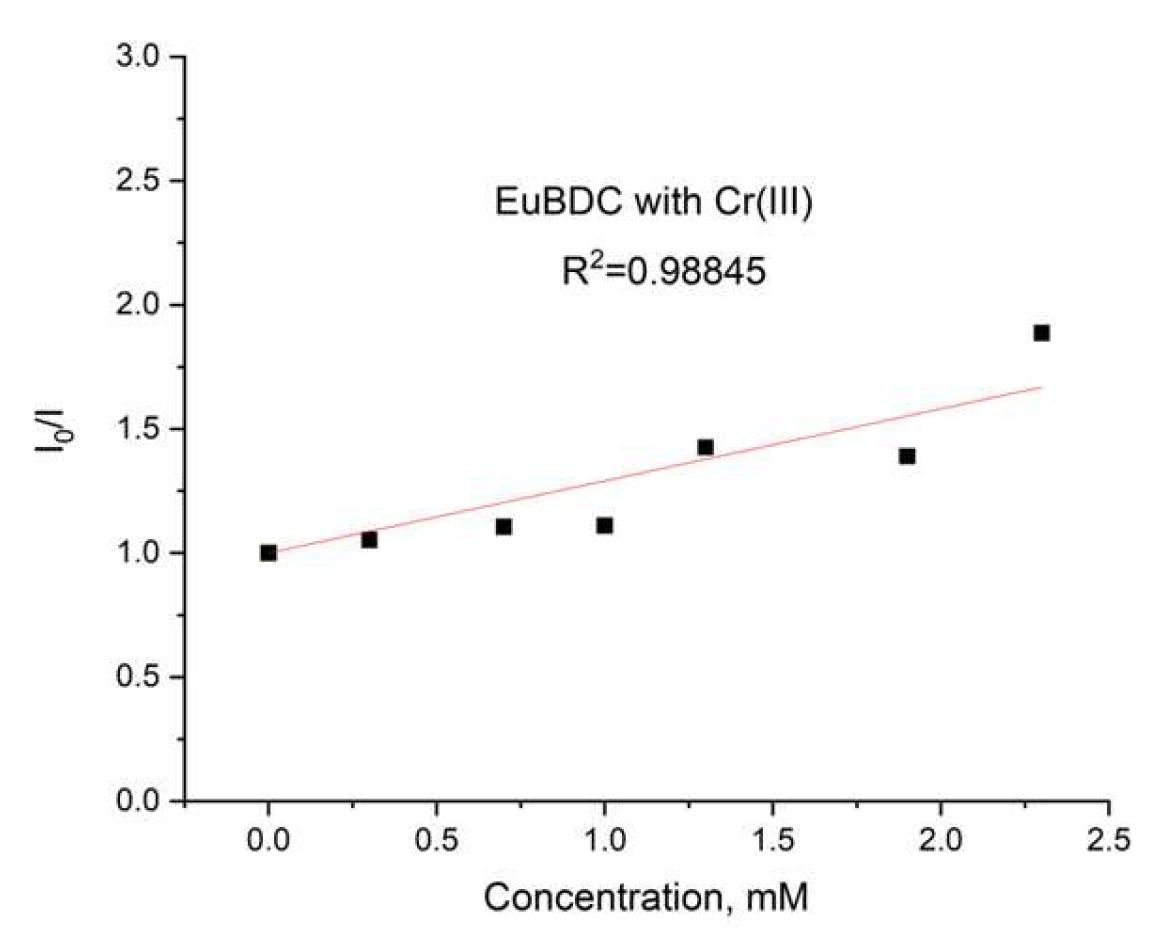
2.5.6. Chromium (III) Sensitivity of EuBDC
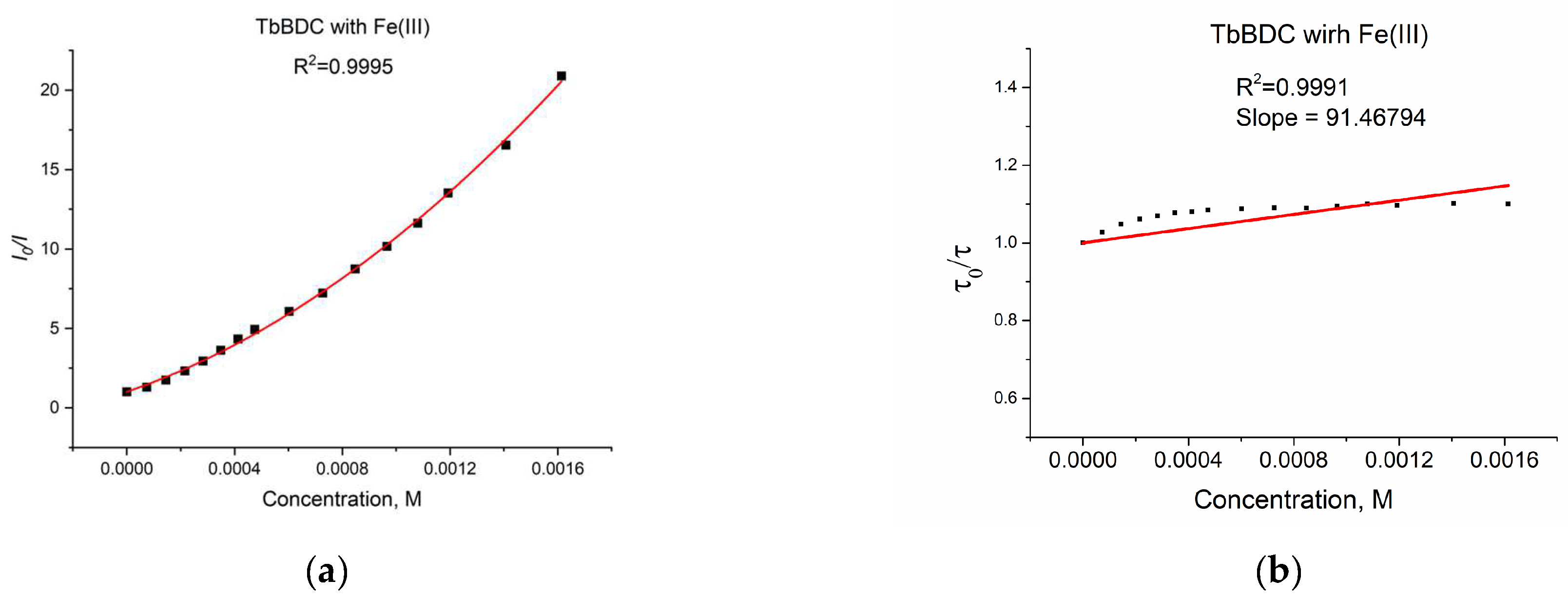
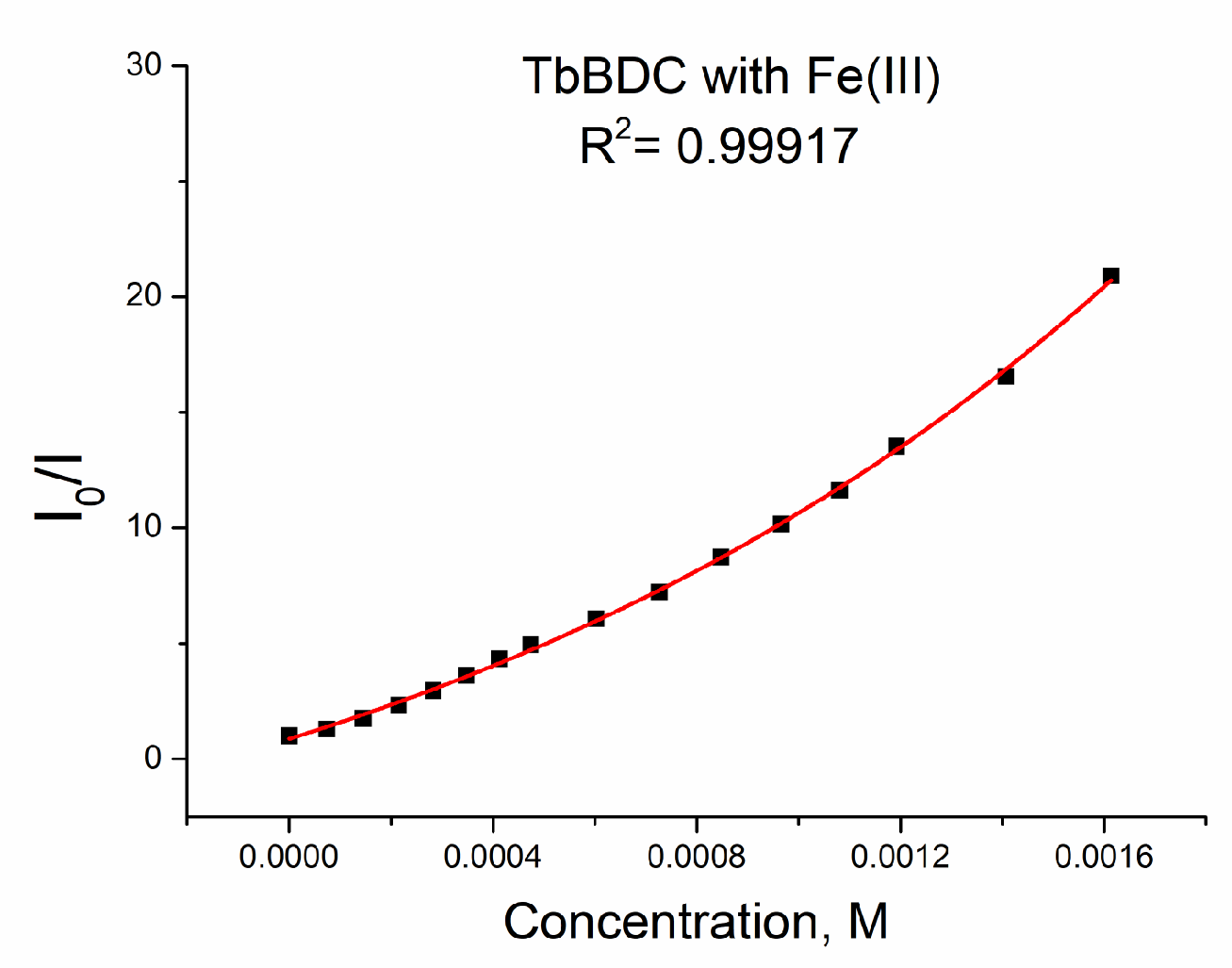
2.5.7. Iron (III) Sensitivity of TbBDC
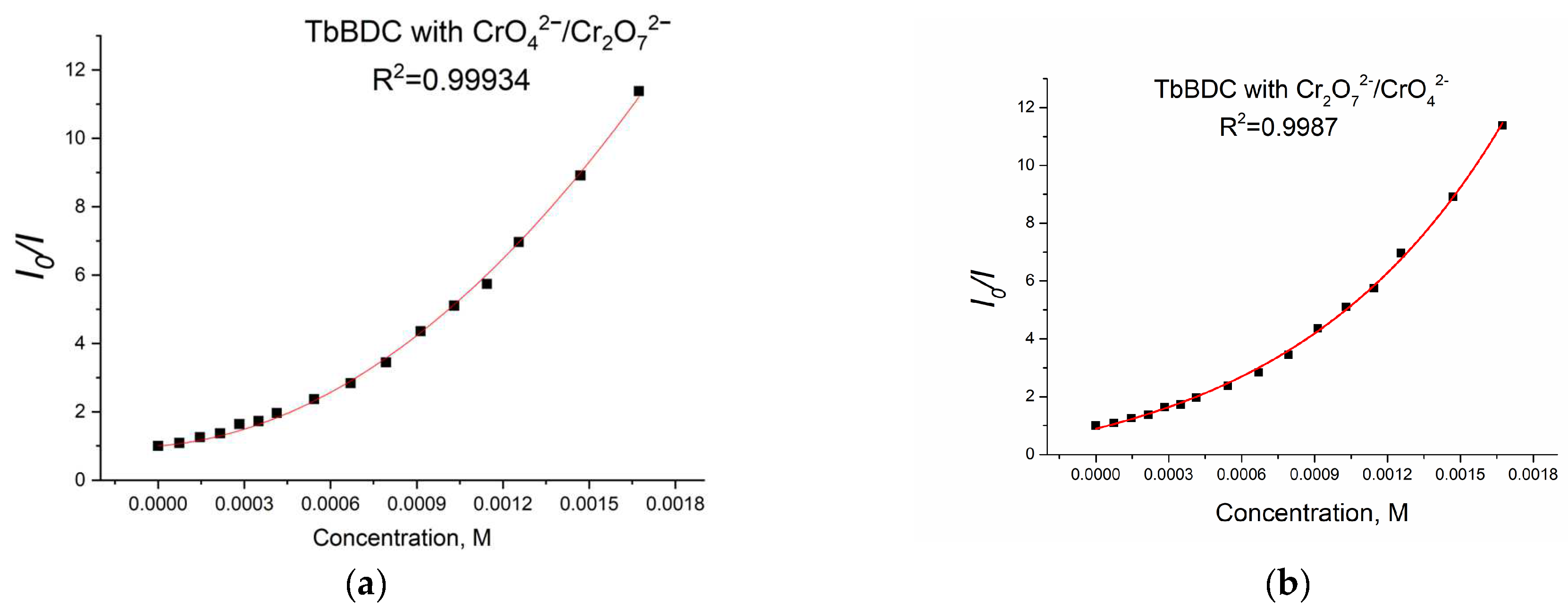
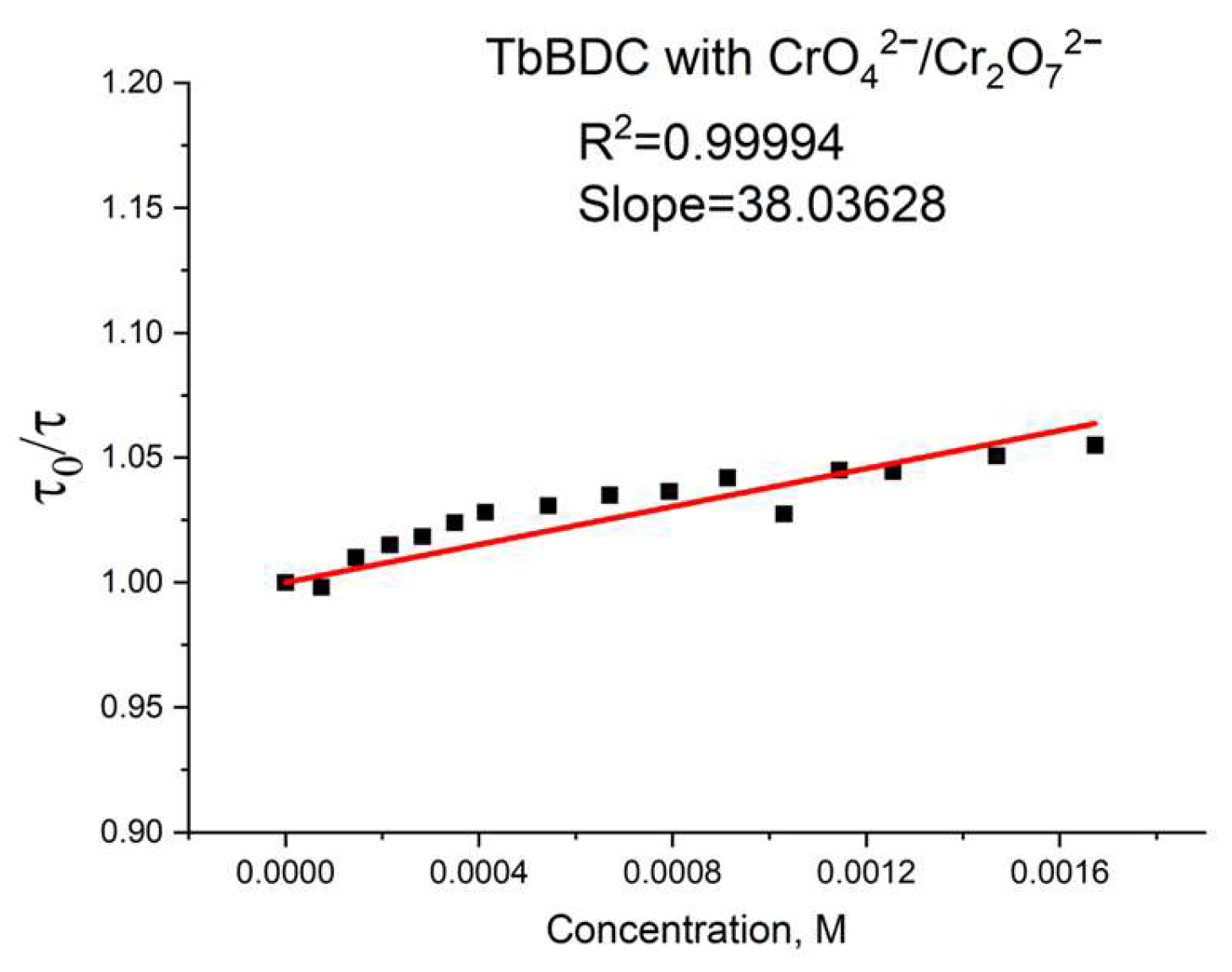
2.5.8. Chromium (VI) sensitivity of TbBDC
2.5.9. Possible Quenching Mechanisms
3. Materials and Methods
3.1. Materials Used
3.2. Synthesis of the Powder Samples SmBDC, EuBDC, TbBDC, and DyBDC
3.3. Single-Crystal Growth of DyBDC-SC
3.4. Characterization
3.5. Fluorescent Measurements in Suspension
3.5.1. Suspension Concentration
3.5.2. Procedure for pH Measurements of the Suspensions
3.5.3. Procedure for Cation and Anion Sensing Experiments
3.5.4. Procedure for Stern–Volmer Data Collections and Data Management
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helwig, N.E.; Hong, S.; Hsiao-wecksler, E.T. Rare Earth Metal-Organic Framework Hybrid Materials for Luminescence Responsive Chemical Sensors; Yan, B., Ed.; Woodhead Pulblishing: Sawson, UK, 2022; ISBN 9780323912365. [Google Scholar]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of Metal–Organic Frameworks and Coordination Polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Nann, T. Lanthanide Luminescence; Hänninen, P., Härmä, H., Eds.; Springer Series on Fluorescence; Springer: Berlin/Heidelberg, Germany, 2011; Volume 7, ISBN 978-3-642-21022-8. [Google Scholar]
- Le Natur, F.; Calvez, G.; Freslon, S.; Daiguebonne, C.; Bernot, K.; Guillou, O. Extending the Lanthanide-Terephthalate System: Isolation of an Unprecedented Tb(III)-Based Coordination Polymer with High Potential Porosity and Luminescence Properties. J. Mol. Struct. 2015, 1086, 34–42. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, L.; Gao, L.X.; Zhu, P.P.; Chen, Q.; Tan, K.J. A Highly Fluorescent Lanthanide Metal-Organic Framework as Dual-Mode Visual Sensor for Berberine Hydrochloride and Tetracycline. Anal. Bioanal. Chem. 2019, 411, 5963–5973. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, J.; Zheng, J.; Chen, X.; Shan, L.; Gao, L.; Wang, L.; Yu, M.; Fan, Y. Lanthanide Coordination Polymer Constructed from 2,2′-Bipyridyl-4,4′-Dicarboxylic Acid: Structure, Catalysis and Fluorescence. Inorganica Chim. Acta 2015, 437, 81–86. [Google Scholar] [CrossRef]
- Li, Z.; Liu, G.; Fan, C.; Pu, S. Ratiometric Fluorescence for Sensitive Detection of Phosphate Species Based on Mixed Lanthanide Metal Organic Framework. Anal. Bioanal. Chem. 2021, 413, 3281–3290. [Google Scholar] [CrossRef] [PubMed]
- Le Natur, F.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Bernot, K.; Ledoux, J.; Le Polleìs, L.; Roiland, C. Coordination Polymers Based on Heterohexanuclear Rare Earth Complexes: Toward Independent Luminescence Brightness and Color Tuning. Inorg. Chem. 2013, 52, 6720–6730. [Google Scholar] [CrossRef] [PubMed]
- Reineke, T.M.; Eddaoudi, M.; Fehr, M.; Kelley, D.; Yaghi, O.M. From Condensed Lanthanide Coordination Solids to Microporous Frameworks Having Accessible Metal Sites. J. Am. Chem. Soc. 1999, 121, 1651–1657. [Google Scholar] [CrossRef]
- Haquin, V.; Gumy, F.; Daiguebonne, C.; Bünzli, J.; Guillou, O. Structural and Near-IR Luminescent Properties of Erbium-Containing Coordination Polymers. Eur. J. Inorg. Chem. 2009, 2009, 4491–4497. [Google Scholar] [CrossRef]
- Haquin, V.; Etienne, M.; Daiguebonne, C.; Freslon, S.; Calvez, G.; Bernot, K.; Le Pollès, L.; Ashbrook, S.E.; Mitchell, M.R.; Bünzli, J.; et al. Color and Brightness Tuning in Heteronuclear Lanthanide Terephthalate Coordination Polymers. Eur. J. Inorg. Chem. 2013, 2013, 3464–3476. [Google Scholar] [CrossRef]
- Daiguebonne, C.; Kerbellec, N.; Guillou, O.; Bünzli, J.-C.; Gumy, F.; Catala, L.; Mallah, T.; Audebrand, N.; Gérault, Y.; Bernot, K.; et al. Structural and Luminescent Properties of Micro- and Nanosized Particles of Lanthanide Terephthalate Coordination Polymers. Inorg. Chem. 2008, 47, 3700–3708. [Google Scholar] [CrossRef]
- Kerbellec, N.; Kustaryono, D.; Haquin, V.; Etienne, M.; Daiguebonne, C.; Guillou, O. An Unprecedented Family of Lanthanide-Containing Coordination Polymers with Highly Tunable Emission Properties. Inorg. Chem. 2009, 48, 2837–2843. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Fu, W.; Zou, G. Urothermal Synthesis of an Unprecedented Pillar-Layered Metal–Organic Framework. J. Coord. Chem. 2012, 65, 4108–4114. [Google Scholar] [CrossRef]
- Zehnder, R.A.; Renn, R.A.; Pippin, E.; Zeller, M.; Wheeler, K.A.; Carr, J.A.; Fontaine, N.; McMullen, N.C. Network Dimensionality and Ligand Flexibility in Lanthanide Terephthalate Hydrates. J. Mol. Struct. 2011, 985, 109–119. [Google Scholar] [CrossRef]
- Cadiau, A.; Brites, C.D.S.; Costa, P.M.F.J.; Ferreira, R.A.S.; Rocha, J.; Carlos, L.D. Ratiometric Nanothermometer Based on an Emissive Ln3+-Organic Framework. ACS Nano 2013, 7, 7213–7218. [Google Scholar] [CrossRef] [PubMed]
- Serwy, I.B.; Wanderley, K.A.; Lucena, M.A.M.; Maldaner, A.O.; Talhavini, M.; Rodrigues, M.O.; Weber, I.T. [Ln2(BDC)3(H2O)4]n: A Low Cost Alternative for GSR Luminescent Marking. J. Lumin. 2018, 200, 24–29. [Google Scholar] [CrossRef]
- Alammar, T.; Hlova, I.Z.; Gupta, S.; Biswas, A.; Ma, T.; Zhou, L.; Balema, V.; Pecharsky, V.K.; Mudring, A.-V. Mechanochemical Synthesis, Luminescent and Magnetic Properties of Lanthanide Benzene-1,4-Dicarboxylate Coordination Polymers (Ln0.5Gd0.5)2(1,4-BDC)3(H2O)4; Ln = Sm, Eu, Tb. New J. Chem. 2020, 44, 1054–1062. [Google Scholar] [CrossRef]
- Elenkova, D.K.; Gagashev, D.A.; Encheva, E.D.; Tsvetkov, M.P. Effect of Different Lanthanide Ions on the Catalytic Activation of Peroxymonosulfate with Lanthanide Metal-Organic Frameworks (Ln-MOFs) with Terephthalic Acid. IOP Conf. Ser. Earth Environ. Sci. 2024, 1305, 012013. [Google Scholar] [CrossRef]
- Shi, L.; Li, N.; Wang, D.; Fan, M.; Zhang, S.; Gong, Z. Environmental Pollution Analysis Based on the Luminescent Metal Organic Frameworks: A Review. TrAC - Trends Anal. Chem. 2021, 134, 116131. [Google Scholar] [CrossRef]
- Yu, H.; Fan, M.; Liu, Q.; Su, Z.; Li, X.; Pan, Q.; Hu, X. Two Highly Water-Stable Imidazole-Based Ln-MOFs for Sensing Fe3+,Cr2O72−/CrO42− in a Water Environment. Inorg. Chem. 2020, 59, 2005–2010. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; Gai, Y.; Zhang, B.; Chen, L. Recent Progresses in Lanthanide Metal-Organic Frameworks (Ln-MOFs) as Chemical Sensors for Ions, Antibiotics and Amino Acids. Jiegou Huaxue 2022, 41, 2211045–2211070. [Google Scholar] [CrossRef]
- Metal-Organic Frameworks (MOFs) for Enviromental Applications; Ghosh, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128146330. [Google Scholar]
- Yang, D.; Lu, L.; Feng, S.; Zhu, M. First Ln-MOF as a Trifunctional Luminescent Probe for the Efficient Sensing of Aspartic Acid, Fe3+and DMSO. Dalt. Trans. 2020, 49, 7514–7524. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhao, D.; Zhang, H.; Wang, F.; Li, B.; Yang, L.; Deng, Y.; Zhang, X. A Hydrolytically Stable Amino-Functionalized Zinc(II) Metal-Organic Framework Containing Nanocages for Selective Gas Adsorption and Luminescent Sensing. Microporous Mesoporous Mater. 2021, 326, 111396. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, D.; Li, B.; Wang, F.; Deng, Y.; Peng, Y.; Wang, X.; Zhang, X. Luminescent Binuclear Zinc(II) Organic Framework as Bifunctional Water-Stable Chemosensor for Efficient Detection of Antibiotics and Cr(VI) Anions in Water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120232. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, W.; Li, W.; Liu, X.; Yang, J.; Lu, F.; Zhang, X.; Fan, L. Eu(III) Functionalized ZnMOF Based Efficient Dual-Emission Sensor Integrated with Self-Calibrating Logic Gate for Intelligent Detection of Epinephrine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 315, 124254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, S.; Nie, S.; Luo, J.; Lin, S.; Wang, Y.; Yang, H. Waste PET as a Reactant for Lanthanide MOF Synthesis and Application in Sensing of Picric Acid. Polymers 2019, 11, 2015. [Google Scholar] [CrossRef] [PubMed]
- Donghan, W.; Han, K.; Xinrui, W.; Wei, Z. Fluorescence Turn Off-on Continuous Response of Dual Lanthanide Metal Organic Frameworks for Selective Detecting Fluoroquinolone Antibiotics. J. Solid State Chem. 2024, 333, 124635. [Google Scholar] [CrossRef]
- Sheldrick, G.M. TWINABS Version 2012/01 ‘Empirical Correction for Absorption Anisotropy Applied to Twinned Crystals’; Universität Göttingen: Göttingen, Germany, 2012. [Google Scholar]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide Luminescence for Functional Materials and Bio-Sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Carnall, W.T.; Goodman, G.L.; Rajnak, K.; Rana, R.S. A Systematic Analysis of the Spectra of the Lanthanides Doped into Single Crystal LaF3. J. Chem. Phys. 1989, 90, 3443–3457. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Yin, C.; Huang, Q.; Zhu, G.; Liu, L.; Li, S.; Yang, X.; Wang, S. High-Performance Lanthanum-Based Metal–Organic Framework with Ligand Tuning of the Microstructures for Removal of Fluoride from Water. J. Colloid Interface Sci. 2022, 607, 1762–1775. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Kirillov, A.M.; Dou, W.; Xu, C.; Xu, C.; Yang, L.; Fang, R.; Liu, W. Multifunctional Ln-MOF Luminescent Probe for Efficient Sensing of Fe3+, Ce3+, and Acetone. ACS Appl. Mater. Interfaces 2018, 10, 23976–23986. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, S.; Dao, X.; Ni, Y. Fluorescent Zn-PDC/Tb3+ Coordination Polymer Nanostructure: A Candidate for Highly Selective Detections of Cefixime Antibiotic and Acetone in Aqueous System. Inorg. Chem. 2018, 57, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.-Y.; Yang, W.; Sun, Z.-M. Highly Selective Acetone Fluorescent Sensors Based on Microporous Cd(II) Metal–Organic Frameworks. J. Mater. Chem. 2012, 22, 23201. [Google Scholar] [CrossRef]
- Gao, L.; Jiao, C.; Chai, H.; Ren, Y.; Zhang, G.; Yu, H.; Tang, L. A Highly Sensitive Multifunctional Eu-MOF Sensor with Pentacarboxylate for Fluorescence Detecting Acetone, Cu2+ and Cr2O72−, and Electrochemical Detection of TNP. J. Solid State Chem. 2020, 284, 121199. [Google Scholar] [CrossRef]
- Vasile Scaeteanu, G.; Manole, M.S.; Bedivan, M.S.; Penescu, A. Evaluation of Water Quality in Lakes from Bucharest. Agric. Agric. Sci. Procedia 2012, 10, 328–339. [Google Scholar] [CrossRef]
- Manganese in Drinking-Water. In Background Document for Development of WHO Guidelines for Drinking-Water Guality; WHO/SDE/WSH/03.04/104/Rev/1; World Health Organization: Geneva, Switzerland, 2011.
- Silver in drinking-water. In Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO/SDE/WSH/03.04/14; World Health Organization: Geneva, Switzerland, 2003.
- Sun, D.; Cao, R.; Bi, W.; Weng, J.; Hong, M.; Liang, Y. Syntheses and Characterizations of a Series of Silver-Carboxylate Polymers. Inorganica Chim. Acta 2004, 357, 991–1001. [Google Scholar] [CrossRef]
- Chromium in drinking-water. In Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO/HEP/ECH/WSH/2020.3; World Health Organization: Geneva, Switzerland, 2020.
- Iron in drinking-water. In Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO/SDE/WSH/03.04/08; World Health Organization: Geneva, Switzerland, 2003.
- Chen, W.; Li, L.; Li, X.X.; Lin, L.D.; Wang, G.; Zhang, Z.; Li, L.; Yu, Y. Layered Rare Earth-Organic Framework as Highly Efficient Luminescent Matrix: The Crystal Structure, Optical Spectroscopy, Electronic Transition, and Luminescent Sensing Properties. Cryst. Growth Des. 2019, 19, 4754–4764. [Google Scholar] [CrossRef]
- Yin, J.-C.; Li, N.; Qian, B.-B.; Yu, M.-H.; Chang, Z.; Bu, X.-H. Highly Stable Zn-MOF with Lewis Basic Nitrogen Sites for Selective Sensing of Fe3+ and Cr2O72− Ions in Aqueous Systems. J. Coord. Chem. 2020, 73, 2718–2727. [Google Scholar] [CrossRef]
- Wu, K.-Y.; Qin, L.; Fan, C.; Cai, S.-L.; Zhang, T.-T.; Chen, W.-H.; Tang, X.-Y.; Chen, J.-X. Sequential and Recyclable Sensing of Fe3+ and Ascorbic Acid in Water with a Terbium(III)-Based Metal–Organic Framework. Dalt. Trans. 2019, 48, 8911–8919. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Lin, R.; Yao, Z.; Lin, Q.; Wang, L.; Zhang, Z.; Xiang, S. Two Water-Stable Lanthanide Metal–Organic Frameworks with Oxygen-Rich Channels for Fluorescence Sensing of Fe(III) Ions in Aqueous Solution. Dalt. Trans. 2018, 47, 16190–16196. [Google Scholar] [CrossRef]
- Wu, P.; Xia, L.; Huangfu, M.; Fu, F.; Wang, M.; Wen, B.; Yang, Z.; Wang, J. Lanthanide-Based Metal–Organic Frameworks Containing “V-Shaped” Tetracarboxylate Ligands: Synthesis, Crystal Structures, “Naked-Eye” Luminescent Detection, and Catalytic Properties. Inorg. Chem. 2020, 59, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, P.; Liu, J.; Chen, X.; Guo, X.; Jin, H.; Chai, J.; Wang, L.; Fan, Y. Multi-Responsive Luminescent Sensor Based on Three Dimensional Lanthanide Metal–Organic Framework. New J. Chem. 2018, 42, 19485–19493. [Google Scholar] [CrossRef]
- Xue, Y.-S.; Ding, J.; Sun, D.-L.; Cheng, W.-W.; Chen, X.-R.; Huang, X.-C.; Wang, J. 3D Ln-MOFs as Multi-Responsive Luminescent Probes for Efficient Sensing of Fe3+, Cr2O72−, and Antibiotics in Aqueous Solution. CrystEngComm 2021, 23, 3838–3848. [Google Scholar] [CrossRef]
- Jin, J.; Xue, J.; Liu, Y.; Yang, G.; Wang, Y.-Y. Recent Progresses in Luminescent Metal–Organic Frameworks (LMOFs) as Sensors for the Detection of Anions and Cations in Aqueous Solution. Dalt. Trans. 2021, 50, 1950–1972. [Google Scholar] [CrossRef]
- Wang, S.; Sun, B.; Su, Z.; Hong, G.; Li, X.; Liu, Y.; Pan, Q.; Sun, J. Lanthanide-MOFs as Multifunctional Luminescent Sensors. Inorg. Chem. Front. 2022, 9, 3259–3266. [Google Scholar] [CrossRef]
- Dong, J.P.; Li, B.; Jin, Y.J.; Wang, L.Y. Efficient Detection of Fe(III) and Chromate Ions in Water Using Two Robust Lanthanide Metal-Organic Frameworks. CrystEngComm. 2021, 23, 1677–1683. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Guan, Q.L.; Liu, C.H.; Li, B.; Zhang, K.Y.; Li, G.H.; Xing, Y.H.; Bai, F.Y.; Sun, L.X. Sensing of Fe3+ and Cr2O72− in Water and White Light: Synthesis, Characterization, and Fluorescence Properties of a Crystalline Bismuth-1,3,5-Benzenetricarboxylic Acid Framework. Cryst. Growth Des. 2019, 19, 7217–7229. [Google Scholar] [CrossRef]
- Tunnicliff, D.D. Solvents for Ultraviolet Spectrophotometry. Talanta 1959, 2, 341–347. [Google Scholar] [CrossRef]
- Mahata, P.; Mondal, S.K.; Singha, D.K.; Majee, P. Luminescent Rare-Earth-Based MOFs as Optical Sensors. Dalt. Trans. 2017, 46, 301–328. [Google Scholar] [CrossRef]
- Téllez S, C.A.; Hollauer, E.; Mondragon, M.; Castaño, V.M. Fourier Transform Infrared and Raman Spectra, Vibrational Assignment and Ab Initio Calculations of Terephthalic Acid and Related Compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 993–1007. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The Genesis of a Modern Open-Source All Purpose Crystallography Software Package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Laws, W.R.; Contino, P.B. [21] Fluorescence Quenching Studies: Analysis of Nonlinear Stern-Volmer Data. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1992; Volume 210, pp. 448–463. [Google Scholar]
- Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- de la Torre, A.; Medina-Rodríguez, S.; Segura, J.C.; Fernández-Sánchez, J.F. A Polynomial-Exponent Model for Calibrating the Frequency Response of Photoluminescence-Based Sensors. Sensors 2020, 20, 4635. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, A.S.; Parui, R.; Garai, R.; Chanu, M.A.; Iyer, P.K. Dual “Static and Dynamic” Fluorescence Quenching Mechanisms Based Detection of TNT via a Cationic Conjugated Polymer. ACS Meas. Sci. Au 2022, 2, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Lu, R.; Tang, S.; Liu, X. Highly Cross-Linked Fluorescent Poly(Cyclotriphosphazene-Co-Curcumin) Microspheres for the Selective Detection of Picric Acid in Solution Phase. J. Mater. Chem. A 2015, 3, 4604–4611. [Google Scholar] [CrossRef]
- Chakraborty, D.; Bej, S.; Sahoo, S.; Chongdar, S.; Ghosh, A.; Banerjee, P.; Bhaumik, A. Novel Nanoporous Ti-Phosphonate Metal–Organic Framework for Selective Sensing of 2,4,6-Trinitrophenol and a Promising Electrode in an Energy Storage Device. ACS Sustain. Chem. Eng. 2021, 9, 14224–14237. [Google Scholar] [CrossRef]
- Han, Z.; Wang, K.; Guo, Y.; Chen, W.; Zhang, J.; Zhang, X.; Siligardi, G.; Yang, S.; Zhou, Z.; Sun, P.; et al. Cation-Induced Chirality in a Bifunctional Metal-Organic Framework for Quantitative Enantioselective Recognition. Nat. Commun. 2019, 10, 5117. [Google Scholar] [CrossRef]
- Wu, S.; Lin, Y.; Liu, J.; Shi, W.; Yang, G.; Cheng, P. Rapid Detection of the Biomarkers for Carcinoid Tumors by a Water Stable Luminescent Lanthanide Metal–Organic Framework Sensor. Adv. Funct. Mater. 2018, 28, 1707169. [Google Scholar] [CrossRef]
- Sun, X.; He, J.; Meng, Y.; Zhang, L.; Zhang, S.; Ma, X.; Dey, S.; Zhao, J.; Lei, Y. Microwave-Assisted Ultrafast and Facile Synthesis of Fluorescent Carbon Nanoparticles from a Single Precursor: Preparation, Characterization and Their Application for the Highly Selective Detection of Explosive Picric Acid. J. Mater. Chem. A 2016, 4, 4161–4171. [Google Scholar] [CrossRef]




| Sample | Lifetime in the Solid State, µs | Lifetime in a Water Suspension, µs |
|---|---|---|
| SmBDC | UDL 1 | UDL 1 |
| EuBDC | 390 ± 3 | 389 ± 3 |
| TbBDC | 901 ± 1 | 885 ± 1 |
| DyBDC | UDL1 | UDL1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elenkova, D.; Dimitrova, Y.; Tsvetkov, M.; Morgenstern, B.; Milanova, M.; Todorovsky, D.; Zaharieva, J. Investigation of the Sensing Properties of Lanthanoid Metal–Organic Frameworks (Ln-MOFs) with Terephthalic Acid. Molecules 2024, 29, 3713. https://doi.org/10.3390/molecules29153713
Elenkova D, Dimitrova Y, Tsvetkov M, Morgenstern B, Milanova M, Todorovsky D, Zaharieva J. Investigation of the Sensing Properties of Lanthanoid Metal–Organic Frameworks (Ln-MOFs) with Terephthalic Acid. Molecules. 2024; 29(15):3713. https://doi.org/10.3390/molecules29153713
Chicago/Turabian StyleElenkova, Denitsa, Yana Dimitrova, Martin Tsvetkov, Bernd Morgenstern, Maria Milanova, Dimitar Todorovsky, and Joana Zaharieva. 2024. "Investigation of the Sensing Properties of Lanthanoid Metal–Organic Frameworks (Ln-MOFs) with Terephthalic Acid" Molecules 29, no. 15: 3713. https://doi.org/10.3390/molecules29153713
APA StyleElenkova, D., Dimitrova, Y., Tsvetkov, M., Morgenstern, B., Milanova, M., Todorovsky, D., & Zaharieva, J. (2024). Investigation of the Sensing Properties of Lanthanoid Metal–Organic Frameworks (Ln-MOFs) with Terephthalic Acid. Molecules, 29(15), 3713. https://doi.org/10.3390/molecules29153713











