Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles
Abstract
1. Introduction
2. Drug Solubility and Biological Variability
2.1. Impact of Drug Solubility on Absorption and Therapeutic Outcomes
2.2. Linking Physiological pH Conditions to Drug Solubility and GI Absorption
2.3. Exploring Case Studies: Variability in Drug Efficacy Due to Solubility Challenges
3. Nanotechnology in Drug Design
3.1. Harnessing Nanotechnology: Enhancing Drug Solubility and Delivery
3.2. Engineering MNPs for Improved Solubility and Targeted Delivery in the GI Tract
3.2.1. Enhancing Drug Solubility Using MNPs
3.2.2. Targeted Delivery within the GI Tract
3.2.3. Engineering Considerations for MNPs in Drug Delivery

3.3. Breakthroughs in Drug Formulations: Success Cases with MNPs
3.3.1. Advances in MNPs Synthesis and Functionalization
3.3.2. MNPs in Cancer Therapy
3.3.3. MNPs in Neurological Disorders
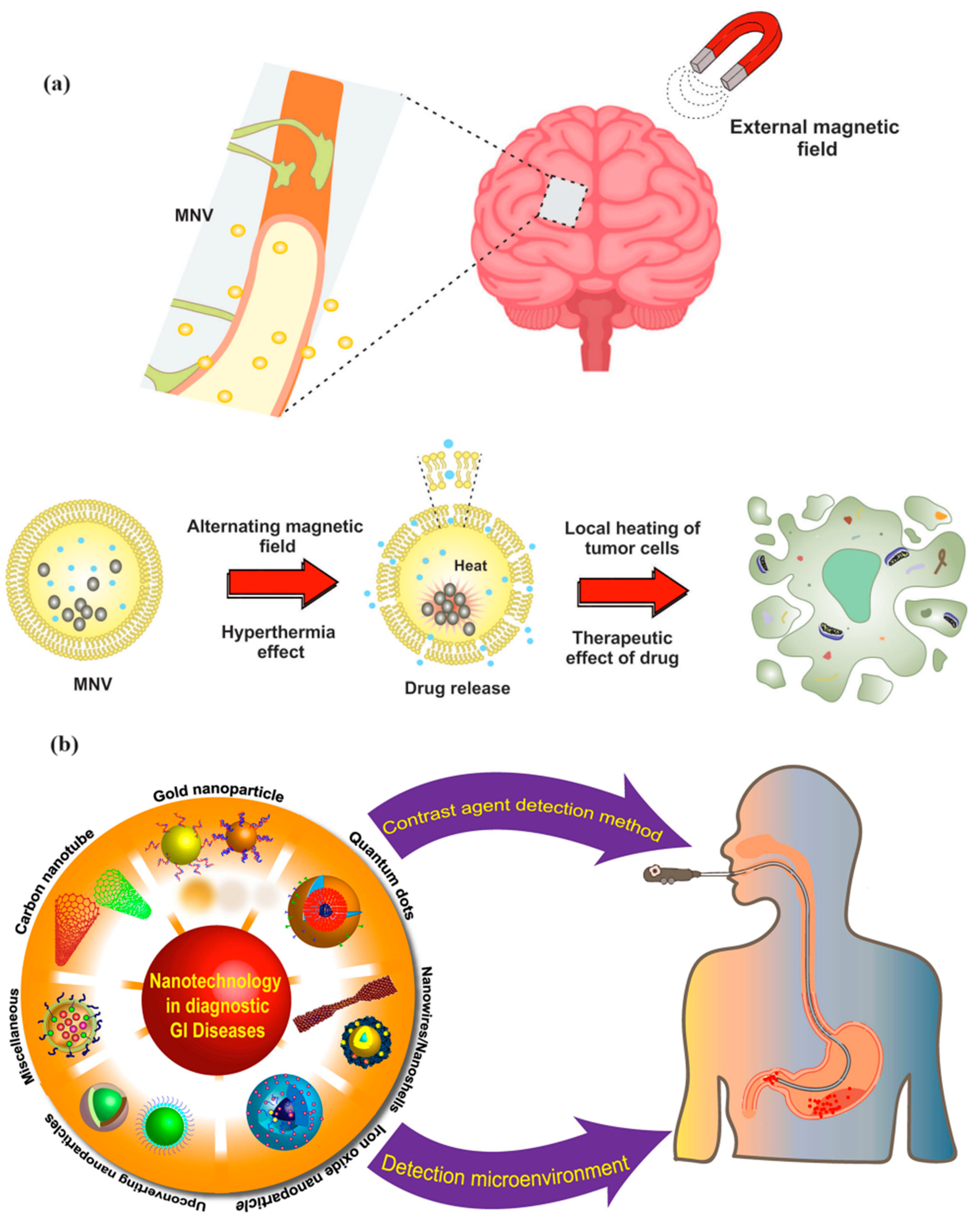
3.3.4. MNPs in GI Disorders
3.3.5. MNPs in Cardiovascular Disorders
4. Challenges and Future Directions
4.1. Overcoming Hurdles in Integrating Nanotechnology and MNPs into Drug Design
4.1.1. Synthesis and Functionalization Challenges
4.1.2. Biocompatibility and Safety Concerns
4.1.3. Regulatory and Manufacturing Challenges
4.1.4. Overcoming Biological Barriers
4.2. Reducing Biological Variability in Drug Response through Advanced Nanotechnologies
4.2.1. Precision Drug Delivery Systems
4.2.2. Genetic Influence on Drug Response
4.2.3. Computational Models and Nanotechnology Integration
4.2.4. Personalized Nanomedicine
4.3. Charting the Future: Interdisciplinary Research and Innovation in Nanotechnology for Therapeutics
4.3.1. The Role of Interdisciplinary Collaboration
4.3.2. Innovation in Nanotechnology for Cancer Therapeutics
4.3.3. Expanding the Scope of Nanotechnology in Therapeutics
4.3.4. The Economic and Societal Impact of Nanotechnology
4.3.5. Future Directions in Interdisciplinary Research
5. Conclusions
5.1. Nanotechnology and MNPs: Addressing Drug Solubility Amidst Biological Variability
5.1.1. Enhancing Drug Solubility with MNPs
5.1.2. Addressing Biological Variability
5.2. The Imperative for Ongoing Innovation and Research to Develop Universal Therapeutic Solutions
5.2.1. Advancing Universal Therapeutic Solutions
5.2.2. Addressing Complex Health Challenges
5.2.3. Innovation Ecosystems and Collaborative Research
5.2.4. The Future of Nanotechnology in Therapeutics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Serajuddin, A.T.M. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Ramos Campos, E.V.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Shin, H.-S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Naz, S.; Shamoon, M.; Wang, R.; Zhang, L.; Zhou, J.; Chen, J. Advances in Therapeutic Implications of Inorganic Drug Delivery Nano-Platforms for Cancer. Int. J. Mol. Sci. 2019, 20, 965. [Google Scholar] [CrossRef]
- Ahmad Panahi, H.; Nourbakhsh, S.; Siami, F. Synthesis of functionalized magnetic nanoparticles as a nanocarrier for targeted drug delivery. Adv. Polym. Technol. 2018, 37, 3659–3664. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Savadkouhi, N.; Ebrahimzadeh, M. Drug Design Strategies That Aim to Improve the Low Solubility and Poor Bioavailability Conundrum in Quercetin Derivatives. Expert Opin. Drug Discov. 2023, 18, 1117–1132. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, D.; Wang, H.; Jiao, L.; Zhao, X.; Song, J.; Yang, D.; Yang, S.; Du, G.; Lu, Y. Cocrystal of Apixaban–Quercetin: Improving Solubility and Bioavailability of Drug Combination of Two Poorly Soluble Drugs. Molecules 2021, 26, 2677. [Google Scholar] [CrossRef]
- Murakami, T.; Bodor, E.T.; Bodor, N. Factors and Dosage Formulations Affecting the Solubility and Bioavailability of P-glycoprotein Substrate Drugs. Expert Opin. Drug Metab. Toxicol. 2021, 17, 555–580. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.; Paiva-Santos, A.C.; Veiga, F. Nano and Microemulsions for the Treatment of Depressive and Anxiety Disorders: An Efficient Approach to Improve Solubility, Brain Bioavailability and Therapeutic Efficacy. Pharmaceutics. 2022, 14, 2825. [Google Scholar] [CrossRef] [PubMed]
- Dressman, J.B.; Reppas, C. In vitro–in vivo correlations for lipophilic, poorly water-soluble drugs. Eur. J. Pharm. Sci. 2000, 11 (Suppl. S2), S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Varum, F.J.O.; Veiga, F.; Sousa, J.S.; Basit, A.W. Mucus thickness in the gastrointestinal tract of laboratory animals. J. Pharm. Pharmacol. 2012, 64, 218–227. [Google Scholar] [CrossRef]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Lennernäs, H.; Brisander, M.; Liljebris, C.; Jesson, G.; Andersson, P. Enhanced Bioavailability and Reduced Variability of Dasatinib and Sorafenib with a Novel Amorphous Solid Dispersion Technology Platform. Clin. Pharmacol. Drug Dev. 2024, 13, 985–999. [Google Scholar] [CrossRef]
- Ghurghure, S.; Ingale, P.L.; Dhange, A.A.; Javalgikar, A.S.; Kore, R.; Birajdar, D.M. Itraconazole Self-Nano Emulsifying Drug Delivery System for Enhancement of Solubility. J. Assoc. Zool. 2023, 44, 150–155. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, S.; Zuo, J.; Sun, J.; Wang, J. Reduced the Food Effect and Enhanced the Oral Bioavailability of Ivacaftor by Self-Nanoemulsifying Drug Delivery System (SNEDDS) Using a New Oil Phase. Drug Des. Dev. Ther. 2022, 16, 1531–1546. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Benet, L.Z.; Hebert, M.F.; Gupta, S.K.; Rowland, M.; Gomez, D.Y.; Wacher, V.J. Differentiation of absorption and first-pass gut and hepatic metabolism in humans: Studies with cyclosporine. Clin. Pharmacol. Ther. 1995, 58, 412–417. [Google Scholar] [CrossRef]
- Sura, R.S.; Subrahmanyam, C.V.S.; Rachamalla, S.S. Development and evaluation of self micro emulsifying drug delivery system (SMEDDS) for nebivolol hydrochloride. Int. J. Life Sci. Pharma Res. 2021, 11, 83–97. [Google Scholar] [CrossRef]
- Pu, L. Polymer Functionalized Nanostructures for Antibacterial Application. Doctoral Thesis, Nanyang Technological University, Singapore, 2017. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef]
- Bourang, S.; Asadian, S.; Noruzpour, M.; Mansuryar, A.; Azizi, S.; Ebrahimi, H.A.; Hooshyar, V.A. PLA-HA/Fe3O4 magnetic nanoparticles loaded with curcumin: Physicochemical characterization and toxicity evaluation in HCT116 colorectal cancer cells. SN Appl. Sci. 2024, 6, 186. [Google Scholar] [CrossRef]
- Asgari, S.; Farasati Far, B.; Charmi, G.; Haji Maghsoudi, P.; Keihankhadiv, S.; Seyedhamzeh, M.; Kaushik, A. Chitosan-grafted-poly(N-vinylcaprolactam)-decorated Fe3O4@SiO2 core-shell nano formulation as an efficient drug delivery system for poorly soluble drugs. ACS Appl. Bio Mater. 2023, 6, 5809–5827. [Google Scholar] [CrossRef]
- Ahmadi, F.; Saeedi, M.; Akbari, J.; Seyedabadi, M.; Ebrahimnejad, P.; Morteza-Semnani, K.; Ghasemi, S.; Moalem-Banhangi, M.; Babaei, A.; Hashemi, S.M.H.; et al. Nanohybrid based on (Mn, Zn) ferrite nanoparticles functionalized with chitosan and sodium alginate for loading of curcumin against human breast cancer cells. AAPS PharmSciTech 2023, 24, 222. [Google Scholar] [CrossRef]
- Ansari, S.R.; Hempel, N.-J.; Asad, S.; Svedlindh, P.; Bergström, C.A.S.; Löbmann, K.; Teleki, A. Hyperthermia-induced in situ drug amorphization by superparamagnetic nanoparticles in oral dosage forms. ACS Appl. Mater. Interfaces 2022, 14, 21978–21988. [Google Scholar] [CrossRef]
- Aghaei, A.; Sadiqi, H.; Mohammad, A.A.K.; Gulmohammad, A.W.; Likozar, B.; Nosrati, H.; Danafar, H.; Shaterian, M. Magnetic ferrite nanoparticles coated with bovine serum albumin and glycine polymers for controlled release of curcumin as a model. J. Biomater. Sci. Polym. Ed. 2023, 34, 2537–2550. [Google Scholar] [CrossRef]
- Idris, A.H.; Abdullah, C.A.C.; Yusof, N.A.; Asmawi, A.A.; Rahman, M.B.A. Nanostructured lipid carrier co-loaded with docetaxel and magnetic nanoparticles: Physicochemical characterization and in vitro evaluation. Pharmaceutics 2023, 15, 1319. [Google Scholar] [CrossRef]
- Mathes, N.; Comas, M.; Bleul, R.; Everaert, K.; Hermle, T.; Wiekhorst, F.; Knittel, P.; Sperling, R.A.; Vidal, X. Nitrogen-vacancy center magnetic imaging of Fe3O4 nanoparticles inside the gastrointestinal tract of Drosophila melanogaster. Nanoscale Adv. 2023, 6, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zheng, L.; He, J.; Lin, J.; Chen, Y.; Yu, H.; Wang, Y.; Zhong, W.; Handschuh-Wang, S.; Niu, S.; et al. Liquid-metal soft electronics coupled with multi-legged robots for targeted delivery in the gastrointestinal tract. Device 2024, 2, 100181. [Google Scholar] [CrossRef]
- Farjadian, F.; Faghih, Z.; Fakhimi, M.; Iranpour, P.; Mohammadi-Samani, S.; Doroudian, M. Glucosamine-modified mesoporous silica-coated magnetic nanoparticles: A “Raisin-Cake”-like structure as an efficient theranostic platform for targeted methotrexate delivery. Pharmaceutics 2023, 15, 2491. [Google Scholar] [CrossRef] [PubMed]
- Hasani, M.; Jafari, S.; Akbari Javar, H.; Abdollahi, H.; Rashidzadeh, H. Cell-penetrating peptidic GRP78 ligand-conjugated iron oxide magnetic nanoparticles for tumor-targeted doxorubicin delivery and imaging. ACS Appl. Bio Mater. 2023, 6, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, G.; Jafari-Gharabaghlou, D.; Farhoudi-Sefidan-Jadid, M.; Alizadeh, E.; Fathi, M.; Zarghami, N. Targeted delivery of silibinin via magnetic niosomal nanoparticles: Potential application in treatment of colon cancer cells. Front. Pharmacol. 2023, 14, 1174120. [Google Scholar] [CrossRef]
- Mirzaghavami, P.S.; Khoei, S.; Khoee, S.; Shirvalilou, S. Folic acid-conjugated magnetic triblock copolymer nanoparticles for dual targeted delivery of 5-fluorouracil to colon cancer cells. Cancer Nanotechnol. 2022, 13, 12. [Google Scholar] [CrossRef]
- Zhou, H.; Alici, G. A magnetically actuated novel robotic capsule for site-specific drug delivery inside the gastrointestinal tract. IEEE Trans. Syst. Man Cybern. Syst. 2022, 52, 4010–4020. [Google Scholar] [CrossRef]
- Xie, M.; Meng, F.; Wang, P.; Díaz-García, A.M.; Parkhats, M.; Santos-Oliveira, R.; Asim, M.; Bostan, N.; Gu, H.; Yang, L.; et al. Surface engineering of magnetic iron oxide nanoparticles for breast cancer diagnostics and drug delivery. Int. J. Nanomed. 2024, 19, 8437–8461. [Google Scholar] [CrossRef]
- Kjeldsen, R.B.; Ghavami, M.; Thamdrup, L.H.; Boisen, A. Magnetic and/or radiopaque functionalization of self-unfolding foils for improved applicability within oral drug delivery. ACS Biomater. Sci. Eng. 2023, 9, 6773–6782. [Google Scholar] [CrossRef]
- Lodi, M.B.; Corda, E.M.A.; Desogus, F.; Fanti, A.; Mazzarella, G. Modeling of magnetic scaffolds as drug delivery platforms for tissue engineering and cancer therapy. Bioengineering 2024, 11, 573. [Google Scholar] [CrossRef]
- Hussain, A.; Dar, M.N.R.; Cheema, W.K.; Kanwal, R.; Han, Y. Investigating hybrid nanoparticles for drug delivery in multi-stenosed catheterized arteries under magnetic field effects. Sci. Rep. 2024, 14, 1170. [Google Scholar] [CrossRef] [PubMed]
- Slavu, L.M.; Antonelli, A.; Scarpa, E.; Abdalla, P.; Wilhelm, C.; Silvestri, N.; Pellegrino, T.; Scheffler, K.; Magnani, M.; Rinaldi, R.; et al. Optimization of magnetic nanoparticles for engineering erythrocytes as theranostic agents. Biomater. Sci. 2023, 11, 3252–3268. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, K.; Gangadharan, A.; Martirosyan, K.S.; Sahu, N.K.; Manogaran, P.; Kreedapathy, G.E. Fabrication of multifunctional drug loaded magnetic phase supported calcium phosphate nanoparticle for local hyperthermia combined drug delivery and antibacterial activity. ACS Appl. Bio Mater. 2022, 6, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Alishiri, M.; Ebrahimi, S.; Shamloo, A.; Boroumand, A.; Mofrad, M.R.K. Drug delivery and adhesion of magnetic nanoparticles coated nanoliposomes and microbubbles to atherosclerotic plaques under magnetic and ultrasound fields. Eng. Appl. Comput. Fluid Mech. 2021, 15, 1703–1725. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef]
- Hosseini, V.; Ghoreishi Mokri, S.M.; Viktorovna, K.A. Targeted drug delivery through the synthesis of magnetite nanoparticle by co-precipitation method and creating a silica coating on it. Int. J. Innov. Sci. Res. Technol. 2024, 9, 1–7. [Google Scholar] [CrossRef]
- Mehraji, S.; DeVoe, D. Microfluidic synthesis of lipid-based nanoparticles for drug delivery: Recent advances and opportunities. Lab Chip 2024, 24, 1154–1174. [Google Scholar] [CrossRef]
- Comanescu, C. Recent advances in surface functionalization of magnetic nanoparticles. Coatings 2023, 13, 1772. [Google Scholar] [CrossRef]
- Iranshahy, M.; Hanafi-Bojd, M.Y.; Aghili, S.H.; Iranshahi, M.; Nabavi, S.; Saberi, S.; Filosa, R.; Nezhad, I.F.; Hasanpour, M. Curcumin-loaded mesoporous silica nanoparticles for drug delivery: Synthesis, biological assays and therapeutic potential—A review. RSC Adv. 2023, 13, 22250–22267. [Google Scholar] [CrossRef]
- Nori, Z.; Bahadori, M.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Mohammadpoor-Baltork, I.; Jafari, S.; Emamzadeh, R.; Alem, H. Synthesis and characterization of a new gold-coated magnetic nanoparticle decorated with a thiol-containing dendrimer for targeted drug delivery, hyperthermia treatment and enhancement of MRI contrast agent. J. Drug Deliv. Sci. Technol. 2023, 81, 104216. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.-P.; Gómez-Pastora, J.; et al. Magnetic nanoparticles: A review on synthesis, characterization, functionalization, and biomedical applications. Small 2024, 20, 2304848. [Google Scholar] [CrossRef]
- Ko, M.J.; Min, S.; Hong, H.; Yoo, W.; Joo, J.; Zhang, Y.S.; Kang, H.; Kim, D.-H. Magnetic nanoparticles for ferroptosis cancer therapy with diagnostic imaging. Bioact. Mater. 2023, 32, 66–97. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, C.; Bohidar, H. Visible laser light mediated cancer therapy via photothermal effect of tannin-stabilized magnetic iron oxide nanoparticles. Nanomaterials 2023, 13, 1456. [Google Scholar] [CrossRef]
- Kovrigina, E.; Poletaeva, Y.; Zheng, Y.-M.; Chubarov, A.; Dmitrienko, E. Nylon-6-coated doxorubicin-loaded magnetic nanoparticles and nanocapsules for cancer treatment. Magnetochemistry 2023, 9, 106. [Google Scholar] [CrossRef]
- Nicolescu, C.; Schilb, A.L.; Kim, J.; Sun, D.; Hall, R.C.; Gao, S.; Gilmore, H.; Schiemann, W.; Lu, Z.-R. Evaluating dual-targeted ECO/siRNA nanoparticles against an oncogenic lncRNA for triple negative breast cancer therapy with magnetic resonance molecular imaging. ACS Chem. Biol. Mol. Imaging 2023, 1, 461–470. [Google Scholar] [CrossRef]
- Oliveira, R.R.; Cintra, E.R.; Sousa-Junior, A.; Moreira, L.; da Silva, A.C.D.; de Souza, A.L.R.; Valadares, M.; Carrião, M.; Bakuzis, A.; Lima, E. Paclitaxel-loaded lipid-coated magnetic nanoparticles for dual chemo-magnetic hyperthermia therapy of melanoma. Pharmaceutics 2023, 15, 818. [Google Scholar] [CrossRef]
- Brero, F.; Calzolari, P.; Albino, M.; Antoccia, A.; Arosio, P.; Berardinelli, F.; Bettega, D.; Ciocca, M.; Faccoetti, A.; Gallo, S.; et al. Proton therapy, magnetic nanoparticles and hyperthermia as combined treatment for pancreatic BxPC3 tumor cells. Nanomaterials 2023, 13, 791. [Google Scholar] [CrossRef]
- Sisubalan, N.; Shalini, R.; Ramya, S.; Sivamaruthi, B.; Chaiyasut, C. Recent advances in nanomaterials for neural applications: Opportunities and challenges. Nanomedicine 2023, 18, 1979–1994. [Google Scholar] [CrossRef]
- Salatin, S.; Farhoudi, M.; Sadigh-Eteghad, S.; Mahmoudi, J. Magnetic hybrid nanovesicles for the precise diagnosis and treatment of central nervous system disorders. Expert Opin. Drug Deliv. 2024, 21, 521–535. [Google Scholar] [CrossRef]
- Hescham, S.; Chiang, P.-H.; Gregurec, D.; Moon, J.; Christiansen, M.G.; Jahanshahi, A.; Liu, H.; Rosenfeld, D.; Pralle, A.; Anikeeva, P.; et al. Magnetothermal nanoparticle technology alleviates parkinsonian-like symptoms in mice. Nat. Commun. 2021, 12, 5569. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Helsper, S.; Marzano, M.; Chen, X.; Muok, L.; Esmonde, C.; Zeng, C.; Sun, L.; Grant, S.; Li, Y. Human forebrain organoid-derived extracellular vesicle labeling with iron oxides for in vitro magnetic resonance imaging. Biomedicines 2022, 10, 3060. [Google Scholar] [CrossRef] [PubMed]
- Yue, N.; Xu, H.; Xu, J.; Zhu, M.; Zhang, Y.; Tian, C.; Nie, Y.; Yao, J.; Liang, Y.; Wang, L. Application of nanoparticles in the diagnosis of gastrointestinal diseases: A complete future perspective. Int. J. Nanomed. 2023, 18, 4143–4170. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, F.; Busato, L.; Valenti, A.; Cardaccio, S.; Longhi, A.; Catalano, C. Magnetic resonance imaging of the gastrointestinal tract: Current role, recent advancements and future prospectives. Diagnostics 2023, 13, 2410. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Zhang, S.-Y.; Zangeneh, M.; Hemmati, S.; Zangeneh, A.; Saremi, S.G.; Kamangar, S.A.; Pirhayati, M. Synthesis of Fe3O4 nanoparticles encapsulated with orange pectin for the treatment of gastrointestinal cancers. Mater. Express 2022, 12, 1455–1464. [Google Scholar] [CrossRef]
- Giannousi, K.; Koutroumpis, E.; Georgiadou, V.; Karagkounis, V.; Dendrinou-Samara, C. Nanoplatforms of Manganese Ferrite Nanoparticles Functionalized with Anti-Inflammatory Drugs. Eur. J. Inorg. Chem. 2019, 2019, 1701–1710. [Google Scholar] [CrossRef]
- Rahman, M. Magnetic Resonance Imaging and Iron-oxide Nanoparticles in the era of Personalized Medicine. Nanotheranostics 2023, 7, 424–449. [Google Scholar] [CrossRef]
- Gorobets, O.; Gorobets, S.; Polyakova, T.; Zablotskii, V. Modulation of calcium signaling and metabolic pathways in endothelial cells with magnetic fields. Nanoscale Adv. 2024, 6, 1163–1182. [Google Scholar] [CrossRef]
- Setia, A.; Mehata, A.K.; Priya, V.; Pawde, D.; Jain, D.; Mahto, S.K.; Muthu, M.S. Current advances in nano-theranostics for molecular imaging and therapy of cardiovascular disorders. Mol. Pharm. 2023, 20, 4922–4941. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zhang, P.; Ren, C.; Xu, X.; Sun, B.; Yang, Z. A flexible implantable polyimide catheter device for targeted treatment of cardiovascular diseases by aggregating magnetic nanoparticles. IEEE Trans. Compon. Packag. Manuf. Technol. 2021, 11, 911–917. [Google Scholar] [CrossRef]
- Özcan, Z.; Yoruç, A.B.H. Vinorelbine-loaded multifunctional magnetic nanoparticles as anticancer drug delivery systems: Synthesis, characterization, and in vitro release study. Beilstein J. Nanotechnol. 2024, 15, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Malehmir, S.; Esmaili, M.; Mahabady, M.K.; Sobhani-Nasab, A.; Atapour, A.; Ganjali, M.; Hasan-Abad, A.M. A review: Hemocompatibility of magnetic nanoparticles and their regenerative medicine, cancer therapy, drug delivery, and bioimaging applications. Front. Chem. 2023, 11, 1249134. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Basu, S.M.; Qasim, M.; Giri, J. Polypropylene sulphide coating on magnetic nanoparticles as a novel platform for excellent biocompatible, stimuli-responsive smart magnetic nanocarriers for cancer therapeutics. Nanoscale 2023, 15, 7384–7402. [Google Scholar] [CrossRef] [PubMed]
- Sheha, M.; Mostafa, A.; Nasr, D.; Shahin, R. Magnetic nanoparticles: Synthesis, characterization, biomedical applications and challenges. Sphinx J. Pharm. Med. Sci. 2023, 5, 1–32. [Google Scholar] [CrossRef]
- Guigou, C.; Lalande, A.; Millot, N.; Belharet, K.; Grayeli, A.B. Use of super paramagnetic iron oxide nanoparticles as drug carriers in brain and ear: State of the art and challenges. Brain Sci. 2021, 11, 358. [Google Scholar] [CrossRef]
- Wirthl, B.; Janko, C.; Lyer, S.; Schrefler, B.; Alexiou, C.; Wall, W.A. An in silico model of the capturing of magnetic nanoparticles in tumour spheroids in the presence of flow. Biomed. Microdevices 2023, 26, 1. [Google Scholar] [CrossRef]
- Esposito, A.; Cotta Filho, C.K.; Lacchini, R. Beyond eNOS: Genetic influence in NO pathway affecting drug response. Genet. Mol. Biol. 2022, 45, e20220157. [Google Scholar] [CrossRef]
- Narykov, O.; Zhu, Y.; Brettin, T.; Evrard, Y.A.; Partin, A.; Shukla, M.; Xia, F.; Clyde, A.R.; Vasanthakumari, P.; Doroshow, J.H.; et al. Integration of computational docking into anti-cancer drug response prediction models. Cancers 2023, 16, 50. [Google Scholar] [CrossRef]
- Dhoundiyal, S.; Alam, M.A. Advances in Pharmacokinetic Modelling and Computational Approaches for Nanoparticles in Drug Delivery Systems. Curr. Nanomed. 2023, 3, 210–227. [Google Scholar] [CrossRef]
- Pascal, J.; Ashley, C.; Wang, Z.; Brocato, T.; Butner, J.D.; Carnes, E.C.; Koay, E.; Brinker, C.; Cristini, V. Mechanistic Modeling Identifies Drug-Uptake History as Predictor of Tumor Drug Resistance and Nano-Carrier-Mediated Response. ACS Nano 2013, 8, 1439–1447. [Google Scholar] [CrossRef]
- Singh, A.V.; Ansari, M.; Rosenkranz, D.; Maharjan, R.; Kriegel, F.L.; Gandhi, K.; Kanase, A.; Singh, R.; Laux, P.; Luch, A. Artificial Intelligence and Machine Learning in Computational Nanotoxicology: Unlocking and Empowering Nanomedicine. Adv. Healthcare Mater. 2020, 9, 1901862. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, J.; Chen, X.; Zhang, L. NeuMF: Predicting anti-cancer drug response through a neural matrix factorization model. Curr. Pharm. Biotechnol. 2022, 17, 835–847. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, H.; Wang, X. Advances in cancer research: Current and future diagnostic and therapeutic strategies. Biosensors 2024, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. A short appraisal on nano-biosensors for epigenetic changes detection: A transformative innovation. Curr. Nanosci. 2024, 20, 1–10. [Google Scholar] [CrossRef]
- Xu, W.; Liang, M.; Su, W.; Yang, J.; Pu, F.; Xie, Z.; Jin, K.; Polyakov, N.E.; Dushkin, A.V. Self-assembled nanocapsules of celery (Apium graveolens Linn) seed oil: Mechanochemical preparation, characterization, and urate-lowering activity. J. Drug Deliv. Sci. Technol. 2021, 66, 102810. [Google Scholar] [CrossRef]
- Kumar, S.; Singhal, A.; Narang, U.; Mishra, S. Recent progresses in organic-inorganic nanotechnological platforms for cancer therapeutics. Curr. Med. Chem. 2020, 27, 6015–6056. [Google Scholar] [CrossRef]
- Loloi, J.; Babar, M.; Davies, K.; Suadicani, S. Nanotechnology as a tool to advance research and treatment of non-oncologic urogenital diseases. Ther. Adv. Urol. 2022, 14, 1–21. [Google Scholar] [CrossRef]
- Mose, P. The applications of nanotechnology in renewable energy. J. Phys. Sci. 2024, 5, 1–12. [Google Scholar] [CrossRef]
- Leung, A.W.Y.; Amador, C.; Wang, L.C.; Mody, U.V.; Bally, M.B. What drives innovation: The canadian touch on liposomal therapeutics. Pharmaceutics 2019, 11, 124. [Google Scholar] [CrossRef]
- Selim, M.M.; El-Safty, S.; Tounsi, A.; Shenashen, M. A review of magnetic nanoparticles used in nanomedicine. APL Mater. 2024, 12, 010601. [Google Scholar] [CrossRef]
- Sreeharsha, N.; Philip, M.; Krishna, S.S.; Viswanad, V.; Sahu, R.; Shiroorkar, P.; Aasif, A.H.; Fattepur, S.; Asdaq, S.M.; Nair, A.B.; et al. Multifunctional mesoporous silica nanoparticles for oral drug delivery. Coatings 2022, 12, 358. [Google Scholar] [CrossRef]
- Chia, J.J.; Shameli, K.; Yusefi, M.; Ali, R.R.; Vekes, B.; Balasundram, S.; Teow, S.Y. Preparation and application of cross-linked alginate nanoparticles as drug carrier: A review. J. Res. Nanosci. Nanotechnol. 2022, 5, 1–11. [Google Scholar] [CrossRef]
- Mehrizi, T.Z.; Mossafa, N.; Vodjgani, M.; Shahmabadi, H.E. Advances in nanotechnology for improving the targeted delivery and activity of Amphotericin B (2011-2023): A systematic review. Nanotoxicology 2024, 18, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Bora, M. Recent bio-medical applications of iron oxide magnetic nanoparticles. J. Interdiscip. Sci. Arts 2023, 1, 56–72. [Google Scholar] [CrossRef]
- Singh, D. Biomembrane-grafted dendrimer-polymeric conjugates for targeting p53—A pioneer innovation in cancer nanomedicine. J. Nanopart. Res. 2023, 25, 257. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Z.; Wang, Y.; Chen, L.; Wang, Y.; Luo, C. Nanotechnology in inflammation: Cutting-edge advances in diagnostics, therapeutics and theranostics. Theranostics 2024, 14, 2490–2504. [Google Scholar] [CrossRef]
- Hartshorn, C.; Russell, L.M.; Grodzinski, P. National cancer institute alliance for nanotechnology in cancer-catalyzing research and translation toward novel cancer diagnostics and therapeutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1570. [Google Scholar] [CrossRef]
- Chopra, H.; Mohanta, Y.K.; Rauta, P.R.; Ahmed, R.; Mahanta, S.; Mishra, P.; Panda, P.; Rabaan, A.; Alshehri, A.A.; Alqahtani, A.S.; et al. An insight into advances in developing nanotechnology based therapeutics, drug delivery, diagnostics and vaccines: Multidimensional applications in tuberculosis disease management. Pharmaceutics 2023, 16, 581. [Google Scholar] [CrossRef]
- Drori, I.; Lavie, D. How do innovation ecosystems emerge? The case of nanotechnology in Israel. J. Manag. Stud. 2023, 60, 351–384. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, H.; Ma, Y.; Dai, Q.; Tang, Z. Celebrating 20 years of NCNST: Innovation in nanoscience and nanotechnology. ACS Nano 2023, 17, 20715–20722. [Google Scholar] [CrossRef]
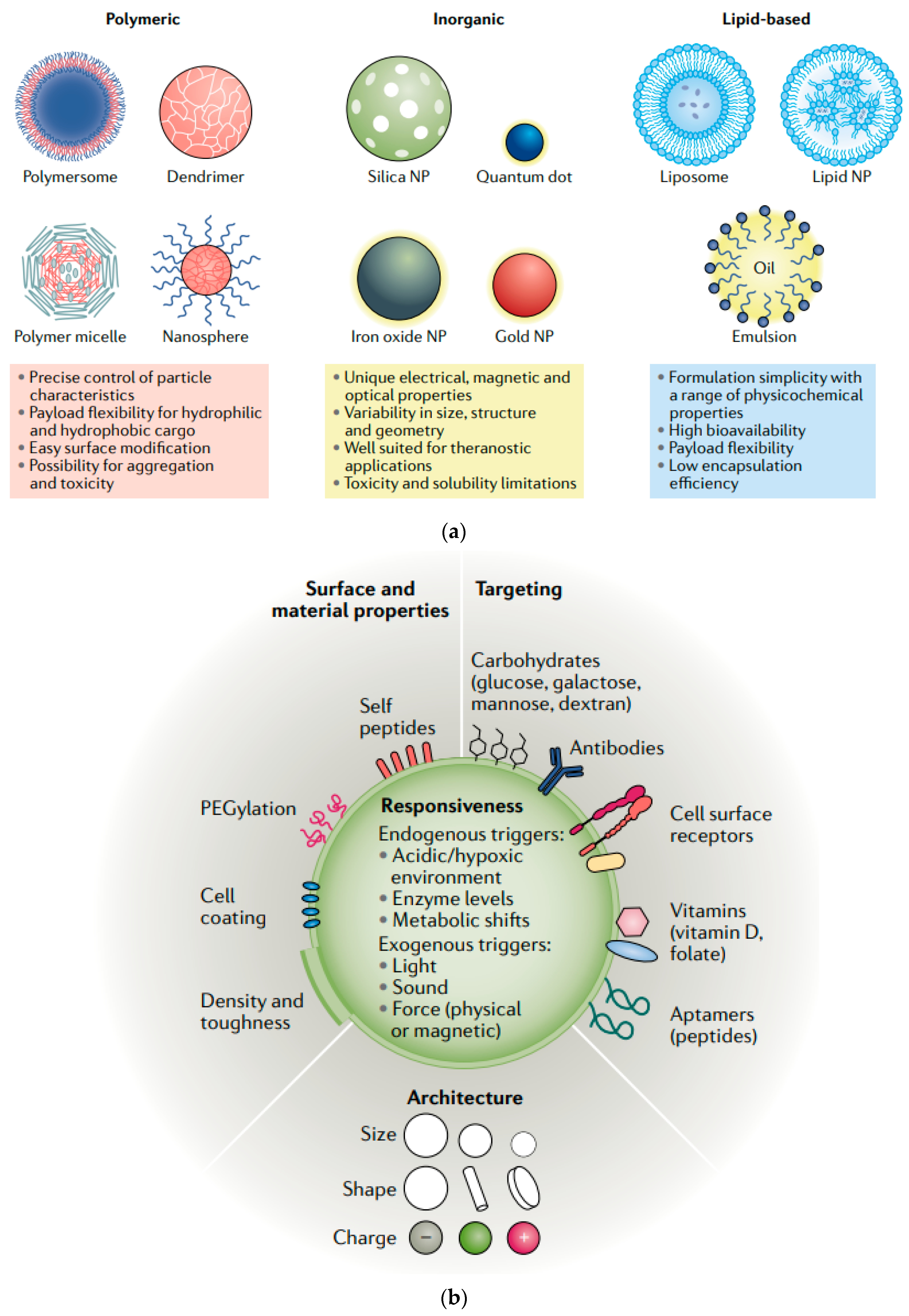
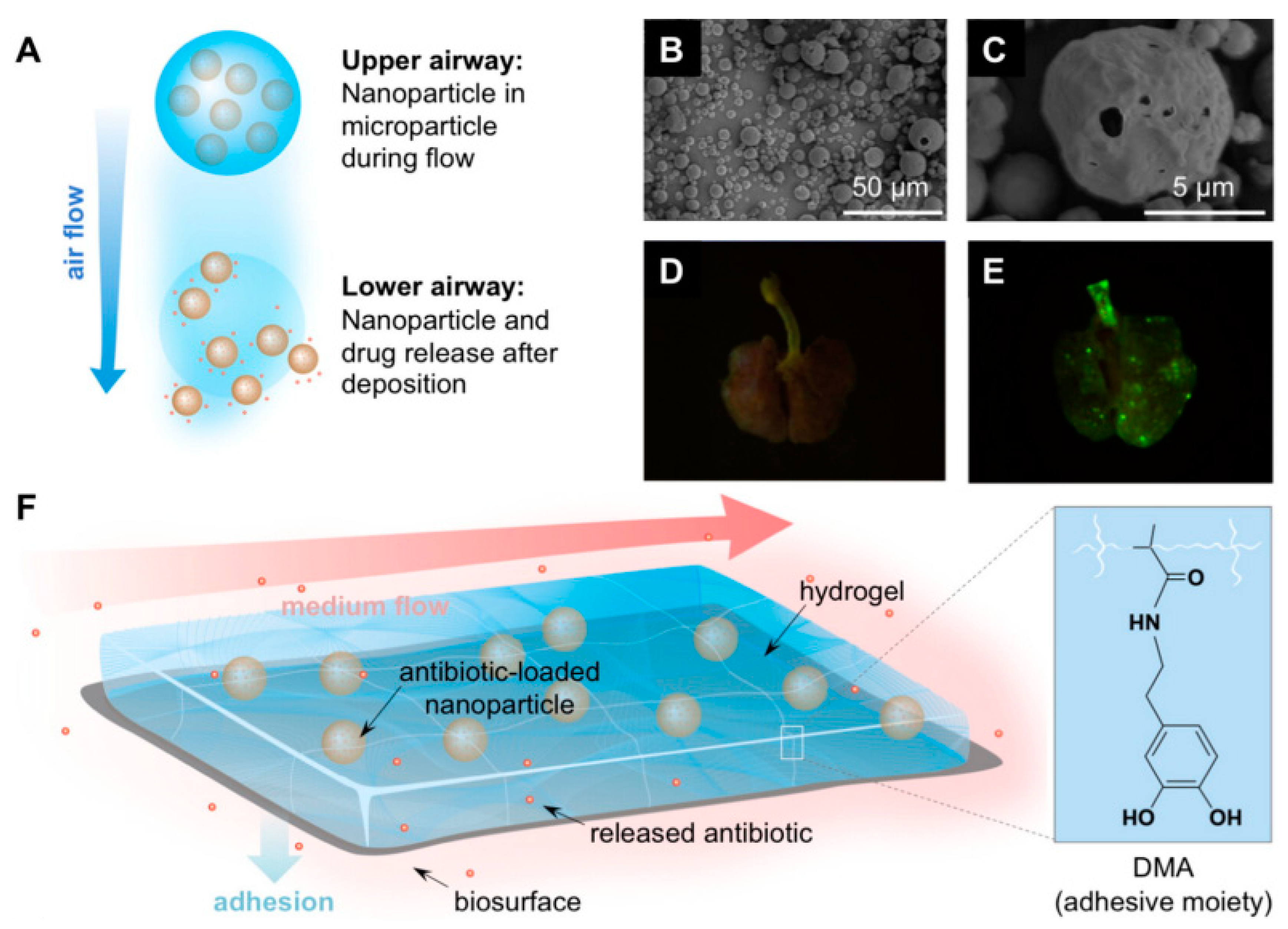
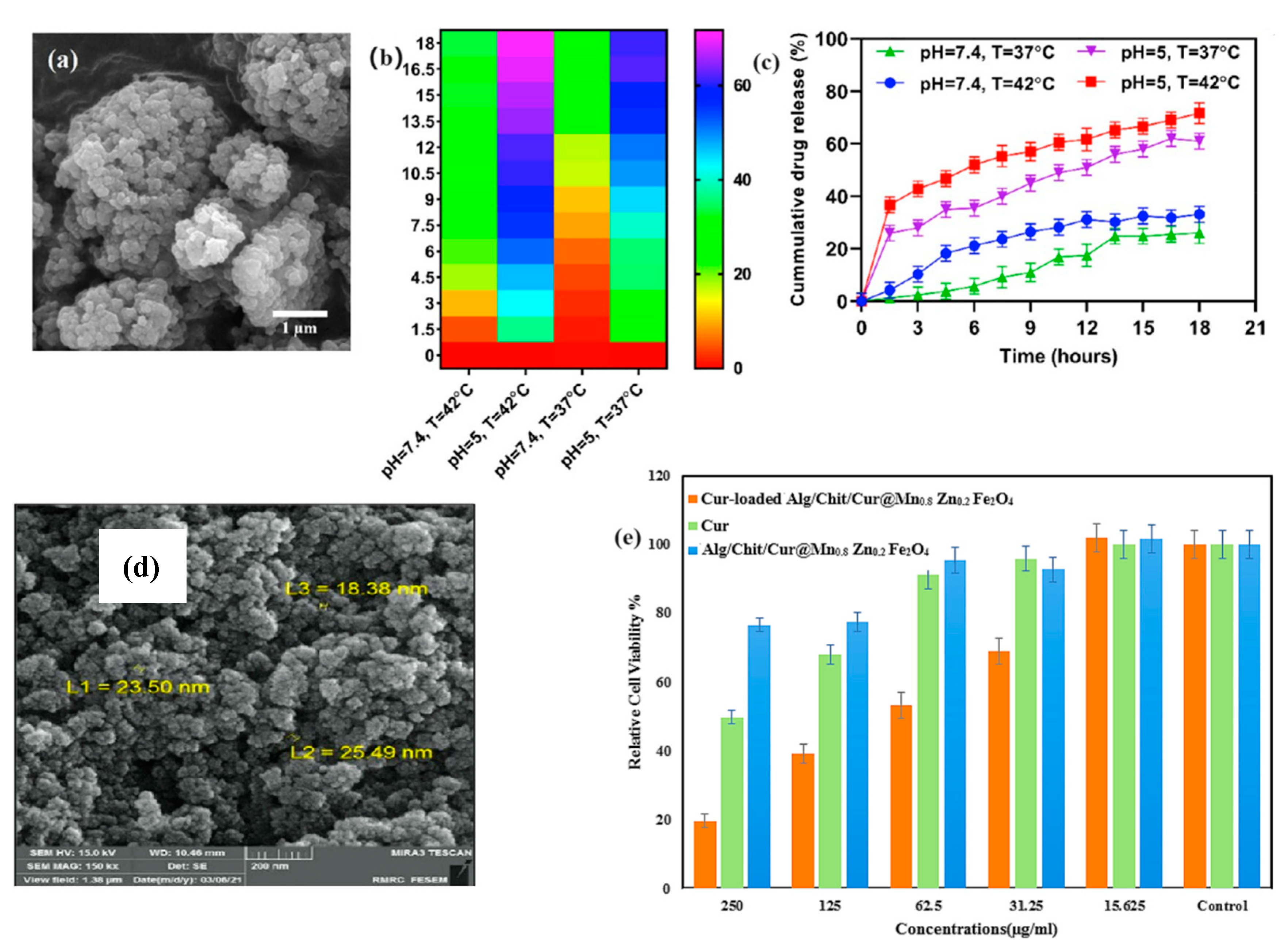
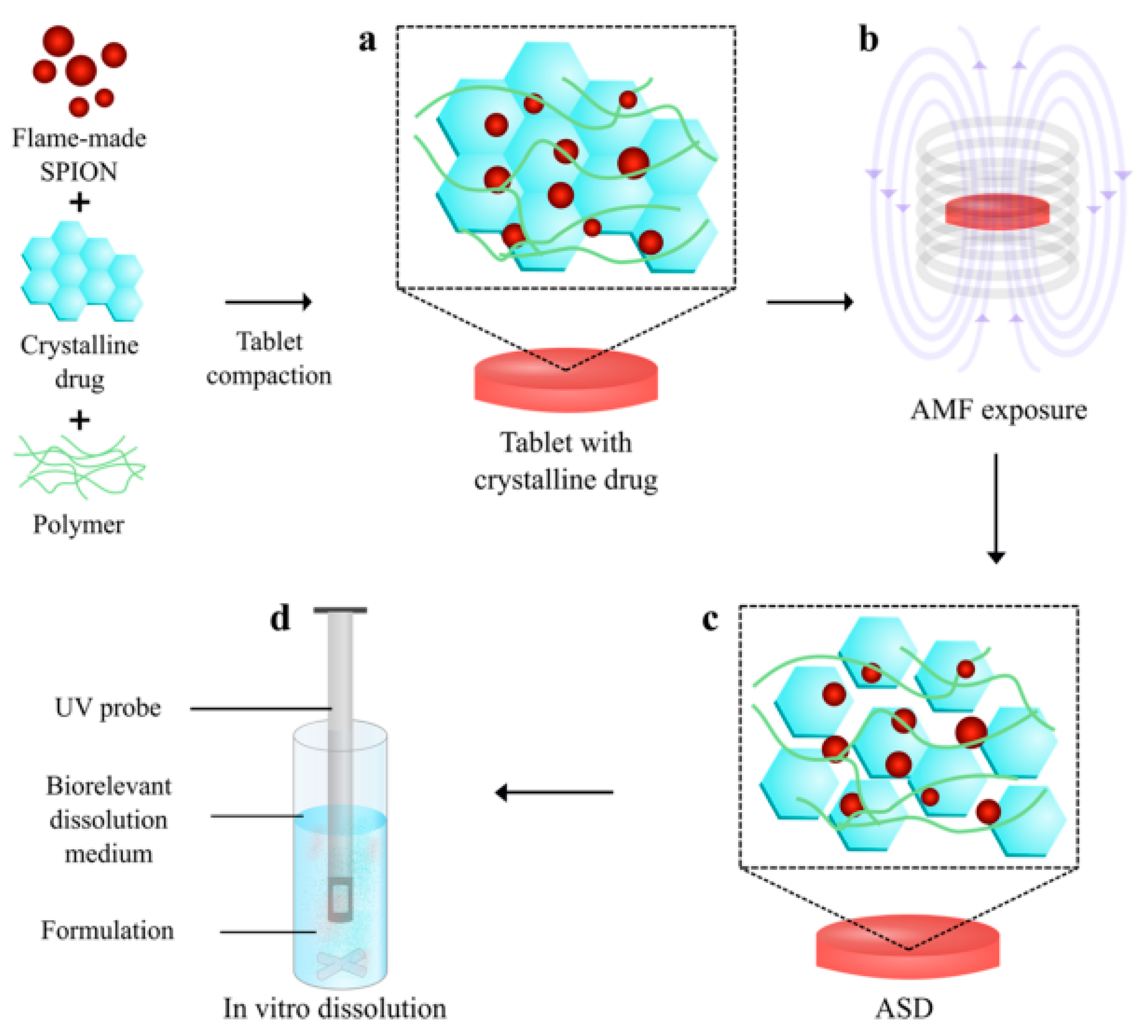

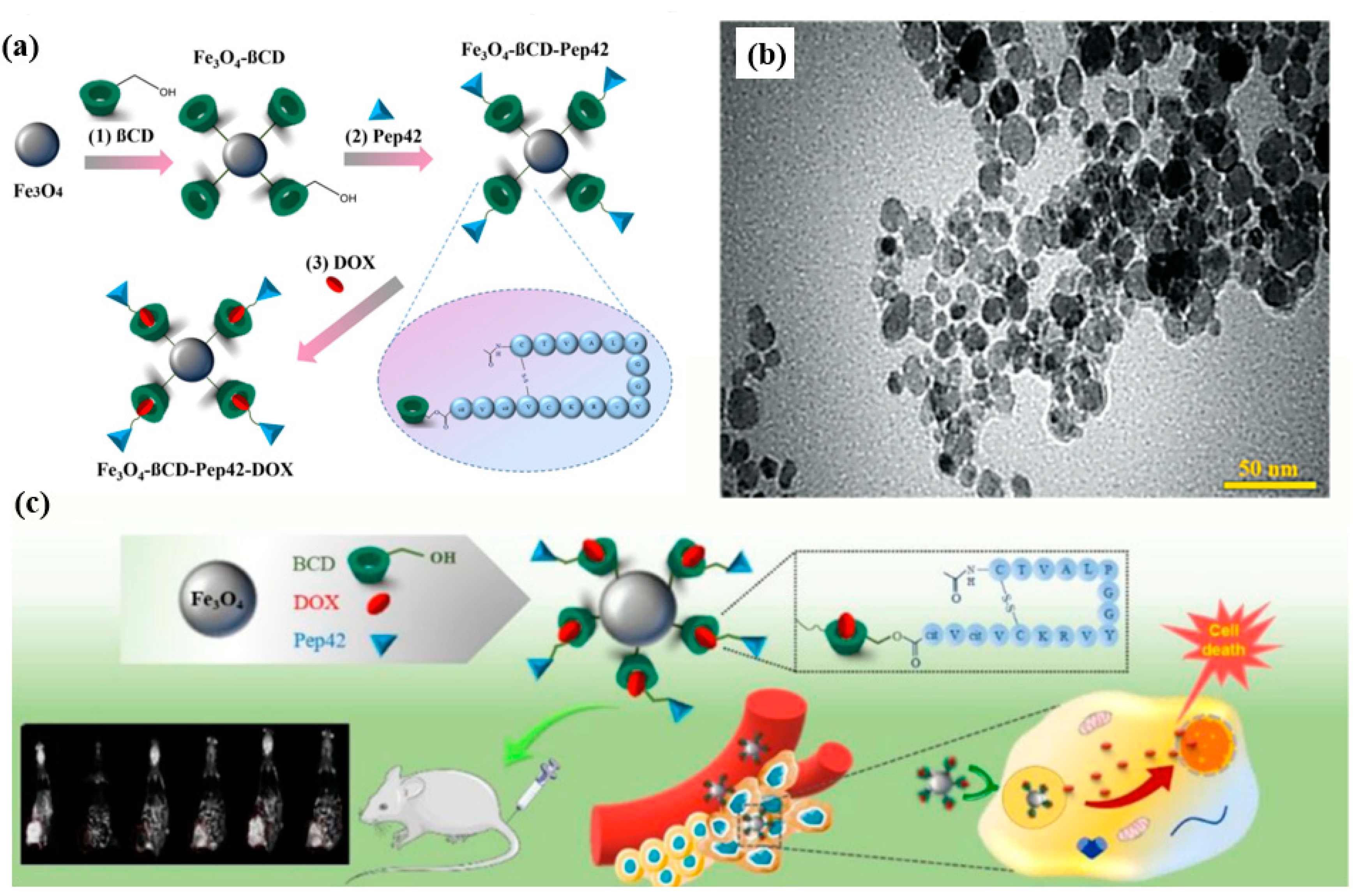
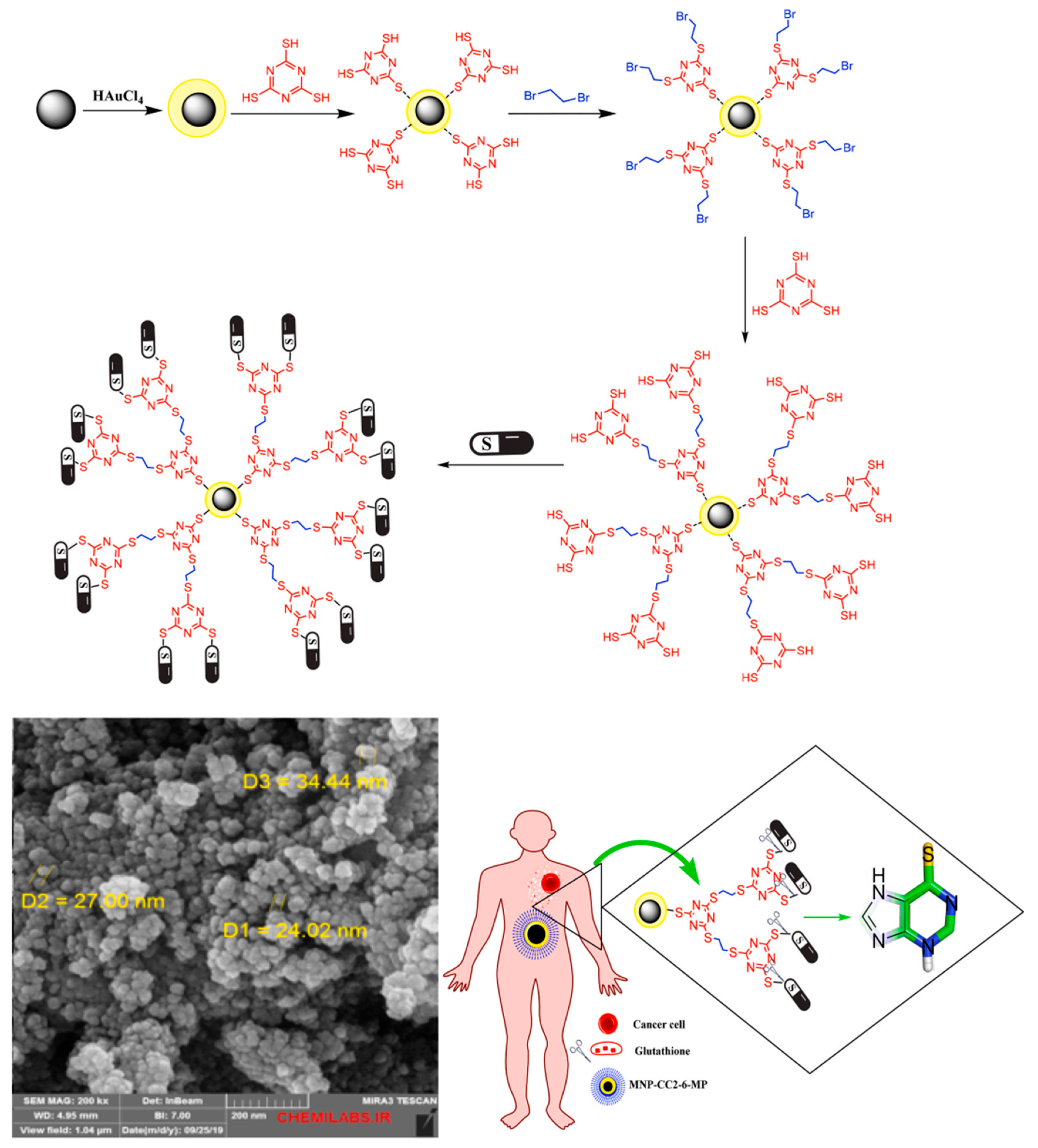
| Drug/Compound | Nanocarrier Type | Size | Route | Effect or Remarks on Solubility/Bioavailability | Reference |
|---|---|---|---|---|---|
| Rifampicin and Thymopentin | Glyceryl Monostearate/Soybean Phosphatidylcholine Nanoparticles | 150–200 nm | Inhalable Drug Delivery | Enhanced solubility and bioavailability in pulmonary systems. | [25] |
| Curcumin | PLA-HA/Fe3O4 Magnetic Nanoparticles | 208 nm | Oral | Enhanced solubility and bioavailability in the GI tract, used for colorectal cancer treatment. | [26] |
| Alginate/Chitosan-Functionalized Mn0.8Zn0.2Fe2O4 Nanoparticles | 20 nm | Oral | Enhanced solubility, controlled release, and improved therapeutic efficacy against breast cancer cells. | [28] | |
| Magnetic Ferrite Nanoparticles Coated with BSA/Glycine Polymers | 50–70 nm | Oral | Improved solubility in the GI tract, stable and sustained release profile, suitable for maintaining therapeutic levels. | [30] | |
| Hydrocortisone | CS-g-PNVCL-Coated Fe3O4@SiO2 Core–Shell Nanoparticles | 45–65 nm | Oral (GI-specific) | Improved solubility and therapeutic efficacy, with pH and temperature-sensitive release profile. | [27] |
| Celecoxib | Superparamagnetic Iron Oxide Nanoparticles (SPIONs) | 15–25 nm | Oral | In situ amorphization under magnetic hyperthermia, increasing solubility by fivefold. | [29] |
| Docetaxel | Magnetic Nanostructured Lipid Carriers (MNLC) | 120–150 nm | Oral | Increased solubility, reduced toxicity, and enhanced efficacy in lung cancer treatment. | [31] |
| N/A (this focuses on the delivery method rather than a specific drug) | Fe3O4 Nanoparticles Embedded in Liquid–Metal Soft Electronics | Diameter of robot legs: 1–2 mm | Oral | The robots are designed to traverse the GI tract effectively and deliver payloads in a minimally invasive manner through controlled magnetic navigation. | [33] |
| Methotrexate | Glucosamine-Modified Mesoporous Silica-Coated Magnetic Nanoparticles | 100–150 nm | IV | Controlled release in tumor environment, efficient theranostic platform for cancer treatment. | [34] |
| Doxorubicin | Fe3O4-ßCD-Pep42-Coated Nanoparticles | 17 nm | IV | Enhanced cancer cell uptake, reduced toxicity to healthy cells, combined use in imaging and therapy. | [35] |
| Silibinin | Magnetic Niosomal Nanoparticles (MNNPs) | 50–70 nm | Oral | Controlled release, increased bioavailability, no significant toxicity to normal cells. | [36] |
| Fe3O4 Nanoparticles (MR-SUFs) | 10–100 nm | Oral | Enhanced GI retention and bioavailability. | [41] | |
| 5-Fluorouracil | Folic-Acid-Conjugated PEG-PCL-PEG-Coated SPIONs | 100–150 nm | Oral | Targeted delivery in colon cancer, enhanced cellular uptake, controlled drug release at tumor sites. | [37] |
| Methotrexate | Glucosamine-Modified Mesoporous Silica-Coated Fe3O4 | 80–100 nm | IV | Enhanced targeting and controlled release of methotrexate. | [39] |
| Camptothecin | Ytterbium Ferrite/PLGA Superparamagnetic Hybrid | 120–150 nm | IV | Enhanced delivery and reduced toxicity, used for targeted cancer therapy. | [43] |
| Disease Type | Type of MNPs | Models | Therapeutic Outcomes | Reference |
|---|---|---|---|---|
| Cancer (ferroptosis-based therapy) | Iron oxide nanoparticles | In vitro (cancer cells) | Enhanced ferroptosis in cancer cells, potential for image-guided therapy combining diagnostic imaging and ferroptosis to treat cancer. | [54] |
| Cancer (photothermal therapy) | Tannin-stabilized superparamagnetic iron oxide | In vitro (cancer cells) | Photothermal effect effectively generated hyperthermia, causing significant cancer cell death under laser light exposure. | [55] |
| Cancer (chemotherapy and hyperthermia) | Nylon-6 coated Fe3O4 nanoparticles with Doxorubicin | In vitro (A549, HEK 293FT cells) | pH-sensitive drug release and significant reduction in cancer cell growth, demonstrating potential for targeted delivery. | [56] |
| Cancer (breast cancer) | ECO/siRNA nanoparticles for DANCR lncRNA inhibition | In vivo (mouse model) | Significant inhibition of tumor growth with image-guided delivery, especially in highly aggressive tumors. | [57] |
| Melanoma | Paclitaxel-loaded lipid-coated Manganese ferrite | In vitro (B16F10 melanoma cells) | Dual chemo-magnetic hyperthermia therapy improved drug delivery and reduced systemic toxicity, with significant melanoma cell death. | [58] |
| Pancreatic cancer | Fe3O4 nanoparticles for hyperthermia | In vitro (pancreatic BxPC3 cells) | Combined hyperthermia and proton therapy reduced cancer cell survival and increased DNA damage, demonstrating synergistic therapeutic effects. | [59] |
| Neurological disorders | SPIONs | In vivo (mouse model) | SPIONs showed promise in treating Alzheimer’s disease by reducing amyloid β-protein clumping and protecting neurons from inflammation and oxidative stress. They also improved cognitive function and immunity by influencing the gut–brain connection. | [60] |
| Central nervous system Disorders | Magnetic hybrid nanovesicles | In vivo (various CNS models) | Enhanced diagnosis and treatment of CNS disorders by crossing the blood–brain barrier (BBB), with potential for improved drug delivery and reduced side effects. | [61] |
| Neurological disorders (Parkinson’s) | Magnetothermal nanoparticles | In vivo (mouse model) | Magnetothermal neuromodulation improved motor behavior in Parkinson’s disease models, offering a non-invasive alternative to deep brain stimulation. | [62] |
| Neurological disorders (Alzheimer’s) | SPIONs functionalized with transferrin | In vitro (brain cells) | Efficient isolation and targeting of brain-derived exosomes for diagnostic and therapeutic applications in neurodegenerative diseases. | [63] |
| Gastrointestinal disorders | SPIONs and quantum dots | In vivo (mouse model) | SPIONs were utilized to improve the diagnosis of colorectal cancer and inflammatory bowel diseases. Quantum dots enhanced imaging in CRC-bearing mice, allowing for better diagnosis and treatment planning. | [64] |
| Gastrointestinal cancers | Fe3O4 encapsulated with orange pectin | In vitro (HT-29, HCT 116, etc.) | Significant cytotoxicity against colorectal, pancreatic, and gastric cancer cells, with reduced side effects on healthy cells. | [65] |
| Cardiovascular diseases (CVDs) | Iron oxide nanoparticles (IONPs) functionalized with targeting ligands | In vitro | Enhanced imaging and therapy of cardiovascular conditions, including drug delivery and real-time monitoring of therapeutic responses, personalized treatment applications. | [68] |
| SPIONs and CLIO nanoparticles | In vivo (various models) | SPIONs, combined with tissue plasminogen activator (tPA), significantly reduced clotting and improved blood flow in cardiovascular diseases. CLIO nanoparticles were developed for fibrin-targeting to improve clot dissolution in thrombotic diseases. | [70] | |
| Drug-loaded MNPs on flexible polyimide catheter | In vivo (rat model) | Effective targeted drug delivery to treat CVDs, demonstrated improved precision of drug release at the target site, reducing stent-induced complications like restenosis. | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuo, Y.; Zhao, Y.-G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. https://doi.org/10.3390/molecules29204854
Zhuo Y, Zhao Y-G, Zhang Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules. 2024; 29(20):4854. https://doi.org/10.3390/molecules29204854
Chicago/Turabian StyleZhuo, Yue, Yong-Gang Zhao, and Yun Zhang. 2024. "Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles" Molecules 29, no. 20: 4854. https://doi.org/10.3390/molecules29204854
APA StyleZhuo, Y., Zhao, Y.-G., & Zhang, Y. (2024). Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules, 29(20), 4854. https://doi.org/10.3390/molecules29204854







