Abstract
Higher alcohol synthesis through the Fischer–Tropsch (F–T) process was considered a promising route for the efficient utilization of fossil resources could be achieved. The CuCo catalysts were proven to be efficient candidates and attracted much interest. Great efforts have been made to investigate the active sites and mechanisms of CuCo catalysts. However, the industrialized application of CuCo catalysts in this process was still hindered. The poor stability of this catalyst was one of the main reasons. This short review summarized the recent development of active sites on the CuCo catalysts for higher alcohol synthesis, including CuCo alloy particles, CuCo core–shell particles, and unsaturated particles. The complex active sites and their continual changes during the reaction led to the poor stability of the catalysts. The effect of active sites on catalytic performance was discussed. Furthermore, the key factors in fabricating stable CuCo catalysts were proposed. Finally, reasonable proposals were proposed for designing efficient and stable CuCo catalysts in higher alcohol synthesis.
1. Introduction
The gradual dwindling of fossil resources in the current world and the issues in the exploitation and utilization process, such as environmental contamination and resource waste, require the development of technologies for the production of valued chemicals from the efficient utilization of fossil resources [1]. In recent years, the concerns about the Fischer–Tropsch (F–T) process have increased as the chemical feedstock, such as hydrocarbons, olefins, and higher alcohols, could be directly converted from syngas (CO+H2) [2]. The production of syngas can be commercially obtained from coal, natural gas, biomass, and even organic waste and has reached commercialization [3,4,5,6]. Thus, the development of the F–T process is considered one of the promising routes for further development of the world.
Among the products in this process, higher alcohols (HA), which contain two or more carbons, have a wide range of applications, which can serve as fuel, fuel additives, and other platform molecules and attract much interest [7,8,9]. The Institut Francais du Petrole (IFP) first proposed that the CuCo catalyst showed excellent HA yield through the F–T process in the 1970s [10]. After that, a large amount of the work focused on facilitating the efficient CuCo catalysts has been made as the low-cost and rich reserves and the moderate operating conditions for high yields of HA [11]. Generally, the Cu and Co species are responsible for the CO non- and dissociative adsorption behaviors in this process, respectively. The combination of intermediates (CO* from Cu and CHx* from Co) is the key step to forming HA with the subsequent hydrogenation process [12]. It is considered that shortening the distance between these two species is the efficient way, which favor decreasing the barriers of CO insertion [13]. Consequently, constructing the bimetallic particles with intimate connections, such as alloy or core–shell particles, was the main strategy in recent years [14].
However, the industrialized application of this process is still hindered by the poor stability of the catalysts [15]. Actually, owing to the low solubility between Cu and Co [16], the preparation parameters have a significant effect on forming the species of components over the catalysts (as shown in Table 1). Moreover, unsaturated particles have been found over the catalysts in recent years, which are formed owing to the incomplete reduction [17,18,19,20], metal–support interaction [21], and carbonization in the reaction process [22], and have been identified as having the ability of CO absorption. Thus, it is implied that the active sites for HA synthesis over the CuCo catalysts are complex and have a tight correlation relationship with the stability of the catalysts.

Table 1.
Effect of preparation parameters on the species of components over CuCo catalysts.
Though the catalyst types [4,26], reaction mechanism [4,27,28], active sites [3,14], and preparation strategy [4,29,30] of the catalysts have been reviewed, the active sites over the CuCo catalysts have not been summarized comprehensively, and the reason for the poor stability of CuCo catalysts have rarely been discussed. Consequently, the objective of this short review was to address this gap by summarizing the active sites over CuCo catalysts from recent works. The overview started with the introduction of HA synthesis over CuCo catalysts and then moved to the classification of active sites for HA synthesis on CuCo catalysts. The emphasis has been placed on the discussion of the relationship between the active sites and stability property.
2. Summary of Active Sites for HA Synthesis over CuCo Catalysts
2.1. Alloy Particles
The CuCo alloy particles were confirmed as typical active sites for HA synthesis [31]. Prieto et al. [17] studied the structure–activity relationship of CuCo catalysts by combining DFT simulations and microkinetic modeling with experimental results. The simulation results showed that monometallic Cu3 and Co3 favor the formation of methanol and hydrocarbon, respectively, and the CuCo alloy phase acts as the active site for HA synthesis. Moreover, their experiment result also was consistent with the simulation results. Cao et al. [13] proposed a plausible reaction mechanism over CuCo alloy particles for HA synthesis, combining density functional theory (DFT) and micro-kinetic modeling. The CuCo alloy particles favored decreasing the required coverage of CHx* and CO, facilitating C-C coupling. Additionally, on the surface of CuCo (211), the low C-O dissociation barrier and the high rate of CHx-CO coupling were responsible for the high selectivity toward ethanol.
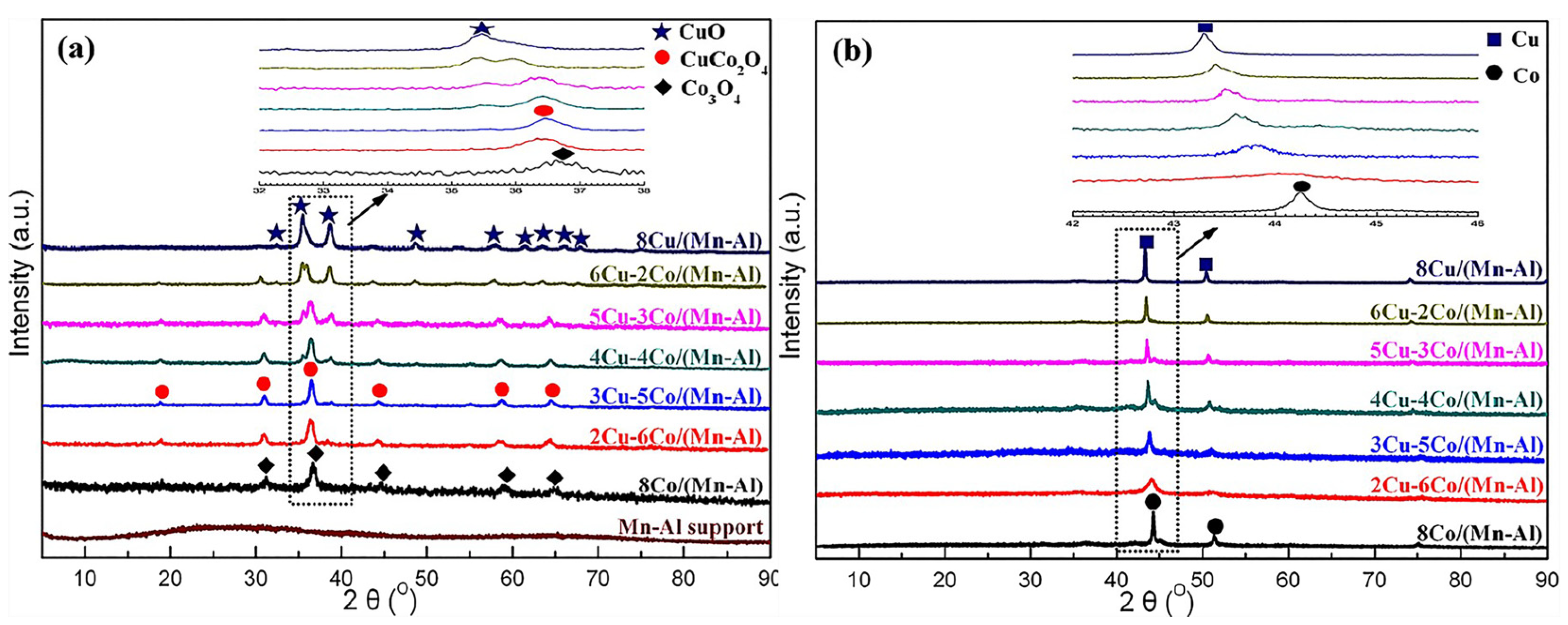
Fabricating the precursor containing Cu and Co was the main route to form CuCo alloy particles as these two species were restrained into atom level [15,25,32,33,34,35,36,37]. The catalytic performance of CuCo alloy particles derived from various precursors is shown in Table 2. Zhao et al. [38] prepared a series of Mn-Al-supported CuCo catalysts with different Cu/Co ratios. Based on the characterization results (Figure 1), the dominant phase was the CuCo2O4 after calcination, and the uniformly CuCo alloy particles were obtained after reduction. Cao et al. [39] studied the catalytic performance of CuCo catalysts derived from layered double hydroxides (LDHs. The characterization results indicated that CuCo mixed oxides were reduced to form CuCo alloys, and the synergistic catalytic effect between Cu and Co in the alloy promoted the formation of HA. Li et al. [25] prepared a series of CuCo catalysts with different Co/Cu ratios encapsulated in KIT-6. After the stepwise pyrolysis, the results indicated that Co and Cu species were anchored into CuxCo3−xO4 spinel oxide and the alloy phase was formed after reduction. The CuCo alloy particles were responsible for HA synthesis as a linear relationship between the surface proportion of alloy and yields of HA was displayed.

Table 2.
Catalytic performance CuCo catalyst with alloy phase derived from precursors.
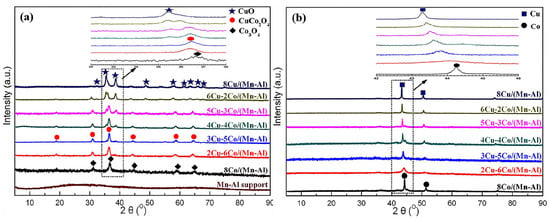
Figure 1.
XRD patterns of (a) the calcinated and (b) reduced catalysts [38]. Copyright (2018) American Chemical Society.
2.2. Core–Shell Particles
The core–shell structure was also responsible for HA synthesis because of the close connection between Cu and Co [43,44]. The catalytic performance of catalysts with core–shell structure is shown in Table 3. Subramanian et al. [43] conducted a DRIFTS study on the CO hydrogenation process over Cu@Co3O4. The results indicated that both dissociative and associative CO adsorption occurred on Cu@Co3O4. They concluded that the higher selectivity of HA was attributed to the existence of Cu@Co3O4.

Table 3.
Catalytic performance of CuCo catalyst with core–shell structure.
Xiang et al. [23] investigated the effect of precursor activation on CuCo catalysts. It was found that the structure of CuCo was closely related to the reduction atmosphere. The Co@Cu particles were formed in H2, whereas a graphite-encapsulated onion-like structure was formed in CO. The catalyst activated by CO exhibited higher CO conversion (27.1%) than the catalyst activated by H2 (5.7%), which was attributed to the stronger CO dissociative adsorption ability of Co-rich species. Liu et al. [24] studied the catalytic performance of CuCo catalysts with Cu@Co and Co@Cu by adjusting reduction conditions. The reduction conditions were as follows: (1) heated in N2 to 600 °C, then reduced in H2 at 600 °C; and (2) heated from room temperature to 600 °C in H2. The characterization results showed that the catalyst reduced by method (1) displayed a Co@Cu structure (Cu shell and Co core), while the catalyst reduced by method (2) displayed a Cu@Co structure (Co shell and Cu core). The authors deemed that the Co@Cu structure was formed because of the aggregation of Cu species, as Cu has a lower surface energy than Co. For Cu@Co, it was ascribed to the lower reduction temperature of Cu and the effect of hydrogen spillover between Cu and Co. Meanwhile, the Co@Cu structure exhibited higher alcohol selectivity but lower CO conversion compared to Cu@Co.
2.3. Unsaturated Particles
In addition to the active sites mentioned above, many works found the presence of unsaturated particles on the CuCo catalysts. For example, Ye et al. [48] studied the catalytic performance of Na-doped catalysts. The X-ray photoelectron spectroscopy (XPS) results suggested that the Cu+, Cu0, Co2+, and Co0 species co-exist over the used catalysts. Meanwhile, the ratio of Cu+/Cu0 and Co2+/Co0 were affected by the additional amount of sodium. In recent years, large amounts of effort have been made to explore the role of unsaturated particles in HA synthesis, and their catalytic performance is shown in Table 4.

Table 4.
Catalytic performance of catalysts with unsaturated particles.
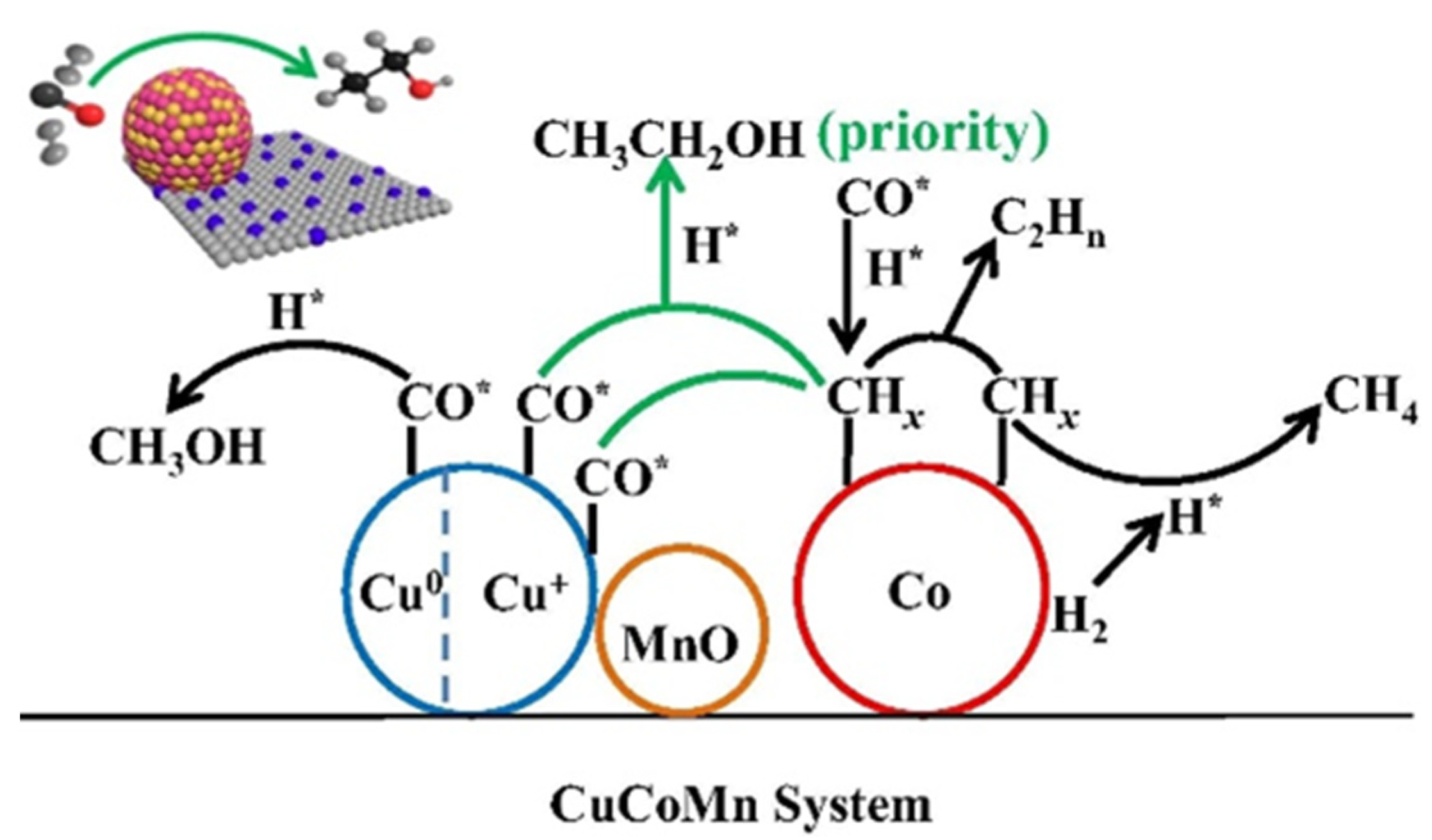
Sun et al. [49] applied Cu-Co-Mn catalysts in HA synthesis and observed significant alcohol selectivity (46.2%) and ethanol fraction (45.4%). The characterization results indicated that the Cu+ species existed on the catalyst owing to the strong interaction between Mn and Cu. They concluded that the outstanding performance was attributed to Cu+ species, which provided the absorbed CO was easier to participate in the CO insertion process than Cu0 [52,53]. They proposed that the synergistic effect between Cu+ and Co0 was responsible for ethanol formation (Figure 2).
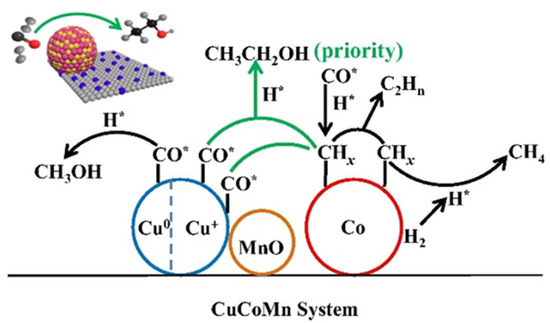
Figure 2.
Schematic diagram of the mechanism of ethanol formation via the synergistic effect of Cu+-Co0 [49]. H* represented the dissociated H, CO* represented the non-dissociative adsorbed CO. Copyright (2019) Elsevier.
The Cu+ and Cu0 also can participate in the process of HAS. Hofstadt et al. [54] suggested that the Cu+ and Cu0 favored the formation of C2+ alcohols. Gong et al. [55] studied the ethanol synthesis from syngas over Cu/SiO2 catalysts. As described, the Cu00 was the sole active site for the activity of the catalysts, while the Cu+ was responsible for the conversion of intermediates. Wang et al. [53] studied the role of Cu+ and Cu0 species in CO hydrogenation reaction over Cu/SiO2 catalysts. They demonstrated that the Cu+ was responsible for the adsorption of methoxy and acyl species, while the Cu0 facilitated the H2 decomposition. Our groups made extensive efforts on the role of Cu species in CO hydrogenation reaction [56,57,58,59,60,61]. Zuo et al. [59] reported that the formation of ethanol needs the coexistence of Cu+ and Cu0 species, which would contribute to forming CH3 species. The DFT calculations suggested that the formed CH3 species were the key intermediate for ethanol formation as it would follow the CO insertion step.
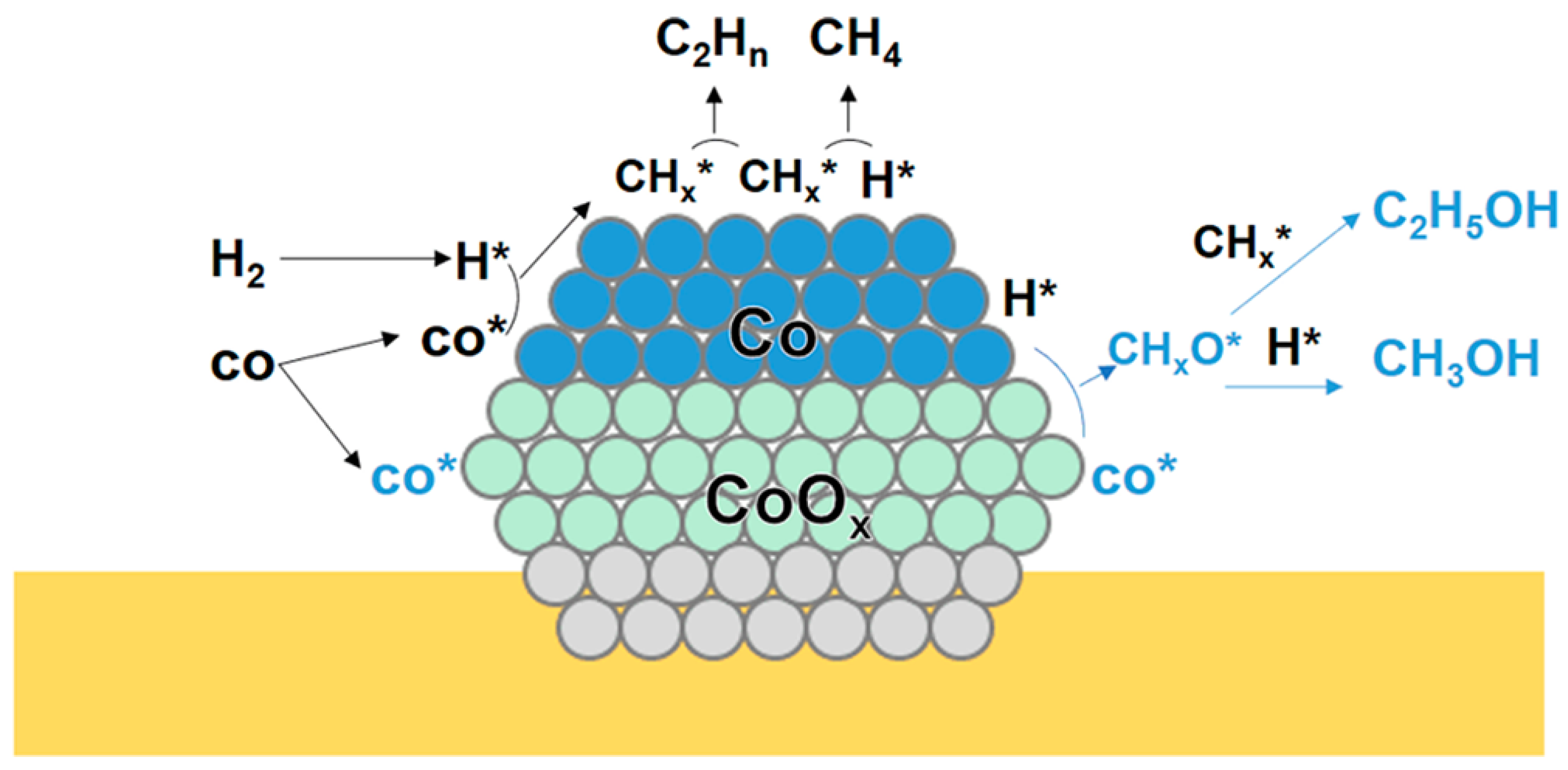
Sun et al. [62] investigated the CuCo catalysts derived from LDHs. A series of CuCoAl catalysts with different Cu/Co ratios were prepared. For the CoAl catalysts, the selectivity of total alcohol was 11.2%. The XPS and TEM results showed that CoO and Co(OH)2 coexist on the CuCo catalysts, which was ascribed to the metal–support interaction. For the CoAl catalysts, the selectivity of total alcohol was 11.2%. They speculated that the formation of HA may attributed to the Co0-Coδ+ pairs. Michel et al. [63] prepared the cobalt oxide-supported cobalt salt catalysts to convert the syngas into alcohols. After the reduction, the final state of the catalyst was Co-Al supported on CoAl2O4. The product formed in the Fischer–Tropsch synthesis was changed, and the production of alcohols was observed. They speculated that the CO insertion would occur on oxidized sites and the C-C coupling would occur on metallic sites. Chen et al. [64] prepared a series of Co/CeO2 with different Co content and applied in HA synthesis. During the reduction process, the characterization results showed that the Co3O4 was reduced into Co2+ and Co0 characteristics, evidenced that the Co0-CoOx pairs were responsible for HA synthesis. The CO-DRIFTS indicated that Co0 favored CO dissociation, producing the CHx. The CO insertion occurred on Coδ+ species. They proposed that the synergistic effect between the Co0 and CoOx species was responsible for HA formation (Figure 3).
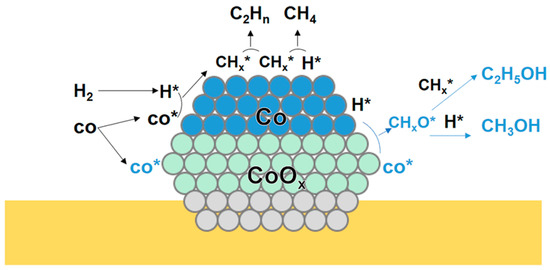
Figure 3.
Schematic diagram of the mechanism of HA synthesis via the synergistic effect of CoOx-Co0 [62]. H* represented the dissociated H species. CO* represented the non-dissociative adsorbed CO species. CHx* represented the intermediates formed by the hydrogenation of dissociative adsorbed CO. Copyright (2018) American Chemical Society.
Moreover, the unsaturated Co species could be formed by doping promoters, such as Ga [51,65,66,67,68,69,70], Mn [71], Ca [72] and Ce [73]. Gao et al. [66] prepared a series of gallium-doped CoGa/AC catalysts and applied them in HA synthesis. With the doping of Ga, the reduction temperature of Co3O4 increased, which favored adjusting the Co2+/Co0 ratio. The catalysts with the optimized ratio exhibited good alcohol selectivity (up to 30.3%). He et al. [68] reported that the formation of Co2+ was ascribed to the strong interaction between Ga and Co, to which the electrons would transform between Ga and Co. Meanwhile, the Co2+/Co0 pairs were responsible for HA formation.
Furthermore, the Co2C species also played an important role in HA synthesis. Nebel et al. [74] pretreated the CuCo catalysts into the CO atmosphere before the reaction. The characterization results illustrated that the Co2C was formed on the catalysts. They concluded that the improved HA selectivity and decreased CO2+methane selectivity contributed to the formation of Co2C. Extensive research has been made to investigate the role of Co2C [22,74,75,76,77,78,79,80,81,82,83,84]. Pei et al. [82] investigated the synergistic effect of Co-Co2C by combining DFT calculations with experimental results. In the experiment section, the Co3O4 sample without any support was prepared. After the carburization in the CO atmosphere, the Co2C was formed. The catalytic results illustrated the Co0-Co2C pairs were the active sites for HA synthesis. The DFT calculation results suggested that the Co2C was responsible for CO non-dissociative adsorption, whereas the Co0 was responsible for CO dissociative adsorption and the subsequent carbon-chain growth.
3. Discussion on Fabricating the Stable CuCo Catalysts
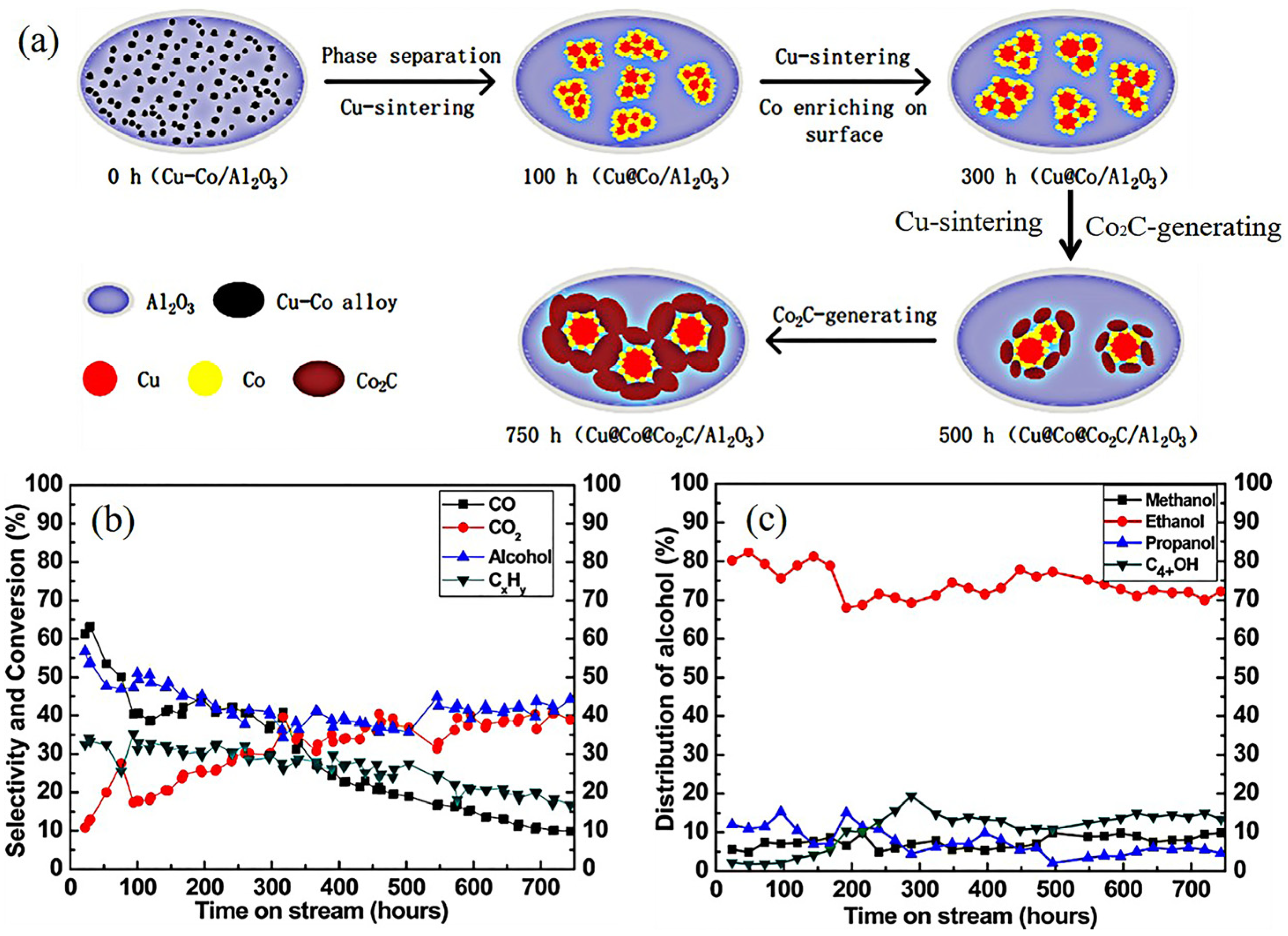
As mentioned above, the active sites on the CuCo catalysts were complex. Research focused on stability studies found that the active sites were not stable during the reaction [85,86,87,88]. The Cu and Co showed poor miscibility in the Cu–Co alloys. Furthermore, the lower surface energy of Cu (1.934 J M2) than Co that of cobalt (2.709 J M2) could result in preferential localization of copper on the particle surface. Yang et al. [86] investigated the structure evolution of CuCo alloy particles in HA synthesis during 800 h (Figure 4). The characterization results show that the alloy particles decomposed into Cu and Co species at the beginning of the reaction (100 h). The core–shell particles gradually formed during 100–300 h. As the reaction continued, the sintering of Cu enlarged the size of the Cu@Co particles. Meanwhile, the Co2C was formed owing to the carbonization of Co. In 500–750 h, the Cu and Co NPs were wrapped by Co2C. The significant deactivation of catalytic performance was also observed in Figure 4b,c. Consequently, the transformation of the active sites during the reaction was the main reason for the poor stability of CuCo catalysts.
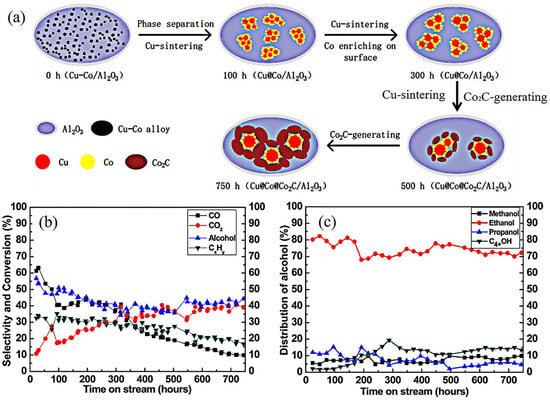
Figure 4.
Structure evolution of CuCo alloy in HA synthesis. (a) The stability test of CuCo catalysts during 800 h [86]; (b) conversion of CO and selectivity of CO2, hydrocarbons, and total alcohols. (c) Distribution of alcohols. Copyright (2017) American Chemical Society.
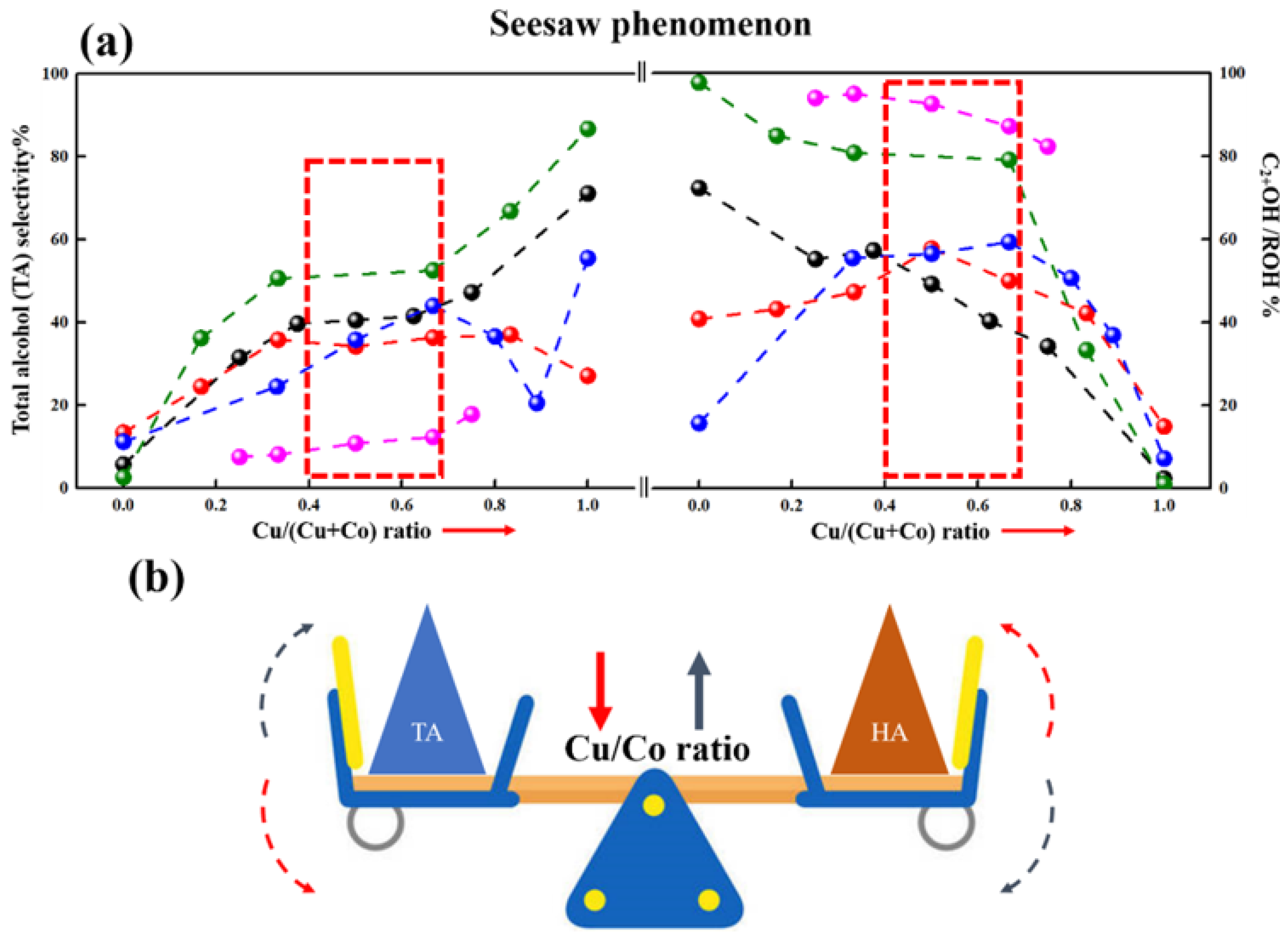
The Cu/Co ratio played an important role in adjusting active sites over CuCo catalysts. Figure 5 summarizes the selectivity of total alcohol (TA) and HA with different Cu/Co ratios from recent studies. A “seesaw” phenomenon is clearly displayed in Figure 5a. For Cu-rich catalysts, TA selectivity was high, while HA selectivity was low. As the Cu/Co ratio decreased, the HA selectivity improved, but the TA selectivity decreased. Meanwhile, the proper TA and HA selectivity were performed over the catalysts with a ratio of nearly 1:1.
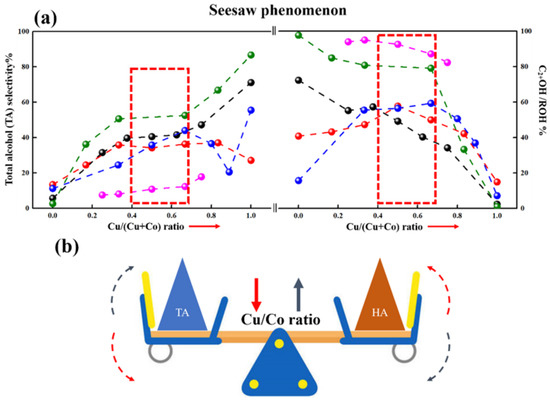
Figure 5.
Catalytic performance of CuCo catalysts varying Cu/Co ratio. (a) Catalytic performance of CuCo catalysts varying Cu/Co ratio, (b) Schematic diagram of “Seesaw” phenomenon. (Note: the red and black arrow in (b) represents the decrease and increase in Cu/Co ratio, respectively).
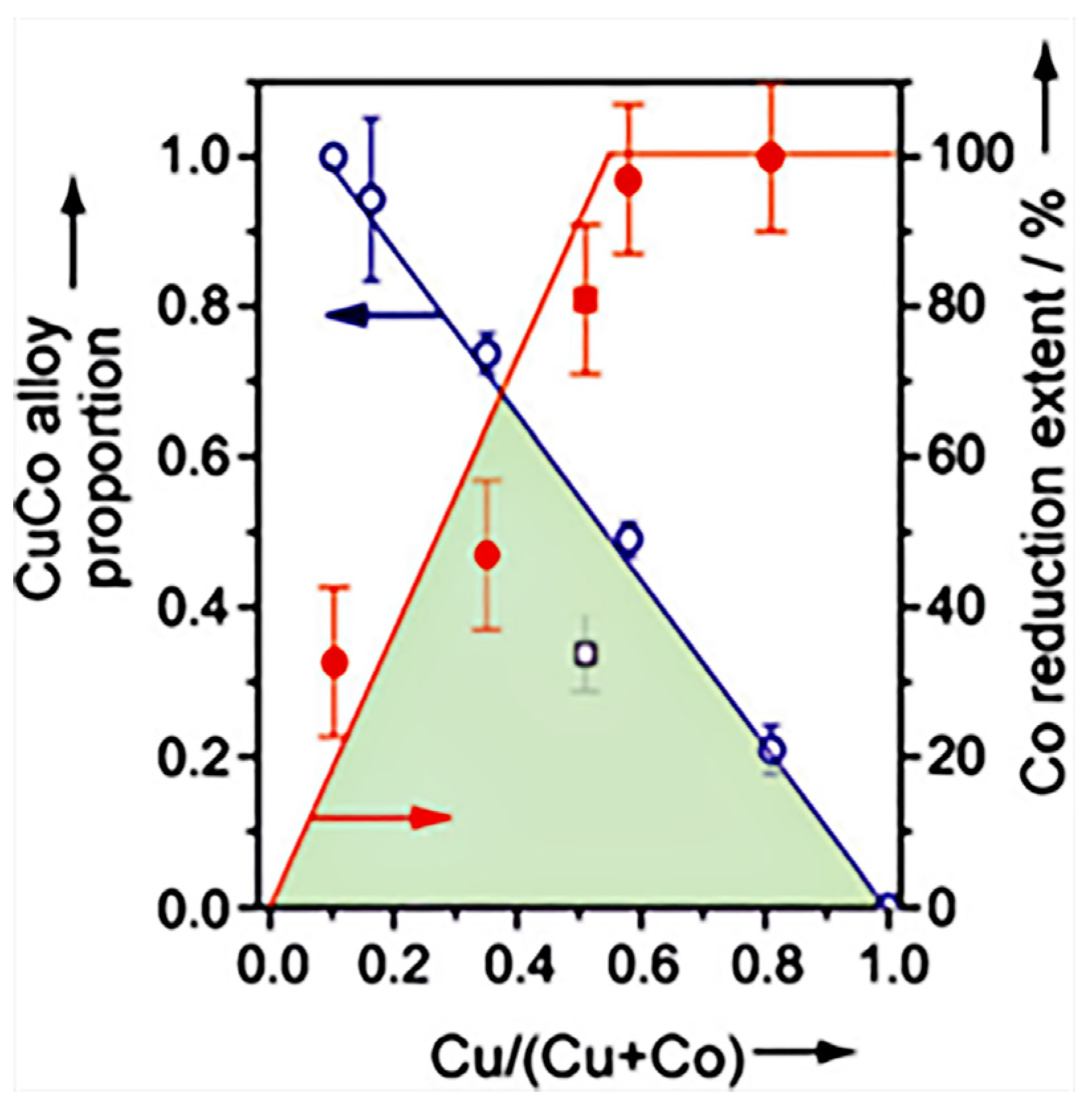
Prieto et al. [17] investigated the effect of the Cu/Co ratio on the active sites over the CuCoMo catalysts, and the evolution of the proportion of CuCo alloy was determined by XRD, and the extent of reduction of surface Co was determined by XPS (Figure 6). Obviously, a linear relationship was displayed between the proportion of the CuCo alloy phase and the Cu content, in which the CuCo alloy phase increased as the Cu content decreased. Conversely, the extent of reduction for Co species showed an increasing trend within the Cu/(Cu + Co) range of 0–0.5. The temperature-resolved X-ray diffraction and H2 consumption results indicated that Cu would be reduced at low temperatures over CuMo, while Co2+ was hard to be reduced over CoMo. However, with the existence of Cu, the Co2+ began to be reduced owing to the H2 dissociation on Cu. Thus, it was suggested that the dominant active sites on Cu-rich and Co-rich catalysts were different, and the effect of Cu/Co ratio needed to be taken into account to improve the stability.
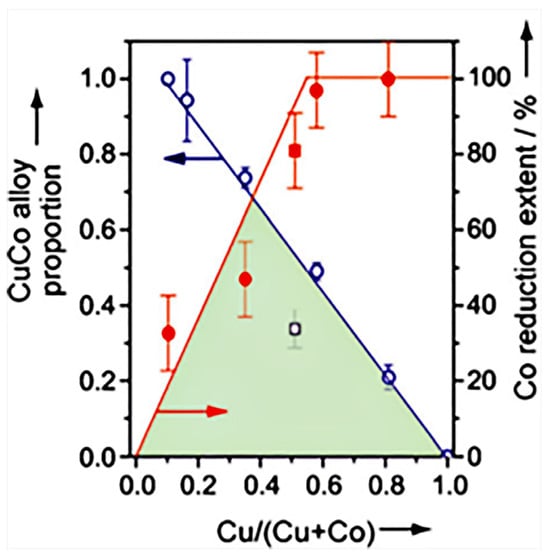
Figure 6.
Effect of Cu/Co ratio on the active sites over Cu-Co catalysts [17]. Copyright (2014) John Wiley and Sons.
For Cu-rich catalysts, owing to the hydrogen spillover effect, the existence of Cu facilitated the reduction of Co [89], which would weaken the role of unsaturated Co particles. However, owing to the different surface energy between Cu and Co, the segregation of Cu occurred. Liu et al. [90] investigated the size effect of Cu on HA synthesis over CuZnAl catalysts. The catalytic results showed that the HA selectivity was 8.9% over the catalysts with a smaller Cu size (11.8 nm), and the HA selectivity increased to 60.5% as the Cu size increased to 38.3 nm. They concluded that the larger Cu sizes favored forming HA. Zhang et al. [91] investigated the size dependence of C2 oxygenate formation from syngas on Cu cluster using the DFT method. The DFT results showed that the overall barrier differences between CHCO/CH3CO and CH4 formation were 58.9 and 11.6 kJ·mol−1 on the Cu13 cluster, and the value between CH2CO and CH4 was 21.9 kJ·mol−1 on Cu38 cluster. The values between CH3CO and CH4 were lower over the Cu55 cluster (−23.0 kJ·mol−1) than the Cu13 cluster. The different dominant products of Cu clusters with different sizes (C2 oxygenates for Cu13 and Cu38, CH4 for Cu55) suggested that Cu cluster size could affect the selectivity toward C2 oxygenates as the adsorption ability of intermediate was affected by adjusting the Cu cluster size. Thus, the crystallite size of Cu played an important role in HA synthesis. Consequently, preventing the segregation of Cu was one of the key factors to improve the stability of Cu-rich catalysts.
For Co-rich catalysts, it was proven that the Co-rich surface favored restraining the segregation of Cu. However, the separation of CuCo alloy particles, as mentioned above, resulted in the deactivation of catalytic performance (Figure 4). Moreover, the formation of Co2C would change the ratio of Co0/Coδ+, for which both Coδ+ and Co0 could be carbonized when exposed to syngas. Li et al. [74] investigated the effect of the Co0/Co2C ratio on the catalytic performance of HA synthesis. By changing the content of Na, a series of catalysts with different Co0/Co2C ratios were successfully prepared. They found that the catalytic activity and paraffin selectivity increased with the increase in Co0/Co2C, while the oxygenates selectivity displayed a volcano variation trend. They concluded that the proper Co0/Co2C ratio favored facilitating the CO insertion. Thus, the ratio of Co0/Coδ+ played an important role in HA synthesis. Consequently, preventing the separation of alloy particles and controlling the proper Co0/Coδ+ ratio were the main factors in improving the stability of Co-rich catalysts.
4. Conclusions and Perspectives
Higher alcohol synthesis through the Fischer–Tropsch (F–T) process was of significant importance for the efficient utilization of fossil resources. CuCo catalysts were proven to be efficient candidates and attracted much interest. Fabricating the stable CuCo catalysts remained a major challenge owing to the complex active sites on the catalysts. In this short review, the recent development of active sites on the CuCo catalysts was summarized, emphasizing the relationship between the active sites and stability. The evolution of active sites varied with the Cu/Co ratio. For Cu-rich catalysts, the role of Coδ+ was weakened owing to the hydrogen spillover effect. However, the segregation of Cu has always occurred. For Co-rich catalysts, the separation of CuCo alloy particles and the change in the Co0/Coδ+ ratio occurred during the reaction. The evolution of the active sites had a significant effect on the catalytic performance of higher alcohol synthesis. Thus, to fabricate the stable CuCo catalysts, the Cu/Co ratio needed to be taken into account. With the development of the catalysts designing concept and reaction mechanism, the following issues are expected to be addressed.
Firstly, the impact of active sites on CO adsorption ability and their continual changes during reactions make it challenging to detect evolution in real time. Since alcohol distribution is closely related to active site behavior, studying the relationship between catalytic performance, alcohol distribution, and active sites is crucial for developing effective methods to explore catalytic mechanisms.
Secondly, the catalytic mechanism remains unclear due to the diverse forms of unsaturated Co species, such as CoO, Co2C, and Coδ+. Advanced characterization techniques should be used to identify the CO adsorption and insertion abilities of these species.
Finally, the “seesaw” phenomenon should be investigated. The Cu-rich catalysts exhibit higher total selectivity and lower HA selectivity. The effect of the Cu0/Cu+ ratio and the crystallite size of Cu should be further revealed. The Co-rich catalysts exhibit lower total selectivity and higher HA selectivity. Studies on preventing the separation of alloy particles and controlling the proper Co0/Coδ+ ratio should be further investigated.
We hope that this review will inspire the designing of efficient and stable CuCo catalysts and foster further advancements in HA synthesis. Moreover, we eagerly anticipate the emergence of innovative research dedicated to overcoming the challenges of HA synthesis.
Author Contributions
Conceptualization, C.H. and W.H; methodology, C.H.; software, J.L.; validation, Z.P.; formal analysis, L.L.; investigation, L.W. and J.H.; resources, W.H.; data curation, C.H., Z.P. and L.L.; writing—original draft preparation, C.H.; writing—review and editing, C.H.; visualization, L.L. and Z.P.; supervision, W.H; project administration, L.L. and W.H.; funding acquisition, W.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project of science and technology cooperation and exchange program of Shanxi province (202104041101018); National Natural Science Youth Fund (NO. 22308245).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 6861, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Xi, X.; Cao, H.; Pei, Y.; Heeres, H.J.; Palkovits, R. Synthesis of mixed alcohols with enhanced C3+ alcohol production by CO hydrogenation over potassium promoted molybdenum sulfide. Appl. Catal. B Environ. 2019, 246, 232–241. [Google Scholar] [CrossRef]
- Ao, M.; Pham, G.H.; Sunarso, J.; Tade, M.O.; Liu, S.M. Active Centers of Catalysts for Higher Alcohol Synthesis from Syngas: A Review. ACS Catal. 2018, 8, 7025–7050. [Google Scholar] [CrossRef]
- Luk, H.T.; Mondelli, C.; Ferre, D.C.; Stewart, J.A.; Perez, R.J. Status and prospects in higher alcohols synthesis from syngas. Chem. Soc. Rev. 2017, 46, 1358–1426. [Google Scholar] [CrossRef] [PubMed]
- Henrik, L. Renewable energy strategies for sustainable development. Energy 2007, 32, 912–919. [Google Scholar] [CrossRef]
- Zhang, G.J.; Dong, Y.; Feng, M.R.; Zhang, Y.F.; Zhao, W.; Cao, H.C. CO2 reforming of CH4 in coke oven gas to syngas over coal char catalyst. Chem. Eng. J. 2010, 156, 519–523. [Google Scholar] [CrossRef]
- Alex Mills, G. Status and future opportunities for conversion of synthesis gas to liquid fuels. Fuel 1994, 73, 1243–1279. [Google Scholar] [CrossRef]
- Thomas, V.; Kwong, A. Ethanol as a lead replacement: Phasing out leaded gasoline in Africa. Energy Policy 2001, 29, 1133–1143. [Google Scholar] [CrossRef]
- Berg, C. World fuel ethanol–Analysis and Outlook. In Sugar Trading Manual; Woodhead Publishing: Sawston, UK, 2004; pp. 1–32. [Google Scholar]
- Verkerk, K.A.N.; Jaeger, B.B.; Finkeldei, C.H.; Keim, W. Recent Developments in Isobutanol Synthesis from Synthesis Gas. Appl. Catal. A-Gen. 1999, 186, 407–431. [Google Scholar] [CrossRef]
- Fang, K.; Li, D.; Lin, M.; Xiang, M.; Wei, W.; Sun, Y. A Short Review of Heterogeneous Catalytic Process for Mixed Alcohols Synthesis via Syngas. Catal. Today 2009, 147, 133–138. [Google Scholar] [CrossRef]
- Xu, X.; Doesburg, E.; Scholten, J. Synthesis of higher alcohols from syngas—Recently patented catalysts and tentative ideas on the mechanism. Catal. Today 1987, 2, 125–170. [Google Scholar] [CrossRef]
- Cao, A.; Schumann, J.L.; Wang, T.; Zhang, L.A.; Xiao, J.P.; Bothra, P.; Liu, Y.; Abild-Pedersen, F.; Nørskov, J.K. Mechanistic Insights into the Synthesis of Higher Alcohols from Syngas on CuCo Alloys. ACS. Catal. 2018, 8, 10148–10155. [Google Scholar] [CrossRef]
- Schmidt, S.; Göbel, C.; Nebel, J.; Wiesmann, T.; Hamel, C.; Reinsdorf, A.; Wolf, D.; Gehrmann, S.; Tenhumberg, N.; Muhler, M.; et al. Recent Developments in the Conversion of Synthesis Gas to Short-Chain Alcohols over Cu-Co-Based Catalysts. Chem. Ing. Tech. 2018, 90, 1465–1475. [Google Scholar] [CrossRef]
- Wang, J.J.; Chernavskii, P.A.; Khodakov, A.Y.; Wang, Y. Structure and catalytic performance of alumina-supported copper–cobalt catalysts for carbon monoxide hydrogenation. J. Catal. 2012, 286, 51–61. [Google Scholar] [CrossRef]
- Rocha, A.L.; Solórzano, I.G.; Vander Sande, J.B. Heterogeneous and homogeneous nanoscale precipitation in dilute Cu–Co alloys. Mater. Sci. Eng. C 2007, 27, 1215–1221. [Google Scholar] [CrossRef]
- Prieto, G.; Beijer, S.; Smith, M.L.; He, M.; Au, Y.; Wang, Z.; Bruce, D.A.; de Jong, K.P.; Spivey, J.J.; de Jongh, P.E. Design and synthesis of copper-cobalt catalysts for the selective conversion of synthesis gas to ethanol and higher alcohols. Angew. Chem. Int. Ed. Engl. 2014, 53, 6397–6401. [Google Scholar] [CrossRef] [PubMed]
- de La Pena O’Shea, V.A.; Homs, N.; Pereira, E.B.; Nafria, R.; Piscina, P.R.d.L. X-ray diffraction study of Co3O4 activation under ethanol steam-reforming. Catal. Today 2007, 126, 148–152. [Google Scholar] [CrossRef]
- Potoczna-Petru, D.; Kpiński, L. Reduction study of Co3O4 model catalyst by electron microscopy. Catal. Lett. 2001, 73, 41–46. [Google Scholar] [CrossRef]
- Llorca, J.; Dalmon, J.A.; de La Piscina, P.R.; Homs, N. In situ magnetic characterisation of supported cobalt catalysts under steam-reforming of ethanol. Appl. Catal. A-Gen. 2003, 243, 261–269. [Google Scholar] [CrossRef]
- Sun, K.; Song, F.; Huang, W.; Tang, Y.; Zhang, Y.; Zhang, J.; Wang, Y.; Tan, Y.S. CO hydrogenation to ethanol and higher alcohols over ultrathin CuCoAl nanosheets derived from LDH precursor. Fuel 2023, 333, 126308. [Google Scholar] [CrossRef]
- Gao, S.; Liu, N.; Liu, J.; Chen, W.K.; Liang, X.L.; Yuan, Y.Z. Synthesis of higher alcohols by CO hydrogenation over catalysts derived from LaCo1−xMnxO3 perovskites: Effect of the partial substitution of Co by Mn. Fuel 2020, 261, 116415. [Google Scholar] [CrossRef]
- Xiang, Y.Z.; Barbosa, R.; Kruse, N. Higher Alcohols through CO Hydrogenation over CoCu Catalysts: Influence of Precursor Activation. ACS Catal. 2014, 4, 2792–2800. [Google Scholar] [CrossRef]
- Liu, G.L.; Geng, Y.X.; Pan, D.M.; Zhang, Y.; Niu, T.; Liu, Y. Bi-metal Cu–Co from LaCo1−xCuxO3 perovskite supported on zirconia for the synthesis of higher alcohols. Fuel Process. Technol. 2014, 128, 289–296. [Google Scholar] [CrossRef]
- Li, Z.S.; Zhuang, Z.; Yao, D.W.; Fan, S.Q.; Guo, S.X.; Lv, J.; Huang, S.Y.; Wang, Y.; Ma, X.B. High-performance CoCu catalyst encapsulated in KIT-6 for higher alcohol synthesis from syngas. ACS Sustain. Chem. Eng. 2020, 8, 200–209. [Google Scholar] [CrossRef]
- Khan, W.U.; Baharudin, L.; Choi, J.; Yip, A.C.K. Recent Progress in CO Hydrogenation over Bimetallic Catalysts for Higher Alcohol Synthesis. ChemCatChem 2020, 13, 111–120. [Google Scholar] [CrossRef]
- Xiao, K.; Bao, Z.H.; Qi, X.Z.; Wang, X.X.; Zhong, L.S.; Fang, K.G.; Lin, M.G.; Sun, Y.H. Advances in bifunctional catalysis for higher alcohol synthesis from syngas. Chin. J. Catal. 2013, 34, 116–129. [Google Scholar] [CrossRef]
- Xue, X.; Weng, Y.; Yang, S.; Meng, S.; Sun, Q.; Zhang, Y. Research progress of catalysts for synthesis of low-carbon alcohols from synthesis gas. RSC Adv. 2021, 11, 6163–6172. [Google Scholar] [CrossRef]
- Medford, A.J.; Lausche, A.C.; Abild-Pedersen, F.; Temel, B. Activity and Selectivity Trends in Synthesis Gas Conversion to Higher Alcohols. Top. Catal. 2014, 57, 135–142. [Google Scholar] [CrossRef]
- Subramani, V.; Gangwal, S.K. A Review of Recent Literature to Search for an Efficient Catalytic Process for the Conversion of Syngas to Ethanol. Energy Fuels 2008, 22, 117–136. [Google Scholar] [CrossRef]
- Su, J.; Zhang, Z.; Fu, D.; Liu, D.; Xu, X.C.; Shi, B.; Wang, X.; Si, R.; Jiang, Z.; Xu, J.; et al. Higher Alcohols Synthesis from Syngas over CoCu/SiO2 Catalysts: Dynamic Structure and the Role of Cu. J. Catal. 2016, 336, 94–106. [Google Scholar] [CrossRef]
- Liu, J.G.; Ding, M.Y.; Wang, T.J.; Ma, L.L. Promoting Effect of Cobalt Addition on Higher Alcohols Synthesis over Copper-Based Catalysts. Adv. Mater. Res. 2012, 550–553, 270–275. [Google Scholar] [CrossRef]
- Li, Z.S.; Luo, G.Y.; Chen, T.; Zeng, Z.; Guo, S.X.; Lv, J.; Huang, S.Y.; Wang, Y.; Ma, X.B. Bimetallic CoCu catalyst derived from in-situ grown Cu-ZIF-67 encapsulated inside KIT-6 for higher alcohol synthesis from syngas. Fuel 2020, 278, 118292. [Google Scholar] [CrossRef]
- Yang, Z.D.; Ma, E.J.; Zhang, Q.; Luan, C.H.; Huang, W. Catalytic performance of CuCoCe supported on nitrogen-doped carbon nanotubes for the synthesis of higher alcohols from syngas. J. Fuel Chem. Technol. 2020, 48, 9. [Google Scholar]
- Tien-Thao, N.; Zahedi-Niaki, M.H.; Alamdari, H.; Kaliaguine, S. Effect of alkali additives over nanocrystalline Co–Cu-based perovskites as catalysts for higher-alcohol synthesis. J. Catal. 2007, 245, 348–357. [Google Scholar] [CrossRef]
- Tien-Thao, N.; Zahedi-Niaki, M.H.; Alamdari, H.; Kaliaguine, S. Conversion of syngas to higher alcohols over nanosized LaCo0.7Cu0.3O3 perovskite precursors. Appl. Catal. A-Gen. 2007, 326, 152–163. [Google Scholar] [CrossRef]
- Cao, A.; Liu, G.L.; Wang, L.F.; Liu, J.G.; Yue, Y.Z.; Zhang, L.H.; Liu, Y. Growing layered double hydroxides on CNTs and their catalytic performance for higher alcohol synthesis from syngas. J. Mater. Sci. 2016, 51, 5216–5231. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, J.N.; Zhang, Q.L.; Li, Y.; Fang, K.G. Preparation, Structural Characteristics, and Catalytic Performance of Cu–Co Alloy Supported on Mn–Al Oxide for Higher Alcohol Synthesis via Syngas. Ind. Eng. Chem. Res. 2018, 57, 14957–14966. [Google Scholar] [CrossRef]
- Cao, A.; Liu, G.L.; Yue, Y.Z.; Zhang, L.H.; Liu, Y. Nanoparticles of Cu–Co alloy derived from layered double hydroxides and their catalytic performance for higher alcohol synthesis from syngas. RSC Adv. 2015, 5, 58804–58812. [Google Scholar] [CrossRef]
- Niu, T.; Liu, G.L.; Chen, Y.; Yang, J.; Wu, J.; Cao, Y.; Liu, Y. Hydrothermal synthesis of graphene-LaFeO3 composite supported with Cu-Co nanocatalyst for higher alcohol synthesis from syngas. Appl. Surf. Sci. 2016, 364, 388–399. [Google Scholar] [CrossRef]
- Wang, L.; Cao, A.; Liu, G.; Zhang, L.; Liu, Y. Bimetallic CuCo Nanoparticles Derived from Hydrotalcite Supported on Carbon Fibers for Higher Alcohols Synthesis from Syngas. Appl. Surf. Sci. 2016, 360, 77–85. [Google Scholar] [CrossRef]
- Sun, K.; Wu, Y.Q.; Tan, M.H.; Wang, L.Y.; Yang, G.H.; Zhang, M.; Zhang, W.; Tan, Y.S. Ethanol and higher alcohols synthesis from syngas over CuCoM (M=Fe, Cr, Ga and Al) nanoplates derived from hydrotalcite-like precursors. ChemCatChem 2019, 11, 2695–2706. [Google Scholar] [CrossRef]
- Subramanian, N.D.; Kumar, C.S.S.R.; Watanabe, K.; Fischer, P.; Tanaka, R.; Spivey, J.J. A DRIFTS study of CO adsorption and hydrogenation on Cu-based core–shell nanoparticles. Catal. Sci. Technol. 2012, 2, 621. [Google Scholar] [CrossRef]
- Lü, D.; Zhu, Y.; Sun, Y.H. Cu nanoclusters supported on Co nanosheets for selective hydrogenation of CO. Chin. J. Catal. 2013, 34, 1998–2003. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, Y.; Chen, H.; Li, Y.; He, S.; Zhang, Y.; Wei, M.; Evans, D.G.; Duan, X. Core-shell Cu@(CuCo-Alloy)/Al2O3 Catalysts for the Synthesis of Higher Alcohols from Syngas. Green Chem. 2015, 17, 1525–1534. [Google Scholar] [CrossRef]
- Subramanian, N.D.; Balaji, G.; Kumar, C.S.S.R.; Spivey, J.J. Development of cobalt–copper nanoparticles as catalysts for higher alcohol synthesis from syngas. Catal. Today 2009, 147, 100–106. [Google Scholar] [CrossRef]
- Ba, R.B.; Zhao, Y.H.; Yu, L.J.; Song, J.J.; Huang, S.S.; Zhong, L.S.; Sun, Y.H.; Zhu, Y. Synthesis of Co-based bimetallic nanocrystals with one-dimensional structure for selective control on syngas conversion. Nanoscale 2015, 7, 12365–12371. [Google Scholar] [CrossRef]
- Ye, T.Q.; Zhang, Z.X.; Xu, Y.; Yan, S.Z.; Zhu, J.F.; Liu, Y.; Li, Q.X. Higher Alcohol Synthesis from Bio·Syngas over Na·Promoted CuCoMn Catalyst. Acta Phys.-Chim. Sin. 2011, 27, 1493–1500. [Google Scholar]
- Sun, K.; Tan, M.H.; Bai, Y.X.; Gao, X.F.; Wang, P.; Gong, N.N.; Zhang, T.; Yang, G.H.; Tan, Y.S. Design and synthesis of spherical-platelike ternary copper-cobalt-manganese catalysts for direct conversion of syngas to ethanol and higher alcohols. J. Catal. 2019, 378, 1–16. [Google Scholar] [CrossRef]
- Liu, Y.J.; Deng, X.; Huang, W. Effect of non-metal promoters on higher alcohols synthesis from syngas over Cu-based catalyst. J. Energy Inst. 2018, 91, 1136–1142. [Google Scholar] [CrossRef]
- Yang, Q.L.; Cao, A.; Kang, N.; An, K.; Liu, Z.T.; Liu, Y. A new catalyst of Co/La2O3-doped La4Ga2O9 for direct ethanol synthesis from syngas. Fuel Process. Technol. 2018, 179, 42–52. [Google Scholar] [CrossRef]
- Bin, F.; Wei, X.; Li, B.; Hui, K.S. Self-sustained combustion of carbon monoxide promoted by the Cu-Ce/ZSM-5 catalyst in CO/O2/N2 atmosphere. Appl. Catal. B-Environ. 2015, 162, 282–288. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.L.; Zhao, Y.J.; Lv, J.; Wang, S.P.; Ma, X.B. Insight into the Balancing Effect of Active Cu Species for Hydrogenation of Carbon–Oxygen Bonds. ACS Catal. 2015, 5, 6200–6208. [Google Scholar] [CrossRef]
- Hofstadt, C.E.; Schneider, M.; Bock, O.; Kochloefl, K. Effect of Preparation Methods and Promoters on Activity and Selectivity of Cu−ZnO−Al2O3−K Catalysts in Aliphatic Alcohols Synthesis from CO and H2. Stud. Surf. Sci. Catal. 1983, 16, 709–721. [Google Scholar] [CrossRef]
- Gong, J.L.; Yue, H.R.; Zhao, Y.J.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.P.; Ma, X.B. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Li, C.; Huang, W. CO Hydrogenation to Higher Alcohols over Cu/Zn/Al Catalysts without Alkalis or Fischer− Tropsch Elements: The Effect of Triethanolamine Content. Catal. Commun. 2016, 76, 29–32. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zuo, Z.J.; Liu, C.B.; Li, C.; Deng, X.; Huang, W. Higher Alcohols Synthesis via CO Hydrogenation on Cu/Zn/Al/Zr Catalysts without Alkalis and F−T Elements. Fuel Process. Technol. 2016, 144, 186–190. [Google Scholar] [CrossRef]
- Zuo, Z.J.; Wang, L.; Liu, Y.J.; Huang, W. The Effect of CuOZnO-Al2O3 Catalyst Structure on the Ethanol Synthesis from Syngas. Catal. Commun. 2013, 34, 69–72. [Google Scholar] [CrossRef]
- Zuo, Z.J.; Wang, L.; Yu, L.M.; Han, P.D.; Huang, W. Experimental and Theoretical Studies of Ethanol Synthesis from Syngas over CuZnAl Catalysts without Other Promoters. J. Phys. Chem. C 2014, 118, 12890–12898. [Google Scholar] [CrossRef]
- Cui, N.; Liu, Y.J.; Jia, P.L.; Luo, P.; Huang, W. Investigation of alkaline complexant on ethanol synthesis from syngas over slurry CuZnAlOOH catalyst. Int. J. Hydrogen Energy 2021, 46, 21889–21900. [Google Scholar] [CrossRef]
- Bai, H.; Ma, M.M.; Bai, B.; Cao, H.J.; Zhang, L.; Gao, Z.H.; Vinokurovc, V.A.; Huang, W. Carbon chain growth by formyl coupling over the Cu/γ-AlOOH(001) surface in syngas conversion. Phys. Chem. Chem. Phys. 2018, 21, 148–159. [Google Scholar] [CrossRef]
- Sun, K.; Gao, X.F.; Bai, Y.X.; Tan, M.H.; Yang, G.H.; Tan, Y.S. Synergetic catalysis of bimetallic copper–cobalt nanosheets for direct synthesis of ethanol and higher alcohols from syngas. Catal. Sci. Technol. 2018, 8, 3936–3947. [Google Scholar] [CrossRef]
- Blanchard, M.; Derule, H.; Canesson, P. Cobalt Catalysts for the Production of Alcohols in the F.T. Synthesis. Synthesis. Catal. Lett. 1989, 2, 319–322. [Google Scholar] [CrossRef]
- Chen, T.Y.; Su, J.; Zhang, Z.C. Structure Evolution of Co-CoOx Interface for Higher Alcohol Synthesis from Syngas over Co/CeO2 Catalysts. ACS Catal. 2018, 8, 8606–8617. [Google Scholar] [CrossRef]
- An, K.; Zhang, S.R.; Wang, J.M.; Liu, Q.; Zhang, Z.Y.; Liu, Y. A highly selective catalyst of Co/La4Ga2O9 for CO2 hydrogenation to ethanol. J. Energy Chem. 2021, 56, 486–495. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.Y.; Li, Y.Y.; Yu, H.B.; Zhang, F.F.; Sun, Y.M.; Fang, H.H.; Zhang, X.B.; Liang, X.L.; Yuan, Y.Z. Effects of gallium as an additive on activated carbon-supported cobalt catalysts for the synthesis of higher alcohols from syngas. Fuel 2018, 230, 194–201. [Google Scholar] [CrossRef]
- Guo, S.X.; Liu, G.L.; Han, T.; Zhang, Z.Y.; Liu, Y. K-Modulated Co Nanoparticles Trapped in La-Ga-O as Superior Catalysts for Higher Alcohols Synthesis from Syngas. Catalysts 2019, 9, 218. [Google Scholar] [CrossRef]
- Ning, X.; An, Z.; He, J. Remarkably efficient CoGa catalyst with uniformly dispersed and trapped structure for ethanol and higher alcohol synthesis from syngas. J. Catal. 2016, 340, 236–247. [Google Scholar] [CrossRef]
- An, Z.; Ning, X.; He, J. Ga-promoted CO insertion and C-C coupling on Co catalysts for the synthesis of ethanol and higher alcohols from syngas. J. Catal. 2017, 356, 157–164. [Google Scholar] [CrossRef]
- Kang, N.; Yang, Q.; An, K.; Li, S.S.; Zhang, L.H.; Liu, Y. Mixed oxides of La-Ga-O modified Co/ZrO2 for higher alcohols synthesis from syngas. Catal. Today 2019, 330, 46–53. [Google Scholar] [CrossRef]
- Paterson, J.; Partington, R.; Peacock, M.; Sullivan, K.; Wilson, J.; Xu, Z. Elucidating the Role of Bifunctional Cobalt-Manganese Catalyst Interactions for Higher Alcohol Synthesis. Eur. J. Inorg. 2020, 2020, 2312–2324. [Google Scholar] [CrossRef]
- Du, H.; Zhu, H.; Chen, X.; Dong, W.; Lu, W.; Luo, W.; Jiang, M.; Liu, T.; Ding, Y. Study on CaO-Promoted Co/AC Catalysts for Synthesis of Higher Alcohols from Syngas. Fuel 2016, 182, 42–49. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Bai, Y.; Gao, X.; Li, X.; Sun, K.; Xie, H.; Yang, G.; Han, Y.; Tan, Y. Effect of the Promoter and Support on Cobalt-Based Catalysts for Higher Alcohols Synthesis through CO Hydrogenation. Fuel 2017, 195, 69–81. [Google Scholar] [CrossRef]
- Nebel, J.; Schmidt, S.; Pan, Q.; Lotz, K.; Kaluza, S.; Muhler, M. On the role of cobalt carbidization in higher alcohol synthesis over hydrotalcite-based Co-Cu catalysts. Chin. J. Catal. 2019, 40, 1731–1740. [Google Scholar] [CrossRef]
- Li, L.S.; Lin, T.J.; Li, X.; Wang, C.Q.; Qin, T.T.; An, Y.L.; Lu, Y.W.; Zhong, L.S.; Sun, Y.H. Control of Co0/Co2C dual active sites for higher alcohols synthesis from syngas. Appl. Catal. A-Gen. 2020, 602, 117704. [Google Scholar] [CrossRef]
- Fan, S.Q.; Wang, Y.; Li, Z.S.; Zeng, Z.; Yao, D.W.; Huang, S.Y.; Ma, X.B. Graphene oxide-ordered mesoporous silica composite supported Co-based catalysts for CO hydrogenation to higher alcohols. Appl. Catal. A-Gen. 2019, 583, 117123. [Google Scholar] [CrossRef]
- Wang, Z.; Kumar, N.; Spivey, J.J. Preparation and characterization of lanthanum-promoted cobalt–copper catalysts for the conversion of syngas to higher oxygenates: Formation of cobalt carbide. J. Catal. 2016, 339, 1–8. [Google Scholar] [CrossRef]
- Qin, T.T.; Lin, T.J.; Qi, X.Z.; Wang, C.Q.; Li, L.S.; Tang, Z.Y.; Zhong, L.S.; Sun, Y.H. Tuning chemical environment and synergistic relay reaction to promote higher alcohols synthesis via syngas conversion. Appl. Catal. B Environ. 2021, 285, 119840. [Google Scholar] [CrossRef]
- Liu, Y.; He, S.; Yang, R.O.; Sun, F.F.; Yang, Y.Q.; Mei, B.B.; Kang, J.C.; Wu, D.S.; Jiang, Z. Tuning the interfaces of Co–Co2C with sodium and its relation to the higher alcohol production in Fischer–Tropsch synthesis. J. Mater. Sci. 2020, 55, 9037–9047. [Google Scholar] [CrossRef]
- Zhao, Z.A.; Lu, W.; Yang, R.O.; Zhu, H.J.; Dong, W.D.; Sun, F.F.; Jiang, Z.; Lyu, Y.; Liu, T.; Du, H.; et al. Insight into the Formation of Co@Co2C Catalysts for Direct Synthesis of Higher Alcohols and Olefins from Syngas. ACS Catal. 2017, 8, 228–241. [Google Scholar] [CrossRef]
- Cui, W.G.; Li, Y.T.; Zhang, H.B.; Wei, Z.C.; Gao, B.H.; Dai, J.J.; Hu, T.L. In situ encapsulated Co/MnOx nanoparticles inside quasi-MOF-74 for the higher alcohols synthesis from syngas. Appl. Catal. B Environ. 2020, 278, 119262. [Google Scholar] [CrossRef]
- Pei, Y.P.; Liu, J.X.; Zhao, Y.H.; Ding, Y.J.; Liu, T.; Dong, W.D.; Zhu, H.J.; Su, H.Y.; Yan, L.; Li, J.L.; et al. High Alcohols Synthesis via Fischer–Tropsch Reaction at Cobalt Metal/Carbide Interface. ACS Catal. 2015, 5, 3620–3624. [Google Scholar] [CrossRef]
- Du, H.; Jiang, M.; Zhao, Z.; Li, Y.H.; Liu, T.; Zhu, H.J.; Zhang, Z.C.; Ding, Y.J. Alcohol Synthesis via Fischer–Tropsch Synthesis over Activated Carbon Supported Alkaline Earth Modified Cobalt Catalyst. Catal. Lett. 2021, 151, 3632–3638. [Google Scholar] [CrossRef]
- Lebarbier, V.M.; Mei, D.; Kim, D.H. Effects of La2O3 on the Mixed Higher Alcohols Synthesis from Syngas over Co Catalysts: A Combined Theoretical and Experimental Study. J. Phys. Chem. C 2011, 115, 17440–17451. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Qi, X.Z.; Wang, X.X.; Lv, D.; Yu, F.; Zhong, L.S.; Wang, H.; Sun, Y.H. Deactivation study of CuCo catalyst for higher alcohol synthesis via syngas. Catal. Today 2016, 270, 101–107. [Google Scholar] [CrossRef]
- Yang, Q.L.; Cao, A.; Kang, N.; Ning, H.Y.; Wang, J.M.; Liu, Z.T.; Liu, Y. Bimetallic Nano Cu–Co Based Catalyst for Direct Ethanol Synthesis from Syngas and Its Structure Variation with Reaction Time in Slurry Reactor. Ind. Eng. Chem. Res. 2017, 56, 2889–2898. [Google Scholar] [CrossRef]
- Göbel, C.; Schmidt, S.; Froese, C.; Fu, Q.; Chen, Y.T.; Pan, Q.S.; Muhler, M. Structural evolution of bimetallic Co-Cu catalysts in CO hydrogenation to higher alcohols at high pressure. J. Catal. 2020, 383, 33–41. [Google Scholar] [CrossRef]
- Liu, G.L.; Niu, T.; Cao, A.; Geng, Y.X.; Zhang, Y.; Liu, Y. The deactivation of Cu–Co alloy nanoparticles supported on ZrO2 for higher alcohols synthesis from syngas. Fuel 2016, 176, 1–10. [Google Scholar] [CrossRef]
- Xiong, M.; Gao, Z.; Qin, Y. Spillover in Heterogeneous Catalysis: New Insights and Opportunities. ACS Catal. 2021, 11, 3159–3172. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, Z.W.; Luo, P.; Cui, N.; Wang, K.J.; Huang, W. Size-dependent and sensitivity of copper particle in ternary CuZnAl catalyst for syngas to ethanol. Appl. Catal. B Environ. 2023, 336, 122949. [Google Scholar] [CrossRef]
- Zhang, R.G.; Peng, M.; Duan, T.; Wang, B.J. Insight into size dependence of C2 oxygenate synthesis from syngas on Cu cluster: The effect of cluster size on the selectivity. Appl. Surf. Sci. 2017, 407, 282–296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).