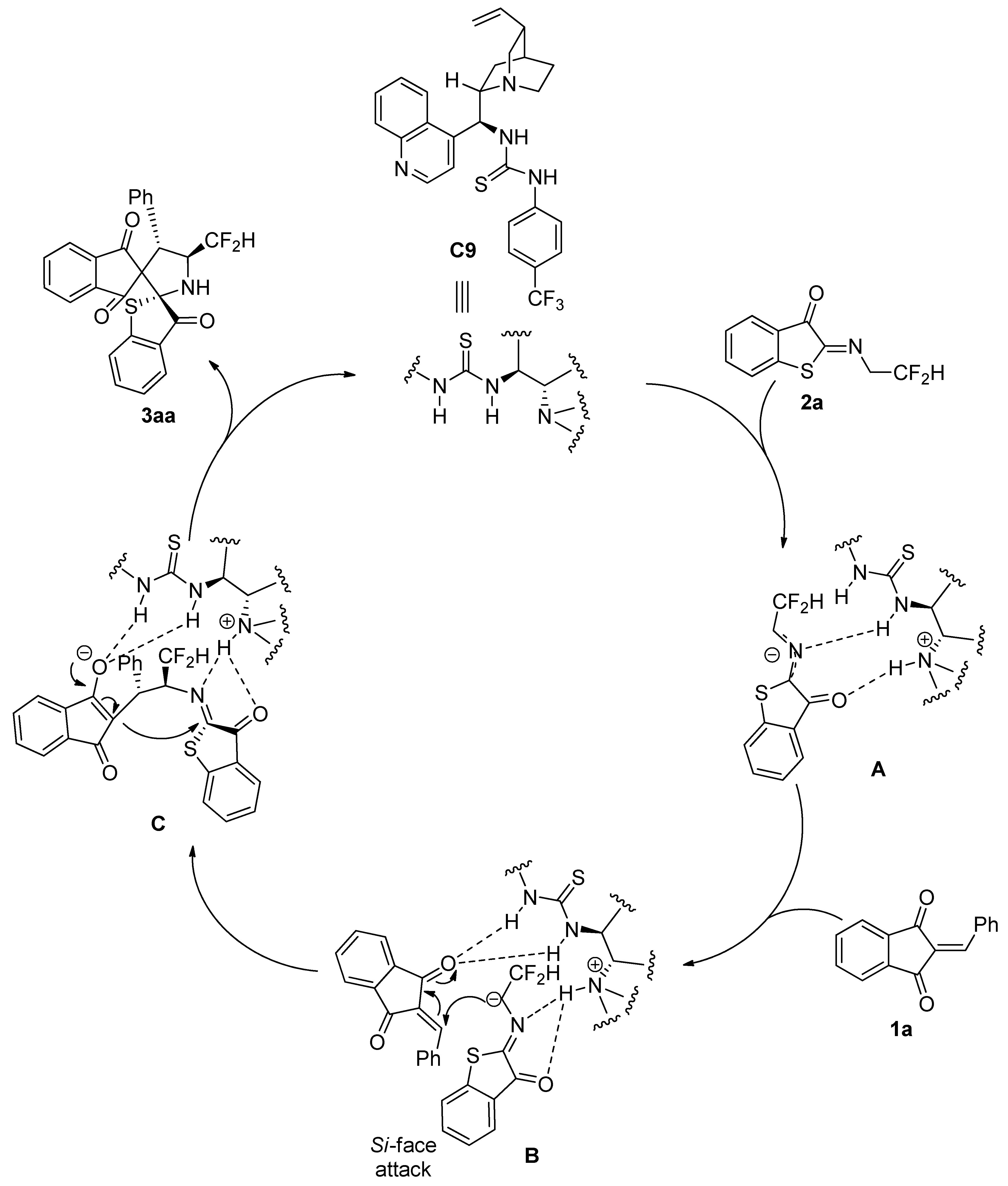
3.3. Procedure for the Asymmetric Synthesis of Compounds 3
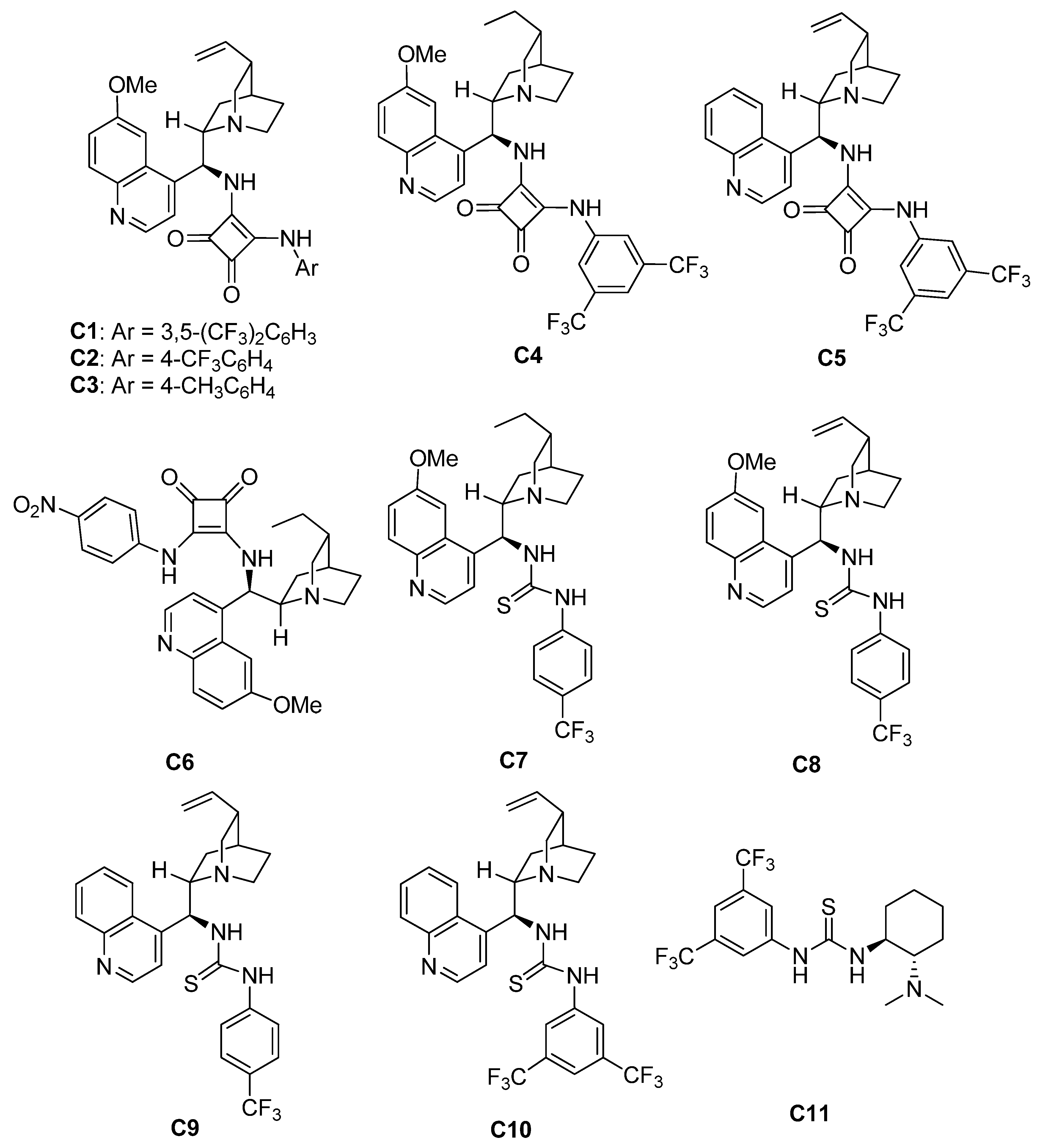
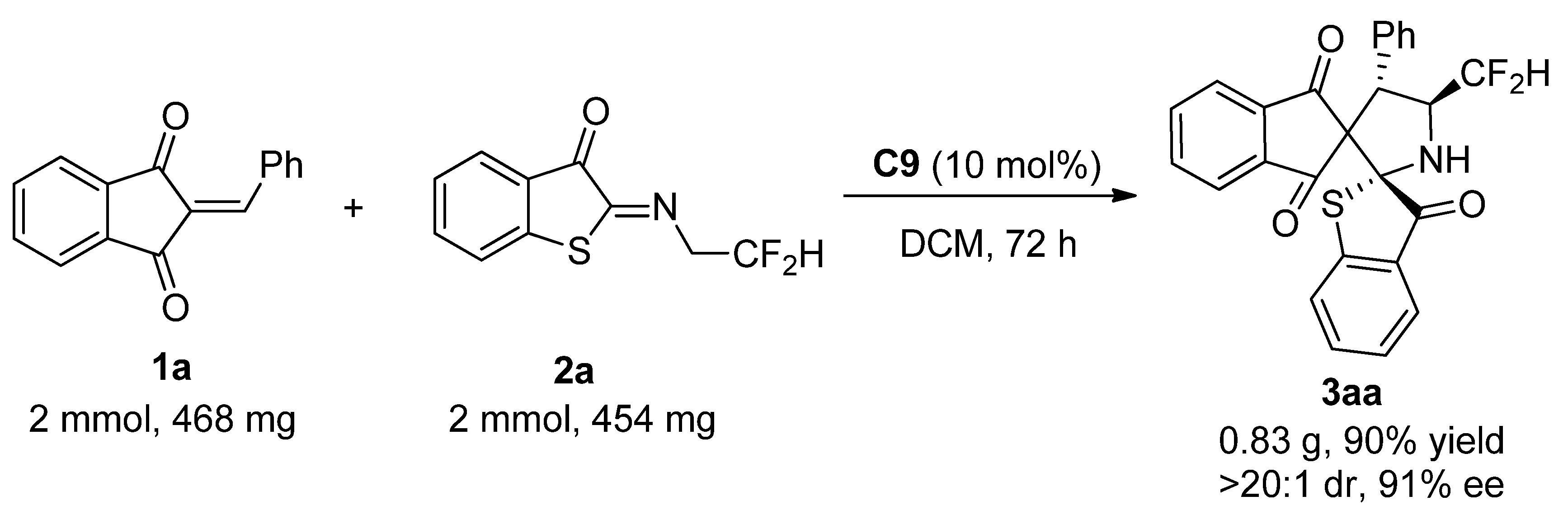
2-Arylidene-1,3-indandione 1 (0.10 mmol), N-2,2-difluoroethylbenzothiophenone imine 2 (0.10 mmol), organocatalyst C9 (4.8 mg, 0.01 mmol, 10 mol%), and CH2Cl2 (2.0 mL) were sequentially added to a small, dried glass bottle. The reaction mixture was stirred at room temperature for 24 h. After completion of the reaction, the solvent was evaporated under reduced pressure, and the residue was purified by silica gel (200–300 mesh) flash column chromatography using ethyl acetate/petroleum ether (1:7) as eluent. The pure products 3 were obtained. The corresponding racemate sample was prepared following a similar procedure with Et3N (10 mol%).
(2S,4′S,5′S)-5′-(Difluoromethyl)-4′-phenyl-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3aa). From 1a (23.4 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3aa (43.8 mg, 95% yield) was obtained as a white solid, m.p. 215–217 °C. HPLC (Daicel Chiralpak IC column, mobile phase n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 9.5 min (minor), tR = 12.0 min (major); 93% ee. [α]D25 = −8.1 (c = 0.54, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.83 (d, J = 7.6 Hz, 1H, ArH), 7.79 (d, J = 7.2 Hz, 1H, ArH), 7.69–7.60 (m, 3H, ArH), 7.45–7.41 (m, 1H, ArH), 7.22 (t, J = 7.6 Hz, 1H, ArH), 7.10 (d, J = 7.2 Hz, 2H, ArH), 7.06–6.98 (m, 4H, ArH), 6.25 (td, J1 = 56.8, J2 = 6.8 Hz, 1H, CF2H), 4.82 (d, J = 10.0 Hz, 1H, CH), 4.72–4.63 (m, 1H, CH), 3.36 (br s, 1H, NH) ppm. 13C NMR (100MHz, CDCl3): δ 200.4, 198.7, 196.1, 147.4, 142.5, 142.1, 136.3, 136.1, 135.8, 132.7, 129.1, 128.5, 128.4, 127.9, 127.6, 125.9, 123.5, 123.21, 123.17, 118.1 (t, 1JC–F = 241.9 Hz), 81.2, 71.2, 62.2 (dd, 2JC–F = 27.3, 21.3 Hz), 51.9 (d, 3JC–F = 7.6 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ −117.2 (d, J = 288.4 Hz, 1F), −121.8 (d, J = 288.8 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C26H18F2NO3S [M + H]+ 462.0970, found 462.0957.
(2S,4′S,5′S)-5′-(Difluoromethyl)-4′-(m-tolyl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3ba). From 1b (24.8 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3ba (42.8 mg, 90% yield) was obtained as a white solid, m.p. 193–195 °C. HPLC (Daicel Chiralpak ADH column, mobile phase n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 11.9 min (major), tR = 23.2 min (minor); 48% ee. [α]D25 = −189.6 (c = 0.75, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.83 (d, J = 7.2 Hz, 1H, ArH), 7.78 (d, J = 7.6 Hz, 1H, ArH), 7.69–7.59 (m, 3H, ArH), 7.45–7.40 (m, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.03 (d, J = 8.0 Hz, 1H, ArH), 6.95–6.86 (m, 3H, ArH), 6.80 (d, J = 6.8 Hz, 1H, ArH), 6.24 (td, J1 = 56.8 Hz, J2 = 6.5 Hz, 1H, CF2H), 4.78 (d, J = 9.6 Hz, 1H, CH), 4.69–4.61 (m, 1H, CH), 3.35 (br s, 1H, NH), 2.11 (s, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 200.5, 198.7, 196.2, 147.4, 142.5, 142.2, 138.0, 136.2, 136.1, 135.7, 132.6, 129.2, 129.1, 128.7, 128.3, 127.6, 125.9, 125.4, 123.5, 123.12, 123.05, 118.1 (t, 1JC–F = 242.0 Hz), 81.2, 71.2, 62.1 (dd, 2JC–F = 27.2, 21.4 Hz), 51.9 (d, 3JC–F = 7.5 Hz), 21.2. 19F NMR (376 MHz, CDCl3): δ −117.2 (d, J = 288.4 Hz, 1F), −121.7 (d, J = 288.0 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C27H20F2NO3S [M + H]+ 476.1127, found 476.1118.
(2S,4′S,5′S)-5′-(Difluoromethyl)-4′-(p-tolyl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3ca). From 1c (24.8 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3ca (42.8 mg, 90% yield) was obtained as a white solid, m.p. 164–165 °C. HPLC (Daicel Chiralpak ADH column, mobile phase n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 13.9 min (major), tR = 29.7 min (minor); 3% ee. [α]D25 = −128.2 (c = 0.29, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.81 (t, J = 7.4 Hz, 2H, ArH), 7.70–7.60 (m, 3H, ArH), 7.42 (td, J1 = 7.6 Hz, J2 = 1.2 Hz, 1H, ArH), 7.21 (t, J = 7.2 Hz, 1H, ArH), 7.02 (d, J = 7.6 Hz, 1H, ArH), 6.98 (d, J = 8.4 Hz, 2H, ArH), 6.84 (d, J = 8.0 Hz, 2H, ArH), 6.23 (dd, J1 = 56.8, J2 = 6.8 Hz, 1H, CF3H), 4.78 (d, J = 9.6 Hz, 1H, CH), 4.68–4.60 (m, 1H, CH), 3.32 (br s, 1H, NH), 2.10 (s, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 200.4, 198.8, 196.2, 147.4, 142.6, 142.2, 137.6, 136.2, 136.1, 135.8, 134.5, 129.64, 129.59, 129.2, 128.3, 127.6, 125.9, 123.5, 123.23, 123.17, 118.1 (t, 1JC–F = 241.8 Hz), 81.3, 71.1, 62.3 (dd, 2JC–F = 27.2, 21.2 Hz), 51.5 (d, 3JC–F = 7.5 Hz), 20.9 ppm. 19F NMR (376 MHz, CDCl3): δ −117.1 (d, J = 288.4 Hz, 1F), −121.8 (d, J = 288.4 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C27H20F2NO3S [M + H]+ 476.1127, found 476.1126.
(2S,4′S,5′S)-5′-(Difluoromethyl)-4′-(4-methoxyphenyl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3da). From 1d (26.4 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3da (46.6 mg, 95% yield) was obtained as a white solid, m.p. 199–201 °C. HPLC (Daicel Chiralpak ADH column, mobile phase n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 16.8 min (major), tR = 32.9 min (minor); 68% ee. [α]D25 = −36.0 (c = 0.17, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.82 (t, J = 7.6 Hz, 2H, ArH), 7.71–7.61 (m, 3H, ArH), 7.43 (td, J1 = 7.6 Hz, J2 = 1.2 Hz, 1H, ArH), 7.23–7.19 (m, 1H, ArH), 7.03 (d, J = 8.0 Hz, 1H, ArH), 6.96 (t, J = 7.8 Hz, 1H, ArH), 6.69 (d, J = 7.6 Hz, 1H, ArH), 6.62 (s, 1H, ArH), 6.54 (dd, J1 = 8.2 Hz, J2 = 2.2 Hz, 1H, ArH), 6.24 (td, J1 = 56.6 Hz, J2 = 6.8 Hz, 1H, CF2H), 4.79 (d, J = 9.6 Hz, 1H, CH), 4.68–4.60 (m, 1H, CH), 3.63 (s, 3H, OCH3), 3.27 (br s, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 200.5, 198.6, 196.1, 159.3, 147.5, 142.5, 142.2, 136.3, 136.2, 135.8, 134.3, 129.5, 129.1, 127.6, 125.9, 123.5, 123.2, 123.1, 120.7, 118.1 (t, 1JC–F = 242.1 Hz), 113.9, 113.8, 81.2, 71.1, 62.2 (dd, 2JC–F = 27.2, 21.4 Hz), 55.1, 51.9 (d, 3JC–F = 7.5 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ −117.3 (d, J = 288.4 Hz, 1F), −121.8 (d, J = 288.4 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C27H20F2NO4S [M + H]+ 492.1076, found 492.1078.
(2S,4′S,5′S)-5′-(Difluoromethyl)-4′-(2-methoxyphenyl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3ea). From 1e (26.4 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3ea (46.2 mg, 94% yield) was obtained as a white solid, m.p. 186–188 °C. HPLC (Daicel Chiralpak IC column, mobile phase n-hexane/2-propanol = 80:20, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 10.6 min (major), tR = 13.2 min (minor); 22% ee. [α]D25 = −2.1 (c = 0.49, CH2Cl2). 1H NMR (700 MHz, CDCl3): δ 7.90 (dd, J1 = 7.7 Hz, J2 = 0.8 Hz, 1H, ArH), 7.70 (d, J = 7.7 Hz, 1H, ArH), 7.63 (td, J1 = 7.7 Hz, J2 = 1.4 Hz, 1H, ArH), 7.59–7.55 (m, 2H, ArH), 7.45 (td, J1 = 7.7 Hz, J2 = 1.4 Hz, 1H, ArH), 7.27 (d, J = 7.7 Hz, 1H, ArH), 7.59–7.22 (m, 1H, ArH), 6.95 (td, J1 = 7.7 Hz, J2 = 1.4 Hz, 1H, ArH), 6.79 (td, J1 = 7.7 Hz, J2 = 0.7 Hz, 1H, ArH), 6.38–6.20 (m, 2H, ArH + CF2H), 5.22 (d, J = 9.8 Hz, 1H, CH), 4.71–4.65 (m, 1H, CH), 3.45 (s, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 201.5, 198.6, 194.9, 156.6, 147.7, 142.8, 141.1, 136.0, 135.43, 135.40, 129.4, 128.7, 128.4, 127.4, 125.7, 123.4, 122.8, 122.2, 121.1, 120.3, 118.1 (t, 1JC–F = 241.7 Hz), 109.2, 80.4, 72.0, 61.5 (dd, 2JC–F = 27.6, 21.5 Hz), 54.1, 45.4 (d, 3JC–F = 7.5 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ −117.1 (d, J = 287.2 Hz, 1F), −122.4 (d, J = 287.2 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C27H20F2NO4S [M + H]+ 492.1076, found 492.1073.
(2S,4′S,5′S)-5′-(Difluoromethyl)-4′-(3-methoxyphenyl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3fa). From 1f (26.4 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3fa (43.7 mg, 89% yield) was obtained as a white solid, m.p. 160–161 °C. HPLC (Daicel Chiralpak ADH column, mobile phase n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 17.3 min (major), tR = 38.0 min (minor); 36% ee. [α]D25 = −117.5 (c = 1.1, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.83–7.80 (m, 2H, ArH), 7.72–7.63 (m, 3H, ArH), 7.45–7.41 (m, 1H, ArH), 7.23–7.19 (m, 1H, ArH), 7.02 (d, J = 8.8 Hz, 3H, ArH), 6.58 (d, J = 8.8 Hz, 2H, ArH), 6.23 (td, J1 = 56.8 Hz, J2 = 6.8 Hz, 1H, CF2H), 4.77 (d, J = 9.6 Hz, 1H, CH), 4.65–4.57 (m, 1H, CH), 3.62 (s, 3H, OCH3), 3.33 (s, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 200.5, 198.9, 196.3, 159.0, 147.5, 142.6, 142.2, 136.23, 136.18, 135.8, 129.6, 129.1, 127.6, 125.9, 124.6, 123.5, 123.23, 123.18, 118.1 (t, 1JC–F = 242.1 Hz), 113.9, 81.3, 71.1, 62.4 (dd, 2JC–F = 27.0, 21.3 Hz), 55.0, 51.2 (d, 3JC–F = 7.5 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ −117.0 (d, J = 288.4 Hz, 1F), −121.8 (d, J = 288.4 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C27H20F2NO4S [M + H]+ 492.1076, found 492.1081.
(2S,4′S,5′S)-5′-(Difluoromethyl)-4′-(4-fluorophenyl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3ga). From 1g (25.2 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3ga (46.0 mg, 96% yield) as a white solid, m.p. 167–170 °C. HPLC (Daicel Chiralpak ADH column, mobile phase n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 13.7 min (major), tR = 27.7 min (minor); 38% ee. [α]D25 = −186.0 (c = 0.23, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.82 (d, J = 7.6 Hz, 2H, ArH), 7.73–7.69 (m, 1H, ArH), 7.67–7.64 (m, 2H, ArH), 7.43 (t, J = 7.4 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.11–7.08 (m, 2H, ArH), 7.02 (d, J = 7.6 Hz, 1H, ArH), 6.75 (t, J = 8.4 Hz, 2H, ArH), 6.24 (td, J1 = 56.8 Hz, J2 = 6.8 Hz, 1H, CF2H), 4.81 (d, J = 10.0 Hz, 1H, CH), 4.67–4.58 (m, 1H, CH), 3.40 (br s, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 200.3, 198.6, 196.0, 162.2 (1JC–F = 245.7 Hz), 147.4, 142.5, 142.1, 136.4, 136.3, 136.0, 130.1 (3JC–F = 8.1 Hz), 129.0, 128.7, 128.7, 127.6, 126.0, 123.6, 123.3 (4JC–F = 3.3 Hz), 118.1 (t, 1JC–F = 242.1 Hz), 115.5 (2JC–F = 21.3 Hz), 81.3, 70.9, 62.4 (dd, 2JC–F = 27.3, 21.6 Hz), 51.0 (d, 3JC–F = 7.5 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ −113.8, −116.9 (d, J = 289.1 Hz, 1F), −121.8 (d, J = 289.1 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C26H17F3NO3S [M + H]+ 480.0876, found 480.0879.
(2S,4′S,5′S)-4′-(4-Chlorophenyl)-5′-(difluoromethyl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3ha). From 1h (26.8 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3ha (49.0 mg, 98% yield) was obtained as a light-yellow solid, m.p. 123–127 °C. HPLC (Daicel Chiralpak IC column, mobile phase n-hexane/2-propanol = 85:15, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 9.4 min (minor), tR = 10.5 min (major); 24% ee. [α]D25 = −41.3 (c = 0.53, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.84–7.80 (m, 2H, ArH), 7.75–7.71 (m, 1H, ArH), 7.67 (d, J = 4.0 Hz, 2H, ArH), 7.43 (td, J1 = 7.6, J2 = 1.2 Hz, 1H, ArH), 7.21 (t, J = 7.5 Hz, 1H, ArH), 7.07–7.01 (m, 5H, ArH), 6.23 (td, J1 = 56.8 Hz, J2 = 6.8 Hz, 1H, CF2H), 4.80 (d, J = 9.6 Hz, 1H, CH), 4.66–4.58 (m, 1H, CH), 3.36 (br s, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 200.2, 198.4, 195.9, 147.3, 142.4, 142.1, 136.4, 136.3, 136.1, 133.8, 131.5, 129.9, 128.9, 128.7, 127.6, 126.0, 123.5, 123.33, 123.27, 118.1 (t, 1JC–F = 242.1 Hz), 81.4, 70.7, 62.3 (dd, 2JC–F = 27.4, 21.6 Hz), 50.9 (d, 3JC–F = 7.6 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ −117.0 (d, J = 289.1 Hz, 1F), −121.8 (d, J = 289.5 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C26H17ClF2NO3S [M + H]+ 496.0581, found 496.0578.
(2S,4′S,5′S)-4′-(4-Bromophenyl)-5′-(difluoromethyl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3ia). From 1i (31.2 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3ia (47.4 mg, 88% yield) was obtained as a light-yellow solid, m.p. 185–187 °C. HPLC (Daicel Chiralpak ADH column, mobile phase n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 19.4 min (major), tR = 39.3 min (minor); 41% ee. [α]D25 = −103.8 (c = 0.67, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.83 (t, J = 8.2 Hz, 2H, ArH), 7.76–7. 72 (m, 1H, ArH), 7.67 (d, J = 3.6 Hz, 2H, ArH), 7.43 (t, J = 7.6 Hz, 1H, ArH), 7.24–7.18 (m, 3H, ArH), 7.03–6.98 (m, 3H, ArH), 6.23 (td, J1 = 56.8 Hz, J2 = 6.8 Hz, 1H, CF2H), 4.78 (d, J = 9.6 Hz, 1H, CH), 4.66–4.57 (m, 1H, CH), 3.35 (br s, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 200.3, 198.6, 196.0, 163.4, 160.9, 147.4, 142.5, 142.1, 136.4, 136.3, 136.0, 130.2, 130.1, 129.0, 127.6, 126.0, 123.6, 123.3, 123.2, 122.1, 118.1 (t, 1JC–F = 242.1 Hz), 115.6, 115.4, 81.3, 70.9, 62.4 (dd, 2JC–F = 27.2, 21.6 Hz), 50.9 (d, 3JC–F = 7.5 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ −117.0 (d, J = 289.5 Hz, 1F), −121.8 (d, J = 289.5 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C26H1779BrF2NO3S [M + H]+ 540.0076, found 540.0062; calculated for C26H1781BrF2NO3S [M + H]+ 542.0055, found 542.0055.
(2S,4′S,5′S)-4′-(3-Bromophenyl)-5′-(difluoromethyl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3ja). From 1j (31.2 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3ja (45.0 mg, 84% yield) as a light-yellow solid, m.p. 207–209 °C. HPLC (Daicel Chiralpak ADH column, mobile phase n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 15.2 min (major), tR = 29.3 min (minor); 81% ee. [α]D25 = −47.6 (c = 0.58, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.83 (t, J = 6.4 Hz, 2H, ArH), 7.74–7.64 (m, 3H, ArH), 7.46–7.42 (m, 1H, ArH), 7.25 (s, 1H, ArH), 7.22 (t, J = 7.4 Hz, 1H, ArH), 7.15 (d, J = 8.0 Hz, 1H, ArH), 7.06 (d, J = 8.0 Hz, 1H, ArH), 7.03 (d, J = 8.0 Hz, 1H, ArH), 6.93 (t, J = 8.0 Hz, 1H, ArH), 6.24 (td, J1 = 56.8 Hz, J2 = 6.8 Hz, 1H, CF2H), 4.78 (d, J = 9.6 Hz, 1H, CH), 4.66–4.56 (m, 1H, CH), 3.37 (br s, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 200.2, 198.3, 195.7, 147.4, 142.4, 142.0, 136.39, 136.35, 136.0, 135.3, 131.6, 131.2, 130.0, 129.0, 127.7, 127.2, 126.0, 123.5, 123.3, 122.5, 118.0 (t, 1JC–F = 242.2 Hz), 81.2, 70.8, 62.2 (dd, 2JC–F = 27.6, 21.8 Hz), 51.1 (d, 3JC–F = 7.6 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ −117.1 (d, J = 289.1 Hz, 1F), −121.7 (d, J = 289.1 Hz, 1F) ppm. HRMS (ESI+): m/z caluclated for C26H1779BrF2NO3S [M + H]+ 540.0076, found 540.0063; calculated for C26H1781BrF2NO3S [M + H]+ 542.0055, found 542.0048.
(2S,4′S,5′S)-4′-(3,5-Dichlorophenyl)-5′-(difluoromethyl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3ka). From 1k (30.2 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3ka (48.7 mg, 92% yield) was obtained as a light-yellow solid, m.p. 206–208 °C. HPLC (Daicel Chiralpak ADH column, mobile phase n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 16.0 min (major), tR = 32.7 min (minor); 14% ee. [α]D25 = −24.2 (c = 0.48, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.87 (d, J = 8.0 Hz, 1H, ArH), 7.81 (dd, J1 = 8.0 Hz, J2 = 1.2 Hz, 1H, ArH), 7.75–7.69 (m, 3H, ArH), 7.45 (td, J = 7.6, J2 = 1.2 Hz, 1H, ArH), 7.30 (d, J = 8.4 Hz, 1H, ArH), 7.25–7.21 (m, 1H, ArH), 7.11 (d, J = 2.0 Hz, 1H, ArH), 7.05 (dd, J = 8.6, 2.2 Hz, 2H, ArH), 6.29 (td, J1 = 56.8 Hz, J2 = 7.0 Hz, 1H, CF2H), 5.59 (d, J = 9.6 Hz, 1H, CH), 4.49–4.41 (m, 1H, CH), 3.29 (br s, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 200.6, 198.7, 194.7, 147.4, 142.4, 141.8, 136.30, 136.28, 135.8, 134.1, 130.4, 129.8, 129.7, 129.1, 127.7, 127.1, 125.9, 123.52, 123.48, 123.2, 117.9 (t, 1JC–F = 242.2 Hz), 81.0, 71.0, 64.1 (dd, 2JC–F = 27.2, 21.4 Hz), 46.5 (d, 3JC–F = 7.8 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ −116.8 (d, J = 289.9 Hz, 1F), −122.6 (d, J = 289.9 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C26H16Cl2F2NO3S [M + H]+ 530.0191, found 530.0191.
(2S,4′S,5′S)-5′-(Difluoromethyl)-4′-(4-nitrophenyl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3la). From 1l (27.9 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3la (45.6 mg, 90% yield) was obtained as a light yellow solid, m.p. 224–226 °C. HPLC (Daicel Chiralpak IC column, mobile phase n-hexane/2-propanol = 80:20, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 21.0 min (minor), tR = 27.4 min (major); 61% ee. [α]D25 = −1.01 (c = 1.38, CH2Cl2). 1H NMR (700 MHz, CDCl3): δ 7.95 (d, J = 9.1 Hz, 2H, ArH), 7.85–7.82 (m, 2H, ArH), 7.76–7.73 (m, 1H, ArH), 7.68 (d, J = 4.2 Hz, 2H, ArH), 7.45 (d, J = 7.4 Hz, 2H, ArH), 7.33 (d, J = 9.1 Hz, 2H, ArH), 7.23 (t, J = 7.7 Hz, 1H, ArH), 7.03 (d, J = 8.4 Hz, 1H, ArH), 6.26 (td, J1 = 56.7, J2 = 7.0 Hz, 1H, CF2H), 4.94 (d, J = 9.8 Hz, 1H, CH), 4.73–4.69 (m, 1H, CH), 3.41 (d, J = 7.7 Hz, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 199.9, 197.9, 195.4, 147.4, 147.2, 142.6, 142.3, 141.9, 140.7, 136.7, 136.5, 136.4, 134.2, 129.6, 128.8, 127.7, 126.1, 124.3, 123.64, 123.60, 123.4, 117.9 (t, 1JC–F = 242.2 Hz), 81.6, 70.4, 62.4 (dd, 2JC–F = 27.9, 22.1 Hz), 50.8 (d, 3JC–F = 7.7 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ −116.9 (d, J = 290.5 Hz, 1F), −121.7 (d, J = 290.4 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C26H17F2N2O5S [M + H]+ 507.0821, found 507.0810.
(2S,4′S,5′S)-5′-(Difluoromethyl)-4′-(thiophen-2-yl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3ma). From 1m (24.0 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3ma (41.1 mg, 88% yield) was obtained as a light-yellow solid, m.p. 208–210 °C. HPLC (Daicel Chiralpak IC column, mobile phase n-hexane/2-propanol = 80:20, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 12.4 min (minor), tR = 16.2 min (major); 81% ee. [α]D25 = −46.1 (c = 0.63, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.88 (d, J = 7.6 Hz, 1H, ArH), 7.82 (d, J = 7.6 Hz, 1H, ArH), 7.76–7.66 (m, 3H, ArH), 7.46–7.41 (m, 1H, ArH), 7.21 (t, J = 7.4 Hz, 1H, ArH), 7.03 (d, J = 7.6 Hz, 1H, ArH), 6.93 (d, J = 4.8 Hz, 1H, ArH), 6.80 (d, J = 3.4 Hz, 1H, ArH), 6.68 (dd, J1 = 5.0 Hz, J2 = 3.8 Hz, 1H, ArH), 6.24 (td, J1 = 56.6 Hz, J2 = 6.8 Hz, 1H, CF2H), 5.11 (d, J = 9.6 Hz, 1H, CH), 4.60–4.51 (m, 1H, CH), 3.37 (s, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 200.3, 198.6, 195.7, 147.5, 142.8, 142.3, 136.4, 136.2, 135.9, 135.3, 128.9, 127.7, 127.1, 126.7, 126.0, 125.1, 123.6, 123.4, 123.3, 117.7 (t, 1JC–F = 242.3 Hz), 81.0, 70.6, 64.4 (dd, 2JC–F = 26.9, 21.6 Hz), 47.0 (d, 3JC–F = 7.6 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ −117.5 (d, J = 289.1 Hz, 1F), −121.9 (d, J = 289.1 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C24H16F2NO3S2 [M + H]+ 468.0535, found 468.0526.
(2S,4′S,5′S)-5′-(Difluoromethyl)-4′-(furan-2-yl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3na). From 1n (22.4 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3na was obtained as a light-yellow solid, m.p. 216–218 °C. HPLC (Daicel Chiralpak ADH column, mobile phase n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 14.0 min (major), tR = 41.9 min (minor); 11% ee. [α]D25 = −36.1 (c = 0.33, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.90–7.86 (m, 1H, ArH), 7.83–7.70 (m, 5H, ArH), 7.46–7.42 (m, 1H, ArH), 7.24–7.19 (m, 1H, ArH), 7.04 (d, J = 8.0 Hz, 1H, ArH), 6.93 (dd, J1 = 1.6, J2 = 0.8 Hz, 1H, ArH), 6.26 (dd, J1 = 56.8 Hz, J2 = 7.2 Hz, 1H, CF2H), 6.10 (d, J = 3.2 Hz, 1H, ArH), 6.03 (dd, J1 = 3.2 Hz, J2 = 2.0 Hz, 1H, ArH), 4.91 (d, J = 9.6 Hz, 1H, CH), 4.53–4.45 (m, 1H. CH), 3.39 (br s, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ 200.3, 197.9, 195.4, 149.0, 147.6, 142.3, 136.3, 136.1, 135.8, 134.9, 129.3, 127.7, 125.9, 123.6, 123.4, 123.3, 122.9, 117.6 (t, 1JC–F = 241.9 Hz), 110.3, 108.9, 80.5, 69.3, 62.1 (dd, 2JC–F = 27.8, 22.2 Hz), 45.5 (d, 3JC–F = 7.9 Hz) ppm. 19F NMR (376 MHz, CDCl3): δ −117.5 (d, J = 288.8 Hz, 1F), −122.0 (d, J = 288.8 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C24H16F2NO4S [M + H]+ 452.0763, found 452.0767.
(2S,4′S,5′S)-5′-(Difluoromethyl)-4′-(pyridin-2-yl)-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3oa). From 1o (23.5 mg, 0.10 mmol) and 2a (22.7 mg, 0.10 mmol), compound 3oa (43.9 mg, 95% yield) was obtained as a white solid, m.p. 207–210 °C. HPLC (Daicel Chiralpak ADH column, mobile phase n-hexane/2-propanol = 60:40, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 19.2 min (major), tR = 22.4 min (minor); 13% ee. [α]D25 = +13.0 (c = 0.71, CH2Cl2). 1H NMR (400 MHz, DMSO-d6): δ 8.27 (dd, J1 = 4.6 Hz, J2 = 1.0 Hz, 1H, ArH), 8.21 (d, J = 2.0 Hz, 1H, ArH), 7.90 (d, J = 7.6 Hz, 2H, ArH), 7.84–7.80 (m, 1H, ArH), 7.72 (d, J = 8.4 Hz, 2H, ArH), 7.58–7.53 (m, 2H, ArH), 7.32–7.25 (m, 2H, ArH), 7.18 (dd, J1 = 8.0 Hz, J2 = 4.8 Hz, 1H, ArH), 6.24 (td, J1 = 56.8 Hz, J2 = 5.8 Hz, 1H, CF2H), 5.15 (d, J = 5.6 Hz, 1H, CH), 4.69–4.50 (m, 2H, CH + NH) ppm. 13C NMR (100 MHz, DMSO-d6): δ 200.2, 196.9, 195.8, 149.2, 149.1, 146.9, 141.5, 141.2, 137.6, 137.0, 136.7, 135.8, 129.1, 128.3, 126.8, 126.0, 123.9, 123.4, 123.2, 123.1, 118.2 (t, 1JC–F = 240.2 Hz), 82.7, 70.0, 60.9 (dd, 2JC–F = 26.8, 20.3 Hz), 54.8 ppm. 19F NMR (376 MHz, DMSO-d6): δ −115.6 (d, J = 285.8 Hz, 1F), −120.7 (d, J = 285.8 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C25H17F2N2O3S [M + H]+ 463.0923, found 463.0917.
(2S,4′S,5′S)-5′-(Difluoromethyl)-7-methyl-4′-phenyl-3H-dispiro[benzo[b]thiophene-2,2′-pyrrolidine-3′,2″-indene]-1″,3,3″-trione (3ab). From 1a (23.4 mg, 0.10 mmol) and 2b (24.1 mg, 0.10 mmol), compound 3ab (43.2 mg, 91% yield) was obtained as a white solid, m.p. 189-191 °C. HPLC (Daicel Chiralpak IC column, mobile phase n-hexane/2-propanol = 90:10, flow rate 1.0 mL/min, detection wavelength 254 nm): tR = 17.0 min (minor), tR = 24.4 min (major); 49% ee. [α]D25 = −155.8 (c = 1.18, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.78 (d, J = 7.6 Hz, 1H, ArH), 7.69–7.58 (m, 4H, ArH), 7.24 (s, 1H, ArH), 7.15 (d, J = 7.6 Hz, 1H, ArH), 7.12–7.08 (m, 2H, ArH), 7.06–6.97 (m, 3H, ArH), 6.25 (td, J1 = 56.8, J2 = 6.8 Hz, 1H, CF2H), 4.85 (d, J = 10.0 Hz, 1H, CH), 4.72–4.64 (m, 1H, CH), 3.39 (s, 1H, NH), 2.02 (s, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 200.8, 198.8, 196.1, 147.1, 142.5, 142.1, 136.5, 136.0, 135.7, 132.8, 132.7, 128.9, 128.44, 128.41, 127.9, 126.0, 125.0, 123.2, 123.1, 118.1 (t, 1JC–F = 241.9 Hz), 81.2, 71.2, 62.2 (dd, 2JC–F = 27.2, 21.3 Hz), 52.0 (d, 3JC–F = 7.5 Hz), 18.4 ppm. 19F NMR (376 MHz, CDCl3): δ −117.1 (d, J = 288.0 Hz, 1F), −121.8 (d, J = 288.4 Hz, 1F) ppm. HRMS (ESI+): m/z calculated for C27H20F2NO3S [M + H]+ 476.1127, found 476.1125.