Density Functional Theory Study on Na+ and K+ Catalysis in the Transformation of Glucose to Fructose and HMF in Hydrothermal Environments
Abstract
1. Introduction
2. Results and Discussion
2.1. Mechanistic Study of Glucose-to-Fructose Conversion Catalyzed by K+ and Na+ Ions
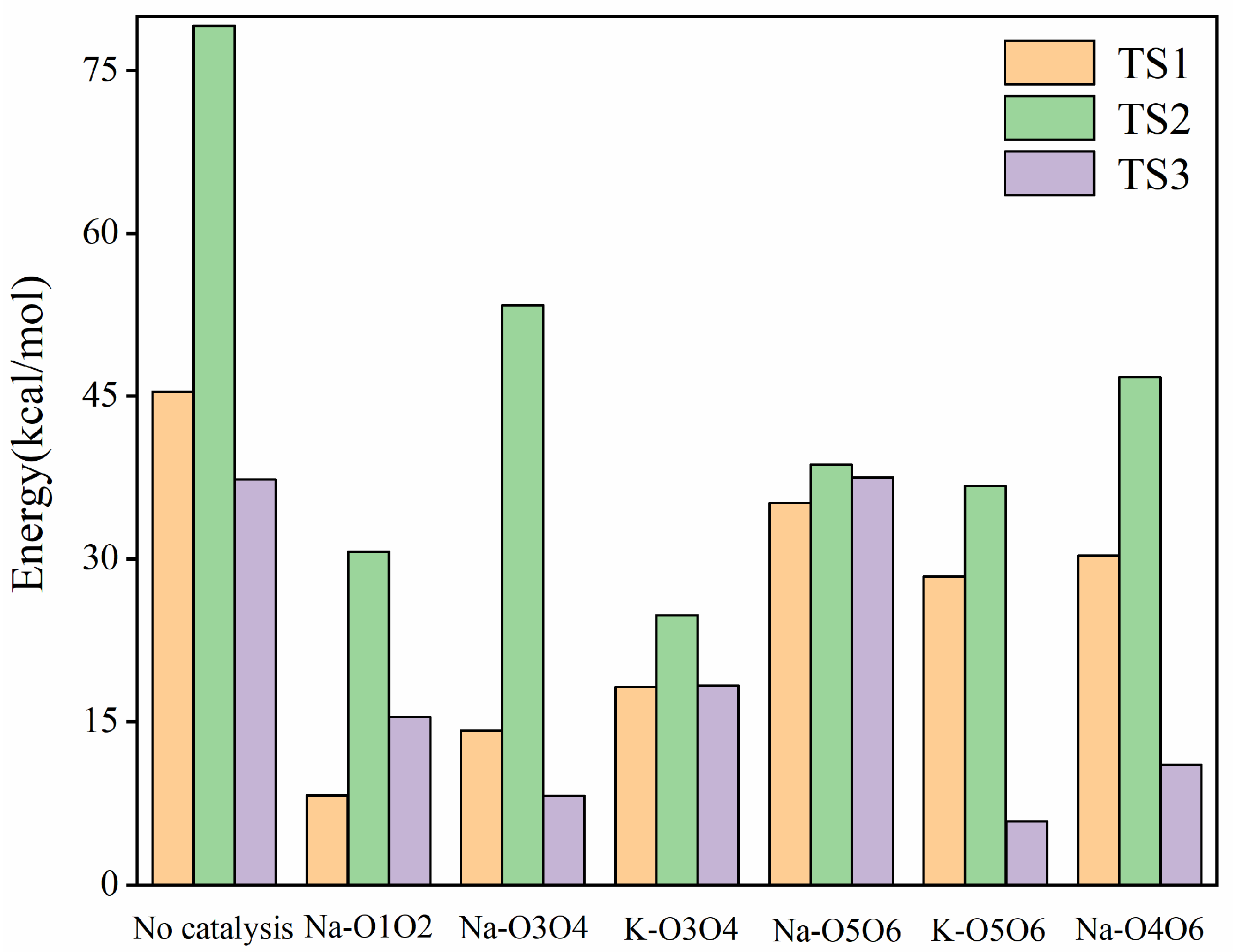
2.1.1. Mechanistic Analysis of Four Distinct Binding Modes of Na+ Ion with Glucose
2.1.2. Mechanistic Analysis of Two Different Binding Modes of K+ Ion with Glucose
2.2. Mechanistic Study of Fructose to HMF Conversion Catalyzed by Na and K Ions
2.2.1. Mechanistic Analysis of Different Binding Modes of Na Ion with Fructose
2.2.2. Mechanistic Analysis of Different Binding Modes of K Ion with Fructose
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Zhang, Z.; Zhang, Y.; Wang, Y.; Yu, Y.; Ji, F.; Ahmad, R.; Dong, R. A Comprehensive Review on Densified Solid Biofuel Industry in China. Renew. Sust. Energ. Rev. 2016, 54, 1412–1428. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, N.; Song, F. Dewaterability Characteristics of Sludge Conditioned with Surfactants Pretreatment by Electrolysis. Bioresour. Technol. 2011, 102, 2308–2315. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Wang, T.; Si, B.; Chen, W.T.; Aierzhati, A.; Zhang, Y. Valorization of Hydrothermal Liquefaction Aqueous Phase: Pathways towards Commercial Viability. Prog. Energ. Combust. 2020, 77, 100819. [Google Scholar] [CrossRef]
- He, C.; Chen, C.; Giannis, A.; Yang, Y.; Wang, J.Y. Hydrothermal Gasification of Sewage Sludge and Model Compounds for Renewable Hydrogen Production: A Review. Renew. Sust. Energ. Rev. 2014, 39, 1127–1142. [Google Scholar] [CrossRef]
- Peng, C.; Zhai, Y.; Zhu, Y.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Production of Char from Sewage Sludge Employing Hydrothermal Carbonization: Char Properties, Combustion Behavior and Thermal Characteristics. Fuel 2016, 176, 110–118. [Google Scholar] [CrossRef]
- Tang, Z.; Boer, D.; Syariati, A.; Enache, M.; Rudolf, P.; Heeres, H.J.; Pescarmona, P.P. Base-Free Conversion of Glycerol to Methyl Lactate Using a Multifunctional Catalytic System Consisting of Au-Pd Nanoparticles on Carbon Nanotubes and Sn-MCM-41-XS. Green. Chem. 2019, 21, 4115–4126. [Google Scholar] [CrossRef]
- Hu, B.; Lu, Q.; Jiang, X.; Dong, X.; Cui, M.; Dong, C.; Yang, Y. Pyrolysis Mechanism of Glucose and Mannose: The Formation of 5-Hydroxymethyl Furfural and Furfural. J. Energy Chem. 2018, 27, 486–501. [Google Scholar] [CrossRef]
- Weiqi, W.; Shubin, W. Experimental and Kinetic Study of Glucose Conversion to Levulinic Acid Catalyzed by Synergy of Lewis and Brønsted Acids. Chem. Eng. J. 2017, 307, 389–398. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, H.; Xiao, H.; Li, W.; Jin, Y.; Wei, W.; Wu, S. Advance in Constructing Acid Catalyst-Solvent Combinations for Efficient Transformation of Glucose into 5-Hydroxymethylfurfural. Mol. Catal. 2020, 498, 111254. [Google Scholar] [CrossRef]
- Arora, J.; Ansari, K.; Chew, J.; Dauenhauer, P.; Mushrif, S. Unravelling the Catalytic Influence of Naturally Occurring Salts on Biomass Pyrolysis Chemistry Using Glucose as a Model Compound: A Combined Experimental and DFT Study. Catal. Sci. Technol. 2019, 9, 3504–3524. [Google Scholar] [CrossRef]
- Guo, W.; Zuo, M.; Zhao, J.; Li, C.; Xu, Q.; Xu, C.; Wu, H.; Sun, Z.; Chu, W. Novel Brønsted–Lewis Acidic Di-Cationic Ionic Liquid for Efficient Conversion Carbohydrate to Platform Compound. Cellulose 2020, 27, 6897–6908. [Google Scholar] [CrossRef]
- Guo, S.; Xiao, W.; Zhao, D.; Liu, Z.; Liu, L.; Xu, D.; Li, X.; Li, G. Water-Catalyzed Conversion of Glucose to Small Molecules during Hydrothermal Carbonization: A Density Functional Theory Study. Sustain. Energ. Fuels 2023, 7, 1322–1332. [Google Scholar] [CrossRef]
- Abdullayev, Y.; Ahmadov, O.; Valadova, G.; Karimli, A.; Autschbach, J. Unveiling the Catalytic Effects of Brønsted Acidic Ionic Liquid on Quantitative α-Glucose Conversion to 5-HMF: Experimental and Computational Studies. Renew. Energ. 2021, 171, 383–390. [Google Scholar] [CrossRef]
- Tian, G.; Tong, X.; Cheng, Y.; Xue, S. Tin-Catalyzed Efficient Conversion of Carbohydrates for the Production of 5-Hydroxymethylfurfural in the Presence of Quaternary Ammonium Salts. Carbohyd Res. 2013, 370, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Moliner, M.; Román-Leshkov, Y.; Davis, M. Tin-Containing Zeolites Are Highly Active Catalysts for the Isomerization of Glucose in Water. Proc. Natl. Acid. Sci. USA 2010, 107, 6164–6168. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Y.; Li, H.; Ma, X. Kinetic Studies on Chromium-Catalyzed Conversion of Glucose into 5-Hydroxymethylfurfural in Alkylimidazolium Chloride Ionic Liquid. Chem. Eng. J. 2014, 237, 55–61. [Google Scholar] [CrossRef]
- Pyo, S.; Glaser, S.; Rehnberg, N.; Hatti-Kaul, R. Clean Production of Levulinic Acid from Fructose and Glucose in Salt Water by Heterogeneous Catalytic Dehydration. ACS Omega 2020, 5, 14275–14282. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Zhang, D.; Liu, C. Theoretical Elucidation of Glucose Dehydration to 5-Hydroxymethylfurfural Catalyzed by a SO3H-Functionalized Ionic Liquid. J. Phys. Chem. B 2015, 119, 13398–13406. [Google Scholar] [CrossRef]
- Parveen, F.; Upadhyayula, S. Efficient Conversion of Glucose to HMF Using Organocatalysts with Dual Acidic and Basic Functionalities-A Mechanistic and Experimental Study. Fuel Process Technol. 2017, 162, 30–36. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Wang, N.; Wang, H.; Song, Z.; Li, W. Effect of Organic Solvent and Broønsted Acid on 5-Hydroxymethylfurfural Preparation from Glucose over CrCl3. RSC Adv. 2015, 5, 27805–27813. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Q.; Zhao, D.; Liu, Z.; Chen, K.; Li, X.; Li, G. Density Functional Theory Study of Acid-Catalyzed Conversion of Glucose to Hydrochar Precursors under Hydrothermal Conditions. Energy 2023, 283, 128547. [Google Scholar] [CrossRef]
- Kang, X.; You, Z.; Huang, Y. Multi-catalytic active site biochar-based catalysts for glucose isomerized to fructose: Experiments and density functional theory study. Adv. Compos. Hybrid. Mater. 2024, 7, 54. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [PubMed]
- Kryachko, E.; Ludeña, E. Density Functional Theory: Foundations Reviewed. Phys. Rep. 2014, 544, 123–239. [Google Scholar] [CrossRef]
- Ferrighi, L.; Pan, Y.; Grönbeck, H.; Hammer, B. Study of Alkylthiolate Self-Assembled Monolayers on Au(111) Using a Semilocal Meta-GGA Density Functional. J. Phys. Chem. C 2012, 116, 7374–7379. [Google Scholar] [CrossRef]













| Bond | Length (Å) | Bond | Length (Å) |
|---|---|---|---|
| C1-O1 | 1.403 | C1-C2 | 1.533 |
| C2-O2 | 1.432 | C2-C3 | 1.525 |
| C3-O3 | 1.437 | C3-C4 | 1.531 |
| C4-O4 | 1.440 | C4-C5 | 1.534 |
| C5-O5 | 1.441 | C5-C6 | 1.530 |
| C6-O6 | 1.434 | C1-O5 | 1.441 |
| Atom | f− | f+ | Atom | f− | f+ |
|---|---|---|---|---|---|
| O1 | 0.026 | 0.009 | C1 | −0.001 | 0.008 |
| O2 | 0.111 | 0.008 | C2 | −0.006 | 0.007 |
| O3 | 0.066 | −0.040 | C3 | −0.007 | 0.002 |
| O4 | 0.055 | −0.052 | C4 | −0.001 | 0.010 |
| O5 | 0.131 | −0.006 | C5 | −0.015 | 0.041 |
| O6 | 0.128 | −0.006 | C6 | −0.006 | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Chen, Q.; Wang, Y.; Che, D.; Sun, B.; Guo, S. Density Functional Theory Study on Na+ and K+ Catalysis in the Transformation of Glucose to Fructose and HMF in Hydrothermal Environments. Molecules 2024, 29, 4849. https://doi.org/10.3390/molecules29204849
Gao L, Chen Q, Wang Y, Che D, Sun B, Guo S. Density Functional Theory Study on Na+ and K+ Catalysis in the Transformation of Glucose to Fructose and HMF in Hydrothermal Environments. Molecules. 2024; 29(20):4849. https://doi.org/10.3390/molecules29204849
Chicago/Turabian StyleGao, Long, Qihao Chen, Yanhong Wang, Deyong Che, Baizhong Sun, and Shuai Guo. 2024. "Density Functional Theory Study on Na+ and K+ Catalysis in the Transformation of Glucose to Fructose and HMF in Hydrothermal Environments" Molecules 29, no. 20: 4849. https://doi.org/10.3390/molecules29204849
APA StyleGao, L., Chen, Q., Wang, Y., Che, D., Sun, B., & Guo, S. (2024). Density Functional Theory Study on Na+ and K+ Catalysis in the Transformation of Glucose to Fructose and HMF in Hydrothermal Environments. Molecules, 29(20), 4849. https://doi.org/10.3390/molecules29204849






