Endophytic Fungus UJ3-2 from Urtica fissa: Antibacterial Activity and Mechanism of Action against Staphylococcus aureus
Abstract
1. Introduction
2. Result
2.1. Identification of Endophytic Fungus UJ3-2
2.2. Metabolome Analysis of UJ3-2 Fermentation Products
2.3. MIC and MBC of Fermentation Product of UJ3-2 against S. aureus
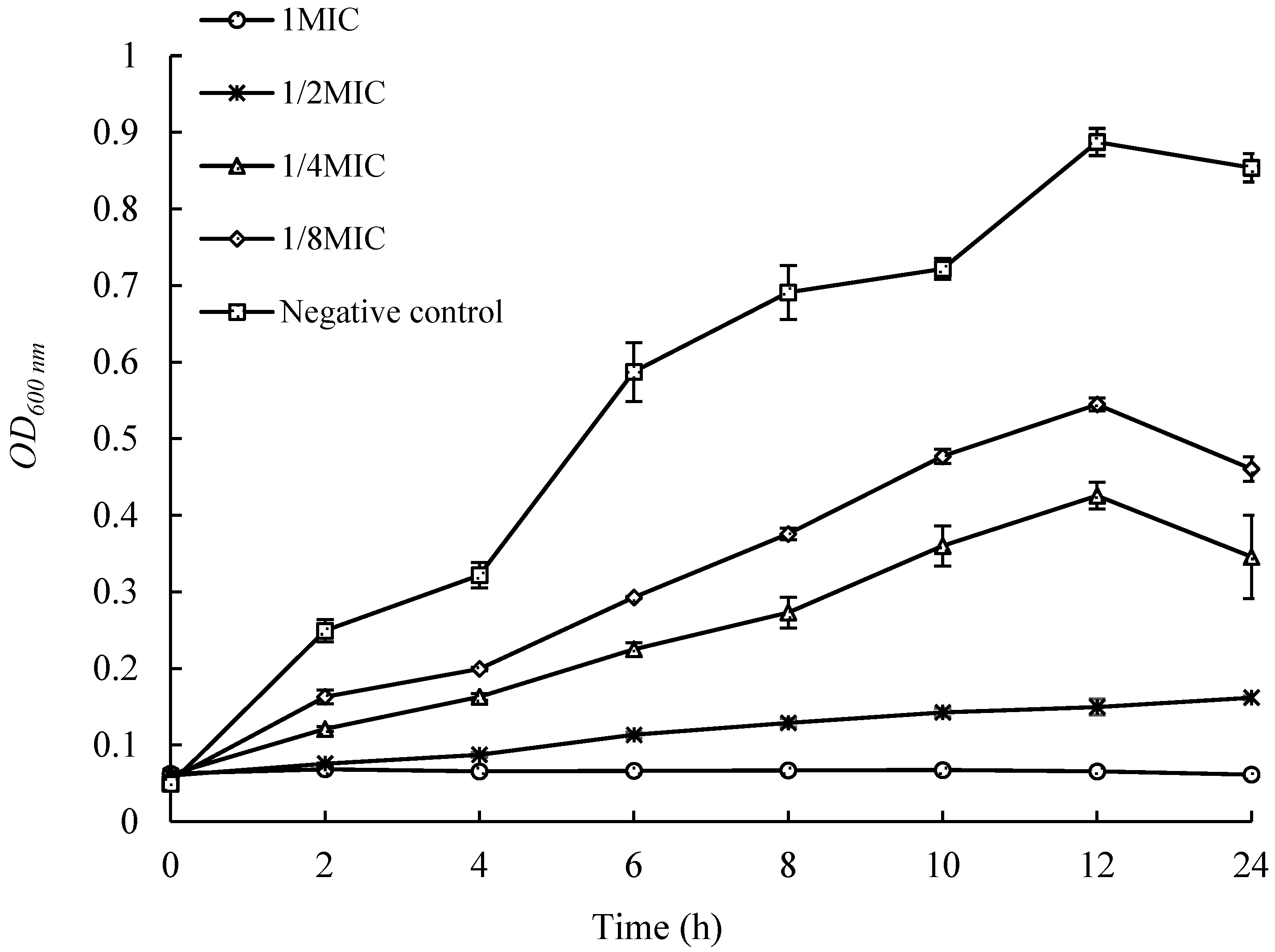
2.4. Effects of UJ3-2 Fermentation Products on Growth of S. aureus
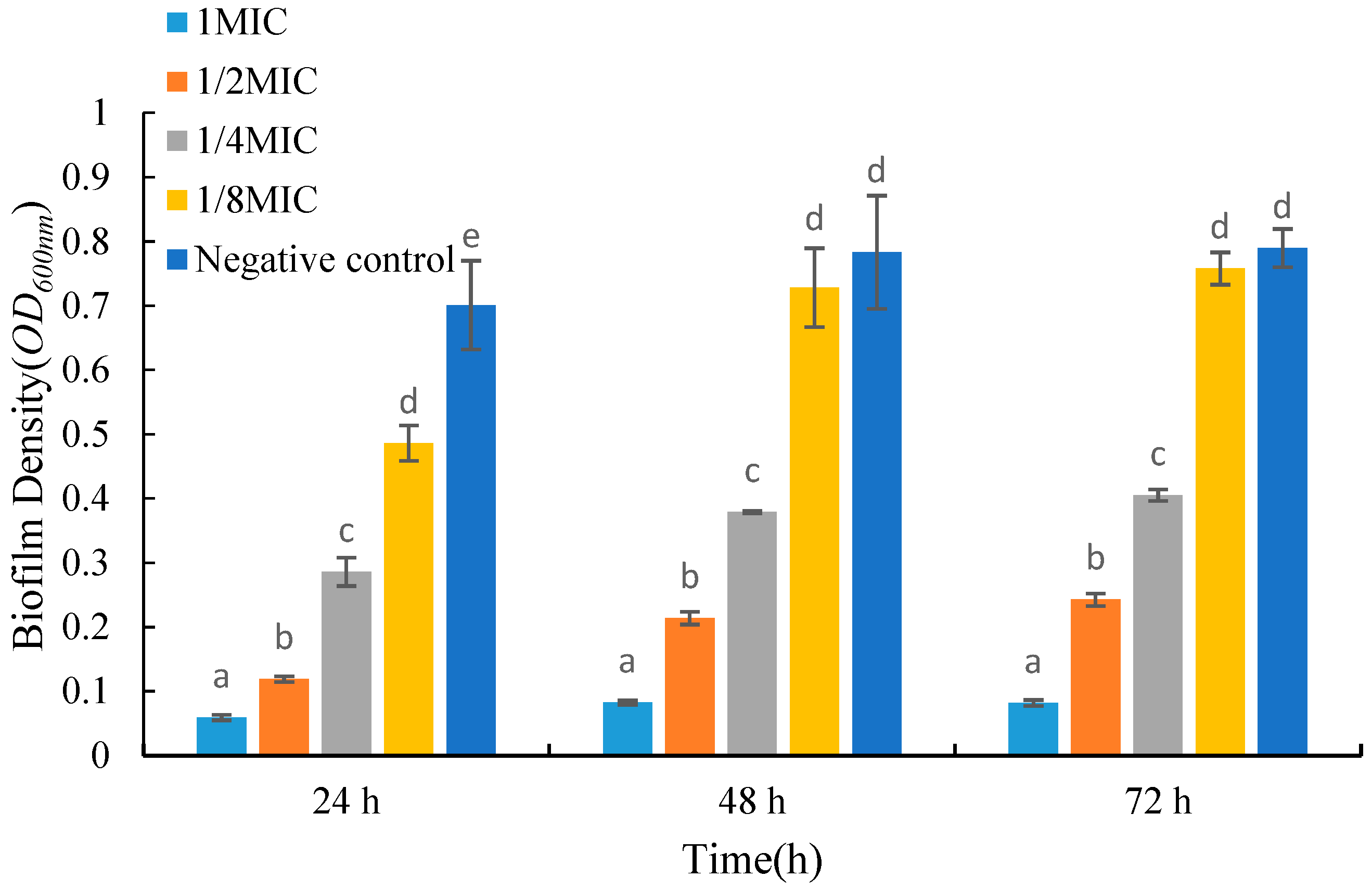
2.5. Effects of UJ3-2 Fermentation Products on Growth of S. aureus Biofilm
2.6. Effects of UJ3-2 Fermentation Products on Extracellular Nucleic Acid and Protein of S. aureus
2.7. Effects of UJ3-2 Fermentation Products on Cell Membrane Integrity of S. aureus
2.8. Effect of Fermentation Products on S. aureus Was Observed by Scanning Electron Microscope
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Identification of Endophytic Fungi
4.2.2. Preparation of UJ3-2 Fermentation Products
4.2.3. Ultra-High-Performance Liquid Chromatography–Mass Spectrometry (UPLC-MS)
4.2.4. Measurement of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.2.5. Determination of Growth Curve of S. aureus by Fermentation Extract of UJ3-2
4.2.6. Determination of the Biofilm of S. aureus by Fermentation Extract of UJ3-2
4.2.7. Detection of Extracellular Nucleic Acid and Protein of S. aureus by Fermentation Extract of UJ3-2
4.2.8. Effects of UJ3-2 Fermentation Extract on Membrane Integrity of S. aureus
4.2.9. Cell Membrane Integrity of S. aureus
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanselman, B.A.; Kruth, S.A.; Rousseau, J.; Weese, J.S. Coagulase positive staphylococcal colonization of humans and their household pets. Can. Vet. J. 2009, 50, 954–958. [Google Scholar] [PubMed]
- Balcucho, J.; Narváez, D.M.; Castro-Mayorga, J.L. Antimicrobial and Biocompatible Polycaprolactone and Copper Oxide Nanoparticle Wound Dressings against Methicillin-Resistant Staphylococcus aureus. Nanomaterials 2020, 10, 1692. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.P.; Molle, V. Staphylococcus aureus Toxins: An Update on Their Pathogenic Properties and Potential Treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef]
- Huang, S.S.; Hinrichsen, V.L.; Datta, R.; Spurchise, L.; Miroshnik, I.; Nelson, K.; Platt, R. Methicillin-resistant Staphylococcus aureus infection and hospitalization in high-risk patients in the year following detection. PLoS ONE 2011, 6, e24340. [Google Scholar] [CrossRef]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.S.; Jain, R.; Bhardwaj, P.; Thakur, A.; Kumari, M.; Bhushan, S.; Kumar, S. Plant probiotics—Endophytes pivotal to plant health. Microbiol. Res. 2022, 263, 127148. [Google Scholar] [CrossRef]
- Gakuubi, M.M.; Munusamy, M.; Liang, Z.X.; Ng, S.B. Fungal Endophytes: A Promising Frontier for Discovery of Novel Bioactive Compounds. J. Fungi 2021, 7, 786. [Google Scholar] [CrossRef]
- Tiwari, P.; Srivastava, Y.; Bae, H. Trends of Pharmaceutical Design of Endophytes as Anti-infective. Curr. Top. Med. Chem. 2021, 21, 1572–1586. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Verekar, S.A.; Bhave, S.V. Endophytic fungi: A reservoir of antibacterials. Front. Microbiol. 2015, 5, 715. [Google Scholar] [CrossRef] [PubMed]
- Matias, R.R.; Sepúlveda, A.M.G.; Batista, B.N.; de Lucena, J.; Albuquerque, P.M. Degradation of Staphylococcus aureus Biofilm Using Hydrolytic Enzymes Produced by Amazonian Endophytic Fungi. Appl. Biochem. Biotechnol. 2021, 193, 2145–2161. [Google Scholar] [CrossRef]
- Sadrati, N.; Zerroug, A.; Demirel, R.; Harzallah, D. Anti-multidrug-resistant Staphylococcus aureus and anti-dermatophyte activities of secondary metabolites of the endophytic fungus Penicillium brevicompactum ANT13 associated with the Algerian endemic plant Abies numidica. Arch. Microbiol. 2023, 205, 110. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, R.; Zhang, S.; Xu, T.; Hu, K.; Wu, S. Antimicrobial secondary metabolites from an endophytic fungus Aspergillus polyporicola. Fitoterapia 2022, 162, 105297. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Ma, S.; Shi, X.; Xue, W.; Liu, C.; Ding, H. Isolation, diversity, and antimicrobial activity of fungal endophytes from Rohdea chinensis (Baker) N.Tanaka (synonym Tupistra chinensis Baker) of Qinling Mountains, China. PeerJ 2020, 17, e9342. [Google Scholar] [CrossRef] [PubMed]
- Perrine-Walker, F.; Le, K. Propidium iodide enabled live imaging of Pasteuria sp.-Pratylenchus zeae infection studies under fluorescence microscopy. Protoplasma 2021, 258, 279–287. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Zhu, G.; Zuo, M.; Gong, Q.; He, W.; Li, M.; Yuan, C.; Hao, X.; Zhu, W. New phenylpyridone derivatives from the Penicillium sumatrense GZWMJZ-313, a fungal endophyte of Garcinia multiflora. Chin. Chem. Lett. 2019, 30, 431–434. [Google Scholar] [CrossRef]
- Zhong, F.; Fan, X.; Ji, W.; Hai, Z.; Hu, N.; Li, X.; Liu, G.; Yu, C.; Chen, Y.; Lian, B.; et al. Soil Fungal Community Composition and Diversity of Culturable Endophytic Fungi from Plant Roots in the Reclaimed Area of the Eastern Coast of China. J. Fungi 2022, 8, 124. [Google Scholar] [CrossRef]
- Gupta, S.; Chaturvedi, P.; Kulkarni, M.G.; Van Staden, J. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol. Adv. 2020, 39, 107462. [Google Scholar] [CrossRef]
- Li, K.; Zhang, H.; Shi, M.; Zhang, Y.; Cong, C.; Chang, X.; Duan, L.; Ding, Y. The complete chloroplast genome sequence of Urtica fissa. Mitochondrial DNA B Resour. 2022, 7, 1005–1007. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Guo, Z.; Li, X.; Wang, M. Chemical constituents from Urtica fissa stem and their inhibitory effects on α-glucosidase activity. Nat. Prod. Res. 2021, 35, 3011–3017. [Google Scholar] [CrossRef]
- Feng, X.; Wang, M.; Cheng, J.; Li, X. Two new secolignans with in vitro anti-inflammatory activities from Urtica fissa rhizomes. J. Nat. Med. 2017, 71, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Bao, T.; Tao, G.; Hu, Y.; Han, C. In vitro evaluation of the composition and acaricidal efficacy of Urtica fissa leaf ethyl acetate extract against Sarcoptes scabiei mites. Vet. Med. 2023, 68, 200–207. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Wang, M.Y.; Chen, S.; Li, X.B. The chemical constituents with cytotoxicity from Urtica fissa seed. J. Asian Nat. Prod. Res. 2019, 21, 1068–1074. [Google Scholar] [CrossRef]
- Cong, G.A.O.; Jun, L.U.O.; Xia, L.I.U.; Lin, M.A.; Xiao-Hong, Y. Recent advances in the studies of chemical compositions and bioactivities of the genus Xylaria. Mycosystema 2016, 35, 767–781. [Google Scholar] [CrossRef]
- Becker, K.; Stadler, M. Recent progress in biodiversity research on the Xylariales and their secondary metabolism. J. Antibiot. 2021, 74, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jeon, J.; Kim, J.A.; Jeon, M.J.; Yu, N.H.; Kim, S.; Park, A.R.; Kim, J.C.; Lee, Y.; Kim, Y.; et al. Draft Genome Sequence of Xylaria grammica EL000614, a Strain Producing Grammicin, a Potent Nematicidal Compound. Mycobiology 2021, 49, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Park, A.R.; Duraisamy, K.; Vo, D.D.; Kim, J.C. Elucidation of the nematicidal mode of action of grammicin on Caenorhabditis elegans. Pestic. Biochem. Physiol. 2022, 188, 105244. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Azevedo, N.F.; Ivask, A. Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Rep. 2019, 9, 6483. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Chen, L.; Tao, D.; Yu, F.; Wang, T.; Qi, M.; Xu, S. Cineole regulates Wnt/β-catenin pathway through Nrf2/keap1/ROS to inhibit bisphenol A-induced apoptosis, autophagy inhibition and immunosuppression of grass carp hepatocytes. Fish Shellfish Immunol. 2022, 131, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tao, D.; Qi, M.; Wang, T.; Jiang, Z.; Xu, S. Cineole alleviates the BPA-inhibited NETs formation by regulating the p38 pathway-mediated programmed cell death. Ecotoxicol. Environ. Saf. 2022, 237, 113558. [Google Scholar] [CrossRef]
- Lindner, S.; Geu-Flores, F.; Bräse, S.; Sherden, N.H.; O’Connor, S.E. Conversion of substrate analogs suggests a Michael cyclization in iridoid biosynthesis. Chem. Biol. 2014, 21, 1452–1456. [Google Scholar] [CrossRef]
- Grover, P.; Mehta, L.; Malhotra, A.; Kapoor, G.; Nagarajan, K.; Kumar, P.; Chawla, V.; Chawla, P.A. Exploring the Multitarget Potential of Iridoids: Advances and Applications. Curr. Top. Med. Chem. 2023, 23, 371–388. [Google Scholar]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research Advances in Their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef]
- da Silva, A.R.P.; Costa, M.D.S.; Araújo, N.J.S.; de Freitas, T.S.; de Almeida, R.S.; Barbosa Filho, J.M.; Tavares, J.F.; de Souza, E.O.; de Farias, P.A.M.; Pinheiro, J.C.A.; et al. Potentiation of Antibiotic Action and Efflux Pump Inhibitory Effect on Staphylococcus aureus Strains by Solasodine. Antibiotics 2022, 11, 1309. [Google Scholar] [CrossRef]
- Navabharath, M.; Srivastava, V.; Gupta, S.; Singh, S.V.; Ahmad, S. Ursolic Acid and Solasodine as Potent Anti-Mycobacterial Agents for Combating Paratuberculosis: An Anti-Inflammatory and in Silico Analysis. Molecules 2022, 28, 274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xie, Y.; Qiu, W.; Mei, J.; Xie, J. Antibacterial and Antibiofilm Efficacy and Mechanism of Ginger (Zingiber officinale) Essential Oil against Shewanella putrefaciens. Plants 2023, 12, 1720. [Google Scholar] [CrossRef]
- Shin, B.; Park, C.; Imlay, J.A.; Park, W. 4-Hydroxybenzaldehyde sensitizes Acinetobacter baumannii to amphenicols. Appl. Microbiol. Biotechnol. 2018, 102, 2323–2335. [Google Scholar] [CrossRef]
- Uçar, K.; Göktaş, Z. Biological activities of naringenin: A narrative review based on in vitro and in vivo studies. Nutr. Res. 2023, 119, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Zhang, W.; Wu, C.; Feng, H.; Peng, Y.; Shahid, H.; Cui, Z.; Ding, P.; Shan, T. Diversity and antibacterial activity of fungal endophytes from Eucalyptus exserta. BMC Microbiol. 2021, 21, 155. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The Antibacterial Mechanism of Terpinen-4-ol Against Streptococcus agalactiae. Curr. Microbiol. 2018, 75, 1214–1220. [Google Scholar] [CrossRef]
- Yang, X.; Lan, W.; Xie, J. Antimicrobial and anti-biofilm activities of chlorogenic acid grafted chitosan against Staphylococcus aureus. Microb. Pathog. 2022, 173, 105748. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; Huai, R.; Yang, D.; Lei, L.; Ouyang, Y.; Xiong, L. Isolation, identification and antibacterial activity of endophytes of Coptis chinensis Franch. Nat. Prod. Res. Dev. 2022, 34, 1456–1471. [Google Scholar]
- Zhou, Y.; Yao, Q.; Zhang, T.; Chen, X.; Wu, Z.; Zhang, N.; Shao, Y.; Cheng, Y. Antibacterial activity and mechanism of green tea polysaccharide conjugates against Escherichia coli. Ind. Crops Prod. 2020, 152, 112464. [Google Scholar] [CrossRef]
- Gowrishankar, S.; Kamaladevi, A.; Ayyanar, K.S.; Balamurugan, K.; Pandian, S.K. Bacillus amyloliquefaciens-secreted cyclic dipeptide—cyclo(l-leucyl-l-prolyl) inhibits biofilm and virulence production in methicillin-resistant Staphylococcus aureus. RSC Adv. 2015, 5, 95788–95804. [Google Scholar] [CrossRef]

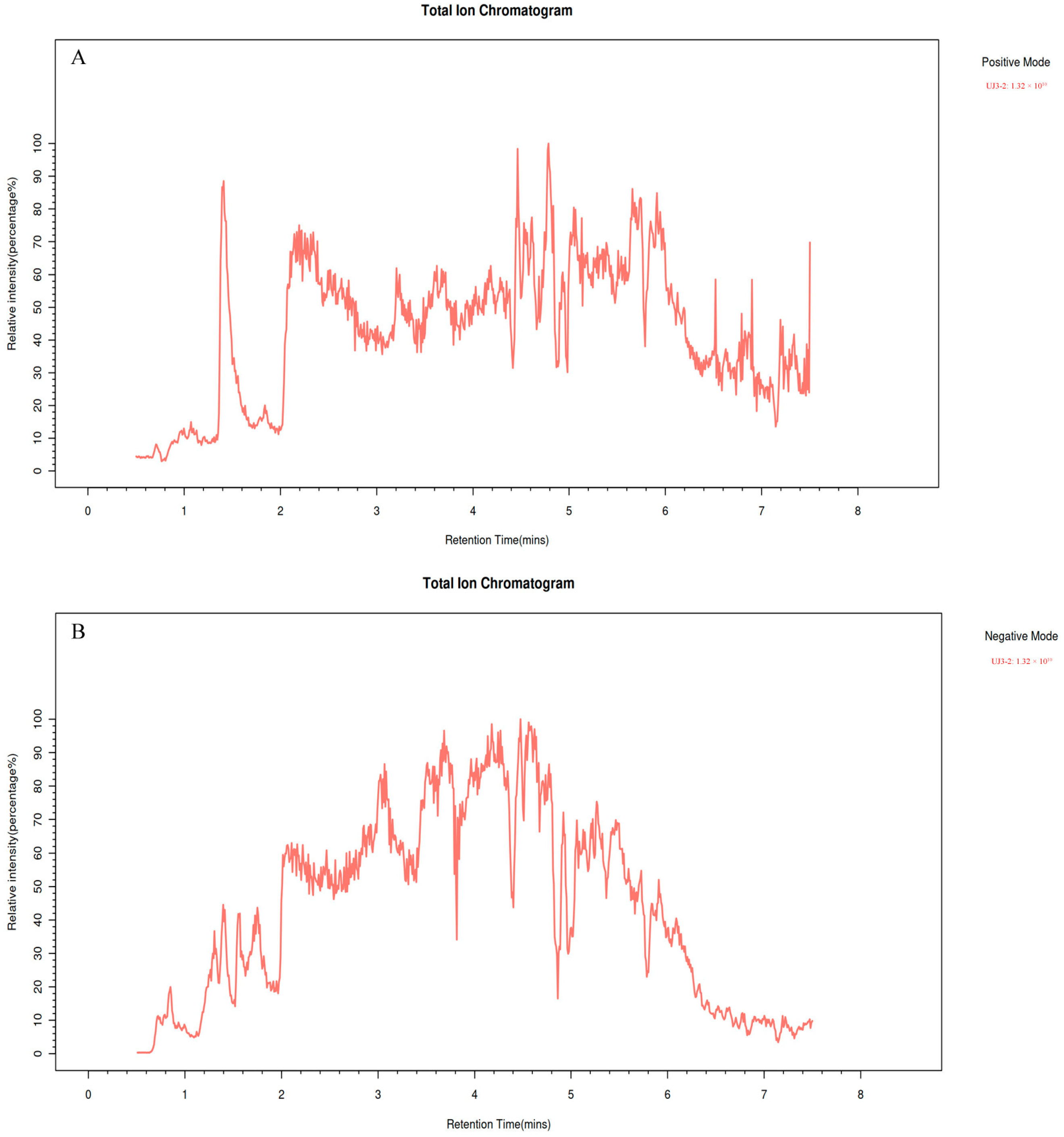



| Compound | Formula | Mz | Rt (S) | Relative Content (%) |
|---|---|---|---|---|
| 6-oxocineole | C10H16O2 | 213.1127 | 275.7 | 17.92% |
| (S)-2-acetolactate | C5H8O4 | 171.1375 | 297.6 | 9.91% |
| 3-methyl-cis,cis-muconate | C7H8O4 | 137.0228 | 161.6 | 4.36% |
| 8-oxogeranial | C10H14O2 | 211.095 | 271.4 | 3.17% |
| 7-hydroxy-4-isopropenyl-7-methyloxepan-2-one | C10H16O3 | 185.117 | 218 | 3.11% |
| (S)-4,5-dihydroxypentane-2,3-dione | C5H8O4 | 113.023 | 56.2 | 2.58% |
| 4-hydroxybenzaldehyde | C7H6O2 | 121.0273 | 255.3 | 2.26% |
| Anandamide | C22H37NO2 | 304.2991 | 341.4 | 2.19% |
| 2-oxohept-3-enedioate | C7H8O5 | 153.0198 | 83.7 | 1.74% |
| 3,5-dihydroxybenzoic acid | C7H6O4 | 307.0443 | 136.2 | 1.65% |
| Coniferaldehyde | C10H10O3 | 177.0531 | 233.9 | 1.52% |
| L-dopa | C9H11NO4 | 179.0351 | 271.4 | 1.26% |
| Methyl gibberellin A9 | C20H26O4 | 311.1681 | 324.1 | 1.21% |
| 4-hydroxy-5-methyl-2-methylene-3(2H)-furanone | C6H6O3 | 125.0229 | 156.1 | 1.10% |
| p-hydroxyphenylacetic acid | C8H8O3 | 135.044 | 184.8 | 1.08% |
| Gentisic acid | C7H6O4 | 307.0461 | 131.2 | 1.07% |
| 7-chlorotryptophan | C11H11CIN2O2 | 237.0399 | 211.2 | 1.01% |
| Aesculetin | C9H6O4 | 355.0451 | 202.8 | 0.94% |
| Tridecanoic acid | C13H26O2 | 427.2343 | 284.2 | 0.93% |
| Norfuraneol | C5H6O3 | 227.0496 | 88.3 | 0.89% |
| 4-acetoxy-2-hexyltetrahydrofuran | C12H22O3 | 237.1117 | 222.5 | 0.84% |
| Solasodine | C27H43NO2 | 396.3254 | 270.8 | 0.76% |
| Bombykol | C16H30O | 256.2628 | 433 | 0.68% |
| 4-hydroxy-3-methylbenzoic acid | C8H8O3 | 151.0383 | 195.4 | 0.63% |
| Bornyl isovalerate | C15H26O2 | 221.1897 | 338 | 0.59% |
| latiluciferin | C15H24O2 | 237.1844 | 325.2 | 0.58% |
| Pyruvic acid | C3H4O3 | 133.0138 | 154.9 | 0.54% |
| 3-hydroxymandelic acid | C8H8O4 | 167.034 | 127.3 | 0.52% |
| Alpha-curcumene | C15H22 | 203.179 | 268.9 | 0.51% |
| Chrysanthemic acid | C10H16O2 | 167.0533 | 300 | 0.49% |
| 8-epiiridodial | C10H16O2 | 169.1209 | 289.1 | 0.49% |
| 3,4-dimethoxybenzaldehyde | C9H10O3 | 165.0537 | 172.8 | 0.47% |
| Erythronic acid | C4H8O5 | 117.018 | 370.3 | 0.46% |
| 2,5-furandicarbaldehyde | C6H4O3 | 142.0496 | 101.7 | 0.45% |
| FA 6_2;O | C6H8O3 | 109.0279 | 202.7 | 0.44% |
| FA 10_2;O | C10H16O3 | 229.1078 | 239.3 | 0.43% |
| Bisphenol A | C15H16O2 | 212.0947 | 126.2 | 0.39% |
| Nepetalactone trans-cis-form | C10H14O2 | 184.1692 | 96.5 | 0.37% |
| 10-OPDA | C18H28O3 | 337.2035 | 350.5 | 0.37% |
| Naringenin | C15H12O5 | 271.0604 | 271.6 | 0.37% |
| 1,2-dimethyl-4-(6-methyl-4-heptenyl)-1,3-cyclohexadiene | C16H26 | 219.1732 | 382 | 0.36% |
| Total | 70.66% |
| Project | Concentration (mg/mL) | Control | MIC (mg/mL) | MBC (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50.00 | 25.00 | 12.50 | 6.250 | 3.125 | 1.563 | 0.781 | 0.391 | ||||
| Growing states | − | − | − | − | − | + | + | + | + | 3.125 | |
| − | − | − | − | − | + | + | + | + | 3.125 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, F.; He, J.; Li, R.; Hu, Y. Endophytic Fungus UJ3-2 from Urtica fissa: Antibacterial Activity and Mechanism of Action against Staphylococcus aureus. Molecules 2024, 29, 4850. https://doi.org/10.3390/molecules29204850
Liao F, He J, Li R, Hu Y. Endophytic Fungus UJ3-2 from Urtica fissa: Antibacterial Activity and Mechanism of Action against Staphylococcus aureus. Molecules. 2024; 29(20):4850. https://doi.org/10.3390/molecules29204850
Chicago/Turabian StyleLiao, Fei, Jie He, Renjun Li, and Yanchun Hu. 2024. "Endophytic Fungus UJ3-2 from Urtica fissa: Antibacterial Activity and Mechanism of Action against Staphylococcus aureus" Molecules 29, no. 20: 4850. https://doi.org/10.3390/molecules29204850
APA StyleLiao, F., He, J., Li, R., & Hu, Y. (2024). Endophytic Fungus UJ3-2 from Urtica fissa: Antibacterial Activity and Mechanism of Action against Staphylococcus aureus. Molecules, 29(20), 4850. https://doi.org/10.3390/molecules29204850






