Studying the Influence of Salt Concentrations on Betalain and Selected Physical and Chemical Properties in the Lactic Acid Fermentation Process of Red Beetroot
Abstract
1. Introduction
2. Results and Discussion
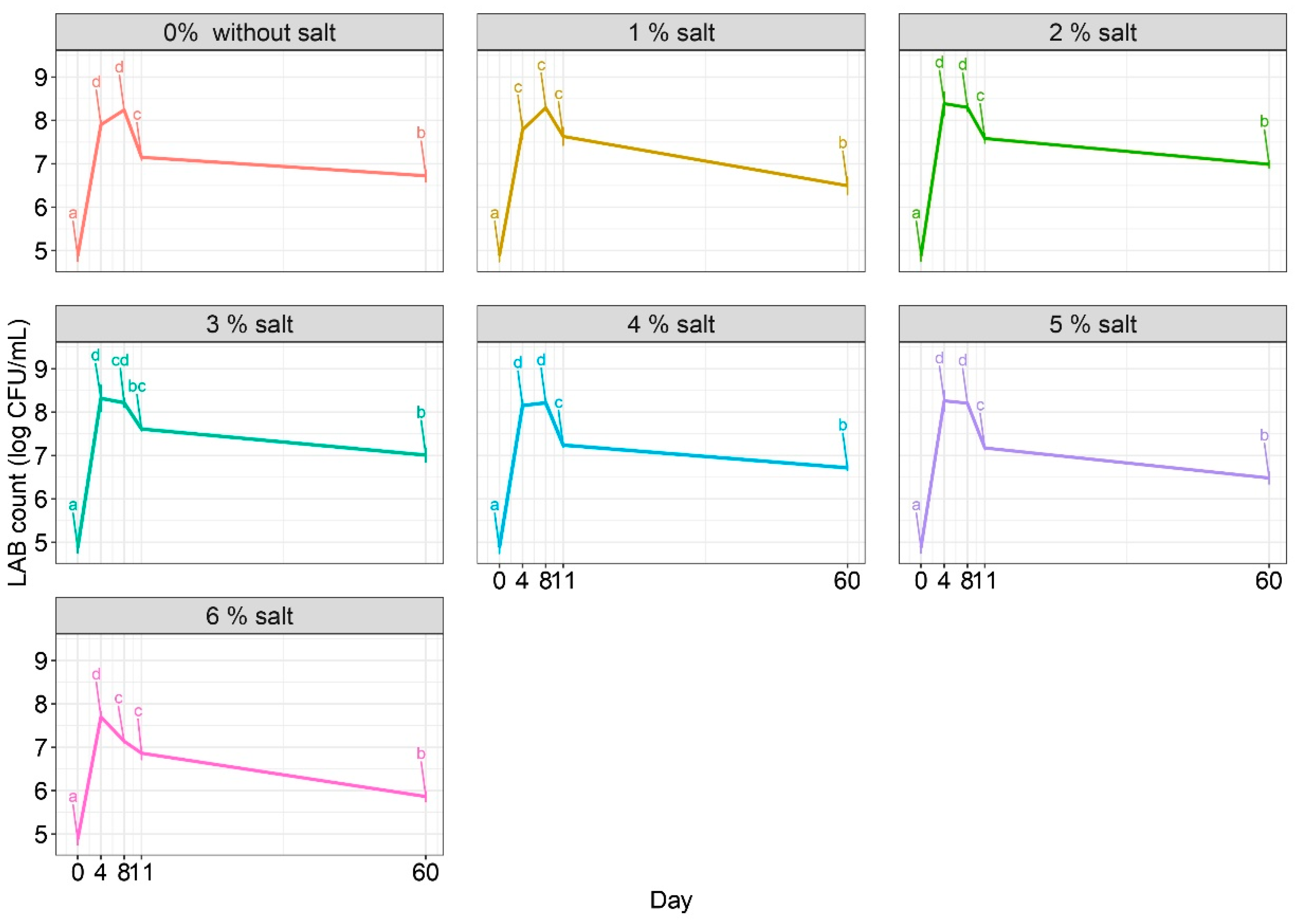
2.1. Microbiology
2.2. Physical Properties of Fermented Beetroot
2.2.1. pH and Total Acidity
2.2.2. Dry Matter and Salt Content
2.2.3. Color of the Fermented Beetroots
2.3. Pigment Content
2.4. Pigment Identification
2.5. Correlations
3. Materials and Methods
3.1. Materials
3.2. Fermentation
3.3. Analytical Methods
3.3.1. Determination of the Number of Lactic Acid Bacteria
3.3.2. Dry Matter
3.3.3. pH
3.3.4. Total Acidity
3.3.5. Salt Content
3.3.6. Color
3.3.7. Betalain Content and Identification
3.4. Data and Statistical Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janiszewska-Turak, E.; Walczak, M.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Witrowa-Rajchert, D. Influence of Fermentation Beetroot Juice Process on the Physico-Chemical Properties of Spray Dried Powder. Molecules 2022, 27, 1008. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Rybak, K.; Grzybowska, E.; Konopka, E.; Witrowa-Rajchert, D. The Influence of Different Pretreatment Methods on Color and Pigment Change in Beetroot Products. Molecules 2021, 26, 3683. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Wajs, J.; Stobiecka, M. Wpływ mlecznych produktów fermentowanych na zdrowie człowieka. Zdr. I Style Życia. Wrocław Uniw. Wrocławski 2020, 13, 133–152. [Google Scholar] [CrossRef]
- Pimentel, T.C.; de Assis, B.B.T.; dos Santos Rocha, C.; Marcolino, V.A.; Rosset, M.; Magnani, M. Prebiotics in non-dairy products: Technological and physiological functionality, challenges, and perspectives. Food Biosci. 2022, 46, 101585. [Google Scholar] [CrossRef]
- Pimentel, T.C.; da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110033. [Google Scholar] [CrossRef]
- Torres, S.; Verón, H.; Contreras, L.; Isla, M.I. An overview of plant-autochthonous microorganisms and fermented vegetable foods. Food Sci. Hum. Wellness 2020, 9, 112–123. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Arruda, H.S.; Geraldi, M.V.; Maróstica Júnior, M.R.; Pastore, G.M. Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Curr. Opin. Food Sci. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Rybak, K.; Pobiega, K.; Nikodem, A.; Gramza-Michałowska, A. Sustainable Production and Characteristics of Dried Fermented Vegetables. Fermentation 2022, 8, 659. [Google Scholar] [CrossRef]
- Gokhale, S.V.; Lele, S.S. Betalain Content and Antioxidant Activity of Beta vulgaris: Effect of Hot Air Convective Drying and Storage. J. Food Process. Preserv. 2014, 38, 585–590. [Google Scholar] [CrossRef]
- Chawla, H.; Parle, M.; Sharma, K.; Yadav, M. Beetroot: A health promoting functional food. Inven. Rapid Nutraceuticals 2016, 1, 0976–3872. [Google Scholar]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, J.; Xie, S.Y.; Zhang, T.Y.; Soladoye, O.P.; Aluko, R.E. Red Beetroot Betalains: Perspectives on Extraction, Processing, and Potential Health Benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef]
- Bangar, S.P.; Nitya, S.; Nikita, S.; Jose, M.L.; Sahu, J.K. Bioactive potential of beetroot (Beta vulgaris). Food Res. Int. 2022, 158, 111556. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Betalains—Emerging prospects for food scientists. Trends Food Sci. Technol. 2007, 18, 514–525. [Google Scholar] [CrossRef]
- Sionek, B. Ocena możliwości produkcji fermentowanego soku z buraka ćwikłowego z dodatkiem szczepów bakterii probiotycznych i potencjalnie probiotycznych rodzaju Lactobacillus®. Postępy Tech. Przetwórstwa Spożywczego 2020, 1, 74–81. [Google Scholar]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Nair, M.R.; Chouhan, D.; Sen Gupta, S.; Chattopadhyay, S. Fermented foods: Are they tasty medicines for Helicobacter pylori associated peptic ulcer and gastric cancer? Front. Microbiol. 2016, 7, 1148. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic delivery through fermentation: Dairy vs. non-dairy beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Bontsidis, C.; Mallouchos, A.; Terpou, A.; Nikolaou, A.; Batra, G.; Mantzourani, I.; Alexopoulos, A.; Plessas, S. Microbiological and chemical properties of chokeberry juice fermented by novel lactic acid bacteria with potential probiotic properties during fermentation at 4 degrees C for 4 weeks. Foods 2021, 10, 768. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P. Red beetroot juice fermented by water kefir grains: Physicochemical, antioxidant profile and anticancer activity. Eur. Food Res. Technol. 2023, 249, 939–950. [Google Scholar] [CrossRef]
- Aljahani, A.H. Microbiological and physicochemical quality of vegetable pickles. J. Saudi Soc. Agric. Sci. 2020, 19, 415–421. [Google Scholar] [CrossRef]
- Tarapata, K.; Lesiów, T. Zmiany zachodzące w owocach i warzywach w łańcuchu logistycznym i sposoby ich ograniczania. Część 1. Nauk. Inżynierskie I Technologie. Pr. Nauk. Uniw. Ekon. We Wrocławiu 2021, 37, 182–205. [Google Scholar]
- Durack, E.; Alonso-Gomez, M.; Wilkinson, M.G. The effect of salt reduction on the growth of food spoilage bacteria in model broth systems and salt-adjusted ready meals. J. Food Saf. 2013, 33, 302–312. [Google Scholar] [CrossRef]
- Rath, K.M.; Maheshwari, A.; Rousk, J. The impact of salinity on the microbial response to drying and rewetting in soil. Soil Biol. Biochem. 2017, 108, 17–26. [Google Scholar] [CrossRef]
- Nieto Gutiérrez, J.J.; Ventosa Ucero, A.; Oren, A. Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 1998, 62, 504–544. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Joghee, N.N.; Jayaraman, G. Biochemical changes induced by salt stress in halotolerant bacterial isolates are media dependent as well as species specific. Prep. Biochem. Biotechnol. 2016, 46, 8–14. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. Reducing population salt intake worldwide: From evidence to implementation. Prog. Cardiovasc. Dis. 2010, 52, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Gallego, J.; Rantsiou, K.; Garrido-Fernandez, A.; Cocolin, L.; Arroyo-Lopez, F.N. Salt reduction in vegetable fermentation: Reality or desire? J. Food Sci. 2013, 78, R1095–R1100. [Google Scholar] [CrossRef]
- Campo, R.; Rosato, P.; Giagnacovo, D. Less salt, same taste: Food marketing strategies via healthier products. Sustainability 2020, 12, 3916. [Google Scholar] [CrossRef]
- Mani, A.; Paul, P.K. Effect of sodium substitution on sensory and quality parameters in mango pickle. Int. Res. J. Pure Appl. Chem. 2020, 21, 45–55. [Google Scholar] [CrossRef]
- Lin, X.; Tang, Y.; Hu, Y.; Lu, Y.; Sun, Q.; Lv, Y.; Zhang, Q.; Wu, C.; Zhu, M.; He, Q. Sodium reduction in traditional fermented foods: Challenges, strategies, and perspectives. J. Agric. Food Chem. 2021, 69, 8065–8080. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, G.; Shen, W.; Wang, Y.; Zhang, W.; Chi, Y. Microbial safety and sensory quality of instant low-salt Chinese paocai. Food Control 2016, 59, 575–580. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Jiang, A.; Xiu, Z.; Ji, Y.; Guan, Y.; Yang, X. Effect of salt concentration on quality of Chinese northeast sauerkraut fermented by Leuconostoc mesenteroides and Lactobacillus plantarum. Food Biosci. 2019, 30, 100421. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Musielik, N. Wpływ stężenia NaCl w zalewie na strukturę i teksturę kiszonego buraka żółtego/Influence of NaCl concentration in brine on the structure and texture of fermented yellow beetroots. Zywnosc. Nauka. Technologia. Jakosc/Food Sci. Technol. Qual. 2024, 31, 67–87. [Google Scholar] [CrossRef]
- Tang, J.; Wu, X.; Lv, D.; Huang, S.; Zhang, Y.; Kong, F. Effect of salt concentration on the quality and microbial community during pickled peppers fermentation. Food Chem. X 2024, 23, 101594. [Google Scholar] [CrossRef]
- Mi, T.; Wang, D.; Yao, S.; Yang, H.; Che, Y.; Wu, C. Effects of salt concentration on the quality and microbial diversity of spontaneously fermented radish paocai. Food Res. Int. 2022, 160, 111622. [Google Scholar] [CrossRef]
- Joshi, V.; Chauhan, A.; Devi, S.; Kumar, V. Application of response surface methodology in optimization of lactic acid fermentation of radish: Effect of addition of salt, additives and growth stimulators. J. Food Sci. Technol. 2015, 52, 4935–4944. [Google Scholar] [CrossRef][Green Version]
- Casciano, F.; Mayr, H.; Nissen, L.; Putti, A.; Zoli, F.; Gianotti, A.; Conterno, L. Red Beetroot Fermentation with Different Microbial Consortia to Develop Foods with Improved Aromatic Features. Foods 2022, 11, 3055. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Hornowska, Ł.; Pobiega, K.; Gniewosz, M.; Witrowa-Rajchert, D. The influence of Lactobacillus bacteria type and kind of carrier on the properties of spray-dried microencapsules of fermented beetroot powders. Int. J. Food Sci. Technol. 2021, 56, 2166–2174. [Google Scholar] [CrossRef]
- Lamba, J.; Goomer, S.; Saxena, S.K. Study the lactic acid bacteria content in traditional fermented Indian drink: Kanji. Int. J. Gastron. Food Sci. 2019, 16, 100143. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, C.; Wu, B.; Wang, T.; Yang, L.; Guan, J.; Yi, Y.; Deng, J.; Wu, H. Effect of Different Salt Additions on the Flavor Profile of Fermented Ciba Pepper. Fermentation 2024, 10, 111. [Google Scholar] [CrossRef]
- Choińska, R.; Piasecka-Jóźwiak, K.; Woźniak, Ł.; Świder, O.; Bartosiak, E.; Bujak, M.; Roszko, M.Ł. Starter culture-related changes in free amino acids, biogenic amines profile, and antioxidant properties of fermented red beetroot grown in Poland. Sci. Rep. 2022, 12, 20063. [Google Scholar] [CrossRef]
- Świder, O.; Wójcicki, M.; Bujak, M.; Juszczuk-Kubiak, E.; Szczepańska, M.; Roszko, M.Ł. Time evolution of microbial composition and metabolic profile for biogenic amines and free amino acids in a model cucumber fermentation system brined with 0.5 to 5.0% sodium chloride. Molecules 2021, 26, 5796. [Google Scholar] [CrossRef]
- Dimakopoulou-Papazoglou, D.; Lazaridou, A.; Biliaderis, C.G.; Katsanidis, E. Effect of Process Temperature on the Physical State of Beef Meat Constituents—Implications on Diffusion Kinetics during Osmotic Dehydration. Food Bioprocess Technol. 2022, 15, 706–716. [Google Scholar] [CrossRef]
- Kuria, M.W.; Matofari, J.W.; Nduko, J.M. Physicochemical, antioxidant, and sensory properties of functional mango (Mangifera indica L.) leather fermented by lactic acid bacteria. J. Agric. Food Res. 2021, 6, 100206. [Google Scholar] [CrossRef]
- Adha, F.A.; Rusdianto, A.S.; Novijanto, N.; Belgis, M. The Potential of Local Garlic (Allium sativum L.) to Become a Flavor Enhancer through Lacto-Fermented Garlic. In Proceedings of the International Conference on Science, Health, and Technology, Surakarta, Indonesia, 6–7 April 2021; pp. 346–351. [Google Scholar]
- Ahmed, I.; Qazi, I.M.; Jamal, S. Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov. Food Sci. Emerg. Technol. 2016, 34, 29–43. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Mereddy, R.; Maqsood, S. Recent developments in emerging technologies for beetroot pigment extraction and its food applications. Food Chem. 2021, 356, 129611. [Google Scholar] [CrossRef] [PubMed]
- Czyżowska, A.; Siemianowska, K.; Śniadowska, M.; Nowak, A. Bioactive Compounds and Microbial Quality of Stored Fermented Red Beetroots and Red Beetroot Juice. Pol. J. Food Nutr. Sci. 2020, 70, 35–44. [Google Scholar] [CrossRef]
- Fijałkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. Wpływ obróbki wstępnej ultradźwiękami na przebieg suszenia oraz barwę i zawartość betalain w buraku ćwikłowym. Zesz. Probl. Postępów Nauk Rol. 2015, 581, 11–20. [Google Scholar]
- Janiszewska-Turak, E.; Kołakowska, W.; Pobiega, K.; Gramza-Michałowska, A. Influence of Drying Type of Selected Fermented Vegetables Pomace on the Natural Colorants and Concentration of Lactic Acid Bacteria. Appl. Sci. 2021, 11, 7864. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Patras, A.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem. 2010, 17, 598–604. [Google Scholar] [CrossRef]
- Barbu, V.; Cotârleț, M.; Bolea, C.A.; Cantaragiu, A.; Andronoiu, D.G.; Bahrim, G.E.; Enachi, E. Three Types of Beetroot Products Enriched with Lactic Acid Bacteria. Foods 2020, 9, 786. [Google Scholar] [CrossRef]
- Thippeswamy, B.; Joshi, A.; Sethi, S.; Dahuja, A.; Kaur, C.; Tomar, B.S.; Varghese, E. Chemical Additives for Preserving the Betalain Pigment and Antioxidant Activity of Red Beetroot. Sugar Tech. 2022, 24, 890–899. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Vieira Teixeira da Silva, D.; Dos Santos Baiao, D.; de Oliveira Silva, F.; Alves, G.; Perrone, D.; Mere Del Aguila, E.; Paschoalin, V.M.F. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological effects of red beetroot and betalains: A review. Phytother. Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Dadan, M.; Janowicz, M.; Wiktor, A.; Witrowa-Rajchert, D.; Mandal, R.; Pratap-Singh, A.; Janiszewska-Turak, E. Effect of nonthermal treatments on selected natural food pigments and color changes in plant material. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5097–5144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Karl, T.; Lewis, D.H.; McGhie, T.K.; Arathoon, S.; Davies, K.M.; Ryan, K.G.; Gould, K.S.; Schwinn, K.E. Production of Betacyanins in Transgenic Nicotiana tabacum Increases Tolerance to Salinity. Front. Plant Sci. 2021, 12, 653147. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Romaszko, E.; Szawara-Nowak, D.; Piskula, M.K. The impact of the matrix of red beet products and interindividual variability on betacyanins bioavailability in humans. Food Res. Int. 2018, 108, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Wilkowska, A.; Ambroziak, W.; Czyżowska, A.; Adamiec, J. Effect of Microencapsulation by Spray Drying and Freeze Drying Technique on the Antioxidant Properties of Blueberry (Vaccinium myrtillus) Juice Polyphenolic Compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11–16. [Google Scholar] [CrossRef]
- Czyżowska, A.; Klewicka, E.; Libudzisz, Z. The influence of lactic acid fermentation process of red beet juice on the stability of biologically active colorants. Eur. Food Res. Technol. 2006, 223, 110–116. [Google Scholar] [CrossRef]
- Coy-Barrera, E. Chapter 17—Analysis of betalains (betacyanins and betaxanthins). In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 593–619. [Google Scholar]
- Khan, M.I. Stabilization of betalains: A review. Food Chem. 2016, 197 Pt B, 1280–1285. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain Stability and Degradation—Structural and Chromatic Aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Karwacka, M.; Ciurzyńska, A.; Galus, S.; Janowicz, M. Freeze-dried snacks obtained from frozen vegetable by-products and apple pomace–Selected properties, energy consumption and carbon footprint. Innov. Food Sci. Emerg. Technol. 2022, 77, 102949. [Google Scholar] [CrossRef]
- Tyl, C.; Sadler, G.D. pH and Titratable Acidity. In Food Analysis; Nielsen, S.S., Ed.; Food Science Text Series; Springer: Cham, Switzerland, 2017; pp. 389–406. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Rybak, K.; Witrowa-Rajchert, D.; Pobiega, K.; Wierzbicka, A.; Ossowski, S.; Sękul, J.; Kufel, A.; Wiśniewska, A.; Trych, U.; et al. Influence of Heat Treatment and Lactic Acid Fermentation on the Physical and Chemical Properties of Pumpkin Juice. Molecules 2024, 29, 4519. [Google Scholar] [CrossRef]
- Nielsen, S.S. Sodium Determination Using Ion-Selective Electrodes, Mohr Titration, and Test Strips. In Food Analysis Laboratory Manual; Food Science Text Series; Springer: Cham, Switzerland, 2017; pp. 161–170. [Google Scholar] [CrossRef]
- Matys, A.; Dadan, M.; Witrowa-Rajchert, D.; Parniakov, O.; Wiktor, A. Response surface methodology as a tool for optimization of pulsed electric field pretreatment and microwave-convective drying of apple. Appl. Sci. 2022, 12, 3392. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Pobiega, K.; Rybak, K.; Synowiec, A.; Woźniak, Ł.; Trych, U.; Gniewosz, M.; Witrowa-Rajchert, D. Changes in Physical and Chemical Parameters of Beetroot and Carrot Juices Obtained by Lactic Fermentation. Appl. Sci. 2023, 13, 6113. [Google Scholar] [CrossRef]
- Kruszewski, B.; Domian, E.; Nowacka, M. Influence of High-Pressure Homogenization on the Physicochemical Properties and Betalain Pigments of Red Beetroot (Beta vulgaris L.) Juice. Molecules 2023, 28, 2018. [Google Scholar] [CrossRef] [PubMed]




| Salt (% w/v) | Day | Dry Matter (%) | Real Salt Content in Samples (%) | Color Coefficient | dE (-) | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| Fresh * | 0 | 14.0 ± 1.1 a | 0.49 ± 0.08 a | 11.5 ± 0.6 cd | 16.3 ± 0.4 a | 3.6 ± 0.3 a | - |
| 0 | 4 | 7.7 ± 0.0 bD | 0.41 ± 0.12 abE | 16.8 ± 2.2 bA | 16.0 ± 1.6 aC | −0.6 ± 0.3 bcD | 7.2 ± 1.0 cdC |
| 6 | 6.9 ± 0.2 bE | 0.17 ± 0.01 cF | 23.4 ± 1.9 aA | 11.5 ± 2.6 bE | −1.5 ± 0.6 cdE | 14.0 ± 2.1 aA | |
| 8 | 7.5 ± 0.0 bE | 0.16 ± 0.01 cG | 15.1 ± 4.4 bcA | 9.1 ± 3.4 bB | −1.8 ± 0.6 dC | 10.8 ± 2.7 bA | |
| 11 | 7.3 ± 0.1 bE | 0.23 ± 0.05 bcG | 17.3 ± 4.2 bAB | 13.2 ± 6.0 abC | −1.3 ± 0.7 cdD | 10.3 ± 3.5 bcAB | |
| 60 | 7.0 ± 0.0 bD | 0.20 ± 0.02 bcG | 11.2 ± 2.0 dA | 14.8 ± 2.0 aA | 0.1 ± 1.2 bC | 4.7 ± 1.3 dB | |
| Fresh * | 0 | 14.0 ± 1.1 a | 0.49 ± 0.08 c | 11.5 ± 0.6 bc | 16.3 ± 0.4 ab | 3.6 ± 0.3 a | - |
| 1 | 4 | 7.8 ± 0.0 bD | 0.71 ± 0.10 bE | 19.7 ± 3.2 aA | 16.7 ± 3.0 abC | −0.2 ± 0.2 cdCD | 9.4 ± 3.1 bBC |
| 6 | 7.7 ± 0.1 bD | 1.16 ± 0.01 aE | 20.8 ± 4.2 aABC | 20.4 ± 6.5 aB | −1.0 ± 0.3 dDE | 12.9 ± 1.9 aBC | |
| 8 | 7.8 ± 0.2 bDE | 0.71 ± 0.03 bF | 13.2 ± 2.3 bAB | 13.2 ± 4.4 bAB | −2.0 ± 0.7 eC | 7.9 ± 2.4 bAB | |
| 11 | 7.4 ± 0.1 bE | 0.70 ± 0.01 bF | 19.3 ± 3.6 aA | 19.9 ± 4.2 aB | 1.1 ± 1.0 bC | 10.1 ± 2.7 abAB | |
| 60 | 7.4 ± 0.1 bD | 0.52 ± 0.02 cF | 8.6 ± 1.5 cA | 15.6 ± 1.8 abA | 0.4 ± 0.2 bcBC | 4.8 ± 0.5 cB | |
| Fresh * | 0 | 14.0 ± 1.1 a | 0.49 ± 0.08 e | 11.5 ± 0.6 c | 16.3 ± 0.4 bc | 3.6 ± 0.3 a | - |
| 2 | 4 | 8.6 ± 0.1 bCD | 1.39 ± 0.10 aD | 19.3 ± 1.2 abA | 20.1 ± 1.4 aBC | 0.7 ± 0.1 bC | 9.3 ± 1.0 abBC |
| 6 | 8.0 ± 0.1 bCD | 1.24 ± 0.00 abE | 18.9 ± 1.2 abBC | 13.2 ± 2.0 cdDE | −0.6 ± 0.3 cD | 9.2 ± 1.6 abC | |
| 8 | 8.2 ± 0.0 bC | 1.04 ± 0.01 cdE | 11.9 ± 3.2 cABC | 15.8 ± 4.0 bcA | −0.5 ± 0.4 cB | 6.2 ± 1.8 cBC | |
| 11 | 8.2 ± 0.0 bD | 1.10 ± 0.00 bcE | 17.6 ± 1.8 bAB | 18.5 ± 0.8 bcBC | −0.6 ± 0.4 cD | 7.8 ± 1.8 bcB | |
| 60 | 8.5 ± 0.4 bC | 0.85 ± 0.03 dE | 20.6 ± 1.8 aBC | 11.0 ± 2.6 dBC | 1.1 ± 0.8 bBC | 10.9 ± 1.8 aA | |
| Fresh * | 0 | 14.0 ± 1.1 a | 0.49 ± 0.08 d | 11.5 ± 0.6 bc | 16.3 ± 0.4 c | 3.6 ± 0.3 a | - |
| 3 | 4 | 9.1 ± 0.3 bC | 1.89 ± 0.14 aC | 18.4 ± 3.0 aA | 23.5 ± 4.1 abAB | 2.5 ± 1.1 bB | 11.1 ± 1.1 bAB |
| 6 | 8.3 ± 0.0 bC | 1.85 ± 0.10 aD | 17.1 ± 3.4 aC | 28.1 ± 3.5 aA | 2.3 ± 0.7 bcB | 13.8 ± 1.6 aA | |
| 8 | 9.1 ± 0.0 bB | 1.70 ± 0.00 abD | 9.9 ± 3.0 bcBC | 16.1 ± 4.0 cA | −0.7 ± 0.4 dB | 6.4 ± 1.8 cBC | |
| 11 | 9.0 ± 0.0 bC | 1.56 ± 0.02 bcD | 13.2 ± 2.4 bC | 22.1 ± 4.9 bAB | 1.4 ± 0.7 bcBC | 7.5 ± 3.0 cB | |
| 60 | 9.0 ± 0.0 bBC | 1.22 ± 0.04 cD | 7.1 ± 1.8 cA | 17.9 ± 3.9 bcA | 1.3 ± 0.7 bcBC | 6.2 ± 1.0 cB | |
| Fresh * | 0 | 14.0 ± 1.1 a | 0.49 ± 0.08 d | 11.5 ± 0.6 cd | 16.3 ± 0.4 c | 3.6 ± 0.3 a | - |
| 4 | 4 | 10.6 ± 0.0 bA | 2.42 ± 0.18 aB | 19.0 ± 3.0 abA | 21.5 ± 3.6 aB | 2.1 ± 1.0 bB | 10.3 ± 1.0 aB |
| 6 | 9.9 ± 0.1 bA | 2.10 ± 0.01 bC | 20.2 ± 2.2 aABC | 17.3 ± 0.3 bcBC | 1.1 ± 0.2 cdcC | 9.1 ± 2.1 abC | |
| 8 | 9.8 ± 0.1 bA | 1.90 ± 0.02 bcC | 9.8 ± 2.3 dBC | 10.3 ± 1.5 dB | −0.9 ± 0.4 eB | 8.0 ± 1.1 bAB | |
| 11 | 9.6 ± 0.1 bB | 2.00 ± 0.02 bcC | 17.0 ± 2.6 abAB | 21.0 ± 5.1 abB | 1.4 ± 0.7 bcBC | 9.2 ± 1.4 abAB | |
| 60 | 9.4 ± 0.2 bB | 1.77 ± 0.04 cC | 16.2 ± 3.6 bcC | 9.0 ± 1.4 dC | 0.7 ± 0.6 dBC | 9.7 ± 1.2 abA | |
| Fresh * | 0 | 14.0 ± 1.1 a | 0.49 ± 0.08 e | 11.5 ± 0.6 c | 16.3 ± 0.4 c | 3.6 ± 0.3 b | - |
| 5 | 4 | 8.5 ± 0.1 bcBC | 2.63 ± 0.06 AB | 20.1 ± 2.4 aA | 26.1 ± 3.0 aA | 4.5 ± 0.9 aA | 13.4 ± 2.4 aA |
| 6 | 9.0 ± 0.0 bcB | 2.59 ± 0.03 abB | 21.0 ± 3.0 aAB | 14.8 ± 3.1 cC | 1.2 ± 0.7 dCD | 10.5 ± 2.5 bBC | |
| 8 | 8.0 ± 0.1 cCD | 2.45 ± 0.03 bcB | 8.9 ± 0.5 cC | 17.0 ± 1.7 cA | 0.3 ± 0.2 eA | 4.5 ± 0.5 cC | |
| 11 | 10.1 ± 0.1 bA | 2.38 ± 0.00 cB | 16.8 ± 2.7 bABC | 21.7 ± 2.4 bB | 2.2 ± 0.1 cAB | 8.2 ± 1.9 bB | |
| 60 | 9.8 ± 0.1 bAB | 2.11 ± 0.02 dB | 18.7 ± 0.8 abBC | 10.8 ± 1.5 cBC | 1.8 ± 0.5 cdB | 9.4 ± 0.7 bA | |
| Fresh * | 0 | 14.0 ± 1.1 a | 0.49 ± 0.08 d | 11.5 ± 0.6 d | 16.3 ± 0.4 c | 3.6 ± 0.3 abc | - |
| 6 | 4 | 10.7 ± 0.1 bB | 3.00 ± 0.04 bA | 19.4 ± 2.2 abA | 22.9 ± 3.4 bAB | 2.7 ± 0.7 cB | 10.9 ± 2.0 bcB |
| 6 | 9.3 ± 0.0 bB | 3.20 ± 0.02 abA | 16.9 ± 0.5 bcC | 29.8 ± 0.5 aA | 3.9 ± 0.2 abA | 14.5 ± 0.4 aA | |
| 8 | 9.8 ± 0.1 bA | 3.20 ± 0.08 aA | 15.8 ± 3.9 cA | 10.4 ± 3.3 dB | 0.3 ± 0.2 dA | 8.7 ± 3.8 cAB | |
| 11 | 10.1 ± 0.1 bA | 3.29 ± 0.02 aA | 14.9 ± 1.0 cBC | 27.8 ± 3.0 aA | 2.8 ± 0.8 bcA | 12.1 ± 2.7 abA | |
| 60 | 10.4 ± 0.1 bA | 2.59 ± 0.06 cA | 20.5 ± 2.3 aAB | 14.8 ± 5.3 cdAB | 4.4 ± 2.6 aA | 10.7 ± 1.4 abcA | |
| Day | Salt (%) | Indexes | ||
|---|---|---|---|---|
| I/B | V/B | O/B | ||
| 0 | 0 | 0.5 | 0.1 | 0.0 |
| 8 | 0 | 1.3 | 0.0 | 0.0 |
| 1 | 0.9 | 0.0 | 0.0 | |
| 2 | 0.7 | 0.0 | 0.0 | |
| 3 | 0.7 | 0.0 | 0.0 | |
| 4 | 0.8 | 0.1 | 0.0 | |
| 5 | 0.7 | 0.0 | 0.0 | |
| 6 | 0.5 | 0.0 | 0.0 | |
| 60 | 0 | 0.7 | 0.0 | 0.9 |
| 1 | 0.8 | 0.0 | 0.5 | |
| 2 | 0.8 | 0.0 | 0.2 | |
| 3 | 0.7 | 0.0 | 0.3 | |
| 4 | 0.7 | 0.0 | 0.3 | |
| 5 | 0.8 | 0.0 | 0.2 | |
| 6 | 0.8 | 0.0 | 0.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiszewska-Turak, E.; Wierzbicka, A.; Rybak, K.; Pobiega, K.; Synowiec, A.; Woźniak, Ł.; Trych, U.; Krzykowski, A.; Gramza-Michałowska, A. Studying the Influence of Salt Concentrations on Betalain and Selected Physical and Chemical Properties in the Lactic Acid Fermentation Process of Red Beetroot. Molecules 2024, 29, 4803. https://doi.org/10.3390/molecules29204803
Janiszewska-Turak E, Wierzbicka A, Rybak K, Pobiega K, Synowiec A, Woźniak Ł, Trych U, Krzykowski A, Gramza-Michałowska A. Studying the Influence of Salt Concentrations on Betalain and Selected Physical and Chemical Properties in the Lactic Acid Fermentation Process of Red Beetroot. Molecules. 2024; 29(20):4803. https://doi.org/10.3390/molecules29204803
Chicago/Turabian StyleJaniszewska-Turak, Emilia, Anna Wierzbicka, Katarzyna Rybak, Katarzyna Pobiega, Alicja Synowiec, Łukasz Woźniak, Urszula Trych, Andrzej Krzykowski, and Anna Gramza-Michałowska. 2024. "Studying the Influence of Salt Concentrations on Betalain and Selected Physical and Chemical Properties in the Lactic Acid Fermentation Process of Red Beetroot" Molecules 29, no. 20: 4803. https://doi.org/10.3390/molecules29204803
APA StyleJaniszewska-Turak, E., Wierzbicka, A., Rybak, K., Pobiega, K., Synowiec, A., Woźniak, Ł., Trych, U., Krzykowski, A., & Gramza-Michałowska, A. (2024). Studying the Influence of Salt Concentrations on Betalain and Selected Physical and Chemical Properties in the Lactic Acid Fermentation Process of Red Beetroot. Molecules, 29(20), 4803. https://doi.org/10.3390/molecules29204803









