Microfluidics-Assisted Polymer Vesicle Budding in Emulsion Systems: A Promising Approach for the Preparation and Application of Polymer Vesicles
Abstract
1. Introduction
2. Results and Discussion
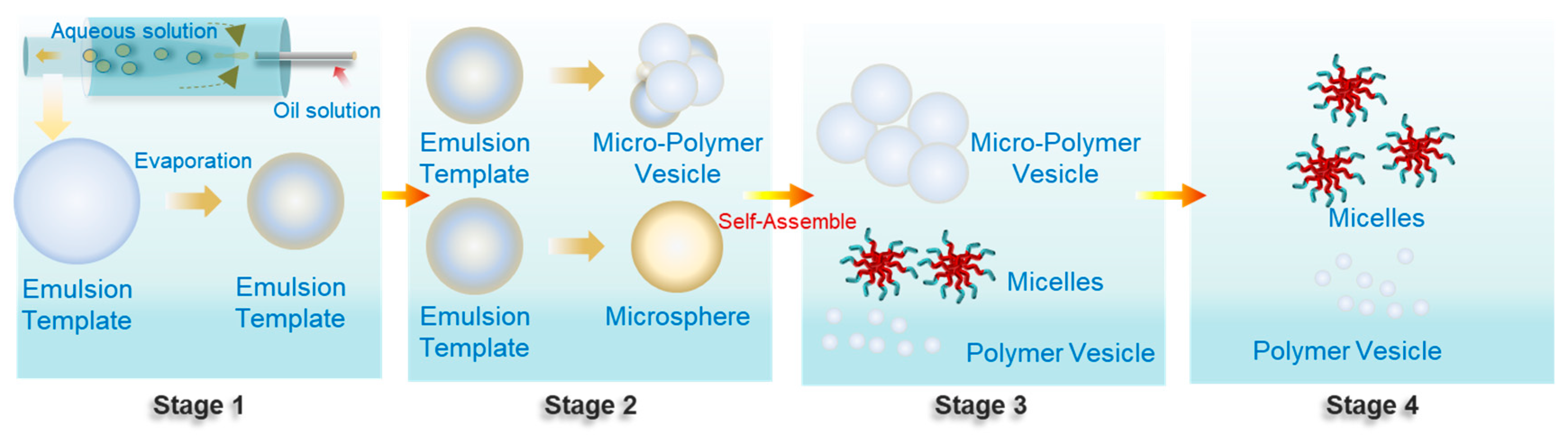
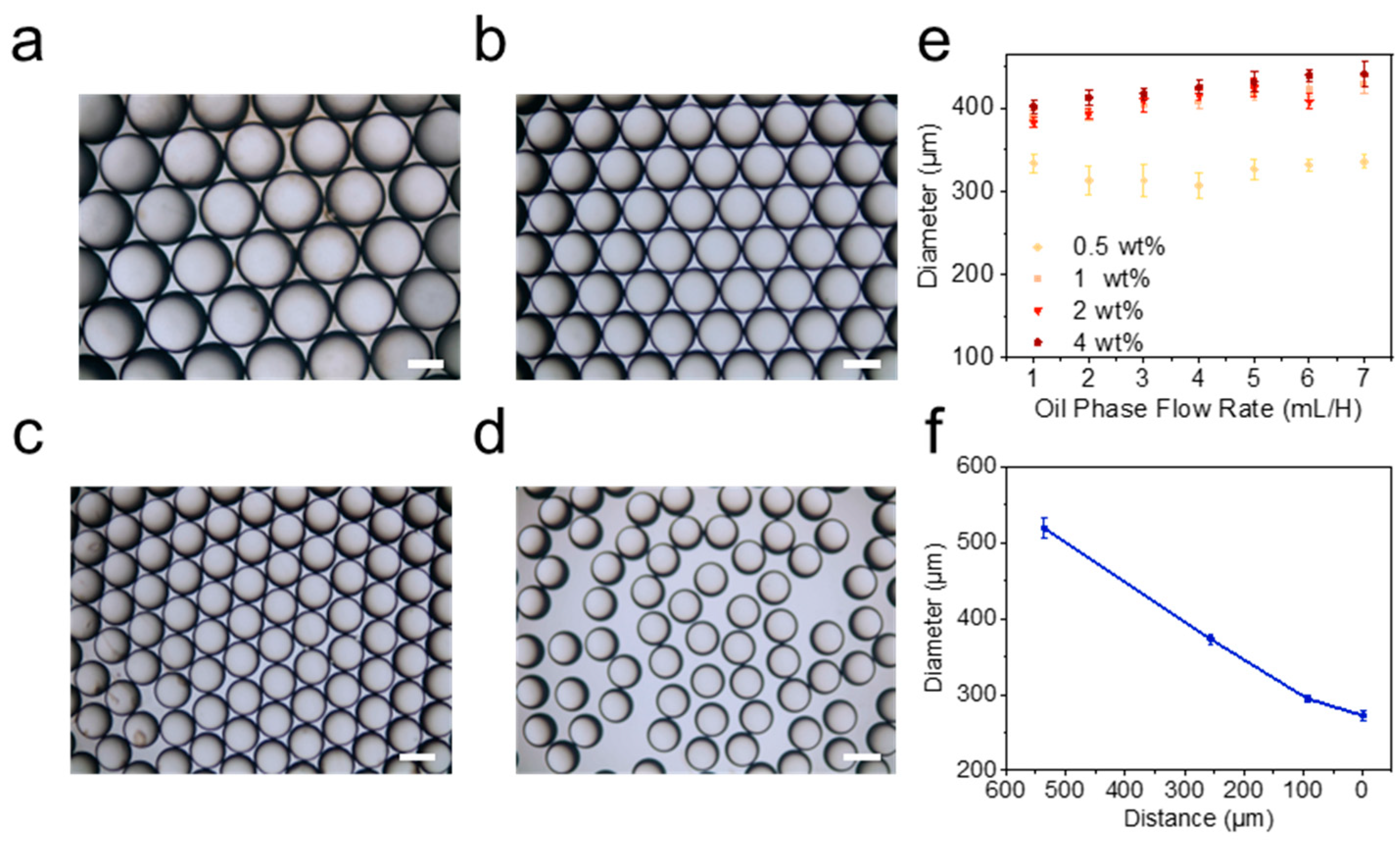
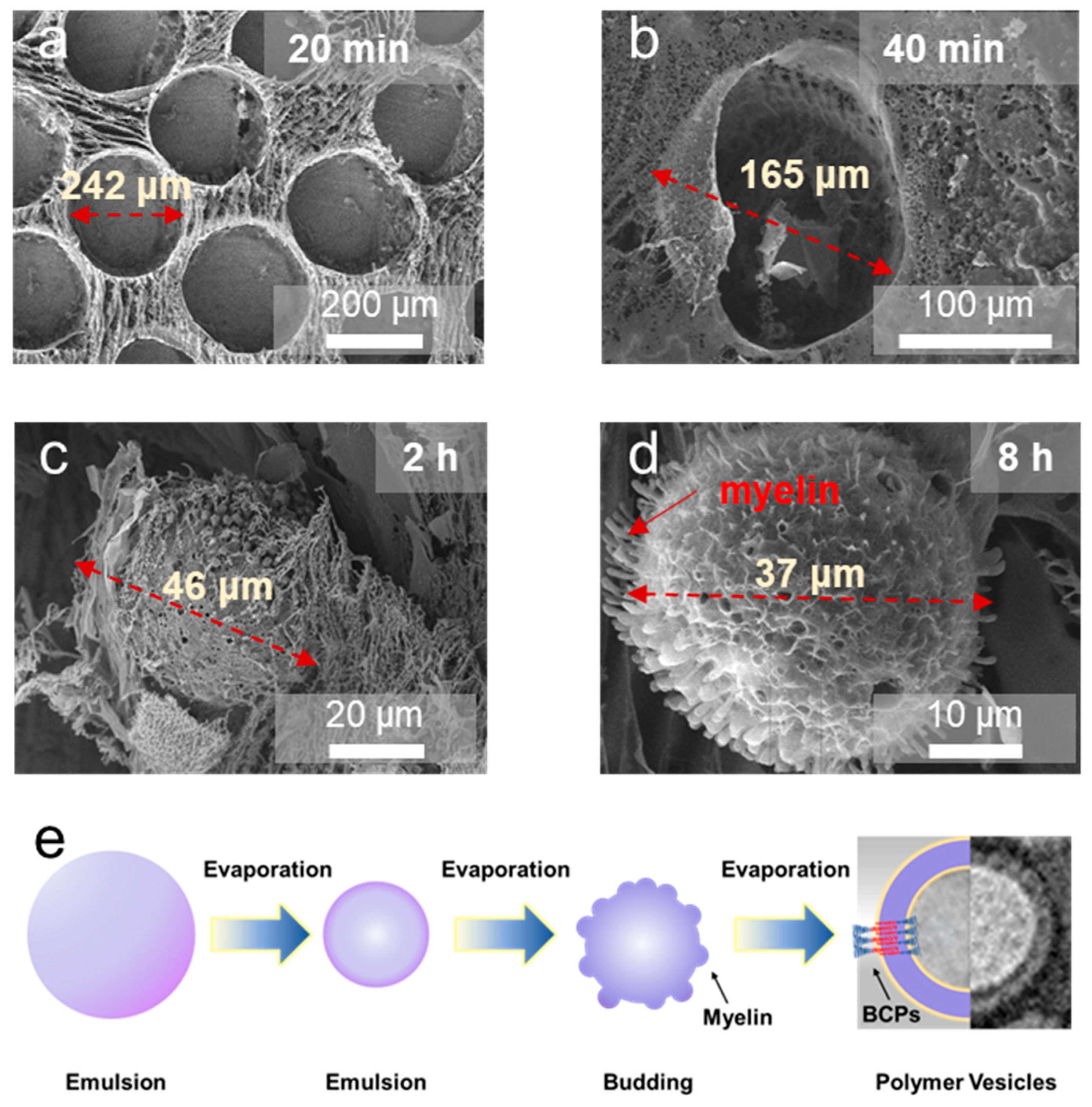
2.1. Preparation of Emulsion Templates
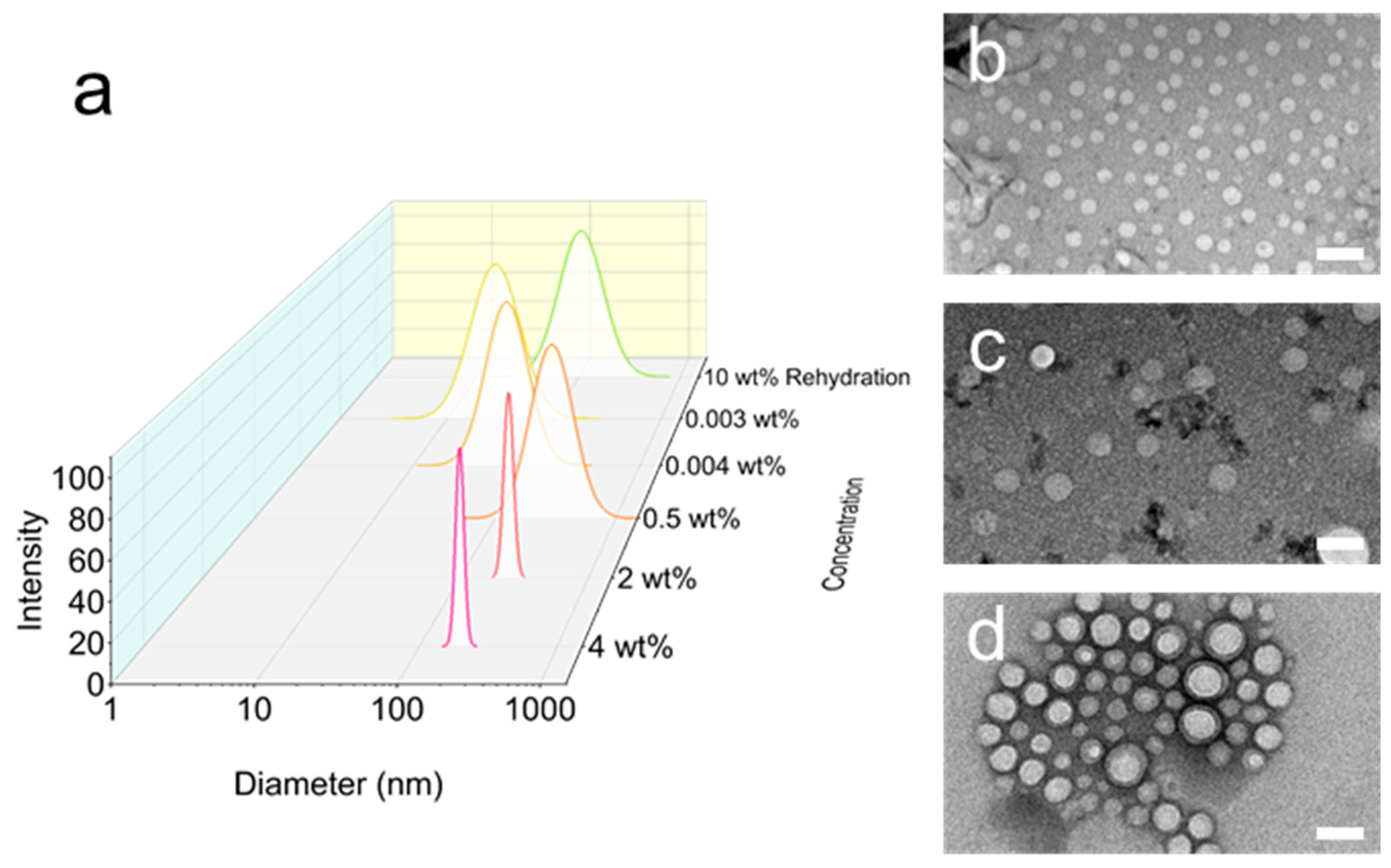
2.2. Self-Assembly and Polymer Vesicle Preparation
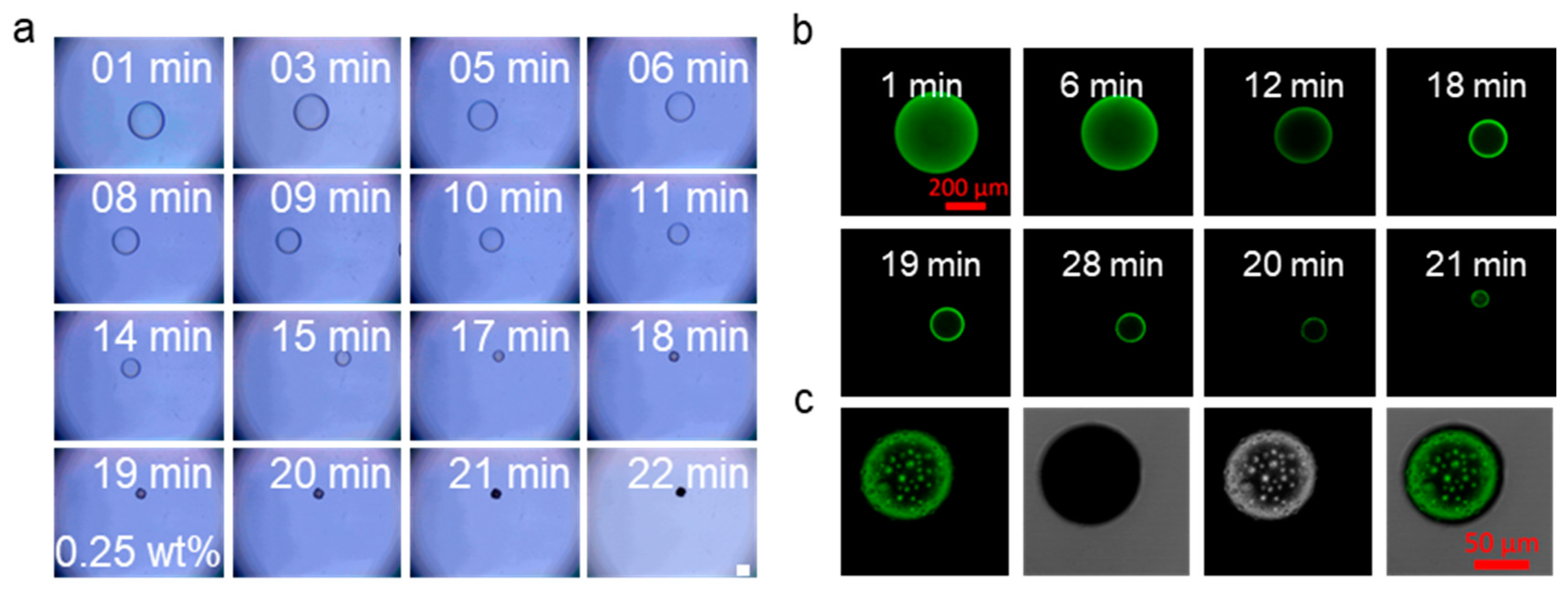
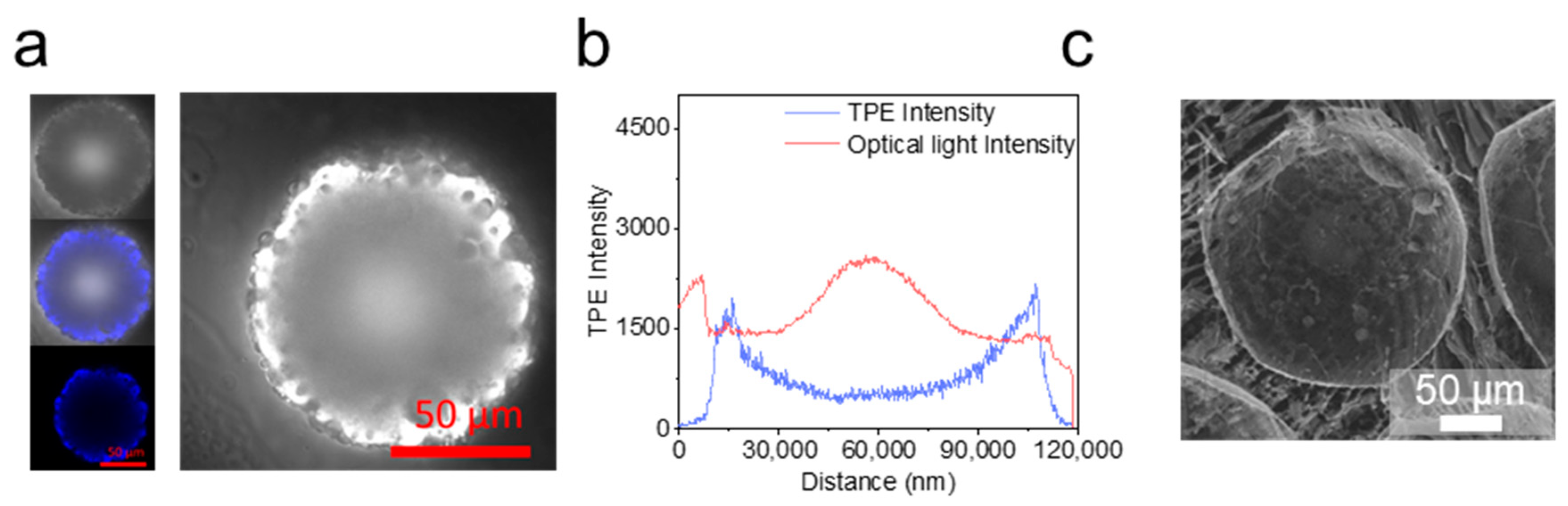
2.3. Evaporation and Self-Assembly in Aqueous Solution
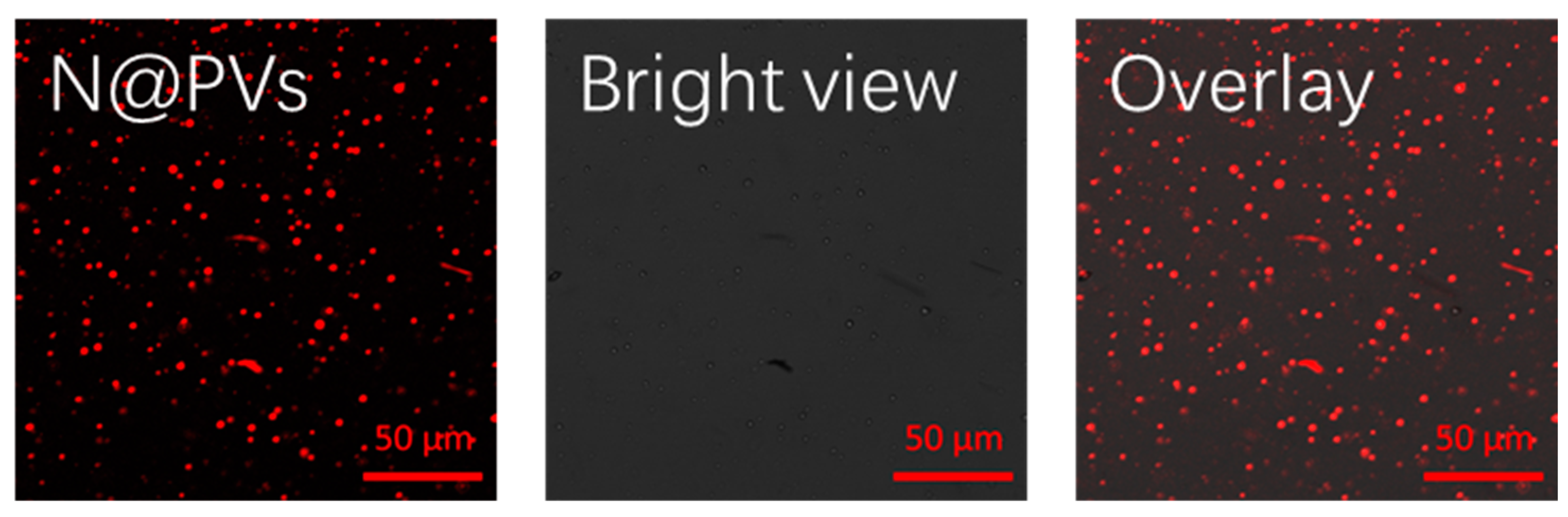
2.4. Drug Loading and Cell Biocompatibility
3. Experimental Design
3.1. Materials
3.2. Microfluidic Equipment
3.3. Single-Emulsion Template Preparation
3.4. Sample Preparation and Aggregation Studies
3.5. Characterization of Emulsion Templates and Polymer Vesicles
3.6. Drug Loading Studies
3.7. Cell Culture and Real-Time Living Cell Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Discher, B.M.; Won, Y.-Y.; Ege, D.S.; Lee, J.C.-M.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Christian, D.A.; Cai, S.; Bowen, D.M.; Kim, Y.; Pajerowski, J.D.; Discher, D.E. Polymersome carriers: From self-assembly to siRNA and protein therapeutics. Eur. J. Pharm. Biopharm. 2009, 71, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zheng, M.; Yang, W.; Meng, F.; Miyata, K.; Kim, H.J.; Kataoka, K.; Zhong, Z. Virus-Mimicking Chimaeric Polymersomes Boost Targeted Cancer siRNA Therapy In Vivo. Adv. Mater. 2017, 29, 1703285. [Google Scholar] [CrossRef]
- Fanalista, F.; Birnie, A.; Maan, R.; Burla, F.; Charles, K.; Pawlik, G.; Deshpande, S.; Koenderink, G.H.; Dogterom, M.; Dekker, C. Shape and Size Control of Artificial Cells for Bottom-Up Biology. ACS Nano 2019, 13, 5439–5450. [Google Scholar] [CrossRef]
- Houbrechts, M.; Caire da Silva, L.; Ethirajan, A.; Landfester, K. Formation of giant polymer vesicles by simple double emulsification using block copolymers as the sole surfactant. Soft Matter 2021, 17, 4942–4948. [Google Scholar] [CrossRef]
- Che, H.L.; van Hest, J.C.M. Adaptive Polymersome Nanoreactors. ChemNanoMat 2019, 5, 1092–1109. [Google Scholar] [CrossRef]
- Vriezema, D.M.; Garcia, P.M.; Sancho Oltra, N.; Hatzakis, N.S.; Kuiper, S.M.; Nolte, R.J.; Rowan, A.E.; Van Hest, J.C. Positional assembly of enzymes in polymersome nanoreactors for cascade reactions. Angew. Chem.-Int. Ed. 2007, 46, 7378–7382. [Google Scholar] [CrossRef]
- Che, H.; Cao, S.; van Hest, J.C.M. Feedback-Induced Temporal Control of “Breathing” Polymersomes to Create Self-Adaptive Nanoreactors. J. Am. Chem. Soc. 2018, 140, 5356–5359. [Google Scholar] [CrossRef]
- Xiao, W.; Cao, X.; Yao, P.; Garamus, V.M.; Chen, Q.; Cheng, J.; Zou, A. Enhanced Insecticidal Effect and Interface Behavior of Nicotine Hydrochloride Solution by a Vesicle Surfactant. Molecules 2022, 27, 6916. [Google Scholar] [CrossRef]
- Ke, W.; Li, J.; Mohammed, F.; Wang, Y.; Tou, K.; Liu, X.; Wen, P.; Kinoh, H.; Anraku, Y.; Chen, H.; et al. Therapeutic Polymersome Nanoreactors with Tumor-Specific Activable Cascade Reactions for Cooperative Cancer Therapy. ACS Nano 2019, 13, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-P.; Su, C.-H.; Chang, Y.-C.; Lin, Y.-J.; Yeh, C.-S. Ultrasound-Induced Reactive Oxygen Species Mediated Therapy and Imaging Using a Fenton Reaction Activable Polymersome. ACS Nano 2016, 10, 2017–2027. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Xu, M.; Hu, F.; Yu, Q.; Wang, L. Polymersomes as virus-surrogate particles for evaluating the performance of air filter mate-rials. Giant 2022, 10, 100104. [Google Scholar] [CrossRef] [PubMed]
- Kala, C.; Asif, M.; Gilani, S.J.; Imam, S.S.; Khan, N.A.; Taleuzzaman, M.; Zafar, A.; Ahmed, M.M.; Alshehri, S.; Ghoneim, M.M. Formulation of Isopropyl Isothiocyanate Loaded Nano Vesicles Delivery Systems: In Vitro Characterization and In Vivo Assessment. Molecules 2022, 27, 2876. [Google Scholar] [CrossRef]
- Li, W.; Liu, S.; Yao, H.; Liao, G.; Si, Z.; Gong, X.; Ren, L.; Wang, L. Microparticle templating as a route to nanoscale polymer vesicles with controlled size distribution for anticancer drug delivery. J. Colloid Interface Sci. 2017, 508, 145–153. [Google Scholar] [CrossRef]
- Battaglia, G.; Ryan, A.J. Bilayers and interdigitation in block copolymer vesicles. J. Am. Chem. Soc. 2005, 127, 8757–8764. [Google Scholar] [CrossRef]
- Kita-Tokarczyk, K.; Grumelard, J.; Haefele, T.; Meier, W. Block copolymer vesicles—Using concepts from polymer chemistry to mimic biomembranes. Polymer 2005, 46, 3540–3563. [Google Scholar] [CrossRef]
- Reeves, J.P.; Dowben, R.M. Formation and properties of thin-walled phospholipid vesicles. J. Cell. Physiol. 1969, 73, 49–60. [Google Scholar] [CrossRef]
- Contini, C.; Pearson, R.; Wang, L.; Messager, L.; Gaitzsch, J.; Rizzello, L.; Ruiz-Perez, L.; Battaglia, G. Bottom-Up Evolution of Vesicles from Disks to High-Genus Polymersomes. iScience 2018, 7, 132–144. [Google Scholar] [CrossRef]
- Soo, P.L.; Eisenberg, A. Preparation of block copolymer vesicles in solution. J. Polym. Sci. Part B-Polym. Phys. 2004, 42, 923–938. [Google Scholar]
- Le Berre, M.; Yamada, A.; Reck, L.; Chen, Y.; Baigl, D. Electroformation of giant phospholipid vesicles on a silicon substrate: Advantages of controllable surface properties. Langmuir 2008, 24, 2643–2649. [Google Scholar] [CrossRef]
- Howse, J.R.; Jones, R.A.; Battaglia, G.; Ducker, R.E.; Leggett, G.J.; Ryan, A.J. Templated formation of giant polymer vesicles with controlled size distributions. Nat. Mater. 2009, 8, 507–511. [Google Scholar] [CrossRef]
- Bergström, M.; Eriksson, J.C. Size distribution of reversibly formed bilayer vesicles. Langmuir 1998, 14, 288–299. [Google Scholar] [CrossRef]
- Pereira-Lachataignerais, J.; Pons, R.; Panizza, P.; Courbin, L.; Rouch, J.; Lopez, O. Study and formation of vesicle systems with low polydispersity index by ultrasound method. Chem. Phys. Lipids 2006, 140, 88–97. [Google Scholar] [CrossRef]
- Chen, W.; Du, J. Ultrasound and pH Dually Responsive Polymer Vesicles for Anticancer Drug Delivery. Sci. Rep. 2013, 3, 2162. [Google Scholar] [CrossRef]
- Frisken, B.J.; Asman, C.; Patty, P.J. Studies of Vesicle Extrusion. Langmuir 2000, 16, 928–933. [Google Scholar] [CrossRef]
- Rusli, W.; van Herk, A.M. Effect of salts on size and morphology of extruded dimethyldioctadecylammonium bromide or chloride vesicle for polymeric nanocapsules synthesis via templating emulsion polymerization. J. Colloid Interface Sci. 2021, 587, 393–401. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, W.; Qu, X.; Wu, H.; Qu, L.; Zhang, X.; Mäkilä, E.; Salonen, J.; Zhu, Y.; Yang, Z.; et al. Photothermal-responsive nanosized hybrid polymersome as versatile therapeutics codelivery nanovehicle for effective tumor suppression. Proc. Natl. Acad. Sci. USA 2019, 116, 7744–7749. [Google Scholar] [CrossRef]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef]
- Shum, H.C.; Kim, J.-W.; Weitz, D.A. Microfluidic fabrication of monodisperse biocompatible and biodegradable polymersomes with controlled permeability. J. Am. Chem. Soc. 2008, 130, 9543–9549. [Google Scholar] [CrossRef]
- Nguyen, X.D.; Park, D.H.; Paik, H.-J.; Jeon, H.J.; Huh, J.; Go, J.S. Microfluidic Tracking of the Growth of Polymeric Vesicles in Hydrodynamic Flow. ACS Appl. Polym. Mater. 2020, 2, 5845–5850. [Google Scholar] [CrossRef]
- Shum, H.C.; Santanach-Carreras, E.; Kim, J.-W.; Ehrlicher, A.; Bibette, J.; Weitz, D.A. Dewetting-Induced Membrane Formation by Adhesion of Amphiphile-Laden Interfaces. J. Am. Chem. Soc. 2011, 133, 4420–4426. [Google Scholar] [CrossRef]
- Matosevic, S.; Paegel, B.M. Stepwise Synthesis of Giant Unilamellar Vesicles on a Microfluidic Assembly Line. J. Am. Chem. Soc. 2011, 133, 2798–2800. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Kawano, R.; Osaki, T.; Akiyoshi, K.; Takeuchi, S. Cell-sized asymmetric lipid vesicles facilitate the investigation of asymmetric membranes. Nat. Chem. 2016, 8, 881–889. [Google Scholar] [CrossRef]
- Stachowiak, J.C.; Richmond, D.L.; Li, T.H.; Brochard-Wyart, F.; Fletcher, D.A. Inkjet formation of unilamellar lipid vesicles for cell-like encapsulation. Lab Chip 2009, 9, 2003–2009. [Google Scholar] [CrossRef]
- Thiele, J.; Steinhauser, D.; Pfohl, T.; Förster, S. Preparation of Monodisperse Block Copolymer Vesicles via Flow Focusing in Mi-crofluidics. Langmuir 2010, 26, 6860–6863. [Google Scholar] [CrossRef] [PubMed]
- Jahn, A.; Vreeland, W.N.; Gaitan, M.; Locascio, L.E. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J. Am. Chem. Soc. 2004, 126, 2674–2675. [Google Scholar] [CrossRef]
- Deshpande, S.; Dekker, C. On-chip microfluidic production of cell-sized liposomes. Nat. Protoc. 2018, 13, 856–874. [Google Scholar] [CrossRef]
- Deshpande, S.; Spoelstra, W.K.; van Doorn, M.; Kerssemakers, J.; Dekker, C. Mechanical Division of Cell-Sized Liposomes. ACS Nano 2018, 12, 2560–2568. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, B.; Chen, S.; Du, J. Polymer vesicles: Mechanism, preparation, application, and responsive behavior. Prog. Polym. Sci. 2017, 64, 1–22. [Google Scholar] [CrossRef]
- Bej, R.; Achazi, K.; Haag, R.; Ghosh, S. Polymersome Formation by Amphiphilic Polyglycerol-b-polydisulfide-b-polyglycerol and Glutathione-Triggered Intracellular Drug Delivery. Biomacromolecules 2020, 21, 3353–3363. [Google Scholar] [CrossRef]
- Shen, H.W.; Eisenberg, A. Morphological phase diagram for a ternary system of block copolymer PS310-b-PAA(52)/dioxane/H2O. J. Phys. Chem. B 1999, 103, 9473–9487. [Google Scholar] [CrossRef]
- Battaglia, G.; Ryan, A.J. Neuron-like tubular membranes made of diblock copolymer amphiphiles. Angew. Chem.-Int. Ed. 2006, 45, 2052–2056. [Google Scholar] [CrossRef]
- Battaglia, G.; Ryan, A.J. The evolution of vesicles from bulk lamellar gels. Nat. Mater. 2005, 4, 869–876. [Google Scholar] [CrossRef]
- Julicher, F.; Lipowsky, R. Domain-induced budding of vesicles. Phys. Rev. Lett. 1993, 70, 2964–2967. [Google Scholar] [CrossRef]
- Jain, S.; Bates, F.S. Consequences of nonergodicity in aqueous binary PEO−PB micellar dispersions. Macromolecules 2004, 37, 1511–1523. [Google Scholar] [CrossRef]
- Thiele, J.; Chokkalingam, V.; Ma, S.; Wilson, D.A.; Huck, W.T.S. Vesicle budding from polymersomes templated by microfluidically prepared double emulsions. Mater. Horiz. 2014, 1, 96–101. [Google Scholar] [CrossRef]
- Yang, Z.; Qin, W.; Leung, N.L.C.; Arseneault, M.; Lam, J.W.Y.; Liang, G.; Sung, H.H.Y.; Williams, I.D.; Tang, B.Z. A mechanistic study of AIE processes of TPE luminogens: Intramolecular rotation vs. configurational isomerization. J. Mater. Chem. C 2016, 4, 99–107. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.; Fan, Y.; Zhou, L.; Trépout, S.; Guo, J.; Li, M.-H. Fluorescent Polymersomes with Aggregation-Induced Emission. ACS Nano 2018, 12, 4025–4035. [Google Scholar] [CrossRef]
- Guan, W.; Zhou, W.; Lu, C.; Tang, B.Z. Synthesis and Design of Aggregation-Induced Emission Surfactants: Direct Observation of Micelle Transitions and Microemulsion Droplets. Angew. Chem. Int. Ed. 2015, 54, 15160–15164. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.-H. Recent Progress in Fluorescent Vesicles with Aggregation-induced Emission. Chin. J. Polym. Sci. 2019, 37, 352–371. [Google Scholar] [CrossRef]
- Liao, G.; Chen, L.; Zhang, Y.; Mykhaylyk, O.O.; Topham, P.D.; Toolan, D.T.; Derry, M.J.; Howse, J.R.; Yu, Q.; Feng, G.; et al. Solvent selectivity governed self-assembly of block copolymer in nanofabrication. Polymer 2023, 283, 126205. [Google Scholar] [CrossRef]
- Dinic, J.; Sharma, V. Flexibility, Extensibility, and Ratio of Kuhn Length to Packing Length Govern the Pinching Dynamics, Coil-Stretch Transition, and Rheology of Polymer Solutions. Macromolecules 2020, 53, 4821–4835. [Google Scholar] [CrossRef]
- Cheng, S.; Kogut, D.; Zheng, J.; Patil, S.; Yang, F.; Lu, W. Dynamics of polylactic acid under ultrafine nanoconfinement: The collective interface effect and the spatial gradient. J. Chem. Phys. 2024, 160, 114904. [Google Scholar] [CrossRef]
- He, J.; Wang, L.; Wei, Z.; Yang, Y.; Wang, C.; Han, X.; Nie, Z. Vesicular Self-Assembly of Colloidal Amphiphiles in Microfluidics. ACS Appl. Mater. Interfaces 2013, 5, 9746–9751. [Google Scholar] [CrossRef]
- Pecora, R. Dynamic light scattering measurement of nanometer particles in liquids. J. Nanopart. Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, D.; Zhan, J.; Liao, G.; Zhu, T.; Yu, Q.; Zhang, W.; Wang, L. Microfluidics-Assisted Polymer Vesicle Budding in Emulsion Systems: A Promising Approach for the Preparation and Application of Polymer Vesicles. Molecules 2024, 29, 4802. https://doi.org/10.3390/molecules29204802
Dong D, Zhan J, Liao G, Zhu T, Yu Q, Zhang W, Wang L. Microfluidics-Assisted Polymer Vesicle Budding in Emulsion Systems: A Promising Approach for the Preparation and Application of Polymer Vesicles. Molecules. 2024; 29(20):4802. https://doi.org/10.3390/molecules29204802
Chicago/Turabian StyleDong, Donghua, Jilai Zhan, Guoxing Liao, Tong Zhu, Qianqian Yu, Wei Zhang, and Linge Wang. 2024. "Microfluidics-Assisted Polymer Vesicle Budding in Emulsion Systems: A Promising Approach for the Preparation and Application of Polymer Vesicles" Molecules 29, no. 20: 4802. https://doi.org/10.3390/molecules29204802
APA StyleDong, D., Zhan, J., Liao, G., Zhu, T., Yu, Q., Zhang, W., & Wang, L. (2024). Microfluidics-Assisted Polymer Vesicle Budding in Emulsion Systems: A Promising Approach for the Preparation and Application of Polymer Vesicles. Molecules, 29(20), 4802. https://doi.org/10.3390/molecules29204802







