A Review of Recent Advances in Chromatographic Quantification Methods for Cyanogenic Glycosides
Abstract
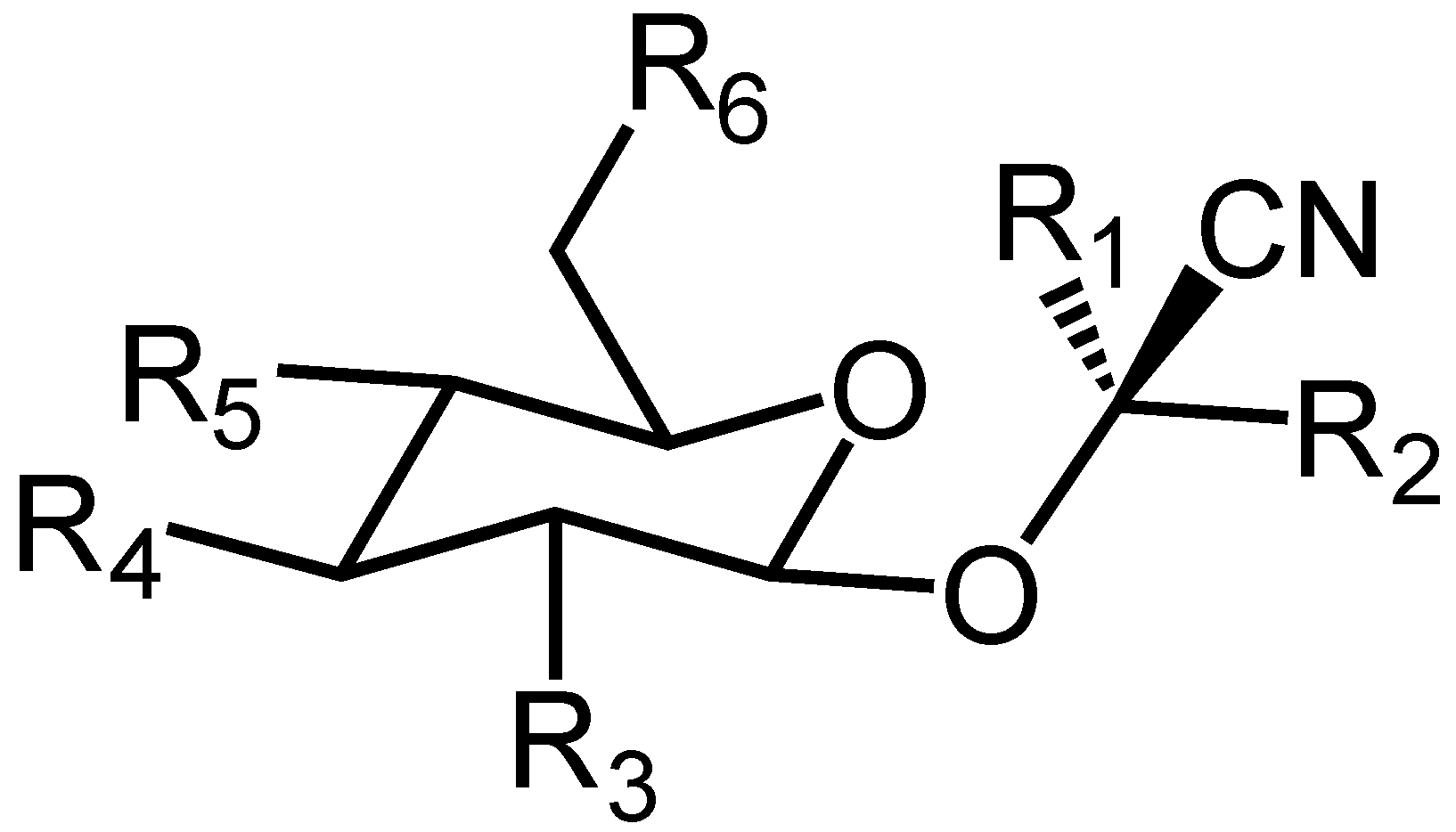
1. Introduction
2. Toxicity of Cyanogenic Glycosides in Plants
2.1. Amygdalin
2.2. Linamarin
2.3. Prunasin
2.4. Dhurrin
3. Determination of Cyanogenic Glycosides Using HPLC/UPLC
4. Determination of Cyanogenic Glycosides Using LC-MS
5. Determination of Cyanogenic Glycosides without HPLC Separation
6. Future Perspectives
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolarinwa, I.F.; Oke, M.O.; Olaniyan, S.A.; Ajala, A.S. A review of cyanogenic glycosides in edible plants. In Toxicology–New Aspects to This Scientific Conundrum; BoD–Books on Demand: Norderstedt, Germany, 2016. [Google Scholar]
- Francisco, I.A.; Pinotti, M.H.P. Cyanogenic glycosides in plants. Braz. Arch. Biol. Technol. 2000, 43, 487–492. [Google Scholar] [CrossRef]
- Gleadow, R.; Bjarnholt, N.; Jørgensen, K.; Fox, J.; Miller, R. Cyanogenic glycosides. Res. Methods Plant Sci. 2011, 1, 283–310. [Google Scholar]
- Gleadow, R.M.; Møller, B.L. Cyanogenic glycosides: Synthesis, physiology, and phenotypic plasticity. Annu. Rev. Plant Biol. 2014, 65, 155–185. [Google Scholar] [CrossRef]
- Tewe, O.O.; Iyayi, E.A. Cyanogenic glycosides. In Toxicants of Plant Origin; CRC Press: Boca Raton, FL, USA, 2023; pp. 43–60. [Google Scholar]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Schwerdtle, T.; EFSA Panel on Contaminants in the Food Chain (CONTAM). Evaluation of the health risks related to the presence of cyanogenic glycosides in foods other than raw apricot kernels. EFSA J. 2019, 17, e05662. [Google Scholar]
- Majak, W. Mammalian metabolism of toxic glycosides from plants. J. Toxicol. Toxin Rev. 1992, 11, 1–40. [Google Scholar] [CrossRef]
- Rawat, K.; Nirmala, C.; Bisht, M.S. Processing techniques for reduction of cyanogenic glycosides from bamboo shoots. In Proceedings of the 10th World Bamboo Congress, Gwangju, Republic of Korea, 17–22 September 2015; pp. 1–12. [Google Scholar]
- Ologhobo, A.D.; Fetuga, B.L.; Tewe, O.O. The cyanogenic glycoside contents of raw and processed limabean varieties. Food Chem. 1984, 13, 117–128. [Google Scholar] [CrossRef]
- Lei, V.; Amoa-Awua, W.K.A.; Brimer, L. Degradation of cyanogenic glycosides by Lactobacillus plantarum strains from spontaneous cassava fermentation and other microorganisms. Int. J. Food Microbiol. 1999, 53, 169–184. [Google Scholar] [CrossRef]
- Mosayyebi, B.; Imani, M.; Mohammadi, L.; Akbarzadeh, A.; Zarghami, N.; Edalati, M.; Rahmati, M. An update on the toxicity of cyanogenic glycosides bioactive compounds: Possible clinical application in targeted cancer therapy. Mater. Chem. Phys. 2020, 246, 122841. [Google Scholar] [CrossRef]
- Parker-Cote, J.L.; Rizer, J.; Vakkalanka, J.P.; Rege, S.V.; Holstege, C.P. Challenges in the diagnosis of acute cyanide poisoning. Clin. Toxicol. 2018, 56, 609–617. [Google Scholar]
- Park, H.; Chung, H.; Choi, S.; Bahn, Y.S.; Son, J. Evaluation of exposure to cyanogenic glycosides and potential hydrogen cyanide release in commercially available foods among the Korean population. Food Chem. 2024, 456, 139872. [Google Scholar] [CrossRef]
- Yildirim, F.A.; Askin, M.A. Variability of amygdalin content in seeds of sweet and bitter apricot cultivars in Turkey. Afr. J. Biotechnol. 2010, 9, 6522–6524. [Google Scholar]
- Ghiulai, V.M.; Socaciu, C.; Jianu, I.; Ranga, F.; Fetea, F. Identification and quantitative evaluation of amygdalin from apricot, plum and peach oils and kernels. Buletin USAMV-CN 2006, 62, 246–253. [Google Scholar] [CrossRef]
- Song, L.; García Martín, J.F.; Zhang, Q.A. Encapsulation of benzaldehyde produced by the eco-friendly degradation of amygdalin in the Apricot Kernel debitterizing wastewater. Foods 2024, 13, 437. [Google Scholar] [CrossRef]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef]
- Manoj, K.M.; Ramasamy, S.; Parashar, A.; Gideon, D.A.; Soman, V.; Jacob, V.D.; Pakshirajan, K. Acute toxicity of cyanide in aerobic respiration: Theoretical and experimental support for murburn explanation. Biomol. Concepts 2020, 11, 32–56. [Google Scholar] [CrossRef]
- García, I.; Arenas-Alfonseca, L.; Moreno, I.; Gotor, C.; Romero, L.C. HCN regulates cellular processes through posttranslational modification of proteins by S-cyanylation. Plant Physiol. 2019, 179, 107–123. [Google Scholar] [CrossRef]
- Laurena, A.C.; Revilleza, M.J.R.; Mendoza, E.M.T. Polyphenols, phytate, cyanogenic glycosides, and trypsin inhibitor activity of several Philippine indigenous food legumes. J. Food Compos. Anal. 1994, 7, 194–202. [Google Scholar] [CrossRef]
- Cereda, M.P.; de Vasconcellos, S.P. Cassava cyanogenic glycosides: Importance, toxicity, and dosage methods. In Varieties and Landraces: Cultural Practices and Traditional Uses; Academic Press: Cambridge, MA, USA, 2023; pp. 179–209. [Google Scholar]
- Rufino, M.N.; Cereda, M.P.; Barreto, W.T.G.; Silva, A.R.D.; Andrade, G.B.D.; Herrera, H.M. Pathological effects of acetone cyanohydrin in swiss rats. Cienc. Agrotecnol. 2016, 40, 577–584. [Google Scholar] [CrossRef][Green Version]
- Tomishima, H.; Luo, K.; Mitchell, A.E. The almond (Prunus dulcis): Chemical properties, utilization, and valorization of coproducts. Annu. Rev. Food Sci. Technol. 2022, 13, 145–166. [Google Scholar] [CrossRef]
- Tezcan, S.; Evrenosoğlu, Y.; Misirli, A.; Gülcan, R.; Gülperçin, N. Prunasin contents of Turkish apricot cultivars and artificial infestation of rootstocks by Capnodis tenebrionis (Linnaeus, 1758) and Capnodis carbonaria (Klug, 1829)(Coleoptera: Buprestidae). Türk. Entomol. Derg. 2011, 35, 407–421. [Google Scholar]
- Newmark, J.; Brady, R.O.; Grimley, P.M.; Gal, A.E.; Waller, S.G.; Thistlethwaite, J.R. Amygdalin (Laetrile) and prunasin beta-glucosidases: Distribution in germ-free rat and in human tumor tissue. Proc. Natl. Acad. Sci. USA 1981, 78, 6513–6516. [Google Scholar] [CrossRef] [PubMed]
- Krothapalli, K.; Buescher, E.M.; Li, X.; Brown, E.; Chapple, C.; Dilkes, B.P.; Tuinstra, M.R. Forward genetics by genome sequencing reveals that rapid cyanide release deters insect herbivory of Sorghum bicolor. Genetics 2013, 195, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kuliahsari, D.E.; Sari, I.N.I.; Estiasih, T. Cyanide detoxification methods in food: A review. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 733, p. 012099. [Google Scholar]
- Kahn, R.A.; Bak, S.; Svendsen, I.; Halkier, B.A.; Moller, B.L. Isolation and reconstitution of cytochrome P450ox and in vitro reconstitution of the entire biosynthetic pathway of the cyanogenic glucoside dhurrin from sorghum. Plant Physiol. 1997, 115, 1661–1670. [Google Scholar] [CrossRef]
- Arrázola, G.; Sánchez, P.R.; Dicenta, F.; Grané, N. Content of the cyanogenic glucoside amygdalin in almond seeds related to the bitterness genotype. Agron. Colomb. 2012, 30, 260–265. [Google Scholar]
- Wang, T.; Lu, S.; Xia, Q.; Fang, Z.; Johnson, S. Separation and purification of amygdalin from thinned bayberry kernels by macroporous adsorption resins. J. Chromatogr. B 2015, 975, 52–58. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lee, J.H.; Hong, S.P. Improvement of the extraction efficiency of d-amygdalin from Armeniacae Semen powder through inactivating emulsin and suppressing the epimerization of d-amygdalin. Arch. Pharmacal Res. 2010, 33, 81–86. [Google Scholar] [CrossRef]
- Xu, S.; Xu, X.; Yuan, S.; Liu, H.; Liu, M.; Zhang, Y.; Li, X. Identification and analysis of amygdalin, neoamygdalin and amygdalin amide in different processed bitter almonds by HPLC-ESI-MS/MS and HPLC-DAD. Molecules 2017, 22, 1425. [Google Scholar] [CrossRef]
- Mazza, G.; Cottrell, T. Carotenoids and cyanogenic glucosides in saskatoon berries (Amelanchier alnifolia Nutt.). J. Food Compos. Anal. 2008, 21, 249–254. [Google Scholar] [CrossRef]
- Wu, H.; Cao, C.; Zhou, C. Determination of amygdalin in the fruit of Eriobotrya japonica Lindl by high performance liquid chromatography. Biomed. Res. 2017, 28, 9028–9032. [Google Scholar]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R. Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem. 2015, 170, 437–442. [Google Scholar] [CrossRef]
- Havlíková, L.; Parmová, M.; Chocholouš, P.; Solich, P. Sensitive monitoring of amygdalin and 5-hydroxytryptamine in food supplements using HILIC OH5 chromatography. Food Anal. Method. 2016, 9, 1849–1856. [Google Scholar] [CrossRef]
- Wang, C.X.; Li, Y.P.; Chang, J.; Zhou, B.; Han, X.; Wu, J.T.; Sun, L.Z. Rapid determination of amygdalin and its novel degradation product in plasma of mice by SPE-HPLC. Adv. Mater. Res. 2013, 781, 83–88. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Reggiani, R. Variation in the content of cyanogenic glycosides in flaxseed meal from twenty-one varieties. Food Nutr. Sci. 2014, 5, 1456–1462. [Google Scholar] [CrossRef]
- Savic, I.M.; Nikolic, V.D.; Savic-Gajic, I.M.; Nikolic, L.B.; Ibric, S.R.; Gajic, D.G. Optimization of technological procedure for amygdalin isolation from plum seeds (Pruni domesticae semen). Front. Plant Sci. 2015, 6, 276. [Google Scholar] [CrossRef]
- Robakowski, P.; Bielinis, E.; Stachowiak, J.; Mejza, I.; Bułaj, B. Seasonal changes affect root prunasin concentration in Prunus serotina and override species interactions between P. serotina and Quercus petraea. J. Chem. Ecol. 2016, 42, 202–214. [Google Scholar] [CrossRef]
- Appenteng, M.K.; Krueger, R.; Johnson, M.C.; Ingold, H.; Bell, R.; Thomas, A.L.; Greenlief, C.M. Cyanogenic glycoside analysis in American elderberry. Molecules 2021, 26, 1384. [Google Scholar] [CrossRef]
- Li, X.B.; Liu, C.H.; Zhang, R.; Huang, X.T.; Li, Y.Y.; Han, L.; Wang, N.S. Determination and pharmacokinetics of amygdalin in rats by LC–MS-MS. J. Chromatogr. Sci. 2014, 52, 476–481. [Google Scholar] [CrossRef]
- Li, X.; Shi, F.; Gu, P.; Liu, L.; He, H.; Ding, L. A sensitive LC–MS/MS method for simultaneous determination of amygdalin and paeoniflorin in human plasma and its application. J. Pharm. Biomed. Anal. 2014, 92, 160–164. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liu, Z.J.; Liu, Q.; Ma, J.N.; Hattori, M.; Ma, C.M. Simultaneous quantification of secoisolariciresinol diglucoside and cyanogenic glycosides in flaxseed products by various processing methods. Food Anal. Methods 2014, 7, 1526–1529. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Transition of phenolics and cyanogenic glycosides from apricot and cherry fruit kernels into liqueur. Food Chem. 2016, 203, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Sendker, J.; Ellendorff, T.; Hölzenbein, A. Occurrence of benzoic acid esters as putative catabolites of prunasin in senescent leaves of Prunus laurocerasus. J. Nat. Prod. 2016, 79, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Bjarnholt, N.; Nakonieczny, M.; Kędziorski, A.; Debinski, D.M.; Matter, S.F.; Olsen, C.E.; Zagrobelny, M. Occurrence of sarmentosin and other hydroxynitrile glucosides in Parnassius (papilionidae) butterflies and their food plants. J. Chem. Ecol. 2012, 38, 525–537. [Google Scholar] [CrossRef]
- Del Cueto, J.; Ionescu, I.A.; Pičmanová, M.; Gericke, O.; Motawia, M.S.; Olsen, C.E.; Sánchez-Pérez, R. Cyanogenic glucosides and derivatives in almond and sweet cherry flower buds from dormancy to flowering. Front. Plant Sci. 2017, 8, 800. [Google Scholar] [CrossRef]
- Song, S.; Chen, F.; Xing, X.; Ren, M.; Ma, Q.; Xie, Y.; Luo, J. Concurrent quantification and comparative pharmacokinetic analysis of bioactive compounds in the Herba Ephedrae-Semen Armeniacae Amarum herb pair. J. Pharm. Biomed. Anal. 2015, 109, 67–73. [Google Scholar] [CrossRef]
- Watanabe-Ishitsuka, A.; Akiyama, H.; Kondo, K.; Obitsu, S.; Kawahara, N.; Teshima, R.; Goda, Y. Determination of cyanogenic glycoside linamarin in cassava flour using liquid chromatography-tandem mass spectrometry. Jpn. J. Food Chem. Saf. 2012, 19, 38–43. [Google Scholar]
- Kasote, D.M.; Lei, Z.; Kranawetter, C.D.; Conway-Anderson, A.; Sumner, B.W.; Sumner, L.W. A Novel UHPLC-MS/MS based method for isomeric separation and quantitative determination of cyanogenic glycosides in American elderberry. Metabolites 2024, 14, 360. [Google Scholar] [CrossRef]
- Makovi, C.M.; Parker, C.H.; Zhang, K. Determination of amygdalin in apricot kernels and almonds using LC-MS/MS. J. AOAC Int. 2023, 106, 457–463. [Google Scholar] [CrossRef]
- Tsukioka, J.; Ohki, Y.; Nakao, M.; Nakamura, S. Quantitative analysis of taxiphyllin, a cyanogenic glycoside, in the leaves of Hydrangea macrophylla var. thunbergii. J. Nat. Med. 2023, 77, 978–985. [Google Scholar] [CrossRef]
- Akatsuka, R.; Ito, M. Content and distribution of prunasin in Perilla frutescens. J. Nat. Med. 2023, 77, 207–218. [Google Scholar] [CrossRef]
- Cortés, V.; Talens, P.; Barat, J.M.; Lerma-García, M.J. Potential of NIR spectroscopy to predict amygdalin content established by HPLC in intact almonds and classification based on almond bitterness. Food Control 2018, 91, 68–75. [Google Scholar] [CrossRef]
- Heraud, P.; Cowan, M.F.; Marzec, K.M.; Møller, B.L.; Blomstedt, C.K.; Gleadow, R. Label-free Raman hyperspectral imaging analysis localizes the cyanogenic glucoside dhurrin to the cytoplasm in sorghum cells. Sci. Rep. 2018, 8, 2691. [Google Scholar] [CrossRef]
- Ngugi, M.P.; Murugi, N.J.; Oduor, R.O.; Mgutu, A.J.; Ombori, R.O.; Cheriyot, R.C. Determination of cyanogenic compounds content in transgenic acyanogenic Kenyan cassava (Manihot esculenta Crantz) genotypes: Linking molecular analysis to biochemical analysis. J. Anal. Bioanal. Technol. 2015, 6, 1000264. [Google Scholar]
- Tivana, L.D.; Francisco, J.D.C.; Zelder, F.; Bergenståhl, B.; Dejmek, P. Straightforward rapid spectrophotometric quantification of total cyanogenic glycosides in fresh and processed cassava products. Food Chem. 2014, 158, 20–27. [Google Scholar] [CrossRef]
- Ortiz, D.; Sanchez, T.; Acosta, H.; Pachon, H. Validation of an analytical methodology to quantify CYANOGENIC GLUCOSIDES in commercial cassava products. Vitae 2011, 18, 319–324. [Google Scholar] [CrossRef]
- Drochioiu, G.; Arsene, C.; Murariu, M.; Oniscu, C. Analysis of cyanogens with resorcinol and picrate. Food Chem. Toxicol. 2008, 46, 3540–3545. [Google Scholar] [CrossRef]
- Tanaka, R.; Nitta, A.; Nagatsu, A. Application of a quantitative 1 H-NMR method for the determination of amygdalin in Persicae semen, Armeniacae semen, and Mume fructus. J. Nat. Med. 2014, 68, 225–230. [Google Scholar] [CrossRef]
- Cho, H.J.; Do, B.K.; Shim, S.M.; Kwon, H.; Lee, D.H.; Nah, A.H.; Lee, S.Y. Determination of cyanogenic compounds in edible plants by ion chromatography. Toxicol. Res. 2013, 29, 143–147. [Google Scholar] [CrossRef] [PubMed]
| Analytes | Matrices | Columns | Eluent | Flow Rate (mL/mL) | Retention Time (min) | Detector | Absorption Wavelength (nm) | LOD | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Amygdalin | Almond seeds | Waters Symmetry column 250 mm × 4.6 mm | Acetonitrile and water (20:80, v:v) | 1.5 | - | UVD | 218 | - | [29] |
| Amygdalin | Thinned bayberry kernels | Symmetry®C18 MG column (4.6 mm × 250 mm, 5 µm) | Acetonitrile and water (13:87, v:v) | 1 | - | DAD | 214 | - | [30] |
| D-Amygdalin | Armeniacae Semen powder | Luna 3u C18 (2) 100A (4.6 mm × 150 mm, 3 μm, Phenomenex) | 10 mM sodium phosphate buffer (pH 2.3) containing 13.5% acetonitrile | 0.6 | ~12 | UVD | 214 | 5 μM per amount injected | [31] |
| Capcell Pak C18 MG (4.6 mm × 250 mm, 5 μm, Shiseido) | 10 mM sodium phosphate buffer (pH 3.1) containing 8.5% acetonitrile | 1.2 | ~31 | ||||||
| Amygdalin amide, D-amygdalin, and L-amygdalin | Processed bitter almonds | Eclipse EXTEND C18 column | Methanol and 0.1% formic acid in water | 1 | 5.4, 11.5 and 16.0 | DAD | 254 | 2 µg/mL | [32] |
| Prunasin and amygdalin | Saskatoon berries | Zorbax SB C18 150 mm × 2.1 mm with a 12.5 mm × 2.1 mm guard column of the same material | 2 mM trifluoroacetic acid and methanol | 0.3 | 5.13 and 6.64 | DAD | 210 | 0.5 mg/kg and 1.5 mg/kg FW of berries | [33] |
| Amygdalin | Organs and tissues of loquat fruit | ODS C18 column (250 mm × 4.6 mm, 5 μm) | Methanol and 0.03 mol/L phosphate buffer (pH 2.8) (25:75, v:v) | 1 | - | UVD | 210 | - | [34] |
| Amygdalin | Apple seeds, fresh apples, and processed apple juices | Phenomenex C18, Type Nucleosile 3, 120 A (150 mm × 4.60 mm, 3 µm) | Methanol and water (25:75, v:v) | 1 | ~4 | DAD | 214 | 0.1 µg/mL | [35] |
| Amygdalin | Food supplements | Ascentis Express OH5 column (100 mm × 3.0 mm; 2.7 μm) | Acetonitrile and 10 mM ammonium acetate pH 3.8 (90:10, v:v) | 0.8 | 2.6 | UVD | 215 | 0.150 mg/L | [36] |
| Amygdalin and prunasin | Mouse plasma | C18 5μ ODS column | 0.05% formic acid in acetonitrile, v/v; and 0.05% formic acid in water, v/v | 1 | 3.14 and 6.82 | UVD | 215 | - | [37] |
| Amygdalin | Fruit kernels, seeds, and processed products | Phenomenex C18, Type Nucleosile 3, 120 A (150 mm × 4.60 mm, 3 µm) | Methanol and water (25:75, v:v) | 1 | ~4 | DAD | 214 | 0.1 µg/mL | [38] |
| Linamarin, linustatin, and neolinustatin | Flaxseed meal from twenty-one varieties | Kinetex 4.6 mm × 125 mm, 2.6 micron C-18 | 0.05% Phosphoric acid in HPLC grade water and methanol | - | 4.9, 8.9 and 18.8 | UVD | 230 | - | [39] |
| Amygdalin | Plum seeds | SUPELCO Analytical HS-C18 column | Acetonitrile and water (75:25, v:v) | 0.9 | - | UVD | 215 | - | [40] |
| Prunasin | Roots of Prunus serotina and Quercus petraea | SunFireTM C18 column (4.6 mm × 250 mm, 5 μm) together with the precolumn Waters (4.6 mm × 20 mm) | Methanol and water (15:85, v:v) | 1.5 | - | UVD | 218 | - | [41] |
| Analytes | Matrices | Sample Processing | Columns | Eluent | Flow Rate (mL/mL) | Retention Time (min) | Detector | LOD | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Amygdalin amide, D-amygdalin, and L-amygdalin | Processed bitter almonds | Extraction with 70% aqueous methanol | Eclipse EXTEND C18 column | Methanol and 0.1% formic acid in water | 1 | 5.4, 11.5 and 16.0 | ESI- using MS/MS | - | [32] |
| Amygdalin, dhurrin, prunasin, and linamarin | American elderberry | Extraction with 75% methanol | C18 column (Acquity BEH, 1.7 μM, 2.1 mm × 50 mm, Waters) | Water with 0.1% formic acid and acetonitrile with 0.1% formic acid | 0.2 | 4.61, 2.54, 5.37, and 1.18 | ESI+ using SIR | 3, 3, 3, and 1 ng/mL | [42] |
| Amygdalin | Rat plasma | SPE | Gemini C18 analytical column (50 mm × 2.0 mm, 5 µm; Phenomenex) | Methanol and water (85:15; v/v) | 0.25 | 1.68 | ESI- using MRM | 1.25 ng/mL | [43] |
| Amygdalin and paeoniflorin | Human plasma | Protein precipitation with methanol | Hedera ODS-2 analytical column (2.1 mm × 150 mm, 5 µm; Hanbon Science and Technology) | Acetonitrile and 5 mM ammonium acetate buffer solution containing 0.05% formic acid (20:80, v/v) | 0.3 | 2.14 and 3.18 | - | - | [44] |
| Linustatin and neolinustatin | Flaxseed powder | Extraction with methanol and purification with a silica gel column | Agilent ZORBAX Eclipse Plus C18 (2.1 mm × 50 mm; particle size, 1.8 μm) | Water containing 0.1% formic acid (v/v) and acetonitrile | 0.4 | 1.8 and 2.7 | ESI- using MRM | - | [45] |
| Amygdalin and prunasin | Apricot kernel, apricot liqueur, cherry kernel, and cherry liqueur | Extraction with 70% methanol | HYPERSIL GOLD aQ column (Thermo Scientific) | Water with 0.1% formic acid with 3% methanol (v/v/v) and methanol with 0.1% formic acid with 3% water (v/v/v) | 0.8 | 3.85 and 4.4 | ESI+ using MRM | - | [46] |
| Prunasin and related compounds | Leaves of Prunus laurocerasus | Extraction with 70% acetonitrile | Dionex Acclaim RSLC 120 C18 column (2.1 mm × 100 mm, 2.2 μm) | Water with 0.1% formic acid and acetonitrile with 0.1% formic acid | 0.8 | - | ESI+ | - | [47] |
| Linamarin | Parnassius butterflies and their food plants | Extraction with 55% methanol containing 0.1% formic acid | Zorbax SB-C18 column (Agilent; 1.8 μM, 2.1 mm × 50 mm) | Water with 0.1% (v/v) HCOOH and 50 mM NaCl and acetonitrile with 0.1% (v/v) HCOOH | 0.2 | 2.6 | ESI+ | - | [48] |
| Prunasin and amygdalin | Almond and sweet cherry | Extraction with 85% methanol | Zorbax SB-C18 column (Agilent; 1.8 mm, 2.1 mm × 50 mm) | Water with 0.1% (v/v) HCOOH and 50 mM NaCl and acetonitrile with 0.1% (v/v) HCOOH | 0.2 | 7 and 6.6 | ESI+ | - | [49] |
| Epimers of amygdalin and prunasin | Rat plasma | Protein precipitation with acetonitrile | Zorbax SB-C18 column (100 mm × 3.0 mm, 1.8 μm; Agilent Technologies) | Acetonitrile and 0.1% formic acid aqueous solution | 0.4 | - | ESI+ using MRM | - | [50] |
| Linamarin | Cassava flour and tapioca | Extraction with acetonitrile | CAPCELL PAK AQ column (2.1 mm × 250 mm, Shiseido) | Water containing 0.01% acetic acid and methanol (70:30; v/v) | 0.2 | 5.5 | ESI- using MRM | 0.75 and 0.84 µg/g | [51] |
| Linamarin, dhurrin, amygdalin, prunasin, and sambunigrin | American elderberry | Extraction with 80% methanol | ACQUITY UPLC HSS T3 column (1.8 μM, 2.1 mm × 100 mm) | Water with 2 mM ammonium formate and ACN | 0.5 | 3.34, 6.40, 9.89, 11.14, and 11.34 | ESI+ using MRM | 1.2, 1.2, 8.2, 2.0, and 0.9 ng/L | [52] |
| Amygdalin | Apricot Kernels and Almonds | Extraction with methanol | Phenomenex Kinetex XB-C18 (2.6 μM, 2.1 mm × 100 mm) | 10 mM ammonium formate/0.1% formic acid/water and 10 mM ammonium formate/0.1% formic acid/methanol | 0.3 | 3.3 | ESI- using MRM | 0.8 µg/g | [53] |
| taxiphyllin | Leaves of Hydrangea macrophylla var. thunbergii | Extraction with methanol | Cosmosil C18-PAQ column (5 μM, 4.6 mm × 250 mm) | acetonitrile and water | 1 | 8.5 min | ESI+ using Q1 scan | [54] | |
| prunasin | Perilla frutescens | Extraction with methanol | COSMOSIL 5C18 MS II column (250 × 4.6 mm) | Methanol and water (14:86) | 0.4 | ~34 min | ESI- | 0.00157 mg/mL (LOQ) | [55] |
| Reference Standard | Matrices | Sample Processing | Principle | Detector Condition | LOD | Ref. |
|---|---|---|---|---|---|---|
| Amygdalin | Plum seeds | Extraction with ethanol | IR absorbance | absorbance measured over 4000–400 cm−1 | [40] | |
| Amygdalin | Intact almonds | NIR absorbance | absorbance measured over 4000–400 cm−1 | [56] | ||
| Dhurrin | Cytoplasm in sorghum cells | Raman microspectroscopic mapping | [57] | |||
| Cyanohydrin and HCN contents | Transgenic Acyanogenic Kenyan cassava genotypes | Ectraction with acid extraction medium | RNA interference approach based on the König reaction | absorbance measured at 600 nm | [58] | |
| Total cyanogenic glycosides | Fresh and processed cassava products | Extraction with 0.1 M phosphoric acid | Aquacyanocobyrinic acid chemosensor | absorbance measured at 578 nm | [59] | |
| HCN | Commercial food products prepared from cassava | Homogenization with 0.1 M (25%, v/v, ethanol) phosphoric acid | IR absorbance | absorbance measured at 605 nm | [60] | |
| HCN | Plants with resorcinol and picrate | Hydrolysis with 2 M sulfuric acid | IR absorbance | absorbance measured at 488 nm | 0.05 µg/mL cyanide | [61] |
| Amygdalin | Persicae semen, Armeniacae semen, and Mume fructus | Sonication with methanol | 1H-NMR spectrometry | Concentration corrected with certified reference material-grade bisphenol A | [62] | |
| Total cyanide | Edible plants from 14 genera | Homogenization with 0.1 M phosphoric acid | Electrode cell Silver working electrode | 0.00 V vs. Ag/AgCl reference | 0.005 mg CN−/L. | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wen, S.; Wang, Y.; Zhang, W.; Xu, X.; Mou, Y. A Review of Recent Advances in Chromatographic Quantification Methods for Cyanogenic Glycosides. Molecules 2024, 29, 4801. https://doi.org/10.3390/molecules29204801
Zhao Y, Wen S, Wang Y, Zhang W, Xu X, Mou Y. A Review of Recent Advances in Chromatographic Quantification Methods for Cyanogenic Glycosides. Molecules. 2024; 29(20):4801. https://doi.org/10.3390/molecules29204801
Chicago/Turabian StyleZhao, Yao, Shuai Wen, Yan Wang, Wenshuo Zhang, Xiangming Xu, and Yi Mou. 2024. "A Review of Recent Advances in Chromatographic Quantification Methods for Cyanogenic Glycosides" Molecules 29, no. 20: 4801. https://doi.org/10.3390/molecules29204801
APA StyleZhao, Y., Wen, S., Wang, Y., Zhang, W., Xu, X., & Mou, Y. (2024). A Review of Recent Advances in Chromatographic Quantification Methods for Cyanogenic Glycosides. Molecules, 29(20), 4801. https://doi.org/10.3390/molecules29204801




