Density Functional Calculation and Evaluation of the Spectroscopic Properties and Luminescent Material Application Potential of the N-Heterocyclic Platinum(II) Tetracarbene Complexes
Abstract
1. Introduction
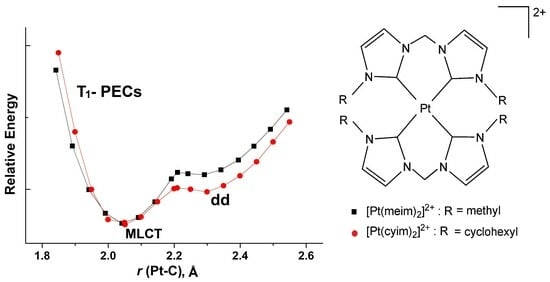
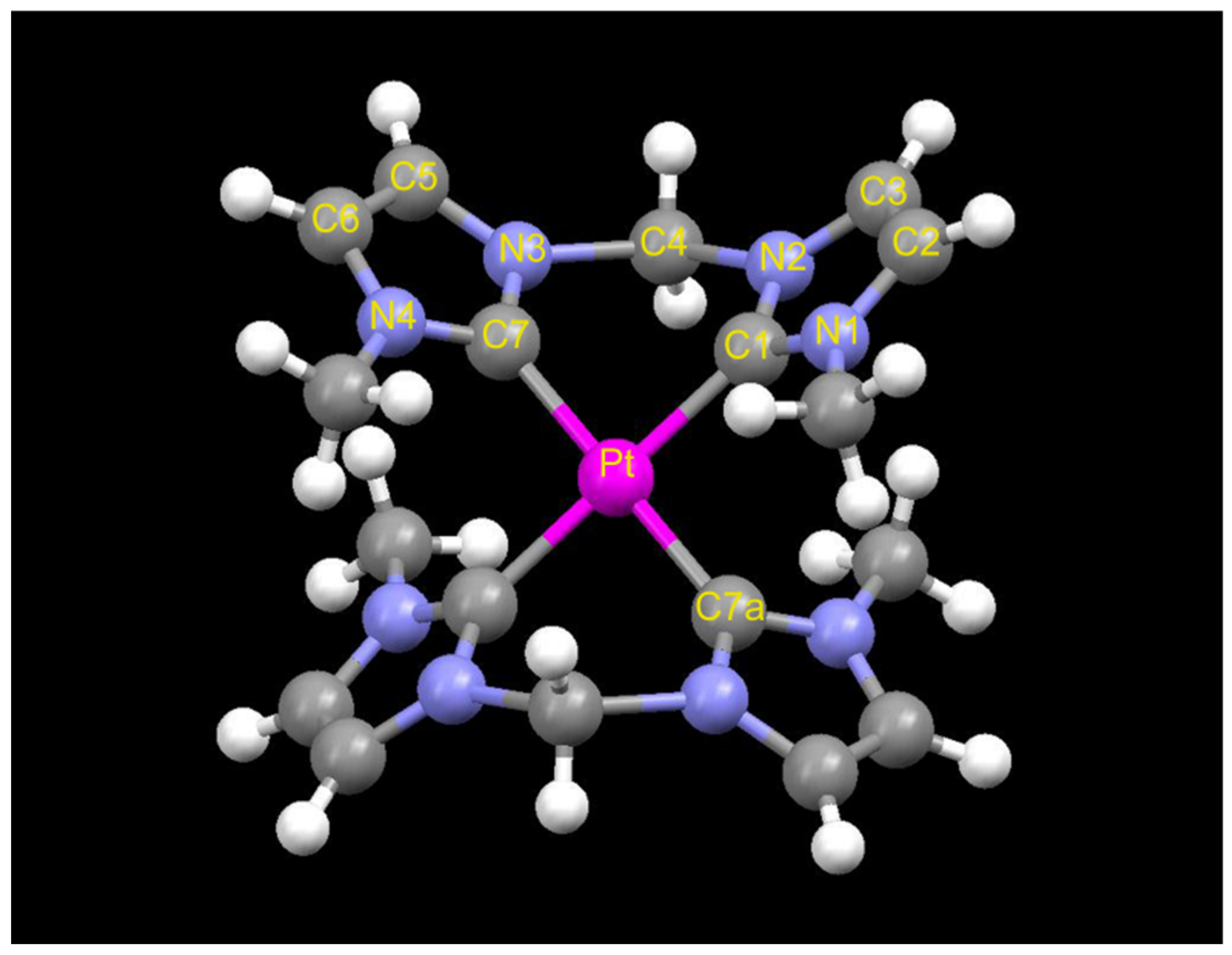
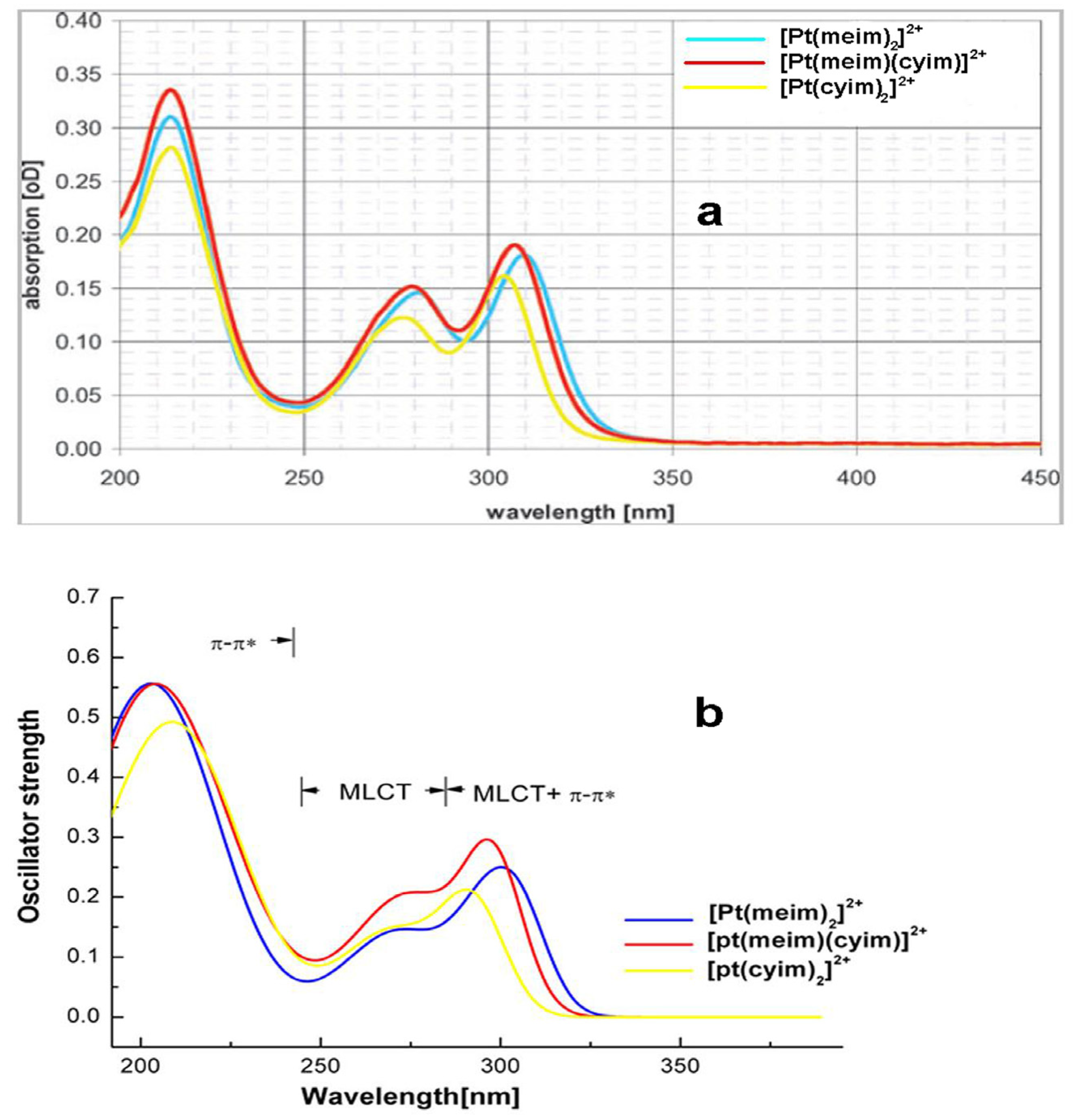
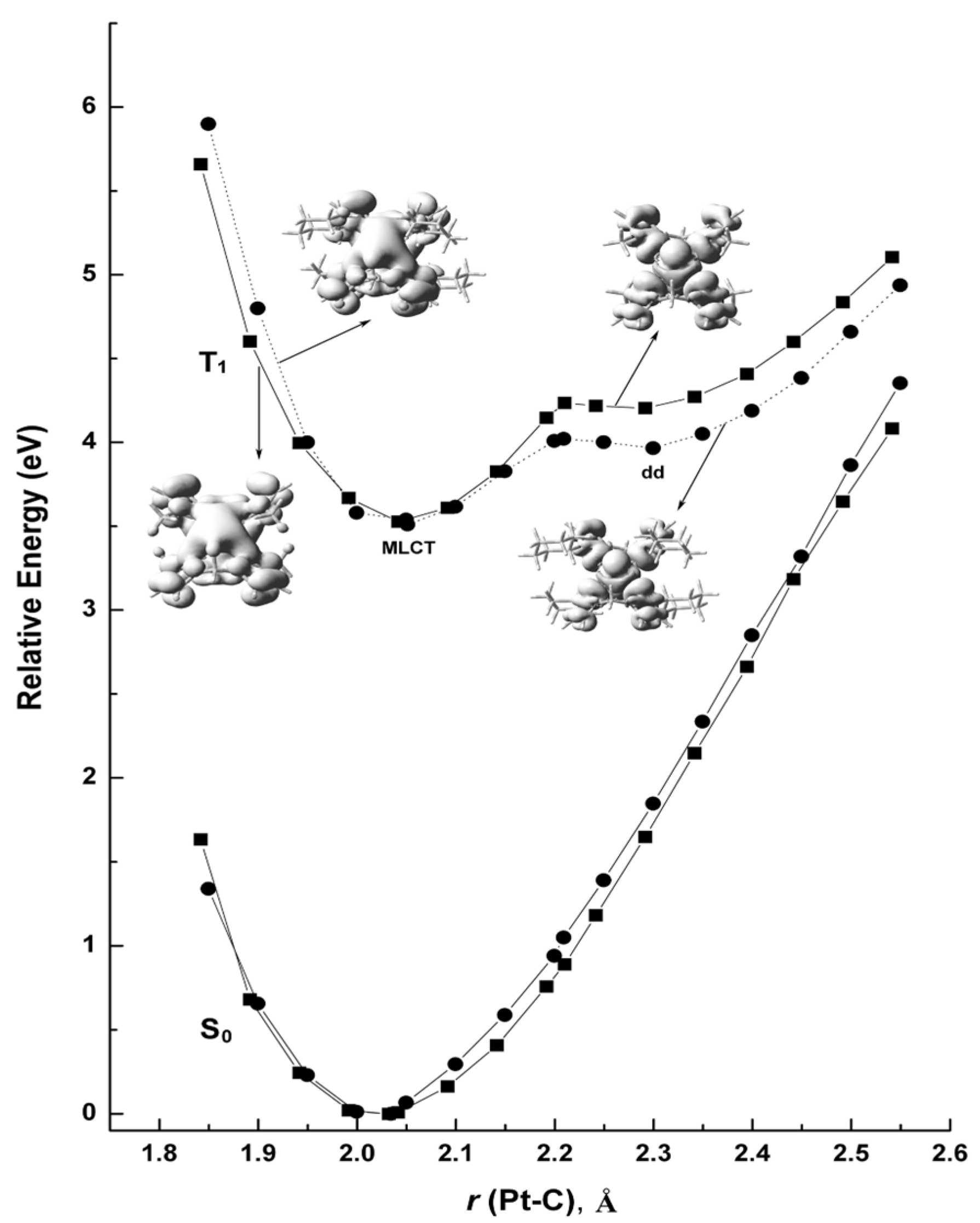
2. Results and Discussion
3. Computational Details
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hahn, F.E.; Jahnke, M.C. Heterocyclic Carbenes: Synthesis and Coordination Chemistry. Angew. Chem. Int. Ed. 2008, 47, 3122–3172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Blacque, O.; Venkatesan, K. Highly Efficient Deep-Blue Emitters Based on cis and trans N-Heterocyclic Carbene PtII Acetylide Complexes: Synthesis, Photophysical Properties, and Mechanistic Studies. Chem. Eur. J. 2013, 19, 15689–15701. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.K.; Schwarz, J.; Frey, G.D.; Herdtweck, E.; Herrmann, W.A. Chiral, Bridged Bis(imidazolin-2-ylidene) Complexes of Palladium. J. Organomet. Chem. 2007, 692, 4560–4568. [Google Scholar] [CrossRef]
- Huh, J.-S.; Lee, D.Y.; Park, K.H.; Kwon, S.-K.; Kim, Y.-H.; Kim, J.-J. Control of the horizontal dipole ratio and emission color of deep blue tetradentate Pt(II) complexes using aliphatic spacer groups. Chem. Eng. J. 2022, 450, 137836. [Google Scholar] [CrossRef]
- McKie, R.; Murphy, J.A.; Park, S.R.; Spicer, M.D.; Zhou, S. Homoleptic Crown N-Heterocyclic Carbene Complexes. Angew. Chem. Int. Ed. 2007, 46, 6525–6528. [Google Scholar] [CrossRef] [PubMed]
- Tenne, M.; Strassner, T. Neutral platinum(II) N-heterocyclic carbene complexes with tetrazolide-tethered imidazolin-2-ylidene ligands. J. Organomet. Chem. 2016, 821, 100–105. [Google Scholar] [CrossRef]
- Albrecht, M. C4-bound Imidazolylidenes: From Curiosities to High-Impact Carbene Ligands. Chem. Commun. 2008, 3601–3610. [Google Scholar] [CrossRef]
- Hudson, Z.M.; Sun, C.; Helander, M.G.; Chang, Y.L.; Lu, Z.H.; Wang, S.N. Highly Efficient Blue Phosphorescence from Triarylboron-Functionalized Platinum(II) Complexes of N-Heterocyclic Carbenes. J. Am. Soc. Chem. 2012, 134, 13930–13933. [Google Scholar] [CrossRef]
- Shen, Y.; Kong, X.; Yang, F.; Bian, H.-D.; Cheng, G.; Cook, T.R.; Zhang, Y. Deep Blue Phosphorescence from Platinum Complexes Featuring Cyclometalated N-Pyridyl Carbazole Ligands with Monocarborane Clusters (CB11H12−). Inorg. Chem. 2022, 61, 16707–16717. [Google Scholar] [CrossRef]
- Unger, Y.; Zeller, A.; Ahrens, S.; Strassner, T. Blue Phosphorescent Emitters: New N-Heterocyclic Platinum(II) Tetracarbene Complexes. Chem. Commun. 2008, 3263–3265. [Google Scholar] [CrossRef]
- Unger, Y.; Zeller, A.; Taige, M.A.; Strassner, T. Near-UV Phosphorescent Emitters: N-Heterocyclic Platinum(II) Tetracarbene Complexes. Dalton Trans. 2009, 38, 4786–4794. [Google Scholar] [CrossRef] [PubMed]
- Bárta, O.; Pinter, P.; Císařová, I.; Strassner, T.; Štěpnička, P. Synthesis and Characterization of Cationic Platinum(II) Complexes with Two Chelating Ligands. Eur. J. Inorg. Chem. 2020, 2020, 575–580. [Google Scholar] [CrossRef]
- Bai, F.-Q.; Zhou, X.; Xia, B.-H.; Liu, T.; Zhang, J.-P.; Zhang, H.-X. Electronic structures and optical properties of neutral substituted fluorene-based cyclometalated platinum(II)—Acetylide complexes: A DFT exploration. J. Organomet. Chem. 2009, 694, 1848–1860. [Google Scholar] [CrossRef]
- Fan, H.-W.; Bai, F.-Q.; Zhang, Z.-X.; Wang, Y.; Qu, Z.-X.; Zhong, R.-L.; Zhang, H.-X. Theoretical investigation on the effect of ancillary ligand modification for highly efficient phosphorescent platinum(II) complex design. RSC Adv. 2017, 7, 17368–17376. [Google Scholar] [CrossRef]
- Feng, T.-T.; Bai, F.-Q.; Xie, L.-M.; Tang, Y.; Zhang, H.-X. Theoretical study and design of highly efficient platinum(II) complexes bearing tetradentate ligands for OLED. RSC Adv. 2016, 6, 11648–11656. [Google Scholar] [CrossRef]
- Strassner, T.; Taige, M.A. Evaluation of Functionals O3LYP, KMLYP, and MPW1K in Comparison to B3LYP for Selected Transition-Metal Compounds. J. Chem. Theory Comput. 2005, 1, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Bolink, H.J.; Cappelli, L.; Cheylan, S.; Coronado, E.; Costa, R.D.; Lardiés, N.; Nazeeruddinb, M.K.; Ortí, E. Origin of the Large Spectral Shift in Electroluminescence in a Blue Light Emitting Cationic Iridium(III) Complex. J. Mater. Chem. 2007, 17, 5032–5041. [Google Scholar] [CrossRef]
- Jamorski, C.; Casida, M.E.; Salahub, D.R. Dynamic Polarizabilities and Excitation Spectra from A Molecular Implementation of Time-Dependent Density-Functional Response Theory: N2 as a Case Study. J. Chem. Phys. 1996, 104, 5134–5147. [Google Scholar] [CrossRef]
- Petersilka, M.; Grossmann, U.J.; Gross, E.K.U. Excitation Energies from Time-Dependent Density-Functional Theory. Phys. Rev. Lett. 1996, 76, 1212–1215. [Google Scholar] [CrossRef]
- Tong, G.S.M.; Che, C.M. Emissive or Nonemissive? A Theoretical Analysis of the Phosphorescence Efficiencies of Cyclometalated Platinum(II) Complexes. Chem.-A Eur. J. 2009, 15, 7225–7237. [Google Scholar] [CrossRef] [PubMed]
- Siddique, Z.A.; Yamamoto, Y.; Ohno, T.; Nozaki, K. Structure-Dependent Photophysical Properties of Singlet and Triplet Metal-to-Ligand Charge Transfer States in Copper(I) Bis(diimine) Compounds. Inorg. Chem. 2003, 42, 6366–6378. [Google Scholar] [CrossRef] [PubMed]
- Gareth Williams, J.A. Photochemistry and Photophysics of Coordination Compounds: Platinum. Top. Curr. Chem. 2007, 281, 205–268. [Google Scholar]
- Islam, A.; Ikeda, N.; Nozaki, K.; Ohno, T. Role of Higher Excited States in Radiative and Nonradiative Processes of Coordination Compounds of Ru(II) and Rh(III) in Crystal. Chem. Phys. Lett. 1996, 263, 209–214. [Google Scholar] [CrossRef]
- Durham, B.; Casper, J.V.; Nagle, J.K.; Meyer, T.J. Photochemistry of Tris(2,2′-bipyridine)ruthenium(2+) Ion. J. Am. Chem. Soc. 1982, 104, 4803–4810. [Google Scholar] [CrossRef]
- Allen, G.H.; White, R.P.; Rillema, D.P.; Meyer, T.J. Synthetic Control of Excited-State Properties. Tris-Chelate Complexes Containing the Ligands 2,2′-bipyrazine, 2,2′-bipyridine, and 2,2′-bipyrimidine. J. Am. Chem. Soc. 1984, 106, 2613–2620. [Google Scholar] [CrossRef]
- Salassa, L.; Garino, C.; Salassa, G.; Gobetto, R.; Nervi, C. Mechanism of Ligand Photodissociation in Photoactivable [Ru(bpy)2L2]2+ Complexes: A Density Functional Theory Study. J. Am. Chem. Soc. 2008, 130, 9590–9597. [Google Scholar] [CrossRef]
- Saito, K.; Nakao, Y.; Sakaki, S. Theoretical Study of Pyrazolate-Bridged Dinuclear Platinum(II) Complexes: Interesting Potential Energy Curve of the Lowest Energy Triplet Excited State and Phosphorescence Spectra. Inorg. Chem. 2008, 47, 4329–4337. [Google Scholar] [CrossRef]
- Yang, L.; Okuda, F.; Kobayashi, K.; Nozaki, K.; Tanabe, Y.; Ishii, Y.; Haga, M. Syntheses and Phosphorescent Properties of Blue Emissive Iridium Complexes with Tridentate Pyrazolyl Ligands. Inorg. Chem. 2008, 47, 7154–7165. [Google Scholar] [CrossRef]
- Yu, J.-K.; Hu, Y.-H.; Cheng, Y.-M.; Chou, P.-T.; Peng, S.-M.; Lee, G.-H.; Carty, A.J.; Tung, Y.-L.; Lee, S.-W.; Chi, Y.; et al. A Remarkable Ligand Orientational Effect in Osmium-Atom-Induced Blue Phosphorescence. Chem. Eur. J. 2004, 10, 6255–6264. [Google Scholar] [CrossRef]
- De Angelis, F.; Fantacci, S.; Evans, N.; Klein, C.; Zakeeruddin, S.M.; Moser, J.-E.; Kalyanasundaram, K.; Bolink, H.J.; Grätzel, M.; Nazeeruddin, M.K. Controlling Phosphorescence Color and Quantum Yields in Cationic Iridium Complexes: A Combined Experimental and Theoretical Study. Inorg. Chem. 2007, 46, 5989–6001. [Google Scholar] [CrossRef] [PubMed]
- Fazzi, D.; Grancini, G.; Maiuri, M.; Brida, D.; Cerullo, G.; Lanzani, G. Ultrafast Internal Conversion in A Low Band Gap Polymer for Photovoltaics: Experimental and Theoretical Study. Phys. Chem. Chem. Phys. 2012, 14, 6367–6374. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Harriman, A.; Mayeux, A. The Triplet Excited State of Ruthenium(II) Bis(2,2:6,2-terpyridine): Comparison Between Experiment and Theory. Phys. Chem. Chem. Phys. 2004, 6, 1157–1164. [Google Scholar] [CrossRef]
- Benniston, A.C.; Chapman, G.; Harriman, A.; Mehrabi, M.; Sams, C.A. Electron Delocalization in a Ruthenium(II) Bis(2,2′:6′,2″-terpyridyl) Complex. Inorg. Chem. 2004, 43, 4227–4233. [Google Scholar] [CrossRef]
- Hutchison, G.H.; Ratner, M.A.; Marks, T.J. Hopping Transport in Conductive Heterocyclic Oligomers: Reorganization Energies and Substituent Effects. J. Am. Chem. Soc. 2005, 127, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.C.; Cheng, C.P.; Lao, Z.P.M. Reorganization Energies in the Transports of Holes and Electrons in Organic Amines in Organic Electroluminescence Studied by Density Functional Theory. J. Phys. Chem. A 2003, 107, 5241–5251. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09, Revision D. 01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange-Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Peach, M.J.G.; Benfield, P.; Helgaker, T.; Tozer, D.J. Excitation Energies in Density Functional Theory: An Evaluation and A Diagnostic Test. J. Chem. Phys. 2008, 128, 044118. [Google Scholar] [CrossRef]
- Runge, E.; Gross, E.K.U. Density-Functional Theory for Time-Dependent Systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of The Colle-Salvetti Correlation-Energy Formula into A Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results Obtained with the Correlation Energy Density Functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for Main Group Elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for K to Au Including the Outermost Core Orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. Accuracy of AHn Equilibrium Geometries by Single Determinant Molecular Orbital Theory. Mol. Phys. 1974, 27, 209–214. [Google Scholar] [CrossRef]
- Li, P.; Feng, L.; Li, G.; Bai, F.-Q. Effects of Electron Donating Ability of Substituents and Molecular Conjugation on the Electronic Structures of Organic Radicals. Chem. Res. Chin. Univ. 2023, 39, 202–207. [Google Scholar] [CrossRef]
- Lam, W.H.; Lam, E.S.-H.; Yam, V.W.-W. Computational Studies on the Excited States of Luminescent Platinum (II) Alkynyl Systems of Tridentate Pincer Ligands in Radiative and Nonradiative Processes. J. Am. Soc. Chem. 2013, 135, 15135–15143. [Google Scholar] [CrossRef] [PubMed]
- Vlček, A.; Zališ, S. Modeling of Charge-Transfer Transitions and Excited States in d6 Transition Metal Complexes by DFT Techniques. Coord. Chem. Rev. 2007, 251, 258–287. [Google Scholar] [CrossRef]
- Andrae, D.; Haussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-Adjusted Ab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Marcus, R.A. On the Theory of Oxidation-Reduction Reactions Involving Electron Transfer. I. J. Chem. Phys. 1965, 24, 966–978. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron Transfer Reactions in Chemistry. Theory and Experiment. Rev. Mod. Phys. 1993, 65, 599–610. [Google Scholar] [CrossRef]

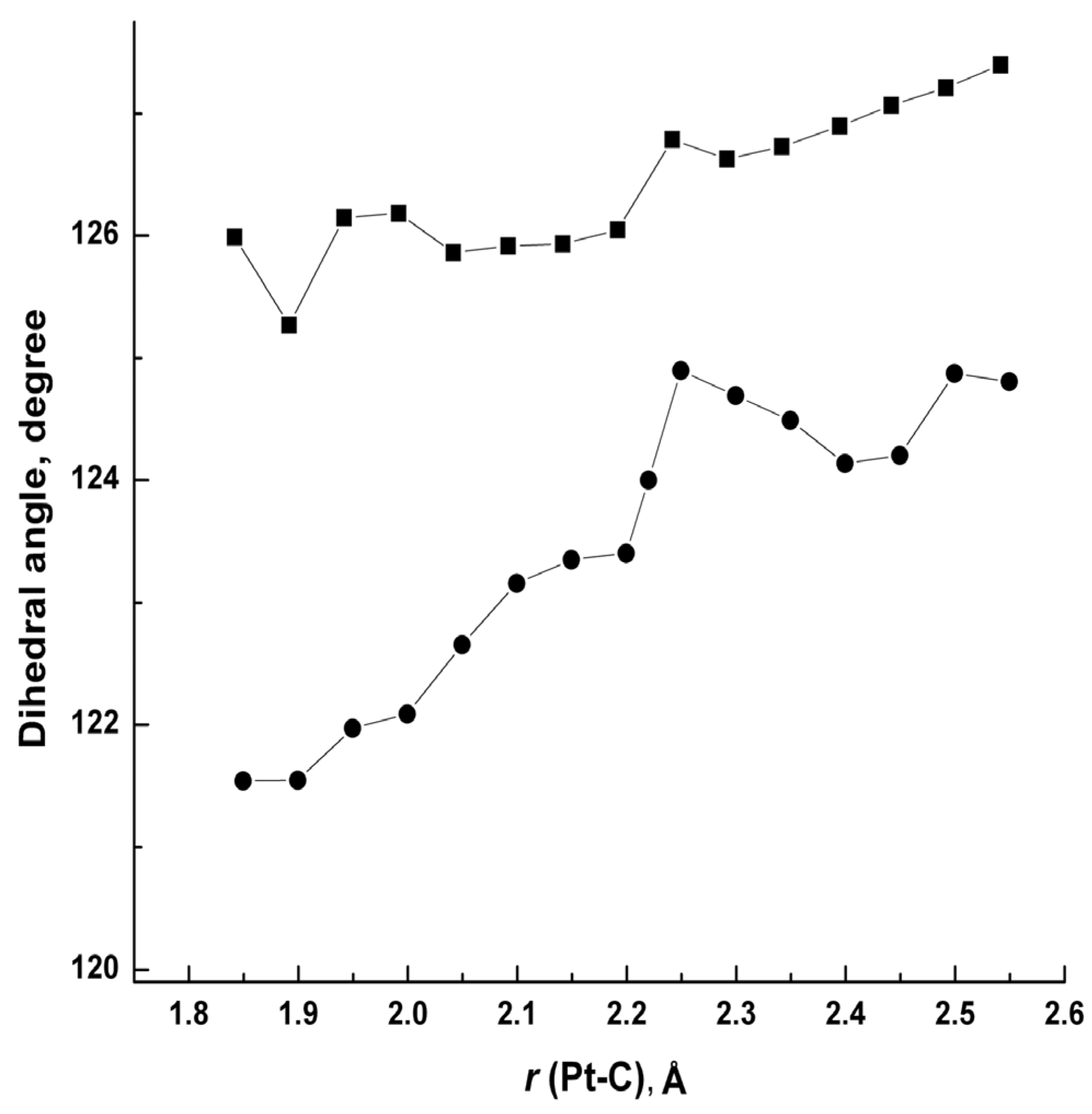
| [Pt(meim)2]2+ | [Pt(cyim)2]2+ | [Pt(meim)(cyim)]2+ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Exp10 | S0/1Ag | T1/3Bu | MC State/3Bg | S0/1Ag | T1/3Bu | MC State/3Bg | S0/1A′ | T1/3A″ | |
| Bond length (Å) | |||||||||
| r (Pt–C1) | 2.026 | 2.032 | 2.042 | 2.292 | 2.034 | 2.051 | 2.293 | 2.031/2.036 | 2.036/2.058 |
| r (C1–N1) | 1.345 | 1.353 | 1.378 | 1.357 | 1.355 | 1.382 | 1.357 | 1.352/1.354 | 1.389/1.370 |
| r (C1–N2) | 1.352 | 1.361 | 1.384 | 1.365 | 1.364 | 1.381 | 1.366 | 1.363/1.364 | 1.395/1.376 |
| r (N2–C4) | 1.454 | 1.458 | 1.451 | 1.458 | 1.458 | 1.450 | 1.457 | 1.455/1.458 | 1.449/1.453 |
| Bite angle (degree) | |||||||||
| a (C1–Pt–C7) | 84.2 | 83.3 | 84.5 | 81.6 | 82.9 | 84.0 | 81.0 | 83.1/83.0 | 83.8/84.0 |
| a (C1–Pt–C7a) | 95.9 | 96.7 | 95.5 | 98.4 | 97.1 | 95.8 | 99.0 | 96.5/96.6 | 96.1/96.1 |
| a (N1–C1–Pt) | 133.0 | 134.2 | 133.2 | 134.6 | 134.7 | 134.2 | 134.9 | 133.9/134.7 | 132.9/134.1 |
| a (N2–C1–Pt) | 122.2 | 121.1 | 122.7 | 120.2 | 120.0 | 121.3 | 119.2 | 121.2/120.1 | 123.1/121.2 |
| a (N1–C1–N2) | 104.8 | 104.6 | 103.8 | 104.0 | 105.0 | 104.1 | 104.4 | 104.7/105.0 | 103.5/104.4 |
| Dihedral angle (degree) | |||||||||
| d (C7–C1–Pt–C7a) | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 179.6 | 178.2 |
| d (C1–C4–Pt–C7) | 124.7 | 122.2 | 125.9 | 126.6 | 118.4 | 122.7 | 123.7 | 122.0/121.1 | 124.7/123.0 |
| [Pt(meim)2]2+ | [Pt(cyim)2]2+ | TPD [36] | ||
|---|---|---|---|---|
| S0 | LUMO | 0.2% | 0.3% | |
| HOMO | 35.3% | 33.1% | ||
| T1-MLCT | HSOMO | 0.3% | 0.1% | |
| HSOMO-1 | 20.9% | 19.8% | ||
| T1-dd | HSOMO | 34.2% | 31.3% | |
| HSOMO-1 | 10.3% | 8.7% | ||
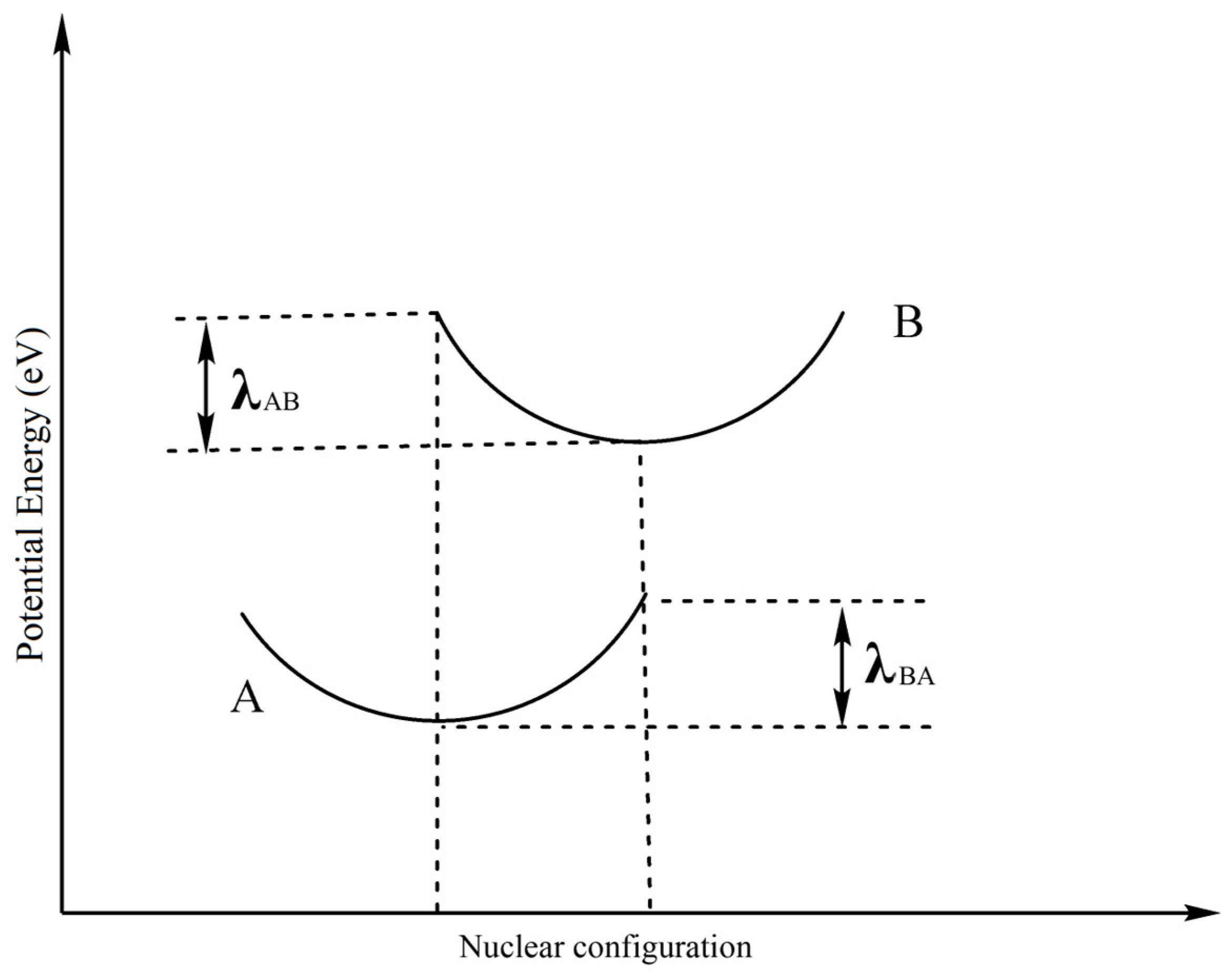
| λ(d-d)/eV | 1.456 | 1.419 | ||
| λi(h)/eV | 0.127 | 0.140 | 0.270 | |
| λi(e)/eV | 0.326 | 0.284 | 0.690 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, B.-H.; Ma, Y.-S.; Bai, F.-Q. Density Functional Calculation and Evaluation of the Spectroscopic Properties and Luminescent Material Application Potential of the N-Heterocyclic Platinum(II) Tetracarbene Complexes. Molecules 2024, 29, 524. https://doi.org/10.3390/molecules29020524
Xia B-H, Ma Y-S, Bai F-Q. Density Functional Calculation and Evaluation of the Spectroscopic Properties and Luminescent Material Application Potential of the N-Heterocyclic Platinum(II) Tetracarbene Complexes. Molecules. 2024; 29(2):524. https://doi.org/10.3390/molecules29020524
Chicago/Turabian StyleXia, Bao-Hui, Yin-Si Ma, and Fu-Quan Bai. 2024. "Density Functional Calculation and Evaluation of the Spectroscopic Properties and Luminescent Material Application Potential of the N-Heterocyclic Platinum(II) Tetracarbene Complexes" Molecules 29, no. 2: 524. https://doi.org/10.3390/molecules29020524
APA StyleXia, B.-H., Ma, Y.-S., & Bai, F.-Q. (2024). Density Functional Calculation and Evaluation of the Spectroscopic Properties and Luminescent Material Application Potential of the N-Heterocyclic Platinum(II) Tetracarbene Complexes. Molecules, 29(2), 524. https://doi.org/10.3390/molecules29020524