Silver and Copper Complexes with Ibuprofen and Caffeine—Preparation and Evaluation of Their Selected Biological Effects
Abstract
1. Introduction
2. Results and Discussion
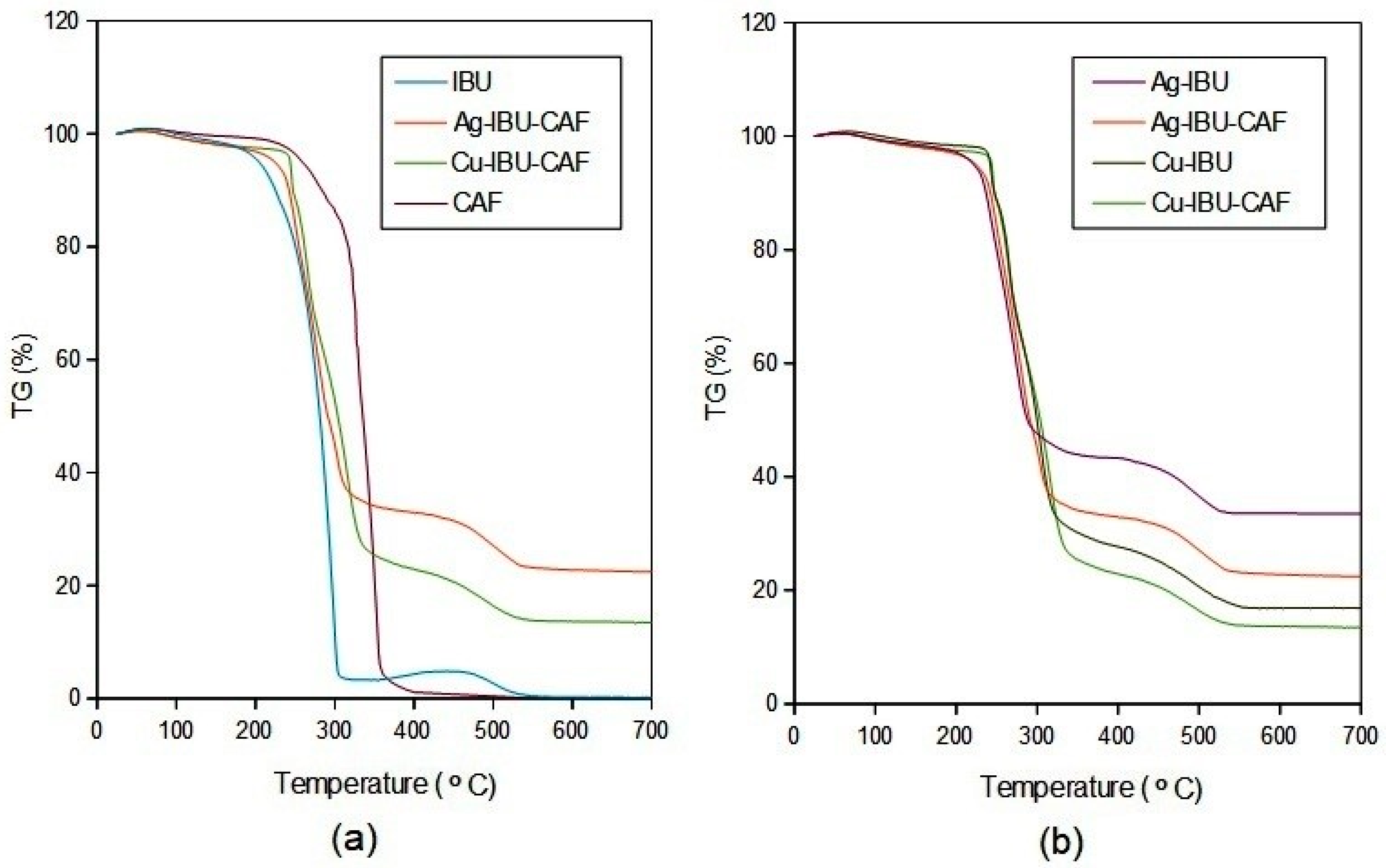
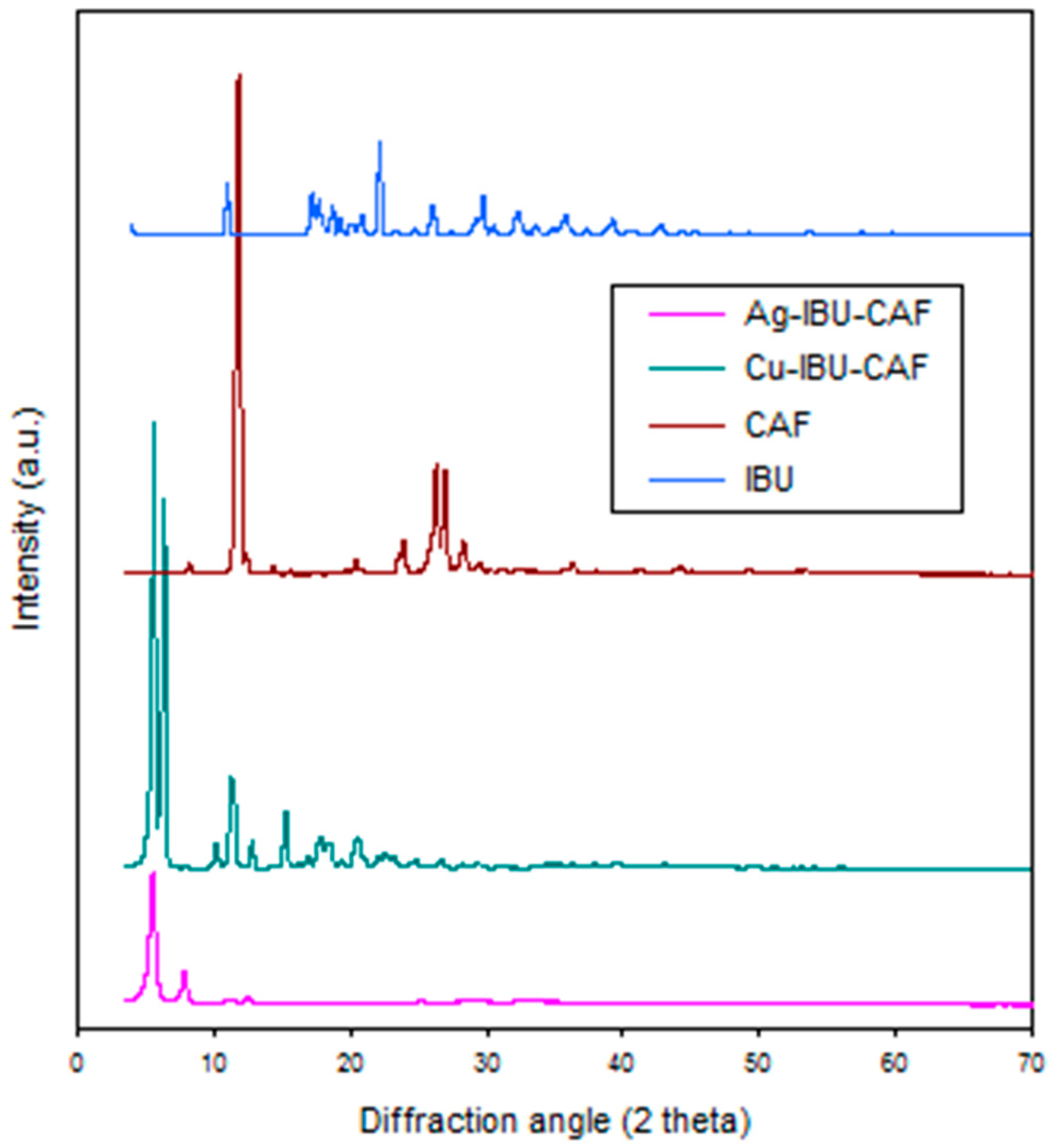
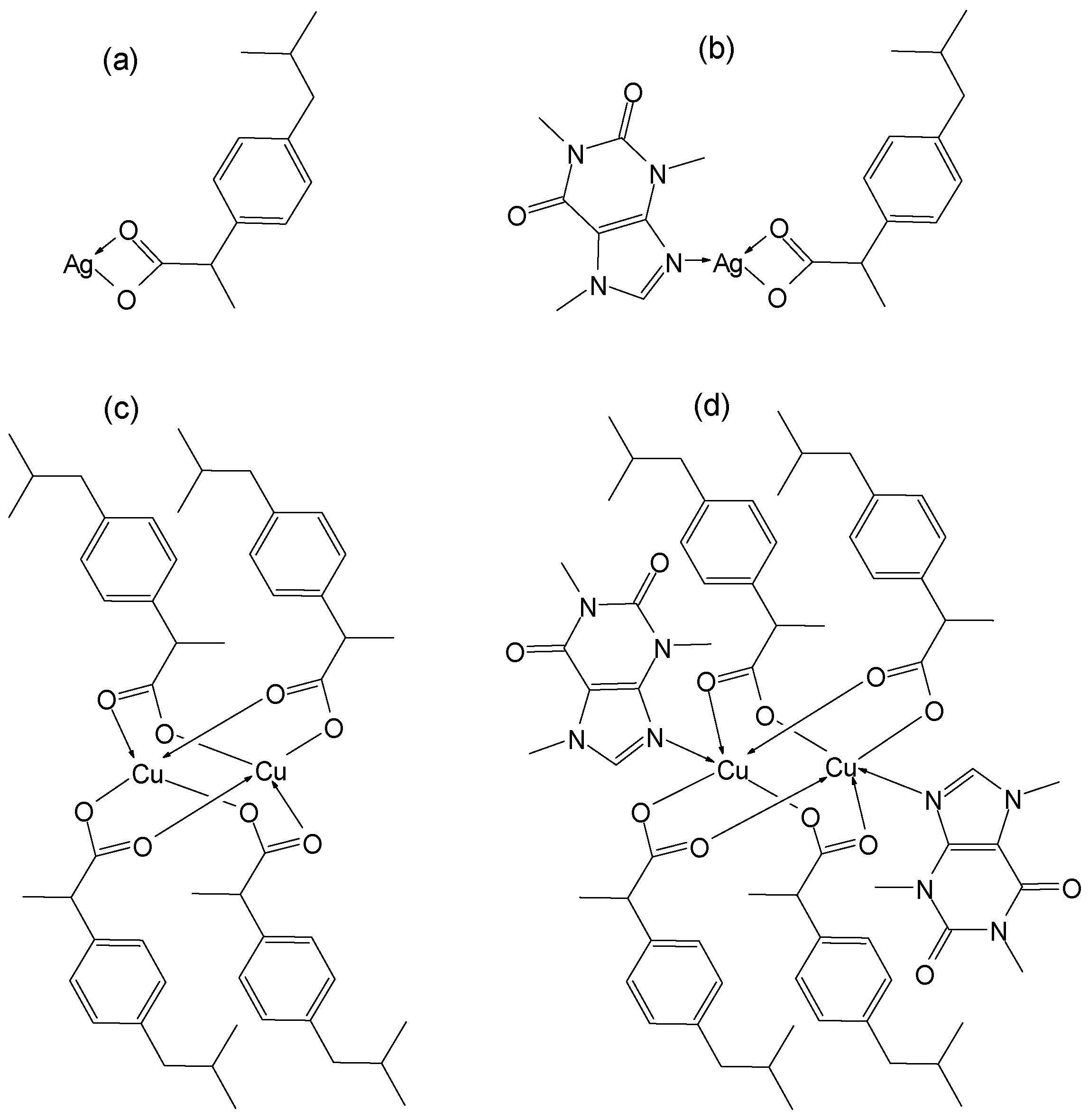
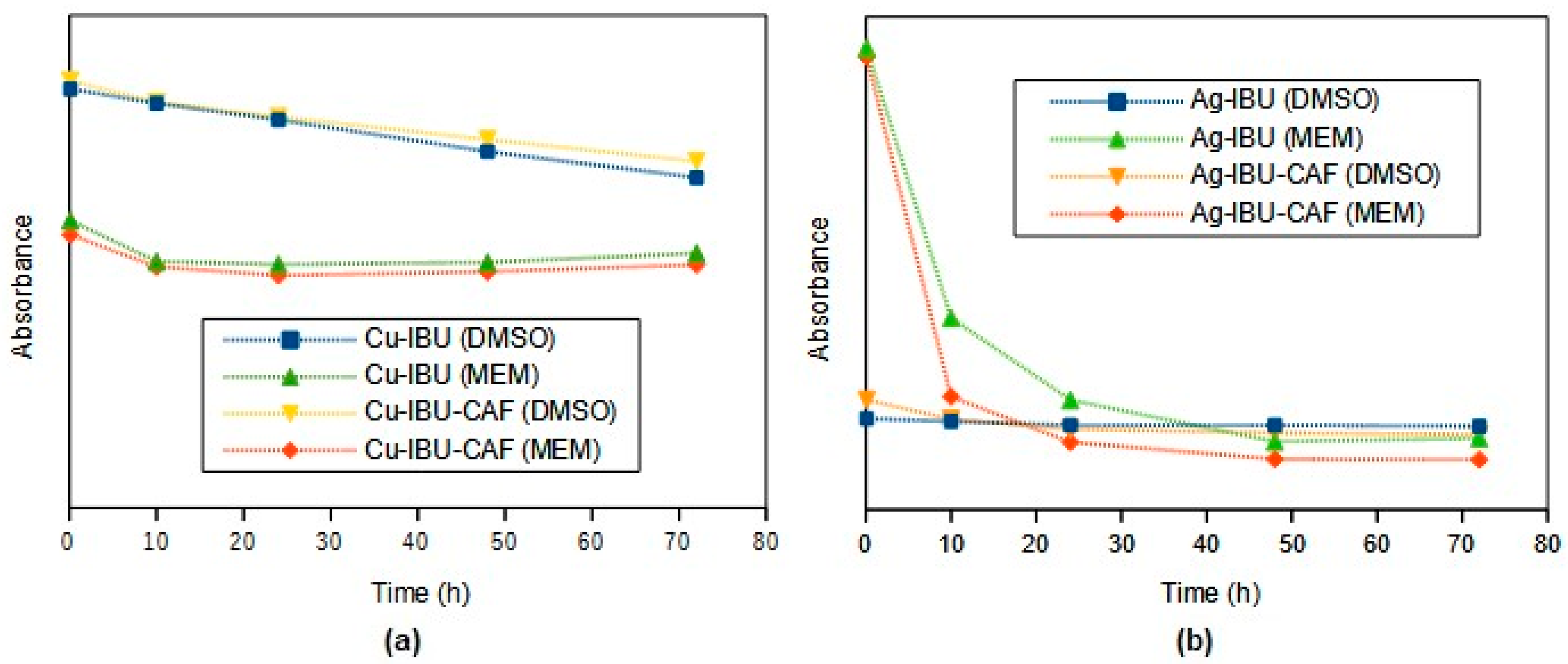
2.1. Synthesis and Characterization
2.2. In Silico ADME Prediction
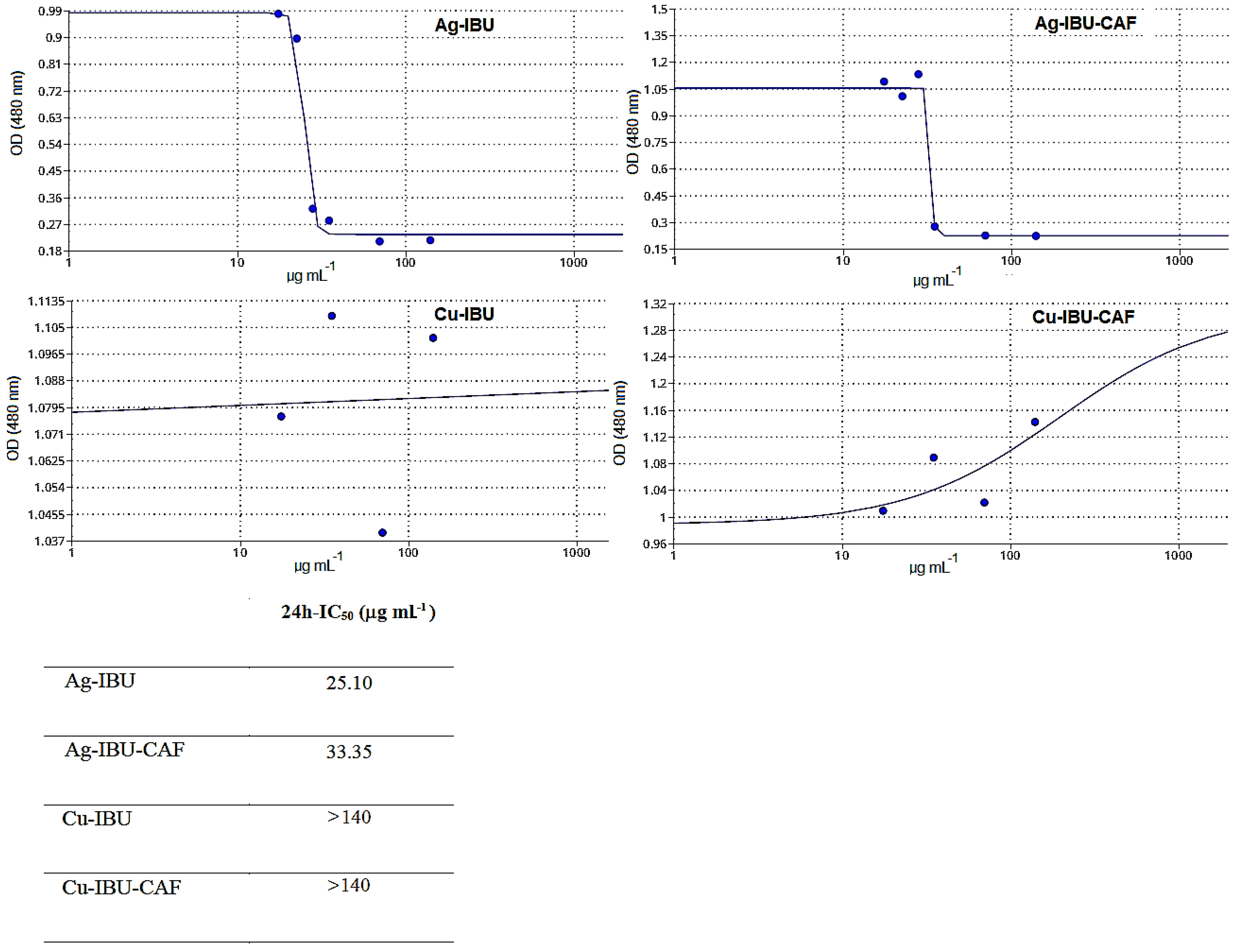
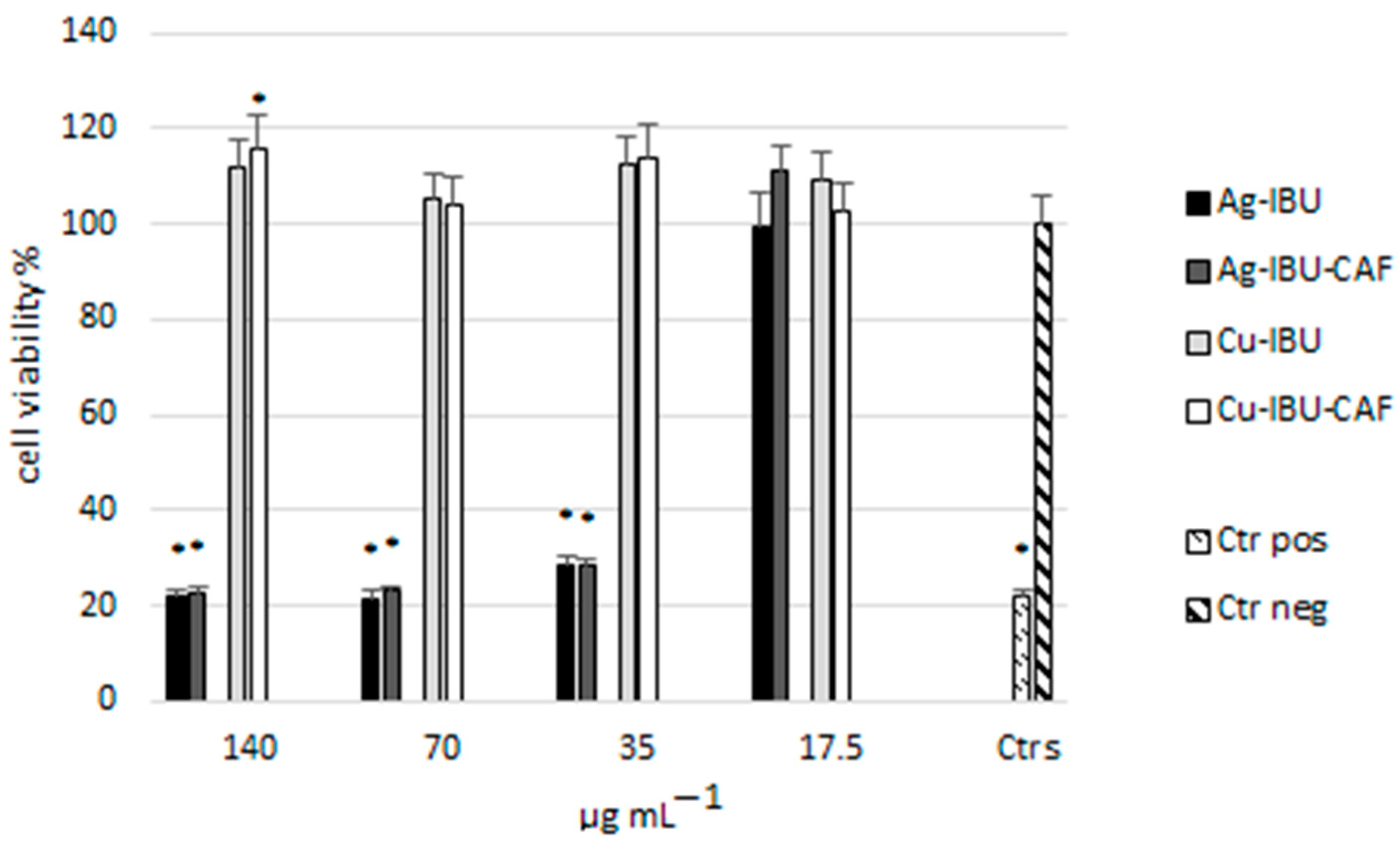
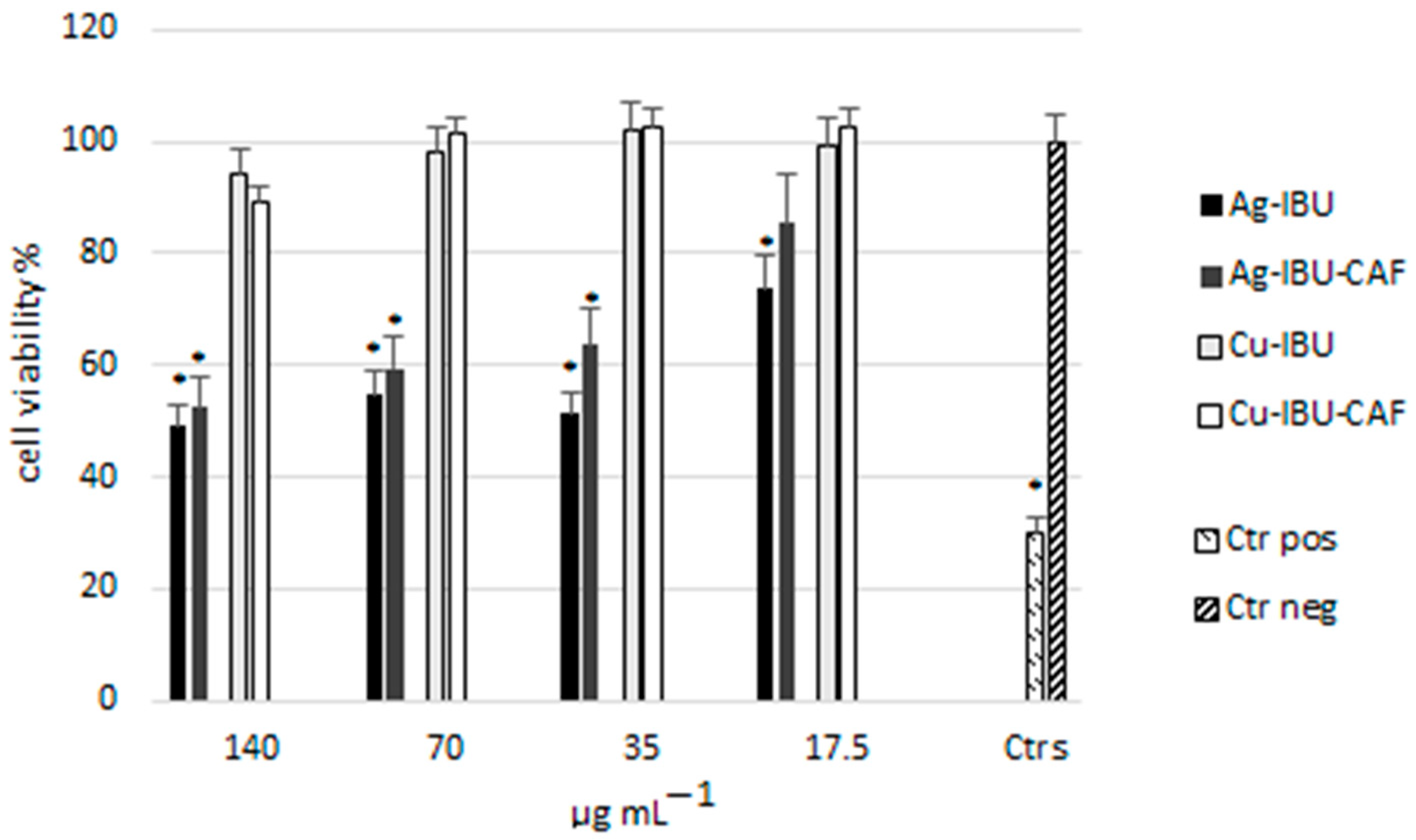
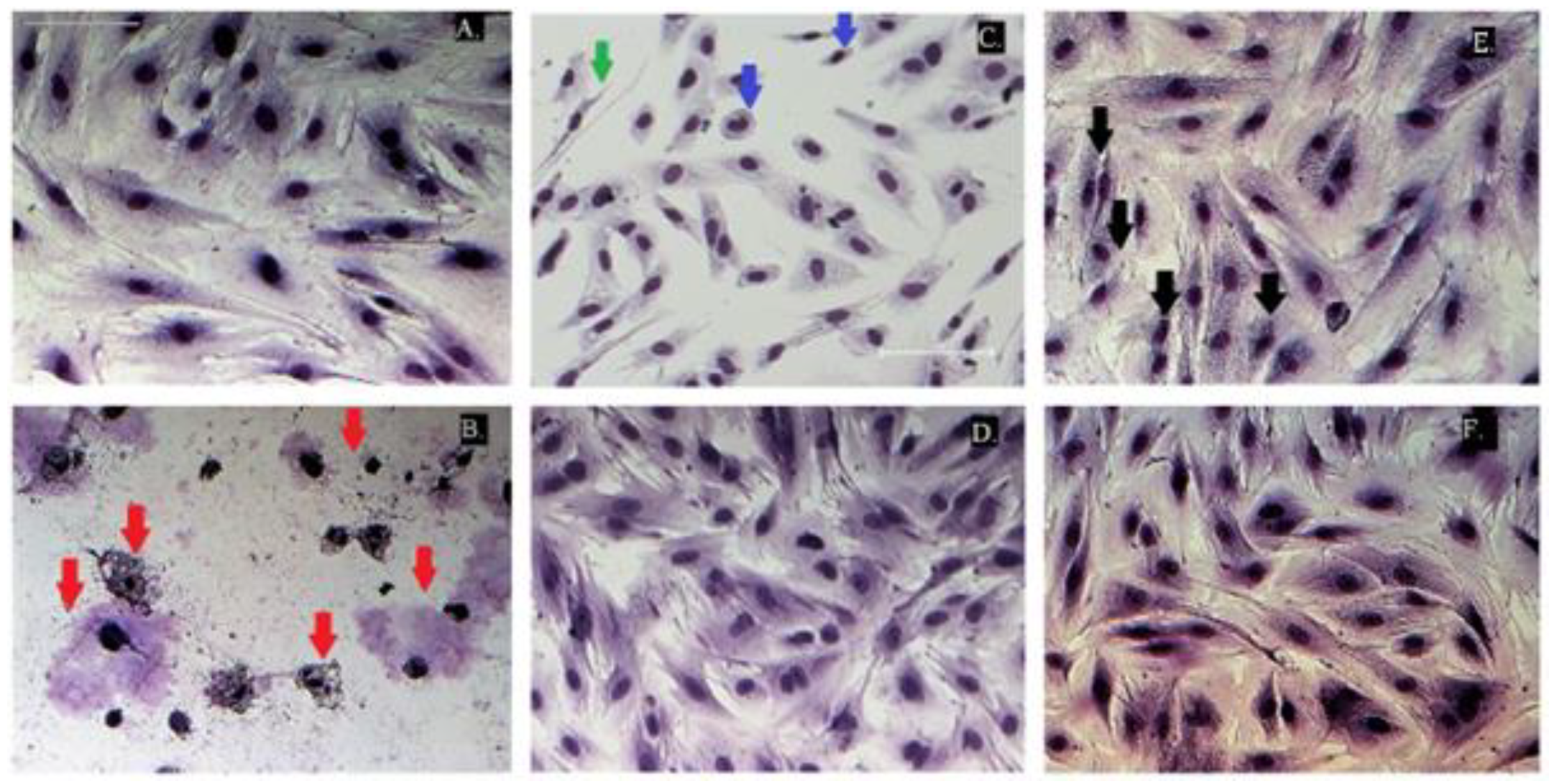
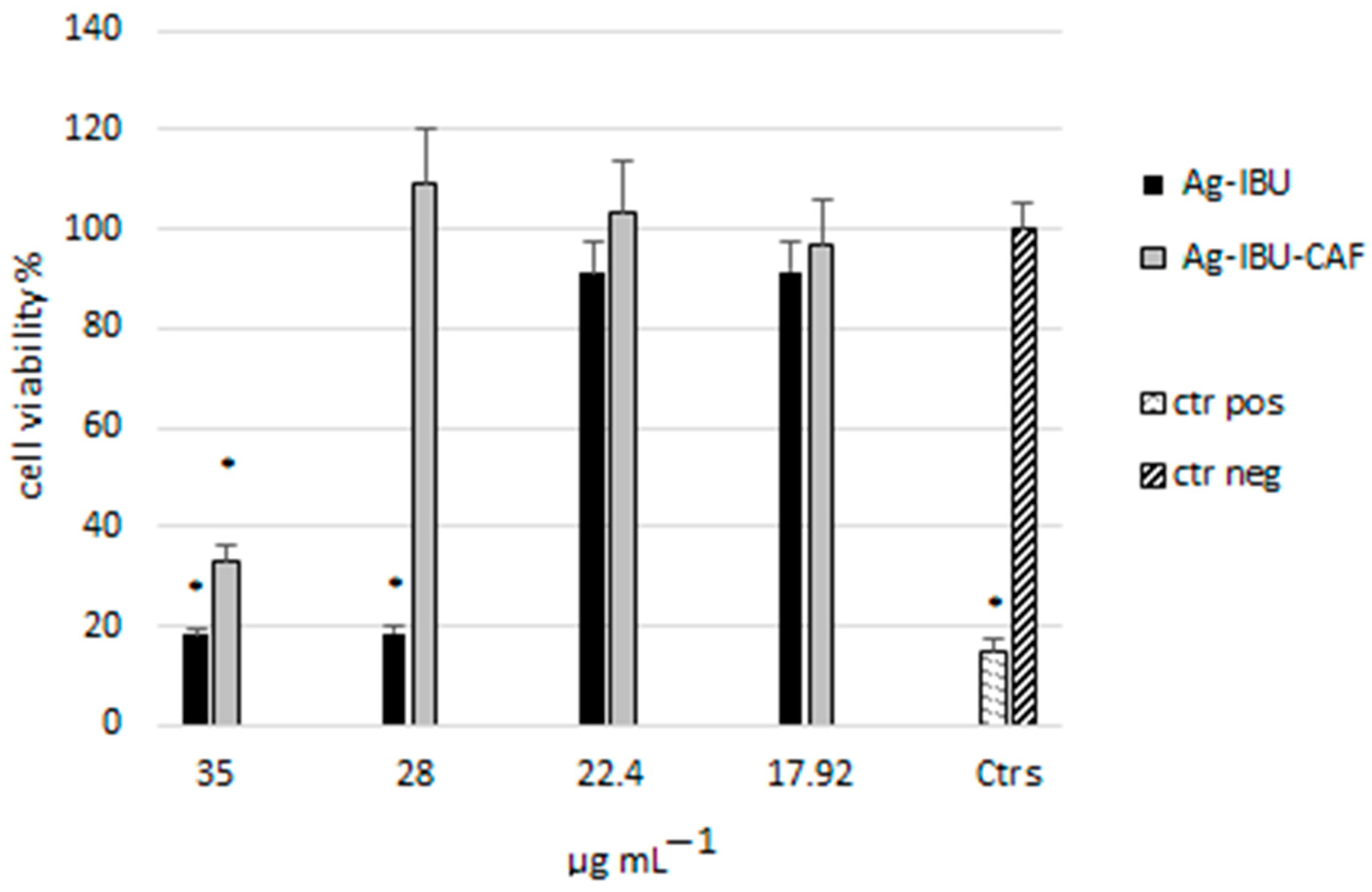
2.3. Cytotoxicity Evaluation
2.4. Antibacterial Effect
3. Materials and Methods
3.1. Synthesis Procedure
3.1.1. Cu-IBU
3.1.2. Cu-IBU-CAF
3.1.3. Ag-IBU
3.1.4. Ag-IBU-CAF
3.2. Material Characterization
3.3. In Silico ADME (Absorption, Distribution, Metabolism, Excretion) Prediction
3.4. Cytotoxicity
3.4.1. Cells and Culture Conditions
3.4.2. Cytotoxicity Assays
3.4.3. Pro- and Anti-Inflammatory Assays
3.5. Antibacterial Assays
3.5.1. Bacterial Strains
3.5.2. Minimal Inhibitory Concentration
3.5.3. Disc Diffusion Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Enhancement of ibuprofen solubility and skin permeation by conjugation with l-valine alkyl esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef] [PubMed]
- Nugrahani, I.; Kartini, K.A. Study of physical interaction between ibuprofen and caffeine and its influence on solubility and hygroscopicity of ibuprofen. Int. J. Pharm. Sci. 2015, 7, 223–227. [Google Scholar]
- Belayneh, A.; Molla, F. The Effect of Coffee on Pharmacokinetic Properties of Drugs: A Review. BioMed Res. Int. 2020, 2020, 7909703. [Google Scholar] [CrossRef]
- Caffeine—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519490/ (accessed on 22 July 2023).
- Trosko, J.E.; Chu, E.H.Y. Inhibition of repair of UV-damaged DNA by caffeine and mutation induction in Chinese hamster cells. Chem. Biol. Interact. 1973, 5, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Sugimoto, N.; Shirai, T.; Hayashi, K.; Nishida, H.; Ohnari, I.; Takeuchi, A.; Yachie, A.; Tsuchiya, H. Caffeine activates tumor suppressor PTEN in sarcoma cells. Int. J. Oncol. 2011, 39, 465–472. [Google Scholar]
- Okano, J.; Nagahara, T.; Matsumoto, K.; Murawaki, Y. Caffeine inhibits the proliferation of liver cancer cells and activates the MEK/ERK/EGFR signaling pathway Basic Clin. Pharmacol. Toxicol. 2008, 102, 543–551. [Google Scholar]
- Al-Ansari, M.M.; Aboussekhra, A. Caffeine mediates sustained inactivation of breast cancer-associated myofibroblasts via up-regulation of tumor suppressor genes. PLoS ONE 2014, 9, 90907. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Lieb, K.; Hüll, M.; Aicher, B.; van Ryn, J.; Pairet, M.; Engelhardt, G. Effects of caffeine and paracetamol alone or in combination with acetylsalicylic acid on prostaglandin E(2) synthesis in rat microglial cells. Neuropharmacology 2000, 39, 2205–2213. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Puranik, R.; Bao, S.; Bonin, A.M.; Kaur, R.; Weder, J.E.; Casbolt, L.; Hambley, T.W.; Lay, P.A.; Barter, P.J.; Rye, K.-A. A novel class of copper(II)- and zinc(II)-bound non-steroidal anti-inflammatory drugs that inhibits acute inflammation in vivo. Cell Biosci. 2016, 6, 6–9. [Google Scholar] [CrossRef]
- Franz, K.J.; Nils, M.-N. Introduction: Metals in medicine. Chem. Rev. 2019, 119, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Banti, C.N.; Hadjikakou, S.K. Non-steroidal anti-inflammatory drugs (NSAIDs) in metal complexes and their effect at the cellular level. Eur. J. Inorg. Chem. 2016, 2016, 3048–3071. [Google Scholar] [CrossRef]
- Omar, S.N.; Abu Ali, H. New complexes of Zn(II) with the anti-inflammatory non-steroidal drug, ibuprofen and nitrogen donor ligands. Synthesis, characterization and biological activity. J. Coord. Chem. 2017, 70, 2436–2452. [Google Scholar] [CrossRef]
- Kisku, T.; Paul, K.; Singh, B.; Das, S.; Mukherjee, S.; Kundu, A.; Rath, J.; Das, R.S. Synthesis of Cu(II)-caffeine complex as potential therapeutic agent: Studies on antioxidant, anticancer and pharmacological activities. J. Mol. Liq. 2022, 364, 119897. [Google Scholar] [CrossRef]
- Crisponi, G.; Nurchi, V.M.; Fanni, D.; Gerosa, C.; Nemolato, S.; Faa, G. Copper-related diseases: From chemistry to molecular pathology. Coord. Chem. Rev. 2010, 254, 876–889. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Trinchero, A.; Bonora, S.; Tinti, A.; Fini, G. Spectroscopic behaviour of copper complexes of nonsteroidal anti-inflammatory drugs. Biopolymers 2004, 74, 120–124. [Google Scholar] [CrossRef]
- Shahabadi, N.; Shiri, F. Multispectroscopic studies on the interaction of a copper(II) complex of ibuprofen drug with calf thymus DN. Nucleosides Nucleotides Nucleic Acids. 2017, 36, 83–106. [Google Scholar] [CrossRef]
- Kowcun, K.; Frańska, M.; Frański, R. Binuclear copper complexes with non-steroidal anti-inflammatory drugs as studied by electrospray ionization mass spectroscopy. Cent. Eur. J. Chem. 2012, 10, 320–326. [Google Scholar]
- Kaur, H.; Puri, J.K.; Singla, A. Metal ion interactions with drugs: Electrochemical study of complexation of various bivalent metal ions with nimesulide and ibuprofen. J. Mol. Liq. 2013, 182, 39–42. [Google Scholar] [CrossRef]
- Tita, B.; Bandur, G.; Tita, D. Novel Cu(II) complex with non-steroidal anti-inflammatory drugs. Rev. Chim. 2013, 64, 569–573. [Google Scholar]
- Abbas, A.M.; Aboelmagd, A.; Kishk, S.M.; Nasrallah, H.H.; Boyd, W.C.; Kalil, H.; Orabi, A.S. A novel ibuprofen derivative and its complexes: Physicochemical characterization, DFT modeling, docking, in vitro anti-inflammatory studies, and DNA interactions. Molecules 2022, 27, 7540. [Google Scholar] [CrossRef]
- Malis, G.; Geromichalou, E.; Geromichalou, G.D.; Hatzidimitriou, A.G.; Psomas, G. Copper(II) complexes with non-steroidal anti-inflammatory drugs: Structural characterization, in vitro and in silico biological profile. J. Inorg. Biochem. 2021, 224, 111563. [Google Scholar] [CrossRef] [PubMed]
- Djokić, S. Treatment of various surfaces with silver and its compounds for topical wound dressings, catheter and other biomedical applications. ECS Trans. 2008, 11, 1–12. [Google Scholar] [CrossRef]
- Amin, S.; Sher, M.; Ali, A.; Rehman, M.F.; Hayat, A.; Ikram, M.; Abbas, A.; Amin, H.M.A. Sulfonamide-functionalized silver nanoparticles as an analytical nanoprobe for selective Ni(II) sensing with synergistic antimicrobial activity. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100735. [Google Scholar] [CrossRef]
- Pereira e Silva, I.M.; De Moraes Profirio, D.; De Paiva, R.E.F.; Lancellotti, M.; Barboza Formiga, A.L.; Corbi, P.P. A silver complex with ibuprofen: Synthesis, solid state characterization, DFT calculations and antibacterial assays. J. Mol. Struct. 2013, 1049, 1–6. [Google Scholar] [CrossRef]
- Kafarska, K.; Wolf, W.M. Novel silver complexes with popular non-steroidal anti-inflammatory drugs. Acta Innov. 2016, 21, 51–59. [Google Scholar]
- Naglah, A.M.; Al-Omar, M.A.; El-Megharbel, S.M.; Refat, M.S. Structural, conductometric and antimicrobial investigations of ibuprofen analgesic drug complex with certain metal ions. Int. J. Pharm. 2015, 11, 773–785. [Google Scholar] [CrossRef]
- Nunez, C.; Fernandez-Lodeiro, A.; Fernandez-Lodeiro, J.; Carballo, J.; Capelo, J.L.; Lodeiro, C. Synthesis, spectroscopic studies and in vitro antibacterial activity of ibuprofen and its derived metal complexes. Inorg. Chem. Commun. 2014, 45, 61–65. [Google Scholar] [CrossRef]
- Fiori, A.T.M.; Lustri, W.R.; Magalhaes, A.; Corbi, P.P. Chemical, spectroscopic characterization and antibacterial activities of a novel gold(I)-ibuprofen complex. Inorg. Chem. Commun. 2011, 14, 738–740. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Nilnumkhum, A.; Kanlaya, R.; Yoodee, S.; Thongboonkerd, V. Caffeine inhibits hypoxia-induced renal fibroblast activation by antioxidant mechanism. Cell Adh. Migr. 2019, 13, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.O.; Hjemdahl, P. Lessons from 20 years with COX-2 inhibitors: Importance of dose-response considerations and fair play in comparative trials. J. Intern. Med. 2022, 292, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Akarasereenont, P.; Thiemermann, C.; Flower, R.J.; Vane, J.R. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc. Natl. Acad. Sci. USA 1993, 90, 11693–11697. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-F.; Chen, Y.; Brown, P.H.; Lyle, B.J.; Black, R.M.; Cheng, I.H.; Ou, B.; Prior, R.L. Bioactivities of crude caffeine: Antioxidant activity, cyclooxygenase-2 inhibition, and enhanced glucose uptake. Food Chem. 2012, 131, 564–568. [Google Scholar] [CrossRef]
- Krisnamurti, G.C.; Fatchiyah, F. Interaction of acetaminophen and caffeine towards cyclooxygenase-2 (COX-2) in inhibition of prostaglandin (PGH2) synthesis. J. Phys. Conf. Ser. 2019, 1146, 012004. [Google Scholar] [CrossRef]
- Chen, J.F.; Eltzschig, H.; Fredholm, B. Adenosine receptors as drug targets—What are the challenges? Nat. Rev. Drug Discov. 2013, 12, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Karges, J.; Stokes, R.W.; Cohen, S.M. Metal Complexes for Therapeutic Applications. Trends Chem. 2021, 3, 523–534. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability enhancement techniques for poorly aqueous soluble drugs and therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef]
- Jurca, T.; Marian, E.; Vicas, L.G.; Mureşan, M.E.; Fritea, L. Metal complexes of pharmaceutical substances. In Spectroscopic Analyses—Developments and Applications; Sharmin, E., Zafar, F., Eds.; In Tech: London, UK, 2017; pp. 123–142. [Google Scholar]
- Ameh, T.; Gibb, M.; Stevens, D.; Pradhan, S.H.; Braswell, E.; Sayes, C.M. Silver and copper nanoparticles induce oxidative stress in bacteria and mammalian cells. Nanomaterials 2022, 12, 2402. [Google Scholar] [CrossRef]
- Raju, S.K.; Karunakaran, A.; Kumar, S.; Sekar, P.; Murugesan, M.; Karthikeyan, M. Silver complexes as anticancer agents: A perspective review. Ger. J. Pharm. Biomater. 2022, 1, 6–28. [Google Scholar] [CrossRef]
- Kalinowska-Lis, U.; Felczak, A.; Chęcińska, L.; Szabłowska-Gadomska, I.; Patyna, E.; Małecki, M.; Lisowska, K.; Ochocki, J. Antibacterial activity and cytotoxicity of silver(I) complexes of pyridine and (benz)imidazole derivatives. X-ray crystal structure of [Ag(2,6-di(CH2OH)py)2]NO3. Molecules 2016, 21, 87. [Google Scholar] [CrossRef] [PubMed]
- Radko, L.; Stypuła-Trębas, S.; Posyniak, A.; Żyro, D.; Ochocki, J. Silver(I) complexes of the pharmaceutical agents metronidazole and 4-hydroxymethylpyridine: Comparison of cytotoxic profile for potential clinical application. Molecules 2019, 24, 1949. [Google Scholar] [CrossRef] [PubMed]
- Pellei, M.; Santini, C.; Bagnarelli, L.; Caviglia, M.; Sgarbossa, P.; De Franco, M.; Zancato, M.; Marzano, C.; Gandin, V. Novel silver complexes based on phosphanes and ester derivatives of bis(pyrazol-1-yl)acetate ligands targeting TrxR: New promising chemotherapeutic tools relevant to SCLC management. Int. J. Mol. Sci. 2023, 24, 4091. [Google Scholar] [CrossRef] [PubMed]
- Korman, D.; Ostrovskaya, L.; Bluhterova, N.; Rikova, V.A.; Fomina, M.M. Cytotoxicity of silver compounds. Biophysics 2022, 67, 565–570. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Tests for in Vitro Cytotoxicity (ISO 10993-5:2009). International Organization for Standardization: Geneva, Switzerland, 2009.
- Śliwa, E.I.; Śliwińska-Hill, U.; Bażanów, B.; Siczek, M.; Kłak, J.; Smoleński, P. Synthesis, structural, and cytotoxic properties of new water-soluble copper(II) complexes based on 2,9-dimethyl-1,10-phenanthroline and their one derivative containing 1,3,5-triaza-7-phosphaadamantane-7-oxide. Molecules 2020, 25, 741. [Google Scholar] [CrossRef]
- Marczewska, J.; Koziorowska, J.H.; Anuszewska, E.L. Influence of ascorbic acid on cytotoxic activity of copper an iron ions in vitro. Acta Pol. Pharm. 2000, 57, 415–417. [Google Scholar]
- Hussain, A.; AlAjmi, M.F.; Rehman, M.T.; Amir, S.; Husain, F.M.; Alsalme, A.; Siddiqui, M.A.; AlKhedhairy, A.A.; Khan, R.A. Copper(II) complexes as potential anticancer and nonsteroidal anti-inflammatory agents: In vitro and in vivo studies. Sci. Rep. 2019, 9, 5237. [Google Scholar] [CrossRef]

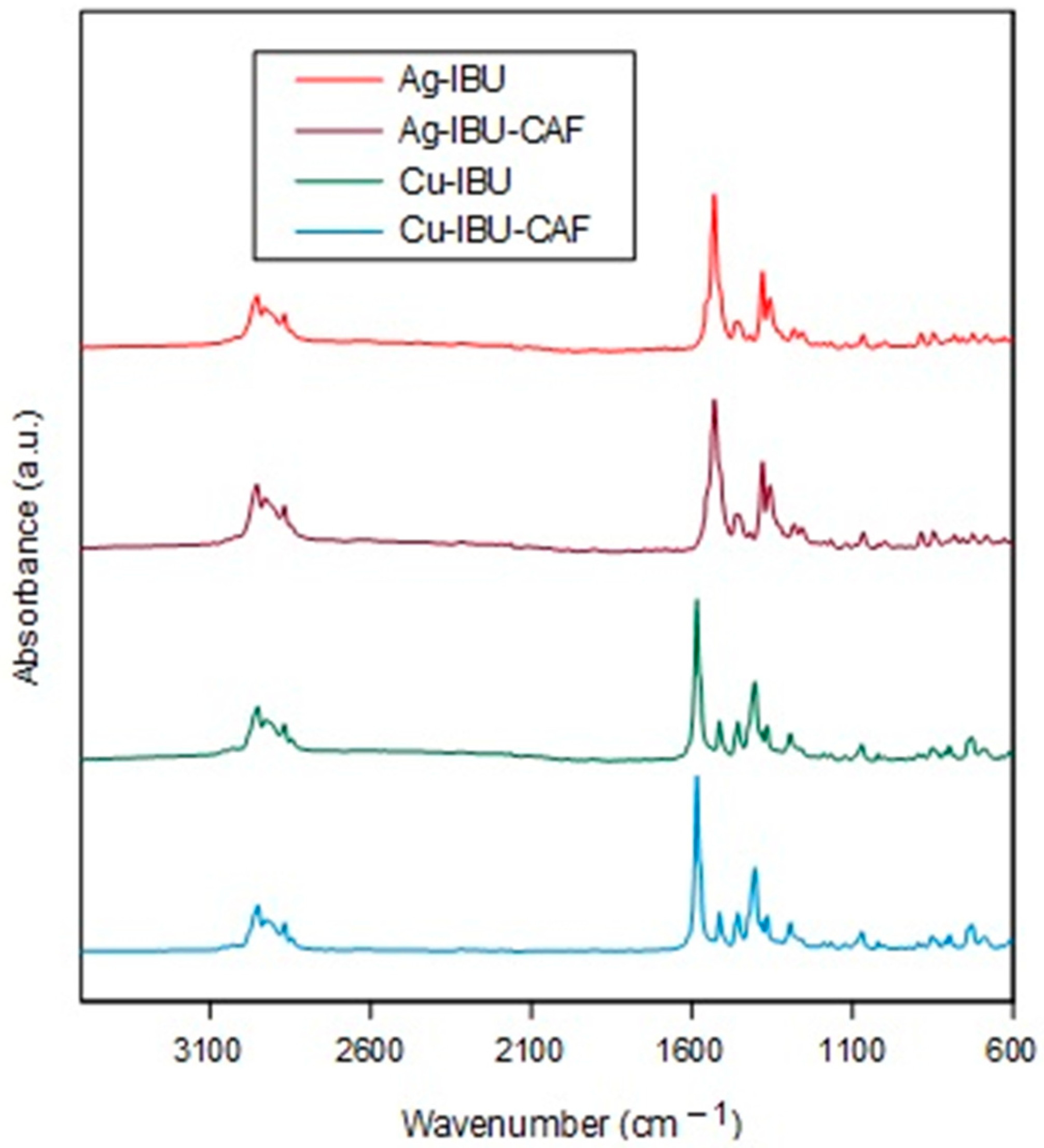
| Sample | Wavenumber (cm−1) | ||
|---|---|---|---|
| νasym | νsym | Δν | |
| Na-IBU | 1581 | 1400 | 181 |
| Ag-IBU | 1531 | 1380 | 151 |
| Ag-IBU-CAF | 1530 | 1380 | 150 |
| Cu-IBU | 1584 | 1404 | 180 |
| Cu-IBU-CAF | 1584 | 1404 | 180 |
| Sample | Molecular Weight (g mol−1) | Heavy Atoms | Fraction Csp3 | Rotatable Bonds | H-Bond Donors | H-Bond Acceptors | Molar Refractivity | TPSA (Å2) |
|---|---|---|---|---|---|---|---|---|
| Cu-IBU | 952.22 | 62 | 0.54 | 16 | 0 | 8 | 241.22 | 73.84 |
| Cu-IBU-CAF | 1346.64 | 90 | 0.59 | 18 | 0 | 16 | 368.50 | 168.04 |
| Ag-IBU | 314.15 | 16 | 0.54 | 4 | 0 | 2 | 60.47 | 18.46 |
| Ag-IBU-CAF | 508.34 | 30 | 0.48 | 4 | 0 | 5 | 112.50 | 80.28 |
| Sample | Solubility Log S (ESOL) | MLOGP | GI Absorption | BBB Permeant | Drug-Likeness (Lipinski) |
|---|---|---|---|---|---|
| Cu-IBU | −14.90 | 5.49 | Low | No | No |
| Cu-IBU-CAF | −16.43 | 2.69 | Low | No | No |
| Ag-IBU | −4.28 | 2.42 | High | Yes | Yes |
| Ag-IBU-CAF | −5.31 | 2.21 | High | No | Yes |
| Concentration (μg mL−1) | Ag-IBU | Ag-IBU-CAF | Cu-IBU | Cu-IBU-CAF |
|---|---|---|---|---|
| 140 | 4 | 4 | 1 | 1 |
| 70 | 4 | 3 | 1 | 1 |
| 35 | 3 | 2 | 0 | 0 |
| 17.5 | 2 | 1 | 0 | 0 |
| Sample | IC50 (μg mL−1) | SI * | |
|---|---|---|---|
| COX-1 | COX-2 | ||
| Ag-IBU | 64.6 | 65.5 | 1 |
| Ag-IBU-CAF | 62.5 | 26.5 | 2.4 |
| Cu-IBU | 72.6 | 78.8 | 0.9 |
| Cu-IBU-CAF | 78.8 | 65.2 | 1.2 |
| Bacterial Strain | MIC (µg mL−1) | Inhibition Halo (mm) * | ||
|---|---|---|---|---|
| Ag-IBU | Ag-IBU-CAF | Ag-IBU | Ag-IBU-CAF | |
| Pseudomonas aeruginosa ATCC 27853 | 10 | 10 | 10 | 15 |
| Bacteroides fragilis ATCC 25285 | 20 | 20 | 7 | 10 |
| Escherichia coli ATCC 25218 | 10 | 5 | 9 | 17 |
| Clostridium bifermentans ATCC 638 | 20 | 20 | 8 | 10 |
| Bacillus subtilis ATCC 6637 | 10 | 10 | 11 | 13 |
| Enterococcus faecalis ATCC 29212 | 10 | 5 | 14 | 16 |
| Staphylococcus aureus ATCC 6538 | 10 | 5 | 14 | 15 |
| Staphylococcus aureus ATCC 25923 | 10 | 5 | 14 | 16 |
| Staphylococcus aureus ATCC 29213 | 5 | 5 | 7 | 12 |
| Staphylococcus aureus ATCC 43300 | 20 | 10 | 13 | 15 |
| Staphylococcus epidermidis ATCC 12228 | 20 | 10 | 10 | 11 |
| Grade | Effect | Cell Morphology |
|---|---|---|
| 0 | None | No substantial changes in cell morphology or number |
| 1 | Slight | Up to 20% round or detached cells, and/or slight changes in the morphology of a small part of the cells, and/or slight growth inhibition |
| 2 | Mild | Up to 50% round or detached cells, and/or some changes in the morphology of part of the cells, and/or less than 50% growth inhibition, no extensive cell lysis |
| 3 | Moderate | Up to 70% of cells rounded or lysed, more than 50% growth inhibition, but cells not completely destroyed |
| 4 | Severe | Nearly complete or complete destruction of the cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borówka, A.; Sierosławska, A.; Baier, A.; Rymuszka, A.; Olszewska, E. Silver and Copper Complexes with Ibuprofen and Caffeine—Preparation and Evaluation of Their Selected Biological Effects. Molecules 2024, 29, 506. https://doi.org/10.3390/molecules29020506
Borówka A, Sierosławska A, Baier A, Rymuszka A, Olszewska E. Silver and Copper Complexes with Ibuprofen and Caffeine—Preparation and Evaluation of Their Selected Biological Effects. Molecules. 2024; 29(2):506. https://doi.org/10.3390/molecules29020506
Chicago/Turabian StyleBorówka, Anna, Anna Sierosławska, Andrea Baier, Anna Rymuszka, and Elżbieta Olszewska. 2024. "Silver and Copper Complexes with Ibuprofen and Caffeine—Preparation and Evaluation of Their Selected Biological Effects" Molecules 29, no. 2: 506. https://doi.org/10.3390/molecules29020506
APA StyleBorówka, A., Sierosławska, A., Baier, A., Rymuszka, A., & Olszewska, E. (2024). Silver and Copper Complexes with Ibuprofen and Caffeine—Preparation and Evaluation of Their Selected Biological Effects. Molecules, 29(2), 506. https://doi.org/10.3390/molecules29020506






