Visible Light Photoactivity of g-C3N4/MoS2 Nanocomposites for Water Remediation of Hexavalent Chromium
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization
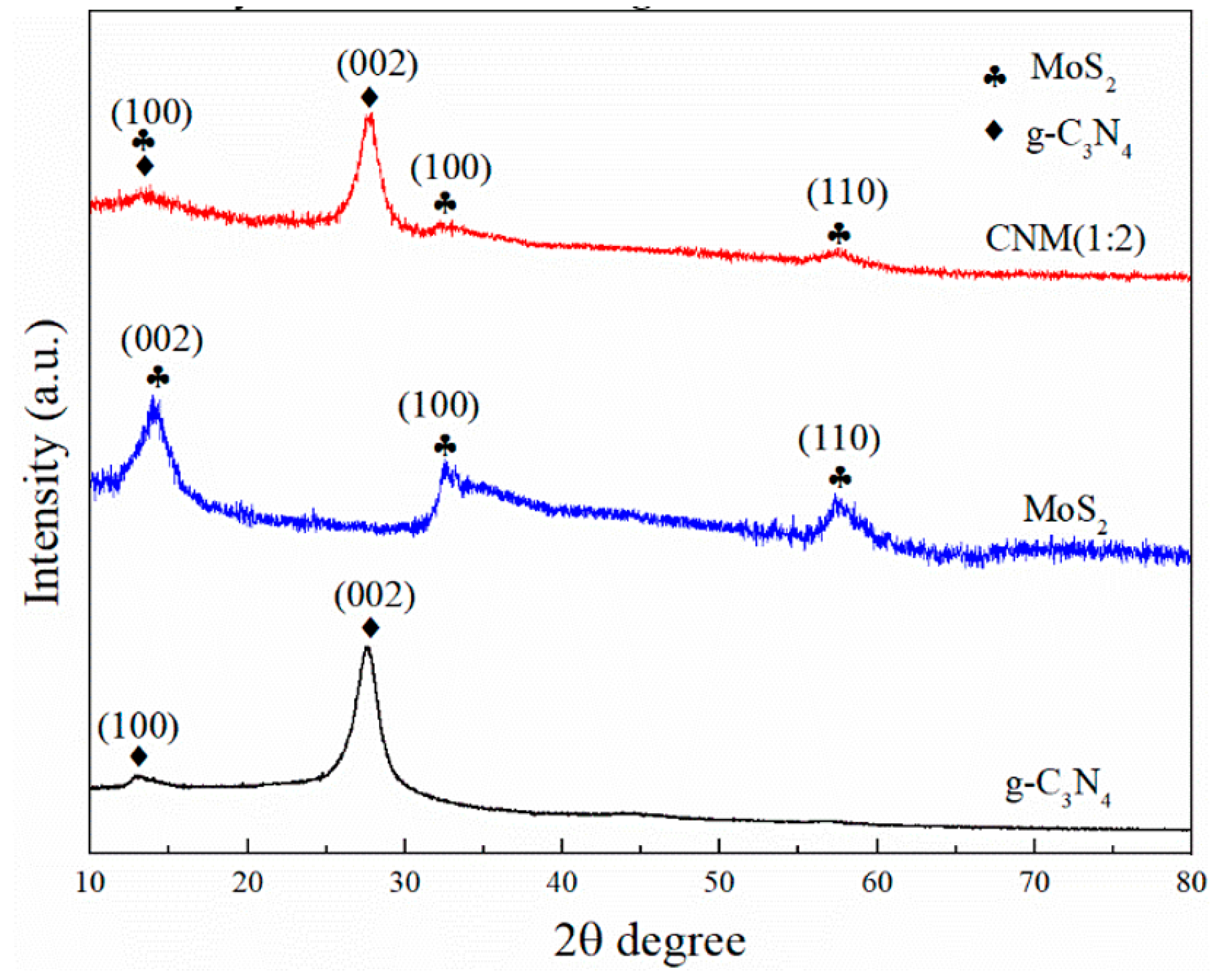
2.1.1. XRD
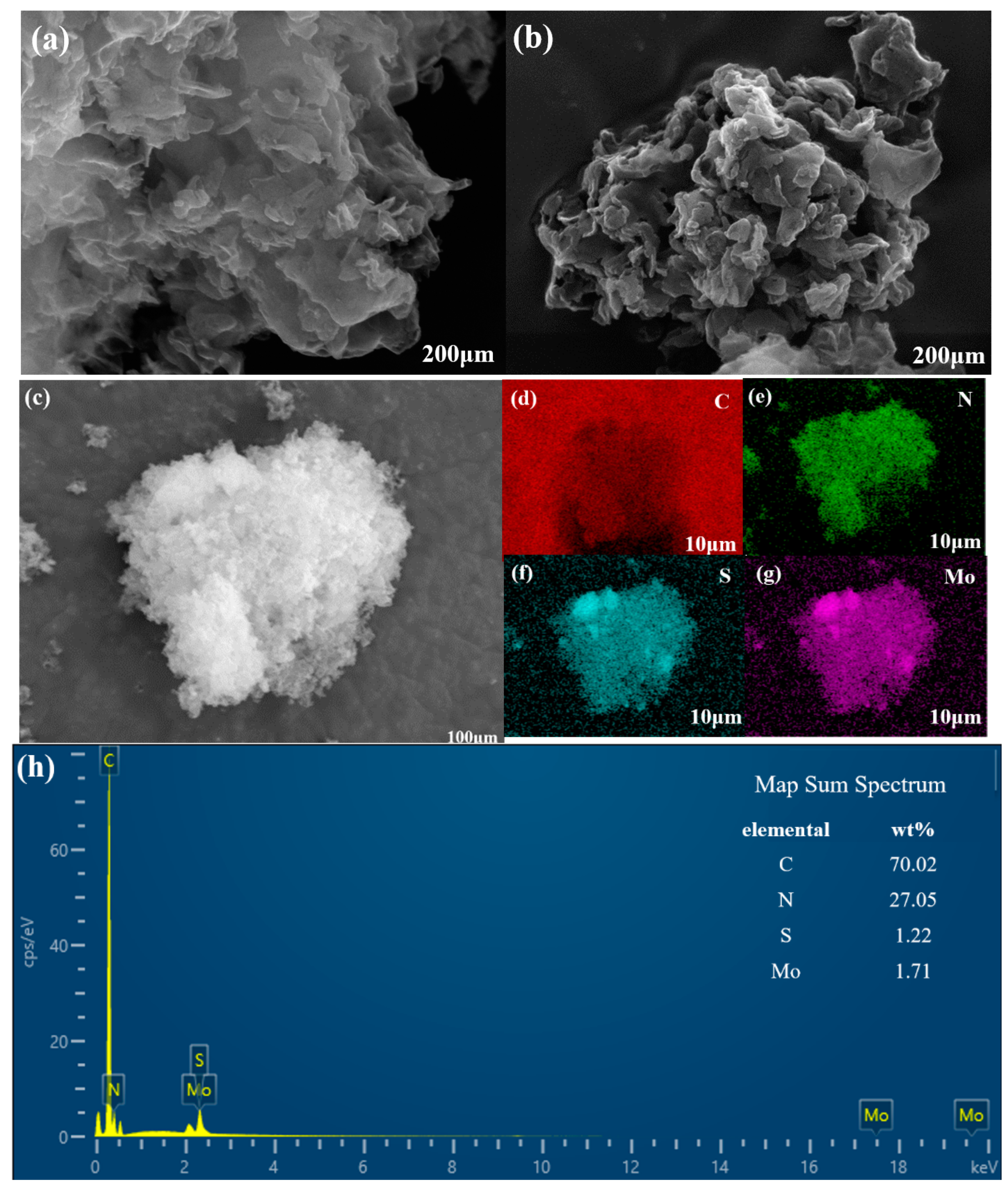
2.1.2. SEM
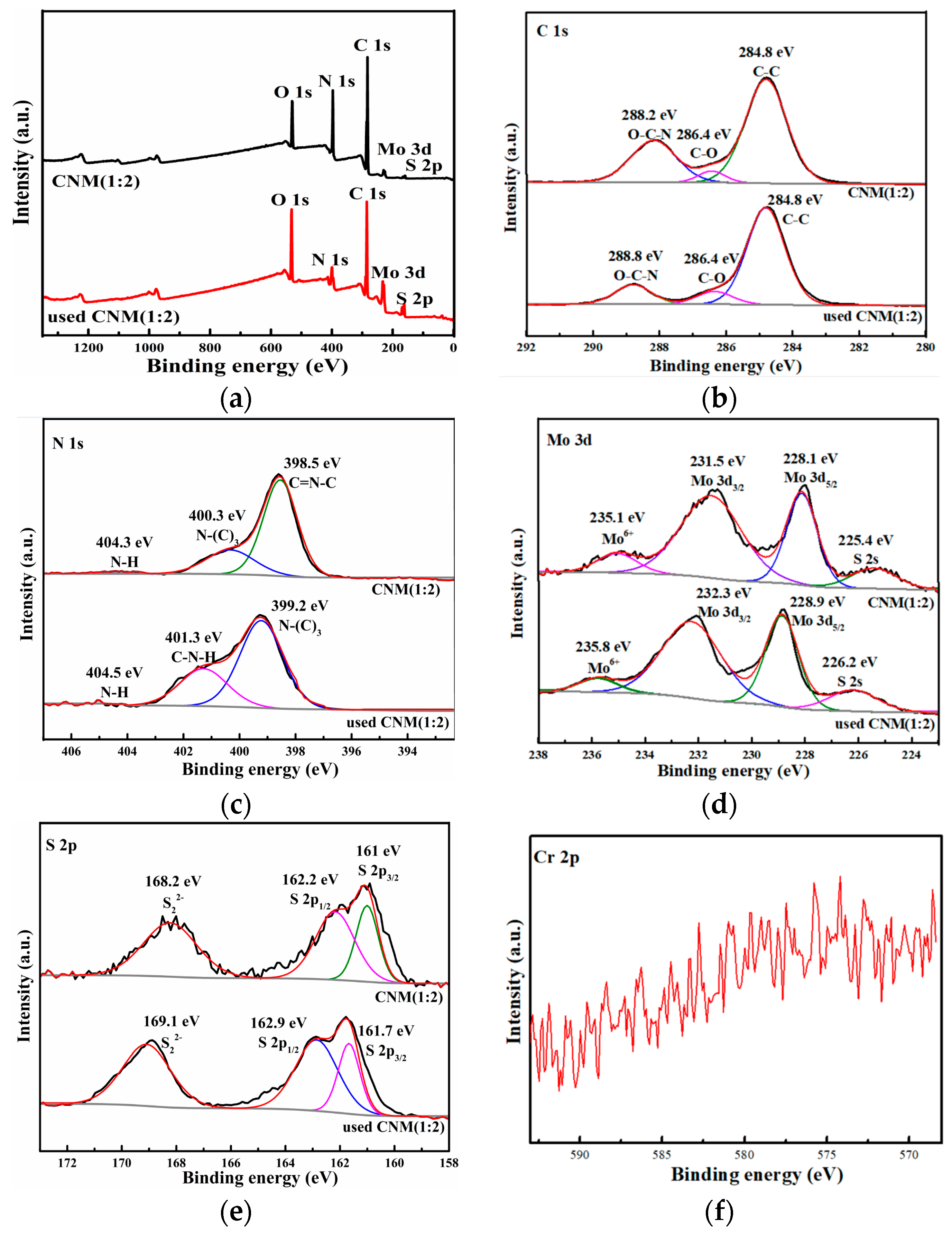
2.1.3. XPS
2.1.4. BET
2.1.5. UV–Vis Diffuse Reflectance Spectra
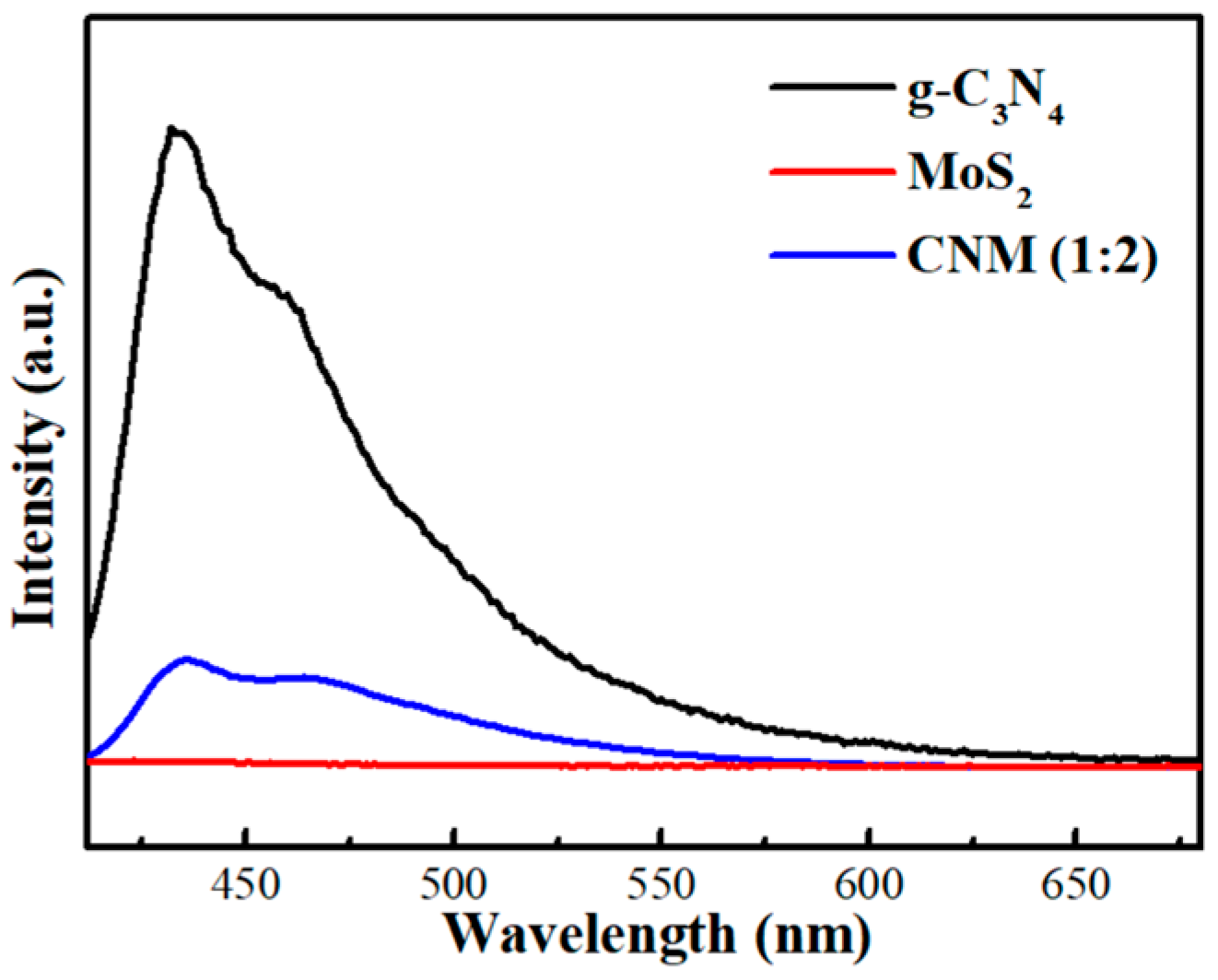
2.1.6. PL Spectra
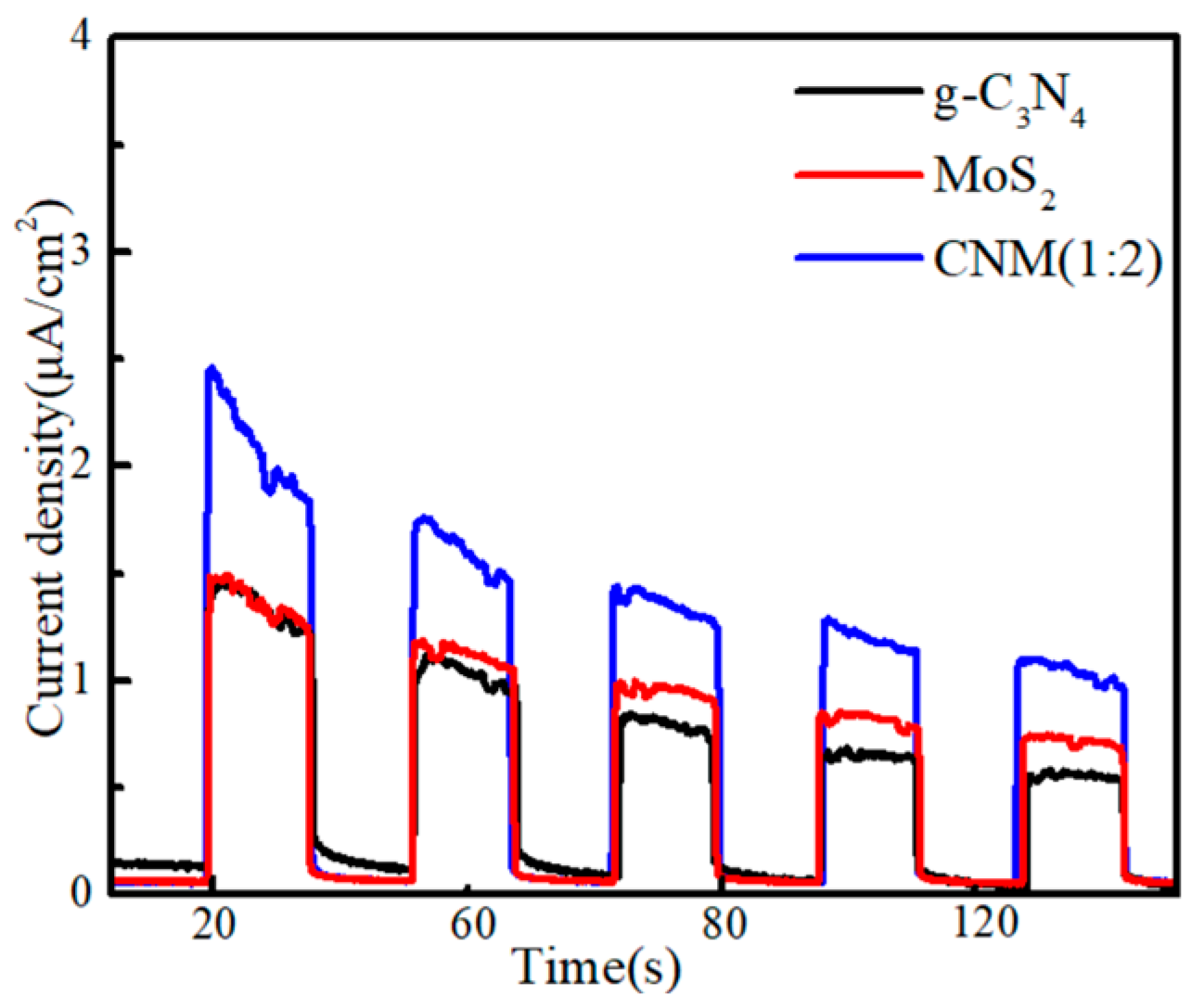
2.1.7. Transient Photocurrent Responses
2.2. Photocatalysis
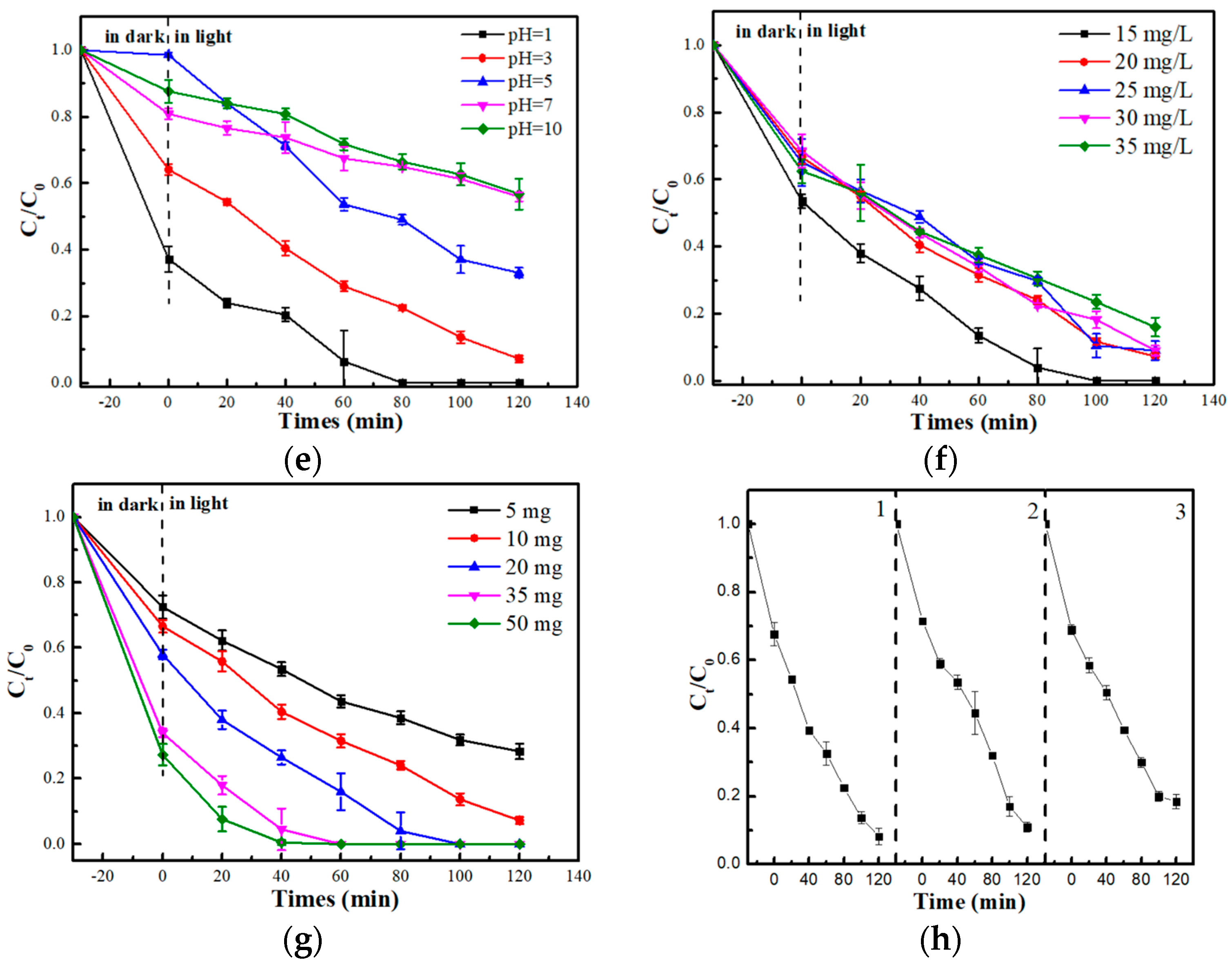
2.2.1. Photocatalytic Performance of Different Photocatalysts
2.2.2. Scavenging Study
2.2.3. Mechanisms of Photocatalysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photocatalysts
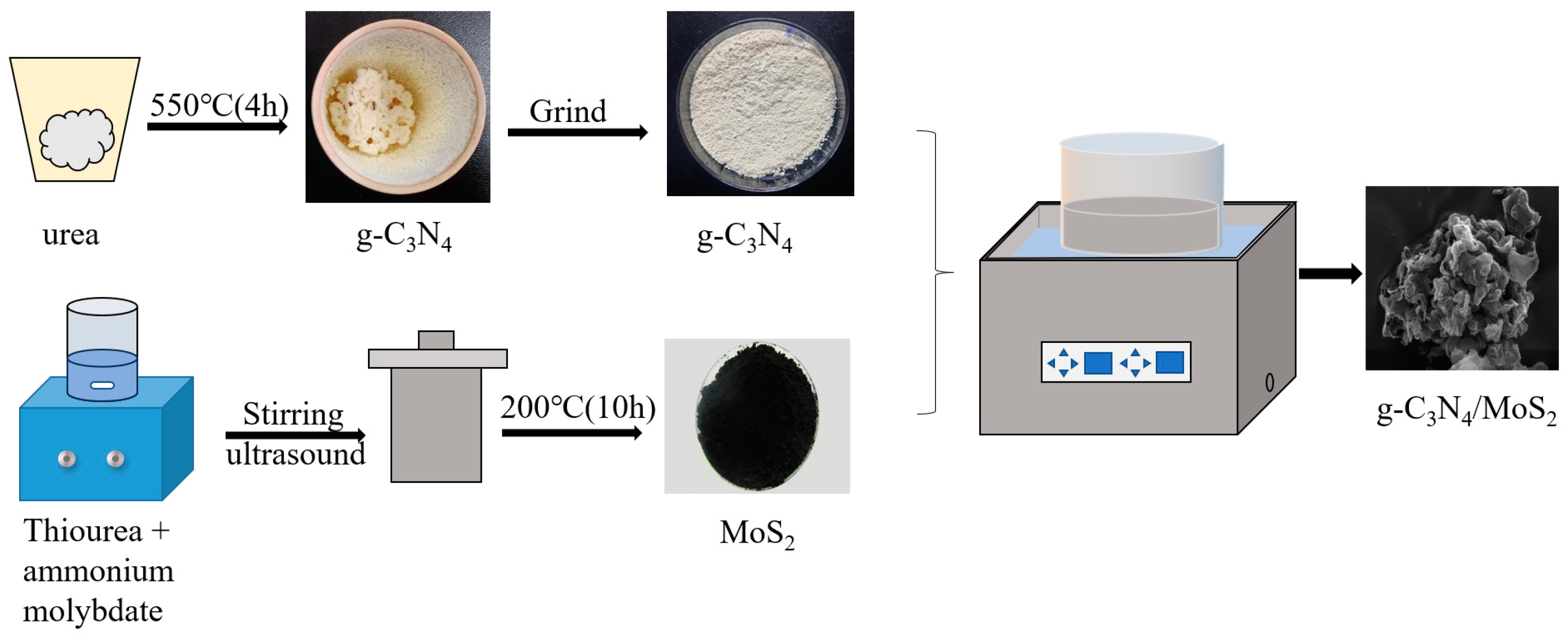
3.2.1. Preparation of g-C3N4
3.2.2. Preparation of MoS2
3.2.3. Preparation of g-C3N4/MoS2 with Different Mass Ratios
3.3. Characterization of Photocatalysts
3.4. Photocatalytic Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Kahn, M.E.; Liu, Y.; Wang, Z. The Consequences of Spatially Differentiated Water Pollution Regulation in China. J. Environ. Econ. Manag. 2018, 88, 468–485. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Z. Industrial Water Pollution, Water Environment Treatment, and Health Risks in China. Environ. Pollut. 2016, 218, 358–365. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A Review of Chemical, Electrochemical and Biological Methods for Aqueous Cr(VI) Reduction. J. Hazard. Mater. 2012, 223–224, 1–12. [Google Scholar] [CrossRef]
- Schroeder, H.A. The Ce: Role of Chromium in Mammalian Nutrition. Am. J. Clin. Nutr. 1968, 21, 230–244. [Google Scholar] [CrossRef]
- Chen, Y.; Qian, Y.; Ma, J.; Mao, M.; Qian, L.; An, D. New Insights into the Cooperative Adsorption Behavior of Cr(VI) and Humic Acid in Water by Powdered Activated Carbon. Sci. Total Environ. 2022, 817, 153081. [Google Scholar] [CrossRef]
- Hansen, M.B.; Johansen, J.D.; Menné, T. Chromium Allergy: Significance of Both Cr(III) and Cr(VI). Contact Dermat. 2003, 49, 206–212. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, M.; Cui, Y.-L.; Zhao, L.; Liu, S. Novel Reduction of Cr(VI) from Wastewater Using a Naturally Derived Microcapsule Loaded with Rutin–Cr(III) Complex. J. Hazard. Mater. 2015, 285, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Y.; Liu, S.; Yin, Y.; Zeng, G.; Tan, X.; Hu, X.; Hu, X.; Jiang, L.; Ding, Y.; et al. Investigation of the Adsorption-Reduction Mechanisms of Hexavalent Chromium by Ramie Biochars of Different Pyrolytic Temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.; Sahu, J.N.; Sahoo, B.K.; Mohanty, C.R.; Meikap, B.C. Removal of Chromium(VI) from Wastewater by Activated Carbon Developed from Tamarind Wood Activated with Zinc Chloride. Chem. Eng. J. 2009, 150, 25–39. [Google Scholar] [CrossRef]
- Attia, A.A.; Khedr, S.A.; Elkholy, S.A. Adsorption of Chromium Ion (VI) by Acid Activated Carbon. Braz. J. Chem. Eng. 2010, 27, 183–193. [Google Scholar] [CrossRef]
- Jiang, B.; Gong, Y.; Gao, J.; Sun, T.; Liu, Y.; Oturan, N.; Oturan, M.A. The Reduction of Cr(VI) to Cr(III) Mediated by Environmentally Relevant Carboxylic Acids: State-of-the-Art and Perspectives. J. Hazard. Mater. 2019, 365, 205–226. [Google Scholar] [CrossRef]
- Liu, X.; Chu, G.; Du, Y.; Li, J.; Si, Y. The Role of Electron Shuttle Enhances Fe(III)-Mediated Reduction of Cr(VI) by Shewanella Oneidensis MR-1. World J. Microbiol. Biotechnol. 2019, 35, 64. [Google Scholar] [CrossRef]
- Liu, F.; Hua, S.; Wang, C.; Qiu, M.; Jin, L.; Hu, B. Adsorption and Reduction of Cr(VI) from Aqueous Solution Using Cost-Effective Caffeic Acid Functionalized Corn Starch. Chemosphere 2021, 279, 130539. [Google Scholar] [CrossRef]
- Yang, H.; Kim, N.; Park, D. Ecotoxicity Study of Reduced-Cr(III) Generated by Cr(VI) Biosorption. Chemosphere 2023, 332, 138825. [Google Scholar] [CrossRef]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Wang, C.; Liu, Y. Ta3N5/CdS Core–Shell S-Scheme Heterojunction Nanofibers for Efficient Photocatalytic Removal of Antibiotic Tetracycline and Cr(VI): Performance and Mechanism Insights. Adv. Fiber Mater. 2023, 5, 994–1007. [Google Scholar] [CrossRef]
- Wen, X.-J.; Shen, C.-H.; Fei, Z.-H.; Fang, D.; Liu, Z.-T.; Dai, J.-T.; Niu, C.-G. Recent Developments on AgI Based Heterojunction Photocatalytic Systems in Photocatalytic Application. Chem. Eng. J. 2020, 383, 123083. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Yan, R.; Chen, X. Constructing Cd0.5Zn0.5S/Bi2WO6 S-Scheme Heterojunction for Boosted Photocatalytic Antibiotic Oxidation and Cr(VI) Reduction. Adv. Powder Mater. 2023, 2, 100073. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Fabrication of Bi2MoO6/ZnO Hierarchical Heterostructures with Enhanced Visible-Light Photocatalytic Activity. Appl. Catal. B Environ. 2019, 250, 313–324. [Google Scholar] [CrossRef]
- Meng, A.; Zhu, B.; Zhong, B.; Zhang, L.; Cheng, B. Direct Z-Scheme TiO2/CdS Hierarchical Photocatalyst for Enhanced Photocatalytic H2-Production Activity. Appl. Surf. Sci. 2017, 422, 518–527. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, W.; Cao, S.; Piao, L. Recent Progress for Hydrogen Production by Photocatalytic Natural or Simulated Seawater Splitting. Nano Res. 2020, 13, 2313–2322. [Google Scholar] [CrossRef]
- Liu, X.; Chen, T.; Xue, Y.; Fan, J.; Shen, S.; Hossain, M.S.A.; Amin, M.A.; Pan, L.; Xu, X.; Yamauchi, Y. Nanoarchitectonics of MXene/Semiconductor Heterojunctions toward Artificial Photosynthesis via Photocatalytic CO2 Reduction. Coord. Chem. Rev. 2022, 459, 214440. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, Y.; Liu, Y.; Cai, M.; Zhao, W.; Duan, X. S-Scheme MIL-101(Fe) Octahedrons Modified Bi2WO6 Microspheres for Photocatalytic Decontamination of Cr(VI) and Tetracycline Hydrochloride: Synergistic Insights, Reaction Pathways, and Toxicity Analysis. Chem. Eng. J. 2023, 455, 140943. [Google Scholar] [CrossRef]
- He, R.; Wang, Z.; Deng, F.; Li, X.; Peng, Y.; Deng, Y.; Zou, J.; Luo, X.; Liu, X. Tunable Bi-Bridge S-Scheme Bi2S3/BiOBr Heterojunction with Oxygen Vacancy and SPR Effect for Efficient Photocatalytic Reduction of Cr(VI) and Industrial Electroplating Wastewater Treatment. Sep. Purif. Technol. 2023, 311, 123176. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; He, D.; Situ, Y.; Huang, H. Construction of Heterostructured G-C3N4/Ag/TiO2 Microspheres with Enhanced Photocatalysis Performance under Visible-Light Irradiation. ACS Appl. Mater. Interfaces 2014, 6, 14405–14414. [Google Scholar] [CrossRef]
- Han, Q.; Hu, C.; Zhao, F.; Zhang, Z.; Chen, N.; Qu, L. One-Step Preparation of Iodine-Doped Graphitic Carbon Nitride Nanosheets as Efficient Photocatalysts for Visible Light Water Splitting. J. Mater. Chem. A 2015, 3, 4612–4619. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Chougule, S.S.; Vidyasagar, D.; Bak, N.; Jung, N.; Kim, Y.-H.; Lee, J.-H.; Kim, S.-G.; Kim, M.-D. UV Light Driven High-Performance Room Temperature Surface Acoustic Wave NH3 Gas Sensor Using Sulfur-Doped g-C3N4 Quantum Dots. Nano Res. 2023, 16, 7682–7695. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, Z.; Liu, P.; Antonietti, M.; Fu, X.; Wang, X. Metal-Free Activation of H2O2 by g-C3N4 under Visible Light Irradiation for the Degradation of Organic Pollutants. Phys. Chem. Chem. Phys. 2012, 14, 1455–1462. [Google Scholar] [CrossRef]
- Preeyanghaa, M.; Vinesh, V.; Sabarikirishwaran, P.; Rajkamal, A.; Ashokkumar, M.; Neppolian, B. Investigating the Role of Ultrasound in Improving the Photocatalytic Ability of CQD Decorated Boron-Doped g-C3N4 for Tetracycline Degradation and First-Principles Study of Nitrogen-Vacancy Formation. Carbon 2022, 192, 405–417. [Google Scholar] [CrossRef]
- Yu, H.; Ma, Q.; Gao, C.; Liao, S.; Zhang, Y.; Quan, H.; Zhai, R. Petal-like g-C3N4 Enhances the Photocatalyst Removal of Hexavalent Chromium. Catalysts 2023, 13, 641. [Google Scholar] [CrossRef]
- Mo, F.; Liu, Y.; Xu, Y.; He, Q.; Sun, P.; Dong, X. Photocatalytic Elimination of Moxifloxacin by Two-Dimensional Graphitic Carbon Nitride Nanosheets: Enhanced Activity, Degradation Mechanism and Potential Practical Application. Sep. Purif. Technol. 2022, 292, 121067. [Google Scholar] [CrossRef]
- Sun, H.; Guo, F.; Pan, J.; Huang, W.; Wang, K.; Shi, W. One-Pot Thermal Polymerization Route to Prepare N-Deficient Modified g-C3N4 for the Degradation of Tetracycline by the Synergistic Effect of Photocatalysis and Persulfate-Based Advanced Oxidation Process. Chem. Eng. J. 2021, 406, 126844. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A Critical Review on Graphitic Carbon Nitride (g-C3N4)-Based Materials: Preparation, Modification and Environmental Application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Cheng, C.; Dong, C.-L.; Shi, J.; Mao, L.; Huang, Y.-C.; Kang, X.; Zong, S.; Shen, S. Regulation on Polymerization Degree and Surface Feature in Graphitic Carbon Nitride towards Efficient Photocatalytic H2 Evolution under Visible-Light Irradiation. J. Mater. Sci. Technol. 2022, 98, 160–168. [Google Scholar] [CrossRef]
- Song, X.; Wang, M.; Liu, W.; Li, X.; Zhu, Z.; Huo, P.; Yan, Y. Thickness Regulation of Graphitic Carbon Nitride and Its Influence on the Photocatalytic Performance towards CO2 Reduction. Appl. Surf. Sci. 2022, 577, 151810. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z. Effect of Oxygen-Doped C3N4 on the Separation Capability of the Photoinduced Electron-Hole Pairs Generated by O-C3N4@TiO2 with Quasi-Shell-Core Nanostructure. Electrochim. Acta 2014, 144, 42–49. [Google Scholar] [CrossRef]
- Cao, L.; Wang, R.; Wang, D. Synthesis and Characterization of Sulfur Self-Doped g-C3N4 with Efficient Visible-Light Photocatalytic Activity. Mater. Lett. 2015, 149, 50–53. [Google Scholar] [CrossRef]
- Ranjbakhsh, E.; Izadyar, M.; Nakhaeipour, A.; Habibi-Yangjeh, A. Quantum Chemistry Calculations of S, P, and O-Doping Effect on the Photocatalytic Molecular Descriptors of g-C3N4 Quantum Dots. J. Iran. Chem. Soc. 2022, 19, 3513–3528. [Google Scholar] [CrossRef]
- Li, K.; Huang, Z.; Zeng, X.; Huang, B.; Gao, S.; Lu, J. Synergetic Effect of Ti 3+ and Oxygen Doping on Enhancing Photoelectrochemical and Photocatalytic Properties of TiO2/g-C3N4 Heterojunctions. ACS Appl. Mater. Interfaces 2017, 9, 11577–11586. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yan, Q.; Yan, T.; Li, M.; Wei, D.; Wang, Z.; Wei, Q.; Du, B. Facile Fabrication of 3D Flower-like Heterostructured g-C3 N4/SnS2 Composite with Efficient Photocatalytic Activity under Visible Light. RSC Adv. 2014, 4, 31019–31027. [Google Scholar] [CrossRef]
- Wang, X.; Yang, W.; Li, F.; Xue, Y.; Liu, R.; Hao, Y. In Situ Microwave-Assisted Synthesis of Porous N-TiO2/g-C3N4 Heterojunctions with Enhanced Visible-Light Photocatalytic Properties. Ind. Eng. Chem. Res. 2013, 52, 17140–17150. [Google Scholar] [CrossRef]
- Li, B.; Fang, Q.; Si, Y.; Huang, T.; Huang, W.-Q.; Hu, W.; Pan, A.; Fan, X.; Huang, G.-F. Ultra-Thin Tubular Graphitic Carbon Nitride-Carbon Dot Lateral Heterostructures: One-Step Synthesis and Highly Efficient Catalytic Hydrogen Generation. Chem. Eng. J. 2020, 397, 125470. [Google Scholar] [CrossRef]
- Rajendran, R.; Vignesh, S.; Raj, V.; Palanivel, B.; Ali, A.M.; Sayed, M.A.; Shkir, M. Designing of TiO2/α-Fe2O3 Coupled g-C3N4 Magnetic Heterostructure Composite for Efficient Z-Scheme Photo-Degradation Process under Visible Light Exposures. J. Alloys Compd. 2022, 894, 162498. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; ALOthman, Z.A.; Iqbal, J.; Bathula, C. High Interfacial Charge Separation in Visible-Light Active Z- Scheme g-C3N4/MoS2 Heterojunction: Mechanism and Degradation of Sulfasalazine. Chemosphere 2022, 308, 136162. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; Daniel Thangadurai, T.; Manjubaashini, N.; Nataraj, D. Two-Dimensional z-Type MoS2/g-C3N4 Semiconductor Heterojunction Nanocomposites for Industrial Methylene Blue Dye Degradation under Daylight. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130090. [Google Scholar] [CrossRef]
- Sima, L.; Li, D.; Dong, L.; Zhang, F. Facile Preparation of Porous G-C3N4/MoS2 Heterojunction for Hydrogen Production under Simulated Sunlight. Mater. Today Sustain. 2022, 20, 100217. [Google Scholar] [CrossRef]
- Lyu, H.; Zhu, W.; Chen, K.; Gao, J.; Xie, Z. 3D Flower-Shaped g-C3N4/MoS2 Composite with Structure Defect for Synergistic Degradation of Dyes. J. Water Process Eng. 2024, 57, 104656. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, L.; Tang, Y.; Zhou, J.; Shi, B. Novel G-C3N4/C/Fe2O3 Composite for Efficient Photocatalytic Reduction of Aqueous Cr(VI) under Light Irradiation. Ind. Eng. Chem. Res. 2021, 60, 13594–13603. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Liu, E.; Fan, J. Novel S-Scheme 2D/2D BiOBr/g-C3N4 Heterojunctions with Enhanced Photocatalytic Activity. Chin. J. Catal. 2021, 42, 1519–1529. [Google Scholar] [CrossRef]
- Wu, Z.; He, X.; Xue, Y.; Yang, X.; Li, Y.; Li, Q.; Yu, B. Cyclodextrins Grafted MoS2/g-C3N4 as High-Performance Photocatalysts for the Removal of Glyphosate and Cr (VI) from Simulated Agricultural Runoff. Chem. Eng. J. 2020, 399, 125747. [Google Scholar] [CrossRef]
- Xue, Y.; Ji, Y.; Wang, X.; Wang, H.; Chen, X.; Zhang, X.; Tian, J. Heterostructuring Noble-Metal-Free 1T’ Phase MoS2 with g-C3N4 Hollow Nanocages to Improve the Photocatalytic H2 Evolution Activity. Green Energy Environ. 2023, 8, 864–873. [Google Scholar] [CrossRef]
- Bashal, A.H.; Alkanad, K.; Al-Ghorbani, M.; Ben Aoun, S.; Bajiri, M.A. Synergistic Effect of Cocatalyst and S-Scheme Heterojunction over 2D/2D g-C3N4/MoS2 Heterostructure Coupled Cu Nanoparticles for Selective Photocatalytic CO2 Reduction to CO under Visible Light Irradiation. J. Environ. Chem. Eng. 2023, 11, 109545. [Google Scholar] [CrossRef]
- Yuan, H.; Fang, F.; Dong, J.; Xia, W.; Zeng, X.; Shangguan, W. Enhanced Photocatalytic Hydrogen Production Based on Laminated MoS2/g-C3N4 Photocatalysts. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128575. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Vidyasagar, D.; Ambadi, L.N.; Bak, N.; Kim, S.-G.; Kim, M.-D. UV Light Activated G-C3N4 Nanoribbons Coated Surface Acoustic Wave Sensor for High Performance Sub-Ppb Level NO2 Detection at Room Temperature. Sens. Actuators B Chem. 2023, 394, 134471. [Google Scholar] [CrossRef]
- GB 7467-87; Water Quality-Determination of Chromiun(VI)-1,5 Dtphenylcarbohydrazide Spectrophotometric Method. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 1987.
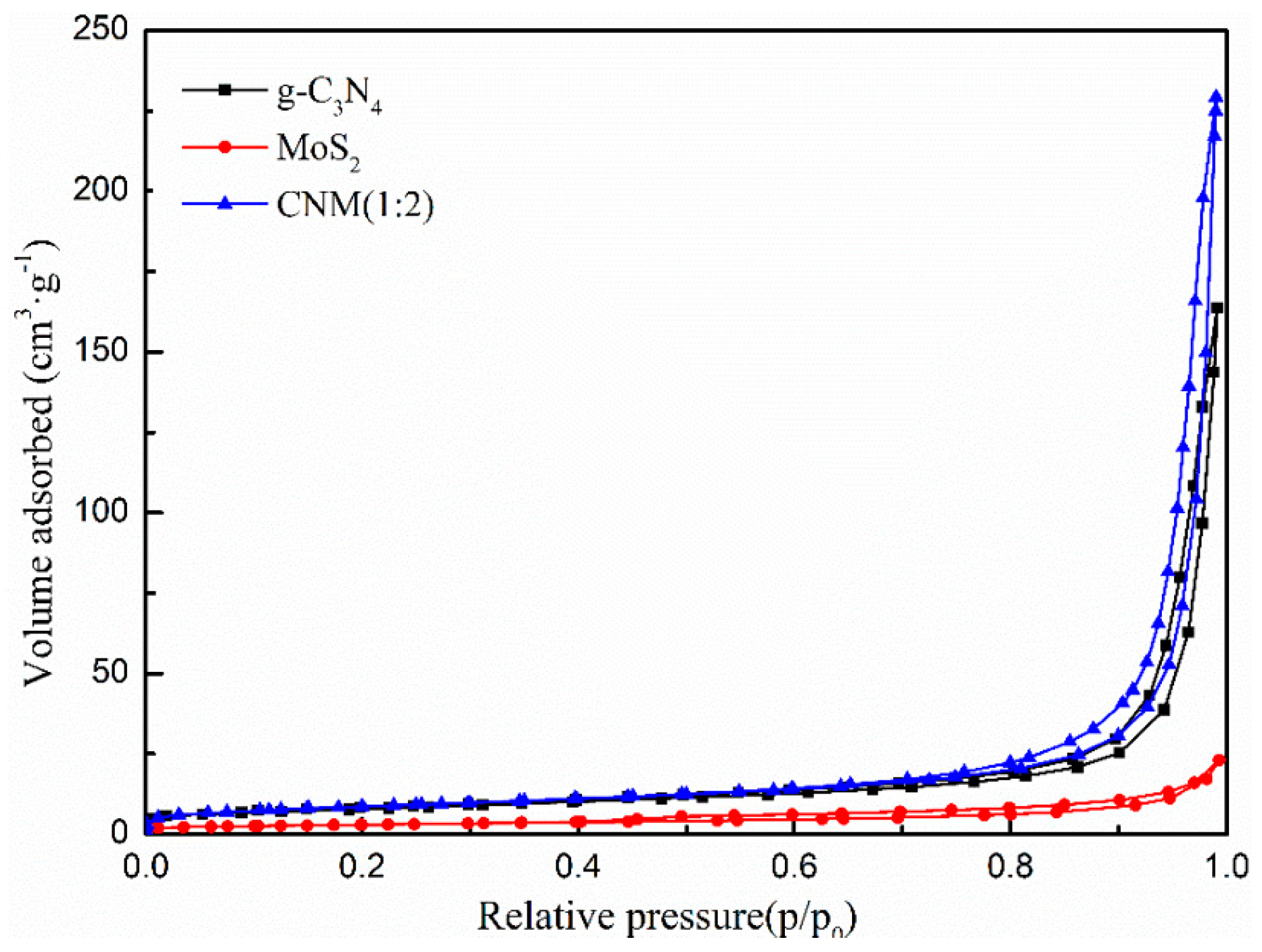




| Samples | SBET (m2·g−1) | VPore (cm3·g−1) |
|---|---|---|
| g-C3N4 | 27.3882 | 0.2385 |
| MoS2 | 10.5045 | 0.0332 |
| CNM (1:2) | 30.7214 | 0.3498 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, C.; Yu, H.; Zhai, R.; Zhang, J.; Gao, C.; Qi, K.; Zhang, Y.; Ma, Q.; Guo, M. Visible Light Photoactivity of g-C3N4/MoS2 Nanocomposites for Water Remediation of Hexavalent Chromium. Molecules 2024, 29, 637. https://doi.org/10.3390/molecules29030637
Tian C, Yu H, Zhai R, Zhang J, Gao C, Qi K, Zhang Y, Ma Q, Guo M. Visible Light Photoactivity of g-C3N4/MoS2 Nanocomposites for Water Remediation of Hexavalent Chromium. Molecules. 2024; 29(3):637. https://doi.org/10.3390/molecules29030637
Chicago/Turabian StyleTian, Chunmei, Huijuan Yu, Ruiqi Zhai, Jing Zhang, Cuiping Gao, Kezhen Qi, Yingjie Zhang, Qiang Ma, and Mengxue Guo. 2024. "Visible Light Photoactivity of g-C3N4/MoS2 Nanocomposites for Water Remediation of Hexavalent Chromium" Molecules 29, no. 3: 637. https://doi.org/10.3390/molecules29030637
APA StyleTian, C., Yu, H., Zhai, R., Zhang, J., Gao, C., Qi, K., Zhang, Y., Ma, Q., & Guo, M. (2024). Visible Light Photoactivity of g-C3N4/MoS2 Nanocomposites for Water Remediation of Hexavalent Chromium. Molecules, 29(3), 637. https://doi.org/10.3390/molecules29030637






