Dimethyl Derivatives of 2,2′-Bipyridine as Ligands in [W(CN)6(bpy)]2−-Type Complexes
Abstract
1. Introduction
2. Results and Discussion
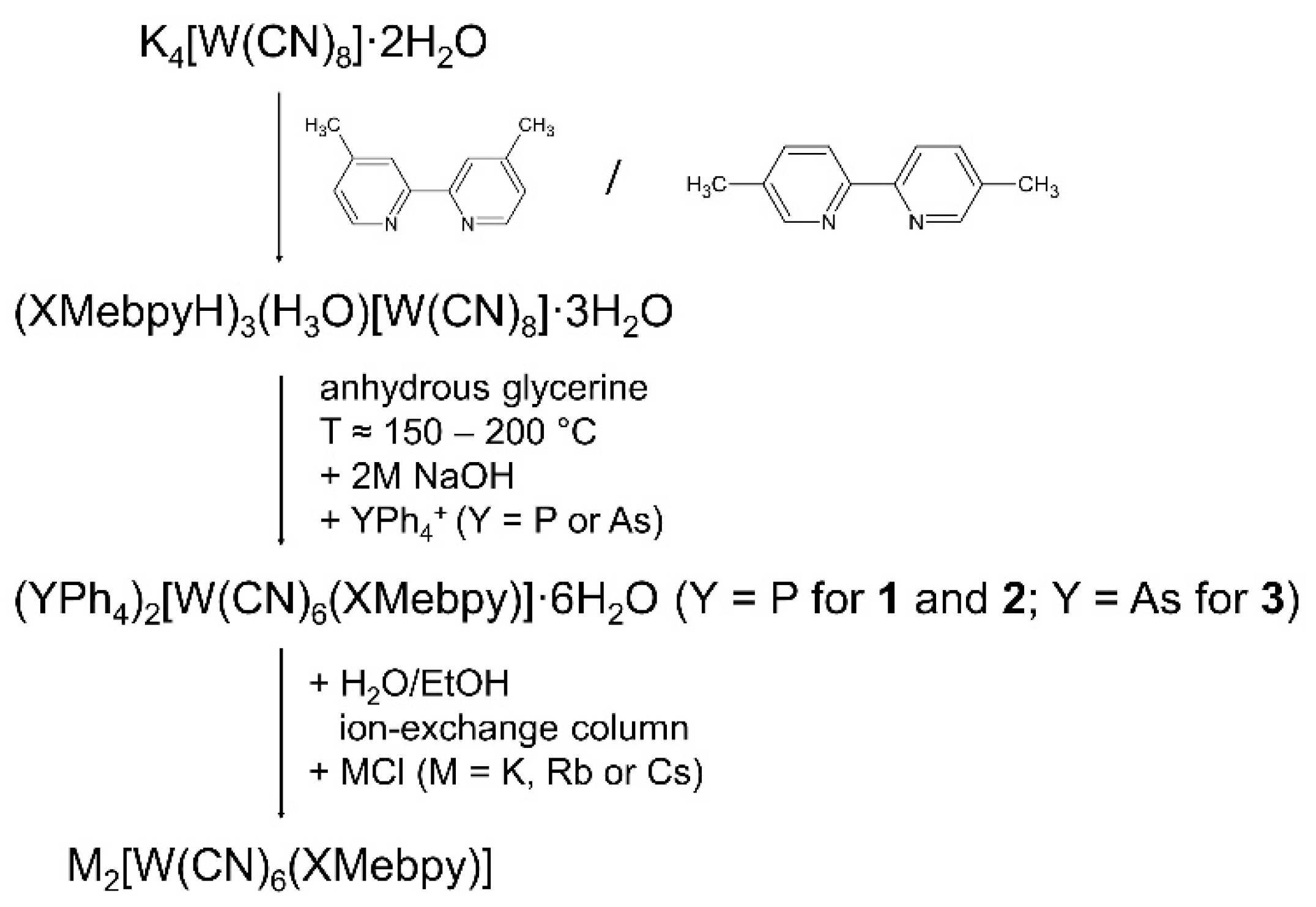
2.1. General Remarks to Syntheses
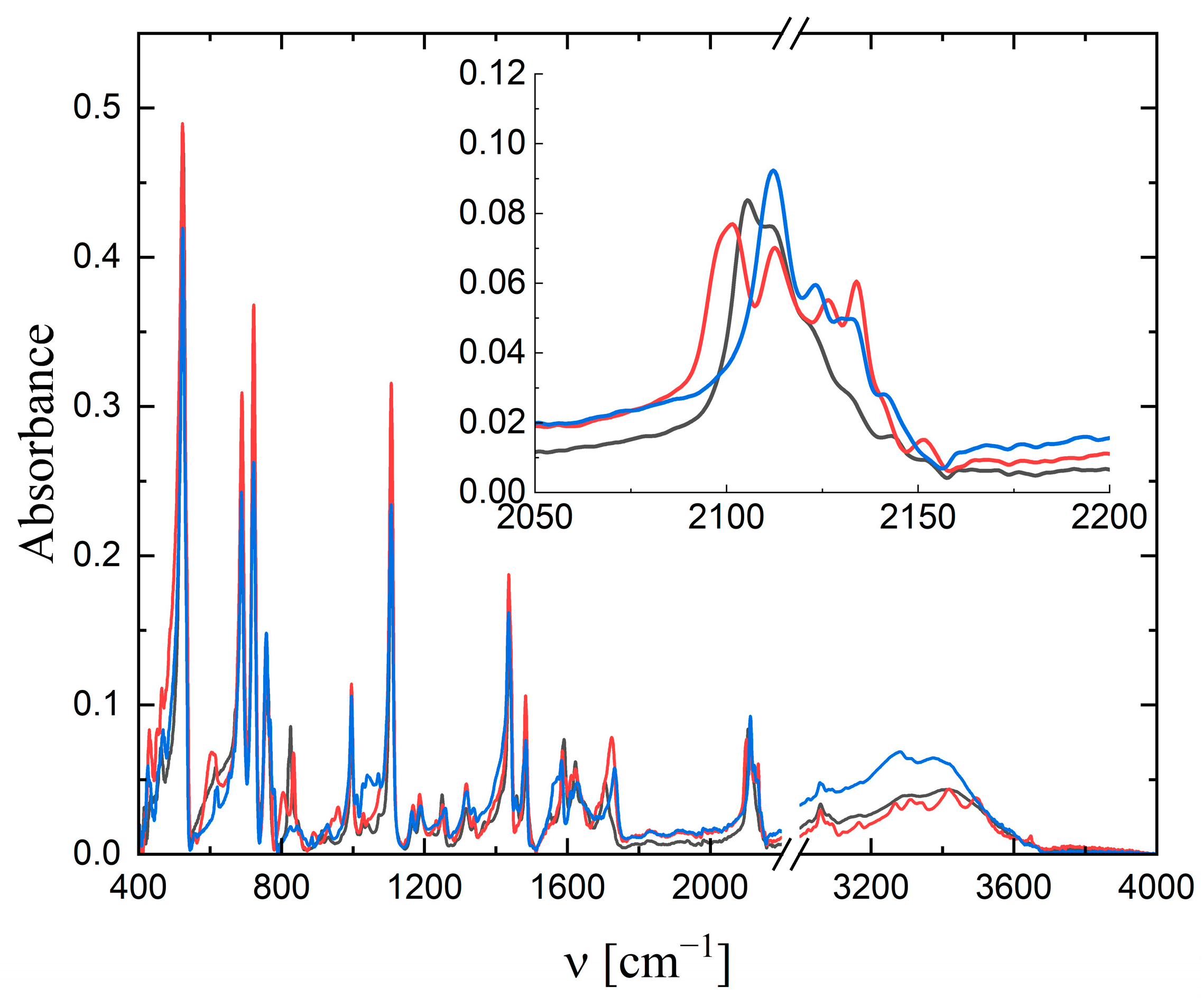
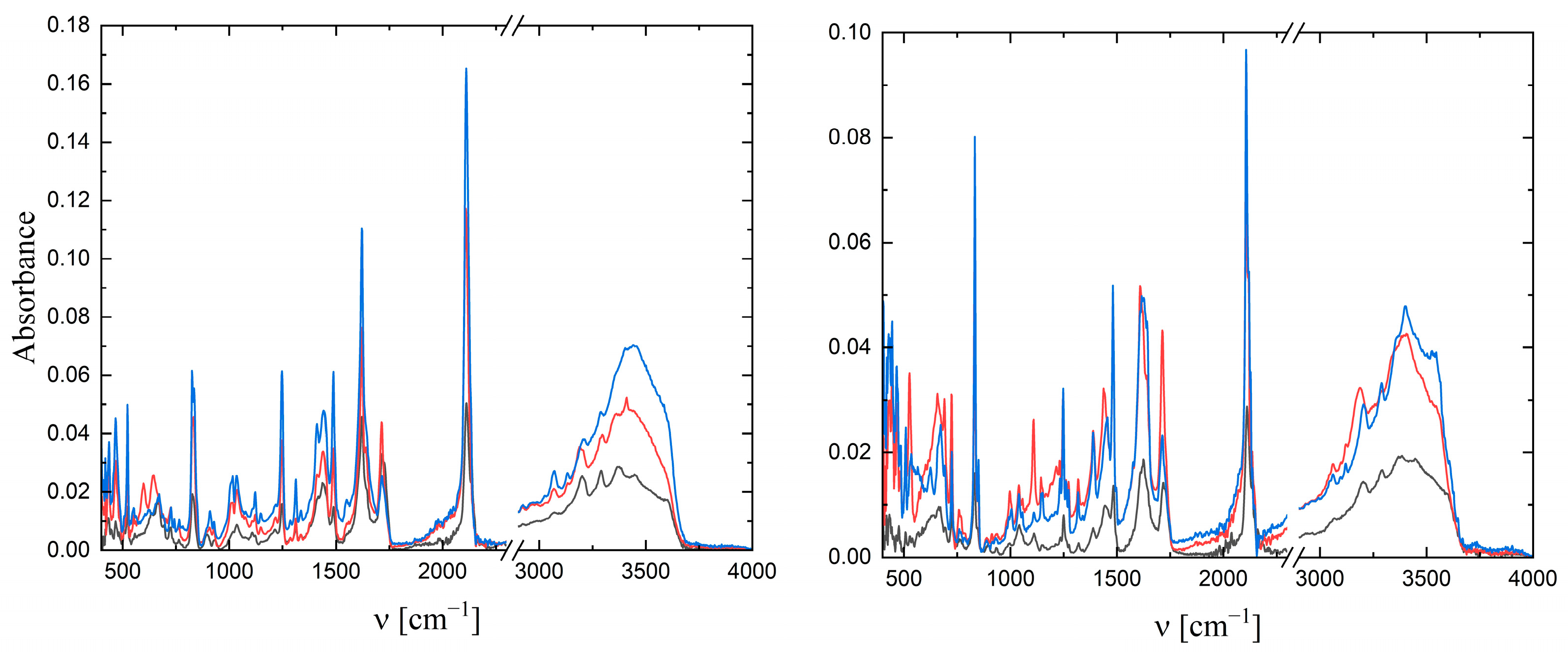
2.2. IR Spectra
2.3. UV-Vis Spectra
2.4. Cyclic Voltammetry Measurements
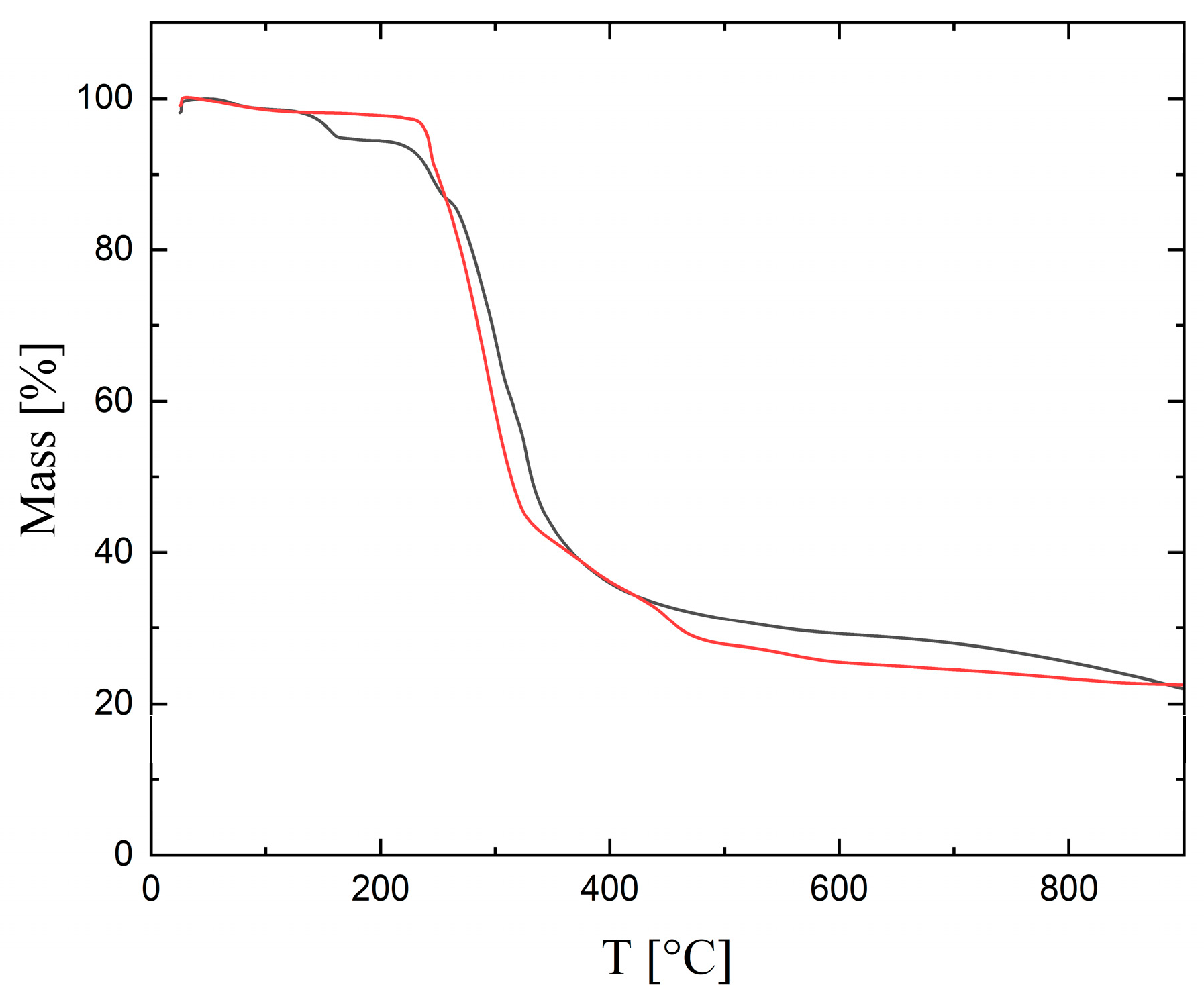
2.5. Thermogravimetric Analysis
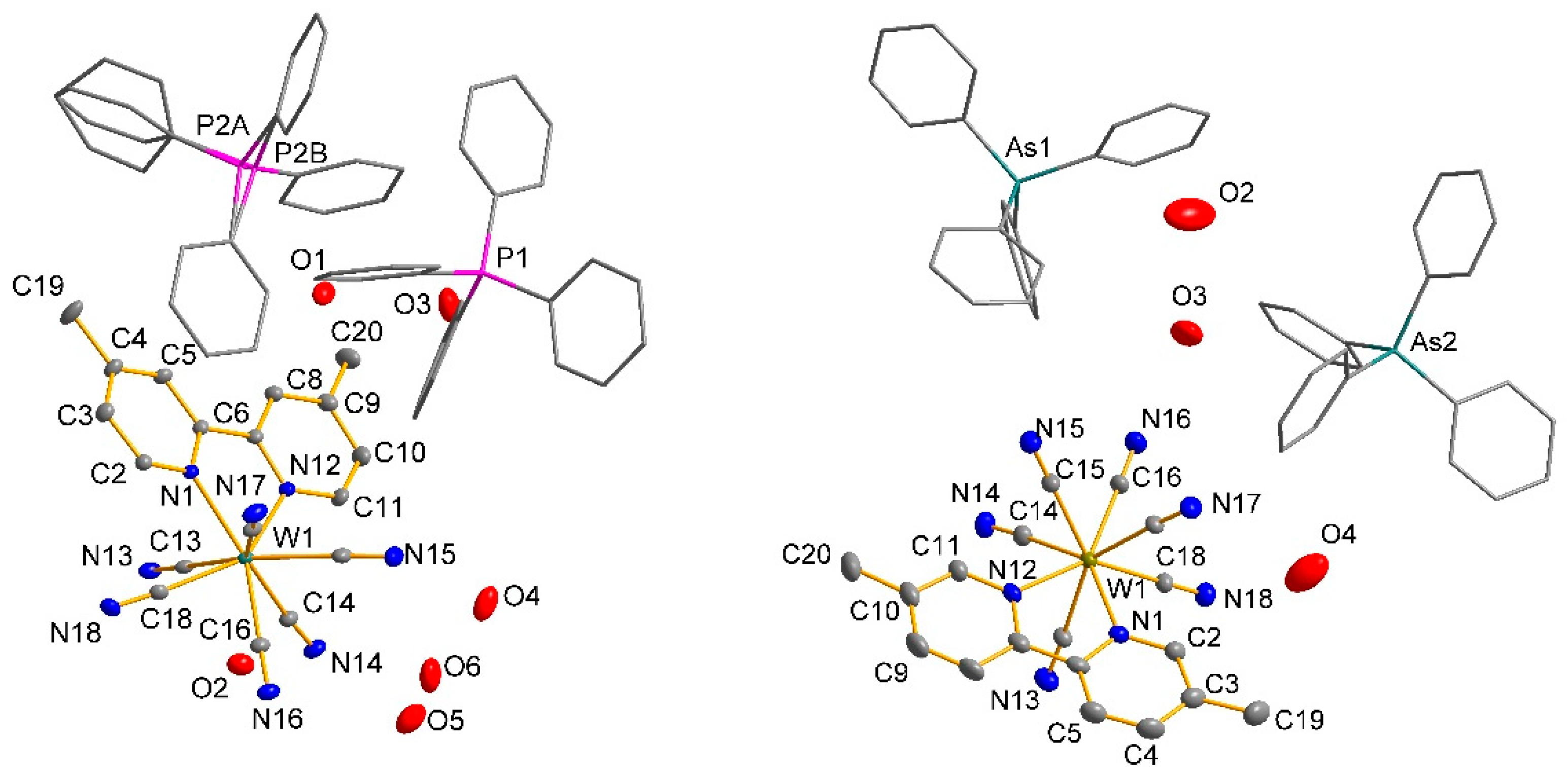
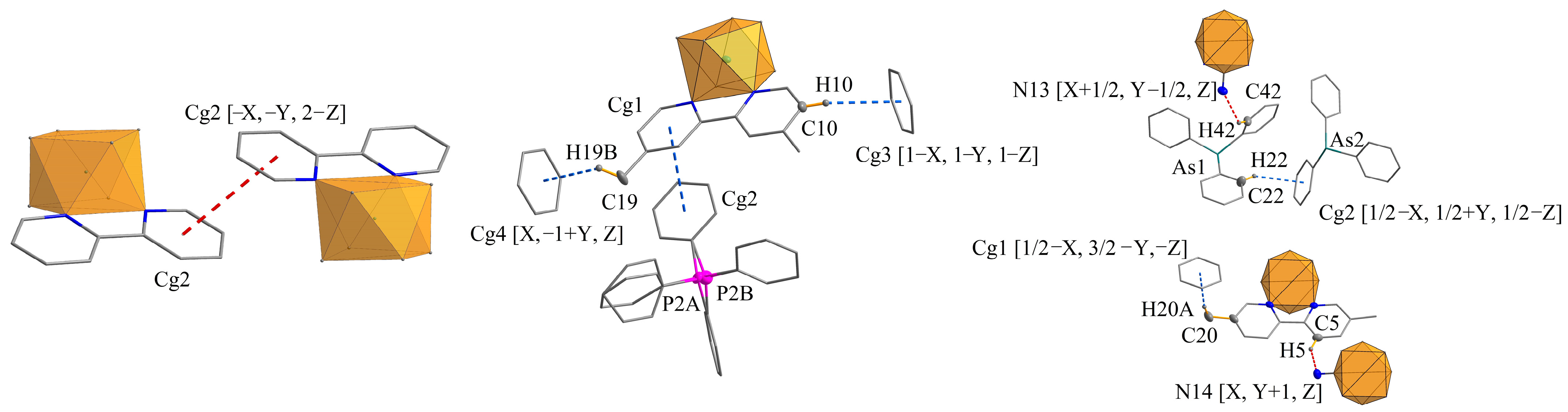
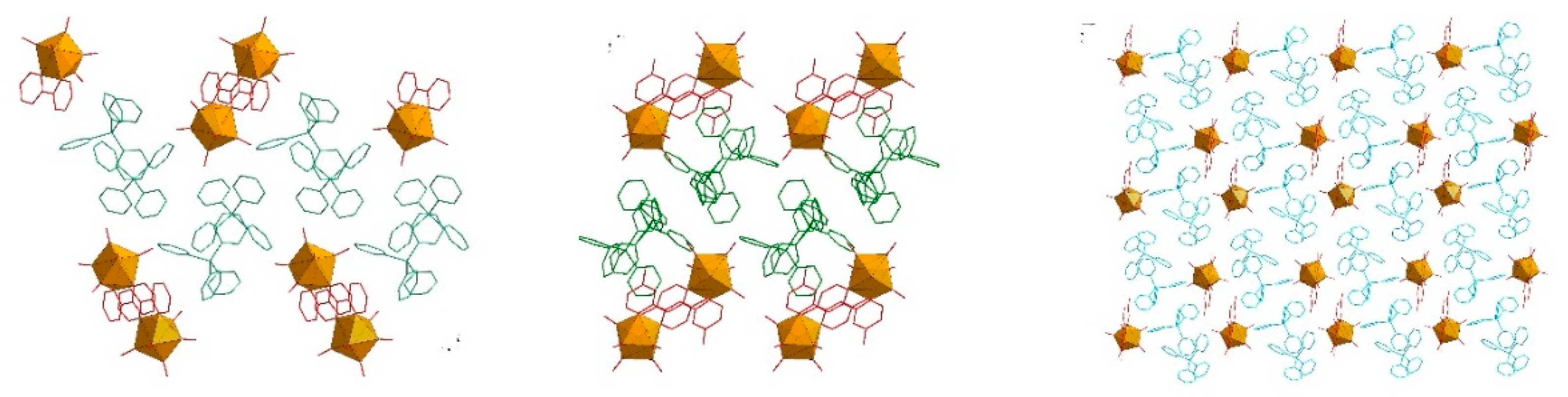
2.6. Crystal Structure of 1 and 3
3. Experimental
3.1. Materials and Methods
3.2. Syntheses
3.2.1. (PPh4)2[W(CN)6(4,4′-Mebpy)]·6H2O (1)
3.2.2. (PPh4)2[W(CN)6(5,5′-Mebpy)]·6H2O (2)
3.2.3. (AsPh4)2[W(CN)6(5,5′-Mebpy)]·5H2O (3)
3.2.4. Syntheses of M2[W(CN)6(XMebpy)] Salts, Where (M = K, Rb, Cs)
3.3. Crystallographic Data Collection and Structure Refinement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Brown, A.J.; Prosvirin, A.V.; Dunbar, K.R. One-dimensional square-and ladder-type architectures incorporating octacyanometallates of molybdenum (V) and tungsten (V). Polyhedron 2013, 64, 321–327. [Google Scholar] [CrossRef]
- Szklarzewicz, J.; Samotus, A. Photochemical reactions of molybdenum and tungsten octacyanometallates (IV) in acidic aqueous media containing 2,2′-bipyridyl or 1,10-phenanthroline. Transit. Met. Chem. 1995, 20, 174–178. [Google Scholar] [CrossRef]
- Semenyshyn, D.; Typilo, I.; Gladyshevskii, R.; Starchevskyy, V. Regularities in the crystal structures of heterocationic octacyanometallates (IV) molybdenum and tungsten. Chem. Chem. Technol. 2013, 7, 369–374. [Google Scholar] [CrossRef]
- Ali, S.; Majid, K. Pyrazine Complexes of Octacyanometallates of Mo (IV) and W (IV) With 8-hydroxyquinoline: Synthesis, characterisation and thermal studies. J. Therm. Anal. Calorim. 1999, 58, 153–165. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, H.; Song, Y.; You, X. Two bimetallic W (IV)-Mn (II) complexes based on octacyanometallates: Structures and magnetic properties. Sci. China Ser. B Chem. 2009, 52, 1801–1807. [Google Scholar] [CrossRef]
- Garde, R.; Desplanches, C.; Bleuzen, A.; Veillet, P.; Verdaguer, M. New Molecule-Based Magnets: From Hexacyano to Octacyanometalates. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 1999, 334, 587–595. [Google Scholar] [CrossRef]
- Hodorowicz, M.; Jurowska, A.; Szklarzewicz, J. X-ray crystal structures of K+ and Rb+ salts of [W(CN)6(bpy)]2− ion. The unusual cation-anion interactions and structure changes going from Li+ to Cs+ salts. CrystEngComm 2021, 23, 1207–1217. [Google Scholar] [CrossRef]
- Qian, J.; Hu, J.; Yoshikawa, H.; Zhang, J.; Awaga, K.; Zhang, C. External-Template-Assisted Formation of Octacyanometalate-Based MV–MnII (M= W, Mo) Bimetallic Coordination Polymers with Magnetic Properties. Eur. J. Inorg. Chem. 2015, 12, 2110–2119. [Google Scholar] [CrossRef]
- Bogdanov, M.; Gryboś, R.; Samotus, A.; Bogolitsyn, K. Electron-transfer kinetics and mechanism of the reduction of octacyanometallates (IV)(M = Mo, W) by hydroxide ion in aqueous solution. Transit. Met. Chem. 1993, 18, 599–603. [Google Scholar] [CrossRef]
- Dhers, S.; Feltham, H.L.; Clérac, R.; Brooker, S. Design of One-Dimensional Coordination Networks from a Macrocyclic {3d-4f} Single-Molecule Magnet Precursor Linked by [W(CN)8]3− Anions. Inorg. Chem. 2013, 52, 13685–13691. [Google Scholar] [CrossRef]
- Rombaut, G.; Mathonière, C.; Guionneau, P.; Golhen, S.; Ouahab, L.; Verelst, M.; Lecante, P. Structural and photo-induced magnetic properties of MII2[WIV(CN)8]·xH2O (M = Fe and x = 8, Cu and x = 5). Comparison with CuII2[MoIV(CN)8]·7.5 H2O. Inorganica Chim. Acta 2001, 326, 27–36. [Google Scholar] [CrossRef]
- Szklarzewicz, J.; Hodorowicz, M.; Jurowska, A. The complex approach to the synthesis of [W(CN)6(bpy)]2−/− ion complexes. The X-ray crystal structures of reaction products. Inorganica Chim. Acta 2023, 546, 121320. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhang, Y.Z.; Southerland, H.; Prosvirin, A.V.; Zhao, H.; Dunbar, K.R. Variations in topology and magnetic properties of hepta-and octacyanometallates of molybdenum with manganese (ii). Dalton Trans. 2014, 43, 6802–6810. [Google Scholar] [CrossRef]
- Chibotaru, L.F.; Mironov, V.S.; Ceulemans, A. Origin of Ferromagnetism in Cyano-Bridged Compounds Containing d1 Octacyanometallates. Angew. Chem. 2001, 113, 4561–4565. [Google Scholar] [CrossRef]
- Takahashi, D.; Nakabayashi, K.; Tanaka, S.; Ohkoshi, S.I. Two-dimensional octacyano-bridged Mn (II)–Nb (IV) bimetal assembly with four different configurations of 3-hydroxypyridines. Inorg. Chem. Commun. 2013, 27, 47–50. [Google Scholar] [CrossRef]
- Akagi, S.; Wang, J.; Imoto, K.; Ohkoshi, S.I.; Tokoro, H. Spin-Flop Transition in a Nickel–Octacyanidotungstate Chain Magnet. Cryst. Growth Des. 2023, 23, 1972–1979. [Google Scholar] [CrossRef]
- Sellin, M.; Rupf, S.M.; Abram, U.; Malischewski, M. Eightfold Electrophilic Methylation of Octacyanotungstate [W(CN)8]4–/3–: Preparation of Homoleptic, Eight-Coordinate Methyl Isocyanide Complexes [W(CNMe)8]4+/5+. Inorg. Chem. 2021, 60, 5917–5924. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Wang, X.; Yang, L.; Si, W.; Zhuang, S.; Liu, H.; Liu, Q.; Zhang, D. Cyanide-bridged polynuclear heterobimetallic complexes: Synthesis, crystal structures, and magnetic properties. Transit. Met. Chem. 2019, 44, 383–389. [Google Scholar] [CrossRef]
- Herrera, J.M.; Bleuzen, A.; Dromzée, Y.; Julve, M.; Lloret, F.; Verdaguer, M. Crystal structures and magnetic properties of two octacyanotungstate (IV) and (V)-cobalt (II) three-dimensional bimetallic frameworks. Inorg. Chem. 2003, 42, 7052–7059. [Google Scholar] [CrossRef]
- Qian, J.; Yoshikawa, H.; Hu, J.; Humphrey, M.G.; Zhang, J.; Awaga, K.; Zhang, C. Auxiliary ligand-induced structural diversities of octacyanometalate-based heterobimetallic coordination polymers towards diverse magnetic properties. Dalton Trans. 2019, 48, 7666–7676. [Google Scholar] [CrossRef]
- Jurowska, A.; Hodorowicz, M.; Kruczała, K.; Szklarzewicz, J. Anion-cation interactions in a series of salts with substituted Hphen, Hbpy and H2bpy cations and [W(CN)8]4- anion: Polymer with a “super-short” N-H···N hydrogen bridges containing exclusively anions and H+. Dalton Trans. 2021, 5, 17981–17987. [Google Scholar] [CrossRef] [PubMed]
- Szklarzewicz, J.; Samotus, A. A novel cyano complex of tungsten(IV) with 2,2’-bipyridyl. Transit. Met. Chem. 1988, 13, 69–71. [Google Scholar] [CrossRef]
- Reichardt, C. Losungsmitteleffekte in der Organischen Chemie; Verlag Chemie: Weinheim, Germany, 1968. [Google Scholar]
- Reichardt, C.; Harbusch-Gornert, E. Über Pyridinium-N-phenolat-Betaine und ihre Verwendung zur Charakterisierung der Polarität von Lösungsmitteln, X. Erweiterung, Korrektur und Neudefinition der ET-Lösungsmittelpolaritätsskala mit Hilfe eines lipophilen penta-tert-butyl-substituierten Pyridinium-N-phenolat-Betainfarbstoffes. Liebigs Ann. Chem. 1983, 1983, 721–743. [Google Scholar]
- Al-Alousy, A.; Burgess, J.; Samotus, A.; Szklarzewicz, J. New solvatochromic inorganic complexes. Spectrochim. Acta Part A Mol. Spectrosc. 1991, 47, 985–989. [Google Scholar] [CrossRef]
- Szklarzewicz, J. New cyano complex of W(V), (AsPh4)[W(bpy)(CN)6]: Reversible redox system W(bpy)(CN)6−/W(bpy)(CN)62−. Inorganica Chim. Acta 1993, 205, 85–89. [Google Scholar] [CrossRef]
- Szklarzewicz, J.; Podgajny, R.; Lewinski, K.; Sieklucka, B. Basket weave-like 2-D coordination polymer generated by the self-assembly of [Mn(H2O)6]2+ and geometrically anisotropic [W(CN)6bpy]2- precursors. CrystEngComm 2002, 4, 199–201. [Google Scholar] [CrossRef]
- Szklarzewicz, J. Synthesis and X-ray crystal structures of Cs2[W(bpy)(CN)6]·2H2O and (AsPh4)2[W(bpy)(CN)6]·3,5H2O. Transit. Met. Chem. 2004, 29, 56–60. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlis PRO; Rigaku Oxford Diffraction: Yarnton, UK, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELX2017, Programs for Crystal Structure Determination; Universität Göttingen: Göttingen, Germany, 2017. [Google Scholar]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Diamond—Crystal and Molecular Structure Visualization Crystal Impact; GbR: Bonn, Germany, 2023. [Google Scholar]
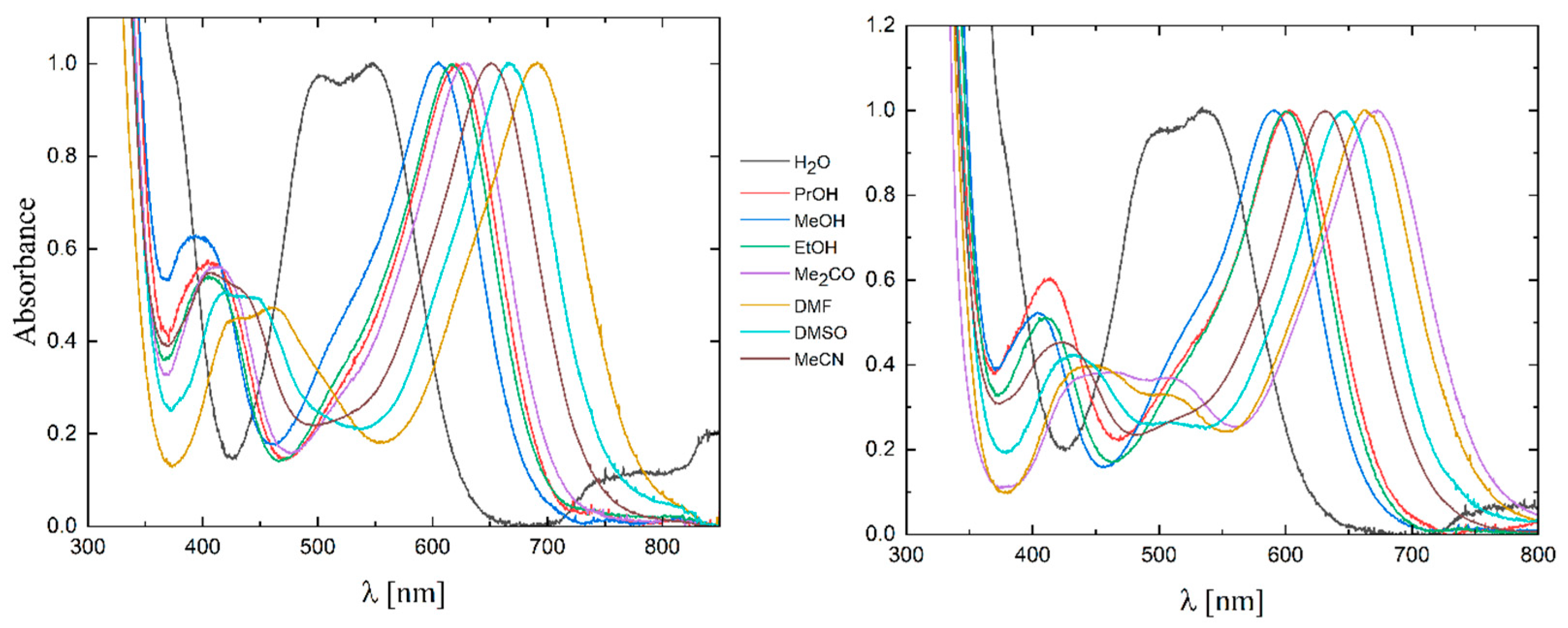
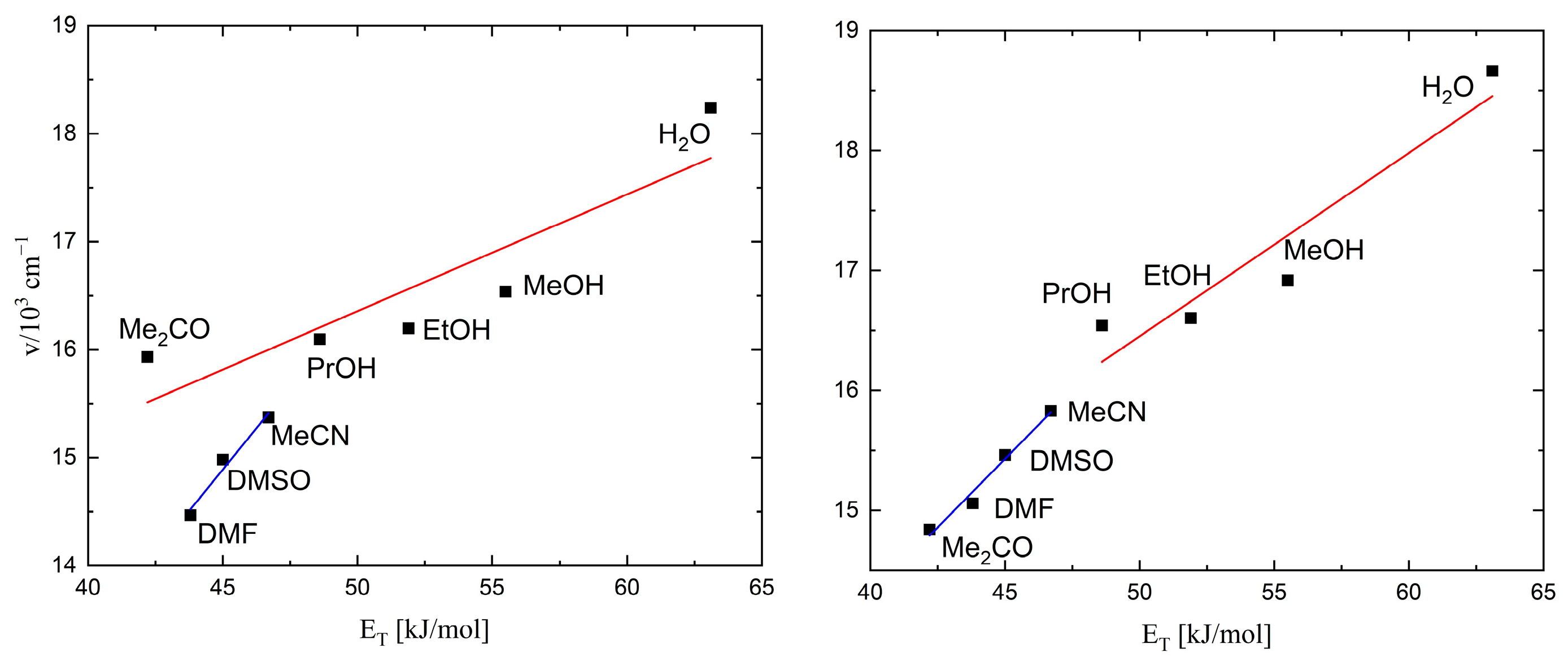


| Solvent | Reichardt Parameter ET (kJ/mol) | Solution Color | Wavelength (nm) | Solution Color | Wavelength (nm) |
|---|---|---|---|---|---|
| 1 | 2 | ||||
| H2O | 63.1 | Violet | 548 | Violet | 536 |
| PrOH | 48.6 | Blue | 621 | Blue | 605 |
| MeOH | 55.5 | Blue | 605 | Blue | 591 |
| EtOH | 51.9 | Blue | 617 | Blue | 602 |
| Me2CO | 42.2 | Green | 628 | Green | 674 |
| DMF | 43.8 | Green | 691 | Green | 664 |
| DMSO | 45.0 | Green | 667 | Green | 647 |
| MeCN | 46.7 | Green | 651 | Green | 632 |
| 1 (4,4′-Mebpy) | 3 (5,5′-Mebpy) | ||
|---|---|---|---|
| Empirical formula | C66H56N8O6P2W | C132H104As4N16O5W2 | |
| Formula weight | 1302.97 | 2661.69 | |
| Temperature [K] | 130(2) | 270(2) | |
| Wavelength [Å] | 0.71073 | 1.54184 | |
| Crystal system | Triclinic | Monoclinic | |
| Space group | P | C 2/c | |
| Unit cell dimensions | a (Å) | 13.3722(3) | 30.70410(10) |
| b (Å) | 14.5777(3) | 9.830 | |
| c (Å) | 16.8548(3) | 39.49840(10) | |
| α (°) | 73.537(2) | 90 | |
| β (°) | 88.941(2) | 91.74 | |
| γ (°) | 77.430(2) | 90 | |
| Volume (Ǻ3) | 3072.11(11) | 11,915.93(5) | |
| Z | 2 | 4 | |
| Density (calculated) (g/cm3) | 1.409 | 1.484 | |
| Absorption coefficient (mm−1) | 1.990 | 5.236 | |
| F(000) | 1320 | 5312 | |
| Crystal size (mm3) | 0.200 × 0.100 × 0.100 | 0.200 × 0.100 × 0.100 | |
| Theta range for data collection (o) | 2.457 to 30.719 | 2.880 to 80.534 | |
| Index ranges | −18 ≤ h ≤ 17 −19 ≤ k ≤ 20 −22 ≤ l ≤ 21 | −39 ≤ h ≤ 39 −12 ≤ k ≤ 10 −50 ≤ l ≤ 50 | |
| Reflections collected | 98,339 | 195,223 | |
| Independent reflections | 16,322 (R(int) = 0.0509) | 12,856 (R(int) = 0.0753) | |
| Completeness to theta | 99.8% | 99.9% | |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | |
| Data/restraints/parameters | 16,322/3/812 | 12,856/0/719 | |
| Goodness-of-fit on F2 | 1.063 | 1.051 | |
| Final R indices (I > 2σ(I)) | R1 = 0.0346, wR2 = 0.0798 | R1 = 0.0324, wR2 = 0.0841 | |
| R indices (all data) | R1 = 0.0407, wR2 = 0.0820 | R1 = 0.0341, wR2 = 0.0852 | |
| Largest diff. peak and hole (e/Ǻ3) | 1.314 and −1.268 | 1.008 and −1.343 | |
| 2,2′-bpyPPh4 [27] | 1 (4,4′-Mebpy) | 2,2′-bpyAsPh4 [28] | 3 (5,5′-Mebpy) | ||||
|---|---|---|---|---|---|---|---|
| Bond | Distance (Å) | Bond | Distance (Å) | Bond | Distance (Å) | Bond | Distance (Å) |
| W(1)-C(69) | 2.147(3) | W(1)-C(14) | 2.149(3) | W(1)-C(3) | 2.139(5) | W(1)-C(14) | 2.147(3) |
| W(1)-C(69) | 2.148(3) | W(1)-C(18) | 2.148(3) | W(1)-C(4) | 2.146(5) | W(1)-C(18) | 2.152(3) |
| W(1)-C(63) | 2.154(3) | W(1)-C(17) | 2.164(3) | W(1)-C(2) | 2.147(5) | W(1)-C(17) | 2.160(3) |
| W(1)-C(73) | 2.159(3) | W(1)-C(16) | 2.153(3) | W(1)-C(6) | 2.155(5) | W(1)-C(16) | 2.160(3) |
| W(1)-C(75) | 2.163(3) | W(1)-C(13) | 2.165(3) | W(1)-C(5) | 2.156(5) | W(1)-C(13) | 2.170(3) |
| W(1)-C(65) | 2.164(3) | W(1)-C(15) | 2.160(3) | W(1)-C(1) | 2.157(5) | W(1)-C(15) | 2.177(3) |
| W(1)-N(48) | 2.215(2) | W(1)-N(12) | 2.224(2) | W(1)-N(8) | 2.206(4) | W(1)-N(12) | 2.225(2) |
| W(1)-N(59) | 2.225(2) | W(1)-N(1) | 2.215(2) | W(1)-N(7) | 2.208(4) | W(1)-N(1) | 2.237(2) |
| π…π | d(π…π) | Shift | Description | |
|---|---|---|---|---|
| 2,2′-bpyPPh4 | Cg1…Cg1 [2 − X, 1 − Y, 2 − Z] | 3.893(2) | 1.349 | Cg1: C91-C92-C93-C94-C95-C96 (PPh4+ cation) Cg2: N7-C8-C9-C10-C11-C12 (bpy ligand) |
| Cg2…Cg2 [−X, −Y, 2 − Z] | 3.8976(18) | 1.889 | ||
| 1 | Cg1…Cg2 | 3.8875(1) | 1.106 | Cg1: N1-C2-C3-C4-C5-C6 (bpy ligand) Cg2: C45-C46-C47-C48-C49-C50 (PPh4+ cation) |
| 2,2′-bpyAsPh4 | Cg1…Cg1 [1 − X, −Y, 1 − Z] | 3.806(3) | 1.274 | Cg1: C29-C30-C31-C32-C33-C34 (AsPh4+ cation) |
| Cg2…Cg2 [2 − X, −Y, 2 − Z] | 3.965(3) | 1.620 | Cg2: N7-C7-C8-C9-C10- C11 (bpy ligand) | |
| Cg3…Cg3 [1 − X, −Y, 2 − Z] | 3.806(3) | 1.800 | Cg3: N8-C12-C13-C14-C15- C16 (bpy ligand) | |
| 3 | no π⋯π-type interactions | |||
| C-H…π | H…Cg | X-H···Cg | X···Cg | Description | |
|---|---|---|---|---|---|
| 2,2′-bpyPPh4 | C94-H94…Cg3 [2 − X, 1 − Y, 2 − Z] | 2.96 | 158 | 3.840(5) | Cg3: C71-C72-C73-C74-C75-C76 (PPh4+ cation) |
| 1 | C10-H10…Cg3 [1 − X, 1 − Y, 1 − Z] | 2.97 | 156 | 3.8627(1) | Cg3: C33-C34-C35-C36-C37-C38 (PPh4+ cation) Cg4: C21-C22-C23-C24-C25-C26 (PPh4+ cation) |
| C19-H19B…Cg4 [X, −1 + Y, Z] | 2.86 | 132 | 3.5884(1) | ||
| 2,2′-bpyAsPh4 | C20-H11…Cg4 [1 + X, Y, Z] | 2.87 | 134 | 3.591(7) | Cg4: C53-C54-C55-C56-C57-C58 (AsPh4+ cation) |
| C32-H21…Cg5 [1 − X, −Y, 1 − Z] | 2.81 | 157 | 3.698(6) | Cg5: C23-C24-C25-C26-C27-C28 (AsPh4+ cation) | |
| 3 | C20-H20A…Cg1 [1/2 − X, 3/2 − Y, −Z] | 2.83 | 168 | 3.7748(1) | Cg1: C21-C22-C23-C24-C25-C26 (AsPh4+ cation) Cg2: C81-C82-C83-C84-C85-C86 (AsPh4+ cation) |
| C22-H22…Cg2 [1/2 − X, 1/2 + Y, 1/2 − Z] | 2.99 | 145 | 3.7890(1) |
| Angle between Ring Planes in Bipyridyl Ligands (°) | The Shift of the W Atom (Å) in Relation to the Plane Defined Based on Nitrogen and Carbon Atoms in Brackets | The Volume of the Coordination Polyhedron around the W Atom (Å3) | |
|---|---|---|---|
| 2,2′-bpyPPh4 [27] | 9.45 | 0.335 (N7, C12, C13, N8) | 17.970 |
| 1 | 6.80 | 0.239 (N1, C6, C7, N12) | 18.021 |
| 2,2′-bpyAsPh4 [28] | 9.77 | 0.344 (N7, C11, C12, N8) | 17.818 |
| 3 | 6.20 | 0.212 (N1, C6, C7, N12) | 18.301 |
| 2,2′-bpyPPh4 [27] | 1 (4,4′-Mebpy) | 2,2′-bpyAsPh4 [28] | 3 (5,5′-Mebpy) | |
|---|---|---|---|---|
| volume (Ǻ3) | 5845.3(1) | 3072.11(11) | 5725.2(8) | 11,915.93(5) |
| density (mg/m3) | 1.417 | 1.409 | 1.538 | 1.484 |
| the smallest W-W distance (Ǻ) | 9.125 | 8.864 | 9.040 | 9.830 |
| smallest W-W distance between layers (Ǻ) | 14.126 | 13.346 | 14.034 | 14.329 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basta, N.; Szklarzewicz, J.; Hodorowicz, M.; Jurowska, A. Dimethyl Derivatives of 2,2′-Bipyridine as Ligands in [W(CN)6(bpy)]2−-Type Complexes. Molecules 2024, 29, 444. https://doi.org/10.3390/molecules29020444
Basta N, Szklarzewicz J, Hodorowicz M, Jurowska A. Dimethyl Derivatives of 2,2′-Bipyridine as Ligands in [W(CN)6(bpy)]2−-Type Complexes. Molecules. 2024; 29(2):444. https://doi.org/10.3390/molecules29020444
Chicago/Turabian StyleBasta, Natalia, Janusz Szklarzewicz, Maciej Hodorowicz, and Anna Jurowska. 2024. "Dimethyl Derivatives of 2,2′-Bipyridine as Ligands in [W(CN)6(bpy)]2−-Type Complexes" Molecules 29, no. 2: 444. https://doi.org/10.3390/molecules29020444
APA StyleBasta, N., Szklarzewicz, J., Hodorowicz, M., & Jurowska, A. (2024). Dimethyl Derivatives of 2,2′-Bipyridine as Ligands in [W(CN)6(bpy)]2−-Type Complexes. Molecules, 29(2), 444. https://doi.org/10.3390/molecules29020444







