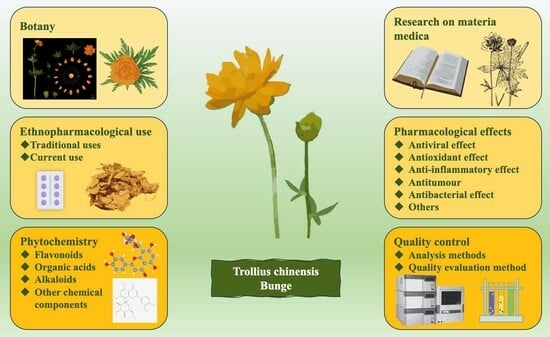
Trollius chinensis Bunge: A Comprehensive Review of Research on Botany, Materia Medica, Ethnopharmacological Use, Phytochemistry, Pharmacology, and Quality Control
Abstract
1. Introduction
2. Materials and Methods
3. Botany
4. Research on Materia Medica
5. Ethnopharmacological Use
5.1. Traditional Uses
5.2. Current Use
6. Phytochemistry
6.1. Flavonoids
6.2. Organic Acids
6.3. Alkaloids
6.4. Other Chemical Components
7. Pharmacological Effects
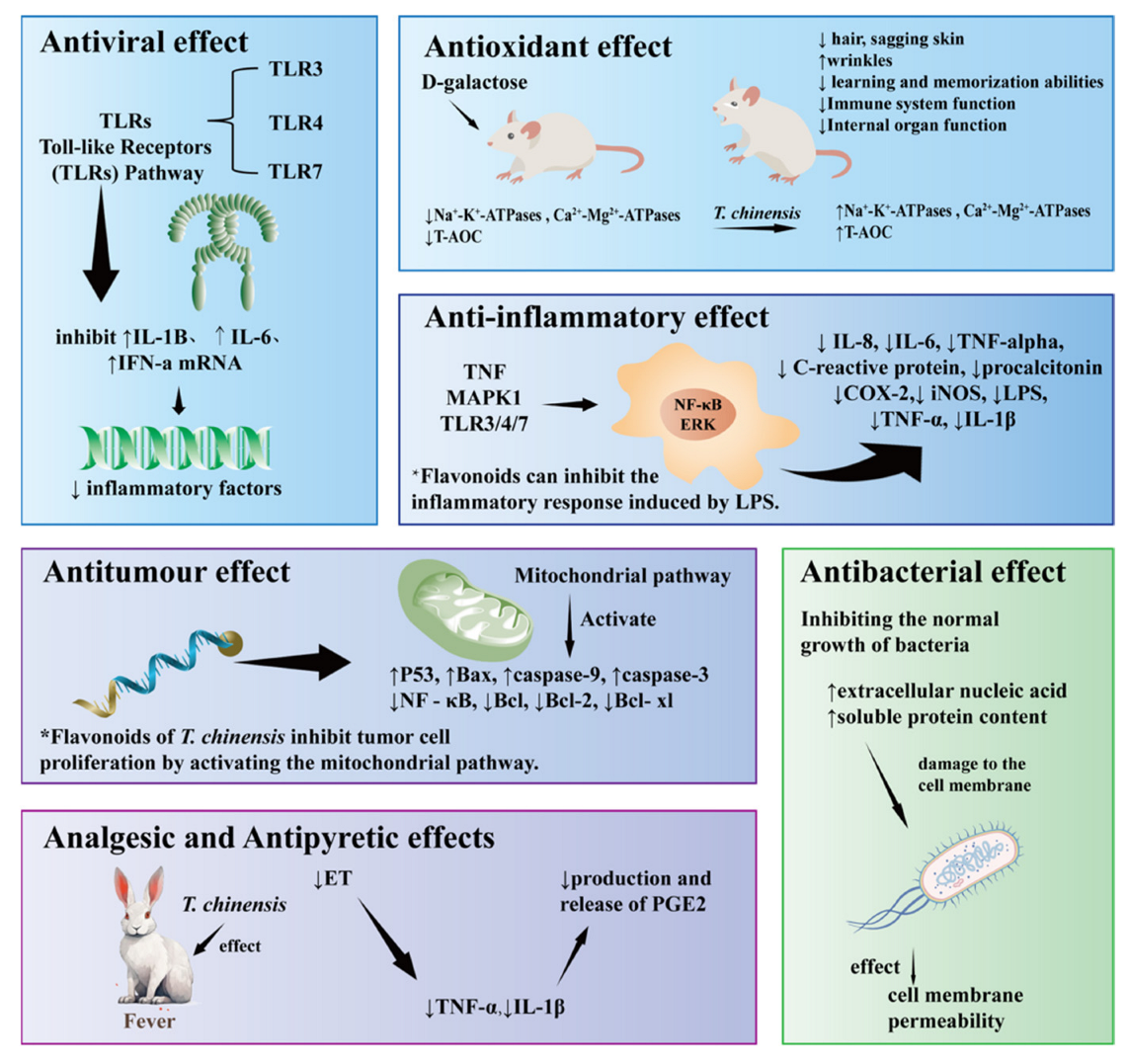
7.1. Antiviral Effect
7.2. Antioxidant Effect
7.3. Anti-Inflammatory Effect
7.4. Antitumour
7.5. Antibacterial Effect
7.6. Others
8. Quality Control
8.1. Analysis Methods
8.2. Quality Evaluation Method
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| ACE2 | Angiotensin-converting enzyme II |
| IL-6 | Interleukin-6 |
| OC43 | HCoV-OC43 |
| HIF-1 | Hypoxia-inducible factor |
| UPLC-DAD-TOF/MS | Ultra-performance liquid chromatography-tandem diode array detector-time-of-flight mass spectrometry |
| TNF | Tumor necrosis factor |
| MAPK1 | Mitogen-activated protein kinase 1 |
| NAFLD | Non-alcoholic fatty liver disease |
| CCK-8 | Cell Counting Kit-8 |
| Annexin-FITC/PI-FCM | AnnexinV-FITCApoptosisDetectionKit |
| PGE2 | Prostaglandin(PG) E2 |
| GSH-Px | glutathione peroxidase |
| RAW264.7 | mononuclear macrophage leukemia |
| MIC | Minimal Inhibitory Concentration |
| TCM | Traditional Chinese Medicine |
| iNOS | Inducible nitric oxide synthase |
| IL-1β | interleukin-1β |
| ERK | extracellular signal-regulated kinase |
| K562 | Leukemia K562 cells |
| A549 | Human non-small cell lung cancer cells A549 |
| MBC | Minimum Bactericidal Concentration |
| A549 | Human non-small cell lung cancer cells A549 |
| GA | Globeflowery acid |
| COVID-19 | Corona Virus Disease 2019 |
| TMPRSS2 | Transmembrane serine two protease |
| IFN-α | Interferon-α |
| TNF-α | Tumor necrosis factor alpha |
| TLR | Toll-like receptors |
| EV71 | Enterovirus 71 |
| ROS | Reactive oxygen species |
| HepG2 | Human hepatocellular carcinomas |
| EC-109 | Human esophageal cancer |
| Hoechest33258 | BisBenzimide H 33258 |
| ET | Gram-negative bacteria ET |
| SOD | Superoxide dismutase |
| MDA | malondialdehyde |
| LPS | lipopolysaccharide |
| MCF-7 | Human Breast Cancer |
| COX-2 | Cyclooxygenase 2 |
| NF-κB | nuclear factor-κB |
| NLRP3 | nucleotide-bindingdomain-(NOD-)like receptor protein 3 |
| He La | HeLa cells |
| HT-29 | Human Carcinoma Cells HT-29 |
| NCI-H446 | Lung cancer cells NCI-H446 |
| PA | Proglobeflowery acid |
| TS | Trolloside |
References
- Liang, Y.; Liu, X.; Hu, J.; Huang, S.; Ma, X.; Liu, X.; Wang, R.; Hu, X. The Crude Extract from the Flowers of Trollius chinensis Bunge Exerts Anti-Influenza Virus Effects through Modulation of the TLR3 Signaling Pathway. J. Ethnopharmacol. 2023, 300, 115743. [Google Scholar] [CrossRef]
- Hou, R.; Yang, L.; Wuyun, T.; Chen, S.; Zhang, L. Genes Related to Osmoregulation and Antioxidation Play Important Roles in the Response of Trollius chinensis Seedlings to Saline-Alkali Stress. Front. Plant Sci. 2023, 14, 1080504. [Google Scholar] [CrossRef]
- Lei, R.; Feng, L.; Liu, Y.; Duan, J. Research progress of Trollius chinensis. J. Chin. Med. Mater. 2015, 38, 1085–1091. [Google Scholar]
- Li, H.; Fan, R.; Zhao, J.; Su, L. Research situation of Trollius plants. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 239–250. [Google Scholar]
- Li, J.; Du, Y.; Xie, L.; Jin, X.; Zhang, Z.; Yang, M. Comparative Plastome Genomics and Phylogenetic Relationships of the Genus Trollius. Front. Plant Sci. 2023, 14, 1293091. [Google Scholar] [CrossRef]
- Witkowska-Banaszczak, E. The Genus Trollius—Review of Pharmacological and Chemical Research. Phytother. Res. 2015, 29, 475–500. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, X.; Zheng, L.; Zhang, Y.; Zhou, H. Herbal Drink Formulation Optimization of Trollius chinensis Bunge by Sensory Fuzzy Comprehensive Evaluation. Acta Sci. Pol. Technol. Aliment. 2020, 19, 185–194. [Google Scholar]
- Cai, H.; Liu, H.; Zheng, G.; Zhan, Z.; Hu, J. Research progress on chemical composition and pharmacological activity of Trollius chinensis Bunge. World Sci. Technol.-Mod. Tradit. Chin. Med. 2021, 23, 2340–2352. [Google Scholar]
- Yuan, M.; Wang, R.; Wu, X.; An, Y.; Yang, X. Investigation on Flos Trollii: Constituents and Bioactivities. Chin. J. Nat. Med. 2013, 11, 449–455. [Google Scholar] [CrossRef]
- Li, Y.-L.; Ma, S.-C.; Yang, Y.-T.; Ye, S.-M.; But, P.P.-H. Antiviral Activities of Flavonoids and Organic Acid from Trollius chinensis Bunge. J. Ethnopharmacol. 2002, 79, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Liu, R.; Zhang, T.; Wu, T. A New Natural Ceramide from Trollius chinensis Bunge. Molecules 2010, 15, 7467–7471. [Google Scholar]
- Liu, J.-Y.; Li, S.-Y.; Feng, J.-Y.; Sun, Y.; Cai, J.-N.; Sun, X.-F.; Yang, S.-L. Flavone C-Glycosides from the Flowers of Trollius chinensis and Their Anti-Complementary Activity. J. Asian Nat. Prod. Res. 2013, 15, 325–331. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, Z.; Shui, G.; Lam, S.M. Extensive Profiling of Polyphenols from Two Trollius Species Using a Combination of Untargeted and Targeted Approaches. Metabolites 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.-L.; Xu, H.-T.; Yang, J.-J.; Chou, G.-X. Diterpenoid Glycosides from the Flower of Trollius chinensis Bunge and Their Nitric Oxide Inhibitory Activities. Bioorg. Chem. 2021, 116, 105312. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Li, X.; Kang, Y.; Chen, Y.; Guo, S.; Li, Y.; Zhang, J.; Liu, S.; Xue, T.; et al. Study on antibacterial and antioxidant activities of flavonoids from Trollius chinensis Bge. J. Pharm. Res. 2023, 42, 228–231+242. [Google Scholar]
- Sun, P.; Li, X.; Xue, T.; Xin, J.; Chen, Y.; Guo, S.; Zhang, B. Progress of pharmacological effects and clinical application of goldenseal flower (Rosa canina). China Pharm. 2022, 33, 507–512. [Google Scholar]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Wu, Q.; Li, P. Chinese Herbal Medicine; Series of Chinese Herbal Medicine Resource Dictionary; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Huang, J.; Feng, J.; Liu, Z.; Su, L.; Fan, R. Decipherment of ancient literature about Trollius chinensis. J. Liaoning Univ. Tradit. Chin. Med. 2023, 25. [Google Scholar]
- Li, L. The Geographical Distribution of Subfam. Helleboroideae (Ranunculaceae). J. Syst. Evol. 1995, 33, 537–555. [Google Scholar]
- Zhu, D.; Ding, W.; Chen, S. Research progress of Trollius plants. World Sci. Technol./Mod. Tradit. Chin. Med. Mater. Medica 2006, 8, 26–33. [Google Scholar]
- Zhao, X. Supplement to Compendium of Materia Medica; China Press of Traditional Chinese Medicine Co., Ltd.: Beijing, China, 1998; ISBN 978-7-80089-671-2. [Google Scholar]
- Editorial Committee of Flora of China, Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1979; Volume 27, ISBN 978-7-03-048169-6. [Google Scholar]
- Shen, Z. Chinese Medicinal Source and Application of Trollius chinensis Bunge. Lishizhen Med. Mater. Medica Res. 2000, 12, 1110. [Google Scholar]
- Zhang, T.; Guo, W.; Zhao, H.; LI, S.; Wang, L. Study on Anti-enterovirus 71 Material Basis of Jinlianhua (Trollius chinensis Bge. Based on Spectrum-effect Correlation). J. Shandong Univ. Tradit. Chin. Med. 2022, 46, 386–392. [Google Scholar]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China (One Part); China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Wang, T. Fundamental Studies on the Anti-Influenza Virus and Pharmacodynamic Substances in Golden Lotus Flower Soup. Master’s Thesis, Beijing Unviersity of Chinese Medicime, Beijing, China, 2018. [Google Scholar]
- Zheng, Y. Summary on “Handbook of Folk Chinese Herbal Medicines Commonly Used in Guangxi”(1,2 Set); National Committee on the Assessment of the Protected Tradtional Chinese Medicinal Products: Beijing, China, 2009.
- Geng, D.; Pang, Y.; Tao, Z.; Wang, S.; Liu, X.; Wang, R. Investigation on Potential of Jinlianhua Decoction against Novel Coronavirus(2019-nCoV)Based on Molecular Docking. Mod. Chin. Med. 2020, 22, 522–532. [Google Scholar]
- Liu, Q.; Wang, Y.; Li, J.; Ma, D.; Wang, L.; Liang, Y.; Liu, J. Chemical components and pyrolysis regularity study of medicine and foodhomology Trollius chinensis Bge. by UPLC-MS. China Food Addit. 2022, 33, 51–61. [Google Scholar]
- Fu, X.; Li, L. Research progress on the extraction and application of yellow pigment of Trollius chinensis. Agric. Technol. 2022, 42, 4–6. [Google Scholar]
- Hebei People’s Publishing House. Guang Qun Fang Pu; Beijing Ancient Books Publishing House Co., Ltd.: Beijing, China, 1989; ISBN 978-7-20200-448-7. [Google Scholar]
- Cha, X. A Sea Record of Cha Shenxing; Beijing Ancient Books Publishing House Co., Ltd.: Beijing, China, 1989; ISBN 978-7-53000-002-1. [Google Scholar]
- Science Press. Hebei Traditional Chinese Medicine Manual; Hebei Province Revolutionary Committee Commercial Bureau Medical Supply Station Co., Ltd.: Beijing, China, 1970.
- Meng, Z. Mongolian medicine training class. In Compilation of Mongolian Medicine Prescriptions; Beijing Ancient Books Publishing House Co., Ltd.: Beijing, China, 2004; ISBN 9787538012521. [Google Scholar]
- Inner Mongolia Revolutionary Committee Health Bureau. Inner Mongolia Chinese Herbal Medicine; Inner Mongolia Autonomous Region Press, Ltd.: Hohhot, China, 1989. [Google Scholar]
- Wang, Z.; Yang, S.; Gao, Y.; Huang, J. Extraction and Purification of Antioxidative Flavonoids from Chionanthus retusa Leaf. Front. Bioeng. Biotechnol. 2022, 10, 1085562. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, Y. Progress on natural flavonoid carbon glycosides and their activities. Pharm. J. Chin. People’s Lib. Army 2005, 21, 135–138. [Google Scholar]
- Sun, P.; Li, X.; Xue, T.; Xin, J.; Liu, Y.; Chen, Y.; Guo, S.; Zhang, B. Research progress of authenticity identification and quality evaluation methods of Trollius chinensis. Mod. Chin. Med. 2022, 24, 2048–2054. [Google Scholar]
- Witkowska-Banaszczak, E. Flavonoids from Trollius Europaeus Flowers and Evaluation of Their Biological Activity. J. Pharm. Pharmacol. 2018, 70, 550–558. [Google Scholar] [CrossRef]
- Li, D.-Y.; Wei, J.-X.; Hua, H.-M.; Li, Z.-L. Antimicrobial Constituents from the Flowers of Trollius chinensis. J. Asian Nat. Prod. Res. 2014, 16, 1018–1023. [Google Scholar] [CrossRef]
- Li, Z.; Li, D.; Hua, H.; Chen, X.; Kim, C. Three New Acylated Flavone C-Glycosides from the Flowers of Trollius chinensis. J. Asian Nat. Prod. Res. 2009, 11, 426–432. [Google Scholar] [CrossRef]
- Qin, Y.; Liang, Y.; Ren, D.; Qiu, X.; Li, X. Separation of Phenolic Acids and Flavonoids from Trollius chinensis Bunge by High Speed Counter-Current Chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1001, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-X.; Li, D.-Y.; Li, Z.-L. Acylated Flavone 8-C-Glucosides from the Flowers of Trollius chinensis. Phytochem. Lett. 2018, 25, 156–162. [Google Scholar] [CrossRef]
- Wu, L.-Z.; Zhang, X.-P.; Xu, X.-D.; Zheng, Q.-X.; Yang, J.-S.; Ding, W.-L. Characterization of Aromatic Glycosides in the Extracts of Trollius Species by Ultra High-Performance Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-Flight Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2013, 75, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sang, L.; Yan, R.; Zhao, Q.; Zhang, Y.; Zhao, G.; Li, Y.; Chen, X.; Zhang, C.; Qiao, H.; et al. Two New Compounds from Trollius chinensis Bunge. J. Nat. Med. 2017, 71, 281–285. [Google Scholar]
- Song, Z.; Wang, H.; Ren, B.; Zhang, B.; Hashi, Y.; Chen, S. On-Line Study of Flavonoids of Trollius chinensis Bunge Binding to DNA with Ethidium Bromide Using a Novel Combination of Chromatographic, Mass Spectrometric and Fluorescence Techniques. J. Chromatogr. A 2013, 1282, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.-Q.; Wang, R.; Yang, X.; Shang, M.; Ma, C.; Shoyama, Y. Antiviral Flavonoid-Type C-Glycosides from the Flowers of Trollius chinensis. Chem. Biodivers. 2006, 3, 343–348. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, R.; Zhang, P.; Shi, S.; Chen, X.; Zhang, G. Isolation and identification of flavonoid chemical components in Lotus japonica. J. Shenyang Pharm. Univ. 2018, 35, 344–347+373. [Google Scholar]
- Yan, R.; Cui, Y.; Deng, B.; Bi, J.; Zhang, G. Flavonoid Glucosides from the Flowers of Trollius chinensis Bunge. J. Nat. Med. 2019, 73, 297–302. [Google Scholar] [CrossRef]
- Wu, W. Intestinal Absorption of Chemical Constituents from T. chinensis Based on Caco-2 Cell Modeling. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2020. [Google Scholar]
- Du, R.; Shi, Z.; Zhan, Z.; Hu, J.; Zheng, G. Optimization of the extraction process of total phenolic acid from Trollii Flos by response surface methodlogy and study on its whitening activity. Nat. Prod. Res. Dev. 2023, 35, 915–924. [Google Scholar]
- Wang, R.; Yang, X.; Ma, C.; Chai, S.; Xu, T. Analysis of Fatty Acids from the Flowers of Trollius chinensis. J. Chin. Med. Mater. 2010, 33, 1579–1581. [Google Scholar]
- Wu, X.-W.; Wang, R.-F.; Liu, L.-J.; Guo, L.-N.; Zhao, C. Absorbability, Mechanism and Structure-Property Relationship of Three Phenolic Acids from the Flowers of Trollius chinensis. Molecules 2014, 19, 18129–18138. [Google Scholar] [CrossRef]
- Qiu, Y. Study on the Extraction Process and Antibacterial Activity Evaluation of Trollius chinensis Bunge. Master’s Thesis, South China University of Technology, Guangzhou, China, 2022. [Google Scholar]
- Shi, S.; Zhang, J.; Liu, T.; Ma, Q.; Liu, C.; Zhang, G. Isolation and structural identification of chemical constituents from Trollius chinensis Bunge. J. Shenyang Pharm. Univ. 2017, 34, 297–301+316. [Google Scholar]
- Zhao, D. Study on Chemical Composition and Biological Activity of Trollius chinensis Bunge. Master’s Thesis, Xiamen University, Xiamen, China, 2019. [Google Scholar]
- Zuo, J. Extraction of Flavonoids from Short-Petaled Goldenseal Flower by Natural Low-Eutectic Solvents and Molecular Blotting Technique. Master’s Thesis, Inner Moncolia University, Hohhot, China, 2022. [Google Scholar]
- Liu, S.; Zhao, C.; Ding, P.; Tao, Z.; Geng, D.; Fan, R. Study on the Metabolites of Goldenseal Extract. Mod. Chin. Med. 2020, 22, 1027–1047. [Google Scholar]
- Liu, Y.; Guo, Q.; Zhang, S.; Bao, Y.; Chen, M.; Gao, L.; Zhang, Y.; Zhou, H. Polysaccharides from Discarded Stems of Trollius chinensis Bunge Elicit Promising Potential in Cosmetic Industry: Characterization, Moisture Retention and Antioxidant Activity. Molecules 2023, 28, 3114. [Google Scholar] [CrossRef] [PubMed]
- Yu, L. Distribution of T. chinensis resources, current cultivation status, main chemical constituents, and pharmacological effects of T. chinensis in Mongolian medicine. J. Med. Pharm. Chin. Minor. 2016, 22, 39–41. [Google Scholar]
- Zhang, L.; Wu, D.; Zhang, A. ICP-AES for primary morphological analysis of inorganic elements in Trollius chinensis Bunge and its dissolution characterization. Chin. J. Spectrosc. Lab. 2011, 28, 739–742. [Google Scholar]
- Li, G.; Liu, J. Determination of trace elements in Trollius chinensis Bunge of different origins. Lishizhen Med. Mater. Medica Res. 2014, 25, 2533–2534. [Google Scholar]
- Zhang, H.; Wang, L.; Chai, C.; Wang, W.; Song, C. Study on the ultrasonic extraction process of nasturtium pigment optimized by response surface method. China Condiment 2012, 37, 102–107. [Google Scholar]
- Mao, X.; Gu, S.; Liu, S.; Huang, W. The Inhibitory Effect of Jinlianhua Soft Capsule Against Human Coronavirus OC43. J. Pharm. Today 2021, 31, 914–917. [Google Scholar]
- Zhou, Q.; Yang, C.; Zhang, Y.; Jin, Z.; Zhou, J.; Zhang, G. Exploring the anti-influenza virus mechanism of action of Golden Lotus based on network pharmacology and molecular docking. J. Shenyang Pharm. Univ. 2022, 39, 1100–1110. [Google Scholar]
- Xie, C.; Ren, J. Network pharmacology research and reflection on the efficacy of traditional Chinese medicine and compound formulas. Chin. J. Exp. Tradit. Med. Formulae 2024, 30, 198–207. [Google Scholar]
- Zhao, H.; Zhao, Y. Studies on the in vitro anti-influenza A virus effect of an alcoholic extract of Amaryllis flowers. China Pharm. 2010, 19, 10–11. [Google Scholar]
- Liu, X.; Liang, Y.; Wang, R.; Hu, X. Experimental study on the protection of the lungs of mice modeled with H1N1 virus infection by extracts of Auricularia auriculae. Chin. Pharmacol. Bull. 2017, 33, 1034–1035. [Google Scholar]
- Wen, Y.; Lin, Y.; Huang, H.; Liu, X.; Wei, G.; Fu, F.; Wu, P.; Yang, C. Experimental study on the antiviral activity of aqueous extracts of goldenseal flowers. Chin. J. Microbiol. Immunol. 1999, 01, 25. [Google Scholar]
- Liang, Y.; Liu, X.; Zhang, L.; Wang, R.; Hu, X. Effects of different extracts of Golden Lotus Flower Soup on cell proliferation and in vitro antiviral activity studies. J. Med. Res. 2018, 47, 100–106. [Google Scholar]
- Wang, T.; Liu, S.; Ding, P.; LIU, S.; Wang, Q.; Pang, Y.; Hu, X.; Wang, R. Study on the anti-influenza virus activity of Golden Lotus Flower Soup. Mod. Chin. Med. 2020, 22, 202–206. [Google Scholar]
- Liu, L.-J.; Li, D.-I.; Fang, M.-Y.; Liu, S.-Y.; Wang, Q.-Q.; Liang, Y.-X.; Hu, X.-H.; Wang, R.-F. The Antiviral Mechanism of the Crude Extract from the Flowers of Trollius chinensis Based on TLR 3 Signaling Pathway. Pak. J. Pharm. Sci. 2021, 34, 1743–1748. [Google Scholar]
- Shi, D. Study on the Anti-Influenza Virus H1N1 Mechanism of Goldenseal Based on TLRs Pathway. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2018. [Google Scholar]
- Qu, H.; Yang, G.; Jiang, W.; Yuan, D.; Wang, S.; An, F. Dynamic effects of Orientin and Bauhinia glycosides from Goldenseal on serum and tissue antioxidant activities in D-galactose-induced senescent mice. Chin. J. Gerontol. 2015, 35, 443–446. [Google Scholar]
- Liang, W.; Yang, Z.; Huang, Y. Mitochondrial Ca2+ transport and regulation of cellular metabolism. Prog. Physiol. Sci. 2000, 04, 357–360. [Google Scholar]
- Yang, G.; Rao, N.; Tian, J.; An, F.; Wang, S. Studies on the antioxidant effects of Orientin and Oryza sativa glycosides in goldenseal flowers. Lishizhen Med. Mater. Medica Res. 2011, 22, 2172–2173. [Google Scholar]
- Tian, J.; Yuan, B.; Zhu, D.; Wang, S.; An, F. Dynamic antioxidant effects of Orientin and Bauhinia glycosides from Goldenseal on D-galactose-induced senescence in mice. Chin. J. Gerontol. 2014, 34, 5512–5513. [Google Scholar]
- Cai, H. Study on the Commodity Specification Grade of Goldenseal Flower. Master’s Thesis, Hubei University of Chinese Medicine, Wuhan, China, 2022. [Google Scholar]
- Liu, C. Study on the Anti-Inflammatory and Antibacterial Potency Sites of T. chinensis Bunge Decoction. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2016. [Google Scholar]
- Zhong, L.; Yang, J.; Feng, M.; Dai, J.; Wang, Z.; Jiang, Q.; Pan, C. The mechanism of Jinlianhua series preparations in the treatment of upper respiratory tract infection based on data mining. J. Chengdu Univ. (Nat. Sci.) 2021, 40, 1–7. [Google Scholar]
- Wang, R.; Geng, D.; Wu, X.; An, Y. Studies on the anti-inflammatory activities of four major components of goldenseal flowers. Lishizhen Med. Mater. Medica Res. 2012, 23, 2115–2116. [Google Scholar]
- Yang, N.; Dong, Z.; Tian, G.; Zhu, M.; Li, C.; Bu, W.; Chen, J.; Hou, X.; Liu, Y.; Wang, G.; et al. Protective Effects of Organic Acid Component from Taraxacum Mongolicum Hand.-Mazz. against LPS-Induced Inflammation: Regulating the TLR4/IKK/NF-κB Signal Pathway. J. Ethnopharmacol. 2016, 194, 395–402. [Google Scholar] [CrossRef]
- Lin, Q.; Zhao, N.; Sun, Q.; Wang, L.; Meng, F. Clinical efficacy of Trollius chinensis preparation against upper respiratory tract infection: A Meta analysis. Chin. J. Pharmacovigil. 2022, 19, 893–896+907. [Google Scholar]
- Gu, Z.; Jin, Y. Observation on the effect of gold lotus granules combined with cefixime granules in the treatment of pediatric acute respiratory tract infections. J. Clin. Med. Pract. 2019, 23, 79–82. [Google Scholar]
- Li, H. Efficacy of gold lotus granules combined with ribavirin in the treatment of pediatric acute upper respiratory tract infection and its effect on serum inflammatory markers. China Med. Pharm. 2019, 9, 21–24. [Google Scholar]
- Su, L.; Cai, L.; Wang, J. Study on the clinical efficacy of gold lotus granules in the treatment of patients with upper respiratory tract infections. Strait Pharm. J. 2019, 31, 227–228. [Google Scholar]
- Zeng, X.; Lin, L.; Mai, L. Clinical study of gold lotus granules combined with cefixime in the treatment of pediatric acute respiratory tract infections. Drugs Clin. 2018, 33, 84–87. [Google Scholar]
- Guo, Y.; Liu, W.; Ju, A.; Ma, W. Study on the anti-inflammatory mechanism of the active ingredients of goldenseal flower based on network pharmacology. Acta Chin. Med. Pharmacol. 2020, 48, 25–28. [Google Scholar]
- Wan, S.; Liu, L.; Liu, M.; Huang, X. Studies on the pharmacological mechanism of action of Orientin. J. Med. Res. 2018, 47, 183–186. [Google Scholar]
- Xiao, Q.; Qu, Z.; Yang, L.; Zhao, Y.; Gao, P. Orientin Ameliorates LPS-Induced Inflammatory Responses through the Inhibitory of the NF-κB Pathway and NLRP3 Inflammasome. Evid.-Based Complement. Altern. Med. 2017, 2017, 2495496. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, F.; Liu, H.; Luo, Q.; An, F. The effects of Trollius flavonoids on human breast cancer cells. Chin. J. Gerontol. 2009, 29, 1098–1099. [Google Scholar]
- Wang, S.; Tian, Q.; An, F. Growth Inhibition and Apoptotic Effects of Total Flavonoids from Trollius chinensis on Human Breast Cancer MCF-7 Cells. Oncol. Lett. 2016, 12, 1705–1710. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Ma, Y.; Zhang, H.; Chen, W. Prediction and Verification of the Active Ingredients and Potential Targets of Erhuang Quzhi Granules on Non-Alcoholic Fatty Liver Disease Based on Network Pharmacology. J. Ethnopharmacol. 2023, 311, 116435. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Yang, J.; Chen, X.; Mao, Q.; Wei, X.; Wen, X.; Wang, Q. Triterpenoid-Rich Fraction from Ilex Hainanensis Merr. Attenuates Non-Alcoholic Fatty Liver Disease Induced by High Fat Diet in Rats. Am. J. Chin. Med. 2013, 41, 487–502. [Google Scholar] [CrossRef]
- Fan, R.; Li, W.; Liu, Y.; Li, H. Effects of Total Flavonoids from Flos Trolliion the Abnormal Function o of in vitro HEPGCells Induced by High Concentration of Glucos. J. Med. Res. 2021, 50, 124–129+112. [Google Scholar]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-Alcoholic Fatty Liver Disease (NAFLD): A Review of Pathophysiology, Clinical Management and Effects of Weight Loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Wang, X.; Lin, C.; Meng, L.; Zhou, J.; Liang, X.; Yang, H.; Qin, Y. Effect of high free fatty on E2F1 expression and invasion and migration of HepG2 cells. J. Guangxi Med. Univ. 2022, 39, 276–281. [Google Scholar]
- Zhu, D.; An, F.; Wang, S. Effects of orientin and vitexin in Trollius chinensis Bunge on growth and apoptosis of human esophageal cancer cells. Chin. J. Gerontol. 2013, 33, 4472–4475. [Google Scholar]
- Liu, R. Research on the Key Technology of Containerized Seedling and Grassland Transplanting of Amaryllis Japonica. Master’s Thesis, Ninner Mongolia Agricultural University, Hohhot, China, 2022. [Google Scholar]
- Li, Y.; Ding, W. Genetic diversity Analysis of partial Chinese Trollius populations Based onRAPD markers. J. Plant Genet. Resour. 2010, 11, 535–539. [Google Scholar]
- Tian, J.; Yang, G.; Rao, N.; An, F.; Wang, S. Effect of Orientin and Vitexin in Trollius chinesis on cell membrane transport activity of aging mice induced by D-galactose. Chin. J. Gerontol. 2012, 32, 3945–3947. [Google Scholar]
- Ye, S.; Li, Y.; Yang, Y.; Cen, Y. Extraction technology of Trollius chinensis Bunge. China J. Chin. Mater. Medica 2002, 6, 66–67. [Google Scholar]
- Ye, Y.; Peng, Y.; Fu, G.; Miu, J. New progress in the research of medicinal goldenseal flower. Mod. Chin. Med. 2007, 3, 29–33. [Google Scholar]
- Tian, P.; Liu, R.; Deng, Y.; Zhong, L.; Lei, S.; Wu, L. Re-evaluation of goldenseal series of preparations based on the mechanism of antibacterial effect. World Notes Antibiot. 2023, 44, 263–268. [Google Scholar]
- Liu, P.; Chen, G.; Deng, S.; Liu, Y.; Dong, J. Experimental study on the antimicrobial effect of total flavonoids of Amaryllis flowers. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 207–210. [Google Scholar]
- Peng, Y.; Liu, L.; Zhao, C.; Guo, L.; Wang, R. First isolation of a phenolic acid compound and its anti-inflammatory and bacteriostatic activities from Lotus corniculatus flower. Chin. Arch. Tradit. Chin. Med. 2015, 33, 1349–1351. [Google Scholar]
- Lin, Q.; Feng, S.; Li, Y.; Cen, Y.; Yang, Y.; Wang, L. Study on the antibacterial and antiviral activity compositions of Trollius chinensis Bunge. J. Zhejiang Univ. (Sci. Ed.) 2004, 31, 412–415. [Google Scholar]
- Wang, X.; Guo, R.; Nie, X.; Chen, H.; Zhi, W.; Tian, M.; Liu, F. Studies on the antibacterial activity of Forsythia and the mechanism of action of its pharmacodynamic component hesperidin against Staphylococcus aureus. Chin. J. Antibiot. 2021, 46, 437–441. [Google Scholar]
- Liu, P.; Hu, N.; Chen, G.; Wang, Y.; Liu, Y.; Tong, J. Antipyretic Effect of Flavonoids from Trollius ledebouri Reichb on Endotoxin-induced Fever and Levels of TNF-α, IL-1β and PGE2 in Rabbits. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 189–191. [Google Scholar]
- Su, L.; Zhao, W.; Nan, Y.; Xing, R. Experimental study on anti-inflammatory and analgesic effects of extracts from stems and leaves of Trollius chinensis. Chin. J. Tradit. Med. Sci. Technol. 2012, 19, 31. [Google Scholar]
- You, S.; Liu, X.; NaiMaiTi, A.; Chen, W.; Zhu, Q.; Qi, X.; Zhao, J.; Liu, T. Experimental study on the antitussive, expec etorant, anti-inflammatory and analgesic effects of total flavonoids extracted from Trollius chinensis Bunge. J. Xinjiang Med. Univ. 2019, 42, 462–466. [Google Scholar]
- Fang, J.; Meng, X.; Liu, Y. Protective effects of flavone of Trollius chinensis Bunge on myocardial ischemia reperfusion injury in rats. J. Chongqing Med. Univ. 2014, 39, 1391–1395. [Google Scholar]
- Fan, C.; LI, J.; Sun, H. Study on the technology of supercritical CO2 extraction of total flavonoids from Trollius chinensis Bunge and the antioxidant effect of the extract. J. Chin. Med. Mater. 2016, 39, 2828–2832. [Google Scholar]
- Liu, X.; Liang, Y.; Wang, R.; Hu, X. Studies on the fatty acid composition of Auricularia auricula. J. Chin. Med. Mater. 2017, 33, 1034–1035. [Google Scholar]
- Shi, D.; Chen, M.; Liu, L.; Wang, Q.; Liu, S.; Wang, L.; Wang, R. Anti-Influenza A Virus Mechanism of Three Representative Compounds from Flos Trollii via TLRs Signaling Pathways. J. Ethnopharmacol. 2020, 253, 112634. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Ma, G. Radical Scavenging Activity of Flavonoids from Trollius chinensis Bunge. Nutrition 2011, 27, 1061–1065. [Google Scholar] [CrossRef]
- An, F.; Yang, G.; Tian, J.; Wang, S. Antioxidant Effects of the Orientin and Vitexin in Trollius chinensis Bunge in D-Galactose-Aged Mice. Neural Regen. Res. 2012, 7, 2565–2575. [Google Scholar]
- Liu, L.-J.; Hu, X.-H.; Guo, L.-N.; Wang, R.-F.; Zhao, Q.-T. Anti-Inflammatory Effect of the Compounds from the Flowers of Trollius chinensis. Pak. J. Pharm. Sci. 2018, 31, 1951–1957. [Google Scholar] [PubMed]
- An, F.; Wang, S.; Tian, Q.; Zhu, D. Effects of Orientin and Vitexin from Trollius chinensis on the Growth and Apoptosis of Esophageal Cancer EC-109 Cells. Oncol. Lett. 2015, 10, 2627–2633. [Google Scholar] [CrossRef]
- Jiang, M.; Yan, L.; Li, K.-A.; Ji, Z.-H.; Tian, S.-G. Evaluation of Total Phenol and Flavonoid Content and Antimicrobial and Antibiofilm Activities of Trollius chinensis Bunge Extracts on Streptococcus Mutans. Microsc. Res. Tech. 2020, 83, 1471–1479. [Google Scholar] [CrossRef]
- Bai, Y.; Qi, X.; Gao, Y. Pharmacognostical studies on Trollius chinensis Bunge. J. Shanxi Med. Univ. 2001, 1, 27–28. [Google Scholar]
- Nie, B.; Zhang, G.; Sun, S.; Zhou, Q.; Ding, W. IR identification study of different nasturtium medicinal materials. J. Chin. Med. Mater. 2006, 04, 323–326. [Google Scholar]
- Pan, Y.; Xiao, P.; Zhang, G.; Lu, J.; Sun, S. Fourier infrared spectroscopy identification and characterization of pharmacodynamic components of nasturtium. China J. Chin. Mater. Medica 2006, 12, 1024–1026. [Google Scholar]
- Wang, H.; Ye, H.; Xu, Q.; Liu, J.; Xie, Z. Study on the Quality of Nasturtium Chinese Medicine Tablets. Chin. J. Mod. Appl. Pharm. 2020, 37, 963–966. [Google Scholar]
- Yuan, Q.; Zhang, W.; Jiang, D. On the Methods and Principles of Molecular Identification of Chinese Herbs. Plant Divers. 2012, 34, 607. [Google Scholar] [CrossRef]
- Zhang, L. Identification, Characterization and Activity Study of Goldenseal Proteins; Beijing University of Chinese Medicine: Beijing, China, 2006. [Google Scholar]
- Guan, Y.; Ding, X.; Wang, W.; Di, L.; Wang, X. X-Ray Fluorescence Analysis and X-ray Diffraction of Goldenseal Flowers. Chin. J. Pharm. Anal. 2006, 26, 1623–1625. [Google Scholar]
- Huang, R.; Zhang, G.; Pan, Y.; Cui, H.; Zhao, Y. Analysis and Identification on Characterization of Chemical Components in Trollii Flos by HPLC-MS. Zhongcaoyao Chin. Tradit. Herb. Drugs 2012, 43, 670–672. [Google Scholar]
- Sun, Y.; Li, Q.; Zhang, Q. Determination of Orientin, Vitexin and Total Flavonoids in Trollius chinensis from Different Areas and Optimization of Extraction Process. Northwest Pharm. J. 2019, 34, 596–601. [Google Scholar]
- Song, Z.; Hashi, Y.; Sun, H.; Liang, Y.; Lan, Y.; Wang, H.; Chen, S. Simultaneous Determination of 19 Flavonoids in Commercial Trollflowers by Using High-Performance Liquid Chromatography and Classification of Samples by Hierarchical Clustering Analysis-ScienceDirect. Fitoterapia 2013, 91, 272–279. [Google Scholar] [CrossRef]
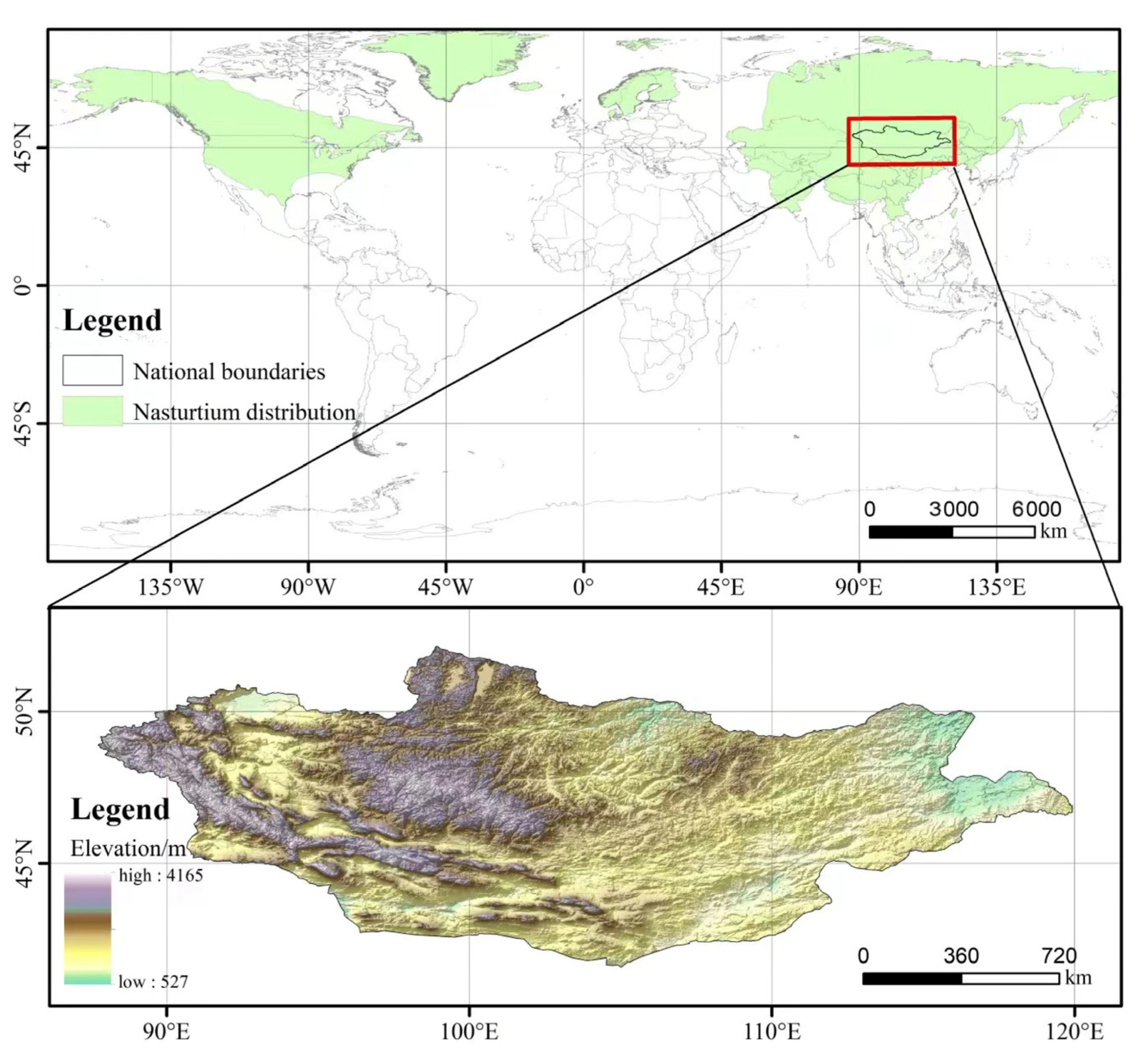


| Num | Latin Name | Distribution Area | Altitude |
|---|---|---|---|
| 1 | T. chinensis Bunge | Shanxi, N. Henan, Hebei, E. Inner Mongolia, W. Liaoning and Jilin provinces of China | 1000–2200 m |
| 2 | Trollius altaicus C. A. Mey. | N. Xinjiang (Tacheng, Altai, etc.), China; W. Inner Mongolia, China; Siberia, Russia; People’s Republic of Mongolia | 1200–2650 m |
| 3 | Trollius asiaticus L. | Heilongjiang, China (Shangzhi); Xinjiang, China (Hami); Siberia, Russia; Mongolia | Not applicable |
| 4 | Trollius buddae Schipcz. | N. Sichuan, China; S. Gansu, China; S. Shaanxi, China | 1780–2400 m |
| 5 | Trollius buddae f. dolichopetalus P. L. Liu and C. Du | Not applicable | Not applicable |
| 6 | Trollius dschungaricus Regel | Tianshan and Zhaosu, Xinjiang, China; Central Asia, Russia | 1800–3100 m |
| 7 | Trollius farreri Stapf | Qinghai, China | 2000–4700 m |
| 8 | Trollius farreri Stapf var/. major W. T. Wang | NW Yunnan, China (Deqin); SE Tibet, China (Tsatsumi) | 3500–4200 m |
| 9 | Trollius japonicus Miq. | Changbai Mountain, Jilin, China; Sakhalin Island (Kuril Islands); Japan | 1200–2300 m |
| 10 | Trollius ledebourii Rchb. | Heilongjiang, China; NE Inner Mongolia, China; E. Siberia, Russia; Far East | 110–900 m |
| 11 | Trollius macropetalus Fr. | Liaoning, China; Jilin, China; Heilongjiang, China, etc.; Russian Far East; N Korea; | 450–600 m; |
| 12 | Trollius micranthus Hand.-Mazz. | NW Yunnan (Deqin); E. Tibet (Motuo) | 3900–4200 m |
| 13 | Trollius pumilus D. Don | Southern Tibet, China; Nepal; Sikkim | 4100–4800 m |
| 14 | Trollius pumilus D. Don var. foliosus (W. T. Wang) W. T. Wang | Min County, S. Gansu, China | 3000–3400 m |
| 15 | Trollius pumilus D. Don var. tanguticus Brühl | NE Tibet, China; NW Sichuan, China; S. and E. Qinghai, China; SW Gansu, China. | 2300–3700 m |
| 16 | Trollius pumilus D. Don var. tehkehensis (W. T. Wang) W. T. Wang | Dege, Sichuan, China | Not applicable |
| 17 | Trollius ranunculoides Hemsl. | NW Yunnan, E Xizang, W Sichuan, S and E Qinghai, S Gansu, China. | 2900–4100 m |
| 18 | Trollius taihasenzanensis Masam. | Taiwan, China | 3400–3900 m |
| 19 | Trollius vaginatus Hand.-Mazz. | NW Yunnan (Zhongdian), China; SW Sichuan (Muli), China. | 3000–4200 m |
| 20 | Trollius yunnanensis (Franch.) Ulbr. | W. and NW Yunnan, China; W. Sichuan, China. | 2700–3600 m |
| 21 | Trollius yunnanensis (Franch.) Ulbr. var. anemonifolius (Brühl) W. T. Wang | W. Sichuan and S. Gansu, China. | 3050–3800 m |
| 22 | Trollius yunnanensis (Franch.) Ulbr. var. eupetalus (Stapf) W. T. Wang | Gonshan and Deqin, NW Yunnan, Sichuan, China | 3300–3900 m |
| 23 | Trollius yunnanensis (Franch.) Ulbr. var. peltatus W. T. Wang | Emei area, Sichuan, China | 1900 m |
| 24 | Trollius lilacinus Bunge | Tian Shan, Xinjiang, China; W. Siberia, USSR; Central Asia | 2600–3500 m |
| 25 | Trollius laxus | the United States in Conn.(Connecticut), Del.(Delaware)NJ.(New Jersey)N.Y.(New York)Pa.(Pennsylvania), Ohio.(Ohio) | Not applicable |
| 26 | Trollius europaeus | N. Europe, Central Europe and W. Asia | Not applicable |
| NO | Ethnopharmacological Use | References |
|---|---|---|
| 1 | Ornamental: The whole flower is golden yellow. It blooms in June. In autumn, the flowers are dry, and the fruit is like millet. | Guang Qun Fang Pu Kangxi (AD. 1708) [32] |
| 2 | Drink: Dry long-term preservation, to spend some tea, a pot of one, boiled water. Medicinal use: taste smooth and bitter, non-toxic, cold, cure sore throat, heat flotation tooth declaration, ear pain, eye pain, and fry this generation of Ming. | A sea record of Cha Shenxing Kangxi (AD. 1713) [33] |
| 3 | Medicinal use: bitter taste, cold, non-toxic, treating mouth sore throat swelling, ear pain, eye pain, sore throat, fever from a cold, eyesight. | Bencao Gangmu Shiyi: A Supplement to Compendium of Materia Medica (AD. 1765) [22] |
| 4 | Medicinal use: treatment of furunculosis big poison, bias wind, wind heat, wind hysteria, and wind arthralgia, et al. | Mountain sea grass letter (Qing dynasty) [22] |
| 5 | Medicinal use: clearing away heat and toxic materials, treatment of chronic/acute tonsillitis, acute otitis media, acute tympanitis, acute conjunctivitis, and acute lymphangitis. | Hebei Traditional Chinese Medicine Manual (1970) [34] |
| 6 | Medicinal use: for treating blade wounds and pulse wound sores; for swollen lymph glands and sore throats. | Compilation of Mongolian medical formulas (In 2004) [35] |
| 7 | Medicinal use: cure fever from an ear infection or eye disease. | Inner Mongolia Herbal Medicine (1972) [36] |
| 8 | Jinlianhua Mixture: clearing heat and removing toxins for upper respiratory tract infections, pharyngitis, and tonsillitis. | 2020 Edition of Chinese Pharmacopoeia (CP) (2020) [26] |
| 9 | Jinlianhua tablets: clearing heat and removing toxins for upper respiratory tract infections, pharyngitis, and tonsillitis. | 2020 Edition of Chinese Pharmacopoeia (CP) (2020) [26] |
| 10 | Jinlianhua capsules: Clearing heat and removing toxins, relieving pharynx and swelling. Suitable for treating inflammation of the upper Jiao, etc. | 2020 Edition of Chinese Pharmacopoeia (CP) (2020) [26] |
| 11 | Jinlianhua granules: Treats upper respiratory tract infections, pharyngitis, and tonsillitis. Relieves inflammation and pain. | 2020 Edition of Chinese Pharmacopoeia (CP) (2020) [26] |
| 12 | Jinlianhua Runhou tablets: clearing heat, removing toxins, reducing swelling, relieving pain, and improving the taste of the throat. | 2020 Edition of Chinese Pharmacopoeia (CP) (2020) [26] |
| 13 | Jinlianhua granules: It is effective in clearing heat and removing toxins, promoting the production of body fluids, improving the pharynx, and relieving cough and expectoration. It is suitable for symptoms of heat and toxicity caused by colds, including high fever, thirst, and dry throat, and for the above symptoms caused by influenza and upper respiratory tract infections. | 2020 Edition of Chinese Pharmacopoeia (CP) (2020) [26] |
| No | Names | Molecular Formula | Parent Nucleus | Substituent | CAS | Molecular Weight | Refs. |
|---|---|---|---|---|---|---|---|
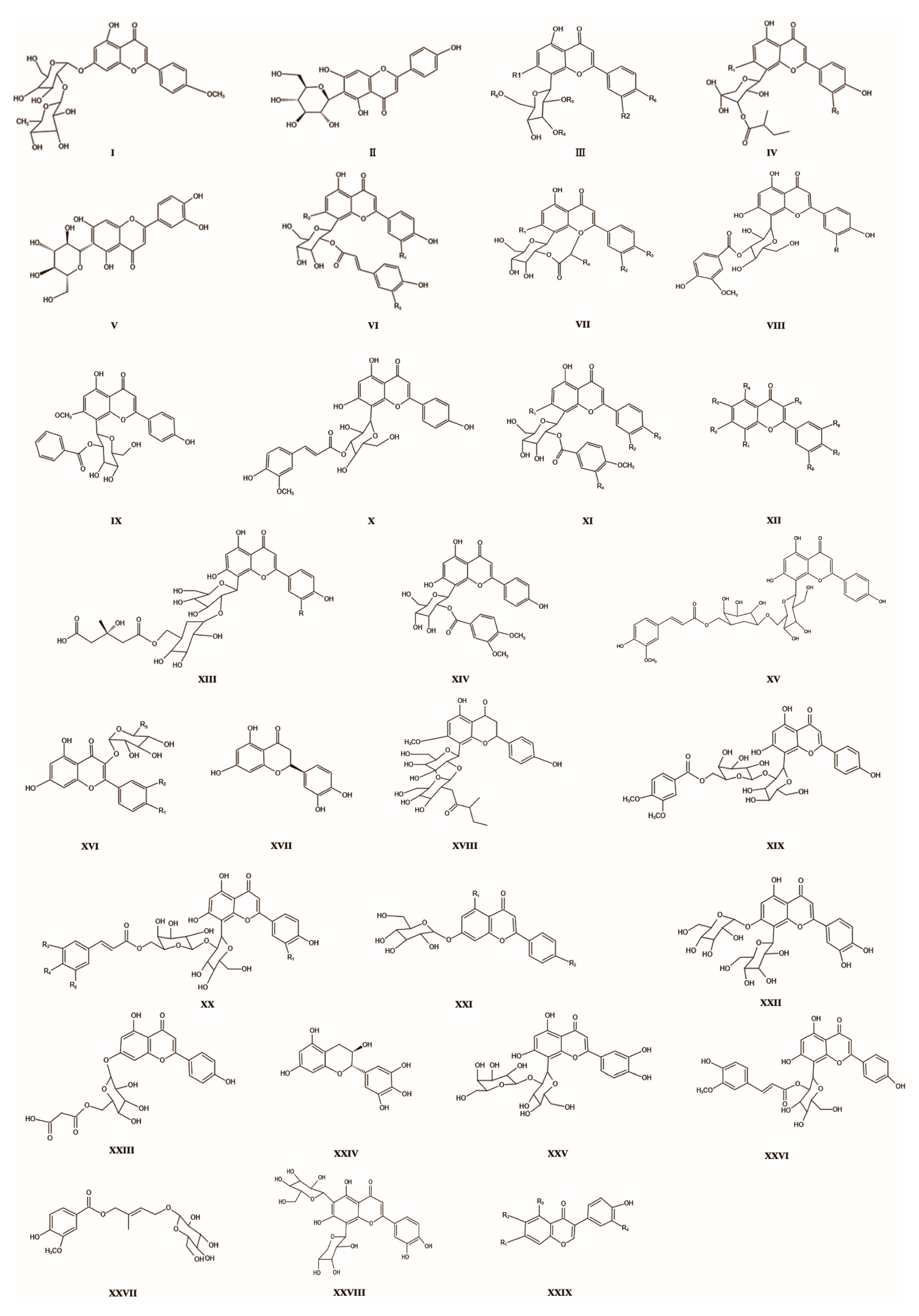
| 1 | 3″-O-Acetylquercetin | C28H32O14 | I | nothing | nothing | 592.50 | [8] |
| 2 | Isorhamnetin | C21H20O10 | II | nothing | 480-19-3 | 432.38 | [40] |
| 3 | Icariin | C21H20O10 | III | R1 = OH; R2 = H; R3 = H; R4 = H; R5 = H; R6 = OH | 489-32-7 | 432.38 | [8] |
| 4 | Apigenin | C21H20O11 | III | R1 = OH; R2 = OH; R3 = H; R4 = H; R5 = H; R6 = OH | 520-36-5 | 448.41 | [40] |
| 5 | Isoswertisin | C22H22O10 | III | R1 = OCH3; R2 = H; R3 = H; R4 = H; R5 = H; R6 = OH | 6980-40-1 | 446.40 | [40] |
| 6 | Isoswertiajaponin | C22H22O11 | III | R1 = OCH3; R2 = OH; R3 = H; R4 = H; R5 = H; R6 = OH | nothing | 462.40 | [8] |
| 7 | Trollisin I | C22H22O10 | III | R1 = OCH3; R2 = H; R3 = H; R4 = H; R5 = H; R6 = OH | nothing | 446.40 | [8] |
| 8 | Cyanidin 2″-O-(β-d-xyranosyl)-β-d-glucoside | C26H28O16 | III | R1 = OH; R2 = OH; R3 = D-xyl; R4 = H; R5 = H; R6 = OH | nothing | 596.49 | [8] |
| 9 | Cyanidin 2″-O-(β-d-pyranosyl)-β-d-glucoside | C26H27O15N | III | R1 = OH; R2 = OH; R3 = D-glu; R4 = H; R5 = H; R6 = OH | nothing | 593.50 | [8] |
| 10 | Cyanidin 2-prime-O-beta-pyranosyl-arabinoside | C26H28O16 | III | R1 = OH; R2 = OH; R3 = D-ara; R4 = H; R5 = H; R6 = OH | nothing | 596.50 | [8] |
| 11 | Cyanidin 2″-O-beta-l-rhamnoside | C27H30O16 | III | R1 = OH; R2 = OH; R3 = L-gal; R4 = H; R5 = H; R6 = OH | nothing | 609.15 | [8] |
| 12 | Cyanidin 3-O-beta-d-glucoside-6″-O-alpha-l-rhamnoside | C26H27O15N | III | R1 = OH; R2 = OH; R3 = H; R4 = H; R5 = D-glu; R6 = OH | nothing | 593.50 | [8] |
| 13 | 6″-O-Acetyl cyanidin | C32H27O11N3 | III | R1 = OH; R2 = OH; R3 = H; R4 = H; R5 = Ac; R6 = OH | nothing | 629.58 | [8] |
| 14 | 3″-O-Acetyl cyanidin | C32H27O11N | III | R1 = OH; R2 = OH; R3 = H; R4 = Ac; R5 = H; R6 = OH | nothing | 629.58 | [8] |
| 15 | 2″-O-Acetyl cyanidin | C32H27O11N3 | III | R1 = OH; R2 = OH; R3 = Ac; R4 = H; R5 = H; R6 = OH | nothing | 629.58 | [8] |
| 16 | Quercetin 2″-O-(β-d-xyranosyl)-β-d-glucoside | C26H28O15 | III | R1 = OH; R2 = H; R3 = D-xyl; R4 = H; R5 = H; R6 = OH | nothing | 580.50 | [8] |
| 17 | Quercetin 2″-O-(β-d-arabinopyranoside) | C26H28O15 | III | R1 = OH; R2 = H; R3 = D-ara; R4 = H; R5 = H; R6 = OH | nothing | 580.50 | [8] |
| 18 | Rhamnetin 2″-O-β-l-rhamnoside | C27H30O16 | III | R1 = OH; R2 = H; R3 = L-gal; R4 = H; R5 = H; R6 = OH | nothing | 610.53 | [8] |
| 19 | Kaempferol 2″-O-β-d-glucopyranoside | C27H30O15 | III | R1 = OH; R2 = H; R3 = D-glu; R4 = H; R5 = H; R6 = OH | nothing | 609.15 | [8] |
| 20 | Kaempferol 6″-O-glucopyranoside | C26H27O14N | III | R1 = OH; R2 = H; R3 = H; R4 = H; R5 = D-glu; R6 = OH | nothing | 577.50 | [8] |
| 21 | 6″-O-acetylkaempferol | C32H27O10N3 | III | R1 = OH; R2 = H; R3 = H; R4 = H; R5 = Ac; R6 = OH | nothing | 613.58 | [8] |
| 22 | 3″-O-acetylkaempferol | C32H27O10N3 | III | R1 = OH; R2 = H; R3 = H; R4 = Ac; R5 = H; R6 = OH | nothing | 613.58 | [8] |
| 23 | 2″-O-acetylkaempferol | C32H27O10N3 | III | R1 = OH; R2 = H; R3 = Ac; R4 = H; R5 = H; R6 = OH | nothing | 613.58 | [8] |
| 24 | Genistein-7-O-β-d-pyranosylglucoside | C22H22O10 | III | R1 = H; R2 = H; R3 = H; R4 = H; R5 = H; R6 = OCH3 | nothing | 446.41 284.26 | [8] |
| 25 | 3″-O-(2‴-methylbutanoyl)resveratrol | C26H28O12 | IV | R1 = OH; R2 = OH | nothing | 532.15 | [8] |
| 26 | 3″-O-(2‴-methylbutanoyl)quercetin | C27H30O11 | IV | R1 = OCH3; R2 = H | nothing | 530.52 | [41] |
| 27 | 3″-O-(2‴-methylbutanoyl) luteolin | C26H28O11 | IV | R1 = OH; R2 = H | nothing | 517.17 | [42] |
| 28 | 3″-O-(2‴-methylbutanoyl) chrysoeriol | C27H30O12 | IV | R1 = OCH3; R2 = OH | nothing | 547.18 | [42] |
| 29 | Isoorientin | C21H20O11 | V | nothing | 28608-75-5 | 448.38 | [40] |
| 30 | 2″-O-feruloylharpagoside | C24H31O18 | VI | R1 = H; R2 = OH; R2 = OCH3 | nothing | 607.14 | [43] |
| 31 | 2″-O-feruloylverbascoside | C31H27O14 | VI | R1 = OH; R2 = OH; R3 = OCH3 | nothing | 623.13 | [43] |
| 32 | 2″-O-feruloylisovitexin | C31H27O11 | VI | R1 = H; R2 = CH3 O; R3 = OCH3 | nothing | 575.16 | [44] |
| 33 | 2″-O-(3‴-methoxycaffeoyl)luteolin | C30H26O13 | VI | R1 = H; R2 = OH; R3 = OH | nothing | 594.14 | [44] |
| 34 | 2″-O-feruloylgenistin | C32H30O13 | VI | R1 = OH; R2 = CH3 O; R3 = OCH3 | nothing | 622.17 | [8] |
| 35 | 2″-O-(2‴-methylbutanoyl)quercetin | C26H28O11 | VII | R1 = OH; R2 = H; R3 = OH; R4 = CH2 CH3 | nothing | 515.15 | [45] |
| 36 | 2″-O-(2‴-methylbutanoyl)kaempferol | C26H28O12 | VII | R1 = OH; R2 = OH; R3 = OH; R4 = CH2 CH3 | nothing | 531.14 | [45] |
| 37 | 4′-methoxy-2”-O-(2‴-methylbutanoyl)luteolin | C32H30O13 | VII | R1 = OH; R2 = H; R3 = OCH3; R4 = CH2CH3 | nothing | 623.17 | [44] |
| 38 | 4′-methoxy-2″-O-(2‴-methylbutanoyl)apigenin | C32H30O14 | VII | R1 = OH; R2 = OH; R3 = OCH3; R4 = CH2CH3 | nothing | 639.17 | [44] |
| 39 | 2″-O-(2‴-methylbutanoyl)isogenistin | C20H33 O17 | VII | R1 = OCH3; R2 = OH; R3 = OH; R4 = CH2CH3 | nothing | 545.17 | [43] |
| 40 | 2″-O-(2‴-methylbutanoyl)isokanamycin A | C27H29 O11 | VII | R1 = OCH3; R2 = H; R3 = OH; R4 = CH2CH3 | nothing | 529.17 | [43] |
| 41 | 2″-O-isopropylbenzoyl-isokanamycin A | C32H31O14 | VII | R1 = OCH3; R2 = OH; R3 = OH; R4 = CH3 | nothing | 639.17 | [46] |
| 42 | 3″-O-veratroyl orientin | C29H26O14 | VIII | R = OH | nothing | 599.13 | [44] |
| 43 | 3″-O-veratroyl vitexin | C31H28O13 | VIII | R = H | nothing | 608.56 | [44] |
| 44 | 2″-O-benzoylisorhamnetin | C29H26O12 | IX | nothing | nothing | 567.14 | [44] |
| 45 | 3″-O-Acetylquercetin | C31H28O13 | X | nothing | nothing | 609.16 | [44] |
| 46 | 2″-O-Vanilloylquercetin | C29H26O14 | XI | R1 = OH; R2 = OH; R3 = OH; R4 = OH | nothing | 599.13 | [44] |
| 47 | 2″-O-(3‴,4‴-dimethoxybenzoyl)isorhamnetin | C31H30O14 | XI | R1 = OCH3; R2 = OH; R3 = OH; R4 = OCH3 | nothing | 626.16 | [45] |
| 48 | 2″-O-(3‴,4‴-dimethoxybenzoyl) isoswertisin | C31H30O13 | XI | R1 = OCH3; R2 = H; R3 = OH; R4 = OCH3 | nothing | 610.17 | [45] |
| 49 | 2″-O-(3‴,4‴-dimethoxybenzoyl)isodaidzein | C30H28O13 | XI | R1 = OH; R2 = H; R3 = OH; R4 = OCH3 | nothing | 595.14 | [47] |
| 50 | 2″-O-(3‴,4‴-dimethoxybenzoyl)quercetin | C30H28O14 | XI | R1 = OH; R2 = OH; R3 = OH; R4 = OCH3 | nothing | 611.14 | [47] |
| 51 | 2″-O-vanilloylisorhamnetin | C30H28O13 | XI | R1 = OCH3; R2 = H; R3 = OH; R4 = OH | nothing | 597.16 | [48] |
| 52 | 2″-O-vanilloylquercetin | C29H26O13 | XI | R1 = OH; R2 = H; R3 = OH; R4 = OH | nothing | 582.5 | [45] |
| 53 | Salvigenin | C18H16O6 | XII | R1 = H; R2 = OCH3; R3 = OCH3; R4 = OH; R5 = H; R6 = H; R7 =OCH3; R8 = H | 19103-54-9 | 328.31 | [8] |
| 54 | Acacetin | C16H12O5 | XII | R1 = H; R2 = OH; R3 = H; R4 = OH; R5 = H; R6 = H; R7 = OCH3; R8 = H | 480-44-4 | 284.26 | [8] |
| 55 | Apigenin | C15H10O5 | XII | R1 = H; R2 = OH; R3 = H; R4 = OH; R5 = H; R6 = H; R7 = OH; R8 = H | 520-36-5 | 270.24 | [8] |
| 56 | Pectolinarigenin | C17H14O6 | XII | R1 = H; R2 = OH; R3 = OCH3; R4 = OH; R5 = H; R6 = H; R7 = OCH3; R8 = H | 520-12-7 | 314.29 | [8] |
| 57 | Cirsimaritin | C17H14O6 | XII | R1 = H; R2 = OCH3; R3 = OCH3; R4 = OH; R5 = H; R6 = H; R7 = OH; R8 = H | 6601-62-3 | 314.29 | [8] |
| 58 | Luteolin | C15H10O6 | XII | R1 = H; R2 = OH; R3 = H; R4 = OH; R5 = H; R6 = OH; R7 = OH; R8 = H | 491-70-3 | 286.24 | [8] |
| 59 | Quercetin | C15H10O7 | XII | R1 = H; R2 = OH; R3 = H; R4 = OH; R5 = OH; R6 = OH; R7 = OH; R8 = H | 73123-10-1 | 302.23 | [8] |
| 60 | Naringenin | C15H12O5 | XII | R1 = H; R2 = OH; R3 = H; R4 = OH; R5 = H; R6 = H; R7 = OH; R8 = H | 480-41-1 | 272.25 | [8] |
| 61 | Chrysoeriol | C16H12O6 | XII | R1 = H; R2 = OH; R3 = H; R4 = OH; R5 = H; R6 = OCH3; R7 = OH; R8 = H | 491-71-4 | 300.26 | [8] |
| 62 | Diosmetin | C16H12O6 | XII | R1 = H; R2 = OH; R3 = H; R4 = OH; R5 = H; R6 = OH; R7 = OCH3; R8 = H | 520-34-3 | 300.26 | [8] |
| 63 | Farnisin | C16H12O5 | XII | R1 = H; R2 = OH; R3 = H; R4 = H; R5 = H; R6 = OH; R7 = OCH3; R8 = H | 54867-60-6 | 284.26 | [8] |
| 64 | Kaempferol | C15H10O6 | XII | R1 = H; R2 = OH; R3 = H; R4 = OH; R5 = OH; R6 = H; R7 = OH; R8 = H | 520-18-3 | 286.24 | [8] |
| 65 | Myricetin | C15H10O8 | XII | R1 = H; R2 = OH; R3 = H; R4 = OH; R5 = OH; R6 = OH; R7 = OH; R8 = OH | 529-44-2 | 318.23 | [8] |
| 66 | Neodiosmin | C28H32O15 | XII | R1 = H; R2 = O-rutinoside; R3 = H; R4 = OH; R5 = H; R6 = H; R7 = OCH3; R8 = H | 38665-01-9 | 608.54 | [8] |
| 67 | 8-C-β-d-pyranosyl catechin | C21H20O10 | XII | R1 = D-xyl; R2 = H; R3 = H; R4 = OH; R5 = H; R6 = OH; R7 = OCH3; R8 = H | nothing | 432.38 | [9] |
| 68 | 7-O-viciafuranosyl quercetin | C28H32O14 | XII | R1 = H; R2 = O-rutinoside; R3 = H; R4 = OH; R5 = H; R6 = OH; R7 = OCH3; R8 = H | nothing | 593.19 | [47] |
| 69 | 7-O-naringenin rutinoside | C28H32O14 | XII | R1 = H; R2 = O-neohesperidoside; R3 = H; R4 = OH; R5 = H; R6 = OH; R7 = OCH3; R8 = H | 20633-93-6 | 607.16 | [45] |
| 70 | Quercetin-3-O-β-l-rhamnoside | C21H20O11 | XII | R1 = H; R2 = OH; R3 = H; R4 = OH; R5 = O-β-l-rhamnoside; R6 = OH; R7 = OH; R8 = H | 522-12-3 | 448.38 | [9] |
| 71 | Quercetin-3-O-β-d-glucopyranoside | C21H20O11 | XII | R1 = H; R2 = OH; R3 = H; R4 = OH; R6 = OH; R7 = OH; R5 = O-β-d-glucopyrano- side; R8 = H | 21637-25-2 | 448.37 | [9] |
| 72 | 5-Hydroxy-4′,7,8-trimethoxyflavone | C18H16O6 | XII | R1 = OCH3; R2 = OCH3; R3 = H; R4 = OH; R5 = H; R6 = H; R7 = OCH3; R8 = H | 57096-03-4 | 328.09 | [8] |
| 73 | 4′,5-Dihydroxy-7,8-dimethoxyflavone | C17H14O6 | XII | R1 = OCH3; R2 = OCH3; R3 = H; R4 = OH; R5 = H; R6 = H; R7 = OH; R8 = H | 6608-33-9 | 314.08 | [8] |
| 74 | 6‴-(3-hydroxy-3-methylbutanoyl)-2″-O-β-d-pyranosyl-hongcaoside | C33H38O20 | XIII | R = OH | nothing | 777.18 | [12] |
| 75 | 6‴-(3-hydroxy-3-methylbutanoyl)-2″-O-β-d-pyranosylmatrine | C33H38O19 | XIII | R = H | nothing | 761.19 | [12] |
| 76 | 2″-O-veratroylvitexin | C30H28O13 | XIV | nothing | nothing | 596.15 | [8] |
| 77 | Isodaphnetin-2″-O-(6-O-feruloyl)-β-l-lactoside | C37H38O18 | XV | nothing | nothing | 771.21 | [49] |
| 78 | Hyperoside | C21H20O12 | XVI | R1 = OH; R2 = OH; R3 = CH2OH | 482-36-0 | 464.40 | [8] |
| 79 | Naringenin 3-(6″-ethyl glucuronide) | C23H24O10 | XVI | R1 = OH; R2 = H; R3 = COOCH2CH3 | nothing | 460.14 | [8] |
| 80 | Astragalin | C21H20O11 | XVI | R1 = OH; R2 = H; R3 = CH2OH | 480-10-4 | 448.40 | [8] |
| 81 | Eriodictyol | C15H12O6 | XVII | nothing | 552-58-9 | 288.25 | [8] |
| 82 | 2″-O-(2‴-O-methybutyryl)-glucopyranosyl isoswertisin | C33H40O16 | XVIII | nothing | nothing | 691.22 | [50] |
| 83 | 2″-O-(6‴-O-veratroyl)-galactopyranosyl vitexin | C36H38O18 | XIX | nothing | nothing | 759.21 | [50] |
| 84 | 2″-O-(6‴-O-caffeoyl)-galactopyranosyl vitexin | C36H36O18 | XX | R1 = H; R2 = OH; R3 = H; R4 = OH | nothing | 757.19 | [50] |
| 85 | 2″-O-(6‴-O-feruloyl)-galactopyranosyl orientin | C37H38O19 | XX | R1 = OH; R2 = OCH3; R3 = H; R4 = OH | nothing | 787.20 | [50] |
| 86 | Trollichinenside A(3″-O-veratroylvitexin) | C36H35O18 | XX | R1 = OH; R2 = OH; R3 = H; R4 = OH | nothing | 755.18 | [8] |
| 87 | Trollichinenside B (3″-O- feruloylvitexin) | C38H40O20 | XX | R1 = OH; R2 = OCH3; R3 = OCH3; R4 = OH | nothing | 816.21 | [8] |
| 88 | Trollichinenside C (6″-O-veratroylvitexin) | C38H40O19 | XX | R1 = OH; R2 = H; R3 = OCH3; R4 = OCH3 | nothing | 800.22 | [8] |
| 89 | Daidzin | C21H20O9 | XXI | R1 = H; R2 = OH | 552-66-9 | 416.41 | [8] |
| 90 | Kaempferol-7-O-β-d-glucoside | C22H22O10 | XXI | R1 = OH; R2 = OCH3 | nothing | 446.12 | [8] |
| 91 | Glucosylorientin | C27H30O17 | XXII | nothing | 76135-83-6 | 626.5 | [8] |
| 92 | 6″-Malonylcosmosiin | C24H22O13 | XXIII | nothing | 86546-87-4 | 518.4 | [8] |
| 93 | (-)-Gallocatechi | C15H14O7 | XXIV | nothing | nothing | 306.27 | [8] |
| 94 | Quercetin-2″-O-β-l-arabinopyranoside | C27H30O16 | XXV | nothing | 861691-37-4 | 610.15 | [8] |
| 95 | Apigenin-8-C-(2″-O-feruloyl)-β-d-glucoside | C31H28O13 | XXVI | nothing | nothing | 608.15 | [8] |
| 96 | (2E)-2-methyl-1-O-vanilloyl-4-β-d-glucopyrano-side-2-butene | C19H26O10 | XXVII | nothing | nothing | 437.14 | [46] |
| 97 | Neocarlinoside | C26H28O15 | XXVIII | nothing | 83151-89-7 | 580.5 | [8] |
| 98 | 4′,5-dihydroxy-3′,7-dimethoxy-isoflavone | C17H14O6 | XXIX | R1 = OCH3; R2 = H; R3 = OH; R4 = OCH3 | nothing | 314.08 | [8] |
| 99 | Glycitein | C16H12O5 | XXIX | R1 = OH; R2 = OCH3; R3 = H; R4 = H | 40957-83-3 | 284.26 | [8] |
| 100 | Daidzein | C15H10O4 | XXIX | R1 = OH; R2 = H; R3 = H; R4 = H | 486-66-8 | 254.23 | [8] |
| No | Names | Molecular Formula | Parent Nucleus | Substituent | CAS | Molecular Weight | Refs. |
|---|---|---|---|---|---|---|---|
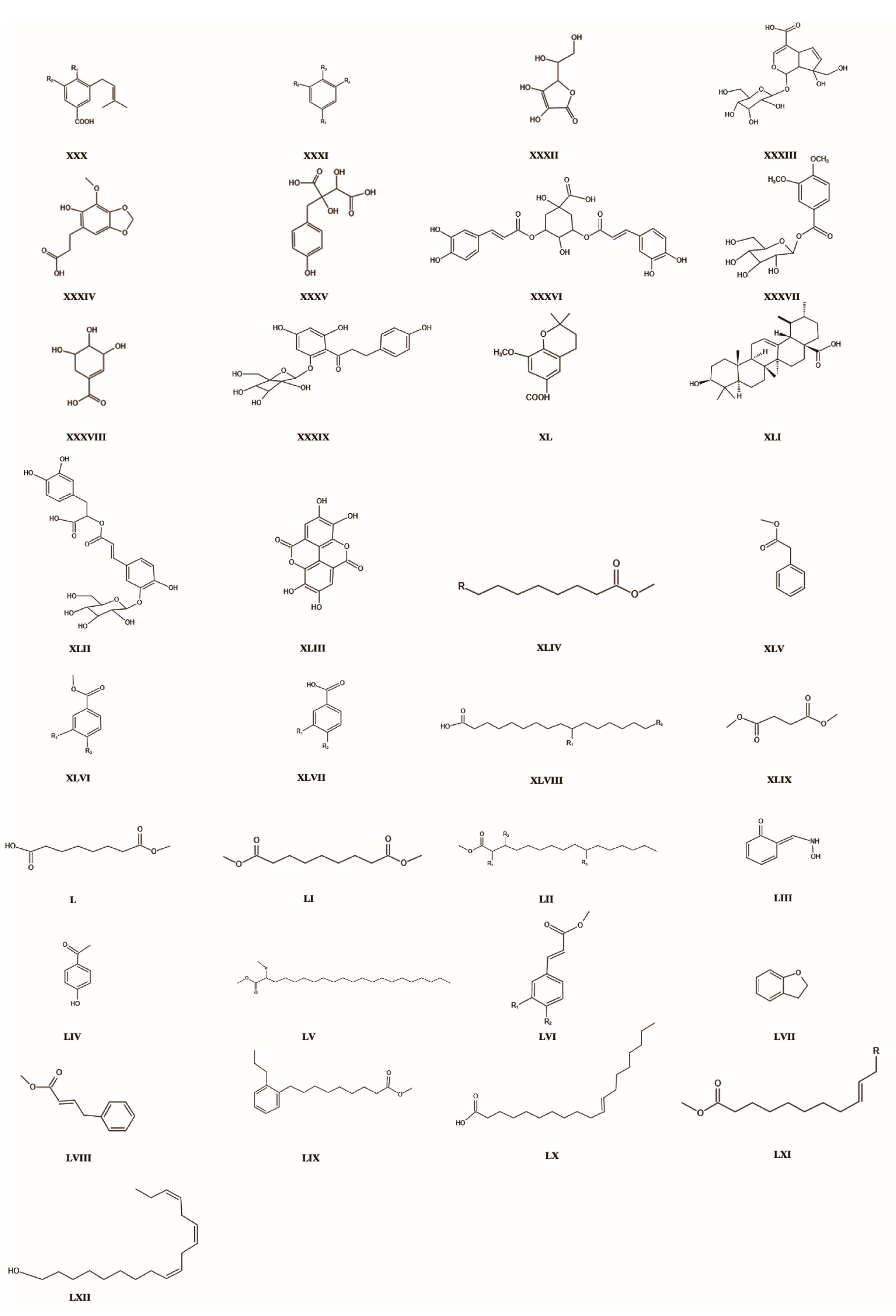
| 101 | Trollioside | C19H26O9 | XXX | R1 = O-β-d-glucopyranosyl; R2 = OCH3 | nothing | 399.16 | [9] |
| 102 | Proglobellowery acid | C7H6O2 | XXX | R1 = OH; R2 = OCH3 | nothing | 235.00 | [9] |
| 103 | 4-(β-d-glucopyranosyloxy)-3- (3-methyl-2-butenyl)benzoic acid | C18H24O8 | XXX | R1 = O-β-d-glucopyranosyl; R2 = H | nothing | 368.38 | [9] |
| 104 | 4-Hydroxybenzoic acid | C7H6O3 | XXXI | R1 = COOH; R2 = H; R3 = OH; R4 = H | 99-96-7 | 138.03 | [9] |
| 105 | 3,4-dihydroxybenzoic acid methyl ester | C8H8O2 | XXXI | R1 = COOCH3; R2 = OH; R3 = OH; R4 = H | 2150-43-8 | 152.05 | [9] |
| 106 | Methylparaben | C6H4O3 | XXXI | R1 = COOCH3; R2 = H; R3 = OH; R4 = H | 35816-31-0 | 152.05 | [9] |
| 107 | Protocatechuic acid | C7H6O4 | XXXI | R1 = COOH; R2 = OH; R3 = OH; R4 = H | 99-50-3 | 154.12 | [9] |
| 108 | Methyl veratrate | C10H12O4 | XXXI | R1 = COOCH3; R2 = OCH3; R3 = OCH3; R4 = H | 2150-38-1 | 196.20 | [9] |
| 109 | Benzoic acid | C7H6O2 | XXXI | R1 = COOH; R2 = H; R3 = H; R4 = H | 117500-35-3 | 122.12 | [9] |
| 110 | Veratric acid | C9H10O4 | XXXI | R1 = COOH; R2 = OCH3; R3 = OCH3; R4 = H | 93-07-2 | 182.17 | [9] |
| 111 | Vanillic acid | C8H8O4 | XXXI | R1 = COOH; R2 = OCH3; R3 = OH; R4 = H | 121-34-6 | 168.14 | [8] |
| 112 | Gallic acid | C7H6O5 | XXXI | R1 = COOH; R2 = OH; R3 = OH; R4 = OH | 149-91-7 | 170.12 | [8] |
| 113 | 4-Hydroxy-2,6-dimethoxybenzaldehyde | C9H10O4 | XXXI | R1 = CHO; R2 = OCH3; R3 = OH; R4 = OCH3 | 22080-96-2 | 182.17 | [8] |
| 114 | Monotropein | C16H22O11 | XXXII | nothing | 5945-50-6 | 390.33 | [8] |
| 115 | Ascorbic acid | C6H8O6 | XXXIII | nothing | 299-36-5 | 176.13 | [8] |
| 116 | 3-(6-hydroxy-7-methoxy-2H-1,3-benzodioxol-5-yl)propanoic acid | C11H12O6 | XXXIV | nothing | nothing | 240.06 | [8] |
| 117 | (2R,3S)-Piscidic acid | C11H12O7 | XXXV | nothing | 469-65-8 | 256.06 | [8] |
| 118 | Isochlorogenic acid A | C25H24O12 | XXXVI | nothing | 2450-53-5 | 516.46 | [8] |
| 119 | Tecomin | C15H20O9 | XXXVII | nothing | 31002-27-4 | 344.31 | [8] |
| 120 | Shikimic acid | C7H10O5 | XXXVIII | nothing | 138-59-0 | 174.15 | [8] |
| 121 | Phlorizin dihydrate | C21H26O11 | XXXIX | nothing | 7061-54-3 | 454.43 | [8] |
| 122 | Globeflowery acid | C13H16O4 | XL | nothing | 4041-28-5 | 236.26 | [8] |
| 123 | Ursolic acid | C30H48O3 | XLI | nothing | 77-52-1 | 456.71 | [8] |
| 124 | Salviaflaside | C24H26O13 | XLII | nothing | 178895-25-5 | 522.46 | [8] |
| 125 | Rhynchophylline | C14H6O8 | XLIII | nothing | 76-66-4 | 302.19 | [8] |
| 126 | Methyl dodecanoate | C13H26O2 | XLIV | R = H | 111-82-0 | 214.34 | [53] |
| 127 | Methyl tridecanoate | C14H2802 | XLIV | R = CH2CH3 | 1731-88-0 | 228.37 | [53] |
| 128 | Methyl tetradecanoate | C15H30O2 | XLIV | R = (CH2)3CH3 | 124-10-7 | 242.40 | [53] |
| 129 | Methyl pentadecanoate | C16H32O2 | XLIV | R = (CH2)4CH3 | 7132-64-1 | 256.42 | [53] |
| 130 | Methyl hexadecanoate | C17H34O2 | XLIV | R = (CH2)7CH | 112-39-0 | 270.45 | [53] |
| 131 | Methyl heptadecanoate | C18H36O2 | XLIV | R = (CH2)8CH3 | 1731-92-6 | 284.47 | [53] |
| 132 | Methyl octadecanoate | C19H38O2 | XLIV | R = (CH2)9CH3 | 112-61-8 | 298.50 | [53] |
| 133 | Methyl eicosanoate | C21H42O2 | XLIV | R = (CH2)11CH3 | 22589-04-4 | 326.55 | [53] |
| 134 | Methyl docosanoate | C23H46O2 | XLIV | R = (CH2)13CH3 | 929-77-1 | 354.61 | [53] |
| 135 | Methyl tetracosanoate | C25H50O2 | XLIV | R = (CH2)15CH3 | 2442-49-1 | 382.66 | [53] |
| 136 | Methyl decanoate | C11H22O2 | XLIV | R = CH2CH3 | 110-42-9 | 186.29 | [53] |
| 137 | Methyl octanoate | C9H18O2 | XLIV | R = H | 111-11-5 | 158.23 | [53] |
| 138 | Methyl benzeneacetate | C9H10O2 | XLV | nothing | 143390-89-0 | 150.17 | [53] |
| 139 | Methyl benzoate | C8H8O2 | XLVI | R1 = H, R2 = H | 36712-21-7 | 136.15 | [53] |
| 140 | 3,4-dimethoxybenzoic acid methyl ester | C10H12O4 | XLVI | R1 = OCH3; R2 = OCH3 | 2150-38-1 | 196.2 | [53] |
| 141 | Dimethyl 3-hydroxy-2-methyl-glutarate | C8H1405 | XLVII | nothing | nothing | 190.19 | [53] |
| 142 | N-hexadecanoic acid | C16H32O2 | XLVIII | R1 = H; R2 = H | 57-10-3 | 256.42 | [53] |
| 143 | Hexadecanoic acid,10,16-dihydroxy | C16H32O4 | XLVIII | R1 = OH; R2 = OH | 3233-90-7 | 288.42 | [53] |
| 144 | Dimethyl butanedioate | C6H10O4 | XLIX | nothing | 106-65-0 | 146.14 | [53] |
| 145 | Dimethyl octanedioate | C10H18O4 | L | nothing | 1732-09-8 | 202.24 | [53] |
| 146 | Dimethyl nonanedioate | C11H26O4 | LI | nothing | 1732-10-1 | 216.27 | [53] |
| 147 | 2-hydroxyhexadecanoic acid methyl ester | C17H34O3 | LII | R1 = OH; R2 = H; R3 = H | 78330-57-1 | 286.45 | [53] |
| 148 | 3-hydroxyhexadecanoic acid methyl ester | C17H34O3 | LII | R1 = H; R2 = OH; R3 = H | 51883-36-4 | 286.45 | [53] |
| 149 | 10-hydroxyhexadecanoic acid methyl ester | C17H34O3 | LII | R1 = H; R2 = H; R3 = OH | 56247-30-4 | 286.45 | [53] |
| 150 | 2-hydroxy-benzaldehyde oxime | C7H7NO2 | LIII | nothing | 94-67-7 | 137.14 | [53] |
| 151 | 4-hydroxy-acetophenone | C8H8O2 | LIV | nothing | 99-93-4 | 136.15 | [53] |
| 152 | 2-methoxydocosyl methanoate | C25H50O3 | LV | nothing | nothing | 398.66 | [53] |
| 153 | 3-phenylprop-2-enoic acid methyl ester | C11H12O | LVI | R1 = H; R2 = H | 103-26-4 | 160.00 | [53] |
| 154 | 3-(4-hydroxyphenyl)prop-2-enoic acid methyl ester | C10H10O3 | LVI | R1 = H; R2 = OH | 61240-27-5 | 178.00 | [53] |
| 155 | (4-hydroxy-3-methoxyphenyl)-2-propenoic acid methyl ester | C11H12O4 | LVI | R1 = OCH3, R2 = OH | 34298-89-0 | 208.00 | [53] |
| 156 | 2,3-Dihydrobenzofuran | C8H8O | LVII | nothing | 496-16-2 | 120.15 | [53] |
| 157 | 4-phenyl-2-butenoic acid methyl ester | C11H12O | LVIII | nothing | 54966-43-7 | 176 | [53] |
| 158 | 9-(propoxybenzene)-nonanoic acid methyl ester | C19H30O2 | LIX | nothing | nothing | 290 | [53] |
| 159 | (E)-11-eicosenoic acid methyl ester | C21H40O2 | LX | nothing | nothing | 324 | [53] |
| 160 | (Z)-9-hexadecenoic acid methyl ester | C17H32O2 | LXI | R = (CH2)4CH3 | 1120-25-8 | 268.43 | [53] |
| 161 | (Z)-9-octadecenoic acid methyl ester | C19H36O2 | LXI | R = (CH2)6CH3 | 112-62-9 | 296.48 | [53] |
| 162 | (Z,Z)-9,12-octadecadienoic acid methyl ester | C19H32O4 | LXI | R = CH = CH(CH2)4CH3 | 168482-44-8 | 294.47 | [53] |
| 163 | (Z,Z,Z)-9,12,15-Octadecatrienoic acid methyl ester | C19H32O2 | LXI | R = CH = CHCH2CH = CHCH2CH3 | 301-00-8 | 292.46 | [53] |
| 164 | (Z,Z,Z)-9,12,15-ctadecatrien-1-ol | C18H32O | LXII | nothing | nothing | 264 | [53] |
| No | Names | Molecular Formula | Parent Nucleus | Substituent | CAS | Molecular Weight | Refs. |
|---|---|---|---|---|---|---|---|
| 165 | Senecionine | C18H25NO5 | LXIII | Nothing | 130-01-8 | 335.4 | [56] |
| 166 | Integerrimine | C18H25NO5 | LXIV | Nothing | 480-79-5 | 335.4 | [46] |
| 167 | Trolline | C12H13NO3 | LXV | Nothing | 1021950-79-7 | 219.24 | [9] |
| 168 | (R)-Cyanomethyl-3-hydroxyindole | C10H7O2N2 | LXVI | Nothing | Nothing | 187.05 | [46] |
| 169 | Adenine | C5H5N5 | LXVII | Nothing | 73-24-5 | 135.13 | [8] |
| No | Names | Molecular Formula | Parent Nucleus | Substituent | Characterization Method | Molecular Weight | Refs. |
|---|---|---|---|---|---|---|---|
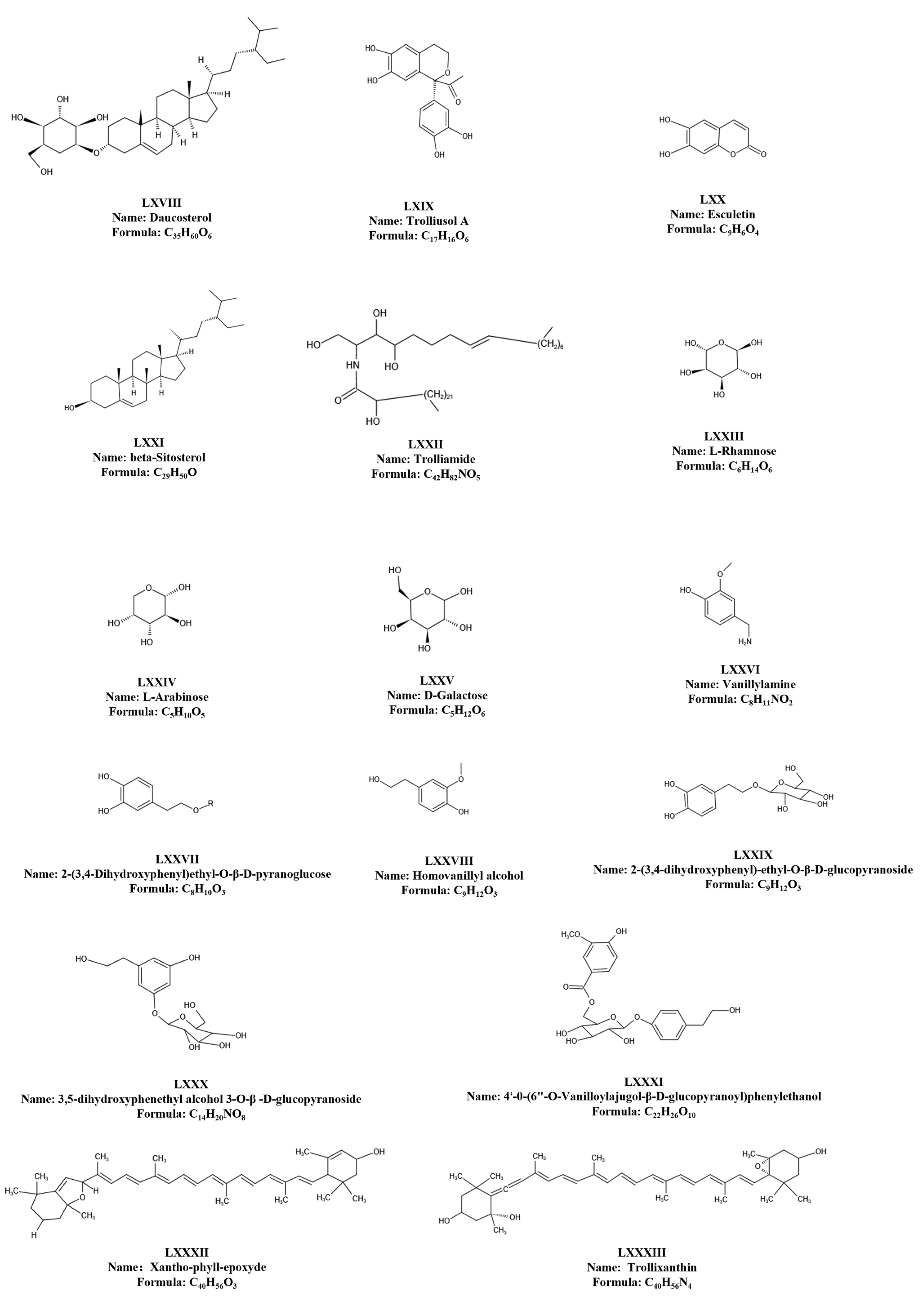
| 168 | Daucosterol | C35H60O6 | LXVIIII | nothing | 474-58-8 | 576.85 | [8] |
| 169 | Trolliusol A | C17H16O6 | LXIX | nothing | nothing | 316.30 | [8] |
| 170 | Esculetin | C9H6O4 | LXX | nothing | 305-01-1 | 178.14 | [8] |
| 171 | β-Sitosterol | C29H50O | LXXI | nothing | 5779-62-4 | 414.71 | [8] |
| 172 | Trolliamide | C42H82NO5 | LXXII | nothing | nothing | 680.62 | [11] |
| 173 | L-Rhamnose | C6H14O6 | LXXIII | nothing | 6155-35-7 | 182.17 | [8] |
| 174 | L-Arabinose | C5H10O5 | LXXIV | nothing | 5328-37-0 | 150.13 | [8] |
| 175 | D-Galactose | C6H12O6 | LXXV | nothing | 59-23-4 | 180.15 | [8] |
| 176 | Vanillylamine | C8H11NO2 | LXXVI | nothing | 1196-92-5 | 153.18 | [8] |
| 177 | 2-(3,4-Dihydroxyphenyl)ethyl-O-β-d-pyranoglucose | C8H10O3 | LXXVII | R = H | 10597-60-1 | 154.16 | [8] |
| 178 | Homovanillyl alcohol | C9H12O3 | LXXVIII | nothing | 2380-78-1 | 168.19 | [8] |
| 179 | 2-(3,4-dihydroxyphenyl)-ethyl-O-β-d-glucopyranoside | C14H20O8 | LXXIX | nothing | nothing | 315.10 | [8] |
| 180 | 3,5-dihydroxyphenethyl alcohol 3-0-β-d-glucopyranoside | C14H20O8 | LXXX | nothing | 52674-86-9 | 315.10 | [8] |
| 181 | 4′-O-(6″-O-Vanilloylajugol-β-d-glucopyranoyl)phenylethanol | C22H26O10 | LXXXI | nothing | 27606-08-2 | 450.44 | [8] |
| 182 | Xantho-phyll-epoxyde | C40H56O3 | LXXXII | nothing | nothing | 584.87 | [64] |
| 183 | Trollixanthin | C40H56N4 | LXXXIII | nothing | 14660-91-4 | 592.90 | [64] |
| Pharmacological Effects | Extracts/Compounds | Animals/Cells | Dosage/Concentration | Effects/Mechanisms | References |
|---|---|---|---|---|---|
| Antiviral | The crude extract from the flowers of T. chinensis | ICR mice | 0.2 mg/g/d | The T. chinensis crude extract treatment resulted in a significant increase in the body weight percentage, a decrease in the number of white blood cells, and a lowered lung index among mice infected with influenza virus A/FM/1/47 (H1N1) virus. | [115] |
| Orientin | Hep 2 cell | 0.1 mL of maintenance medium containing serial two-fold dilutions of the tested compounds 0.1 mL of maintenance medium without the test compound was added | The flavonoids isolated from T. chinensis, Orientin, and Vitexin, possess strong anti-viral activities against Para 3. Proglobeflowery acid showed weak antiviral activity against Para 3. | [10] | |
| Vitexin | |||||
| Proglobeflowery acid | |||||
| The crude extract from the flowers of T. chinensis | ICR mice | 0.2 mg/g/d | The crude extract from the flowers of T. chinensis was found to inhibit the increased expression of TLR3, TBK1, TAK1, and IRF3 induced by the high-dose influenza virus and treat mice infected with influenza virus by activating the TLR3 signaling pathway. | [1] | |
| Veratric acid | RAW264.7 cell | 50, 100, 200, 400, and 800 μmol/L | The three representative compounds play a role in anti-H1N1 viral effects by regulating the TLR 3, 4, and 7 pathways, counteracting the inflammatory damage caused by excessive production of NO, IL-1, IL-6, and TNF induced by viral infection, and promoting the production of IFN- to eliminate the virus. | [116] | |
| Vitexin | |||||
| Trolline | |||||
| Piscidic acid | EV71-infected RD cells | The T. chinensis mother liquor was diluted by a factor of 20, 2−1, 2−2, 2−3, 2−4, and 100 μL was dispensed into each well of the cell culture plate | The viral inhibition rate of T. chinensis ranges from 49.64% to 73.69%. It exhibits a clear inhibitory effect on the EV71 virus, and the three compounds form the foundation of T. chinensis’ anti-EV71 material. | [25] | |
| 2″-Oacetylorientin | |||||
| 2-(4-hydroxybenzyl) malic acid | |||||
| Antioxidant | Orientin | Not applicable | 46/5.64/5.19/3.97 mg/mL | Under in vitro conditions, phenolic and flavonoid compounds could efficiently scavenge a variety of ROS or DPPH-free radicals. | [117] |
| Vitexin | |||||
| Proglobeflowery acid | |||||
| Orientin | KM mice | 40/20/10 mg/kg | Slowing down d-galactose-induced aging by enhancing the activity of antioxidant enzymes, eliminating excessive oxygen free radicals, and mitigating damage to cells and tissues. | [118] | |
| Vitexin | |||||
| Antiinflammatory | Trolliusditerpenosides A-Q (1–17) | RAW 264.7 cells mediated by LPS | Not applicable | The inhibitory effects on LPS-induced NO (pro-inflammatory mediator nitric oxide) release in RAW 264.7 cells by diterpenoid glycosides from T. chinensis. | [14] |
| Orientin | RAW264.7 cells | 0, 25, 50, 100, 200, and 400 µmol/L−1 | The production of NO, IL-6, and TNF-stimulated cells decreased. | [119] | |
| Vitexin | |||||
| Quercetin | |||||
| Isoquercetin | |||||
| Veratric acid | |||||
| Proglobeflowery acid | |||||
| Trollioside | |||||
| 2″-O-β-l-galactopyranosy-orientin | |||||
| Luteolin | RAW264.7 cells | 0, 12.5, 25, 50, 100, 200, and 400 µmol·L−1 | The production of NO, IL-6, and TNF-stimulated cells decreased. | [119] | |
| Trolline | |||||
| Aqueous extract of the stem and leaves of T. chinensis | KM mice | Distilled water: 20 mL/kg Positive drug: 100 mg/kg Aqueous extract of stem and leaves of T. chinensis (low/high): 12 g/kg/24 g/kg Alcohol extract of the stem and leaves of T. chinensis (low/high): 12 g/kg/24 g/kg | T. chinensis has some anti-inflammatory effects on stem and leaf extracts. | [111] | |
| Alcohol extract of the stem and leaves of T. chinensis | |||||
| Antitumor | Total flavonoids | MCF-7 cells | 0/0.0991/0.1982/0.3964/0.7928/1.5856 mg/mL | Flavonoids were found to suppress growth and induce apoptosis in MCF-7 cells. | [93] |
| Orientin | EC-109 cells | 5.0, 10.0, 20.0, 40.0, and 80.0 µM | Orientalin and Vitexin reduce apoptosis in human esophageal cancer EC-109 cells by regulating oncogenes and tumorigenic genes. | [120] | |
| Vitexin | |||||
| Antibacterial | Trolliusol A | M. albicans E. coli P. aeruginosa B. subtilis S. aureus | Drug concentrations: 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, 1:512, 1:1024, 1:2048, and 1:4096 | Minimal Inhibitory Concentration (MIC) was achieved by the microbroth method to achieve inhibition efficiency. | [41] |
| 1-(3′,4′-dihydroxyphenyl)-6,7-dihydroxyisochroman | |||||
| (S)-1-(3′,4′-dihydroxyphenyl)-1-hydroxypropan-2-one | |||||
| 3,4-dihydroxyphenylethanol | |||||
| 2″-O-(2‴-methylbutyryl)isoswertisin | |||||
| 3″-O-(2‴-methylbutyryl)isoswertisin | |||||
| Isoswertisin | |||||
| Orientin | |||||
| Water extracts from T. chinensis | Microorganism S. mutans | 50/25/12.5/6.25/3.125 (mg/mL) | T. chinensis has antibacterial and anti-inflammatory effects and can be used against mutant baculoviruses. Thirty percent ethanol extractexhibited the best antibacterial and antibiofilm effects. | [121] | |
| 30% ethanol extracts fromT. chinensis | |||||
| 60% ethanol extracts from T. chinensis | |||||
| 90% ethanol extracts from T. chinensis | |||||
| Antiaging | Orientin | KM mice | 40/20/10 mg/kg | It can enhance the activity of antioxidant enzymes, eliminate excessive oxygen-free radicals, and reduce the damage to cells and tissues so as to delay the senescence caused by D-galactose. | [118] |
| Vitexin | |||||
| Antipyretic | Flavonoids | New Zealand rabbits | Flavonoids 200 mg·kg−1 group Flavonoids 100 mg·kg−1 group Flavonoids 50 mg·kg−1 group Asprin 100 mg·kg−1 group | By inhibiting the expression of TNF-α and IL-1β in serum and PGE2 in cerebrospinal fluid. | [110] |
| Analgesic | Aqueous extract of stem and leaves of T. chinensis | KM mice | Distilled water: 20 mL/kg Positive drug: 100 mg/kg Aqueous extract of stem and leaves of T. chinensis (low/high): 12 g/kg/24 g/kg Alcohol extract of the stem and leaves of T. chinensis (low/high): 12 g/kg/24 g/kg | T. chinensis extracts from stems and leaves have been shown to have some analgesic effects. | [111] |
| Alcohol extract of stem and leaves of T. chinensis | |||||
| The total flavones in T. chinensis | KM mice | 125, 250, and 2500 mg/kg | It may increase the pain threshold of the hot plate in mice and have analgesic effects. | [112] | |
| Antitussive and Expectorant | The total flavones in T. chinensis | KM mice | 125, 2250, and 2500 mg/kg | The total flavonoid extract of T. chinensis has obvious anti-tussive and expectorant effects. | [112] |
| Myocardial ischemia/reperfusion injury (MI/RI) | The total flavones in T. chinensis | SD rats | 50 mg/(kg·d)–100 mg/(kg·d) | The total flavones in T. chinensis protect the myocardium from MI/RI. | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Wang, Z.; Lu, J.; Qin, C.; He, J.; Ren, W.; Liu, X. Trollius chinensis Bunge: A Comprehensive Review of Research on Botany, Materia Medica, Ethnopharmacological Use, Phytochemistry, Pharmacology, and Quality Control. Molecules 2024, 29, 421. https://doi.org/10.3390/molecules29020421
He L, Wang Z, Lu J, Qin C, He J, Ren W, Liu X. Trollius chinensis Bunge: A Comprehensive Review of Research on Botany, Materia Medica, Ethnopharmacological Use, Phytochemistry, Pharmacology, and Quality Control. Molecules. 2024; 29(2):421. https://doi.org/10.3390/molecules29020421
Chicago/Turabian StyleHe, Lianqing, Zhen Wang, Jiaxin Lu, Chen Qin, Jiajun He, Weichao Ren, and Xiubo Liu. 2024. "Trollius chinensis Bunge: A Comprehensive Review of Research on Botany, Materia Medica, Ethnopharmacological Use, Phytochemistry, Pharmacology, and Quality Control" Molecules 29, no. 2: 421. https://doi.org/10.3390/molecules29020421
APA StyleHe, L., Wang, Z., Lu, J., Qin, C., He, J., Ren, W., & Liu, X. (2024). Trollius chinensis Bunge: A Comprehensive Review of Research on Botany, Materia Medica, Ethnopharmacological Use, Phytochemistry, Pharmacology, and Quality Control. Molecules, 29(2), 421. https://doi.org/10.3390/molecules29020421