Urchin-like Ce(HCOO)3 Synthesized by a Microwave-Assisted Method and Its Application in an Asymmetric Supercapacitor
Abstract
1. Introduction
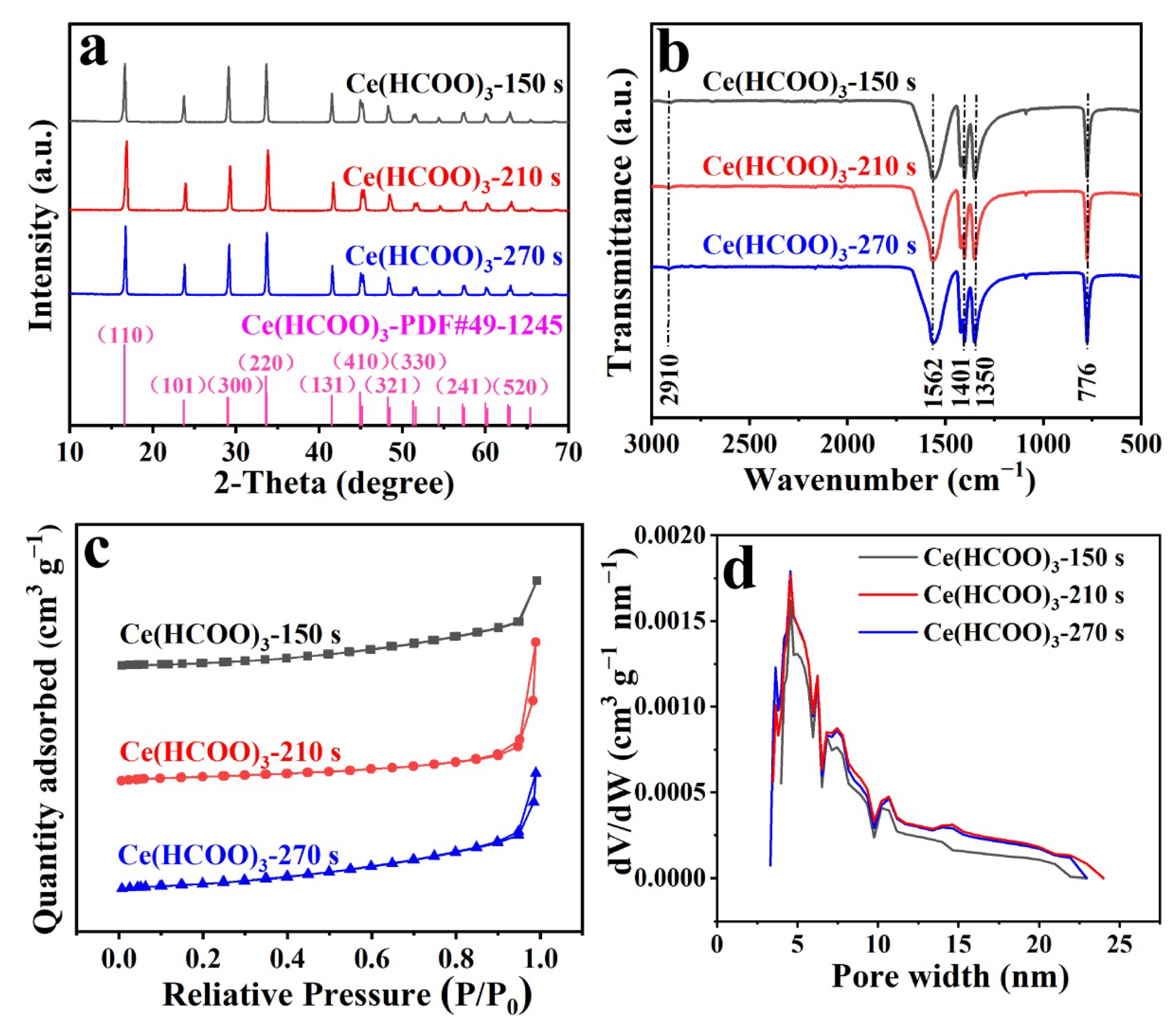
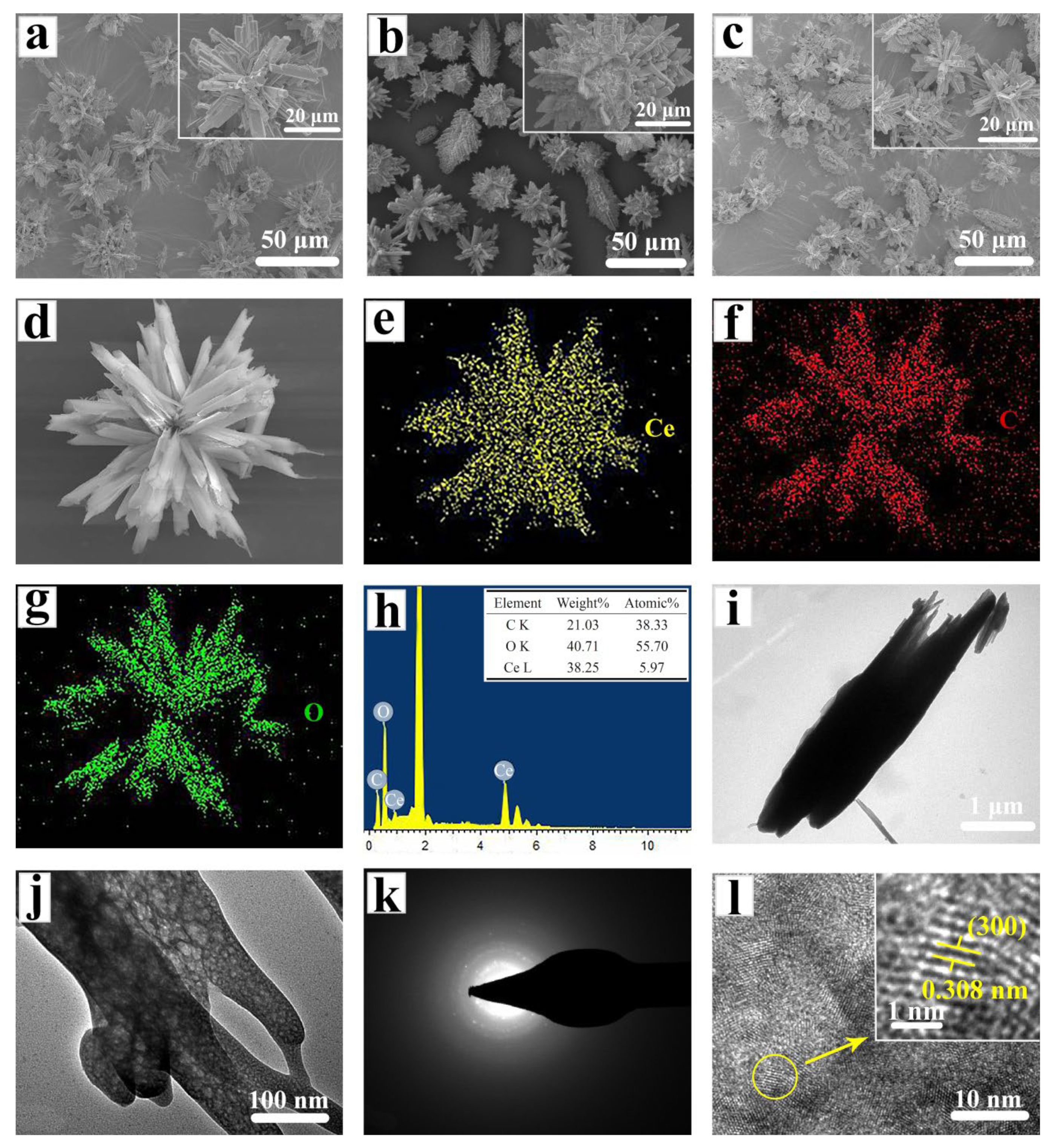
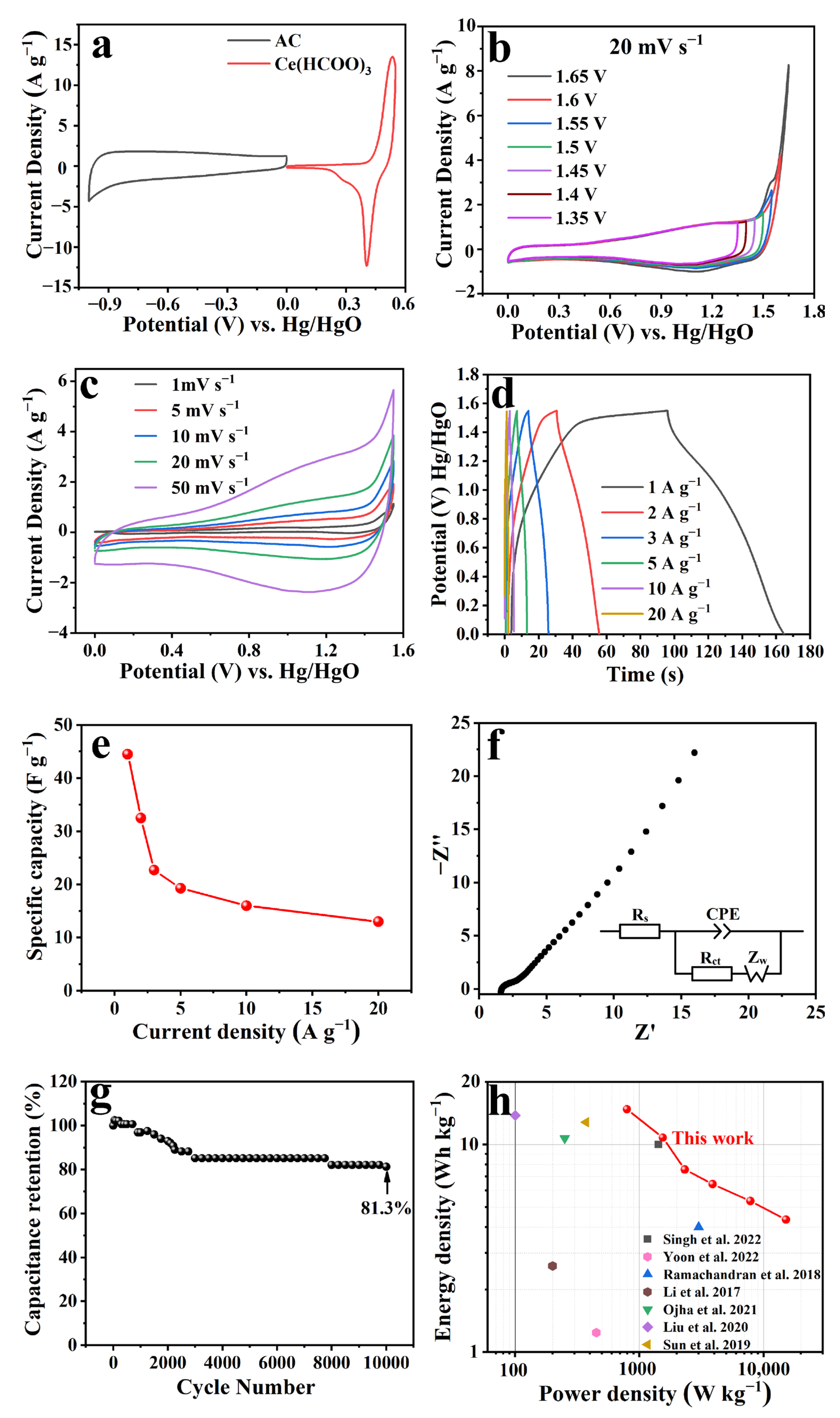
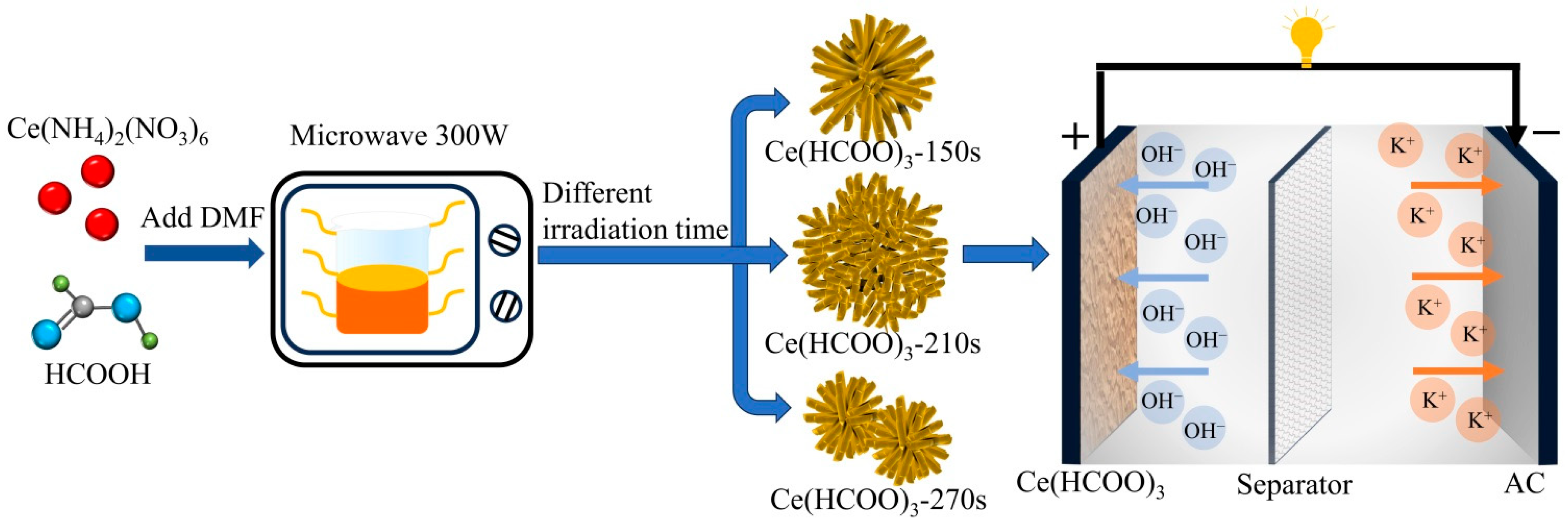
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Ce(HCOO)3 Electrode and Ce(HCOO)3//AC-Based ASC
3.3. Structural Characterization, Morphological Study, and Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Cui, J.; Wang, S.; Wu, A.; Xia, Y.; Jiang, Q.; Guo, J.; He, T.; Chen, P. Deforming lanthanum trihydride for superionic conduction. Nature 2023, 616, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Sulka, G.D. Electrochemistry of Thin Films and Nanostructured Materials. Molecules 2023, 28, 4040–4051. [Google Scholar] [PubMed]
- Li, X.; Pereira-Hernández, X.I.; Chen, Y.; Xu, J.; Zhao, J.; Pao, C.W.; Fang, C.Y.; Zeng, J.; Wang, Y.; Gates, B.C.; et al. Functional CeOx nanoglues for robust atomically dispersed catalysts. Nature 2022, 611, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Cevik, E.; Gunday, S.T.; Akhtar, S.; Bozkurt, A. A comparative study of various polyelectrolyte/nanocomposite electrode combinations in symmetric supercapacitors. Int. J. Hydrogen Energy 2019, 44, 16099–16109. [Google Scholar] [CrossRef]
- Cevik, E.; Gunday, S.T.; Akhtar, S.; Yamani, Z.H.; Bozkurt, A. Sulfonated Hollow Silica Spheres as Electrolyte Store/Release Agents: High-Performance Supercapacitor Applications. Energy Technol. 2019, 7, 1900511–1900519. [Google Scholar]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.L.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285–eaan8296. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.T.; Mei, L.; Liang, J.F.; Zhao, Z.P.; Lee, C.; Fei, H.L.; Ding, M.N.; Lau, J.; Li, M.F.; Wang, C.; et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef]
- Deng, W.N.; Li, Y.H.; Xu, D.F.; Zhou, W.; Xiang, K.X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Deng, W.A.; Liu, W.M.; Zhu, H.; Chen, L.; Liao, H.Y.; Chen, H. Click-chemistry and ionic cross-linking induced double cross-linking ionogel electrolyte for flexible lithium-ion batteries. J. Energy Storage 2023, 72, 108509–108518. [Google Scholar]
- Gao, Y.; Xie, C.; Zheng, Z.J. Textile composite electrodes for flexible batteries and supercapacitors: Opportunities and challenges. Adv. Energy Mater. 2021, 11, 2002838–2002846. [Google Scholar]
- Huang, J.; Xie, Y.P.; You, Y.; Yuan, J.L.; Xu, Q.Q.; Xie, H.B.; Chen, Y.W. Rational design of electrode materials for advanced supercapacitors: From lab research to commercialization. Adv. Funct. Mater. 2023, 33, 2213095–2213126. [Google Scholar] [CrossRef]
- Keum, K.; Kim, J.W.; Hong, S.Y.; Son, J.G.; Lee, S.S.; Ha, J.S. Flexible/stretchable supercapacitors with novel functionality for wearable electronics. Adv. Mater. 2020, 32, 2002180–2002213. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Montemor, M.F. Metal oxide and hydroxide–based aqueous supercapacitors: From charge storage mechanisms and functional electrode engineering to need-tailored devices. Adv. Sci. 2019, 6, 1801797–1801837. [Google Scholar] [CrossRef] [PubMed]
- Sirimanne, D.C.U.; Kularatna, N.; Arawwawala, N. Electrical Performance of Current Commercial Supercapacitors and Their Future Applications. Electronics 2023, 12, 2465–2476. [Google Scholar] [CrossRef]
- Zhai, Z.Z.; Zhang, L.H.; Du, T.M.; Ren, B.; Xu, Y.L.; Wang, S.S.; Miao, J.F.; Liu, Z.F. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017–111035. [Google Scholar]
- Wang, Y.F.; Zhang, L.; Hou, H.Q.; Xu, W.H.; Duan, G.G.; He, S.J.; Liu, K.M.; Jiang, S.H. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Li, W.W.; Liu, J.; Wei, J.N.; Yang, Z.Y.; Ren, C.L.; Li, B.X. Recent progress of conductive hydrogel fibers for flexible electronics: Fabrications, applications, and perspectives. Adv. Funct. Mater. 2023, 33, 2213485–2213512. [Google Scholar] [CrossRef]
- Luo, G.X.; Xie, J.Q.; Liu, J.L.; Zhang, Q.K.; Luo, Y.Y.; Li, M.; Zhou, W.K.; Chen, K.; Li, Z.K.; Yang, P.; et al. Highly conductive, stretchable, durable, breathable electrodes based on electrospun polyurethane mats superficially decorated with carbon nanotubes for multifunctional wearable electronics. Chem. Eng. J. 2023, 451, 138549–138560. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, K.; Hou, Y.; Zhang, Q.; Ye, Z.; Lu, J. Designing flexible, smart and self-sustainable supercapacitors for portable/wearable electronics: From conductive polymers. Chem. Soc. Rev. 2021, 50, 12702–12743. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, X.; Wang, S.L.; Liu, H.K.; Li, L. Transition metal based battery-type electrodes in hybrid supercapacitors: A review. Energy Storage Mater. 2020, 28, 122–145. [Google Scholar]
- Gan, Z.H.; Yin, J.Y.; Xu, X.; Cheng, Y.H.; Yu, T. Nanostructure and advanced energy storage: Elaborate material designs lead to high-rate pseudocapacitive ion storage. ACS Nano 2022, 16, 5131–5152. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.S.; Shehab, M.; Helmy, S.; Soliman, M.; Salama, R.S. Nickel and cobalt oxides supported on activated carbon derived from willow catkin for efficient supercapacitor electrode. J. Energy Storage 2023, 61, 106806–106817. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Cai, P.F.; Wei, Z.H.; Liu, T.; Yu, J.G.; Al-Ghamdi, A.A.; Wageh, S. Synthesis of reduced graphene oxide supported nickel-cobalt-layered double hydroxide nanosheets for supercapacitors. J. Colloid Interf. Sci. 2021, 588, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Jing, C.; Fu, X.; Shen, M.; Li, K.L.; Liu, X.Y.; Yao, H.C.; Zhang, Y.X.; Yao, K.X. Synthesis of porous NiCoS nanosheets with Al leaching on ordered mesoporous carbon for high-performance supercapacitors. Chem. Eng. J. 2020, 384, 123367–123376. [Google Scholar] [CrossRef]
- Chen, B.L.; Xu, L.L.; Xie, Z.Y.; Wong, W.Y. Supercapacitor electrodes based on metal-organic compounds from the first transition metal series. EcoMat 2021, 3, e12106–e12159. [Google Scholar] [CrossRef]
- Zhao, W.B.; Zeng, Y.T.; Zhao, Y.H.; Wu, X.L. Recent advances in metal-organic framework-based electrode materials for supercapacitors: A review. J. Energy Storage 2023, 62, 106934–106955. [Google Scholar] [CrossRef]
- Xu, G.Y.; Nie, P.; Dou, H.; Ding, B.; Li, L.Y.; Zhang, X.G. Exploring metal organic frameworks for energy storage in batteries and supercapacitors. Mater. Today 2017, 20, 191–209. [Google Scholar] [CrossRef]
- Wang, M.; Wu, T.; Bu, Q.; Song, X.; Li, M.; Yuan, S. Rare earth Ce based metal organic framework as efficient synergistic thermal stabilizer for PVC: Preparation and thermal stabilization behavior. Thermochim. Acta 2022, 718, 179365–179372. [Google Scholar] [CrossRef]
- Wang, Z.J.; He, P.J.; Zhang, H.; Zhang, N.; Lü, F. Desalination, nutrients recovery, or products extraction: Is electrodialysis an effective way to achieve high-value utilization of liquid digestate? Chem. Eng. J. 2022, 446, 136996–137009. [Google Scholar] [CrossRef]
- He, Q.; Wang, W.L.; Yang, N.; Chen, W.M.; Yang, X.; Fang, X.; Zhang, Y.X. Ultra-High Cycling Stability of 3D Flower-like Ce(COOH)3 for Supercapacitor Electrode via a Facile and Scalable Strategy. Molecules 2023, 28, 6806–6819. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.L.; Ghosh, T.K.; Mishra, V.; Ramasamy, S.; Sahoo, M.K.; Gangavarapu, R.R. Three-dimensional lanthanide-based nanoporous metal–organic frameworks for high-performance supercapacitors. ACS Appl. Nano Mater. 2022, 5, 15237–15249. [Google Scholar] [CrossRef]
- Kumaresan, L.; Hanamantrao, D.P.; Raj, S.L.S.; Chenrayan, S.; Rangasamy, B.; Vediappan, K. Spherically Structured Ce-Metal-Organic Frameworks with Rough Surfaces and Carbon-Coated Cerium Oxide as Potential Electrodes for Lithium Storage and Supercapacitors. ChemistrySelect 2023, 8, e202204759–e202204769. [Google Scholar] [CrossRef]
- Yoon, J.H.; Jinsoo, B.; Cho, I.; Vinodh, R.; Pollet, B.G.; Babu, R.S.; Kim, H.J. Novel supercapacitor electrode derived from one dimensional cerium hydrogen phosphate (1D-Ce(HPO4)2·xH2O). Molecules 2022, 27, 7691–7703. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.F.; Zhang, H.Q.; Liu, X.H.; Chen, Y.D.; Zhang, W.G.; Chen, H.; Ling, Q.D. Microwave-assisted synthesis of NiCo-LDH/graphene nanoscrolls composite for supercapacitor. Carbon 2022, 190, 57–67. [Google Scholar]
- Kumar, R.; Sahoo, S.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Kar, K.K. Microwave-assisted thin reduced graphene oxide-cobalt oxide nanoparticles as hybrids for electrode materials in supercapacitor. J. Energy Storage 2021, 40, 102724–102733. [Google Scholar]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K. A review on the current research on microwave processing techniques applied to graphene-based supercapacitor electrodes: An emerging approach beyond conventional heating. J. Energy Chem. 2022, 74, 252–282. [Google Scholar]
- Cho, H.Y.; Yang, D.A.; Kim, J.; Jeong, S.Y.; Ahn, W.S. CO2 adsorption and catalytic application of Co-MOF-74 synthesized by microwave heating. Catal. Today 2012, 185, 35–40. [Google Scholar] [CrossRef]
- Huang, C.; Gu, X.Y.; Su, X.Y.; Xu, Z.C.; Liu, R.; Zhu, H.J. Controllable synthesis of Co-MOF-74 catalysts and their application in catalytic oxidation of toluene. J. Solid State Chem. 2020, 289, 121497–121503. [Google Scholar] [CrossRef]
- Luo, J.J.; Yang, X.; Wang, S.M.; Bi, Y.H.; Nautiyal, A.; Zhang, X.Y. Facile synthesis of nickel-based metal organic framework [Ni3(HCOO)6] by microwave method and application for supercapacitor. Funct. Mater. Lett. 2018, 11, 1850030–1850033. [Google Scholar]
- Gou, L.; Liu, P.G.; Liu, D.; Wang, C.Y.; Lei, H.Y.; Li, Z.Y.; Fan, X.Y.; Li, D.L. Rational synthesis of Ni3(HCOO)6/CNT ellipsoids with enhanced lithium storage performance: Inspired by the time evolution of the growth process of a nickel formate framework. Dalton T. 2017, 46, 6473–6482. [Google Scholar]
- Neeraj, N.S.; Mordina, B.; Srivastava, A.K.; Mukhopadhyay, K.; Prasad, N.E. Impact of process conditions on the electrochemical performances of NiMoO4 nanorods and activated carbon based asymmetric supercapacitor. Appl. Surf. Sci. 2019, 473, 807–819. [Google Scholar]
- Pham, N.D.; Tiong, V.T.; Yao, D.S.; Martens, W.; Guerrero, A.; Bisquert, J.; Wang, H.X. Guanidinium thiocyanate selective Ostwald ripening induced large grain for high performance perovskite solar cells. Nano Energy 2017, 41, 476–487. [Google Scholar]
- Yang, Y.Q.; Wu, J.H.; Wang, X.B.; Guo, Q.Y.; Liu, X.P.; Sun, W.H.; Wei, Y.L.; Huang, Y.F.; Lan, Z.; Huang, M.L.; et al. Suppressing vacancy defects and grain boundaries via ostwald ripening for high-performance and stable perovskite solar cells. Adv. Mater. 2020, 32, 1904347–1904353. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.H.; Chuang, C.H.; Gu, Y.J.; Ho, W.H.; Song, Y.D.; Chen, Y.C.; Wang, Y.C.; Kung, C.W. Cerium-based metal-organic framework nanocrystals interconnected by carbon nanotubes for boosting electrochemical capacitor performance. ACS Appl. Mater. Inter. 2021, 13, 16418–16426. [Google Scholar]
- Yuan, N.C.; Mei, Y.X.; Liu, Y.W.; Xie, Y.T.; Lin, B.N.; Zhou, Y.H. Fabrication of UiO-66-NH2/Ce(HCOO)3 heterojunction with enhanced photocatalytic reduction of CO2 to CH4. J. CO2 Util. 2022, 64, 102151–102161. [Google Scholar] [CrossRef]
- Kale, A.M.; Manikandan, R.; Raj, C.J.; Savariraj, A.D.; Voz, C.; Kim, B.C. Protonated nickel 2-methylimidazole framework as an advanced electrode material for high-performance hybrid supercapacitor. Mater. Today Energy 2021, 21, 100736–100747. [Google Scholar]
- Ma, W.J.; Li, W.F.; Li, M.; Mao, Q.H.; Pan, Z.H.; Hu, J.; Li, X.; Zhu, M.F.; Zhang, Y.G. Unzipped Carbon Nanotube/Graphene Hybrid Fiber with Less “Dead Volume” for Ultrahigh Volumetric Energy Density Supercapacitors. Adv. Funct. Mater. 2021, 31, 210095–210103. [Google Scholar]
- Karuppasamy, K.; Jothi, V.R.; Vikraman, D.; Prasanna, K.; Maiyalagan, T.; Sang, B.I.; Yi, S.C.; Kim, H.S. Metal-organic framework derived NiMo polyhedron as an efficient hydrogen evolution reaction electrocatalyst. Appl. Surf. Sci. 2019, 478, 916–923. [Google Scholar]
- Xie, X.B.; Zhang, B.; Wang, Q.; Zhao, X.H.; Wu, D.; Wu, H.T.; Sun, X.Q.; Hou, C.X.; Yang, X.Y.; Yu, R.H.; et al. Efficient microwave absorber and supercapacitors derived from puffed-rice-based biomass carbon: Effects of activating temperature. J. Colloid Interface Sci. 2021, 594, 290–303. [Google Scholar] [CrossRef]
- Sonar, P.A.; Sanjeevagol, S.G.; Manjanna, J.; Patake, V.D.; Nitin, S. Electrochemical behavior of cerium (III) hydroxide thin-film electrode in aqueous and non-aqueous electrolyte for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2022, 33, 25787–25795. [Google Scholar]
- Chen, K.F.; Xue, D.F. In-situ electrochemical route to aerogel electrode materials of graphene and hexagonal CeO2. J. Colloid Interf. Sci. 2015, 446, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Barzoki, M.F.; Fatemi, S.; Ganjali, M. Fabrication and comparison of composites of cerium metal-organic framework/reduced graphene oxide as the electrode in supercapacitor application. J. Energy Storage 2022, 55, 105545–105555. [Google Scholar] [CrossRef]
- Manikandan, R.; Raj, C.J.; Rajesh, M.; Kim, B.C.; Park, S.; Yu, K.H. Vanadium Pentoxide with H2O, K+, and Na+ Spacer between Layered Nanostructures for High-Performance Symmetric Electrochemical Capacitors. Adv. Mater. Interfaces 2018, 5, 1800041–1800053. [Google Scholar]
- He, Q.; He, R.; Zia, A.; Gao, G.H.; Liu, Y.F.; Neupane, M.; Wang, M.; Benedict, Z.; Al-Quraishi, K.K.; Li, L.; et al. Self-Promoting Energy Storage in Balsa Wood-Converted Porous Carbon Coupled with Carbon Nanotubes. Small 2022, 18, 2200272–2200281. [Google Scholar]
- Wen, X.Y.; Luo, J.H.; Xiang, K.X.; Zhou, W.; Zhang, C.F.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Jing, X.X.; Wang, L.; Qu, K.G.; Li, R.; Kang, W.J.; Li, H.B.; Xiong, S.L. KOH Chemical-Activated Porous Carbon Sponges for Monolithic Supercapacitor Electrodes. ACS Appl. Energy Mater. 2021, 4, 6768–6776. [Google Scholar]
- Li, S.K.; Chai, H.R.; Zhang, L.; Xu, Y.C.; Jiao, Y.; Chen, J.R. Constructing oxygen vacancy-rich MXene@Ce-MOF composites for enhanced energy storage and conversion. J. Colloid Interface Sci. 2023, 642, 235–245. [Google Scholar] [PubMed]
- Ramachandran, R.; Xuan, W.; Zhao, C.; Leng, X.; Sun, D.; Luo, D.; Wang, F. Enhanced electrochemical properties of cerium metal-organic framework based composite electrodes for high-performance supercapacitor application. RSC Adv. 2018, 8, 3462–3469. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Ding, K.; Tian, H.R.; Yao, M.S.; Nath, B.; Deng, W.H.; Wang, Y.B.; Xu, G. Conductive metal–organic framework nanowire array electrodes for high-performance solid-state supercapacitors. Adv. Funct. Mater. 2017, 27, 1702067–1702073. [Google Scholar] [CrossRef]
- Ojha, M.; Wu, B.; Deepa, M. Cost-effective MIL-53 (Cr) metal–organic framework-based supercapacitors encompassing fast-ion (Li+/H+/Na+) conductors. ACS Appl Energ. Mater. 2021, 4, 4729–4743. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhou, Y.; Xie, Z.; Li, Y.; Liu, Y.P.; Sun, J.; Ma, Y.H.; Terasaki, O.S.M.; Chen, L. Conjugated copper-catecholate framework electrodes for efficient energy storage. Angew. Chem. Int. Edit. 2020, 59, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Huang, M.J.; Wang, P.C.; Lu, M. Controllable hydrothermal synthesis of Ni/Co MOF as hybrid advanced electrode materials for supercapacitor. J. Electrochem. Soc. 2019, 16, 1799–1805. [Google Scholar] [CrossRef]



| Sample | Crystallinity Index | Average Crystallite Size (nm) |
|---|---|---|
| Ce(HCOO)3-150 s | 39.24% | 34.27 |
| Ce(HCOO)3-210 s | 86.67% | 44.19 |
| Ce(HCOO)3-270 s | 65.93% | 40.85 |
| Sample | Specific Surface Area (m2 g−1) | BJH Pore Volume (cm3 g−1) | Average Pore Size (nm) |
|---|---|---|---|
| Ce(HCOO)3-150 s | 1.93 | 0.015 | 3.796 |
| Ce(HCOO)3-210 s | 2.89 | 0.023 | 3.391 |
| Ce(HCOO)3-270 s | 2.57 | 0.020 | 3.803 |
| Materials | Energy Density (Wh kg−1) | Power Density (W kg−1) | Cyclic Stability (Cycle Numbers) | Ref. |
|---|---|---|---|---|
| Ce-H2L//Ce-H2L | 10 | 1425 | 70% (5000) | [31] |
| Ce(HPO4)2·xH2O//Ce(HPO4)2·xH2O | 1.24 | 449.8 | 92.7% (5000) | [33] |
| Ce-BTC//Ce-BTC | 4 | 3000 | 83% (5000) | [58] |
| Cu–CAT NWAs//Cu–CAT NMAs | 2.6 | 200 | 85% (5000) | [59] |
| Cr-MOF//BPC | 10.7 | 250 | 85% (10,000) | [60] |
| Cu-BDC//Cu-BDC | 13.8 | 100 | 80% (2000) | [61] |
| Ni/Co-MOF//AC | 12.8 | 372.5 | 70.3% (2000) | [62] |
| Ce(HCOO)3//AC | 14.78 | 794.6 | 81.3% (10,000) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Wang, W.; Li, G.; Chen, W.; Yang, X.; Ni, C.; Fang, X. Urchin-like Ce(HCOO)3 Synthesized by a Microwave-Assisted Method and Its Application in an Asymmetric Supercapacitor. Molecules 2024, 29, 420. https://doi.org/10.3390/molecules29020420
He Q, Wang W, Li G, Chen W, Yang X, Ni C, Fang X. Urchin-like Ce(HCOO)3 Synthesized by a Microwave-Assisted Method and Its Application in an Asymmetric Supercapacitor. Molecules. 2024; 29(2):420. https://doi.org/10.3390/molecules29020420
Chicago/Turabian StyleHe, Qing, Wanglong Wang, Guohua Li, Wenmiao Chen, Xing Yang, Chengyuan Ni, and Xing Fang. 2024. "Urchin-like Ce(HCOO)3 Synthesized by a Microwave-Assisted Method and Its Application in an Asymmetric Supercapacitor" Molecules 29, no. 2: 420. https://doi.org/10.3390/molecules29020420
APA StyleHe, Q., Wang, W., Li, G., Chen, W., Yang, X., Ni, C., & Fang, X. (2024). Urchin-like Ce(HCOO)3 Synthesized by a Microwave-Assisted Method and Its Application in an Asymmetric Supercapacitor. Molecules, 29(2), 420. https://doi.org/10.3390/molecules29020420







