Quality Evaluation of Lonicerae Flos Produced in Southwest China Based on HPLC Analysis and Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Validation
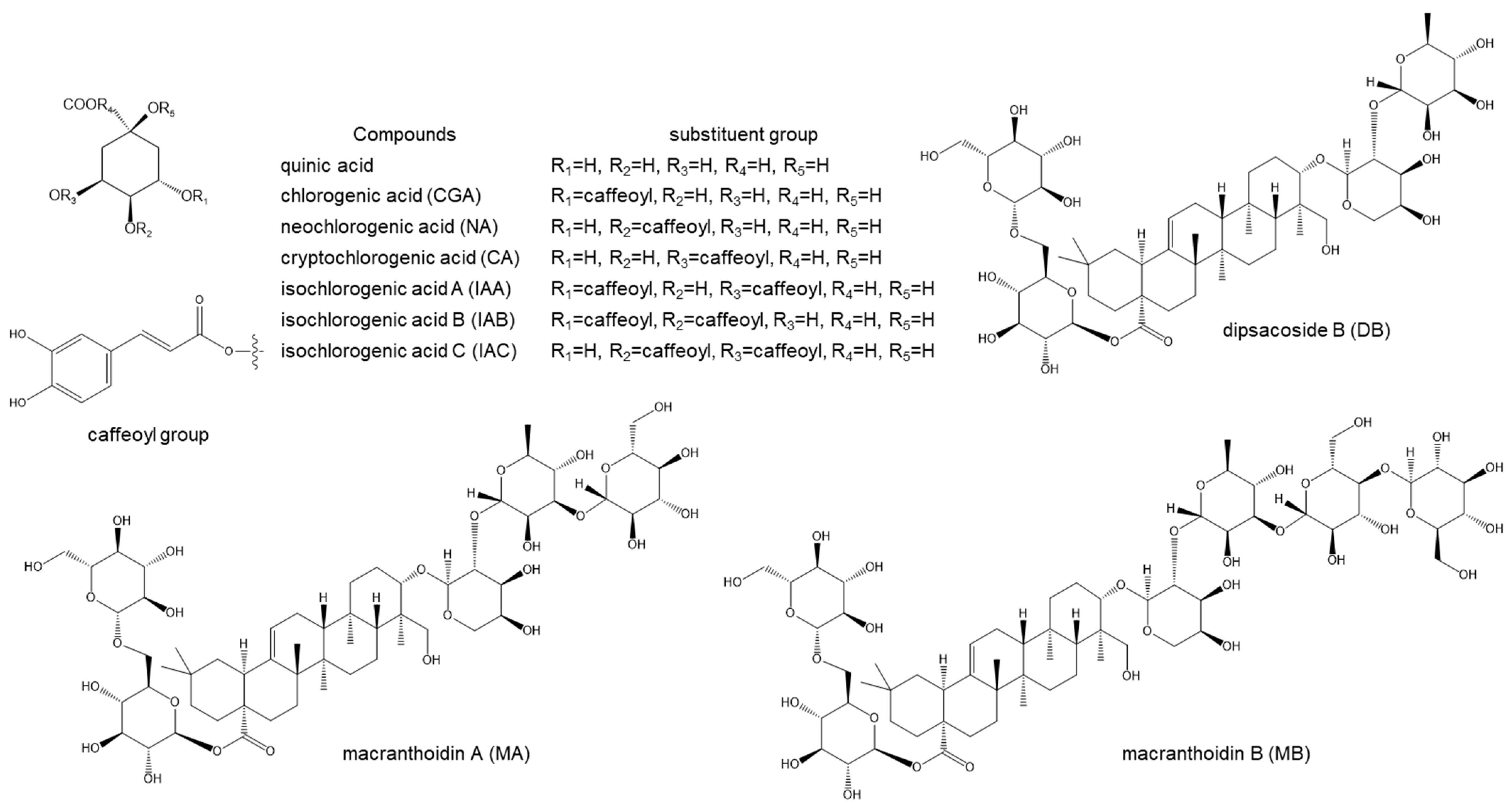
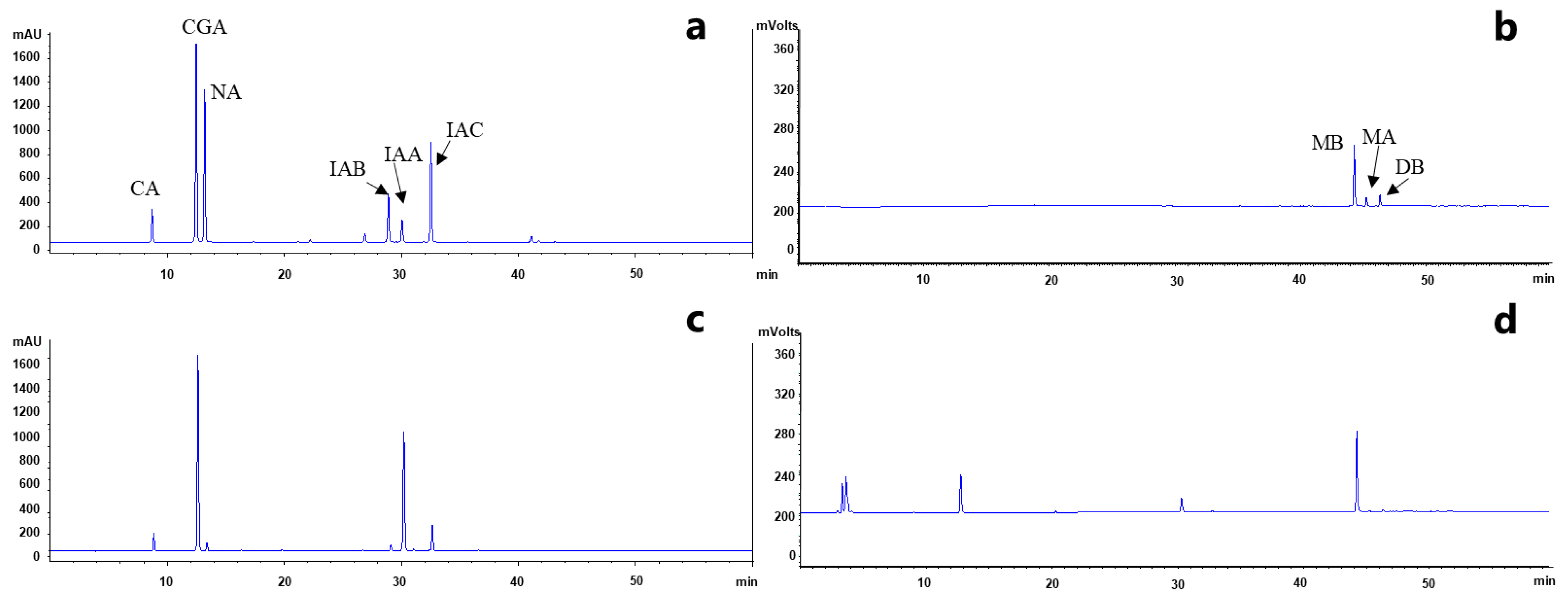
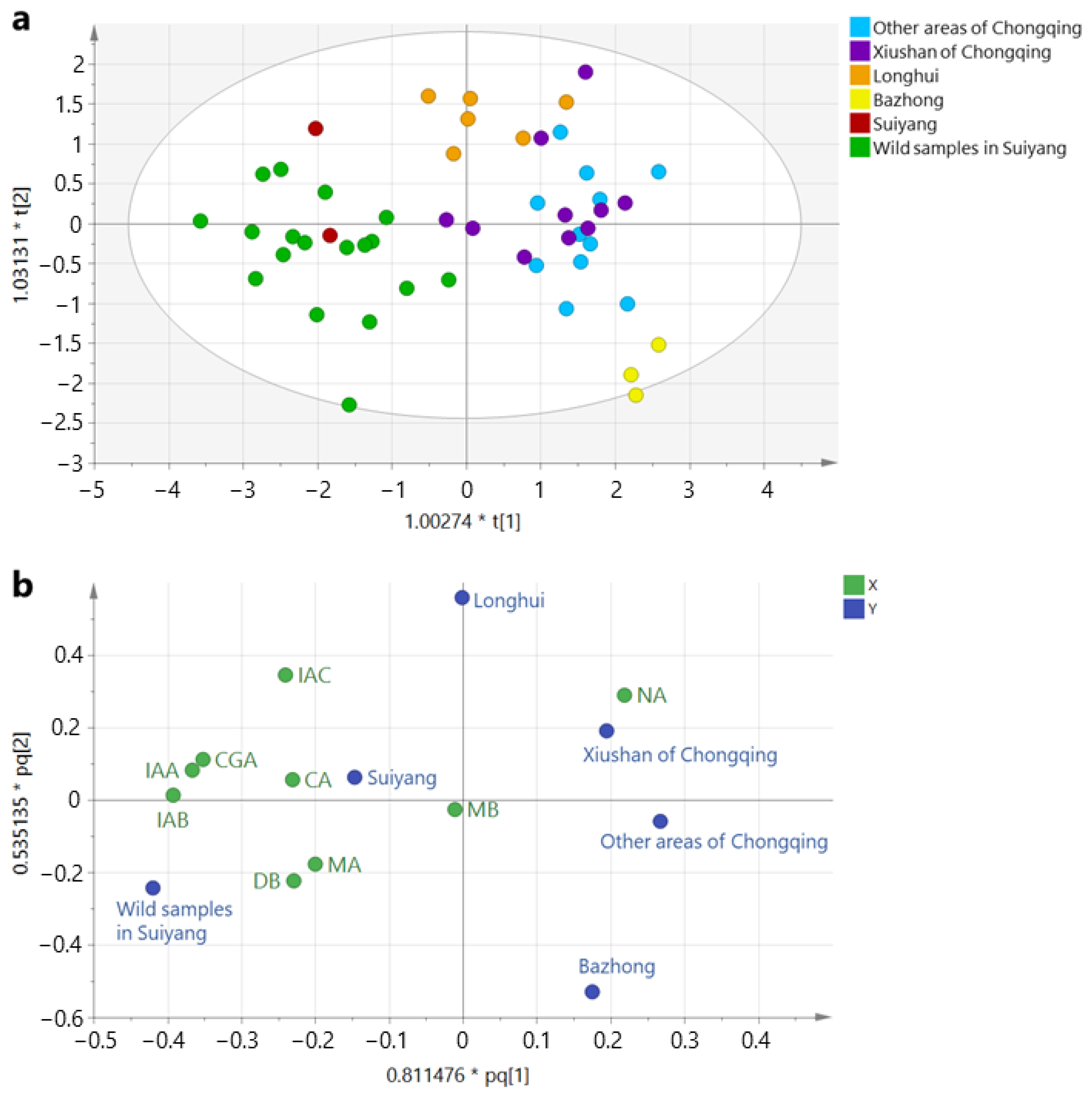
2.2. Metabolite Profiling of L. macranthoides
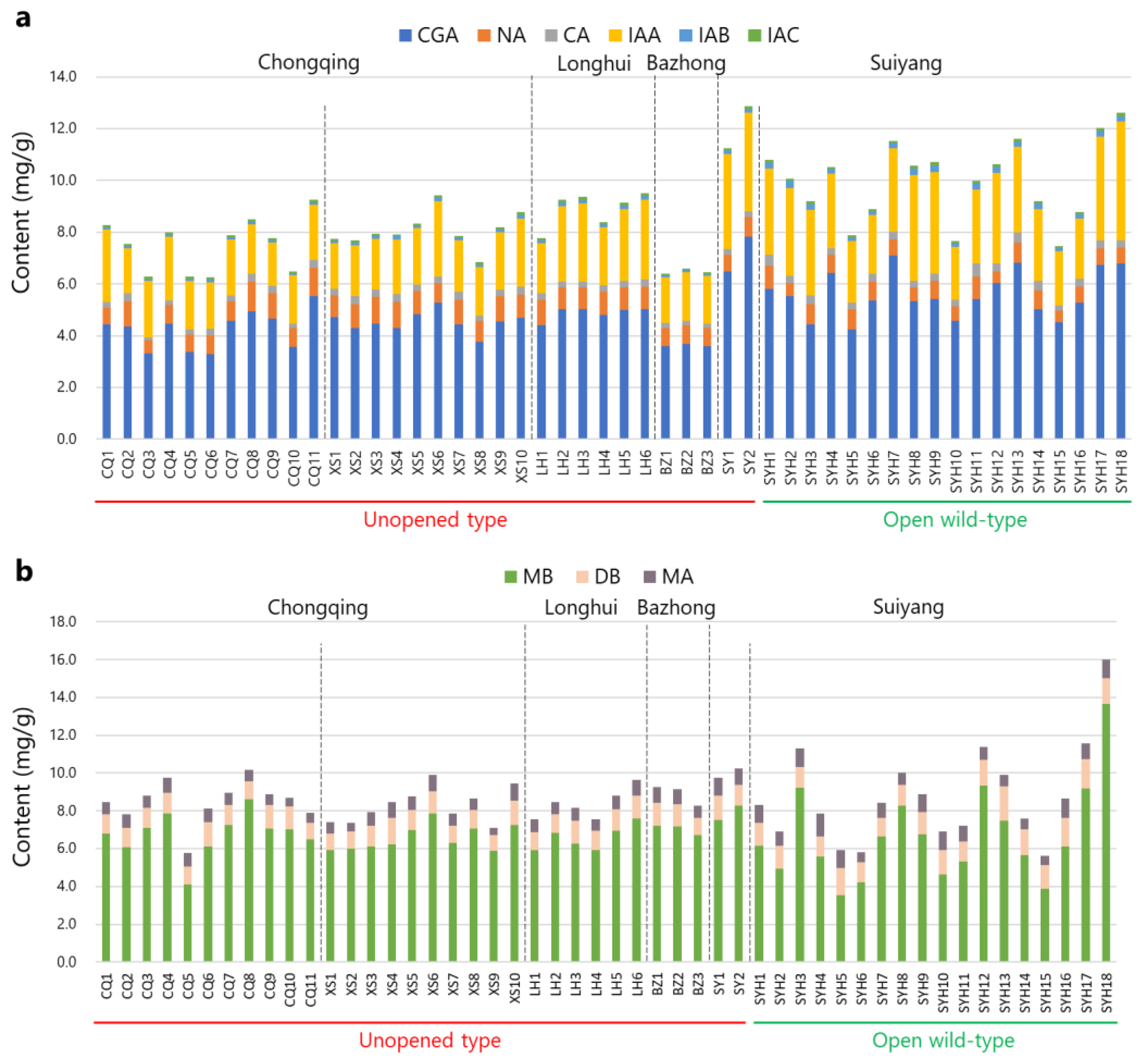
2.3. Quantitative Analysis of the Nine Compounds in L. macranthoides
2.4. Antioxidant Activity
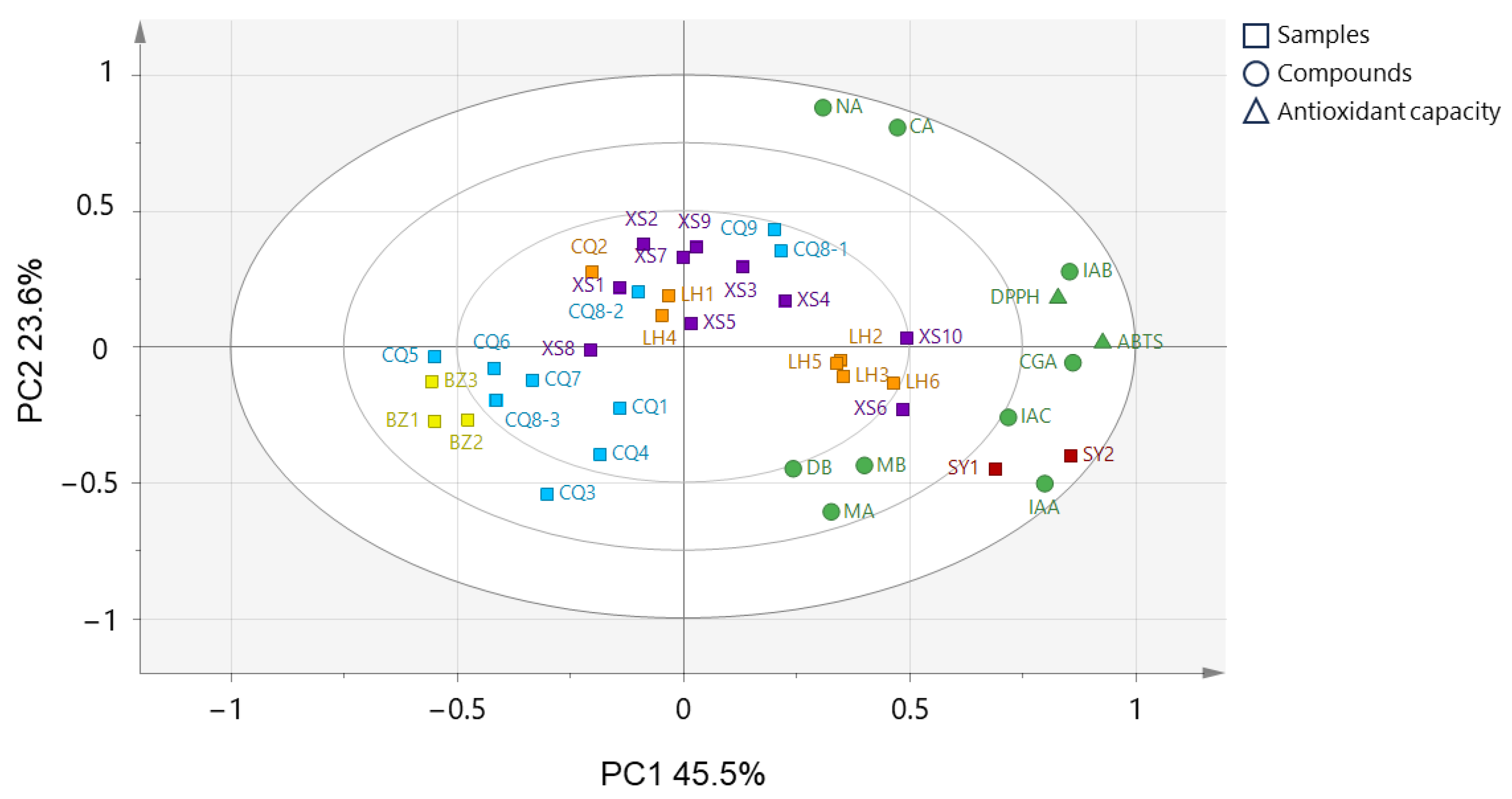
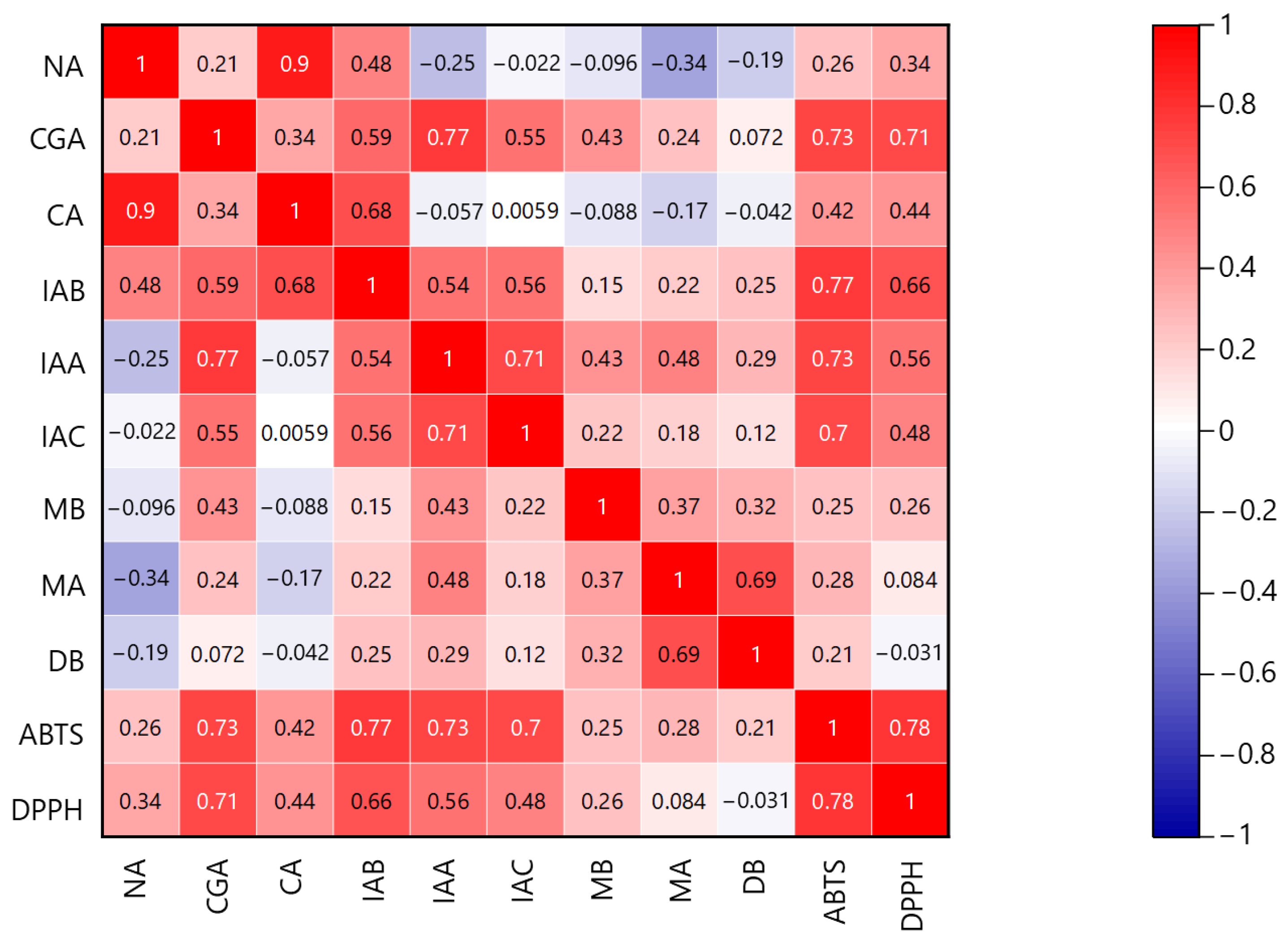
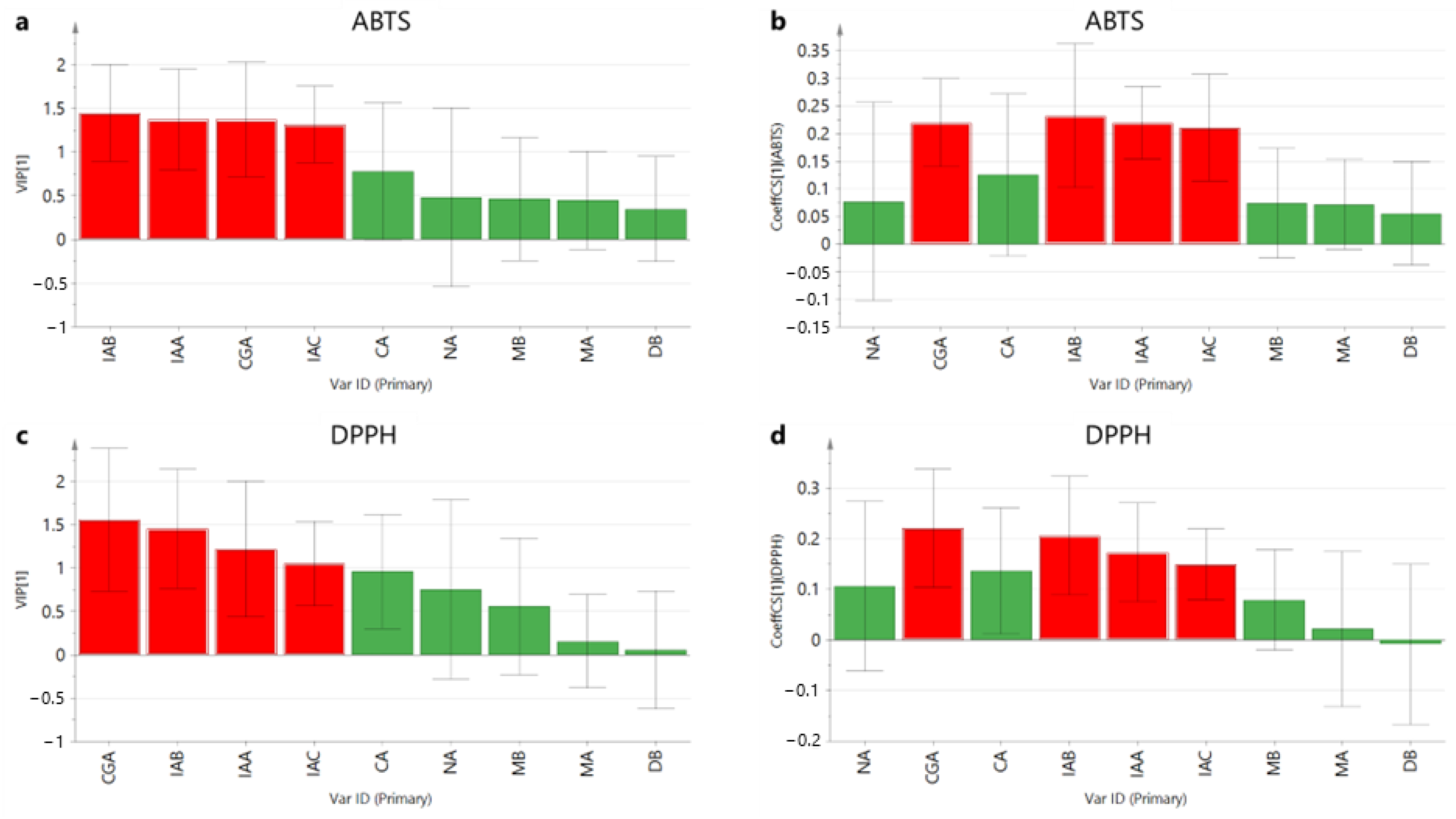
2.5. Relationship between HPLC Chromatograms and Antioxidant Components
3. Materials and Methods
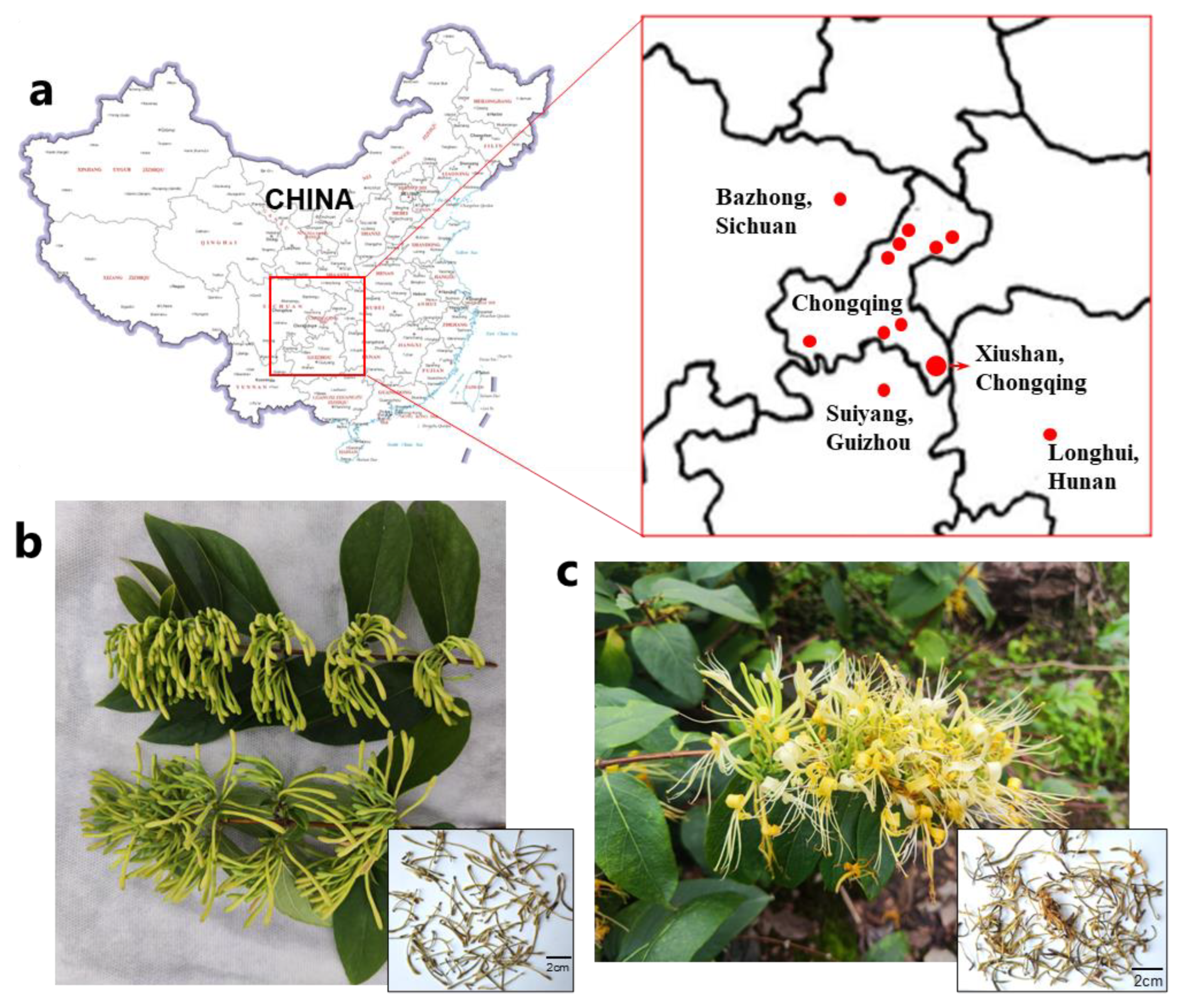
3.1. Materials
3.2. Chemicals and Reagents
3.3. Preparation of Standard and Sample Solutions for HPLC Analysis
3.4. HPLC Conditions
3.5. HPLC Method Validation
3.6. Sample Process for Oxidation Resistance Assay
3.7. ABTS Free Radical Scavenging Assay
3.8. DPPH-Free Radical Scavenging Assay
3.9. Multivariate Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Zhang, J.; Wang, F.; Zou, Y.; Chen, X. Isolation and Characterization of New Minor Triterpenoid Saponins from the Buds of Lonicera macranthoides. Carbohydr. Res. 2013, 370, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, F.; Liu, Q.; Qi, Y.; Wang, Q.; Liu, H. Comparison of Anti-Inflammatory Effects of Lonicerae japonicae flos and Lonicerae flos Based on Network Pharmacology. Chin. Herb. Med. 2021, 13, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Fang, L.; Li, J.; Zhao, Z.; Zhang, H.; Zhang, Y. Separation of Five Iridoid Glycosides from Lonicerae japonicae flos Using High-Speed Counter-Current Chromatography and Their Anti-Inflammatory and Antibacterial Activities. Molecules 2019, 24, 197. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.; Fu, C.; Song, Y.; Fu, Q. Lonicerae japonicae flos and Lonicerae flos: A Systematic Review of Ethnopharmacology, Phytochemistry and Pharmacology. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2020, 19, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tang, N.; You, Y.; Lan, J.; Liu, Y.; Li, Z. Transcriptome Analysis Reveals the Mechanism Underlying the Production of a High Quantity of Chlorogenic Acid in Young Leaves of Lonicera macranthoides Hand.-Mazz. PLoS ONE 2015, 10, e0137212. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of The People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume I, pp. 268–269. [Google Scholar]
- Wen, Q.; Shu, B.; Ding, Y.; Hu, L.; Fang, L. General Situation of Resource Distribution and Development of Planting Technique of Lonicera Japonica Flos and Lonicera Flos. China Pharm. 2018, 27, 004. [Google Scholar]
- Kang, K.B.; Kang, S.-J.; Kim, M.S.; Lee, D.Y.; Han, S.I.; Kim, T.B.; Park, J.Y.; Kim, J.; Yang, T.-J.; Sung, S.H. Chemical and Genomic Diversity of Six Lonicera Species Occurring in Korea. Phytochemistry 2018, 155, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gao, T.; Yao, H.; Shi, L.; Zhu, Y.; Chen, S. Identification of Lonicera Japonica and Its Related Species Using the DNA Barcoding Method. Planta Med. 2011, 77, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L.; He, P.; Li, H.; Pan, X.; Zhang, W.; Xiao, M.; He, F. Traditional Uses, Botany, Phytochemistry, and Pharmacology of Lonicerae japonicae flos and Lonicerae flos: A Systematic Comparative Review. J. Ethnopharmacol. 2024, 322, 117278. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Surucu, B.; Kuhnert, N. Characterization by LC-MS(n) of Four New Classes of Chlorogenic Acids in Green Coffee Beans: Dimethoxycinnamoylquinic Acids, Diferuloylquinic Acids, Caffeoyl-Dimethoxycinnamoylquinic Acids, and Feruloyl-Dimethoxycinnamoylquinic Acids. J. Agric. Food Chem. 2006, 54, 1957–1969. [Google Scholar] [CrossRef]
- Mahesh, V.; Million-Rousseau, R.; Ullmann, P.; Chabrillange, N.; Bustamante, J.; Mondolot, L.; Morant, M.; Noirot, M.; Hamon, S.; De Kochko, A.; et al. Functional Characterization of Two P-Coumaroyl Ester 3′-Hydroxylase Genes from Coffee Tree: Evidence of a Candidate for Chlorogenic Acid Biosynthesis. Plant Mol. Biol. 2007, 64, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, C.; Idehen, E.; Shi, L.; Lv, L.; Sang, S. Novel Theaflavin-Type Chlorogenic Acid Derivatives Identified in Black Tea. J. Agric. Food Chem. 2018, 66, 3402–3407. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Yang, J.; Jiang, Y.; Lu, B.; Hu, Y.; Zhou, F.; Mao, S.; Shen, C. Phenolic Compounds and Antioxidant Capacities of 10 Common Edible Flowers from China. J. Food Sci. 2014, 79, C517–C525. [Google Scholar] [CrossRef]
- Narváez-Cuenca, C.-E.; Vincken, J.-P.; Gruppen, H. Quantitative Fate of Chlorogenic Acid during Enzymatic Browning of Potato Juice. J. Agric. Food Chem. 2013, 61, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Sheng, W.; Song, X.; Song, J.; Li, Y.; Huang, W.; Liu, Y. Chlorogenic Acid from Burdock Roots Ameliorates Oleic Acid-Induced Steatosis in HepG2 Cells through AMPK/ACC/CPT-1 Pathway. Molecules 2023, 28, 7257. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic Acid: A Comprehensive Review of the Dietary Sources, Processing Effects, Bioavailability, Beneficial Properties, Mechanisms of Action, and Future Directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Rivelli, D.P.; Filho, C.A.H.; Almeida, R.L.; Ropke, C.D.; Sawada, T.C.H.; Barros, S.B.M. Chlorogenic Acid UVA–UVB Photostability. Photochem. Photobiol. 2010, 86, 1005–1007. [Google Scholar] [CrossRef]
- Kundu, A.; Vadassery, J. Chlorogenic Acid-Mediated Chemical Defence of Plants against Insect Herbivores. Plant Biol. Stuttg. Ger. 2019, 21, 185–189. [Google Scholar] [CrossRef]
- Martínez, G.; Regente, M.; Jacobi, S.; Rio, M.D.; Pinedo, M.; de la Canal, L. Chlorogenic Acid Is a Fungicide Active against Phytopathogenic Fungi. Pestic. Biochem. Physiol. 2017, 140, 30–35. [Google Scholar] [CrossRef]
- Yang, X.; Lan, W.; Xie, J. Antimicrobial and Anti-Biofilm Activities of Chlorogenic Acid Grafted Chitosan against Staphylococcus aureus. Microb. Pathog. 2022, 173, 105748. [Google Scholar] [CrossRef]
- Xiong, S.; Su, X.; Kang, Y.; Si, J.; Wang, L.; Li, X.; Ma, K. Effect and Mechanism of Chlorogenic Acid on Cognitive Dysfunction in Mice by Lipopolysaccharide-Induced Neuroinflammation. Front. Immunol. 2023, 14, 1178188. [Google Scholar] [CrossRef] [PubMed]
- Bal, E.; Hanalıoğlu, Ş.; Apaydın, A.S.; Bal, C.; Şenat, A.; Gümüşkaya, B.; Bahadır, B.; Türkoğlu, Ö.F. Can Chlorogenic Acid Reduce Oxidative Stress and in an Experimental Spinal Cord Injury? Turk. J. Trauma Emerg. Surg. 2022, 28, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, A.; Iqbal, M.S.; Srivastava, J.K. Therapeutic Promises of Chlorogenic Acid with Special Emphasis on Its Anti-Obesity Property. Curr. Mol. Pharmacol. 2020, 13, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Xiang, Y.; Huang, F.; Chen, Y.; Ding, H.; Du, J.; Chen, X.; Wang, X.; Wei, X.; Cai, Y.; et al. Comparative Genomics of the Medicinal Plants Lonicera macranthoides and L. Japonica Provides Insight into Genus Genome Evolution and Hederagenin-based Saponin Biosynthesis. Plant Biotechnol. J. 2023, 21, 2209–2223. [Google Scholar] [CrossRef]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as Cytotoxic Agents: A Review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, J.; Yang, R.; Fang, L.; Zhang, Y. A Review: The Triterpenoid Saponins and Biological Activities of Lonicera Linn. Molecules 2020, 25, 3773. [Google Scholar] [CrossRef] [PubMed]
- Pirtskhalava, M.; Mittova, V.; Tsetskhladze, Z.R.; Palumbo, R.; Pastore, R.; Roviello, G.N. Georgian Medicinal Plants as Rich Natural Sources of Antioxidant Derivatives: A Review on the Current Knowledge and Future Perspectives. Curr. Med. Chem. 2024, in press. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Lin, X.; Luo, H.; Li, G.; Yao, H. On-Line Screening and Identification of Radical Scavenging Compounds Extracted from Flos Lonicerae by LC-DAD–TOF-MS. Chromatographia 2008, 68, 327–332. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Li, Y.; Li, P. Identification and Quantification of Free Radical Scavengers in the Flower Buds of Lonicera Species by Online HPLC-DPPH Assay Coupled with Electrospray Ionization Quadrupole Time-of-flight Tandem Mass Spectrometry. Biomed. Chromatogr. 2012, 26, 449–457. [Google Scholar] [CrossRef]
- Liu, S.; Qiao, Z.; Liu, X.; Wang, X.; Zeng, H.; Li, Y.; Cai, N. Comparative Transcriptome Analysis of Corolla Opening in Lonicera macranthoides between Bud Type and Common Type. Mol. Plant Breed. 2021, 19, 1822–1829. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Ma, P.; Wu, Y. Study on optimal harvest period of Lonicera Flos (Lonicera macranthoides). China J. Chin. Mater. Medica 2014, 39, 3060–3064. [Google Scholar] [CrossRef]
- Xiao, Z.; Xie, M.; Gan, L.; Fang, M.; Zhou, X.; Zhou, Y.; He, W.; Chen, L.; Huang, J. Determination of chlorogenic acid, total flavones, and anti-oxidant activity of Flos Lonicerae Japonicae and Flos Lonicerae. Chin. Tradit. Herb. Drugs 2019, 50, 210–216. [Google Scholar] [CrossRef]
- Wu, F.; Qing, Z.; Zeng, J. HPLC determination of chlorogenic acid and galuteolin in different parts of Lonicera macranthoides. Mod. Chin. Med. 2014, 16, 614–617. [Google Scholar] [CrossRef]
- Liu, M.; Gao, S.; Liu, L.; Zhang, Y.; Nian, L.; Liu, X. Simultaneous determination of six organic acids in Lonicerae japonicae flos and Lonicerae flos in different habitats by HPLC. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2013, 36, 196–198. [Google Scholar] [CrossRef]
- Sun, L.; Fan, X.; Guo, Q.; Zhang, X.; Chen, L. Simultaneous determination of 5 saponins in Lonicerae flos by QAMS method. China Pharm. 2017, 28, 2546–2549. [Google Scholar] [CrossRef]
- Tian, A.; Zhou, W.; Wen, S.; Huang, G.; Xu, Q.; Li, H. Advances in flue-cured tobacco chlorogenic acid content factors. Anhui Agric. Sci. Bull. 2016, 22, 22–25. [Google Scholar] [CrossRef]
- Zhang, P.; Pu, G. Study on influences of climatic factors to chlorogenic acid content in Flos lonicerae. Shandong Agric. Sci. 2015, 47, 77–79. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Yang, M.; Li, H. Effects of different altitudes on quality of Lonicerae flos. Agric. Res. Appl. 2023, 36, 8–12. [Google Scholar]
- Rivera-Pérez, A.; Romero-González, R.; Garrido Frenich, A. A Metabolomics Approach Based on 1H NMR Fingerprinting and Chemometrics for Quality Control and Geographical Discrimination of Black Pepper. J. Food Compos. Anal. 2022, 105, 104235. [Google Scholar] [CrossRef]
- Galindo-Prieto, B.; Trygg, J.; Geladi, P. A New Approach for Variable Influence on Projection (VIP) in O2PLS Models. Chemom. Intell. Lab. Syst. 2017, 160, 110–124. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, S.; Liu, Q.; Zhang, Y. Spectrum–Effect Relationship Study between HPLC Fingerprints and Antioxidant of Honeysuckle Extract. Biomed. Chromatogr. 2019, 33, e4583. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In Vitro and in Vivo Antioxidant Properties of Chlorogenic Acid and Caffeic Acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, L.; Shi, S.; Cai, P.; Liang, X.; Zhang, S. Antioxidant Capacity and Phenolic Compounds of Lonicerae macranthoides by HPLC–DAD–QTOF-MS/MS. J. Pharm. Biomed. Anal. 2016, 124, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Batsukh, Z.; Toume, K.; Javzan, B.; Kazuma, K.; Cai, S.; Hayashi, S.; Atsumi, T.; Yoshitomi, T.; Uchiyama, N.; Maruyama, T.; et al. Characterization of Metabolites in Saposhnikovia divaricata Root from Mongolia. J. Nat. Med. 2021, 75, 11–27. [Google Scholar] [CrossRef]
- Dong, Y.; Toume, K.; Zhu, S.; Shi, Y.; Tamura, T.; Yoshimatsu, K.; Komatsu, K. Metabolomics Analysis of Peony Root Using NMR Spectroscopy and Impact of the Preprocessing Method for NMR Data in Multivariate Analysis. J. Nat. Med. 2023, 77, 792–816. [Google Scholar] [CrossRef]
| Component | Calibration Curve Equation | r | Linear Range (mg/mL) | Precision (RSD%) n = 6 | Repeatability (RSD%) n = 6 | Stability (RSD%) n = 8 | Recovery (%) n = 6 | RSD% |
|---|---|---|---|---|---|---|---|---|
| CGA | y = 23722x + 494.29 | 0.9993 | 0.098–0.935 | 1.57 | 0.42 | 0.36 | 100.50 | 1.45 |
| NA | y = 23991x + 85.449 | 0.9999 | 0.014–0.271 | 1.95 | 0.24 | 0.36 | 100.67 | 1.13 |
| CA | y = 18329x + 58.61 | 0.9999 | 0.015–0.289 | 1.60 | 2.60 | 1.40 | 99.00 | 1.91 |
| IAA | y = 27927x + 240.89 | 0.9999 | 0.053–0.505 | 1.90 | 1.40 | 0.34 | 93.99 | 1.55 |
| IAB | y = 22053x + 12.829 | 0.9997 | 0.002–0.043 | 1.48 | 1.46 | 1.66 | 99.89 | 1.22 |
| IAC | y = 23732x − 70.103 | 0.9996 | 0.002–0.032 | 1.73 | 1.81 | 1.39 | 99.89 | 1.61 |
| MB | y = 732336x − 79386 | 0.9983 | 0.010–1.903 | 1.14 | 0.13 | 1.14 | 100.41 | 1.53 |
| MA | y = 503752x − 9198.3 | 0.9926 | 0.028–0.527 | 0.79 | 2.13 | 1.09 | 99.56 | 1.47 |
| DB | y = 482044x − 25891 | 0.9978 | 0.033–0.625 | 0.97 | 0.70 | 1.63 | 100.13 | 1.59 |
| Producing Area | Voucher No. | CGA | NA | CA | IAA | IAB | IAC | SUM of Six Phenolic Acids | MB | DB | MA | SUM of MB&DB | SUM of Three Saponins |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chongqing | CQ1 | 4.426 | 0.667 | 0.200 | 2.804 | 0.092 | 0.082 | 8.270 | 6.797 | 1.004 | 0.651 | 7.802 | 8.452 |
| CQ2 | 4.357 | 0.977 | 0.300 | 1.738 | 0.096 | 0.075 | 7.542 | 6.075 | 1.020 | 0.736 | 7.094 | 7.830 | |
| CQ3 | 3.326 | 0.496 | 0.117 | 2.167 | 0.056 | 0.116 | 6.278 | 7.103 | 1.044 | 0.660 | 8.147 | 8.807 | |
| CQ4 | 4.455 | 0.748 | 0.171 | 2.463 | 0.069 | 0.097 | 8.003 | 7.850 | 1.117 | 0.795 | 8.967 | 9.763 | |
| CQ5 | 3.359 | 0.687 | 0.185 | 1.876 | 0.086 | 0.087 | 6.281 | 4.102 | 0.954 | 0.704 | 5.056 | 5.761 | |
| CQ6 | 3.296 | 0.721 | 0.245 | 1.810 | 0.107 | 0.079 | 6.257 | 6.120 | 1.288 | 0.726 | 7.407 | 8.133 | |
| CQ7 | 4.576 | 0.763 | 0.224 | 2.140 | 0.086 | 0.082 | 7.870 | 7.234 | 1.064 | 0.651 | 8.298 | 8.949 | |
| CQ8 | 4.947 | 1.144 | 0.314 | 1.886 | 0.125 | 0.089 | 8.505 | 8.622 | 0.942 | 0.592 | 9.564 | 10.156 | |
| CQ9 | 4.658 | 0.990 | 0.274 | 1.671 | 0.102 | 0.087 | 7.782 | 7.057 | 1.263 | 0.560 | 8.320 | 8.880 | |
| CQ10 | 3.569 | 0.718 | 0.186 | 1.858 | 0.078 | 0.077 | 6.486 | 7.039 | 1.214 | 0.448 | 8.253 | 8.701 | |
| CQ11 | 5.539 | 1.077 | 0.315 | 2.124 | 0.102 | 0.087 | 9.244 | 6.479 | 0.887 | 0.540 | 7.366 | 7.906 | |
| XS1 | 4.711 | 0.837 | 0.268 | 1.746 | 0.098 | 0.080 | 7.739 | 5.923 | 0.878 | 0.610 | 6.801 | 7.411 | |
| XS2 | 4.287 | 0.938 | 0.291 | 1.979 | 0.112 | 0.074 | 7.682 | 6.014 | 0.893 | 0.470 | 6.906 | 7.376 | |
| XS3 | 4.465 | 1.026 | 0.304 | 1.934 | 0.122 | 0.099 | 7.950 | 6.111 | 1.102 | 0.714 | 7.213 | 7.926 | |
| XS4 | 4.292 | 1.026 | 0.301 | 2.095 | 0.126 | 0.082 | 7.923 | 6.220 | 1.413 | 0.849 | 7.632 | 8.481 | |
| XS5 | 4.829 | 0.908 | 0.254 | 2.161 | 0.102 | 0.081 | 8.335 | 6.995 | 1.064 | 0.697 | 8.059 | 8.756 | |
| XS6 | 5.281 | 0.748 | 0.247 | 2.911 | 0.145 | 0.105 | 9.437 | 7.850 | 1.186 | 0.865 | 9.036 | 9.900 | |
| XS7 | 4.431 | 0.955 | 0.298 | 1.992 | 0.112 | 0.075 | 7.864 | 6.291 | 0.939 | 0.612 | 7.230 | 7.842 | |
| XS8 | 3.770 | 0.801 | 0.209 | 1.865 | 0.102 | 0.092 | 6.838 | 7.074 | 0.974 | 0.610 | 8.048 | 8.658 | |
| XS9 | 4.549 | 0.983 | 0.261 | 2.191 | 0.110 | 0.099 | 8.193 | 5.899 | 0.836 | 0.350 | 6.735 | 7.086 | |
| XS10 | 4.677 | 0.917 | 0.297 | 2.622 | 0.156 | 0.098 | 8.768 | 7.240 | 1.311 | 0.895 | 8.551 | 9.446 | |
| Hunan | LH1 | 4.423 | 0.973 | 0.242 | 1.935 | 0.108 | 0.103 | 7.783 | 5.930 | 0.950 | 0.688 | 6.880 | 7.567 |
| LH2 | 5.033 | 0.827 | 0.243 | 2.911 | 0.132 | 0.115 | 9.261 | 6.818 | 1.006 | 0.631 | 7.824 | 8.456 | |
| LH3 | 5.025 | 0.828 | 0.241 | 3.019 | 0.126 | 0.119 | 9.358 | 6.275 | 1.201 | 0.690 | 7.477 | 8.167 | |
| LH4 | 4.805 | 0.896 | 0.250 | 2.237 | 0.108 | 0.097 | 8.393 | 5.923 | 1.019 | 0.601 | 6.942 | 7.543 | |
| LH5 | 4.997 | 0.867 | 0.257 | 2.778 | 0.131 | 0.115 | 9.145 | 6.942 | 1.144 | 0.730 | 8.086 | 8.816 | |
| LH6 | 5.020 | 0.885 | 0.273 | 3.071 | 0.144 | 0.114 | 9.508 | 7.586 | 1.200 | 0.868 | 8.786 | 9.654 | |
| Sichuan | BZ1 | 3.598 | 0.708 | 0.190 | 1.772 | 0.065 | 0.063 | 6.395 | 7.198 | 1.241 | 0.833 | 8.439 | 9.272 |
| BZ2 | 3.687 | 0.709 | 0.186 | 1.884 | 0.067 | 0.065 | 6.598 | 7.160 | 1.173 | 0.821 | 8.332 | 9.153 | |
| BZ3 | 3.608 | 0.681 | 0.181 | 1.848 | 0.064 | 0.061 | 6.442 | 6.712 | 0.914 | 0.652 | 7.626 | 8.278 | |
| Guizhou | SY1 | 6.483 | 0.654 | 0.223 | 3.660 | 0.130 | 0.105 | 11.255 | 7.503 | 1.319 | 0.921 | 8.822 | 9.743 |
| SY2 | 7.839 | 0.753 | 0.228 | 3.797 | 0.127 | 0.120 | 12.864 | 8.264 | 1.108 | 0.871 | 9.371 | 10.243 | |
| SYH1 | 5.808 | 0.906 | 0.416 | 3.332 | 0.234 | 0.109 | 10.805 | 6.158 | 1.206 | 0.946 | 7.364 | 8.310 | |
| SYH2 | 5.533 | 0.487 | 0.287 | 3.404 | 0.264 | 0.105 | 10.080 | 4.951 | 1.216 | 0.747 | 6.167 | 6.914 | |
| SYH3 | 4.447 | 0.770 | 0.337 | 3.297 | 0.232 | 0.103 | 9.185 | 9.215 | 1.110 | 0.984 | 10.325 | 11.308 | |
| SYH4 | 6.425 | 0.691 | 0.274 | 2.869 | 0.170 | 0.095 | 10.525 | 5.583 | 1.069 | 1.202 | 6.652 | 7.854 | |
| SYH5 | 4.241 | 0.790 | 0.254 | 2.375 | 0.139 | 0.088 | 7.887 | 3.551 | 1.438 | 0.939 | 4.989 | 5.928 | |
| SYH6 | 5.358 | 0.725 | 0.305 | 2.273 | 0.156 | 0.079 | 8.896 | 4.220 | 1.057 | 0.520 | 5.277 | 5.797 | |
| SYH7 | 7.095 | 0.630 | 0.289 | 3.239 | 0.182 | 0.093 | 11.528 | 6.647 | 0.974 | 0.816 | 7.621 | 8.438 | |
| SYH8 | 5.332 | 0.532 | 0.262 | 4.088 | 0.248 | 0.118 | 10.581 | 8.282 | 1.103 | 0.624 | 9.385 | 10.008 | |
| SYH9 | 5.404 | 0.713 | 0.290 | 3.914 | 0.263 | 0.118 | 10.700 | 6.757 | 1.192 | 0.940 | 7.949 | 8.889 | |
| SYH10 | 4.587 | 0.550 | 0.252 | 2.037 | 0.141 | 0.083 | 7.650 | 4.619 | 1.319 | 0.957 | 5.939 | 6.896 | |
| SYH11 | 5.408 | 0.879 | 0.490 | 2.880 | 0.249 | 0.085 | 9.991 | 5.331 | 1.050 | 0.827 | 6.381 | 7.207 | |
| SYH12 | 6.033 | 0.450 | 0.308 | 3.500 | 0.233 | 0.105 | 10.629 | 9.345 | 1.343 | 0.698 | 10.689 | 11.387 | |
| SYH13 | 6.831 | 0.778 | 0.393 | 3.294 | 0.203 | 0.108 | 11.608 | 7.492 | 1.789 | 0.635 | 9.281 | 9.915 | |
| SYH14 | 5.038 | 0.728 | 0.363 | 2.770 | 0.217 | 0.090 | 9.206 | 5.671 | 1.361 | 0.550 | 7.032 | 7.583 | |
| SYH15 | 4.507 | 0.464 | 0.194 | 2.100 | 0.122 | 0.076 | 7.464 | 3.888 | 1.230 | 0.514 | 5.118 | 5.632 | |
| SYH16 | 5.287 | 0.606 | 0.295 | 2.339 | 0.166 | 0.074 | 8.766 | 6.119 | 1.529 | 1.010 | 7.648 | 8.658 | |
| SYH17 | 6.738 | 0.627 | 0.329 | 3.993 | 0.229 | 0.111 | 12.027 | 9.168 | 1.584 | 0.813 | 10.751 | 11.565 | |
| SYH18 | 6.793 | 0.607 | 0.285 | 4.582 | 0.219 | 0.139 | 12.625 | 13.652 | 1.348 | 1.016 | 15.000 | 16.015 |
| Voucher No. | ABTS | DPPH | ||
|---|---|---|---|---|
| Clearing Ratio % | SD | Clearing Ratio % | SD | |
| CQ1 | 77.89 | 0.03 | 58.24 | 0.06 |
| CQ2 | 77.07 | 0.02 | 55.50 | 0.02 |
| CQ3 | 81.80 | 0.02 | 58.09 | 0.05 |
| CQ4 | 77.14 | 0.02 | 53.85 | 0.05 |
| CQ5 | 71.89 | 0.02 | 52.65 | 0.07 |
| CQ6 | 72.07 | 0.02 | 48.45 | 0.06 |
| CQ7 | 69.61 | 0.03 | 51.24 | 0.07 |
| CQ8 | 81.74 | 0.03 | 62.59 | 0.06 |
| CQ9 | 77.01 | 0.02 | 57.19 | 0.06 |
| CQ10 | 72.46 | 0.02 | 55.83 | 0.11 |
| CQ11 | 82.62 | 0.01 | 68.18 | 0.02 |
| XS1 | 80.80 | 0.02 | 61.12 | 0.08 |
| XS2 | 81.75 | 0.02 | 61.34 | 0.05 |
| XS3 | 82.34 | 0.05 | 62.24 | 0.04 |
| XS4 | 90.76 | 0.02 | 62.26 | 0.05 |
| XS5 | 77.01 | 0.04 | 65.95 | 0.03 |
| XS6 | 87.44 | 0.01 | 67.07 | 0.05 |
| XS7 | 81.83 | 0.01 | 64.25 | 0.03 |
| XS8 | 73.47 | 0.02 | 62.16 | 0.02 |
| XS9 | 81.62 | 0.01 | 65.69 | 0.04 |
| XS10 | 86.45 | 0.01 | 69.66 | 0.03 |
| LH1 | 79.03 | 0.02 | 61.72 | 0.04 |
| LH2 | 88.38 | 0.01 | 61.69 | 0.03 |
| LH3 | 85.93 | 0.01 | 63.89 | 0.04 |
| LH4 | 79.21 | 0.03 | 56.56 | 0.05 |
| LH5 | 85.85 | 0.02 | 61.76 | 0.02 |
| LH6 | 87.60 | 0.02 | 59.67 | 0.04 |
| BZ1 | 68.39 | 0.01 | 52.86 | 0.04 |
| BZ2 | 72.76 | 0.01 | 53.68 | 0.05 |
| BZ3 | 71.57 | 0.03 | 56.06 | 0.11 |
| SY1 | 92.12 | 0.01 | 71.37 | 0.05 |
| SY2 | 92.52 | 0.01 | 69.86 | 0.06 |
| Producing Area | Voucher No. | L. macranthoides Breeds | Collection Date | |
|---|---|---|---|---|
| Chongqing | Zhong county | CQ1 | unopened type | 2022.6 |
| Jiangjin district | CQ2 | unopened type | 2022.6 | |
| Kaizhou district | CQ3 | unopened type | 2022.7 | |
| Wanzhou district | CQ4 | unopened type | 2022.7 | |
| Pengshui county | CQ5 | unopened type | 2022.7 | |
| Kaizhou district | CQ6 | unopened type | 2022.7 | |
| Wulong district | CQ7 | unopened type | 2022.7 | |
| Fengjie county | CQ8 | unopened type | 2022.7 | |
| Fengjie county | CQ9 | unopened type | 2022.7 | |
| Fengjie county | CQ10 | unopened type | 2022.7 | |
| Yunyang county | CQ11 | unopened type | 2022.7 | |
| Liangshui village, Longfengba town, Xiushan county | XS1 | unopened type | 2022 | |
| Shiban village, Rongxi town, Xiushan county | XS2 | unopened type | 2022 | |
| Shuiyuan village, Longchi town, Xiushan county | XS3 | unopened type | 2022 | |
| Guiluo village, Zhongping town, Xiushan county | XS4 | unopened type | 2022 | |
| Pingyang village, Qingxichang, Xiushan county | XS5 | unopened type | 2022 | |
| Malu, Zhongling town, Xiushan county | XS6 | unopened type | 2022 | |
| Rongxi town, Xiushan county | XS7 | unopened type | 2022 | |
| Pingjian, Pingkai, Xiushan county | XS8 | unopened type | 2022 | |
| Xinqiao village, Cenxi town, Xiushan county | XS9 | unopened type | 2022 | |
| Bamang village, Aikou town, Xiushan county | XS10 | unopened type | 2022 | |
| Hunan | Longhui county, Shaoyang city | LH1 | unopened type | 2022 |
| Longhui county, Shaoyang city | LH2 | unopened type | 2022 | |
| Longhui county, Shaoyang city | LH3 | unopened type | 2022 | |
| Longhui county, Shaoyang city | LH4 | unopened type | 2022 | |
| Longhui county, Shaoyang city | LH5 | unopened type | 2022 | |
| Longhui county, Shaoyang city | LH6 | unopened type | 2022 | |
| Sichuan | Bazhong city | BZ1 | unopened type | 2022.8 |
| Bazhong city | BZ2 | unopened type | 2022.8 | |
| Bazhong city | BZ3 | unopened type | 2022.8 | |
| Guizhou | Suiyang county, Zunyi city | SY1 | unopened type | 2022.9 |
| Suiyang county, Zunyi city | SY2 | unopened type | 2022.9 | |
| Suiyang county, Zunyi city | SYH1 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH2 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH3 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH4 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH5 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH6 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH7 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH8 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH9 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH10 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH11 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH12 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH13 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH14 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH15 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH16 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH17 | open wild type | 2022.7 | |
| Suiyang county, Zunyi city | SYH18 | open wild type | 2022.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Yu, H.; Dong, Y.; Quan, W.; Su, Z.; Li, L. Quality Evaluation of Lonicerae Flos Produced in Southwest China Based on HPLC Analysis and Antioxidant Activity. Molecules 2024, 29, 2560. https://doi.org/10.3390/molecules29112560
Liu Q, Yu H, Dong Y, Quan W, Su Z, Li L. Quality Evaluation of Lonicerae Flos Produced in Southwest China Based on HPLC Analysis and Antioxidant Activity. Molecules. 2024; 29(11):2560. https://doi.org/10.3390/molecules29112560
Chicago/Turabian StyleLiu, Qundong, Huanhuan Yu, Yuzhuo Dong, Wenjing Quan, Zhimin Su, and Longyun Li. 2024. "Quality Evaluation of Lonicerae Flos Produced in Southwest China Based on HPLC Analysis and Antioxidant Activity" Molecules 29, no. 11: 2560. https://doi.org/10.3390/molecules29112560
APA StyleLiu, Q., Yu, H., Dong, Y., Quan, W., Su, Z., & Li, L. (2024). Quality Evaluation of Lonicerae Flos Produced in Southwest China Based on HPLC Analysis and Antioxidant Activity. Molecules, 29(11), 2560. https://doi.org/10.3390/molecules29112560






