Decoding Cosmetic Complexities: A Comprehensive Guide to Matrix Composition and Pretreatment Technology
Abstract
1. Introduction
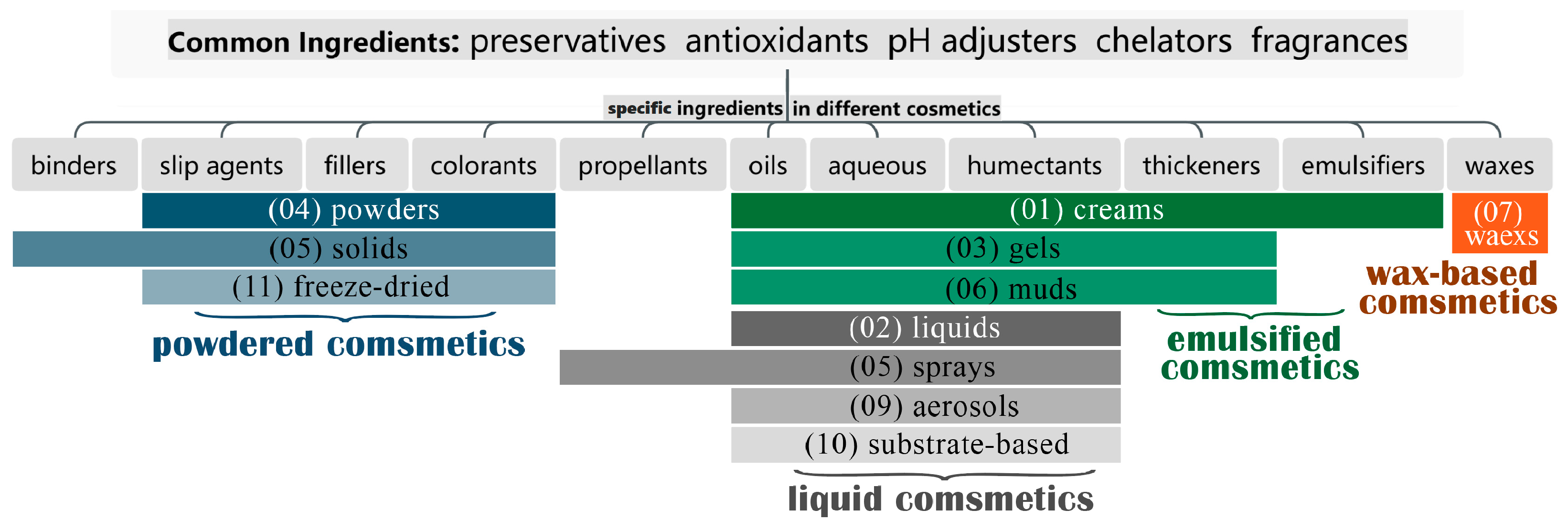
2. Compositions and Representative Compounds of Cosmetics
2.1. Common Ingredients in Cosmetic Formulations
2.1.1. Preservatives
2.1.2. Antioxidants
2.1.3. pH Adjusters
2.1.4. Chelators
2.1.5. Fragrances
2.2. Emulsified Cosmetics
2.2.1. Oils
2.2.2. Aqueous Phases
2.2.3. Emulsifiers
2.2.4. Humectants
2.2.5. Thickeners
2.3. Liquid Cosmetics
2.3.1. Solvents
2.3.2. Propellants
2.4. Powdered Cosmetics
2.4.1. Binders
2.4.2. Slip Agents
2.4.3. Fillers
2.4.4. Colorants
2.5. Wax-Based Cosmetics
2.6. Thresholds for Cosmetic Ingredient Additions
2.7. Illegal Additives in Cosmetics
2.8. Analytical Technologies in Cosmetics
3. Advances in Cosmetic Pretreatment Techniques
3.1. Emulsified Cosmetics
3.2. Liquid Cosmetics
3.3. Powdered Cosmetics
3.4. Wax-Based Cosmetics
| Pretreatment Techniques | Advantages | Disadvantages | Applicable Matrices | Refs. | |
|---|---|---|---|---|---|
| Solid phase extraction | Solid-phase extraction (SPE) | Offers high selectivity, removes complex matrices, and concentrates analytes | Can require multiple processing steps and meticulous optimization of conditions | Water-based or low viscosity cosmetic products | [16] |
| Magnetic SPE | Easy to operate, fast, and amenable to automation | Higher cost of magnetic materials | Products with substantial particulates or for rapid sample processing | [150] | |
| Mechanical stirring SPE | Efficiently mixes the sample, enhancing extraction efficiency | Equipment may be more complex | Samples that require thorough mixing to improve extraction efficiency | [160] | |
| Selective adsorbent SPE | Targeted extraction of specific components using specific adsorbents | Requires precise selection of adsorbents | Cosmetics with specific active ingredients | [151] | |
| Dispersive solid phase extraction | Dispersive solid-phase extraction (dSPE) | Simple, low cost, and quick processing time | May require more solvent | Solid and semi-solid cosmetics, like foundations and eyeshadows | [5] |
| Matrix solid-phase dispersion (MSPD) | Integrates sample dispersion and extraction, improving efficiency | Technically more complex | Solid and semi-solid samples | [156] | |
| Dispersive liquid–liquid microextraction (DLLME) | Rapid, low cost, and requires minimal solvent | High specificity in solvent selection | Extraction of small organic compounds | [13] | |
| Ultrasound-assisted extraction | UA-MSPD | Ultrasound improves extraction efficiency | Potential damage to sensitive components | A broad range of cosmetic products | [134] |
| Ultrasound-assisted extraction (UAE) | Ultrasound speeds up the extraction process, saving time | Requires specific equipment | A broad range of cosmetic products | [129] | |
| Liquid–liquid extraction | Liquid–liquid extraction (LLE) | Traditional method with wide applicability | High solvent consumption, potentially less environmentally friendly | A broad range of cosmetic products | [138] |
| Liquid phase microextraction (LPME) | Low solvent usage, more environmentally friendly | May require specialized equipment | Extracts easily separable from water with organic solvents | [153] | |
| Cloud point extraction | Cloud point extraction (CPE) | Eco-friendly, does not require organic solvents | May need temperature control | Samples containing surfactants. | [142] |
| Co-precipitation-assisted CPE | Enhances extraction efficiency, reduces solvent usage | Potentially more complex operation steps | Samples requiring simultaneous removal of various impurities | [143] | |
| Stir-bar adsorption extraction | Stir-bar sorptive extraction (SBSE) | Reusable, minimal solvent required | Potential for desorption of analytes during processing | Volatile and semi-volatile organic compounds analysis in perfumes and essential oils | [139] |
| Pressurized liquid extraction | Pressurized liquid extraction (PLE) | Increased extraction efficiency and speed due to the use of high pressure | High equipment cost | Solid and semi-solid samples | [157] |
| Accelerated solvent ex-traction (ASE) | Fast, saves solvents and time | Requires specialized equipment | Solid or viscous samples | [158] | |
| Other extraction methods | Digestion | Complete breakdown of samples, suitable for mineral analysis | Can destroy some organic components | Samples requiring complete decomposition, like for mineral content analysis | [152] |
| Ashing | Removes organic matter, useful for inorganic component analysis | Not suitable for organic component analysis | Analysis of inorganic components like heavy metals | [161] | |
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, X.; Li, L.; Dong, Y.; Dong, W.; Cui, H.; Xia, C.; Xu, T.; Wang, C.; Zhang, J.; Liu, T.; et al. Rapid detection of five estrogens added illegally to dietary supplements by combining tlc with raman imaging microscope. Molecules 2022, 27, 2650. [Google Scholar] [CrossRef]
- Chiari, B.G.; Almeida, M.; Corrêa, M.A.; Isaac, V.L.B. Cosmetics’ quality control. In Latest Research into Quality Control; InTech: London, UK, 2012; pp. 337–364. [Google Scholar] [CrossRef][Green Version]
- Su, Z.; Luo, F.-Y.; Pei, X.-R.; Zhang, F.-L.; Xing, S.-X.; Wang, G.-L. Final publication of the “Regulations on the Supervision and Administration of Cosmetics” and new prospectives of cosmetic science in China. Cosmetics 2020, 7, 98. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, S.-S.; Wu, Y.-C.; Liang, Q.-W.; Xie, X.-T.; Luo, Z.-H.; Liang, Z.-H.; Liu, M.-S. Research progress of detection technology for illegal addition of prohibited substances in cosmetics. In Proceedings of the 3rd International Conference on Agricultural and Food Science (ICAFS), Kuala Lumpur, Malaysia, 8–11 December 2020. [Google Scholar]
- Zhan, J.; Ni, M.L.; Zhao, H.Y.; Ge, X.M.; He, X.Y.; Yin, J.Y.; Yu, X.J.; Fan, Y.M.; Huang, Z.Q. Multiresidue analysis of 59 nonallowed substances and other contaminants in cosmetics. J. Sep. Sci. 2014, 37, 3684–3690. [Google Scholar] [CrossRef] [PubMed]
- Gbetoh, M.H.; Amyot, M. Mercury, hydroquinone and clobetasol propionate in skin lightening products in West Africa and Canada. Environ. Res. 2016, 150, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhou, P.; Hu, Y.; Li, G.; Xia, L. Triphenylbenzene functionalized polyhedral oligomeric silsesquioxane fluorescence sensor for the selective analysis of trace nitrofurazone in aquatic product and cosmetics. Anal. Chim. Acta 2022, 1225, 340249. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, B.; Liu, M.; Mao, S. Demand, status, and prospect of antibiotics detection in the environment. Sens. Actuators B Chem. 2022, 369, 132383. [Google Scholar] [CrossRef]
- Pratiwi, R.; Auliya As, N.N.; Yusar, R.F.; Shofwan, A.A.A. Analysis of prohibited and restricted ingredients in cosmetics. Cosmetics 2022, 9, 87. [Google Scholar] [CrossRef]
- Abad-Gil, L.; Lucas-Sánchez, S.; Jesús Gismera, M.; Teresa Sevilla, M.; Procopio, J.R. HPLC method with electrochemical detection on gold electrode for simultaneous determination of different antimicrobial agents in cosmetics. Microchem. J. 2022, 182, 107881. [Google Scholar] [CrossRef]
- Cosmetic Product Sampling Notice. Available online: https://www.nmpa.gov.cn/hzhp/hzhpcjgg/index.html (accessed on 7 January 2024).
- Zangheri, M.; Calabretta, M.M.; Calabria, D.; Fiori, J.; Guardigli, M.; Michelini, E.; Melandri, S.; Maris, A.; Mirasoli, M.; Evangelisti, L. Immunological analytical techniques for cosmetics quality control and process monitoring. Processes 2021, 9, 1982. [Google Scholar] [CrossRef]
- Schettino, L.; Garcia-Juan, A.; Fernandez-Lozano, L.; Benede, J.L.; Chisvert, A. Trace determination of prohibited acrylamide in cosmetic products by vortex-assisted reversed-phase dispersive liquid-liquid microextraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2023, 1687, 463651. [Google Scholar] [CrossRef]
- Schettino, L.; Peris-Pastor, G.; Benedé, J.L.; Chisvert, A. A comprehensive review on the use of microextraction techniques in the analysis of cosmetic products. Adv. Sample Prep. 2022, 3, 100024. [Google Scholar] [CrossRef]
- Trujillo-Rodriguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of ionic liquids in analytical chemistry. Anal. Chem. 2019, 91, 505–531. [Google Scholar] [CrossRef]
- Poole, C.F. New trends in solid-phase extraction. TrAC Trends Anal. Chem. 2003, 22, 362–373. [Google Scholar] [CrossRef]
- Celeiro, M.; Garcia-Jares, C.; Llompart, M.; Lores, M. Recent advances in sample preparation for cosmetics and personal care products analysis. Molecules 2021, 26, 4900. [Google Scholar] [CrossRef]
- Lores, M.; Llompart, M.; Alvarez-Rivera, G.; Guerra, E.; Vila, M.; Celeiro, M.; Lamas, J.P.; Garcia-Jares, C. Positive lists of cosmetic ingredients: Analytical methodology for regulatory and safety controls—A review. Anal. Chim. Acta 2016, 915, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, M.; Meng, X.; Lu, Y.; Zhang, N.; Lian, X.; Wang, C.; Gao, H.; Ma, Q. Research progress in sample pretreatment and analytical techniques for cosmetics. Se Pu 2020, 38, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Cosmetic Classification Rules and Catalogs (No. 49 of 2021). Available online: https://www.nmpa.gov.cn/xxgk/fgwj/xzhgfxwj/20210409160151122.html (accessed on 10 November 2023).
- Aerosols, Foams, & Sprays. Available online: https://pharmlabs.unc.edu/labexercises/compounding/aerosols/ (accessed on 10 November 2023).
- Cosmetics—Personal Care Chemicals. Available online: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Chemistry_for_Changing_Times_%28Hill_and_McCreary%29/21%3A_Household_Chemicals/21.06%3A_Cosmetics_-_Personal_Care_Chemicals (accessed on 10 November 2023).
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.; Barreiro, M.F. Cosmetics preservation: A teview on present strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [PubMed]
- Al-Halaseh, L.K.; Al-Adaileh, S.; Mbaideen, A.; Hajleh, M.N.A.; Al-Samydai, A.; Zakaraya, Z.Z.; Dayyih, W.A. Implication of parabens in cosmetics and cosmeceuticals: Advantages and limitations. J. Cosmet. Dermatol. 2022, 21, 3265–3271. [Google Scholar] [CrossRef]
- Garcia-Hidalgo, E.; Sottas, V.; von Goetz, N.; Hauri, U.; Bogdal, C.; Hungerbühler, K. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermat. 2017, 76, 96–106. [Google Scholar] [CrossRef]
- Lv, C.; Hou, J.; Xie, W.; Cheng, H. Investigation on formaldehyde release from preservatives in cosmetics. Int. J. Cosmet. Sci. 2015, 37, 474–478. [Google Scholar] [CrossRef]
- Ben Ouaghrem, M.; de Vaugelade, S.; Bourcier, S.; Genty, C.; Pirnay, S.; Bouchonnet, S. Characterization of photoproducts and global ecotoxicity of chlorphenesin: A preservative used in skin care products. Int. J. Cosmet. Sci. 2022, 44, 10–19. [Google Scholar] [CrossRef]
- Del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar] [CrossRef] [PubMed]
- Sigg, M.; Daniels, R. Investigations on alkanediols as alternative preservatives in a nonionic hydrophilic cream. Pharmaceutics 2020, 12, 1117. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; Meng, R.; Bu, X.; Liu, Z.; Yan, H.; Zhang, Y.; Guo, N.A. Antibacterial effect of caprylic acid and potassium sorbate in combination against listeria monocytogenes ATCC 7644. J. Food Prot. 2020, 83, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, T.; Ikami, T.; Kikukawa, K.; Kobayashi, M.; Takai, R.; Kozaki, D.; Yamamoto, A. Direct quantitation of the preservatives benzoic and sorbic acid in processed foods using derivative spectrophotometry combined with micro dialysis. Food Chem. 2018, 240, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Ratz-Lyko, A.; Arct, J.; Pytkowska, K. Methods for evaluation of cosmetic antioxidant capacity. Ski. Res. Technol. 2012, 18, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kusumawati, I.; Indrayanto, G. Natural antioxidants in cosmetics. Stud. Nat. Prod. Chem. 2013, 40, 485–505. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Farina, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Lupo, M.P. Antioxidants and vitamins in cosmetics. Clin. Dermatol. 2001, 19, 467–473. [Google Scholar] [CrossRef]
- Vagkidis, N.; Marsh, J.; Chechik, V. The role of polyphenolic antioxidants from tea and rosemary in the hydroxyl radical oxidation of N-acetyl alanine. Molecules 2023, 28, 7514. [Google Scholar] [CrossRef]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards optimal pH of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Blaak, J.; Staib, P. The relation of pH and skin cleansing. Curr. Probl. Dermatol. 2018, 54, 132–142. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of inorganic hydroxides as used in cosmetics. Int. J. Toxicol. 2021, 40, 16S–35S. [Google Scholar] [CrossRef]
- Johnson, W., Jr.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Safety assessment of formic acid and sodium formate as used in cosmetics. Int. J. Toxicol. 2016, 35, 41S–54S. [Google Scholar] [CrossRef] [PubMed]
- West, N.X.; He, T.; Zou, Y.; DiGennaro, J.; Biesbrock, A.; Davies, M. Bioavailable gluconate chelated stannous fluoride toothpaste meta-analyses: Effects on dentine hypersensitivity and enamel erosion. J. Dent. 2021, 105, 103566. [Google Scholar] [CrossRef]
- Kitazawa, M.; Iwasaki, K.; Sakamoto, K. Iron chelators may help prevent photoaging. J. Cosmet. Dermatol. 2006, 5, 210–217. [Google Scholar] [CrossRef]
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int. J. Toxicol. 2002, 21 (Suppl. S2), 95–142. [Google Scholar] [CrossRef]
- Abedi, G.; Talebpour, Z.; Jamechenarboo, F. The survey of analytical methods for sample preparation and analysis of fragrances in cosmetics and personal care products. TrAC Trends Anal. Chem. 2018, 102, 41–59. [Google Scholar] [CrossRef]
- Chisvert, A.; López-Nogueroles, M.; Miralles, P.; Salvador, A. Perfumes in cosmetics: Regulatory aspects and analytical methods. In Analysis of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 225–248. [Google Scholar]
- Heisterberg, M.V.; Menne, T.; Johansen, J.D. Contact allergy to the 26 specific fragrance ingredients to be declared on cosmetic products in accordance with the EU cosmetics directive. Contact Dermat. 2011, 65, 266–275. [Google Scholar] [CrossRef]
- Simoes, A.; Veiga, F.; Vitorino, C. Developing cream formulations: Renewed Interest in an old problem. J. Pharm. Sci. 2019, 108, 3240–3251. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.; Shah, V.; Abriola, L.; Caetano, P.; Flynn, G.L. Release of hydrocortisone from a cream matrix: Dependency of release on suspension concentration and measurement of solubility and diffusivity. Pharm. Dev. Technol. 2001, 6, 373–384. [Google Scholar] [CrossRef]
- Guillaume, D.; Charrouf, Z. Argan oil and other argan products: Use in dermocosmetology. Eur. J. Lipid Sci. Technol. 2011, 113, 403–408. [Google Scholar] [CrossRef]
- Miklavcic, M.B.; Taous, F.; Valencic, V.; Elghali, T.; Podgornik, M.; Strojnik, L.; Ogrinc, N. Fatty acid composition of cosmetic argan oil: Provenience and authenticity criteria. Molecules 2020, 25, 4080. [Google Scholar] [CrossRef] [PubMed]
- Nardello, V.; Chailloux, N.; Poprawski, J.M.; Salager, J.L.; Aubry, J.M. HLD concept as a tool for the characterization of cosmetic hydrocarbon oils. Polym. Int. 2003, 52, 602–609. [Google Scholar] [CrossRef]
- Smaoui, S.; Hlima, H.B.; Jarraya, R.; Kamoun, N.G.; Ellouze, R.; Damak, M. Cosmetic emulsion from virgin olive oil: Formulation and bio-physical evaluation. Afr. J. Biotechnol. 2012, 11, 9664–9671. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S. Triglycerides of medium-chain fatty acids: A concise review. J. Food Sci. Technol. 2023, 60, 2143–2152. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Czaja, T.P.; Bakalis, S.; Zhang, W.; Lametsch, R. Structural characteristics of high-moisture extrudates with oil-in-water emulsions. Food Res. Int. 2022, 158, 111554. [Google Scholar] [CrossRef]
- Luengo, G.; Galliano, A.; Dubief, C. Aqueous Lubrication in Cosmetics; World Scientific Publishing Co Pte Ltd.: Singapore, 2014; pp. 103–144. [Google Scholar] [CrossRef]
- Chandran, G.R.; Dailin, D.J.; Manas, N.H.A.; El-Ensashy, H.A.; Man, M.; Edis, Z.; Fatriasari, W.; Azelee, N.I.W. Antimicrobial properties of deep-sea water towards escherichia coli and staphylococcus aureus. J. Bioprocess. Biomass Technol. 2023, 2, 13–17. [Google Scholar] [CrossRef]
- Glacial Cosmetic. Available online: https://www.trendhunter.com/protrends/spring-cosmetic (accessed on 10 November 2023).
- Hanay, C.; Osterwalder, U. Challenges in formulating sunscreen products. Curr. Probl. Dermatol. 2021, 55, 93–111. [Google Scholar] [CrossRef]
- Moravkova, T.; Filip, P. The influence of emulsifier on rheological and sensory properties of cosmetic lotions. Adv. Mater. Sci. Eng. 2013, 2013, 168503. [Google Scholar] [CrossRef]
- Rahate, A.R.; Nagarkar, J.M. Emulsification of vegetable oils using a blend of nonionic surfactants for cosmetic applications. J. Dispers. Sci. Technol. 2007, 28, 1077–1080. [Google Scholar] [CrossRef]
- Lozano Gorgoso, S. Stabilizing Effect of Polyglycerides as Emulsifiers in Cosmetic Emulsions. 2023. Available online: https://diposit.ub.edu/dspace/bitstream/2445/195002/1/TFG_QU%20Lozano%20Gorgoso%2C%20Sergio.pdf (accessed on 10 November 2023).
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef]
- Flower, C.; Carter, S.; Earls, A.; Fowler, R.; Hewlins, S.; Lalljie, S.; Lefebvre, M.; Mavro, J.; Small, D.; Volpe, N. A method for the determination of N-Nitrosodiethanolamine in personal care products–collaboratively evaluated by the CTPA Nitrosamines Working Group. Int. J. Cosmet. Sci. 2006, 28, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Ye, Y.K.; Ling, M.; Shackman, J.G.; Ileka, K.M.; Raglione, T.V. High-molecular weight impurity screening by size-exclusion chromatography on a reversed-phase column. J. Pharm. Biomed. Anal. 2021, 196, 113908. [Google Scholar] [CrossRef] [PubMed]
- Ó’Fágáin, C.; Cummins, P.M.; O’Connor, B.F. Gel-Filtration Chromatography. Methods Mol. Biol. 2017, 1485, 15–25. [Google Scholar] [CrossRef]
- Burlando, B.; Cornara, L. Honey in dermatology and skin care: A review. J. Cosmet. Dermatol. 2013, 12, 306–313. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of Glycerin as used in cosmetics. Int. J. Toxicol. 2019, 38, 6s–22s. [Google Scholar] [CrossRef]
- Kerdudo, A.; Fontaine-Vive, F.; Dingas, A.; Faure, C.; Fernandez, X. Optimization of cosmetic preservation: Water activity reduction. Int. J. Cosmet. Sci. 2015, 37, 31–40. [Google Scholar] [CrossRef]
- Politi, R.; Sapir, L.; Harries, D. The impact of polyols on water structure in solution: A computational study. J. Phys. Chem. A 2009, 113, 7548–7555. [Google Scholar] [CrossRef]
- Laba, D. How Do I Thicken My Cosmetic Formula? Cosmet. Toilet. 2001, 116, 35–48. Available online: https://img.cosmeticsandtoiletries.com/files/base/allured/all/image/2019/11/ct.CT_116_11_035_09.pdf (accessed on 10 November 2023).
- Karsheva, M.; Georgieva, S.; Handjieva, S. The choice of the thickener—A way to improve the cosmetics sensory properties. J. Univ. Chem. Technol. Metall. 2007, 42, 187–194. Available online: https://www.researchgate.net/profile/Maria-Karsheva/publication/292447286_Thickener_choice_-_A_way_to_improve_cosmetics_sensory_properties/links/56bafbbd08ae2567351ee444/Thickener-choice-A-way-to-improve-cosmetics-sensory-properties.pdf (accessed on 10 November 2023).
- Lochhead, R.Y. The role of polymers in cosmetics: Recent trends. In Cosmetic Nanotechnology; American Chemical Society: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]
- Patil, A.; Ferritto, M.S. Polymers for personal care and cosmetics: Overview. In Polymers for Personal Care and Cosmetics; American Chemical Society: Washington, DC, USA, 2013; pp. 3–11. [Google Scholar] [CrossRef]
- Boran, G.; Regenstein, J.M. Fish gelatin. Adv. Food Nutr. Res. 2010, 60, 119–143. [Google Scholar] [CrossRef]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lindman, B.; Edlund, H.; Norgren, M. Emulsion formation and stabilization by biomolecules: The leading role of cellulose. Polymers 2019, 11, 1570. [Google Scholar] [CrossRef]
- Fagioli, L.; Pavoni, L.; Logrippo, S.; Pelucchini, C.; Rampoldi, L.; Cespi, M.; Bonacucina, G.; Casettari, L. Linear viscoelastic properties of selected polysaccharide gums as function of concentration, pH, and temperature. J. Food Sci. 2019, 84, 65–72. [Google Scholar] [CrossRef]
- Salager, J.-L.; Antón, R.; Bullón, J.; Forgiarini, A.; Marquez, R. How to use the normalized hydrophilic-lipophilic deviation (HLDN) concept for the formulation of equilibrated and emulsified surfactant-oil-water systems for cosmetics and pharmaceutical products. Cosmetics 2020, 7, 57. [Google Scholar] [CrossRef]
- Berthele, H.; Sella, O.; Lavarde, M.; Mielcarek, C.; Pense-Lheritier, A.M.; Pirnay, S. Determination of the influence of factors (ethanol, pH and a(w)) on the preservation of cosmetics using experimental design. Int. J. Cosmet. Sci. 2014, 36, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Baran, R. Nail cosmetics: Allergies and irritations. Am. J. Clin. Dermatol. 2002, 3, 547–555. [Google Scholar] [CrossRef]
- Chou, M.; Dhingra, N.; Strugar, T.L. Contact sensitization to allergens in nail cosmetics. Dermatitis 2017, 28, 231–240. [Google Scholar] [CrossRef]
- Khaiat, A.; Saliou, C. Botanical extracts. In Cosmeceuticals and Active Cosmetics; CRC Press: Boca Raton, FL, USA, 2015; Volume 385, Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/b18895-34/botanical-extracts-alain-khaiat-claude-saliou (accessed on 10 November 2023).
- Zhou, W.; Wang, P.G.; Wittenberg, J.B.; Rua, D.; Krynitsky, A.J. Simultaneous determination of cosmetics ingredients in nail products by fast gas chromatography with tandem mass spectrometry. J. Chromatogr. A 2016, 1446, 134–140. [Google Scholar] [CrossRef]
- Infante, P.F.; Petty, S.E.; Groth, D.H.; Markowitz, G.; Rosner, D. Vinyl chloride propellant in hair spray and angiosarcoma of the liver among hairdressers and barbers: Case reports. Int. J. Occup. Environ. Health 2009, 15, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Abba, M.; Naeem, Z.; Nourian, A.; Nasr, G.G. Effects of pressure decay on Non-Methane Volatile Organic Compounds (NMVOC) species distribution in domestic aerosol sprays with LPG propellants. In Proceedings of the 15th Triennial International Conference on Liquid Atomization and Spray Systems (ICLASS), Edinburgh, UK, 29 August–2 September 2021; Volume 1. [Google Scholar] [CrossRef]
- Emmen, H.H.; Hoogendijk, E.M.; Klopping-Ketelaars, W.A.; Muijser, H.; Duistermaat, E.; Ravensberg, J.C.; Alexander, D.J.; Borkhataria, D.; Rusch, G.M.; Schmit, B. Human safety and pharmacokinetics of the CFC alternative propellants HFC 134a (1,1,1,2-tetrafluoroethane) and HFC 227 (1,1,1,2,3,3,3-heptafluoropropane) following whole-body exposure. Regul. Toxicol. Pharmacol. 2000, 32, 22–35. [Google Scholar] [CrossRef] [PubMed]
- McFarland, M. Application and Emissions of Fluorocarbon Gases: Past, Present, and Prospects for the Future. In Non-CO2 Greenhouse Gases: Scientific Understanding, Control and Implementation, Proceedings of the Second International Symposium, Noordwijkerhout, The Netherlands, 8–10 September 1999; Springer: Dordrecht, The Netherlands, 2000; pp. 65–82. Available online: https://link.springer.com/chapter/10.1007/978-94-015-9343-4_3 (accessed on 10 November 2023).
- Steiling, W.; Almeida, J.F.; Assaf Vandecasteele, H.; Gilpin, S.; Kawamoto, T.; O’Keeffe, L.; Pappa, G.; Rettinger, K.; Rothe, H.; Bowden, A.M. Principles for the safety evaluation of cosmetic powders. Toxicol. Lett. 2018, 297, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Riley, P. Decorative cosmetics. In Poucher’s Perfumes, Cosmetics and Soaps; Springer: Dordrecht, The Netherlands, 2000; pp. 167–216. Available online: https://link.springer.com/chapter/10.1007/978-94-017-2734-1_6 (accessed on 10 November 2023).
- Scott, D.A. A review of ancient Egyptian pigments and cosmetics. Stud. Conserv. 2016, 61, 185–202. [Google Scholar] [CrossRef]
- Watanabe, H.; Takahashi, K.; Kumagai, S. Development of 3D powdery cosmetics with new ‘dry binder’. Int. J. Cosmet. Sci. 2010, 32, 23–28. [Google Scholar] [CrossRef]
- Kaneda, I. Rheology control agents for cosmetics. In Rheology of Biological Soft Matter: Fundamentals and Applications; Springer: Tokyo, Japan, 2017; pp. 295–321. Available online: https://link.springer.com/chapter/10.1007/978-4-431-56080-7_11 (accessed on 10 November 2023).
- Amnuaikit, T.; Chusuit, T.; Raknam, P.; Boonme, P. Effects of a cellulose mask synthesized by a bacterium on facial skin characteristics and user satisfaction. Med. Devices 2011, 4, 77–81. [Google Scholar] [CrossRef]
- Podkowa-Zawadzka, I.; Wasilewski, T.; Zięba, M. Evaluation of the quality of bath cosmetics in powder form depending on the Selection of Fillers. Tenside Surfactants Deterg. 2021, 58, 334–341. [Google Scholar] [CrossRef]
- Lam, H. Factors enhancing adhesion of color cosmetic products toskin: The role of pigments and fillers. In Surface Science and Adhesion in Cosmetics; Wiley: Hoboken, NJ, USA, 2021; pp. 487–541. [Google Scholar] [CrossRef]
- Brudzynska, P.; Sionkowska, A.; Grisel, M. Plant-derived colorants for food, cosmetic and textile industries: A review. Materials 2021, 14, 3484. [Google Scholar] [CrossRef]
- Vigneshwaran, L.; Amritha, P.; Farsana, K.; Khairunnisa, T.; Sebastian, V.; TK, A.B. A review on natural colourants used in cosmetics. Curr. Res. Pharm. Sci. 2023, 13, 83–92. [Google Scholar] [CrossRef]
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Analysis of dyes in cosmetics: Challenges and recent developments. Cosmetics 2018, 5, 47. [Google Scholar] [CrossRef]
- Chuberre, B.; Araviiskaia, E.; Bieber, T.; Barbaud, A. Mineral oils and waxes in cosmetics: An overview mainly based on the current European regulations and the safety profile of these compounds. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Lal, U.R.; Mukhtar, H.M.; Singh, P.S.; Shah, G.; Dhawan, R.K. Phytochemical profile of sugarcane and its potential health aspects. Pharmacogn. Rev. 2015, 9, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Pazyar, N.; Yaghoobi, R.; Ghassemi, M.R.; Kazerouni, A.; Rafeie, E.; Jamshydian, N. Jojoba in dermatology: A succinct review. G. Ital. Dermatol. Venereol. 2013, 148, 687–691. Available online: https://www.minervamedica.it/en/journals/Ital-J-Dermatol-Venereol/article.php?cod=R23Y2013N06A0687&acquista=1 (accessed on 10 November 2023).
- Kurek-Gorecka, A.; Gorecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee products in dermatology and skin care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef]
- Regulation (EC) No 1223/2009 of The European Parliament and of The Council of 30 November 2009 on Cosmetic Products. Available online: https://health.ec.europa.eu/document/download/47f167ec-b5db-4ec9-9d12-3d807bf3e526_en (accessed on 7 January 2024).
- Commission Regulation (EU) 2022/2195 of 10 November 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022R2195&from=EN (accessed on 7 January 2024).
- Announcement of the China National Medical Products Administrationon the Publication of the Technical Specification for Cosmetic Safety (2015 Edition) (No. 268 of 2015). Available online: https://www.nmpa.gov.cn/hzhp/hzhpfgwj/hzhpgzwj/20151223120001986.html?type=pc&m= (accessed on 7 January 2024).
- Announcement of the China National Medical Products Administration on the Catalog of Used Cosmetic Ingredients (2021 Edition) (No. 62 of 2021). Available online: https://www.nmpa.gov.cn/xxgk/ggtg/hzhpggtg/jmhzhptg/20210430162707173.html (accessed on 7 January 2024).
- Linoleamide DEA—Cosmetics Info. Available online: https://www.cosmeticsinfo.org/ingredient/linoleamide-dea/ (accessed on 7 January 2024).
- Span™ 60 Pharma|Croda Pharma. Available online: https://www.crodapharma.com/en-gb/product-finder/product/5545-span_1_60_1_pharma (accessed on 7 January 2024).
- Glyceryl Stearate—Cosmetics Info. Available online: https://www.cosmeticsinfo.org/ingredient/glyceryl-stearate/ (accessed on 7 January 2024).
- Mica—Cosmetics Info. Available online: https://www.cosmeticsinfo.org/ingredient/mica/ (accessed on 7 January 2024).
- Calcium Carbonate—Cosmetics Info. Available online: https://www.cosmeticsinfo.org/ingredient/calcium-carbonate/ (accessed on 7 January 2024).
- Montanov™ 82. Available online: https://www.formulatorsampleshop.com/montanovtm-82.html (accessed on 7 January 2024).
- Talc—Cosmetics Info. Available online: https://www.cosmeticsinfo.org/ingredient/talc/ (accessed on 7 January 2024).
- Water—Cosmetics Info. Available online: https://www.cosmeticsinfo.org/ingredient/water/ (accessed on 7 January 2024).
- Toluene—Cosmetics Info. Available online: https://www.cosmeticsinfo.org/ingredient/toluene/ (accessed on 7 January 2024).
- Iron Oxides—Cosmetics Info. Available online: https://www.cosmeticsinfo.org/ingredient/iron-oxides/ (accessed on 7 January 2024).
- Zinc Oxide—Cosmetics Info. Available online: https://www.cosmeticsinfo.org/ingredient/zinc-oxide/ (accessed on 7 January 2024).
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=73.2496&SearchTerm=mica (accessed on 7 January 2024).
- Safety of Cosmetic Ingredients—Canada.ca. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/labelling/safety-ingredients.html#a4.1 (accessed on 7 January 2024).
- Vitis Vinifera (Grape) Seed Oil—Cosmetics Info. Available online: https://www.cosmeticsinfo.org/ingredient/vitis-vinifera-grape-seed-oil/ (accessed on 7 January 2024).
- Sodium Lactate—Cosmetics Info. Available online: https://www.cosmeticsinfo.org/ingredient/sodium-lactate/ (accessed on 7 January 2024).
- TBHQ in Skin Care: What it Is & Is it Safe? Available online: https://www.paulaschoice.com/ingredient-dictionary/ingredient-tbhq.html (accessed on 7 January 2024).
- Hyaluronic Acid—Cosmetics Info. Available online: https://www.cosmeticsinfo.org/ingredient/hyaluronic-acid/ (accessed on 7 January 2024).
- Announcement of the China National Medical Products Administration on Updating the List of Prohibited Ingredients in Cosmetics (No. 74 of 2021). Available online: https://www.nmpa.gov.cn/xxgk/ggtg/hzhpggtg/jmhzhptg/20210528174051160.html?type=pc&m= (accessed on 7 January 2024).
- Uríčková, V.; Sádecká, J. Determination of geographical origin of alcoholic beverages using ultraviolet, visible and infrared spectroscopy: A review. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 148, 131–137. [Google Scholar] [CrossRef]
- Ablat, H.; Nurmamat, X.; Ma, X.; Xie, Q.; Zhao, Z. Application of infrared spectroscopy and its theoretical simulation to arsenic adsorption processes. Water Environ. Res. 2023, 95, e10867. [Google Scholar] [CrossRef]
- Shang, Y.; Meng, X.; Liu, J.; Song, N.; Zheng, H.; Han, C.; Ma, Q. Applications of mass spectrometry in cosmetic analysis: An overview. J. Chromatogr. A 2023, 1705, 464175. [Google Scholar] [CrossRef]
- Lu, Y.; He, Y.; Wang, X.; Wang, H.; Qiu, Q.; Wu, B.; Wu, X. Screening, characterization, and determination of suspected additives bimatoprost and latanoprost in cosmetics using NMR and LC-MS methods. Anal. Bioanal. Chem. 2023, 415, 3549–3558. [Google Scholar] [CrossRef]
- Alsohaimi, I.H.; Khan, M.R.; Ali, H.M.; Azam, M.; Alammari, A.M. Solvent extraction and gas chromatography-mass spectrometric determination of probable carcinogen 1,4-dioxane in cosmetic products. Sci. Rep. 2020, 10, 5214. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Ziemlewska, A.; Bujak, T.; Nizioł-Łukaszewska, Z.; Hordyjewicz-Baran, Z. Cosmetic and dermatological properties of selected ayurvedic plant extracts. Molecules 2021, 26, 614. [Google Scholar] [CrossRef]
- Bocca, B.; Forte, G.; Pino, A.; Alimonti, A. Heavy metals in powder-based cosmetics quantified by ICP-MS: An approach for estimating measurement uncertainty. Anal. Methods 2013, 5, 402–408. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Pschenitza, M.; Niessner, R.; Li, Y.; Knopp, D.; Deng, A. Highly sensitive and specific determination of mercury(II) ion in water, food and cosmetic samples with an ELISA based on a novel monoclonal antibody. Anal. Bioanal. Chem. 2012, 403, 2519–2528. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, G.; Wu, Y.; Luo, Z.; Zhu, B. Ultrasound-assisted matrix solid-phase dispersive liquid extraction for the determination of intermediates in hair dyes with ion chromatography. Anal. Chim. Acta 2012, 752, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ogunfowokan, A.O.; Torto, N.; Adenuga, A.A.; Okoh, E.K. Survey of levels of phthalate ester plasticizers in a sewage lagoon effluent and a receiving stream. Environ. Monit. Assess. 2006, 118, 457–480. Available online: https://link.springer.com/article/10.1007/s10661-006-1500-z (accessed on 10 November 2023). [CrossRef] [PubMed]
- Cai, Y.; Cai, Y.e.; Shi, Y.; Liu, J.; Mou, S.; Lu, Y. A liquid–liquid extraction technique for phthalate esters with water-soluble organic solvents by adding inorganic salts. Microchim. Acta 2007, 157, 73–79. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, B.; Zhong, X.; Li, S. Determination of 15 volatile organic compound residues in cosmetics by headspace gas chromatography. Se Pu 2010, 28, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.R.; Monteiro-Riviere, N.A.; Riviere, J.E. Trace analysis of fullerenes in biological samples by simplified liquid-liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2006, 1129, 216–222. [Google Scholar] [CrossRef]
- David, F.; Sandra, P. Stir bar sorptive extraction for trace analysis. J. Chromatogr. A 2007, 1152, 54–69. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Kozik, V.; Dabioch, M. Complex-forming organic ligands in cloud-point extraction of metal ions: A review. Talanta 2013, 110, 202–228. [Google Scholar] [CrossRef]
- Prasada Rao, T.; Kala, R. On-line and off-line preconcentration of trace and ultratrace amounts of lanthanides. Talanta 2004, 63, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Soruraddin, M.H.; Heydari, R.; Puladvand, M.; Zahedi, M.M. A new spectrophotometric method for determination of selenium in cosmetic and pharmaceutical preparations after preconcentration with cloud point extraction. Int. J. Anal. Chem. 2011, 2011, 729651. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, X.; Xu, X.; Li, G. Co-precipitation assisted cloud point extraction coupled with high performance liquid chromatography for the determination of estrogens in water and cosmetic samples. Anal. Methods 2013, 5, 6376–6381. [Google Scholar] [CrossRef]
- Azzouz, A.; Kailasa, S.K.; Lee, S.S.; Rascón, A.J.; Ballesteros, E.; Zhang, M.; Kim, K.-H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. TrAC Trends Anal. Chem. 2018, 108, 347–369. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, S.; She, Y.; Zhang, C.; Zheng, L.; Jin, M.; Shao, H.; Jin, F.; Du, X.; Wang, J. Subcritical water extraction combined with molecular imprinting technology for sample preparation in the detection of triazine herbicides. J. Chromatogr. A 2017, 1515, 17–22. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Liu, J.; Huang, K.; Wu, C.; Shao, H.; Chen, H.; Liu, X. Modification of garlic peel by nitric acid and its application as a novel adsorbent for solid-phase extraction of quinolone antibiotics. Chem. Eng. J. 2017, 326, 745–755. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, Y.; Sheng, K.; Jing, W.; Xu, X.; Bao, T.; Wang, S. Effective extraction of fluoroquinolones from water using facile modified plant fibers. J. Pharm. Anal. 2022, 12, 791–800. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Bagheri, A.R.; Guo, X.; Wang, L.; Li, J.; Wang, X.; Li, B.; Chen, L. Strategies of molecular imprinting-based solid-phase extraction prior to chromatographic analysis. TrAC Trends Anal. Chem. 2020, 128, 115923. [Google Scholar] [CrossRef]
- Zhao, F.; She, Y.; Zhang, C.; Wang, S.; Du, X.; Jin, F.; Jin, M.; Shao, H.; Zheng, L.; Wang, J. Selective determination of chloramphenicol in milk samples by the solid-phase extraction based on dummy molecularly imprinted polymer. Food Anal. Methods 2017, 10, 2566–2575. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Liang, N.; Zhao, L. Deep eutectic solvent-based magnetic colloidal gel assisted magnetic solid-phase extraction: A simple and rapid method for the determination of sex hormones in cosmetic skin care toners. Chemosphere 2020, 255, 127004. [Google Scholar] [CrossRef]
- Vicario, A.; Aragón, L.; Wang, C.C.; Bertolino, F.; Gomez, M.R. A simple and highly selective molecular imprinting polymer-based methodology for propylparaben monitoring in personal care products and industrial waste waters. J. Pharm. Biomed. Anal. 2018, 149, 225–233. [Google Scholar] [CrossRef]
- Cha, N.-R.; Lee, J.-K.; Lee, Y.-R.; Jeong, H.-J.; Kim, H.-K.; Lee, S.-Y. Determination of Iron, Copper, Zinc, Lead, Nickel and Cadmium in Cosmetic Matrices by Flame Atomic Absorption Spectroscopy. Anal. Lett. 2010, 43, 259–268. [Google Scholar] [CrossRef]
- Xiao, X.; Yin, Y.; Hu, Y.; Li, G. Determination of Trace Estrogens in Cosmetic Water by Liquid-Phase Microextraction Coupled with High Performance Liquid Chromatography. Se Pu 2007, 25, 234–237. Available online: https://www.chrom-china.com/EN/Y2007/V25/I2/234 (accessed on 7 January 2024).
- Shi, M.-Z.; Yu, Y.-L.; Zhu, S.-C.; Yang, J.; Cao, J. Latest development of matrix solid phase dispersion extraction and microextraction for natural products from 2015–2021. Sep. Purif. Rev. 2023, 52, 262–282. [Google Scholar] [CrossRef]
- Tu, X.; Chen, W. A Review on the recent progress in matrix solid phase dispersion. Molecules 2018, 23, 2767. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Bai, H.; Zhai, J.F.; Meng, X.S.; Guo, X.Y.; Wang, C.; Wang, P.L.; Lei, H.M.; Niu, Z.Y.; Ma, Q. Comprehensive screening of 63 coloring agents in cosmetics using matrix solid-phase dispersion and ultra-high-performance liquid chromatography coupled with quadrupole-Orbitrap high-resolution mass spectrometry. J. Chromatogr. A 2019, 1590, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Barp, L.; Višnjevec, A.M.; Moret, S. Pressurized Liquid Extraction: A powerful tool to implement extraction and purification of food contaminants. Foods 2023, 12, 2017. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ge, X.; Lv, Y.; Wang, A. Application of accelerated solvent extraction in the analysis of organic contaminants, bioactive and nutritional compounds in food and feed. J. Chromatogr. A 2012, 1237, 1–23. [Google Scholar] [CrossRef]
- Dimakopoulou-Papazoglou, D.; Giannakaki, F.; Katsanidis, E. Structural and physical characteristics of mixed-component oleogels: Natural wax and monoglyceride interactions in different edible oils. Gels 2023, 9, 627. [Google Scholar] [CrossRef]
- Wang, C.C.; Masi, A.N.; Fernandez, L. On-line micellar-enhanced spectrofluorimetric determination of rhodamine dye in cosmetics. Talanta 2008, 75, 135–140. [Google Scholar] [CrossRef]
- Brandão, J.D.O.; Okonkwo, O.J.; Sehkula, M.; Raseleka, R.M. Concentrations of lead in cosmetics commonly used in South Africa. Toxicol. Environ. Chem. 2012, 94, 70–77. [Google Scholar] [CrossRef]
- Saravanakumar, K.; De Silva, S.; Santosh, S.S.; Sathiyaseelan, A.; Ganeshalingam, A.; Jamla, M.; Sankaranarayanan, A.; Veeraraghavan, V.P.; MubarakAli, D.; Lee, J.; et al. Impact of industrial effluents on the environment and human health and their remediation using MOFs-based hybrid membrane filtration techniques. Chemosphere 2022, 307, 135593. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, L.; Erny, G.L.; Santos, L.; Alves, A. Applications of molecularly imprinted polymers to the analysis and removal of personal care products: A review. Talanta 2016, 146, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.C.; Hsu, K.C.; Shiea, C.S.; Huang, Y.L. Recent trends in nanomaterial-based microanalytical systems for the speciation of trace elements: A critical review. Anal. Chim. Acta 2015, 884, 1–18. [Google Scholar] [CrossRef]
- Yang, F.; Wang, C.; Yu, H.; Guo, Y.; Cheng, Y.; Yao, W.; Xie, Y. Establishment of the thin-layer chromatography-surface-enhanced Raman spectroscopy and chemometrics method for simultaneous identification of eleven illegal drugs in anti-rheumatic health food. Food Biosci. 2022, 49, 101842. [Google Scholar] [CrossRef]
- Duarte, L.C.; Baldo, T.A.; Silva-Neto, H.A.; Figueredo, F.; Janegitz, B.C.; Coltro, W.K. 3D printing of compact electrochemical cell for sequential analysis of steroid hormones. Sens. Actuators B Chem. 2022, 364, 131850. [Google Scholar] [CrossRef]






| Ingredients | Thresholds | Ingredients | Thresholds | Ingredients | Thresholds | Ingredients | Thresholds | Ingredients | Thresholds |
|---|---|---|---|---|---|---|---|---|---|
| Preservatives | Ubidecarenone (24) | N.D. [f] | Cetylicalcohol (45) | N.D. | Linoleic acid diethanolamide (68) | 10% in rinse-off products [108] | PMMA | N.D. [f] | |
| Methylparaben (1) | 0.4% (as acid) for single [a]; 0.8% (as acid) for mixtures [a] | pH Adjusters | Polyethylene glycol monocetyl ether (69) | 0.4–6.7% [d]; 0.345–6.25% [e] | |||||
| Propylparaben (2) | 0.14% (as acid) for single [a] 0.8% (as acid) for mixtures [a] | Triethanolamine (25) | 8% [c] | Tween 60 (46) | 25% [e] | Cetyl palmitate (70) | 12.5% [e] | Sorbitan esters | 3–20% [d]; 1–20.7% [e] |
| Butylparaben (3) | Citric acid (26) | 0.2% [a] | Span 60 (47) | 5% [109] | Myristyl dimethylamine oxide (71) | 0.39% [d] | Propylene glycol | 89.493% [d]; 66.19% [e] | |
| Methylisothiazolinone (4) | 0.0015% [a] | Sodium citrate (27) | N.D. [f] | Stearyl alcohol polyether-2 (48) | 8.95% [d]; 8% [e] | Hexadecylbetaine (72) | 10% [d] | PEG-8 | 69% [d]; 60% [e] |
| Chloromethylisothiazolinone (5) | Succinic acid (28) | 10% [d]; 1% [e] | Polyethylene glycol octadecyl ether (49) | 1.7% [d][e] | Potassium oleate (73) | 30.4% [d] | Fillers | ||
| Imidazolidinyl urea (6) | 0.6% [a] | Disodium succinate (29) | 1.6% [e] | Glycerine monostearate (50) | No Limits [110] | Cellulose (74) | 18% [e] | Mica | No limits [111] |
| DMDMH (7) | 0.6% [a] | Potassium phosphate (30) | 0.88% [d] | Polyglycerol-10 laurate (51) | 3.5% [d]; 3% [e] | Alginic acid (75) | 0.33915% [e] | Kaolin | 83.333% [d]; 67.742% [e] |
| Chlorphenesin (8) | 0.3% [a] | Chelators | Eumulgin SG (52) | 67.12% [d]; 2% [e] | Hectorite (76) | 5% [d]; 3.8% [e] | Calcium carbonate | No limits [112] | |
| 2-phenoxyethanol (9) | 1.0% [a] | Disodium EDTA (31) | 5% [e] | Montanov™ 82 (53) | 3% [113] | Solvents | Talc | No limits [114] | |
| 1,2-pentanediol (10) | 21.29 [e] | Citric acid (32) | 0.2% [a] | Thickeners | Water | No limits [115] | Silica | 100% [e] | |
| Sorbic acid (11) | 0.6% (as acid) [a] | Phosphoric acid (33) | 2.55% [d]; 0.6 [e] | Glycerin (54) | 98.525% [d]; 62.1% [e] | Ethanol | 5% [a] | Pearl | 49.51% [d]; 0.1% [e] |
| Potassium sorbate (12) | 0.6% (as acid) [a] | Fragrances | 1,2-butanediol (55) | 8% [d]; 6% [e] | Ethyl acetate | N.D. | Nylon | 25% [d]; 3–59.5 [e] | |
| Sodium benzoate (13) | 2.5% (as acid) for rinse-off products; 1.7% for oral care products [a] | Linalool (34) | 1.25% [d]; 1% [e] | 1,3-butanediol (56) | 87.98% [e] | Toluene | 33% [116] | Colorants | |
| Levulinic acid (14) | 5% [e] | Vanillin (35) | 1.2% [e] | Sodium L-pyroglutamate (57) | 20% [e] | Propellants | Iron oxides | No limits [117] | |
| Anisic acid (15) | 0.96% [e] | Cinnamaldehyde (36) | 0.016% [d] | Panthenol (58) | 40% [e] | Propane | 58.7% [d]; 36.544% [e] | Zinc oxide | No limits in non-nano particle form [118] |
| Antioxidants | Eugenol (37) | 0.036% [d]; 0.031 [e] | Sorbitol (59) | 51.46% [d]; 38.849% [e]. | Butane | 70.045% [d]; 56% [e] | Titanium dioxide | 25% [a] | |
| Butylated hydroxytoluene (16) | 0.001% for mouthwash; 0.01% for toothpaste; [b] | Oil-based Constituents | Betaine (60) | 20% [e] | Isobutane | 81.522% [e] | Mica | No limits [119] | |
| Butylated hydroxyanisole (17) | 0.1% [120] | Grape seed oil (38) | No Limits [121] | Sodium lactate (61) | 10% [122] | Carbon dioxide | 50% [e] | Waxes | |
| Tertiary-butylhydroquinone (18) | 0.1% [123] | Petrolatum (39) | 75.175% [d] | Sodium hyaluronate (62) | 74.993% [d]; 1% [e] | Nitrogen | 40.476% [e] | Carnauba | 5% [e] |
| Propyl gallate (19) | 8.0854% [d]; 1.5% [e] | Isopropyl palmitate (40) | 79.69% [e] | Ceramides (63) | 22.5% [e] | Binders | Candelilla | 30% [e] | |
| Vitamin C (20) | N.D. [f] | Isopropyl myristate (41) | 78.278% [d]; 42% [e] | Lauryl alcohols (64) | 15% [d]; 3.5% [e] | Beeswax | 50% [e] | Jojoba | 5% [e] |
| Hyaluronic acid (21) | 2% [124] | Isononyl isononyl ester (42) | 71.4% [e] | Myristyl alcohols (65) | 12% [d]; 7.02% [e] | Keratin | 1% [d] | Bees | 50% [e] |
| Vitamin E (22) | 33.702% [e] | Lecithin (43) | 20.008% [d]; 14% [e] | Linolenic acid (66) | 13.3% [e] | Liquid paraffin | 99.788% [e] | Japan waxes | 8% [e] |
| Squalene (23) | 82% [c]; 2% [e] | Squalane (44) | 48.98% [e] | Coconut monoethanolamide (67) | 1.24% [d] | Silicone oils | N.D. [f] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.-N.; He, Y.; Chen, Y.-W.; Liu, Q.; Sun, L.; Sun, H.-M.; Wu, X.-F.; Lu, Y. Decoding Cosmetic Complexities: A Comprehensive Guide to Matrix Composition and Pretreatment Technology. Molecules 2024, 29, 411. https://doi.org/10.3390/molecules29020411
Du X-N, He Y, Chen Y-W, Liu Q, Sun L, Sun H-M, Wu X-F, Lu Y. Decoding Cosmetic Complexities: A Comprehensive Guide to Matrix Composition and Pretreatment Technology. Molecules. 2024; 29(2):411. https://doi.org/10.3390/molecules29020411
Chicago/Turabian StyleDu, Xiao-Nan, Yu He, You-Wen Chen, Qian Liu, Lei Sun, Hui-Min Sun, Xian-Fu Wu, and Yong Lu. 2024. "Decoding Cosmetic Complexities: A Comprehensive Guide to Matrix Composition and Pretreatment Technology" Molecules 29, no. 2: 411. https://doi.org/10.3390/molecules29020411
APA StyleDu, X.-N., He, Y., Chen, Y.-W., Liu, Q., Sun, L., Sun, H.-M., Wu, X.-F., & Lu, Y. (2024). Decoding Cosmetic Complexities: A Comprehensive Guide to Matrix Composition and Pretreatment Technology. Molecules, 29(2), 411. https://doi.org/10.3390/molecules29020411






