Portable Mid-Infrared Spectroscopy Combined with Chemometrics to Diagnose Fibromyalgia and Other Rheumatologic Syndromes Using Rapid Volumetric Absorptive Microsampling
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of Subjects
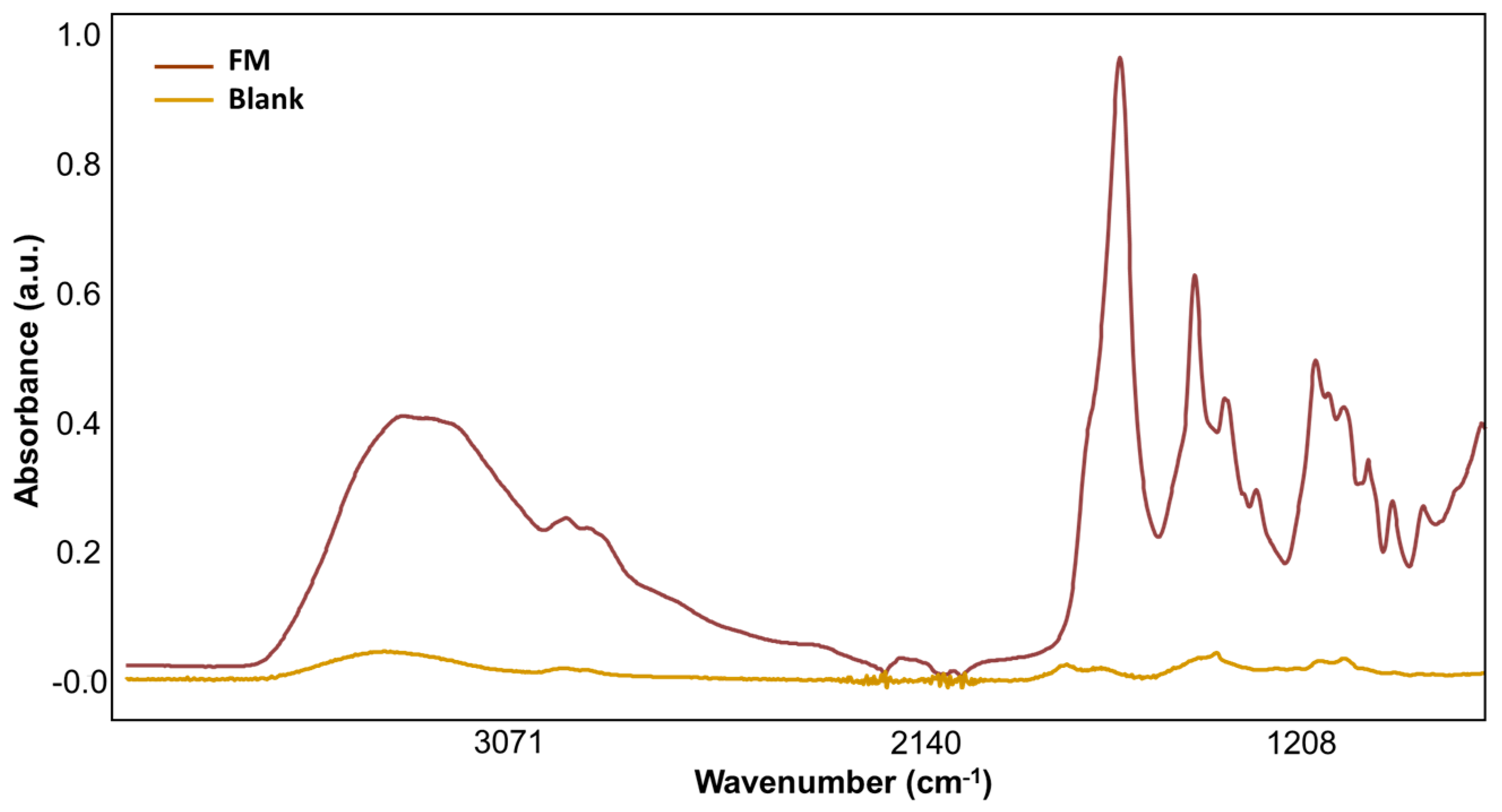
2.2. Mid-Infrared Spectroscopy
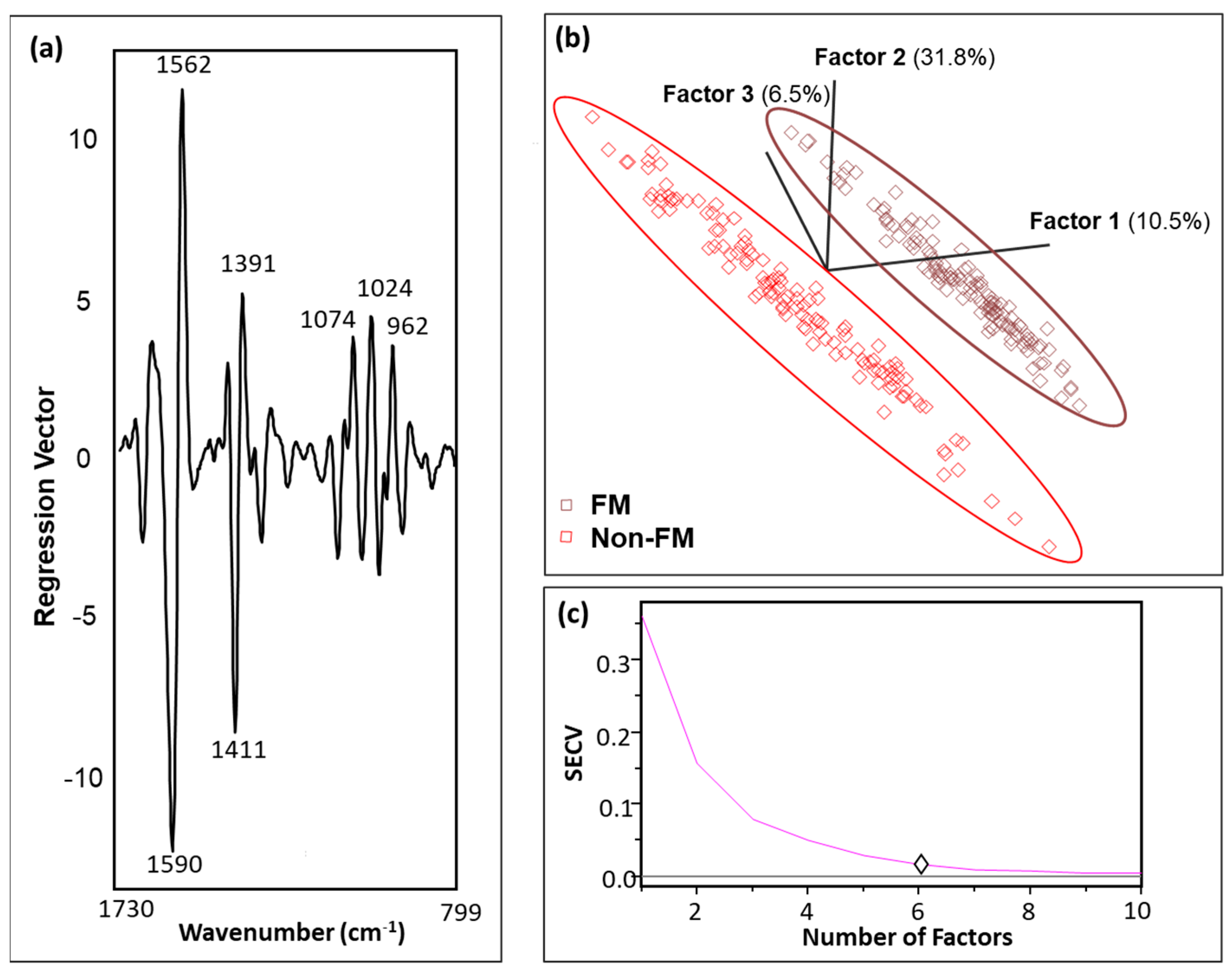
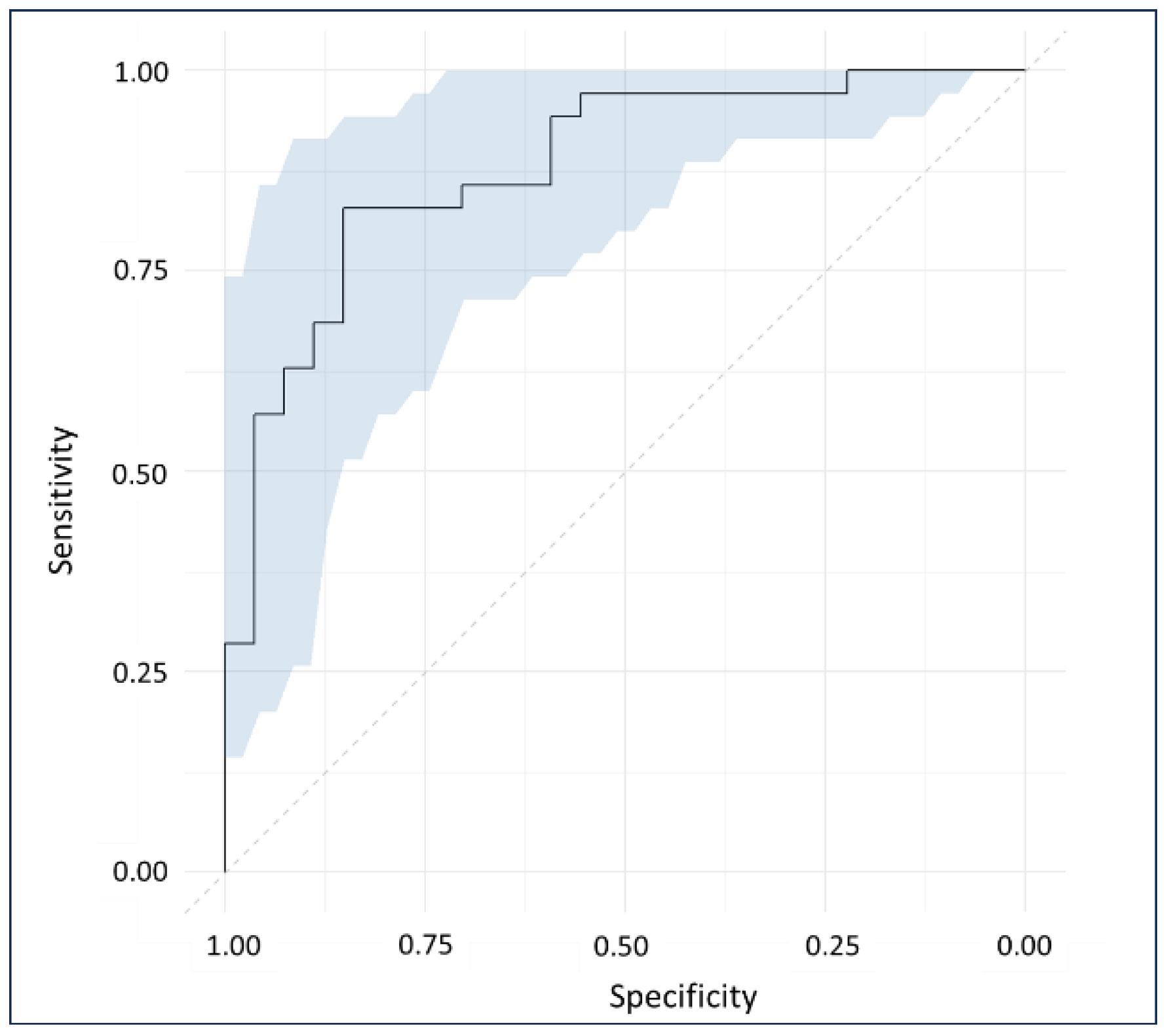
2.3. Classification Analysis
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment and Blood Sampling
4.2. Sample Extraction
4.3. Infrared Spectroscopy Analysis
4.4. Pattern Recognition Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ablin, J.N.; Buskila, D.; Clauw, D.J. Biomarkers in fibromyalgia. Curr. Pain Headache Rep. 2009, 13, 343–349. [Google Scholar] [CrossRef]
- Hackshaw, K.V.; Aykas, D.P.; Sigurdson, G.T.; Plans, M.; Madiai, F.; Yu, L.; Buffington, C.A.T.; Giusti, M.M.; Rodriguez-Saona, L. Metabolic fingerprinting for diagnosis of fibromyalgia and other rheumatologic disorders. J. Biol. Chem. 2019, 294, 2555–2568. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Di Paola, R.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Phillips, K.; Clauw, D.J. Review: Central pain mechanisms in the rheumatic diseases: Future directions. Arthritis Rheum. 2012, 65, 291–302. [Google Scholar] [CrossRef]
- Miller, J.S.; Rodriguez-Saona, L.; Hackshaw, K.V. Metabolomics in Central Sensitivity Syndromes. Metabolites 2020, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.S.; Harris, R.; Clauw, D. Focused Review Fibromyalgia: An Afferent Processing Disorder Leading to a Complex Pain Generalized Syndrome. Available online: www.painphysicianjournal.com (accessed on 5 September 2023).
- Wolfe, F.; Ross, K.; Anderson, J.; Russell, I.J.; Hebert, L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995, 38, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Walitt, B.; Katz, R.S.; Bergman, M.J.; Wolfe, F. Three-Quarters of Persons in the US Population Reporting a Clinical Diagnosis of Fibromyalgia Do Not Satisfy Fibromyalgia Criteria: The 2012 National Health Interview Survey. PLoS ONE 2016, 11, e0157235. [Google Scholar] [CrossRef] [PubMed]
- Lavudi, K.; Harika, V.S.; Kokkanti, R.R.; Patchigolla, S.; Sinha, A.; Patnaik, S.; Penchalaneni, J. 2-Dimensional in vitro culture assessment of ovarian cancer cell line using cost effective silver nanoparticles from Macrotyloma uniflorum seed extracts. Front. Bioeng. Biotechnol. 2022, 10, 978846. [Google Scholar] [CrossRef]
- Lavudi, K.; Nuguri, S.M.; Olverson, Z.; Dhanabalan, A.K.; Patnaik, S.; Kokkanti, R.R. Targeting the retinoic acid signaling pathway as a modern precision therapy against cancers. Front. Cell Dev. Biol. 2023, 11, 1254612. [Google Scholar] [CrossRef]
- Osterberg, E.C.; Laudano, M.A.; Li, P.S. Clinical and investigative applications of Raman spectroscopy in Urology and Andrology. Transl. Androl. Urol. 2014, 3, 84–88. [Google Scholar] [CrossRef]
- Hackshaw, K.V.; Rodriguez-Saona, L.; Plans, M.; Bell, L.N.; Buffington, C.A.T. A bloodspot-based diagnostic test for fibromyalgia syndrome and related disorders. Analyst 2013, 138, 4453–4462. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Bao, H.; Nuguri, S.M.; Yu, L.; Mikulik, Z.; Osuna-Diaz, M.M.; Sebastian, K.R.; Hackshaw, K.V.; Rodriguez-Saona, L. Rapid Biomarker-Based Diagnosis of Fibromyalgia Syndrome and Related Rheumatologic Disorders by Portable FT-IR Spectroscopic Techniques. Biomedicines 2023, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, K.V.; Yao, S.; Bao, H.; Castellvi, S.d.L.; Aziz, R.; Nuguri, S.M.; Yu, L.; Osuna-Diaz, M.M.; Brode, W.M.; Sebastian, K.R.; et al. Metabolic Fingerprinting for the Diagnosis of Clinically Similar Long COVID and Fibromyalgia Using a Portable FT-MIR Spectroscopic Combined with Chemometrics. Biomedicines 2023, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.D. Dried Blood Spots for Global Health Diagnostics and Surveillance: Opportunities and Challenges. Am. J. Trop. Med. Hyg. 2018, 99, 256–265. [Google Scholar] [CrossRef] [PubMed]
- McMahon, R.; Hill, C.; Rudge, J.; Herbert, B.; Karsten, E. Stability of inflammation markers in human blood collected using volumetric absorptive microsampling (VAMS) under typical laboratory storage temperatures. Cytokine 2023, 171, 156355. [Google Scholar] [CrossRef] [PubMed]
- Denniff, P.; Spooner, N. Volumetric Absorptive Microsampling: A Dried Sample Collection Technique for Quantitative Bioanalysis. Anal. Chem. 2014, 86, 8489–8495. [Google Scholar] [CrossRef] [PubMed]
- The Mitra 96-Auto Rack|Laboratory Microsampling Automation Tool. Available online: https://www.neoteryx.com/mitra-laboratory-tools (accessed on 3 December 2023).
- Miller, J.H.; Poston, P.A.; Rutan, S.C.; Karnes, T. An On-card Approach for Assessment of Hematocrit on Dried Blood Spots which Allows for Correction of Sample Volume. J. Anal. Bioanal. Tech. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Kip, A.; Kiers, K.; Rosing, H.; Schellens, J.; Beijnen, J.; Dorlo, T. Volumetric absorptive microsampling (VAMS) as an alternative to conventional dried blood spots in the quantification of miltefosine in dried blood samples. J. Pharm. Biomed. Anal. 2017, 135, 160–166. [Google Scholar] [CrossRef]
- A. Background, 10. Infrared Spectroscopy, (n.d.) 1–7. Available online: https://community.wvu.edu/~josbour1/Labs/F2016/Exp%208%20-%20Infrared%20Spectroscopy.pdf (accessed on 28 September 2023).
- Ando, T.; Ishii, M.; Kamo, M.; Sato, Y. H-D exchange reaction on diamond surfaces studied by diffuse reflectance Fourier transform IR spectroscopy. Diam. Relat. Mater. 1995, 4, 607–611. [Google Scholar] [CrossRef]
- Mayo, D.W. Characteristic Frequencies of Alkanes, Course Notes on the Interpretation of Infrared and Raman Spectra; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 33–72. [Google Scholar] [CrossRef]
- Roy, S.; Perez-Guaita, D.; Bowden, S.; Heraud, P.; Wood, B.R. Spectroscopy goes viral: Diagnosis of hepatitis B and C virus infection from human sera using ATR-FTIR spectroscopy. Clin. Spectrosc. 2019, 1, 100001. [Google Scholar] [CrossRef]
- Liu, K.-Z.; Shaw, R.A.; Man, A.; Dembinski, T.C.; Mantsch, H.H. Reagent-free, Simultaneous Determination of Serum Cholesterol in HDL and LDL by Infrared Spectroscopy. Clin. Chem. 2002, 48, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Interpretation of Infrared Spectra|California State University Stanislaus. Available online: https://www.csustan.edu/chemistry/interpretation-infrared-spectra (accessed on 24 June 2023).
- Petibois, C.; Cazorla, G.; Cassaigne, A.; Déléris, G. Plasma Protein Contents Determined by Fourier-Transform Infrared Spectrometry. Clin. Chem. 2001, 47, 730–738. [Google Scholar] [CrossRef]
- Mann, S.; Webb, J.; Williams, R.J.P.; Falini, G.; Albeck, S.; Weiner, S.; Addadi, L.; Aizenberg, J.; Hanson, J.; Koetzle, T.F.; et al. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Z. Physiol. Chem. 1951, 40, 1832. Available online: https://pubs.acs.org/sharingguidelines (accessed on 29 December 2023).
- Luo, J.; Ying, K.; Bai, J. Savitzky–Golay smoothing and differentiation filter for even number data. Signal Process. 2005, 85, 1429–1434. [Google Scholar] [CrossRef]
- Infometrix Inc. Pirouette Multivariate Data Analysis Software; Infometrix Inc.: Bothell, WA, USA, 2011; p. 506. Available online: http://www.infometrix.com/ (accessed on 7 July 2023).
- Aptula, A.O.; Jeliazkova, N.G.; Schultz, T.W.; Cronin, M.T.D. The Better Predictive Model: High q2 for the Training Set or Low Root Mean Square Error of Prediction for the Test Set? QSAR Comb. Sci. 2005, 24, 385–396. [Google Scholar] [CrossRef]
- Ayvaz, H.; Sierra-Cadavid, A.; Aykas, D.P.; Mulqueeney, B.; Sullivan, S.; Rodriguez-Saona, L.E. Monitoring multicomponent quality traits in tomato juice using portable mid-infrared (MIR) spectroscopy and multivariate analysis. Food Control 2016, 66, 79–86. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. PCA as a Practical Indicator of OPLS-DA Model Reliability. Curr. Metabolomics 2016, 4, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.; Yan, J. Principle Component Analysis and Partial Least Squares: Two Dimension Reduction Techniques for Regression. Appl. Multivar. Stat. Models 2008, 79, 79–90. [Google Scholar]
- Ballabio, D. A MATLAB toolbox for Principal Component Analysis and unsupervised exploration of data structure. Chemom. Intell. Lab. Syst. 2015, 149, 1–9. [Google Scholar] [CrossRef]
- Eusebi, P. Diagnostic Accuracy Measures. Cerebrovasc. Dis. 2013, 36, 267–272. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zeng, N.; Wang, N. Sensitivity, Specificity, Accuracy, Associated Confidence Interval and ROC Analysis with Practical SAS® Implementations. 2010. Available online: https://lexjansen.com/nesug/nesug10/hl/hl07.pdf (accessed on 3 December 2023).
- Hackshaw, K.V. The Search for Biomarkers in Fibromyalgia. Diagnostics 2021, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Favretti, M.; Iannuccelli, C.; Di Franco, M. Pain Biomarkers in Fibromyalgia Syndrome: Current Understanding and Future Directions. Int. J. Mol. Sci. 2023, 24, 10443. [Google Scholar] [CrossRef] [PubMed]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Judge, K.; Brown, C.W.; Hamel, L. Sensitivity of Raman Spectra to Chemical Functional Groups. Appl. Spectrosc. 2008, 62, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Ablin, J.N.; Wolfe, F. A Comparative Evaluation of the 2011 and 2016 Criteria for Fibromyalgia. J. Rheumatol. 2017, 44, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.A. Multivariate Analysis and Artificial Neural Network Approaches of near Infrared Spectroscopic Data for Non-destructive Quality Attributes Prediction of Mango (Mangifera indica L.). Ph.D. Dissertation, Zentralbibliothek, Göttingen, Germany, 2014. Available online: https://ediss.uni-goettingen.de/handle/11858/00-1735-0000-0022-5E52-8 (accessed on 12 August 2022).
- Kokalj, M.; Rihtarič, M.; Kreft, S. Commonly applied smoothing of IR spectra showed unappropriate for the identification of plant leaf samples. Chemom. Intell. Lab. Syst. 2011, 108, 154–161. [Google Scholar] [CrossRef]
- Allegrini, F.; Olivieri, A.C. A new and efficient variable selection algorithm based on ant colony optimization. Applications to near infrared spectroscopy/partial least-squares analysis. Anal. Chim. Acta 2011, 699, 18–25. [Google Scholar] [CrossRef]
- Mayer, T.G.; Neblett, R.; Cohen, H.; Howard, K.J.; Choi, Y.H.; Williams, M.J.; Perez, Y.; Gatchel, R.J. The Development and Psychometric Validation of the Central Sensitization Inventory. Pain Pract. 2011, 12, 276–285. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The american college of rheumatology 1990 Criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheumatol. 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Qaseem, A.; Snow, V.; Casey, D.; Cross, J.T.; Shekelle, P.; Owens, D.K. Diagnosis and Treatment of Low Back Pain: A Joint Clinical Practice Guideline from the American College of Physicians and the American Pain Society. Ann. Intern. Med. 2007, 147, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; Mcshane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; Mcshane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Greening, D.W.; Simpson, R.J. A centrifugal ultrafiltration strategy for isolating the low-molecular weight (≤25 K) component of human plasma proteome. J. Proteom. 2010, 73, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Alexandris, N.; Gupta, S.; Koutsias, N. Remote sensing of burned areas via PCA, Part 1; centering, scaling and EVD vs SVD. Open Geospat. Data Softw. Stand. 2017, 2, 17. [Google Scholar] [CrossRef]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Shaw, A.D.; Winson, M.K.; Woodward, A.M.; McGovern, A.C.; Davey, H.M.; Kaderbhai, N.; Broadhurst, D.; Gilbert, R.J.; Taylor, J.; Timmins, E.M.; et al. Rapid analysis of high-dimensional bioprocesses using multivariate spectroscopies and advanced chemometrics. Adv. Biochem. Eng. Biotechnol. 2000, 66, 83–113. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Sun, X.; Xu, W. Fast Implementation of DeLong’s Algorithm for Comparing the Areas under Correlated Receiver Operating Characteristic Curves. IEEE Signal Process. Lett. 2014, 21, 1389–1393. [Google Scholar] [CrossRef]


| n | Age | (M/F) (%M/%F) | BMI | CSI | SIQR | FIQR | MPQ | BDI | |
|---|---|---|---|---|---|---|---|---|---|
| FM | 179 | 43.1 ± 13.4 | (15/164) (8.3/91.7) | 32.0 ± 9.2 | 58.1 ± 22.9 | 48.2 ± 26.0 | 90.5 ± 51.7 | 19.3 ± 11.8 | |
| Non-FM | 158 | 50.5 ± 15.98 | (36/122) (23/77) | 28.2 ± 11.3 | 27.8 ± 21.9 | 33.5 ± 23.0 | 41.7 ± 49.6 | 9.5 ± 8.6 | |
| NC | 13 | 42.3 ± 15.0 | (5/8) (39/61) | 25.5 ± 3.1 | 7.0 ± 10.5 | 1.7 ± 3.7 | 1.9 ± 3.8 | 1.1 ± 2.8 |
| Age/Range | FIQR/SIQR | BDI | MPQ | CSI | |
|---|---|---|---|---|---|
| FM | 18–73 | * | * | * | * |
| Non-FM | 18–81 | p < 0.05 | p < 0.001 | p < 0.001 | p < 0.001 |
| Figures of Merit | Calibration Set (n = 275) | Validation Set (n = 62) |
|---|---|---|
| SECV/SEP | 0.02 | 0.02 |
| R2 | 0.99 | - |
| Sensitivity (%) | 96 | 83 |
| Specificity (%) | 100 | 85 |
| Accuracy (%) | 98 | 84 |
| Dataset | FM | SLE | OA | RA | CLBP |
|---|---|---|---|---|---|
| Calibration | 144 | 39 | 28 | 48 | 16 |
| Validation | 35 | 7 | 8 | 10 | 2 |
| Total | 179 | 158 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuguri, S.M.; Hackshaw, K.V.; de Lamo Castellvi, S.; Bao, H.; Yao, S.; Aziz, R.; Selinger, S.; Mikulik, Z.; Yu, L.; Osuna-Diaz, M.M.; et al. Portable Mid-Infrared Spectroscopy Combined with Chemometrics to Diagnose Fibromyalgia and Other Rheumatologic Syndromes Using Rapid Volumetric Absorptive Microsampling. Molecules 2024, 29, 413. https://doi.org/10.3390/molecules29020413
Nuguri SM, Hackshaw KV, de Lamo Castellvi S, Bao H, Yao S, Aziz R, Selinger S, Mikulik Z, Yu L, Osuna-Diaz MM, et al. Portable Mid-Infrared Spectroscopy Combined with Chemometrics to Diagnose Fibromyalgia and Other Rheumatologic Syndromes Using Rapid Volumetric Absorptive Microsampling. Molecules. 2024; 29(2):413. https://doi.org/10.3390/molecules29020413
Chicago/Turabian StyleNuguri, Shreya Madhav, Kevin V. Hackshaw, Silvia de Lamo Castellvi, Haona Bao, Siyu Yao, Rija Aziz, Scott Selinger, Zhanna Mikulik, Lianbo Yu, Michelle M. Osuna-Diaz, and et al. 2024. "Portable Mid-Infrared Spectroscopy Combined with Chemometrics to Diagnose Fibromyalgia and Other Rheumatologic Syndromes Using Rapid Volumetric Absorptive Microsampling" Molecules 29, no. 2: 413. https://doi.org/10.3390/molecules29020413
APA StyleNuguri, S. M., Hackshaw, K. V., de Lamo Castellvi, S., Bao, H., Yao, S., Aziz, R., Selinger, S., Mikulik, Z., Yu, L., Osuna-Diaz, M. M., Sebastian, K. R., Giusti, M. M., & Rodriguez-Saona, L. (2024). Portable Mid-Infrared Spectroscopy Combined with Chemometrics to Diagnose Fibromyalgia and Other Rheumatologic Syndromes Using Rapid Volumetric Absorptive Microsampling. Molecules, 29(2), 413. https://doi.org/10.3390/molecules29020413









