Wastewater Characterization: Chemical Oxygen Demand or Total Organic Carbon Content Measurement?
Abstract
1. Introduction
2. Results
2.1. Data Collections, Reliability, and General Observations
2.2. Groups of Organic Molecules
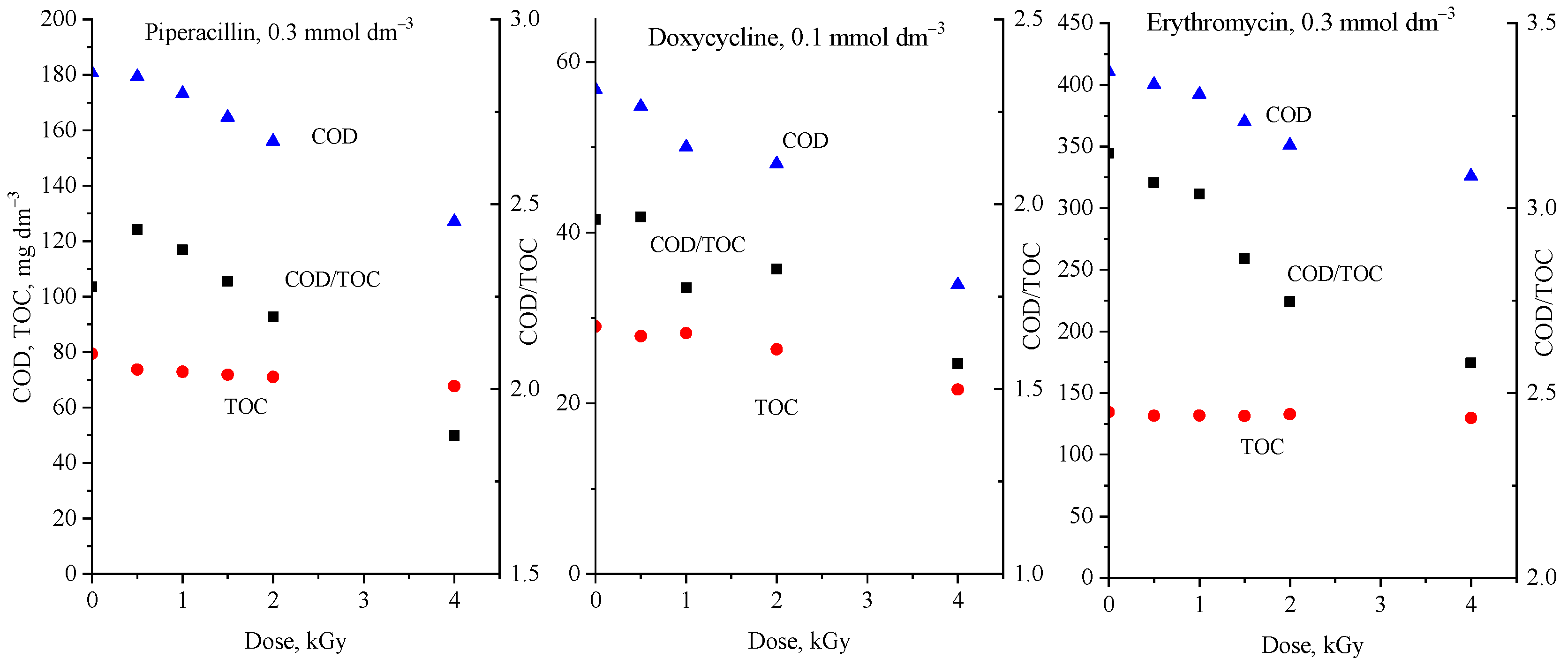
2.3. Application of COD and TOC Values in an AOP
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. CODmeas Determination
4.3. TOCmeas Determination
4.4. γ-Radiolytic Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anagnostopoulos, A.; Hjort, M.; Vaiopoulou, E. Assessment of Chemical Oxygen Demand/Total Organic Carbon (COD/TOC) ratios in refinery effluents. Report: Environmental Science for European Refining, no. 16/22. Brussels, 2022. Available online: https://www.concawe.eu/publications/?_sft_topic=manufacturing-water-wastewater-emissions&_sft_publicationscategory=report (accessed on 16 December 2023).
- Aguilar-Torrejón, J.A.; Balderas-Hernández, P.; Roa-Morales, G.; Barrera-Díaz, C.E.; Rodríguez-Torres, I.; Torres-Blancas, T. Relationship, importance, and development of analytical techniques: COD, BOD, and, TOC in water—An overview through time. SN Appl. Sci. 2023, 5, 118. [Google Scholar] [CrossRef]
- OECD. Guidelines for the Testing of Chemicals Ready Biodegradability Section 3; Test No. 301; OECD: Paris, France, 1992. [Google Scholar]
- DIN EN 1899-1:1998; Water Quality. Determination of the Biochemical Oxygen Demand after N Days (BOD[n]) of Water-Part 1: Dilution and Seeding Method with Allylthiourea Addition. European Committee for Standardization: Brussels, Belgium, 1998.
- Li, J.; Luo, G.; He, L.J.; Xu, J.; Lyu, J. Analytical approaches for determining chemical oxygen demand in water bodies: A review. Crit. Rev. Anal. Chem. 2018, 48, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, Y.; Liu, Y. A review of detection techniques for chemical oxygen demand in wastewater. Am. J. Biochem. Biotechnol. 2022, 18, 23–32. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Sasaki, S.; Yano, K.; Ikebukuro, K.; Hashimoto, K.; Karube, I. Relationship between theoretical oxygen demand and photocatalytic chemical oxygen demand for specific classes of organic chemicals. Analyst 2000, 125, 1915–1918. [Google Scholar] [CrossRef]
- Baker, J.R.; Milke, M.W.; Michelcic, J.R. Relationship between chemical and theoretical oxygen demand for specific classes of organic chemicals. Water Res. 1999, 33, 327–334. [Google Scholar] [CrossRef]
- Janicke, W. Chemische Oxidierbarkeit Organischer Wasserinhaltstoffe; WaBoLu Berichte, Dietrich Reimer Verlag: Berlin, Germany, 1983. [Google Scholar]
- Annex XIV of the REACH Regulation (Regulation on the Registration, Evaluation, Authorisation and Restriction of Chemicals). Available online: https://echa.europa.eu/hu/authorisation-list (accessed on 16 December 2023).
- Choi, I.-W.; Kim, J.-H.; Im, J.-K.; Park, T.-J.; Kim, S.-Y.; Son, D.-H.; Huh, I.-A.; Rhew, D.-H.; Yu, S.-J. Application of TOC standards for managing refractory organic compounds in industrial wastewater. J. Korean Soc. Water Environ. 2015, 31, 29–34. [Google Scholar] [CrossRef][Green Version]
- Tian, X.; Zhao, C.; Ji, X.; Feng, T.; Liu, Y.; Bian, D. The correlation analysis of TOC and CODCr in urban Sewage treatment. E3S Web Conf. 2019, 136, 6010. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, S.Y.; Noh, J.H.; Bae, Y.H.; Lee, J.W.; Maeng, S.K. A shift from chemical oxygen demand to total organic carbon for stringent industrial wastewater regulations: Utilization of organic matter characteristics. J. Environ. Manag. 2022, 305, 114412. [Google Scholar] [CrossRef]
- Homlok, R.; Takács, E.; Wojnárovits, L. Degradation of organic molecules in advanced oxidation processes: Relation between chemical structure and degradability. Chemosphere 2013, 91, 383–389. [Google Scholar] [CrossRef]
- Rácz, G.; Csay, T.; Takács, E.; Wojnárovits, L. Degradation of Triton X-100 surfactant/lipid regulator systems by ionizing radiation in water. J. Radioanal. Nucl. Chem. 2017, 314, 1189–1196. [Google Scholar] [CrossRef]
- Albarrán, G.; Mendoza, E. Radiolysis induced degradation of 1,3-dichlorobenzene and 4-chlorophenol in aqueous solution. Radiat. Phys. Chem. 2020, 182, 109318. [Google Scholar] [CrossRef]
- Albarrán, G.; Mendoza, E. Radiolytic oxidation and degradation of 2,4-dichlorophenol in aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 17055–17065. [Google Scholar] [CrossRef] [PubMed]
- Albarrán, G.; Mendoza, E. Radiolytic degradation of chlorobenzene in aerated and deoxygenated aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 22855–22864. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Okumura, M. Feasibility of mercury-free chemical oxygen demand (COD) test with excessive addition of silver sulfate. J. Water Environ. Technol. 2018, 16, 221–232. [Google Scholar] [CrossRef]
- Sági, G.; Csay, T.; Szabó, L.; Pátzay, G.; Csonka, E.; Takács, E.; Wojnárovits, L. Analytical approaches to the OH radical induced degradation of sulfonamide antibiotics in dilute aqueous solutions. J. Pharm. Biomed. Anal. 2015, 106, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tegze, A.; Sági, G.; Kovács, K.; Homlok, R.; Tóth, T.; Mohácsi-Farkas, C.; Wojnárovits, L.; Takács, E. Degradation of fluoroquinolone antibiotics during ionizing radiation treatment and assessment of antibacterial activity, toxicity and biodegradability of the products. Radiat. Phys. Chem. 2018, 147, 101–105. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Homlok, R.; Kovács, K.; Bezsenyi, A.; Takács, E. Ionizing radiation induced removal of ofloxacin, abatement its toxicity and antibacterial activity in various water matrices. Appl. Sci. 2023, 13, 7211. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Wang, J.; Chu, L.; Tóth, T.; Kovács, K.; Bezsenyi, A.; Szabó, L.; Homlok, R.; Takács, E. Matrix effect in the hydroxyl radical induced degradation of β-lactam and tetracycline type antibiotics. Radiat. Phys. Chem. 2022, 193, 109980. [Google Scholar] [CrossRef]
- Belkheiri, D.; Fourcade, F.; Geneste, F.; Floner, D.; Aït-Amar, H.; Amrane, A. Combined process for removal of tetracycline antibiotic–Coupling pretreatment with a nickel-modified graphite felt electrode and a biological treatment. Int. Biodeter. Biodegr. 2015, 103, 147–153. [Google Scholar] [CrossRef]
- Takács, E.; Wang, J.; Chu, L.; Tóth, T.; Kovács, K.; Bezsenyi, A.; Szabó, L.; Homlok, R.; Wojnárovits, L. Elimination of oxacillin, its toxicity and antibacterial activity by using ionizing radiation. Chemosphere 2022, 286, 131467. [Google Scholar] [CrossRef]
- Kovács, K.; Simon, Á.; Tóth, T.; Wojnárovits, L. Free radical chemistry of atenolol and propranolol investigated by pulse and gamma radiolysis. Radiat. Phys. Chem. 2022, 196, 110141. [Google Scholar] [CrossRef]
- Kovács, K.; Tegze, A.; Bezsenyi, A.; Wojnárovits, L. Hydroxyl radical induced degradation of the ß-blocker Nadolol and comparison with Propranolol. J. Environ. Chem. Eng. 2023, 11, 110330. [Google Scholar] [CrossRef]
- Szabó, L.; Tóth, T.; Homlok, R.; Rácz, G.; Takács, E.; Wojnárovits, L. Hydroxyl radical induced degradation of salicylates in aerated aqueous solution. Radiat. Phys. Chem. 2014, 97, 239–245. [Google Scholar] [CrossRef]
- Albarrán, G.; Mendoza, E. Ionizing radiation induced degradation of salicylic acid. Radiat. Phys. Chem. 2018, 147, 27–34. [Google Scholar] [CrossRef]
- Csay, T.; Rácz, G.; Takács, E.; Wojnárovits, L. Radiation induced degradation of pharmaceutical residues in water: Chloramphenicol. Radiat. Phys. Chem. 2012, 81, 1489–1494. [Google Scholar] [CrossRef]
- Kovács, K.; Simon, Á.; Balogh, G.T.; Tóth, T.; Wojnárovits, L. High energy ionizing radiation induced degradation of amodiaquine in dilute aqueous solution: Radical reactions and kinetics. Free Radic. Res. 2020, 54, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Csay, T.; Rácz, G.; Salik, Á.; Takács, E.; Wojnárovits, L. Reactions of clofibric acid with oxidative and reductive radicals–products, mechanisms, efficiency and toxic effects. Radiat. Phys. Chem. 2014, 102, 72–78. [Google Scholar] [CrossRef]
- Kovács, K.; Mile, V.; Csay, T.; Takács, E.; Wojnárovits, L. Hydroxyl radical-induced degradation of fenuron in pulse and gamma radiolysis: Kinetics and product analysis. Environ. Sci. Pollut. Res. 2014, 21, 12693–12700. [Google Scholar] [CrossRef]
- Kovács, K.; He, S.; Mile, V.; Földes, T.; Pápai, I.; Takács, E.; Wojnárovits, L. Ionizing radiation induced degradation of monuron in dilute aqueous solution. Radiat. Phys. Chem. 2016, 124, 191–197. [Google Scholar] [CrossRef]
- Kovács, K.; He, S.; Mile, V.; Csay, T.; Takács, E.; Wojnárovits, L. Ionizing radiation induced degradation of diuron in dilute aqueous solution. Chem. Cent. J. 2015, 9, 21. [Google Scholar] [CrossRef]
- Hassani, M.; Lázaro, R.; Pérez, C.; Condón, S.; Pagán, R. Thermostability of oxytetracycline, tetracycline, and doxycycline at ultrahigh temperatures. J. Agric. Food Chem. 2008, 56, 2676–2680. [Google Scholar] [CrossRef] [PubMed]
- Hayati, F.; Khodabakhshi, R.K.; Isari, A.A.; Sina Moradi, S.; Kakavandi, B. LED-assisted sonocatalysis of sulfathiazole and pharmaceutical wastewater using N,Fe co-doped TiO2@SWCNT: Optimization, performance and reaction mechanism studies. J. Water Proc. Eng. 2020, 38, 101693. [Google Scholar] [CrossRef]
- Kakavandi, B.; Ahmadi, M. Efficient treatment of saline recalcitrant petrochemical wastewater using heterogeneous UV-assisted sono-Fenton process. Ultrason. Sonochem. 2019, 56, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V. An overview of the radiation chemistry of liquids. In Radiation Chemistry: From Basics to Applications in Material and Life Sciences; Spotheim-Maurizot, M., Mostafavi, M., Douki, T., Belloni, J., Eds.; EDP Sciences: Les Ulis, France, 2008; pp. 3–16. [Google Scholar]
- IAEA. Radiation Processing, Environmental Applications; International Atomic Energy Agency: Vienna, Austria, 2007. [Google Scholar]
- CGN. 20IAEA. 2020. Available online: https://www.iaea.org/newscenter/news/started-with-iaea-support-chinas-electron-beamindustry-opens-worlds-largest-wastewater-treatment-facility (accessed on 6 June 2023).
- Moreira, F.C.; Garcia-Segura, S.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Degradation of the antibiotic trimethoprim by electrochemical advanced oxidation processes using a carbon-PTFE air-diffusion cathode and a boron-doped diamond or platinum anode. Appl. Catal. B 2014, 160–161, 492–505. [Google Scholar] [CrossRef]
- Giannakis, S.; Gamarra Vives, F.A.; Grandjean, D.; Magnet, A.; De Alencastro, L.F.; Pulgarin, C. Effect of advanced oxidation processes on the micropollutants and the effluent organic matter contained in municipal wastewater previously treated by three different secondary methods. Water Res. 2015, 84, 295–306. [Google Scholar] [CrossRef]
- ISO/ASTM 51538:2017; Practice for Use of the Ethanol-Chlorobenzene Dosimetry System. ASTM: West Conshohocken, PA, USA, 2017.

| Compound, Formula | ThODmg/mg | TOCmg/mg | Conc. mmol dm−3 | CODmeas/ ThOD | CODmeas/ TOCmeas | Reference |
|---|---|---|---|---|---|---|
| Laboratory chemicals | ||||||
| Isopropanol, C3H8O | 2.39 | 0.60 | 0.1 | 0.76 | 3.03 | This work |
| Ethylene glycol, C2H6O2 | 1.29 | 0.39 | 0.1 | 1.15 | 3.17 | This work |
| Vinyl acetate, C4H6O2 | 1.67 | 0.56 | 0.1 | 0.76 | 2.73 | This work |
| Maleic acid, C4H4O4 | 0.83 | 0.411 | 4.0 | 0.97 | [14] | |
| 0.99 | [8] | |||||
| Fumaric acid, | 0.83 | 0.411 | 4.0 | 1.07 | [14] | |
| C4H4O4 | 0.1 | 1.10 | 2.25 | This work | ||
| Cyclohexanone, C6H10O | 2.61 | 0.734 | 1.0 | 0.63 | 2.15 | This work |
| Triton X-100 C14H22O(C2O4)n, n = 9–10 | 0.1 | 3.25 | [15] | |||
| Benzoic acid, C7H6O2 | 1.97 | 0.690 | 0.1 | 0.89 | 2.17 | This work |
| Phenol, C6H5OH | 2.38 | 0.765 | 2.0 | 1.01 | 3.15 | [14] |
| 1.0 | 0.99 | 3.08 | This work | |||
| Phenolate, C6H5O− | 2.32 | 0.770 | 5.0 | 1.04 | 2.75 | [14] |
| 1.0 | 1.02 | 2.25 | ||||
| 4-Hydroxyanisol, C7H8O2 | 2.06 | 0.677 | 0.1 | 1.05 | 2.80 | This work |
| o-Cresol, CH3C6H4OH | 2.52 | 0.776 | 1.5 | 0.97 | [14] | |
| m-Cresol, CH3C6H4OH | 2.52 | 0.776 | 1.5 | 1.07 | [14] | |
| p-Cresol, CH3C6H4OH | 2.52 | 0.776 | 1.5 | 0.92 | [14] | |
| o-Chlorophenol, ClC6H4OH | 1.62 | 0.560 | 2.0 | 1.00 | [14] | |
| m-Chlorophenol, ClC6H4OH | 1.62 | 0.560 | 2.0 | 1.06 | [14] | |
| p-Chlorophenol, ClC6H4OH | 1.62 | 0.560 | 2.0 | 0.99 | [14] | |
| 0.3 | 0.67 | [16] | ||||
| 0.1 | 0.95 | 2.28 | This work | |||
| 2,4-dichlorophenol, C6H4Cl2O | 1.18 | 0.442 | 0.1 | 0.72 | [17] | |
| Chlorobenzene, C6H5Cl | 1.99 | 0.640 | 0.58 | [8] | ||
| 0.09 | 0.25 | [18] | ||||
| o-Aminophenol, C6H7NO | 1.91 | 0.660 | 1.0 | 1.04 | [14] | |
| m-Aminophenol, C6H7NO | 1.91 | 0.660 | 1.0 | 1.02 | [14] | |
| p-Aminophenol, C6H7NO, | 1.91 | 0.660 | 1.0 | 1.04 | [14] | |
| 1.5 | 0.92 | |||||
| 1.0 | 1.00 | 2.88 | This work | |||
| 2,6-Dichloraniline, C6H5Cl2N | 1.19 | 0.444 | 0.5 | 0.93 | 2.34 | [14] |
| 1.0 | 0.89 | 2.50 | ||||
| 0.1 | 1.00 | 2.59 | This work | |||
| Benzamine, C7H9N | 2.54 | 0.784 | 0.1 | 0.86 | 2.08 | This work |
| Sulfonamides | ||||||
| Sulfanilic acid, C6H7NO3S | 1.29 | 0.416 | 1.02 | [19] | ||
| Sulfamethoxazole, C10H11N3O3S | 1.33 | 0.474 | 0.1 | 1.00 | 2.92 | [20] |
| Sulfacetamide C8H10N2O3S | 1.34 | 0.448 | 0.1 | 0.99 | 2.97 | [20] |
| Sulfanilamide, C6H8N2O2S | 1.30 | 0.418 | 0.1 | 0.82 | 2.52 | [20] |
| Sulfadiazine, C10H10N4O2S | 1.28 | 0.480 | 0.1 | 1.05 | 2.83 | [20] |
| Sulfaguanidine C7H10N4O2S | 1.05 | 0.392 | 0.1 | 1.00 | 3.14 | [20] |
| Sulfathiazole, C9H9N3O2S2 | 1.38 | 0.423 | 0.1 | 0.98 | 3.28 | [20] |
| Sulfamethazine, C12H14N4O2S | 1.50 | 0.517 | 0.1 | 0.96 | 2.86 | [20] |
| Sulfisoxazole, C11H13N3O3S | 1.44 | 0.494 | 0.1 | 0.90 | 2.58 | [20] |
| Trimethoprim, C14H18N4O3 | 1.54 | 0.579 | 0.1 | 0.86 | 2.24 | This work |
| 0.3 | 1.02 | 2.83 | ||||
| Fluoroquinolones | ||||||
| Ciprofloxacin, C17H18FN3O3 | 1.61 | 0.616 | 0.1 | 1.03 | 1.96 | [21] |
| Norfloxacin, C16H18FN3O3 | 1.57 | 0.601 | 0.1 | 0.89 | 2.32 | [21] |
| Ofloxacin, C18H20FN3O4 | 1.55 | 0.598 | 0.1 | 0.77 | 1.94 | [22] |
| Tetracyclines | ||||||
| Tetracycline, C22H24N2O8 | 1.62 | 0.594 | 0.1 | 0.96 | 2.61 | [23] |
| 0.22 | 0.81 | 2.22 | [24] | |||
| Chlortetracycline, C22H23ClN2O8 | 1.47 | 0.554 | 0.1 | 0.87 | 2.18 | [23] |
| Doxycycline, C22H24N2O8 | 1.62 | 0.594 | 0.1 | 0.79 | 1.96 | This work |
| Oxytetracycline, C22H24N2O9 | 1.53 | 0.573 | 0.1 | 0.77 | 2.04 | This work |
| β-Lactam antibiotics | ||||||
| Amoxicillin, C16H19N3O5S | 1.53 | 0.525 | 0.1 | 0.83 | 2.74 | This work |
| Oxacillin, C19H19N3O5S | 1.63 | 0.568 | 0.1 | 0.96 | 2.31 | [25] |
| Cloxacillin, C19H18ClN3O5S | 1.47 | 0.523 | 0.1 | 0.81 | 2.28 | [23] |
| Piperacillin, C23H27N5O7S | 1.48 | 0.533 | 0.3 | 0.79 | 2.28 | This work |
| Macrolides | ||||||
| Erythromycin, C37H67NO13 | 2.03 | 0.605 | 0.3 | 0.92 | 3.04 | This work |
| Azithromycin, C38H72N2O12 | 2.07 | 0.609 | 0.1 | 0.98 | 3.35 | This work |
| Clarithromycin, C38H69NO13 | 2.05 | 0.610 | 0.1 | 1.06 | 3.35 | This work |
| Blood pressure regulators | ||||||
| Atenolol, C14H22N2O3 | 1.98 | 0.542 | 0.1 | 0.85 | 2.64 | [26] |
| Nadolol, C17H27NO4 | 2.17 | 0.659 | 0.1 | 0.92 | 2.96 | [27] |
| Propranolol, C16H21NO | 2.41 | 0.740 | 0.1 | 0.72 | 2.22 | [26] |
| Metoprolol tartrate, C34H56N2O10 | 2.03 | 0.596 | 0.1 | 0.85 | 2.91 | This work |
| Labetalol, C19H24N2O3 | 2.14 | 0.694 | 0.1 | 0.96 | 3.04 | This work |
| Acebutolol, C18H28N2O4 | 2.04 | 0.642 | 0.3 | 0.95 | 2.99 | This work |
| 0.1 | 0.88 | 2.64 | ||||
| Non-steroidal anti-inflammatory drugs | ||||||
| Salicylic acid, C7H6O3 | 1.62 | 0.608 | 1.0 | 0.97 | 2.48 | [28] |
| 0.1 | 1.02 | [29] | ||||
| Acetylsalicylic acid, C9H8O4 | 1.60 | 0.600 | 1.0 | 0.97 | 2.55 | [28] |
| 1.5 | 0.97 | 2.54 | ||||
| 5-Sulfo-salicylic acid, C7H6O6S | 1.03 | 0.385 | 1.0 | 0.89 | 2.86 | [28] |
| Ketoprofen, C16H14O3 | 2.26 | 0.755 | 0.4 | 0.97 | 2.88 | [14] |
| Diclofenac, C14H11Cl2NO2 | 1.57 | 0.567 | 0.5 | 0.99 | [14] | |
| Paracetamol, C8H9NO2 | 1.80 | 0.635 | 1 | 0.93 | [14] | |
| 0.95 | 2.69 | This work | ||||
| Miscellaneous drugs | ||||||
| Chloramphenicol, C11H12Cl2N2O5 | 0.94 | 0.409 | 1.0 | 0.59 | 1.41 | [30] |
| Amodiaquine, C20H22ClN3O | 2.03 | 0.674 | 0.1 | 1.10 | 2.55 | [31] |
| Clofibric acid, C10H11ClO3 | 1.64 | 0.559 | 1.0 | 0.91 | 2.67 | [32] |
| Pesticides | ||||||
| Fenuron, C9H12N2O | 1.95 | 0.658 | 0.1 | 1.00 | 2.96 | [33] |
| Monuron, C9H11ClN2O | 1.53 | 0.544 | 0.1 | 1.02 | 2.79 | [34] |
| Diuron, C9H10Cl2N2O | 1.24 | 0.463 | 0.1 | 1.05 | 2.84 | [35] |
| Monolinuron, C9H11ClN2O2 | 1.34 | 0.503 | 1.11 | [8] | ||
| Linuron, C9H10Cl2N2O2 | 1.40 | 0.433 | 1.06 | [8] | ||
| Buturon, C12H13ClN2O | 1.76 | 0.608 | 0.90 | [8] | ||
| 2,4-Dichlorophenoxy-acetic acid, C8H6Cl2O3 | 1.09 | 0.434 | 2.0 | 0.92 | 2.45 | [14] |
| Dyes | ||||||
| Acid Red 1, C18H13N3Na2O8S2 | 1.23 | 0.424 | 1.5 | 0.96 | [14] | |
| Chlorophenol red, C19H12Cl2O5S | 1.55 | 0.539 | 0.1 | 1.06 | 3.05 | This work |
| Methylene blue, C16H18N3S+ | 2.22 | 0.675 | 0.3 | 0.70 | 2.93 | This work |
| Tetrazolium violet, C23H17N4+ | 2.22 | 0.790 | 0.3 | 0.98 | 3.17 | This work |
| Ninhydrin, C9H6O4 | 1.53 | 0.606 | 0.1 | 1.03 | 2.74 | This work |
| Thionine, C12H10N3S+ | 1.93 | 0.631 | 0.1 | 0.91 | 2.41 | This work |
| Natural water contaminants | ||||||
| Acetovanillone, C9H10O3 | 1.93 | 0.650 | 0.5 | 0.96 | [14] | |
| 0.5 | 1.19 | |||||
| Gallic acid, C7H6O5 | 1.13 | 0.494 | 2.0 | 0.89 | [14] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojnárovits, L.; Homlok, R.; Kovács, K.; Tegze, A.; Takács, E. Wastewater Characterization: Chemical Oxygen Demand or Total Organic Carbon Content Measurement? Molecules 2024, 29, 405. https://doi.org/10.3390/molecules29020405
Wojnárovits L, Homlok R, Kovács K, Tegze A, Takács E. Wastewater Characterization: Chemical Oxygen Demand or Total Organic Carbon Content Measurement? Molecules. 2024; 29(2):405. https://doi.org/10.3390/molecules29020405
Chicago/Turabian StyleWojnárovits, László, Renáta Homlok, Krisztina Kovács, Anna Tegze, and Ezsébet Takács. 2024. "Wastewater Characterization: Chemical Oxygen Demand or Total Organic Carbon Content Measurement?" Molecules 29, no. 2: 405. https://doi.org/10.3390/molecules29020405
APA StyleWojnárovits, L., Homlok, R., Kovács, K., Tegze, A., & Takács, E. (2024). Wastewater Characterization: Chemical Oxygen Demand or Total Organic Carbon Content Measurement? Molecules, 29(2), 405. https://doi.org/10.3390/molecules29020405