Abstract
The long time (2 h) required for measurement, expensive chemicals (Ag2SO4), and toxic reagents (K2Cr2O7, HgSO4) limit the application of the standard method for measuring the oxygen equivalent of organic content in wastewater (chemical oxygen demand, COD). In recent years, the COD has increasingly been replaced by the total organic carbon (TOC) parameter. Since the limit values of the pollution levels are usually given in terms of the COD, efforts are being made to find the correlation between these parameters. Several papers have published correlation analyses of COD and TOC for industrial and municipal wastewater, but the relationship has not been discussed for individual chemicals. Here, this relationship was investigated using 70 contaminants (laboratory chemicals, pharmaceuticals, and pesticides). The calculated COD values, in most cases, agreed, within ~10%, with the experimental ones; for tetracyclines and some chloroaromatic molecules, the measured values were 20–50% lower than the calculated values. The COD/TOC ratios were between 2 and 3: for macrolides, they were ~3; for fluoroquinolones and tetracyclines, they were ~2. The molecular structure dependence of the ratio necessitates the establishing of the correlation on an individual basis. In advanced oxidation processes (AOPs), the ratio changes during degradation, limiting the application of TOC instead of COD.
1. Introduction
The organic carbon in water/wastewater originates from a variety of organic compounds in various oxidation states. Some of these carbon compounds can be oxidized through chemical or biological processes, and the routinely applied chemical oxygen demand (CODmeas) and biochemical oxygen demand (BOD) analyses are typically used to characterize these fractions [,]. CODmeas measures the oxygen equivalent (mg O2 dm−3) of the organic matter content of the sample, susceptible to oxidation by strong chemical oxidants (potassium permanganate or potassium dichromate) []. Potassium dichromate is mainly used for assessing the water quality in moderately or heavily contaminated water bodies (like in wastewater treatment plants), while potassium permanganate is often used as an oxidant for relatively clean water (like surface water). For the BOD, the digestion is carried out using a mixed microorganism population collected from the purification system of a wastewater treatment plant [].
The classical CODmeas analytical technique using dichromate is based on refluxing a 10 mL sample for 2 h in a sulfuric acidic medium containing potassium dichromate (K2Cr2O7) in the presence of a silver sulfate (Ag2SO4) catalyst (open reflux method). Generally, mercuric sulfate (HgSO4) is also added to the reaction mixture to avoid interference from chloride ions. After cooling, the solution is titrated with a ferrous ammonium sulfate ((NH4)2Fe(SO4)2) solution to determine the residual dichromate, using ferroin ([Fe(C12H8N2)3]SO4) indicator, and compared to the value measured for the untreated sample to determine the concentration of dichromate consumed for oxidation []. This standard method has a few disadvantages that limit its application, such as a long reflux time, expensive chemicals (Ag2SO4), and toxic (K2Cr2O7, HgSO4) and highly corrosive (H2SO4) reagents. Nevertheless, this classic technique seems to be one of the frequently used COD measuring procedures in practice []. In a modified version of the method, the digestion takes place in a closed system, and spectrophotometry is used for analysis (the sealed-tube method). This method applies the smaller sample volume of 2 mL. Over the last 30–40 years, many new analytical approaches have been developed to replace persulfate solution refluxing with other digestion methods in order to avoid its drawbacks [,].
In laboratory investigations on selected organic compounds, the experimental CODmeas values are often compared with the theoretically calculated ones (ThOD) using the chemical composition and molar concentration []. ThOD is the stoichiometric amount of O2 needed to transform a CcHhClclNnNanaOoPpSs compound to CO2, NH3, H2PO4−, and H2O. The ThOD is calculated through the equation []:
where M is the molecular mass. This equation may be used in the interactive mode through the internet. The calculation gives the theoretical COD in mg/mg units (ThODmg/mg) (https://www.aropha.com/thod-calculator.html, accessed on 17 December 2023). We obtain the ThOD in the usual mg O2 dm−3 unit by multiplying the value with 1000 × M × conc; conc is the concentration in mol dm−3 unit. The calculation assumes that, during treatment, N transforms to NH3 (no nitrification).
The CODmeas values can differ considerably from the theoretical ones. Baker et al. [] carried out a detailed comparison of the experimental COD values (using the measurements of Janicke et al. []) and calculated COD values for 565 laboratory organic chemicals, such as simple aromatics, alcohols, and aldehydes. They introduced an empirical constant, a, which was defined as the ratio of the measured and calculated values: a = CODmeas/ThOD. The authors classified the organic compounds into six groups according to the structural characteristics. For instance, in the cases of alcohols, carbohydrates, and unsaturated carboxylic acids, the a values were between 0.95 and 1. However, for some classes of other compounds, e.g., hydrocarbons, several halogenated aromatic hydrocarbons, the correlation between the measured and the calculated values was very poor, with a less than 0.5 for some compounds. This is an important observation, because CODmeas analysis of the wastewater of factories releasing larger amounts of waste with compounds characterized by low a may give misleading results regarding the discharged organics.
The reason for the deviation of a from 1 is not clear; some theories have already been published in the literature. For example, some compounds may not be oxidized completely during digestion, or volatile compounds may leave the flask before complete oxidation. The low CODmeas values measured for some aliphatic compounds may be due to the conversion of terminal methyl groups to acetic acid or methane and not to CO2 []. Methane can be driven out of the solution before oxidation occurs.
Potassium dichromate has been proven to be carcinogenic and mutagenic, and the substance is restricted under Annex XIV of the REACH regulation (Regulation on the Registration, Evaluation, Authorization and Restriction of Chemicals) []. Thus, other analytical methods have been considered to replace the COD measurement to eliminate the use of potassium dichromate. The total organic carbon (TOC) content is a possible candidate: in recent years, the COD has increasingly been replaced by the TOC [,,].
TOCmeas expresses the organic carbon content in mg C dm−3 units, and this amount can be determined using automatic analyzers. TOC analysis takes from 15 to 25 min and, thus, it is much faster than the classical COD measurement. The samples to be analyzed usually contain bicarbonates and carbonates (inorganic carbon (IC)). IC can be removed by acidifying the sample to a pH value of 2 or less to release IC as CO2, which can be measured or vented to waste. The non-purgeable organic carbon (NPOC) remaining in the liquid is then oxidized, releasing CO2, which is sent to the detector for measurement. Unlike COD, TOC is independent of the oxidation state of organic matter. The theoretical TOC value in mg/mg unit is calculated via the equation:
We obtain the value in the traditional mg C dm−3 unit (TOCcalc) by multiplying TOCmg/mg with 1000 × M × conc.
Since the limit values of organic pollution levels in wastewater are usually described in terms of CODmeas, efforts are being made to find a correlation between the two parameters []. To replace CODmeas with TOCmeas, the relationship between the two quantities, i.e., the CODmeas/TOCmeas ratio should be well established. In many papers, we find correlation analysis of CODmeas and TOCmeas parameters in the cases of industrial or municipal wastewater (e.g., [,]). The reported ratios vary in a relatively large range depending on the source of the wastewater. In the chemical industry, the CODmeas/TOCmeas ratios are mostly in the range of 2.5–3.5 [].
Here, we publish (a=) CODmeas/ThOD and CODmeas/TOCmeas values for a large number of individual water contaminants, including simple molecules that may serve as models for more complicated chemicals, and also for more complex molecules that are often detected in wastewater: drugs, pesticides, etc. For most of these compounds, no data are available in the literature. In the evaluation of the CODmeas/ThOD and CODmeas/TOCmeas values, we also use data taken from our previous works, determined using the same technique (described in Section 4, Materials and Methods) as in the present work. In the experimental work, a solute concentration of 0.1–0.3 mmol dm−3 was used in most cases. In the γ-radiolysis of three antibiotics, piperacillin, doxycycline, and erythromycin, we also investigate the changes in the CODmeas/TOCmeas ratio during degradation. With these investigations, we want to answer the question of whether, in studies related to advanced oxidation processes (AOPs), the COD→TOC replacement is applicable or not.
2. Results
2.1. Data Collections, Reliability, and General Observations
Column 1 of Table 1 shows the names and chemical formulae of chemicals whose parameters are discussed here; we collected the chemical structures in the Supplementary Material. Columns 2 and 3 contain the ThODmg/mg and TOCmg/mg values calculated using Equations (1) and (2). Column 4 shows the concentrations for each compound used during the measurement of the CODmeas and TOCmeas values. In the next two columns, the calculated (a=) CODmeas/ThOD and CODmeas/TOCmeas ratios are presented. The table contains data taken from our previous works and from other authors’ publications. In the rows, first, we show the results for some general laboratory chemicals and, then, for different classes of harmful chemical compounds. The ThODmg/mg values are concentration-independent; they are highly characteristic of individual compounds because they vary theoretically in a wide range, from methane (3.99) to oxalic acid (0.18). For saturated hydrocarbons, higher-molecular-mass alcohols, and oxo compounds (e.g., cyclohexanone), these values are around 2.5–3.5 mg/mg, while for the highly oxidized molecules, such as maleic and fumaric acids, gallic acid, and acetovanillone, the values are close to 1. The ThODmg/mg value can also be low for compounds containing several oxygen atoms or oxidizable heteroatoms (e.g., S). The theoretical TOCmg/mg value of oxalic acid is 0.27; for methane, 0.75 is calculated. The theoretical ThODmg/mg/TOCmg/mg ratios change between 0.67 (oxalic acid) and 5.33 (methane) [].

Table 1.
Data collection for chemicals with their chemical formulae, theoretical ThODmg/mg and TOCmg/mg, (a=) CODmeas/ThOD, and experimental CODmeas/TOCmeas values.
According to our observations, the experimental TOC values (TOCmeas) are very close to the calculated ones (TOCcalc): the difference between the two values is usually less than ±4%. This provided an opportunity the check the reliability of the measurements. The agreement of TOCmeas and TOCcalc values gives proof that the disagreement observed in some cases between CODmeas and ThOD, when both measurements were obtained from the same sample solution, is not due to any sample preparation issues or inorganic or organic impurities in the sample, but the disagreement does exist.
Table 1 shows the data measured at different concentrations for certain compounds tested in our laboratory (e.g., phenol, 2,6-dichloroaniline, p-aminophenol, phenolate, paracetamol); the results reasonably agree in most cases. For tetracycline, salicylic acid, and maleic acid, our CODmeas/ThOD ratios also agree with the values calculated based on the results published in the literature. However, for chlorobenzene, there is a large difference between the value published by Baker et al. [] and the value calculated based on the measurements of Albarran and Mendoza []: 0.58 and 0.25, respectively. Column 6 of Table 1 contains the ratios of the experimentally obtained COD and TOC values, CODmeas/TOCmeas. These data vary within a relatively narrow range; most ratios are between 2.0 and 3.0.
2.2. Groups of Organic Molecules
In Table 1, under the heading of laboratory chemicals, we list simple chemical compounds that can serve as starting materials for the synthesis of more complex molecules for pharmaceuticals, plant protection, paints, etc. The calculated ThODmg/mg values strongly depend on the oxidation state of the molecule and on the oxidizable heteroatoms. The smallest values, around 1, were calculated for ethylene glycol, maleic and fumaric acids, 2,4-dichlorophenol, and 2,6-dichloroaniline. Cyclohexanone, cresols, and benzamine had the highest values, around 2.5. Most of these compounds can be measured well using the dichromate/titration method; the ratios of measured and calculated COD values are around 1. A notable exception is cyclohexanone.
Sulfonamide antibiotics, which prevent the multiplication of bacteria, are usually applied in combination with trimethoprim []. The CODmeas/ThOD values for sulfonamides are in the 0.82–1.05 range, and the CODmeas/TOCmeas ratios are between 2.52 and 3.28. As Figure 1 shows, there is some correlation between the CODmeas/TOCmeas and ThODmg/mg for this group: the ratio decreases with the increasing COD values. We measured the COD and TOC values of trimethoprim at 0.1 and 0.3 mmol dm−3 concentrations. The CODmeas/ThOD and CODmeas/TOCmeas values at the two concentrations are different: 0.86 and 1.02, respectively, and 2.24 and 2.83, respectively.
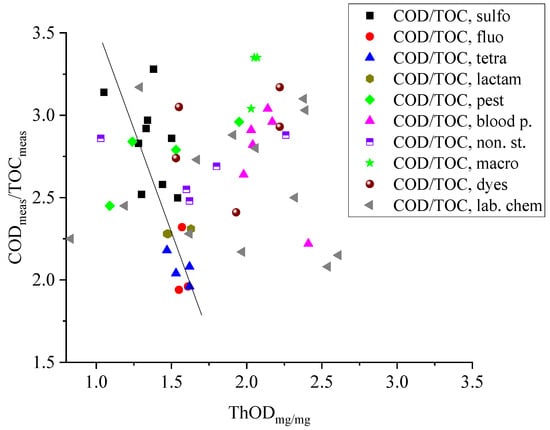
Figure 1.
Relationship between the ThODmg/mg values and the CODmeas/TOCmeas ratios.
The broad-spectrum fluoroquinolone bactericides in Table 1, ciprofloxacin, norfloxacin, and ofloxacin share a 4-quinolone bicyclic core structure; several substituents are attached to this core [,]. The CODmeas/ThOD values for fluoroquinolones vary between 0.77 and 1.03; the smallest value was measured for ofloxacin. In this molecule, the oxazine ring attached to the central core is fragile and may decompose before reaching the digestion temperature of 150 °C. As a result, low-boiling fragments can leave the system without oxidation. The average CODmeas/TOCmeas ratio for fluoroquinolones is low, at 2.07.
The CODmeas/ThOD ratios for the tetracycline antibiotics are low; according to our measurements, they are in the 0.77–0.96 range []. The value calculated for tetracycline, using the data of Belkheiri et al. [] is 0.81, lower than our value, 0.96. The tetracyclines in Table 1 each have three methyl groups. CODmeas/ThOD ratios lower than 1 are consistent with the methyl group theory mentioned in the Introduction, although these molecules are considered thermally very stable []. Their CODmeas/TOCmeas ratios are also low; the average is 2.07.
The four β-lactam antibiotics in Table 1, amoxicillin, oxacillin, cloxacillin, and piperacillin, have similar chemical structures []; their ThODmg/mg values fall in a narrow range between 1.47 and 1.63. Since their CODmeas/ThOD values are also close to each other (0.79–0.96), the CODmeas/TOCmeas ratios are also in a narrow range, with an average of 2.40.
The macrolides in Table 1, erythromycin, azithromycin, and clarithromycin, contain a large number of carbon (37–38) and hydrogen (67–72) atoms, 1 or 2 nitrogen atoms, and 12–13 oxygen atoms. Due to the similar structure, the ThODmg/mg values are in a narrow range, between 2.03 and 2.07. The CODmeas/TOCmeas ratios are high; they are in the 3.03–3.35 range. The ratios of the measured and calculated COD values are close to 1. These molecules have several methyl groups, but their COD-reducing effect is not observed.
The blood pressure regulators in Table 1 (atenolol, nadolol, propranolol, metoprolol tartrate, labetalol, and acebutolol) contain an oxypropanolamine unit and benzene ring(s) [,]. In labetalol, there are two separate rings; in propranolol, the benzene ring is replaced by a naphthalene unit. Metoprolol is mostly used as metoprolol tartrate salt (two metoprolol and one tartrate unit); this combination was used in our experiments. For these molecules, the CODmeas/ThOD ratios are in the 0.85–0.96 range, and the CODmeas/TOCmeas values are between 2.64 and 3.04. The exception is propranolol, which contains a naphthalene moiety. The CODmeas/ThOD and CODmeas/TOCmeas ratios for propranolol are low; they are 0.72 and 2.22, respectively. We assume that this behavior is related to the stability of the naphthalene unit. On the CODmeas/TOCmeas–ThODmg/mg plot, the data points for blood pressure regulators are far from the range of points belonging to other compounds (Figure 1).
Salicylates (salicylic acid, acetylsalicyclic acid, and 5-sulfo-salicyclic acid), ketoprofen, diclofenac, and paracetamol are listed among the non-steroidal anti-inflammatory drugs [,,]. Their CODmeas/ThOD ratios are close to 1.0, and the CODmeas/TOCmeas ratios are between 2.48 and 2.88. In Figure 1, on the CODmeas/TOCmeas–COD plot, the points for the three salicylates are around the straight line. Ketoprofen shows “irregular” behavior; the corresponding point is in the range where we find the points of the blood pressure regulators.
The broad-spectrum antibiotic chloramphenicol was listed among the miscellaneous drugs. Due to the high number of oxygen atoms (5) and heteroatoms (4) in the molecule, it has a very low ThODmg/mg value, 0.94. This is one of the most problematic compounds for COD measurements using the dichromate method. The measured value is significantly lower that the calculated one; the CODmeas/ThOD ratio is 0.59. In a former publication, we reported on radiolytic degradation experiments (a type of advanced oxidation process, AOP) with chloramphenicol and found that COD increased with the progress of degradation, suggesting that the products can be more easily oxidized than the starting molecule []. This was attributed to the “compact” structure (whatever this may mean) of the starting molecule and to the partly oxidized forms of the products. We mention that Barker et al. [] noted that several –NO2 and –Cl containing molecules exhibited measured COD values lower than the theoretical value.
The antimalarial drug amodiaquine is easily oxidized in dichromate solution []. The CODmeas/TOCmeas ratio is in the medium range, 2.55. Clofibric acid is an essential part, and, at the same time, metabolite of several anticholesteremic drugs []. Its CODmeas/TOCmeas ratio (2.67) is similar to that of amodiaquine.
The data for fenuron, monuron, and diuron phenylurea pesticides are from our laboratory [,,]. Their CODmeas data agree well with the ThOD. The same is true for the data for linuron, monolinuron, and buturon reported in the work of Baker et al. []. The average of all six CODmeas/ThOD values is 1.02. The high ratio is surprising, since all molecules have methyl group(s), and, with the exception of fenuron chlorine, atom(s) are attached to the aromatic ring. The CODmeas/TOCmeas ratios of fenuron, monuron, and diuron are very close to each other; the average is 2.86.
The six dyes in Table 1 have considerably different structures; their ThODmg/mg values cover a wide range, between 1.23 and 2.22. Despite the wide range of ThOD, the CODmeas/TOCmeas ratios fall in a narrower range; they are between 2.41 and 3.17.
2.3. Application of COD and TOC Values in an AOP
Chemical oxygen demand (COD) and total organic carbon (TOC) measurements are often used in advanced oxidation processes (AOPs) to monitor the progress of degradation (e.g., [,]). Sometimes, both parameters are followed; sometimes, it is only one of them, which nowadays is mostly the TOC. Figure 2 shows the COD and TOC values and their ratio measured during the γ-radiolytic degradation of three antibiotics: piperacillin, doxycycline, and erythromycin. In the radiolysis of an aerated, dilute aqueous solution, the degradation of organic contaminants is mainly initiated by hydroxyl radicals (•OH); the amount of these radicals injected into the solution is proportional to the absorbed radiation energy (D, absorbed dose, unit J kg−1 (Gray)): [•OH] = 2.8 × 10−7 × D mol dm−3 []. The technology based on the application of ionizing radiation (electron beam irradiation) has already been introduced on an industrial scale, treating 10,000–30,000 m3 day−1 [,]. As Figure 2 shows, both the COD and TOC in all three systems decrease with the absorbed dose. However, the decrease in COD is much faster than that in TOC; their ratio constantly decreases during the process.
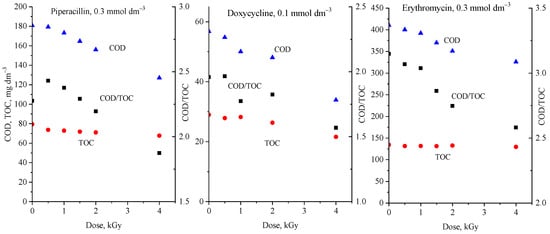
Figure 2.
Dose dependence of the COD (blue triangle), TOC (red circle), and COD/TOC (black square) values in the γ-radiolytic degradation of piperacillin, doxycycline, and erythromycin.
3. Discussion
The experimental COD values, CODmeas, with some exceptions (isopropanol, vinyl acetate, cyclohexanone, 2,4-dichlorophenol, chlorobenzene, tetracyclines, propranolol, chloramphenicol, methylene blue) are close to the theoretically obtained ones (ThOD). Usually, CODmeas is slightly smaller than the ThOD. If the mentioned compounds are disregarded, then the average value of the ratio is CODmeas/ThOD ± σ = 0.944 ± 0.106. The correlation suggests that the ThOD can be used as a good approximation of CODmeas. Our investigations, similarly to other studies, could not explain the significant difference between the two values observed for a few molecules []. It seems that the terminal methyl groups can reduce CODmeas in some cases, while, in others, they have no effect on the values.
In all cases, the experimental TOC values were very close to the theoretical ones. Although, theoretically, the CODmeas/TOCmeas values can vary over a very wide range, for the compounds in Table 1, the values fall in the range of 2.0–3.0, with very few exceptions. For the fluoroquinolones and the tetracyclines, the CODmeas/TOCmeas ratio is low; it is around 2.0. Benzoic acid, p-chlorophenol, cyclohexanone, and chloramphenicol also have low ratios. The ratios are high, at around 3 or above, for isopropanol, ethylene glycol, sulfonamides, and the macrolides.
The structural dependence of the CODmeas/TOCmeas ratios observed for individual compounds, similarly to in investigations with domestic and industrial wastewater [], reflects that a single multiplication factor for the TOC to approximate the COD cannot be applied. The ratio should be determined individually and, in the case of domestic and industrial wastewater, it must be checked regularly.
In the conducted AOP investigations (γ-radiolytic degradation of antibiotics), COD decreased faster than TOC as the degradation progressed. The result shows that the investigated antibiotic molecules gradually disintegrate in many steps, forming smaller, highly oxidized fragments: first of all, small molecular mass organic acids (formic acid, acetic acid, oxalic acid, etc.) and, finally, the carbon and hydrogen atoms transform to CO2 and H2O. These small organic acids were considered “ultimate acids” as they were supposed to mineralize directly to CO2 [,]. This mechanism is supported by the gradual and strong decrease in pH during the treatment generally observed in such experiments []. Since the COD/TOC ratio in AOP experiments changes considerably during degradation, TOC cannot be a good substitute for COD. The change in COD during the process reflects oxidation, while the TOC characterizes the final step, the transformation of fragments to CO2.
4. Materials and Methods
4.1. Chemicals
The organic compounds investigated in the experiments were mainly obtained from Sigma Aldrich (Merck, Darmstadt, Germany). Pure water used in the experiments was prepared using an Adrona B30 system (Adrona SIA, Riga, Latvia), which provides high-quality water with a conductivity of 0.055 μS cm−1 and a total organic carbon content <2 ppb.
4.2. CODmeas Determination
In the experiments, a Behrotest TRS 200 (Behr Labor-Technik GmbH, Düsseldorf, Germany) COD digestion system was used with K2Cr2O7 as oxidant, Ag2SO4 as catalyst, and HgSO4 to eliminate the interference of Cl−. Test mixtures were boiled at 150 °C for 2 h, and the remaining K2Cr2O7 oxidizing agent was determined via titration []. In order to increase the accuracy, and to remain in the recommended COD range (30–700 mg dm−3), in several cases, we added 30 mL solution to the digestion system in contrast to the usual 10 mL. The results are averages of three titrations.
4.3. TOCmeas Determination
For the total organic carbon (TOCmeas) content measurements, Shimadzu (Kyoto, Japan) TOC-LCSH/CSN equipment was used. This assay is based on catalytic combustion of the organic content of samples and analysis of the formed CO2 using non-dispersive infrared detection.
4.4. γ-Radiolytic Experiments
The irradiation experiments were conducted at room temperature, in the panoramic type 1.8 PBq activity 60Co-γ facility of the Institute of Isotopes Co., Ltd. (Budapest, Hungary). The dose rate measured using ethanol-chlorobenzene dosimetry was 2 kGy h−1 []. The time of irradiation was varied between 0.5 and 2 h. The samples were irradiated in open air, under the conditions applied, the warming up of the samples were less than 1 °C. During irradiations, the solutions were gently bubbled with air in order to avoid oxygen depletion. In radiolytic experiments, the reactive intermediates (mainly •OH radicals) are produced during irradiation as a result of the radiolysis of water. Therefore, neither catalyst nor additives are required for the AOP.
5. Conclusions
The sum parameters, chemical oxygen demand (COD) and total organic carbon (TOC) are often used to characterize wastewater. The same parameters are also used in AOP investigations to follow the degradation of organic pollutants. Due to the disadvantages of COD, such as the long reflux time (2 h), expensive chemicals (Ag2SO4), and toxic reagents (K2Cr2O7, HgSO4), the COD has increasingly been replaced by the TOC. The pollution limit values in wastewater treatment are established in the COD values; therefore, efforts are being made to find the correlation between these parameters. This correlation was evaluated and discussed for ~70 laboratory chemicals, pharmaceuticals, pesticides, etc. The measured COD/TOC ratios were found to be between 2.0 and 3.0; for macrolides, they were around 3.0, and for fluoroquinolones and tetracyclines, they were around 2.0. The structure dependence suggests that the ratio should be established individually. In scientific investigations connected to degradation studies in advanced oxidation processes, we do not recommend the COD→TOC replacement. Changes in COD and TOC have different meanings: decreases in COD reflect the progress of oxidation, while in TOC, the changes show the last step, the transformation of organic carbon to CO2. In the case of domestic and industrial wastewater, this replacement is possible when the ratio does not change much over time, but this needs regular checking.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29020405/s1, Scheme S1: Chemical structures of organic molecules whose COD, TOC, and other parameters were discussed in the publication.
Author Contributions
Conceptualization, writing—original draft preparation L.W.; writing—review and editing, E.T.; investigation, data curation R.H., K.K. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the International Atomic Energy Agency (Coordinated Research Project F23034) and the National Research, Development and Innovation Office of Hungary (OTKA FK 146883).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the system applied in our institute insert reason here.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Anagnostopoulos, A.; Hjort, M.; Vaiopoulou, E. Assessment of Chemical Oxygen Demand/Total Organic Carbon (COD/TOC) ratios in refinery effluents. Report: Environmental Science for European Refining, no. 16/22. Brussels, 2022. Available online: https://www.concawe.eu/publications/?_sft_topic=manufacturing-water-wastewater-emissions&_sft_publicationscategory=report (accessed on 16 December 2023).
- Aguilar-Torrejón, J.A.; Balderas-Hernández, P.; Roa-Morales, G.; Barrera-Díaz, C.E.; Rodríguez-Torres, I.; Torres-Blancas, T. Relationship, importance, and development of analytical techniques: COD, BOD, and, TOC in water—An overview through time. SN Appl. Sci. 2023, 5, 118. [Google Scholar] [CrossRef]
- OECD. Guidelines for the Testing of Chemicals Ready Biodegradability Section 3; Test No. 301; OECD: Paris, France, 1992. [Google Scholar]
- DIN EN 1899-1:1998; Water Quality. Determination of the Biochemical Oxygen Demand after N Days (BOD[n]) of Water-Part 1: Dilution and Seeding Method with Allylthiourea Addition. European Committee for Standardization: Brussels, Belgium, 1998.
- Li, J.; Luo, G.; He, L.J.; Xu, J.; Lyu, J. Analytical approaches for determining chemical oxygen demand in water bodies: A review. Crit. Rev. Anal. Chem. 2018, 48, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, Y.; Liu, Y. A review of detection techniques for chemical oxygen demand in wastewater. Am. J. Biochem. Biotechnol. 2022, 18, 23–32. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Sasaki, S.; Yano, K.; Ikebukuro, K.; Hashimoto, K.; Karube, I. Relationship between theoretical oxygen demand and photocatalytic chemical oxygen demand for specific classes of organic chemicals. Analyst 2000, 125, 1915–1918. [Google Scholar] [CrossRef]
- Baker, J.R.; Milke, M.W.; Michelcic, J.R. Relationship between chemical and theoretical oxygen demand for specific classes of organic chemicals. Water Res. 1999, 33, 327–334. [Google Scholar] [CrossRef]
- Janicke, W. Chemische Oxidierbarkeit Organischer Wasserinhaltstoffe; WaBoLu Berichte, Dietrich Reimer Verlag: Berlin, Germany, 1983. [Google Scholar]
- Annex XIV of the REACH Regulation (Regulation on the Registration, Evaluation, Authorisation and Restriction of Chemicals). Available online: https://echa.europa.eu/hu/authorisation-list (accessed on 16 December 2023).
- Choi, I.-W.; Kim, J.-H.; Im, J.-K.; Park, T.-J.; Kim, S.-Y.; Son, D.-H.; Huh, I.-A.; Rhew, D.-H.; Yu, S.-J. Application of TOC standards for managing refractory organic compounds in industrial wastewater. J. Korean Soc. Water Environ. 2015, 31, 29–34. [Google Scholar] [CrossRef][Green Version]
- Tian, X.; Zhao, C.; Ji, X.; Feng, T.; Liu, Y.; Bian, D. The correlation analysis of TOC and CODCr in urban Sewage treatment. E3S Web Conf. 2019, 136, 6010. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, S.Y.; Noh, J.H.; Bae, Y.H.; Lee, J.W.; Maeng, S.K. A shift from chemical oxygen demand to total organic carbon for stringent industrial wastewater regulations: Utilization of organic matter characteristics. J. Environ. Manag. 2022, 305, 114412. [Google Scholar] [CrossRef]
- Homlok, R.; Takács, E.; Wojnárovits, L. Degradation of organic molecules in advanced oxidation processes: Relation between chemical structure and degradability. Chemosphere 2013, 91, 383–389. [Google Scholar] [CrossRef]
- Rácz, G.; Csay, T.; Takács, E.; Wojnárovits, L. Degradation of Triton X-100 surfactant/lipid regulator systems by ionizing radiation in water. J. Radioanal. Nucl. Chem. 2017, 314, 1189–1196. [Google Scholar] [CrossRef]
- Albarrán, G.; Mendoza, E. Radiolysis induced degradation of 1,3-dichlorobenzene and 4-chlorophenol in aqueous solution. Radiat. Phys. Chem. 2020, 182, 109318. [Google Scholar] [CrossRef]
- Albarrán, G.; Mendoza, E. Radiolytic oxidation and degradation of 2,4-dichlorophenol in aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 17055–17065. [Google Scholar] [CrossRef] [PubMed]
- Albarrán, G.; Mendoza, E. Radiolytic degradation of chlorobenzene in aerated and deoxygenated aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 22855–22864. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Okumura, M. Feasibility of mercury-free chemical oxygen demand (COD) test with excessive addition of silver sulfate. J. Water Environ. Technol. 2018, 16, 221–232. [Google Scholar] [CrossRef]
- Sági, G.; Csay, T.; Szabó, L.; Pátzay, G.; Csonka, E.; Takács, E.; Wojnárovits, L. Analytical approaches to the OH radical induced degradation of sulfonamide antibiotics in dilute aqueous solutions. J. Pharm. Biomed. Anal. 2015, 106, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tegze, A.; Sági, G.; Kovács, K.; Homlok, R.; Tóth, T.; Mohácsi-Farkas, C.; Wojnárovits, L.; Takács, E. Degradation of fluoroquinolone antibiotics during ionizing radiation treatment and assessment of antibacterial activity, toxicity and biodegradability of the products. Radiat. Phys. Chem. 2018, 147, 101–105. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Homlok, R.; Kovács, K.; Bezsenyi, A.; Takács, E. Ionizing radiation induced removal of ofloxacin, abatement its toxicity and antibacterial activity in various water matrices. Appl. Sci. 2023, 13, 7211. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Wang, J.; Chu, L.; Tóth, T.; Kovács, K.; Bezsenyi, A.; Szabó, L.; Homlok, R.; Takács, E. Matrix effect in the hydroxyl radical induced degradation of β-lactam and tetracycline type antibiotics. Radiat. Phys. Chem. 2022, 193, 109980. [Google Scholar] [CrossRef]
- Belkheiri, D.; Fourcade, F.; Geneste, F.; Floner, D.; Aït-Amar, H.; Amrane, A. Combined process for removal of tetracycline antibiotic–Coupling pretreatment with a nickel-modified graphite felt electrode and a biological treatment. Int. Biodeter. Biodegr. 2015, 103, 147–153. [Google Scholar] [CrossRef]
- Takács, E.; Wang, J.; Chu, L.; Tóth, T.; Kovács, K.; Bezsenyi, A.; Szabó, L.; Homlok, R.; Wojnárovits, L. Elimination of oxacillin, its toxicity and antibacterial activity by using ionizing radiation. Chemosphere 2022, 286, 131467. [Google Scholar] [CrossRef]
- Kovács, K.; Simon, Á.; Tóth, T.; Wojnárovits, L. Free radical chemistry of atenolol and propranolol investigated by pulse and gamma radiolysis. Radiat. Phys. Chem. 2022, 196, 110141. [Google Scholar] [CrossRef]
- Kovács, K.; Tegze, A.; Bezsenyi, A.; Wojnárovits, L. Hydroxyl radical induced degradation of the ß-blocker Nadolol and comparison with Propranolol. J. Environ. Chem. Eng. 2023, 11, 110330. [Google Scholar] [CrossRef]
- Szabó, L.; Tóth, T.; Homlok, R.; Rácz, G.; Takács, E.; Wojnárovits, L. Hydroxyl radical induced degradation of salicylates in aerated aqueous solution. Radiat. Phys. Chem. 2014, 97, 239–245. [Google Scholar] [CrossRef]
- Albarrán, G.; Mendoza, E. Ionizing radiation induced degradation of salicylic acid. Radiat. Phys. Chem. 2018, 147, 27–34. [Google Scholar] [CrossRef]
- Csay, T.; Rácz, G.; Takács, E.; Wojnárovits, L. Radiation induced degradation of pharmaceutical residues in water: Chloramphenicol. Radiat. Phys. Chem. 2012, 81, 1489–1494. [Google Scholar] [CrossRef]
- Kovács, K.; Simon, Á.; Balogh, G.T.; Tóth, T.; Wojnárovits, L. High energy ionizing radiation induced degradation of amodiaquine in dilute aqueous solution: Radical reactions and kinetics. Free Radic. Res. 2020, 54, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Csay, T.; Rácz, G.; Salik, Á.; Takács, E.; Wojnárovits, L. Reactions of clofibric acid with oxidative and reductive radicals–products, mechanisms, efficiency and toxic effects. Radiat. Phys. Chem. 2014, 102, 72–78. [Google Scholar] [CrossRef]
- Kovács, K.; Mile, V.; Csay, T.; Takács, E.; Wojnárovits, L. Hydroxyl radical-induced degradation of fenuron in pulse and gamma radiolysis: Kinetics and product analysis. Environ. Sci. Pollut. Res. 2014, 21, 12693–12700. [Google Scholar] [CrossRef]
- Kovács, K.; He, S.; Mile, V.; Földes, T.; Pápai, I.; Takács, E.; Wojnárovits, L. Ionizing radiation induced degradation of monuron in dilute aqueous solution. Radiat. Phys. Chem. 2016, 124, 191–197. [Google Scholar] [CrossRef]
- Kovács, K.; He, S.; Mile, V.; Csay, T.; Takács, E.; Wojnárovits, L. Ionizing radiation induced degradation of diuron in dilute aqueous solution. Chem. Cent. J. 2015, 9, 21. [Google Scholar] [CrossRef]
- Hassani, M.; Lázaro, R.; Pérez, C.; Condón, S.; Pagán, R. Thermostability of oxytetracycline, tetracycline, and doxycycline at ultrahigh temperatures. J. Agric. Food Chem. 2008, 56, 2676–2680. [Google Scholar] [CrossRef] [PubMed]
- Hayati, F.; Khodabakhshi, R.K.; Isari, A.A.; Sina Moradi, S.; Kakavandi, B. LED-assisted sonocatalysis of sulfathiazole and pharmaceutical wastewater using N,Fe co-doped TiO2@SWCNT: Optimization, performance and reaction mechanism studies. J. Water Proc. Eng. 2020, 38, 101693. [Google Scholar] [CrossRef]
- Kakavandi, B.; Ahmadi, M. Efficient treatment of saline recalcitrant petrochemical wastewater using heterogeneous UV-assisted sono-Fenton process. Ultrason. Sonochem. 2019, 56, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V. An overview of the radiation chemistry of liquids. In Radiation Chemistry: From Basics to Applications in Material and Life Sciences; Spotheim-Maurizot, M., Mostafavi, M., Douki, T., Belloni, J., Eds.; EDP Sciences: Les Ulis, France, 2008; pp. 3–16. [Google Scholar]
- IAEA. Radiation Processing, Environmental Applications; International Atomic Energy Agency: Vienna, Austria, 2007. [Google Scholar]
- CGN. 20IAEA. 2020. Available online: https://www.iaea.org/newscenter/news/started-with-iaea-support-chinas-electron-beamindustry-opens-worlds-largest-wastewater-treatment-facility (accessed on 6 June 2023).
- Moreira, F.C.; Garcia-Segura, S.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Degradation of the antibiotic trimethoprim by electrochemical advanced oxidation processes using a carbon-PTFE air-diffusion cathode and a boron-doped diamond or platinum anode. Appl. Catal. B 2014, 160–161, 492–505. [Google Scholar] [CrossRef]
- Giannakis, S.; Gamarra Vives, F.A.; Grandjean, D.; Magnet, A.; De Alencastro, L.F.; Pulgarin, C. Effect of advanced oxidation processes on the micropollutants and the effluent organic matter contained in municipal wastewater previously treated by three different secondary methods. Water Res. 2015, 84, 295–306. [Google Scholar] [CrossRef]
- ISO/ASTM 51538:2017; Practice for Use of the Ethanol-Chlorobenzene Dosimetry System. ASTM: West Conshohocken, PA, USA, 2017.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).