Impact of Sprouting Process on the Protein Quality of Yellow and Red Quinoa (Chenopodium quinoa)
Abstract
1. Introduction
2. Results
2.1. Germination Parameters
2.2. Content of Total Saponins during Germination
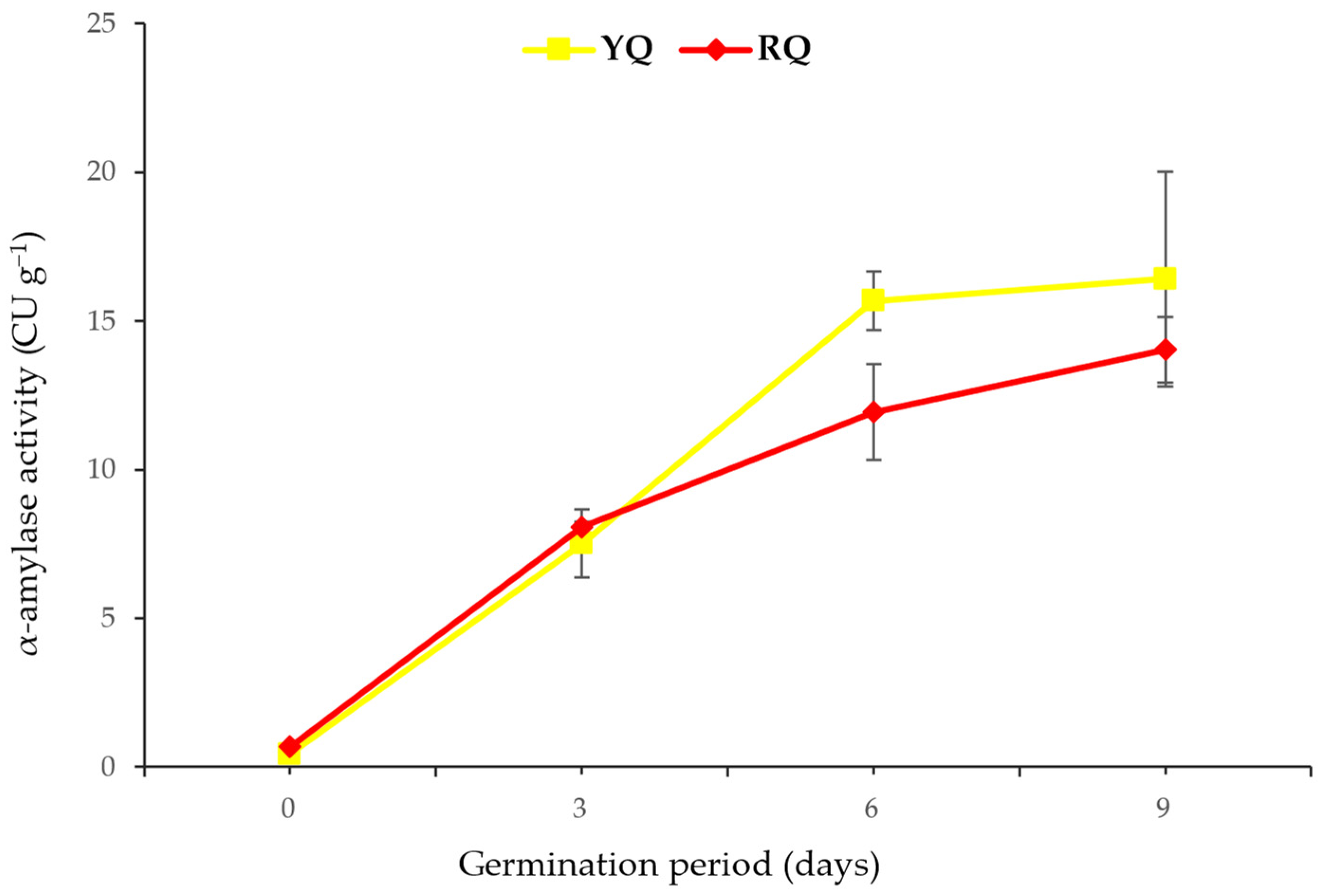
2.3. Content of α-Amylase and Protease during Germination
2.4. Composition of Amino Acids during Germination
2.5. Quality of Protein during Germination
3. Discussion
4. Materials and Methods
4.1. Ingredients
4.2. Sprouting Process of Quinoa Seeds
4.3. Determination of Total Saponins (TS)
4.4. Determination of α-Amylase and Protease Enzyme
4.4.1. Extraction of α-Amylase
4.4.2. Calculations of α-Amylase
4.4.3. Extraction of Protease Enzyme
4.4.4. Calculations of Protease Enzyme
4.5. Determination of Amino Acids
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langyan, S.; Yadava, P.; Khan, F.N.; Dar, Z.A.; Singh, R.; Kumar, A. Sustaining Protein Nutrition through Plant-Based Foods. Front. Nutr. 2022, 8, 772573. [Google Scholar] [CrossRef] [PubMed]
- Hoehnel, A.; Zannini, E.; Arendt, E.K. Targeted Formulation of Plant-Based Protein-Foods: Supporting the Food System’s Transformation in the Context of Human Health, Environmental Sustainability and Consumer Trends. Trends Food Sci. 2022, 128, 238–252. [Google Scholar] [CrossRef]
- Ahnen, R.T.; Jonnalagadda, S.S.; Slavin, J.L. Role of Plant Protein in Nutrition, Wellness, and Health. Nutr. Rev. 2019, 77, 735–747. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef]
- Angeli, V.; Miguel Silva, P.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An Overview of the Potentials of the “Golden Grain” and Socio-Economic and Environmental Aspects of Its Cultivation and Marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef]
- Belton, P.S.; Taylor, J.R. Pseudocereals and Less Common Cereals: Grain Properties and Utilization Potential; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Rosentrater, K.A.; Evers, A. Chapter 1—Introduction to Cereals and Pseudocereals and Their Production, In Kent’s Technology of Cereals, 5th ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 1–76. [Google Scholar]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition Facts and Functional Potential of Quinoa (Chenopodium quinoa Willd.), an Ancient Andean Grain: A Review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Choque-Quispe, D.; Ligarda-Samanez, C.A.; Ramos-Pacheco, B.S.; Leguía-Damiano, S.; Calla-Florez, M.; Zamalloa-Puma, L.M.; Colque-Condeña, L. Phenolic Compounds, Antioxidant Capacity, and Protein Content of Three Varieties of Germinated Quinoa (Chenopodium quinoa Willd). Ing. Investig. 2021, 41, 1–7. [Google Scholar] [CrossRef]
- Pathan, S.; Siddiqui, R.A. Nutritional Composition and Bioactive Components in Quinoa (Chenopodium quinoa Willd.) Greens: A Review. Nutrients 2022, 14, 558. [Google Scholar] [CrossRef]
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Fischer, S.; Wilckens, R.; Jara, J.; Aranda, M.; Valdivia, W.; Bustamante, L.; Graf, F.; Obal, I. Protein and Antioxidant Composition of Quinoa (Chenopodium quinoa Willd.) Sprout from Seeds Submitted to Water Stress, Salinity and Light Conditions. Ind. Crops Prod. 2017, 107, 558–564. [Google Scholar] [CrossRef]
- Poutanen, K.S.; Kårlund, A.O.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N. Grains–a Major Source of Sustainable Protein for Health. Nut. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and Germination Improve Nutritional Value of Cereals and Legumes through Activation of Endogenous Enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Bera, I.; O’Sullivan, M.; Flynn, D.; Shields, D.C. Relationship between Protein Digestibility and the Proteolysis of Legume Proteins During Seed Germination. Molecules 2023, 28, 3204. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Ikram, A.; Saeed, F.; Afzaal, M.; Imran, A.; Niaz, B.; Tufail, T.; Hussain, M.; Anjum, F.M. Nutritional and End-Use Perspectives of Sprouted Grains: A Comprehensive Review. Food Sci. Nutr. 2021, 9, 4617–4628. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.A.; Van den Broeck, H.C.; Brouns, F.J.; De Brier, N. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Food and Agriculture Organization (FAO). Food Policy and Food Science Service. Amino-acid Content of Foods and Biological Data on Proteins. In Amino-Acid Content of Foods and Biological Data on Proteins; FAO: Rome, Italy, 1970. [Google Scholar]
- Sibian, M.S.; Saxena, D.C.; Riar, C.S. Effect of Germination on Chemical, Functional and Nutritional Characteristics of Wheat, Brown Rice and Triticale: A Comparative Study. J. Sci. Food Agric. 2017, 97, 4643–4651. [Google Scholar] [CrossRef]
- Hung, P.V.; Maeda, T.; Yamamoto, S.; Morita, N. Effects of Germination on Nutritional Composition of Waxy Wheat. J. Sci. Food Agric. 2012, 92, 667–672. [Google Scholar] [CrossRef]
- Van Hung, P.; Maeda, T.; Morita, N. Improvement of Nutritional Composition and Antioxidant Capacity of High-Amylose Wheat During Germination. J. Food Sci. Technol. 2015, 52, 6756–6762. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Favela, M.A.; Gutierrez-Dorado, R.; Cuevas-Rodriguez, E.O.; Canizalez-Roman, V.A.; Del Rosario Leon-Sicairos, C.; Milan-Carrillo, J.; Reyes-Moreno, C. Improvement of Chia Seeds with Antioxidant Activity, Gaba, Essential Amino Acids, and Dietary Fiber by Controlled Germination Bioprocess. Plant Foods Hum. Nutr. 2017, 72, 345–352. [Google Scholar] [CrossRef]
- Wenefrida, I.; Utomo, H.S.; Blanche, S.B.; Linscombe, S.D. Enhancing Essential Amino Acids and Health Benefit Components in Grain Crops for Improved Nutritional Values. Recent Pat. DNA Gene Seq. 2009, 3, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, R.A.; Singh, T.P.; Rani, M.; Sogi, D.S.; Bhat, M.A. Diversity in Grain, Flour, Amino Acid Composition, Protein Profiling, and Proportion of Total Flour Proteins of Different Wheat Cultivars of North India. Front. Nutr. 2020, 7, 141. [Google Scholar] [CrossRef]
- Aggarwal, R.; Bains, K. Protein, Lysine and Vitamin D: Critical Role in Muscle and Bone Health. Crit. Rev. Food Sci. Nutr. 2022, 62, 2548–2559. [Google Scholar] [CrossRef]
- Agu, R.; Chiba, Y.; Goodfellow, V.; Mackinlay, J.; Brosnan, J.; Bringhurst, T.; Jack, F.; Harrison, B.; Pearson, S.; Bryce, J. Effect of Germination Temperatures on Proteolysis of the Gluten-Free Grains Rice and Buckwheat During Malting and Mashing. J. Agric. Food Chem. 2012, 60, 10147–10154. [Google Scholar] [CrossRef] [PubMed]
- Kaukovirta-Norja, A.; Wilhelmson, A.; Poutanen, K. Germination: A Means to Improve the Functionality of Oat. Agric. Food Sci. 2004, 13, 100–112. [Google Scholar] [CrossRef]
- Tang, Q.; Tan, P.; Ma, N.; Ma, X. Physiological Functions of Threonine in Animals: Beyond Nutrition Metabolism. Nutrients 2021, 13, 2592. [Google Scholar] [CrossRef] [PubMed]
- Kałużna-Czaplińska, J.; Gątarek, P.; Chirumbolo, S.; Chartrand, M.S.; Bjørklund, G. How Important Is Tryptophan in Human Health? Crit. Rev. Food Sci. Nutr. 2019, 59, 72–88. [Google Scholar] [CrossRef]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Huang, D.; Maulu, S.; Ren, M.; Liang, H.; Ge, X.; Ji, K.; Yu, H. Dietary Lysine Levels Improved Antioxidant Capacity and Immunity Via the Tor and P38 Mapk Signaling Pathways in Grass Carp, Ctenopharyngodon Idellus Fry. Front. Immunol. 2021, 12, 635015. [Google Scholar] [CrossRef]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Unni, U.S.; Raj, T.; Sambashivaiah, S.; Kuriyan, R.; Uthappa, S.; Vaz, M.; Regan, M.M.; Kurpad, A.V. The Effect of a Controlled 8-Week Metabolic Ward Based Lysine Supplementation on Muscle Function, Insulin Sensitivity and Leucine Kinetics in Young Men. Clin. Nutr. 2012, 31, 903–910. [Google Scholar] [CrossRef]
- Hayamizu, K.; Oshima, I.; Nakano, M. Comprehensive Safety Assessment of L-Lysine Supplementation from Clinical Studies: A Systematic Review. J. Nutr. 2020, 150, 2561S–2569S. [Google Scholar] [CrossRef] [PubMed]
- Ruales, J.; Nair, B.M. NAIR. Nutritional Quality of the Protein in Quinoa (Chenopodium quinoa, Willd) Seeds. Plant Foods Hum. Nutr. 1992, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-H.; Rozan, P.; Lambein, F.; Frias, J.; Vidal-Valverde, C. Effects of Different Germination Conditions on the Contents of Free Protein and Non-Protein Amino Acids of Commercial Legumes. Food Chem. 2004, 86, 537–545. [Google Scholar] [CrossRef]
- Pumera, M. Microfluidics in Amino Acid Analysis. Electrophoresis 2007, 28, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant Food Anti-Nutritional Factors and Their Reduction Strategies: An Overview. Food Prod. Process. Nutr. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Wunthunyarat, W.; Seo, H.S.; Wang, Y.J. Effects of Germination Conditions on Enzyme Activities and Starch Hydrolysis of Long-Grain Brown Rice in Relation to Flour Properties and Bread Qualities. J. Food Sci. 2020, 85, 349–357. [Google Scholar] [CrossRef]
- Rezaei, K.; Jenab, E.; Temelli, F. Effects of Water on Enzyme Performance with an Emphasis on the Reactions in Supercritical Fluids. Crit. Rev. Biotechnol. 2007, 27, 183–195. [Google Scholar] [CrossRef]
- Makinen, O.E.; Zannini, E.; Arendt, E.K. Germination of Oat and Quinoa and Evaluation of the Malts as Gluten Free Baking Ingredients. Plant Foods Hum. Nutr. 2013, 68, 90–95. [Google Scholar] [CrossRef]
- Lorenz, K.; Nyanzi, F. Enzyme Activities in Quinoa (Chenopodium quinoa). Int. J. Food Sci. Technol. 1989, 24, 543–551. [Google Scholar] [CrossRef]
- Guzmán-Ortiz, F.A.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Mora-Escobedo, R.; Rojas-León, A.; Rodríguez-Marín, M.L.; Falfán-Cortés, R.N.; Román-Gutiérrez, A.D. Enzyme Activity During Germination of Different Cereals: A Review. Food Rev. Int. 2019, 35, 177–200. [Google Scholar] [CrossRef]
- Helland, M.; Wicklund, T.; Narvhus, J. Effect of Germination Time on Alpha-Amylase Production and Viscosity of Maize Porridge. Food Res. Int. 2002, 35, 315–321. [Google Scholar] [CrossRef]
- Suárez-Estrella, D.; Bresciani, A.; Iametti, S.; Marengo, M.; Pagani, M.A.; Marti, A. Effect of Sprouting on Proteins and Starch in Quinoa (Chenopodium quinoa Willd.). Plant Foods Hum. Nutr. 2020, 75, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Hager, A.-S.; Mäkinen, O.E.; Arendt, E.K. Amylolytic Activities and Starch Reserve Mobilization During the Germination of Quinoa. Eur. Food Res. Technol. 2014, 239, 621–627. [Google Scholar] [CrossRef]
- Lu, S.; Cik, T.-T.; Lii, C.-y.; Lai, P.; Chen, H.-H. Effect of Amylose Content on Structure, Texture and A-Amylase Reactivity of Cooked Rice. LWT-Food Sci. Technol. 2013, 54, 224–228. [Google Scholar] [CrossRef]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The Rice Alpha-Amylase, Conserved Regulator of Seed Maturation and Germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Hilal, M.; Gonzalez, J.A.; Prado, F.E. Changes in Soluble Carbohydrates and Related Enzymes Induced by Low Temperature During Early Developmental Stages of Quinoa (Chenopodium quinoa) Seedlings. J. Plant Physiol. 2004, 161, 683–689. [Google Scholar] [CrossRef]
- Schlick, G.; Bubenheim, D.L. Quinoa an Emerging New Crop with Potential for Celss. NASA Tech. Pap. 1993, 3422, 1–14. [Google Scholar]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed Germination and Vigor: Ensuring Crop Sustainability in a Changing Climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef]
- del Hierro, J.N.; Herrera, T.; Fornari, T.; Reglero, G.; Martin, D. The Gastrointestinal Behavior of Saponins and Its Significance for Their Bioavailability and Bioactivities. J. Funct. Foods 2018, 40, 484–497. [Google Scholar] [CrossRef]
- Ruales, J.; Nair, B.M. Saponins, Phytic Acid, Tannins and Protease Inhibitors in Quinoa (Chenopodium quinoa, Willd) Seeds. Food Chem. 1993, 48, 137–143. [Google Scholar] [CrossRef]
- Brady, K.; Ho, C.-T.; Rosen, R.T.; Sang, S.; Karwe, M.V. Effects of Processing on the Nutraceutical Profile of Quinoa. Food Chem. 2007, 100, 1209–1216. [Google Scholar] [CrossRef]
- Vega-GÁLvez, A.; San MartÍN, R.; Sanders, M.; Miranda, M.; Lara, E. Characteristics and Mathematical Modeling of Convective Drying of Quinoa (Chenopodium quinoa Willd.): Influence of Temperature on the Kinetic Parameters. J. Food Process. Preserv. 2010, 34, 945–963. [Google Scholar] [CrossRef]
- Guajardo-Flores, D.; García-Patiño, M.; Serna-Guerrero, D.; Gutiérrez-Uribe, J.; Serna-Saldívar, S. Characterization and Quantification of Saponins and Flavonoids in Sprouts, Seed Coats and Cotyledons of Germinated Black Beans. Food Chem. 2012, 134, 1312–1319. [Google Scholar] [CrossRef]
- Maldonado-Alvarado, P.; Pavón-Vargas, D.J.; Abarca-Robles, J.; Valencia-Chamorro, S.; Haros, C.M. Effect of Germination on the Nutritional Properties, Phytic Acid Content, and Phytase Activity of Quinoa (Chenopodium quinoa Willd). Foods 2023, 12, 389. [Google Scholar] [CrossRef]
- Al-Qabba, M.M.; El-Mowafy, M.A.; Althwab, S.A.; Alfheeaid, H.A.; Aljutaily, T.; Barakat, H. Phenolic Profile, Antioxidant Activity, and Ameliorating Efficacy of Chenopodium quinoa Sprouts against Ccl(4)-Induced Oxidative Stress in Rats. Nutrients 2020, 12, 2904. [Google Scholar] [CrossRef]
- Barakat, H.; Spielvogel, A.; Hassan, M.; El-Desouky, A.; El-Mansy, H.; Rath, F.; Meyer, V.; Stahl, U. The Antifungal Protein Afp from Aspergillus Giganteus Prevents Secondary Growth of Different Fusarium Species on Barley. Appl. Microbiol. Biotechnol. 2010, 87, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Morillo, A.C.; Manjarres, E.H.; Mora, M.S. Afrosymetric Method for Quantifying Saponins in Chenopodium quinoa Willd. From Colombia. Braz. J. Biol. 2022, 82, e262716. [Google Scholar] [CrossRef]
- McCleary, B.V.; McNally, M.; Monaghan, D.; Mugford, D.C. Measurement of A-Amylase Activity in White Wheat Flour, Milled Malt, and Microbial Enzyme Preparations, Using the Ceralpha Assay: Collaborative Study Barry. J. AOAC Int. 2002, 85, 1096–1102. [Google Scholar] [CrossRef]
- Cupp-Enyard, C. Sigma’s Non-Specific Protease Activity Assay—Casein as a Substrate. J. Vis. Exp. 2008, 19, e899. [Google Scholar] [CrossRef]
- Le, L.; Gong, X.; An, Q.; Xiang, D.; Zou, L.; Peng, L.; Wu, X.; Tan, M.; Nie, Z.; Wu, Q.; et al. Quinoa Sprouts as Potential Vegetable Source: Nutrient Composition and Functional Contents of Different Quinoa Sprout Varieties. Food Chem. 2021, 357, 129752. [Google Scholar] [CrossRef]
- Steel, R.G. Principles and Procedures of Statistics A Biometrical Approach, 3rd ed.; McGraw-Hill: New York, NY, USA, 1997; pp. 139–203. [Google Scholar]



| Item | Germination % | Mean Time of Germination | Germination Index |
|---|---|---|---|
| YQ | 43.33 ± 1.53 b | 2.43 ± 0.19 a | 4.13 ± 0.32 b |
| RQ | 57.76 ± 1.53 a | 2.00 ± 0.02 b | 5.00 ± 0.05 a |
| Amino Acid | YQ | RQ | Eggs (FAO, 1970) [19] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | SE | 0 | 3 | 6 | SE | ||||
| Essential Amino Acids (EAAs) | |||||||||||
| NAAs | Threonine | 0.231 b | 0.257 a | 0.266 a | 0.011 | 0.220 c | 0.233 b | 0.240 a | 0.006 | 0.32 | |
| NAAs | Cystine | 0.182 a | 0.134 b | 0.125 b | 0.018 | 0.104 b | 0.133 b | 0.208 a | 0.031 | 0.152 | |
| HAAs | Valine | 0.309 b | 0.336 a | 0.340 a | 0.010 | 0.360 a | 0.329 b | 0.336 b | 0.009 | 0.428 | |
| HAAs | Isoleucine | 0.243 c | 0.257 b | 0.278 a | 0.010 | 0.232 c | 0.244 b | 0.267 a | 0.010 | 0.393 | |
| HAAs | Leucine | 0.413 b | 0.459 a | 0.470 a | 0.017 | 0.396 c | 0.430 b | 0.480 a | 0.024 | 0.551 | |
| HAAs | Phenylalanine | 0.276 b | 0.280 b | 0.312 a | 0.011 | 0.262 b | 0.271 b | 0.304 a | 0.013 | 0.358 | |
| HAAs | Methionine | 0.176 b | 0.179 a | 0.176 b | 0.001 | 0.152 b | 0.170 a | 0.171 a | 0.006 | ||
| BAAs | Lysine | 0.380 b | 0.392 a | 0.397 a | 0.005 | 0.341 c | 0.366 b | 0.379 a | 0.011 | 0.436 | |
| BAAs | Histidine | 0.198 b | 0.213 a | 0.187 c | 0.008 | 0.171 b | 0.196 a | 0.203 a | 0.010 | 0.152 | |
| Non-Essential Amino Acids (NEAAs) | |||||||||||
| AAAs | Aspartic acid | 0.551 c | 0.565 b | 0.578 a | 0.008 | 0.530 b | 0.600 a | 0.624 a | 0.028 | 0.601 | |
| AAAs | Glutamic acid | 0.904 b | 1.024 a | 1.060 a | 0.047 | 0.835 b | 0.944 a | 0.987 a | 0.045 | 0.796 | |
| NAAs | Serine | 0.237 b | 0.291 a | 0.306 a | 0.021 | 0.244 b | 0.255 a | 0.235 c | 0.006 | 0.478 | |
| NAAs | Tyrosine | 0.231 a | 0.168 b | 0.238 a | 0.022 | 0.238 b | 0.228 b | 0.294 a | 0.021 | 0.26 | |
| HAAs | Proline | 0.237 b | 0.246 a | 0.249 a | 0.004 | 0.220 c | 0.239 b | 0.251 a | 0.009 | 0.26 | |
| HAAs | Glycine | 0.347 a | 0.347 a | 0.334 a | 0.004 | 0.317 c | 0.329 b | 0.336 a | 0.006 | 0.207 | |
| HAAs | Alanine | 0.309 c | 0.330 b | 0.368 a | 0.017 | 0.311 b | 0.302 b | 0.352 a | 0.015 | 0.37 | |
| BAAs | Arginine | 0.562 a | 0.565 | 0.538 b | 0.009 | 0.500 b | 0.541 a | 0.539 a | 0.013 | 0.381 | |
| Total EAAs | 2.409 c | 2.507 b | 2.550 a | 0.042 | 2.238 c | 2.372 b | 2.589 a | 0.102 | 3.218 | ||
| Total NEAAs | 3.379 c | 3.536 b | 3.672 a | 0.085 | 3.195 c | 3.438 b | 3.619 a | 0.123 | 3.093 | ||
| Total amino acids | 5.787 c | 6.043 b | 6.222 a | 0.126 | 5.433 c | 5.810 b | 6.207 a | 0.224 | 6.311 | ||
| Items | TEAA g 16 N−1 | TNEAA g 16 N−1 | EAAs: NEAAs | EAAs: Protein | EAAs: Total AA | EAAI % |
|---|---|---|---|---|---|---|
| YQ-0 | 38.54 c | 54.06 c | 0.71 a | 0.39 b | 0.42 a | 77.84 b |
| YQ-3 | 40.11 b | 56.58 b | 0.71 a | 0.40 ab | 0.41 b | 77.85 b |
| YQ-6 | 40.80 a | 58.75 a | 0.69 b | 0.41 a | 0.41 b | 79.03 a |
| SE | 0.67 | 1.36 | 0.01 | 0.1 | 0.00 | 0.40 |
| RQ-0 | 35.80 c | 51.12 c | 0.70 b | 0.36 c | 0.41 b | 68.95 b |
| RQ-3 | 37.95 b | 55.01 b | 0.69 b | 0.38 b | 0.41 b | 75.41 a |
| RQ-6 | 41.42 a | 57.90 a | 0.72 a | 0.41 a | 0.42 a | 77.84 a |
| SE | 1.64 | 1.97 | 0.01 | 0.01 | 0.00 | 2.66 |
| Egg (FAO, 1970) [19] | 43.709 | 45.571 | 1.179 | 0.537 | 0.451 | 100.00 |
| Beef (FAO, 1970) [19] | 42.724 | 57.276 | 0.746 | 0.427 | 0.427 | 79.55 |
| Amino Acid | YQ | RQ | Eggs (FAO, 1970) [19] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | SE | 0 | 3 | 6 | SE | ||
| Threonine | 96.11 c | 106.87 b | 110.57 a | 4.34 | 91.14 c | 96.93 | 99.72 a | 2.53 | 110.42 |
| Valine | 128.15 b | 139.39 a | 141.16 a | 4.08 | 149.37 a | 136.58 b | 139.61 b | 3.86 | 147.69 |
| Isoleucine | 100.69 c | 106.87 b | 115.28 a | 4.23 | 96.20 c | 101.33 b | 110.80 a | 4.28 | 135.61 |
| Leucine | 171.62 b | 190.50 a | 195.27 a | 7.23 | 164.56 c | 178.43 b | 199.44 a | 10.15 | 190.13 |
| Phenylalanine | 114.42 b | 116.16 b | 129.40 a | 4.74 | 108.86 b | 112.35 b | 126.31 a | 5.34 | 123.53 |
| Lysine | 157.89 c | 162.62 b | 164.69 a | 2.01 | 141.77 b | 152.00 a | 157.34 a | 4.57 | 150.45 |
| Histidine | 82.38 b | 88.28 a | 77.64 c | 3.08 | 70.89 b | 81.51 a | 84.21 a | 4.07 | 52.45 |
| Cystine | 75.51 a | 55.76 b | 51.76 b | 7.35 | 43.04 b | 55.07 b | 86.42 a | 12.95 | 52.45 |
| Methionine | 73.23 c | 74.34 a | 72.93 b | 0.43 | 63.29 b | 70.49 a | 70.91 a | 2.48 | 72.46 |
| Amino Acid | YQ | RQ | Eggs (FAO, 1970) [19] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | SE | 0 | 3 | 6 | SE | ||
| Threonine | 92.59 b | 102.95 a | 106.53 a | 4.18 | 79.37 b | 97.00 a | 99.21 a | 6.28 | 40 |
| Valine | 98.77 b | 107.43 a | 108.79 a | 3.14 | 104.06 b | 109.35 a | 111.11 a | 2.12 | 50 |
| Isoleucine | 97.00 b | 102.95 b | 111.06 a | 4.08 | 83.77 c | 101.41 b | 110.23 a | 7.79 | 40 |
| Leucine | 94.48 b | 104.87 a | 107.50 a | 3.98 | 81.88 c | 102.04 b | 113.38 a | 9.22 | 70 |
| Phenylalanine | 91.86 b | 93.26 b | 103.88 a | 3.80 | 79.00 c | 93.69 b | 104.72 a | 7.46 | 35 |
| Lysine | 108.65 c | 111.91 b | 113.33 a | 1.39 | 88.18 b | 108.65 a | 111.80 a | 7.41 | 48 |
| Histidine | 151.17 b | 162.00 a | 142.47 c | 5.66 | 117.58 b | 155.37 a | 159.57 a | 13.37 | 55 |
| Cystine | 64.67 a | 47.75 b | 44.32 b | 6.30 | 33.31 c | 48.99 b | 76.43 a | 12.62 | 21 |
| Methionine | 62.71 b | 63.66 a | 62.46 b | 0.37 | 48.99 b | 62.71 a | 62.71 a | 4.58 | - |
| Cystine + methionine | 62.71 a | 62.71 a | 60.75 b | 0.65 | 48.99 b | 62.71 a | 62.71 a | 4.58 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, H.; Al-Qabba, M.M.; Algonaiman, R.; Radhi, K.S.; Almutairi, A.S.; Al Zhrani, M.M.; Mohamed, A. Impact of Sprouting Process on the Protein Quality of Yellow and Red Quinoa (Chenopodium quinoa). Molecules 2024, 29, 404. https://doi.org/10.3390/molecules29020404
Barakat H, Al-Qabba MM, Algonaiman R, Radhi KS, Almutairi AS, Al Zhrani MM, Mohamed A. Impact of Sprouting Process on the Protein Quality of Yellow and Red Quinoa (Chenopodium quinoa). Molecules. 2024; 29(2):404. https://doi.org/10.3390/molecules29020404
Chicago/Turabian StyleBarakat, Hassan, Maryam M. Al-Qabba, Raya Algonaiman, Khadija S. Radhi, Abdulkarim S. Almutairi, Muath M. Al Zhrani, and Ahmed Mohamed. 2024. "Impact of Sprouting Process on the Protein Quality of Yellow and Red Quinoa (Chenopodium quinoa)" Molecules 29, no. 2: 404. https://doi.org/10.3390/molecules29020404
APA StyleBarakat, H., Al-Qabba, M. M., Algonaiman, R., Radhi, K. S., Almutairi, A. S., Al Zhrani, M. M., & Mohamed, A. (2024). Impact of Sprouting Process on the Protein Quality of Yellow and Red Quinoa (Chenopodium quinoa). Molecules, 29(2), 404. https://doi.org/10.3390/molecules29020404








