Extraction, Chemical Composition and Insecticidal Activities of Lantana camara Linn. Leaf Essential Oils against Tribolium castaneum, Lasioderma serricorne and Callosobruchus chinensis
Abstract
1. Introduction
2. Results
2.1. Yield and Chemical Composition Analysis by GC-MS/MS
2.2. Contact Toxicity
2.3. Fumigant Toxicity
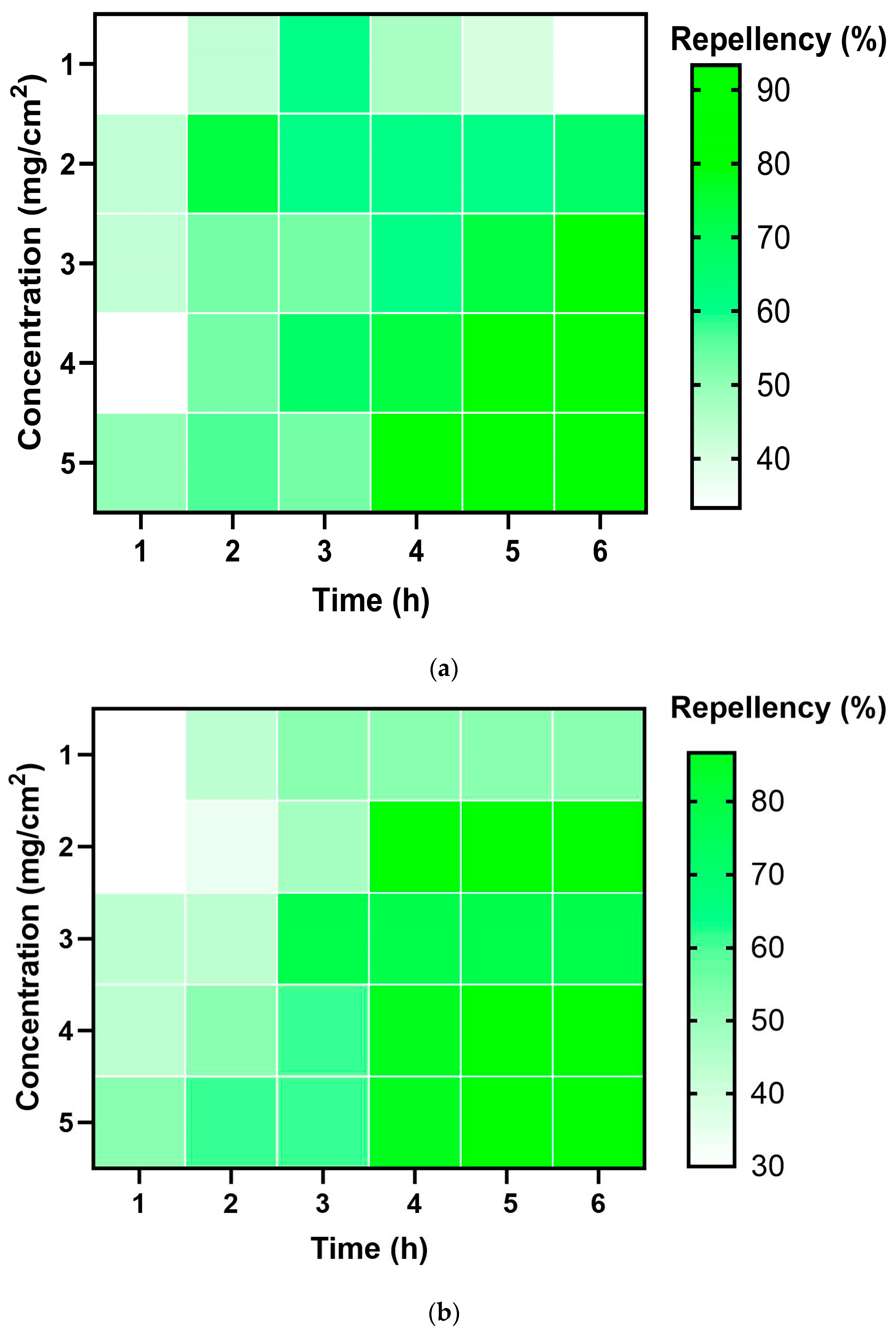
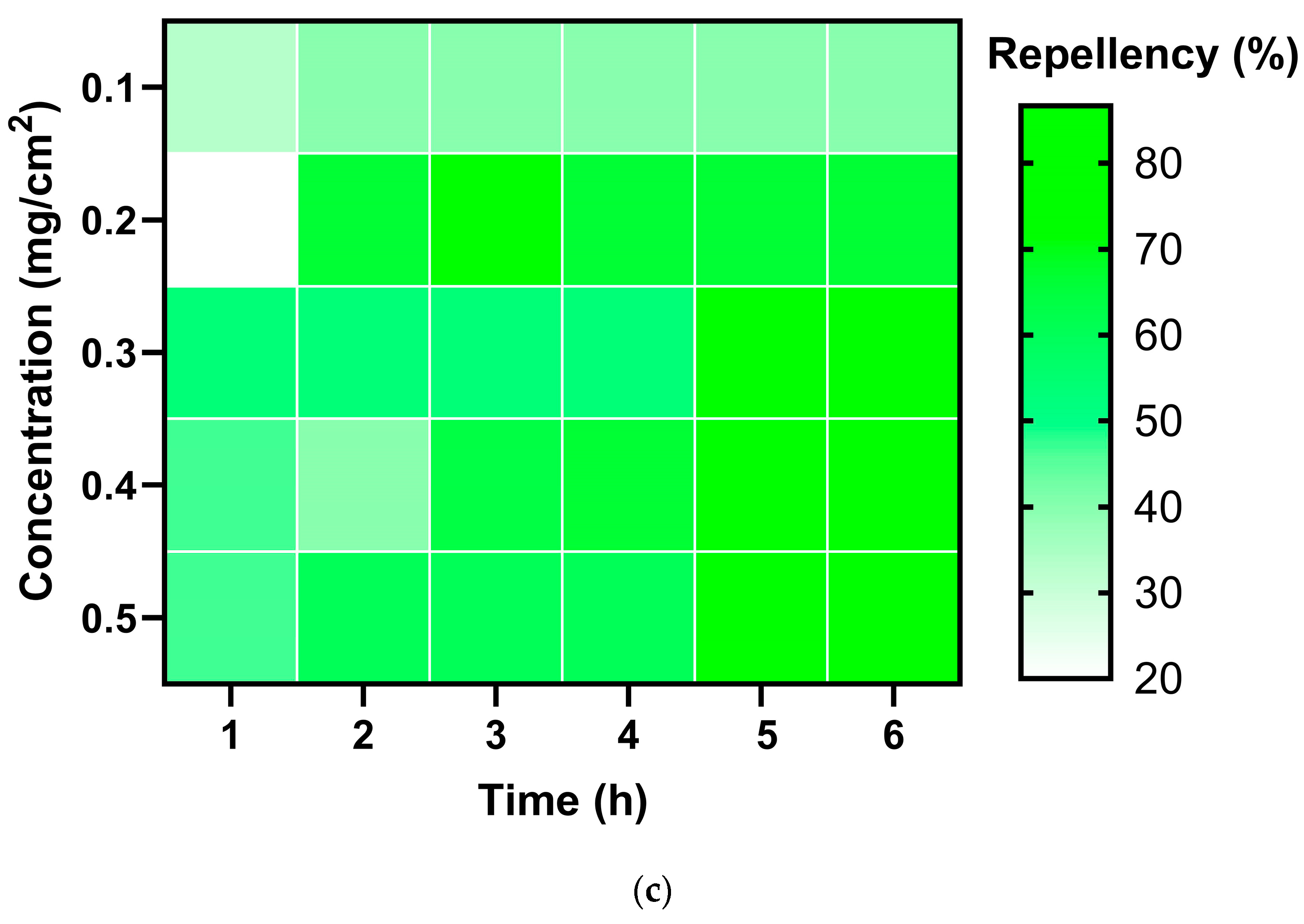
2.4. Repellent Activity
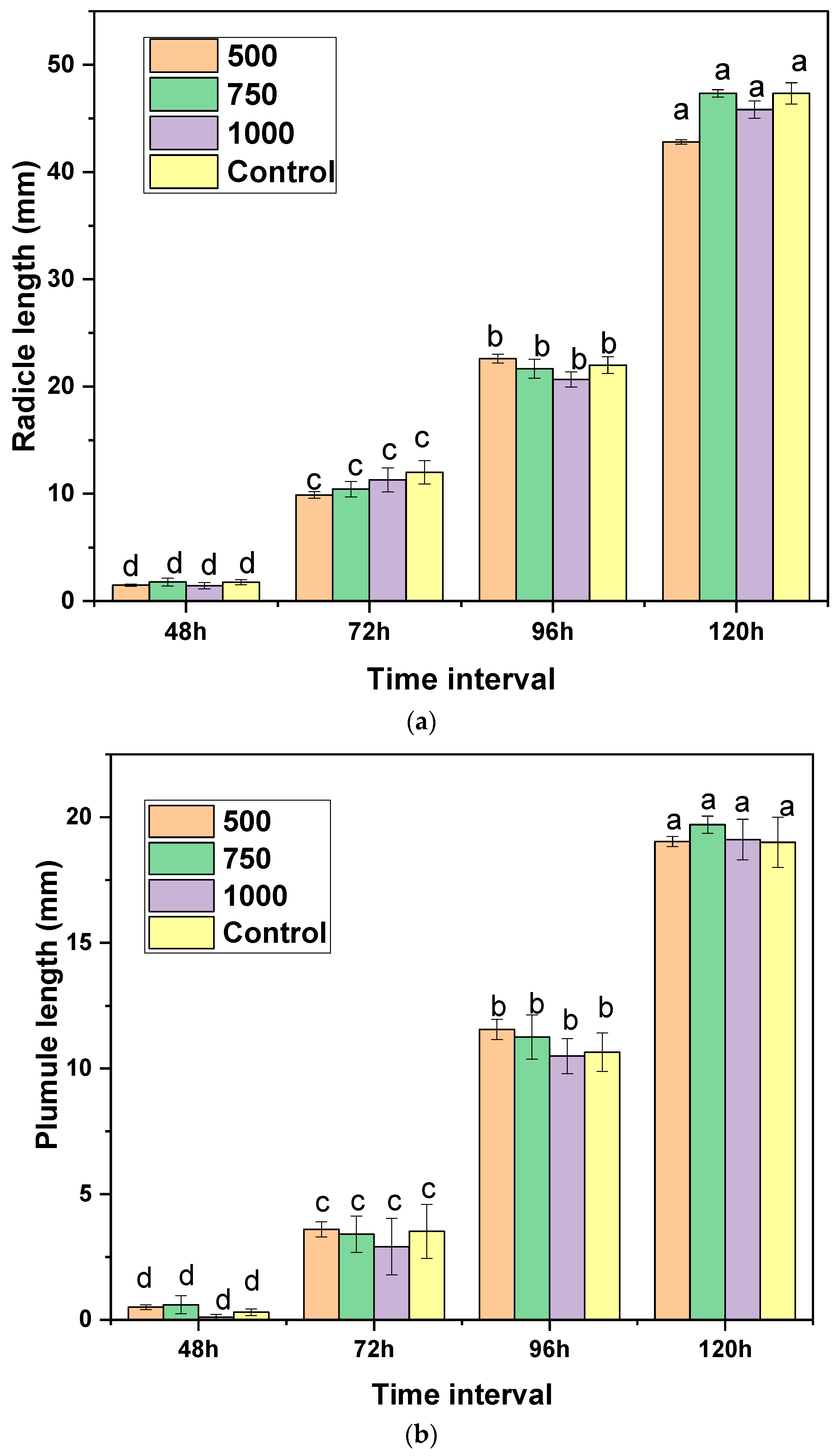
2.5. Phytotoxicity Study of Essential Oil on Grains
3. Discussion
4. Materials and Methods
4.1. Collection of Plant Material and Extraction of Essential Oil
4.2. Essential Oil Chemical Characterization Using GC-MS/MS
4.3. Test Insect Culture
4.4. Insecticidal Activities
4.4.1. Contact Toxicity
4.4.2. Fumigant Toxicity
4.4.3. Repellent Activity
4.5. Phytotoxicity Study on Grains
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, Z.; Zhang, J.E.; DiTommaso, A.; Wang, R.L.; Liang, K.M. Predicting the Potential Distribution of Lantana camara L. Under RCP Scenarios Using ISI-MIP Models. Clim. Change 2016, 134, 193–208. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Witt, A.B.R.; Nunda, W.; Richardson, D.M. Chromolaena odorata (Siam Weed) in Eastern Africa: Distribution and Socio-Ecological Impacts. Biol. Invasions 2016, 19, 1285–1298. [Google Scholar] [CrossRef]
- Negi, G.C.S.; Sharma, S.; Vishvakarma, S.C.R.; Samant, S.S.; Maikhuri, R.K.; Prasad, R.C.; Palni, L.M.S. Ecology and Use of Lantana camara in India. Bot. Rev. 2019, 85, 109–130. [Google Scholar] [CrossRef]
- Priyanka, N.; Joshi, P. A Review of Lantana camara Studies in India. Int. J. Sci. Res. Publ. 2013, 3, 42–52. [Google Scholar]
- Shankar, U.; Abrol, D.P. Integrated Pest Management in Stored Grains; CABI eBooks: Wallingford, UK, 2012; pp. 386–407. [Google Scholar] [CrossRef]
- Satya, S.; Kadian, N.; Kaushik, G.; Sharma, U. Impact of Chemical Pesticides for Stored Grain Protection on Environment and Human Health. Available online: https://www.semanticscholar.org/paper/Impact-of-chemical-pesticides-for-stored-grain-on-Satya-Kadian/846312a3e0a1055a0b6bb77fd2c387a24bd0e62b (accessed on 3 November 2023).
- Zoubiri, S.; Baaliouamer, A. Chemical Composition and Insecticidal Properties of Lantana camara L. Leaf Essential Oils from Algeria. J. Essent. Oil Res. 2012, 24, 377–383. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Visakh, N.U.; Sasidharan, A.; Pathrose, B.; Olatunji, O.J.; Al-Ansari, A.; Alfarhan, A.; Ramesh, V. Chemical Composition, Antioxidant, Anti-Bacterial, and Anti-Cancer Activities of Essential Oils Extracted from Citrus Limetta Risso Peel Waste Remains after Commercial Use. Molecules 2022, 27, 8329. [Google Scholar] [CrossRef] [PubMed]
- Albaqami, J.J.; Hamdi, H.; Narayanankutty, A.; Visakh, N.U.; Sasidharan, A.; Kuttithodi, A.M.; Famurewa, A.C.; Pathrose, B. Chemical Composition and Biological Activities of the Leaf Essential Oils of Curcuma longa, Curcuma aromatica and Curcuma angustifolia. Antibiotics 2022, 11, 1547. [Google Scholar] [CrossRef]
- Kuttithodi, A.M.; Narayanankutty, A.; Visakh, N.U.; Job, J.T.; Pathrose, B.; Olatunji, O.J.; Alfarhan, A.; Ramesh, V. Chemical Composition of the Cinnamomum Malabatrum Leaf Essential Oil and Analysis of Its Antioxidant, Enzyme Inhibitory and Antibacterial Activities. Antibiotics 2023, 12, 940. [Google Scholar] [CrossRef]
- Visakh, N.U.; Pathrose, B.; Narayanankutty, A.; Alfarhan, A.; Ramesh, V. Utilization of Pomelo (Citrus maxima) Peel Waste into Bioactive Essential Oils: Chemical Composition and Insecticidal Properties. Insects 2022, 13, 480. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Khan, G.S.; Mushtaq, Z.; Zubair, M. Essential Oils. In Essential Oil Research; Springer: Cham, Switzerland, 2019; pp. 3–17. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Russo, A.; Palmeri, V.; Zappalà, L. Essential Oils in Stored Product Insect Pest Control. J. Food Qual. 2018, 2018, 6906105. [Google Scholar] [CrossRef]
- Campbell, J.F.; Athanassiou, C.G.; Hagstrum, D.W.; Zhu, K.Y. Tribolium castaneum: A Model Insect for Fundamental and Applied Research. Annu. Rev. Entomol. 2022, 67, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Edde, P.A. Biology, Ecology, and Control of Lasioderma serricorne (F.) (Coleoptera: Anobiidae): A Review. J. Econ. Entomol. 2019, 112, 1011–1031. [Google Scholar] [CrossRef] [PubMed]
- Ramazeame, L.; Adiroubane, D.; Govindan, K.; Jagatheeswari, J. Management of Pulse Beetle, Callosobruchus chinensis Linn. Using Botanicals. Available online: https://www.entomoljournal.com/archives/2014/vol2issue4/PartG/34.pdf (accessed on 10 December 2023).
- Sousa, E.O.; Costa, J.G.M. Genus Lantana: Chemical Aspects and Biological Activities. Rev. Bras. Farmacogn. 2012, 22, 1115–1180. [Google Scholar] [CrossRef]
- Zandi-Sohani, N.; Hojjati, M.; Carbonell-Barrachina, Á.A. Bioactivity of Lantana camara L. Essential Oil against Callosobruchus maculatus (Fabricius). Chil. J. Agric. Res. 2012, 72, 502–506. [Google Scholar] [CrossRef]
- Bouda, H.; Tapondjou, L.A.; Fontem, D.A.; Gumedzoe, M.Y.D. Effect of Essential Oils from Leaves of Ageratum Conyzoides, Lantana camara and Chromolaena odorata on the Mortality of Sitophilus zeamais (Coleoptera, Curculionidae). J. Stored Prod. Res. 2001, 37, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Alexander, A.; Saraf, S.; Saraf, S.; Vishwakarma, U.K.; Nakhate, K.T. Ajazuddin Mosquito Repellent and Larvicidal Perspectives of Weeds Lantana camara L. and Ocimum gratissimum L. Found in Central India. Biocatal. Agric. Biotechnol. 2021, 34, 102040. [Google Scholar] [CrossRef]
- Misra, L.; Saikia, A.K. Chemotypic Variation in Indian Lantana camara Essential Oil. J. Essent. Oil Res. 2011, 23, 1–5. [Google Scholar] [CrossRef]
- Barros, L.M.; Duarte, A.E.; Morais-Braga, M.F.B.; Waczuk, E.P.; Vega, C.; Leite, N.F.; De Menezes, I.R.A.; Coutinho, H.D.M.; Rocha, J.B.T.; Kamdem, J.P. Chemical Characterization and Trypanocidal, Leishmanicidal and Cytotoxicity Potential of Lantana camara L. (Verbenaceae) Essential Oil. Molecules 2016, 21, 209. [Google Scholar] [CrossRef]
- Khan, M.; Mahmood, A.; Alkhathlan, H.Z. Characterization of Leaves and Flowers Volatile Constituents of Lantana camara Growing in Central Region of Saudi Arabia. Arab. J. Chem. 2016, 9, 764–774. [Google Scholar] [CrossRef]
- Nurby Ríos Tesch; Mora, F.; Rojas, L.; Díaz, T.; Velasco, J.; Yánez, C.; Rios, N.; Carmona, J.; Pasquale, S.A. Chemical Composition and Antibacterial Activity of the Essential Oil of Lantana camara Var. Moritziana. Nat. Prod. Commun. 2011, 6, 1934578X1100600727. [Google Scholar] [CrossRef]
- Oyedeji, O.A.; Ekundayo, O.; König, W.A. Volatile Leaf Oil Constituents of Lantana camara L. from Nigeria. Flavour Fragr. J. 2003, 18, 384–386. [Google Scholar] [CrossRef]
- Liambila, N.R.; Wesonga, J.M.; Ngamau, C.N.; Waudo, W. Pesticidal properties of essential oils of Lantana camara L. African J. Hortic. Sci. 2021, 17, 101–114. [Google Scholar]
- de Sena Filho, J.G.; Rabbani, A.R.C.; dos Santos Silva, T.R.; da Silva, A.V.C.; Souza, I.A.; Santos, M.J.B.A.; de Jesus, J.R.; de Lima Nogueira, P.C.; Duringer, J.M. Chemical and Molecular Characterization of Fifteen Species from the Lantana (Verbenaceae) Genus. Biochem. Syst. Ecol. 2012, 45, 130. [Google Scholar] [CrossRef]
- Visakh, N.U.; Pathrose, B.; Chellappan, M.; Ranjith, M.T.; Sindhu, P.V.; Mathew, D. Extraction and Chemical Characterisation of Agro-Waste from Turmeric Leaves as a Source of Bioactive Essential Oils with Insecticidal and Antioxidant Activities. Waste Manag. 2023, 169, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rajashekar, Y.; Ravindra, K.V.; Bakthavatsalam, N. Leaves of Lantana camara Linn. (Verbenaceae) as a Potential Insecticide for the Management of Three Species of Stored Grain Insect Pests. J. Food Sci. Technol. 2012, 51, 3494–3499. [Google Scholar] [CrossRef]
- Rajashekar, Y.; Raghavendra, A.; Bakthavatsalam, N. Acetylcholinesterase Inhibition by Biofumigant (Coumaran) from Leaves of Lantana camara in Stored Grain and Household Insect Pests. BioMed Res. Int. 2014, 2014, 187019. [Google Scholar] [CrossRef]
- Du, S.-S.; Yang, K.; Wang, C.; You, C.; Geng, Z.; Guo, S.-S.; Deng, Z.; Liu, Z. Chemical Constituents and Activities of the Essential Oil from Myristica fragrans against Cigarette Beetle Lasioderma serricorne. Chem. Biodivers. 2014, 11, 1449–1456. [Google Scholar] [CrossRef]
- Murugesan, S.; Senthilkumar, N.; Babu, D.; Rajasugunasekar, D.R. Chemical Constituents and Toxicity Assessment of the Leaf Oil of Lantana camara Linn from Tamilnadu Regions. Asian J. Plant Sci. Res. 2016, 6, 32–42. [Google Scholar]
- Liambila, R.; Wesonga, J.; Ngamau, C.; Waudo, W. Chemical Composition and Bioactivity of Lantana camara L. Essential Oils from Diverse Climatic Zones of Kenya against Leaf Miner (Tuta absoluta Meyrick). Afr. J. Agric. Res. 2021, 17, 1198–1208. [Google Scholar] [CrossRef]
- Chaubey, M. Terpenes in Maize Weevil Management Insecticidal Property of Terpenes against Maize Weevil, Sitophilus Zeamais (Motschulsky). J. Biopestic. 2022, 15, 92–102. [Google Scholar] [CrossRef]
- Esther, O.O.; Robyn, M.; Kim, P.T.; Nelson, N.N. Essential Oil Composition of Different Fractions of Piper Guineense Schumach. Et Thonn from Cameroon Using Gas Chromatography-Mass Spectrometry and Their Insecticidal Effect on Sitophilus oryzae (L.). Afr. J. Biotechnol. 2015, 14, 2662–2671. [Google Scholar] [CrossRef]
- Mahmoudvand, M.; Abbasipour, H.; Basij, M.; Hossein Hosseinpour, M.; Rastegar, F.; Bagher Nasiri, M. Fumigant Toxicity of Some Essential Oils on Adults of Some Stored-Product Pests. Chil. J. Agric. Res. 2011, 71, 83–89. [Google Scholar] [CrossRef]
- Salem, N.; Bachrouch, O.; Sriti, J.; Msaada, K.; Khammassi, S.; Hammami, M.; Selmi, S.; Boushih, E.; Koorani, S.; Abderraba, M.; et al. Fumigant and Repellent Potentials of Ricinus communis and Mentha pulegium Essential Oils against Tribolium castaneum Lasioderma serricorne. Int. J. Food Prop. 2017, 20, S2899–S2913. [Google Scholar] [CrossRef]
- Lee, B.-H.; Annis, P.C.; Tumaalii, F.; Choi, W.-S. Fumigant toxicity of essential oils from the Myrtaceae family and 1,8-cineole against 3 major stored-grain insects. J. Stored Prod. Res. 2004, 40, 553–564. [Google Scholar] [CrossRef]
- Lee, B.-H.; Lee, S.-E.; Annis, P.C.; Pratt, S.J.; Park, B.-S.; Tumaalii, F. Fumigant Toxicity of Essential Oils and Monoterpenes against the Red Flour Beetle, Tribolium castaneum Herbst. J. Asia-Pac. Entomol. 2002, 5, 237–240. [Google Scholar] [CrossRef]
- Visakh, N.U.; Pathrose, B.; Narayanankutty, A. Characterization of Secondary Metabolites from the Leaves of Curry Leaf (Murraya koenigii L.) Essential Oils with Insecticidal Activities against Stored Product Insects. Biocatal. Agric. Biotechnol. 2023, 54, 102973. [Google Scholar] [CrossRef]
- Visakh, N.U.; Pathrose, B.; Chellappan, M.; Ranjith, M.T.; Sindhu, P.V.; Mathew, D. Chemical Characterisation, Insecticidal and Antioxidant Activities of Essential Oils from Four citrus spp. Fruit Peel Waste. Food Biosci. 2022, 50, 102163. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Stashenko, E.E. Repellent Activity of Essential Oils and Some of Their Individual Constituents against Tribolium castaneum Herbst. J. Agric. Food Chem. 2011, 59, 1690–1696. [Google Scholar] [CrossRef]
- Ma, S.; Jia, R.; Guo, M.; Qin, K.; Zhang, L. Insecticidal Activity of Essential Oil from Cephalotaxus sinensis and Its Main Components against Various Agricultural Pests. Ind. Crops Prod. 2020, 150, 112403. [Google Scholar] [CrossRef]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Phytotoxic Effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus Essential Oils in Weeds of Mediterranean Summer Crops. Biochem. Syst. Ecol. 2009, 37, 362–369. [Google Scholar] [CrossRef]
- Jaya; Singh, P.; Prakash, B.; Dubey, N.K. Insecticidal Activity of Ageratum conyzoides L., Coleus aromaticus Benth. and Hyptis suaveolens (L.) Poit Essential Oils as Fumigant against Storage Grain Insect Tribolium castaneum Herbst. J. Food Sci. Technol. 2012, 51, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. Available online: https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1421994 (accessed on 4 November 2023). [CrossRef]
- Debbabi, H.; El Mokni, R.; Nardoni, S.; Chaieb, I.; Maggi, F.; Nzekoue, F.K.; Caprioli, G.; Hammami, S. Chemical Diversity and Biological Activities of Essential Oils from Native Populations of Clinopodium menthifolium Subsp. Ascendens (Jord.) Govaerts. Environ. Sci. Pollut. Res. Int. 2021, 28, 13624–13633. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Parsad, R.; Joseph, B.; Adarsh, V.S. GrapesAgri1: Collection of Shiny Apps for Data Analysis in Agriculture. J. Open Source Softw. 2021, 6, 3437. [Google Scholar] [CrossRef]
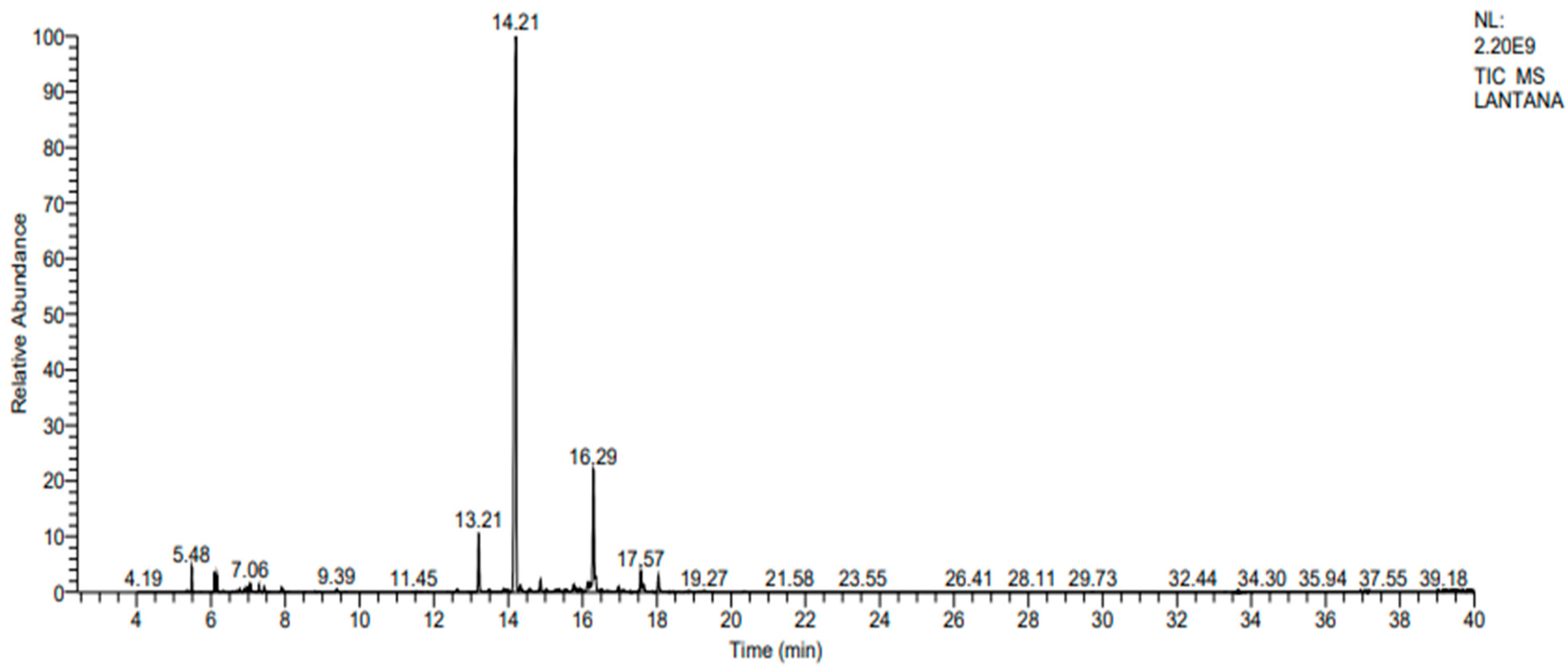
| Peak No. | RT a | Compounds | RSI b | %RA c |
|---|---|---|---|---|
| 1. | 5.48 | α-Pinene | 945 | 1.13 |
| 2. | 6.09 | α-Phellandrene | 919 | 0.91 |
| 3. | 6.97 | Limonene | 892 | 0.35 |
| 4. | 7.06 | α-Ocimene | 917 | 0.53 |
| 5. | 7.29 | 2,7-Dimethyl-2,6-octadien-4-ol | 864 | 0.33 |
| 6. | 7.43 | γ-Terpinene | 910 | 0.32 |
| 7. | 7.90 | (+)-4-Carene | 897 | 0.30 |
| 8. | 9.39 | Terpinen-4-ol | 895 | 0.25 |
| 9. | 12.63 | α-Cubebene | 910 | 0.21 |
| 10. | 13.21 | α-Copaene | 911 | 4.11 |
| 11. | 13.87 | Isocaryophillene | 905 | 0.24 |
| 12. | 14.21 | Caryophyllene | 961 | 69.96 |
| 13. | 14.33 | α-ylangene | 848 | 0.61 |
| 14. | 14.58 | Benzene, 1-(1,5-dimethylhexyl)-4-methyl- | 840 | 0.27 |
| 15. | 14.87 | Humulene | 909 | 0.88 |
| 16. | 15.30 | γ-Muurolene | 881 | 0.24 |
| 17. | 15.76 | Guaia-1(10),11-diene | 895 | 0.91 |
| 18. | 15.93 | Davana ether | 786 | 0.37 |
| 19. | 16.15 | Isoaromadendrene epoxide | 815 | 0.97 |
| 20. | 16.29 | Isoledene | 819 | 12.00 |
| 21. | 16.97 | Aromadendrene oxide-(2) | 817 | 0.42 |
| 22. | 17.57 | Lilac alcohol B, Lilac alcohol D | 809 | 1.80 |
| 23. | 17.65 | Caryophyllene oxide | 914 | 0.73 |
| 24. | 18.04 | Hexanamide, N-(2,6-dimethyl phenyl)- | 875 | 1.37 |
| Insects | Exposure Time (h) | LC50 a (mg/cm2) | LC90 a (mg/cm2) | Slope ± SEM b | χ2 (df) |
|---|---|---|---|---|---|
| T. castaneum | 24 | 8.93 (8.06–9.81) | 13.54 (11.91–17.13) | 0.35 ± 0.18 | 0.40 |
| 48 | 7.92 (7.21–8.61) | 10.47 (9.58–11.90) | 1.07 ± 0.22 | 0.04 | |
| L. serricorne | 24 | 4.82 (3.91–6.32) | 17.47 (11.41–40.31) | 0.73 ± 0.23 | 0.16 |
| 48 | 3.41 (2.62–4.44) | 15.57 (10.01–21.04) | 0.91 ± 0.22 | 0.42 | |
| C. chinensis | 24 | 1.83 (1.21–2.51) | 6.27 (4.59–9.79) | 1.77 ± 0.28 | 0.29 |
| 48 | 0.45 (0.21–0.68) | 5.06 (2.68–8.58) | 1.64 ± 1.22 | 0.62 |
| Insects | Exposure Time (h) | LC50 a (mg/L Air) | LC90 a (mg/L Air) | Slope ± SEM b | χ2 (df) |
|---|---|---|---|---|---|
| T. castaneum | 24 | 16.70 (15.75–18.08) | 23.21 (20.67–29.06) | −10.95 ± 1.87 | 1.98 |
| 48 | 14.47 (13.51–15.45) | 20.97 (18.85–25.67) | −9.23 ± 1.63 | 2.44 | |
| L. serricorne | 24 | 4.14 (3.03–4.98) | 10.91 (8.29–20.94) | −1.87 ± 0.52 | 0.76 |
| 48 | 2.53 (0.82–3.61) | 8.62 (6.53–16.34) | −0.97 ± 0.54 | 0.04 | |
| C. chinensis | 24 | 6.24 (4.87–9.43) | 27.36 (15.05–128.03) | −1.58 ± 0.32 | 1.46 |
| 48 | 3.07 (1.77–5.25) | 25.85 (11.11–426.17) | 0.58 ± 0.18 | 3.88 |
| Insect | Dosage (mg/cm2) | Mean Repellency (%) | Class |
|---|---|---|---|
| T. castaneum | 1 | 42.78 ± 9.98 a | III |
| 2 | 62.22 ± 6.88 a | IV | |
| 3 | 63.33 ± 13.82 a | IV | |
| 4 | 64.44 ± 18.21 a | IV | |
| 5 | 65.56 ± 21.67 a | IV | |
| L. serricorne | 1 | 37.78 ± 18.69 a | II |
| 2 | 56.10 ± 33.56 a | III | |
| 3 | 57.78± 13.77 a | III | |
| 4 | 62.22 ± 17.72 a | IV | |
| 5 | 64.44 ± 15.0 a | IV | |
| C. chinensis | 0.1 | 38.89 ± 2.72 b | II |
| 0.2 | 61.11 ± 20.83 ab | IV | |
| 0.3 | 61.67 ± 12.95 ab | IV | |
| 0.4 | 62.22 ± 16.28 ab | IV | |
| 0.5 | 64.44 ± 13.77 a | IV |
| Seed Germination (%) of Treatments after | ||||
|---|---|---|---|---|
| Concentration (µg/mL) | 48 h | 72 h | 96 h | 120 h |
| 500 | 90.0 ± 5.74 a | 96.67 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| 750 | 90.0 ± 10.0 a | 93.33 ± 5.74 a | 96.67 ± 5.74 a | 96.78 ± 5.74 a |
| 1000 | 96.67 ± 5.74 a | 96.67.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| b Control | 93.33 ± 5.74 a | 96.67 ± 5.74 a | 96.67 ± 5.74 a | 96.67 ± 5.74 a |
| F value | 0.61 | 1.83 | 1.58 | 0.93 |
| p value | 0.62 | 0.21 | 0.24 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aisha, K.; Visakh, N.U.; Pathrose, B.; Mori, N.; Baeshen, R.S.; Shawer, R. Extraction, Chemical Composition and Insecticidal Activities of Lantana camara Linn. Leaf Essential Oils against Tribolium castaneum, Lasioderma serricorne and Callosobruchus chinensis. Molecules 2024, 29, 344. https://doi.org/10.3390/molecules29020344
Aisha K, Visakh NU, Pathrose B, Mori N, Baeshen RS, Shawer R. Extraction, Chemical Composition and Insecticidal Activities of Lantana camara Linn. Leaf Essential Oils against Tribolium castaneum, Lasioderma serricorne and Callosobruchus chinensis. Molecules. 2024; 29(2):344. https://doi.org/10.3390/molecules29020344
Chicago/Turabian StyleAisha, Kolapparamban, Naduvilthara U. Visakh, Berin Pathrose, Nicola Mori, Rowida S. Baeshen, and Rady Shawer. 2024. "Extraction, Chemical Composition and Insecticidal Activities of Lantana camara Linn. Leaf Essential Oils against Tribolium castaneum, Lasioderma serricorne and Callosobruchus chinensis" Molecules 29, no. 2: 344. https://doi.org/10.3390/molecules29020344
APA StyleAisha, K., Visakh, N. U., Pathrose, B., Mori, N., Baeshen, R. S., & Shawer, R. (2024). Extraction, Chemical Composition and Insecticidal Activities of Lantana camara Linn. Leaf Essential Oils against Tribolium castaneum, Lasioderma serricorne and Callosobruchus chinensis. Molecules, 29(2), 344. https://doi.org/10.3390/molecules29020344









