The Effect of Peppermint and Thyme Oils on Stabilizing the Fatty Acid Profile of Sunflower Oil
Abstract
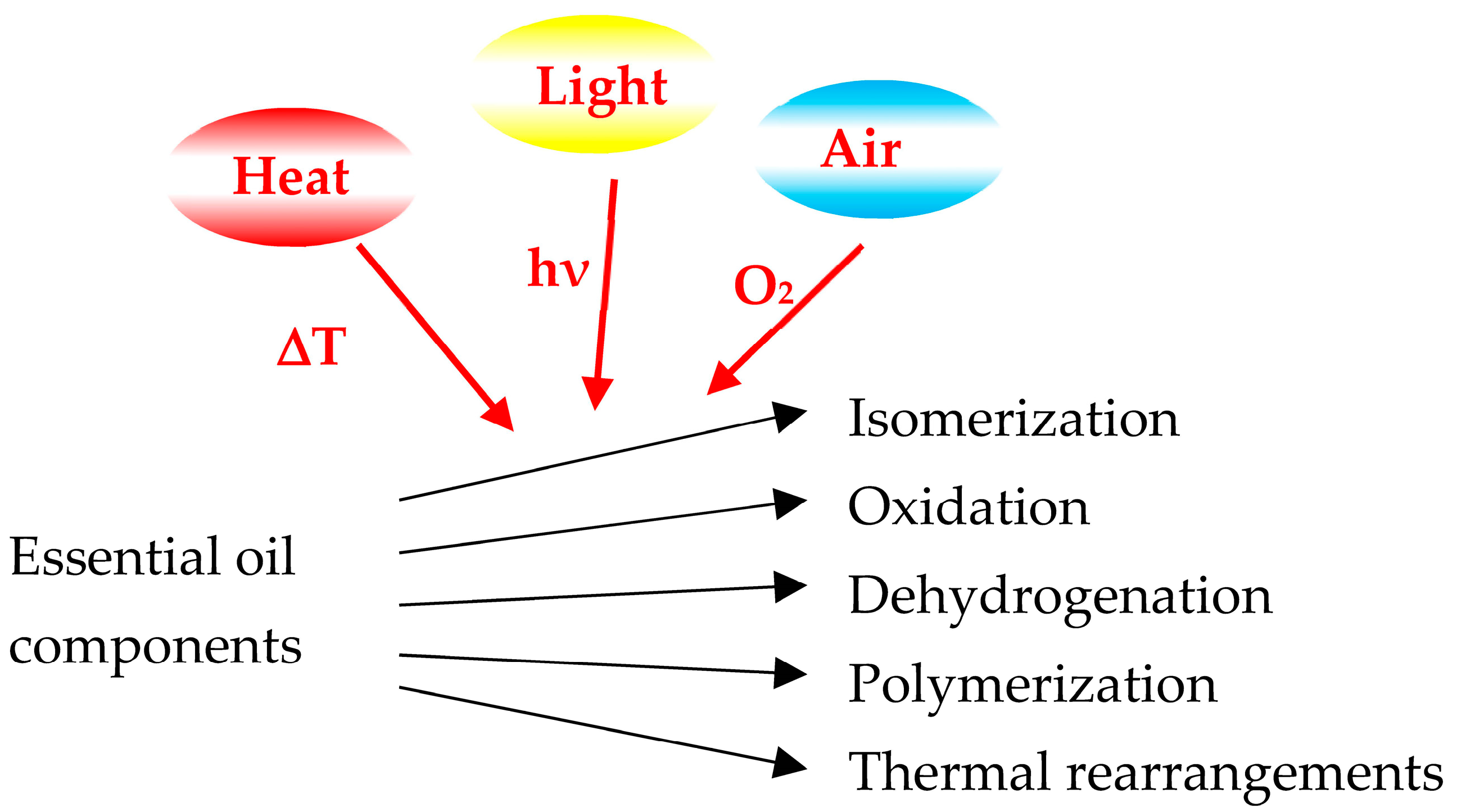
1. Introduction
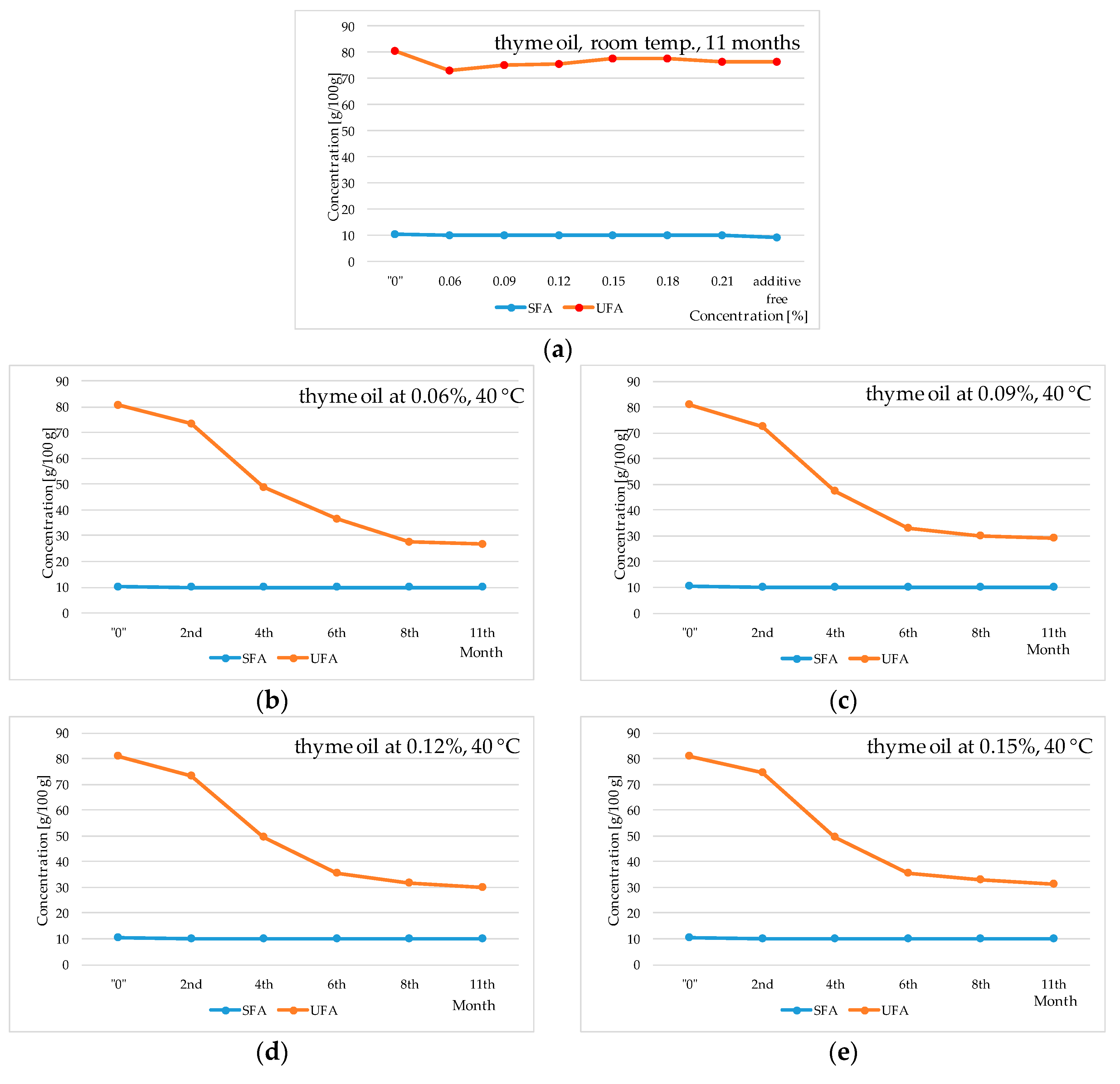
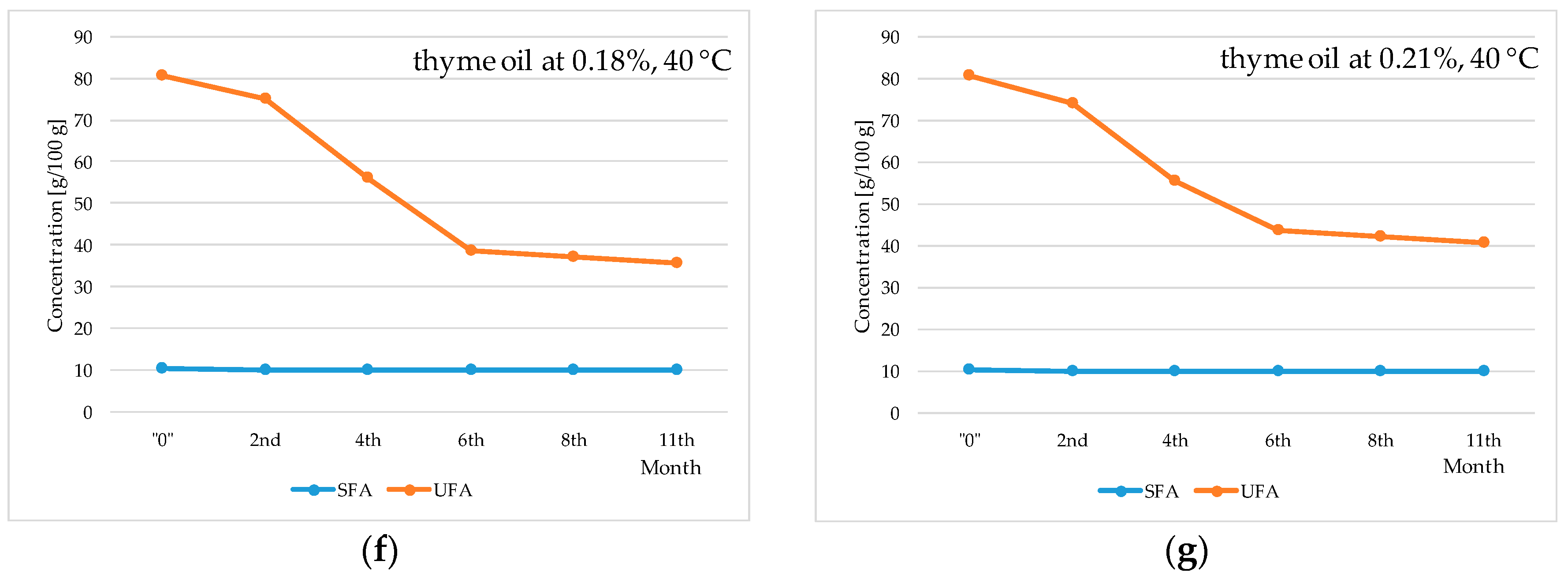
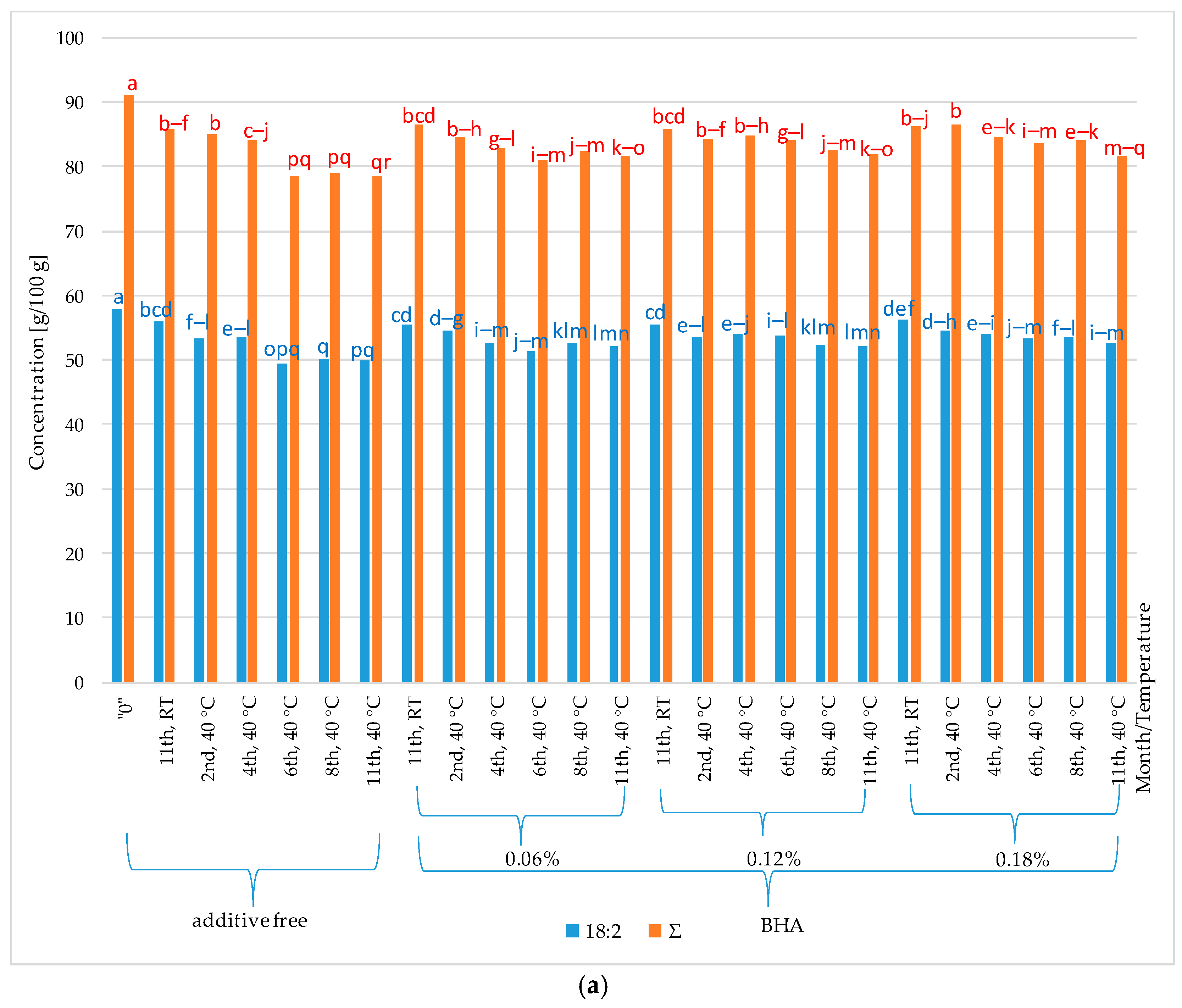
2. Results and Discussion
3. Materials and Methods
3.1. Experimental Material
- -
- Sunflower oil (ZT Bodaczów, Poland) purchased at a supermarket in Lublin. The justification for choosing sunflower oil in the experiment was the fact that fats with a significant content of polyunsaturated acids, such as sunflower or soybean oil, are more susceptible to oxidation.
- -
- Peppermint and thyme oils obtained from peppermint leaves (Mentha × piperita L.) and common thyme herb (Thymus vulgaris L.) produced by Dary Natury (Koryciny, Poland). The essential oil was distilled in accordance with the procedure described in the Polish Pharmacopoeia VIII (2008) [49].
3.2. Addition of Antioxidant (BHA) and Peppermint and Thyme Oils
- BHA: 0.06%, 0.12%, 0.18%;
- Peppermint and thyme oils: 0.06%, 0.09%, 0.12%, 0.15%, 0.18%, 0.21%.
3.3. Storage of Samples and Materials for Analyses
3.4. Determination of Fatty Acids
3.5. Determination of Essential Oils’ Chemical Composition
GC Analysis
- GC/MS
- GC/FID
3.6. Determination of Properties of Peppermint and Thyme Oils Inhibiting Changes in Fatty Acid Composition Compared to BHA
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Billingsley, H.; Carbone, S.; Lavie, C. Dietary Fats and Chronic Noncommunicable Diseases. Nutrients 2018, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- Botella-Martínez, C.; Gea-Quesada, A.; Sayas-Barberá, E.; Pérez-Álvarez, J.Á.; Fernández-López, J.; Viuda-Martos, M. Improving the Lipid Profile of Beef Burgers Added with Chia Oil (Salvia hispanica L.) or Hemp Oil (Cannabis sativa L.) Gelled Emulsions as Partial Animal Fat Replacers. LWT 2022, 161, 113416. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef] [PubMed]
- Temkov, M.; Mureșan, V. Tailoring the Structure of Lipids, Oleogels and Fat Replacers by Different Approaches for Solving the Trans-Fat Issue—A Review. Foods 2021, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Löwe, J.; Gröger, H. Fatty Acid Hydratases: Versatile Catalysts to Access Hydroxy Fatty Acids in Efficient Syntheses of Industrial Interest. Catalysts 2020, 10, 287. [Google Scholar] [CrossRef]
- Kowalski, R.; Kowalska, G.; Pankiewicz, U.; Mazurek, A.; Włodarczyk-Stasiak, M.; Sujka, M.; Wyrostek, J. The Effect of an Addition of Marjoram Oil on Stabilization Fatty Acids Profile of Rapeseed Oil. LWT 2019, 109, 225–232. [Google Scholar] [CrossRef]
- Tomaino, A.; Cimino, F.; Zimbalatti, V.; Venuti, V.; Sulfaro, V.; De Pasquale, A.; Saija, A. Influence of Heating on Antioxidant Activity and the Chemical Composition of Some Spice Essential Oils. Food Chem. 2005, 89, 549–554. [Google Scholar] [CrossRef]
- Farag, R.S.; Badei, A.Z.M.A.; Hewedi, F.M.; El-Baroty, G.S.A. Antioxidant Activity of Some Spice Essential Oils on Linoleic Acid Oxidation in Aqueous Media. J. Am. Oil Chem. Soc. 1989, 66, 792–799. [Google Scholar] [CrossRef]
- Moldão-Martins, M.; Beirão-da-Costa, S.; Neves, C.; Cavaleiro, C.; Salgueiro, L.; Luísa Beirão-da-Costa, M. Olive Oil Flavoured by the Essential Oils of Mentha × Piperita and Thymus mastichina L. Food Qual. Prefer. 2004, 15, 447–452. [Google Scholar] [CrossRef]
- Kowalski, R.; Kowalska, G.; Pankiewicz, U.; Mazurek, A.; Sujka, M.; Włodarczyk-Stasiak, M.; Kałwa, K. Effect of the Method of Rapeseed Oil Aromatisation with Rosemary Rosmarinus officinalis L. on the Content of Volatile Fraction. LWT 2018, 95, 40–46. [Google Scholar] [CrossRef]
- Kowalski, R.; Kowalska, G.; Pankiewicz, U.; Włodarczyk-Stasiak, M.; Sujka, M.; Mazurek, A. Effect of Rapeseed Oil Aromatisation with Marjoram on the Content of Volatile Fraction and Antioxidant Properties. J. Food Sci. Technol. 2020, 57, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Abbas, T.; Ali, T.M.; Hasnain, A. Effect of Storage on Oxidation Stability of Essential Oils Derived from Culinary Herbs and Spices. J. Food Meas. Charact. 2018, 12, 877–883. [Google Scholar] [CrossRef]
- Popa, C.L.; Lupitu, A.; Mot, M.D.; Copolovici, L.; Moisa, C.; Copolovici, D.M. Chemical and Biochemical Characterization of Essential Oils and Their Corresponding Hydrolats from Six Species of the Lamiaceae Family. Plants 2021, 10, 2489. [Google Scholar] [CrossRef] [PubMed]
- Morkeliūnė, A.; Rasiukevičiūtė, N.; Šernaitė, L.; Valiuškaitė, A. The Use of Essential Oils from Thyme, Sage and Peppermint against Colletotrichum acutatum. Plants 2021, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Malik, A. Antimicrobial Potential and Chemical Composition of Mentha piperita Oil in Liquid and Vapour Phase against Food Spoiling Microorganisms. Food Control 2011, 22, 1707–1714. [Google Scholar] [CrossRef]
- Pelter, L.S.W.; Amico, A.; Gordon, N.; Martin, C.; Sandifer, D.; Pelter, M.W. Analysis of Peppermint Leaf and Spearmint Leaf Extracts by Thin-Layer Chromatography. J. Chem. Educ. 2008, 85, 133. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A Review of the Bioactivity and Potential Health Benefits of Peppermint Tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Najda, A. Skład Chemiczny i Działanie Przeciwutleniające Ekstraktów z Mentha × Piperita L. Postępy Fitoter. 2017, 18, 251–258. [Google Scholar] [CrossRef]
- Zawiślak, G. Analysis of Chemical Composition of Essential Oil in the Herb of Thyme (Thymus vulgaris L.) Grown in South-Eastern Poland. Herba Pol. 2007, 53, 241–245. [Google Scholar]
- Wesolowska, A.; Grzeszczuk, M.; Jadczak, D. Comparison of the Chemical Composition of Essential Oils Isolated by Water-Steam Distillation and Hydrodistillation from Garden Thyme (Thymus vulgaris L.). J. Essent. Oil Bear. Plants 2016, 19, 832–842. [Google Scholar] [CrossRef]
- Raal, A.; Arak, E.; Orav, A. Comparative Chemical Composition of the Essential Oil of Thymus vulgaris L. from Different Geographical Sources. Herba Pol. 2005, 51, 10–17. [Google Scholar]
- Yildiz, S.; Dilmen, S.; Turan, S.; Kiralan, M.; Ramadan, M.F. Effects of Natural Phenolics and Synthetic Antioxidants on the Oxidative Thermal Stability of Refined and Purified Sunflower Oils. Riv. Ital. Delle Sostanze Grasse 2021, 98, 93–104. [Google Scholar]
- Yildiz, S.; Turan, S.; Kiralan, M.; Ramadan, M.F. Antioxidant Properties of Thymol, Carvacrol, and Thymoquinone and Its Efficiencies on the Stabilization of Refined and Stripped Corn Oils. J. Food Meas. Charact. 2021, 15, 621–632. [Google Scholar] [CrossRef]
- Kowalski, R. Changes of Linoleic Acid Concentration during Heating of Some Plant-Origin Oils with Polyphenol Addition. J. Food Qual. 2010, 33, 269–282. [Google Scholar] [CrossRef]
- Kowalski, R. GC Analysis of Changes in the Fatty Acid Composition of Sunflower and Olive Oils Heated with Quercetin, Caffeic Acid, Protocatechuic Acid, and Butylated Hydroxyanisole. Acta Chromatogr. 2007, 18, 15–23. [Google Scholar]
- Kowalski, R. Silphium L. Extracts—Composition and Protective Effect on Fatty Acids Content in Sunflower Oil Subjected to Heating and Storage. Food Chem. 2009, 112, 820–830. [Google Scholar] [CrossRef]
- Milczarek, A.; Osek, M. Zmiany Liczby Kwasowej i Nadtlenkowej Tłuszczu Produktów Rzepakowych Przechowywanych w Różnych Warunkach Bez i z Dodatkiem Przeciwutleniacza. Rośliny Oleiste—Oilseed Crops 2013, 33, 295–306. [Google Scholar] [CrossRef]
- PN-EN ISO 12966-2:2011; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2011.
- Besbes, S.; Blecker, C.; Deroanne, C.; Lognay, G.; Drira, N.-E.; Attia, H. Heating Effects on Some Quality Characteristics of Date Seed Oil. Food Chem. 2005, 91, 469–476. [Google Scholar] [CrossRef]
- Bracco, U.; Dieffenbacher, A.; Kolarovic, L. Frying Performance of Palm Oil Liquid Fractions. J. Am. Oil Chem. Soc. 1981, 58, 6–12. [Google Scholar] [CrossRef]
- Stevenson, S.G.; Vaisey-Genser, M.; Eskin, N.A.M. Quality Control in the Use of Deep Frying Oils. J. Am. Oil Chem. Soc. 1984, 61, 1102–1108. [Google Scholar] [CrossRef]
- Halilović, B.; Čorbo, S.; Gavrić, T.; Begić, M. The Effect of Essential Oils on the Quality and Oxidative Stability of Linseed Oil. In Lecture Notes in Bioengineering: Scientific-Expert Conference of Agriculture and Food Industry, Proceedings of the 32nd Scientific-Expert Conference of Agricultureand Food Industry, Sarajevo, Bosnia and Herzegovina, 1–2 December 2022; Springer: Berlin/Heidelberg, Germany, 2023; pp. 218–231. [Google Scholar]
- Micić, D.; Đurović, S.; Riabov, P.; Tomić, A.; Šovljanski, O.; Filip, S.; Tosti, T.; Dojčinović, B.; Božović, R.; Jovanović, D.; et al. Rosemary Essential Oils as a Promising Source of Bioactive Compounds: Chemical Composition, Thermal Properties, Biological Activity, and Gastronomical Perspectives. Foods 2021, 10, 2734. [Google Scholar] [CrossRef]
- Setyaningsih, D.; Siahaan, D.G. The Influence of Essential Oil Addition to Oxidative Stability of Palmoil Biodisel. IOP Conf. Ser. Earth Environ. Sci. 2018, 209, 012007. [Google Scholar] [CrossRef]
- Góra, J.; Lis, A. The Most Valuable Essential Oils I; Wydawnictwo Politechniki Łódzkiej: Łódź, Poland, 2017. [Google Scholar]
- Freire, M.M.; Jham, G.N.; Dhingra, O.D.; Jardim, C.M.; Barcelos, R.C.; Valente, V.M.M. Composition, Antifungal Activity and Main Fungitoxic Components of the Essential Oil of Mentha piperita L. J. Food Saf. 2012, 32, 29–36. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Compounds by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured: Carol Stream, IL, USA, 2001. [Google Scholar]
- Gavrila, A.I.; Chisega-Negrila, C.G.; Maholea, L.; Gavrila, M.L.; Parvulescu, O.C.; Popa, I. Enhancing the Extraction Process Efficiency of Thyme Essential Oil by Combined Ultrasound and Microwave Techniques. Agronomy 2023, 13, 2331. [Google Scholar] [CrossRef]
- Radivojac, A.; Bera, O.; Zeković, Z.; Teslić, N.; Mrkonjić, Ž.; Bursać Kovačević, D.; Putnik, P.; Pavlić, B. Extraction of Peppermint Essential Oils and Lipophilic Compounds: Assessment of Process Kinetics and Environmental Impacts with Multiple Techniques. Molecules 2021, 26, 2879. [Google Scholar] [CrossRef] [PubMed]
- Derwich, E.; Chabir, R.; Taouil, R.; Senhaji, O. In-Vitro Antioxidant Activity and GC/MS Studies on the Leaves of Mentha piperita (Lamiaceae) from Morocco. Int. J. Pharm. Sci. Drug Res. 2011, 3, 130–136. [Google Scholar]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant Activity and Mechanism of Action of Thymol and Carvacrol in Two Lipid Systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Kiralan, M.; Ulaş, M.; Özaydin, A.; Özdemır, N.; Özkan, G.; Bayrak, A.; Ramadan, M.F. Blends of Cold Pressed Black Cumin Oil and Sunflower Oil with Improved Stability: A Study Based on Changes in the Levels of Volatiles, Tocopherols and Thymoquinone during Accelerated Oxidation Conditions. J. Food Biochem. 2017, 41, e12272. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Gopalakrishnan, N. Studies on the Storage Quality of Carbon Dioxide-Extracted Cardamom and Clove Bud Oils. J. Agric. Food Chem. 1994, 42, 796–798. [Google Scholar] [CrossRef]
- Braun, M.; Franz, G. Quality Criteria of Bitter Fennel Oil in the German Pharmacopoeia. Pharm. Pharmacol. Lett. 1999, 9, 48–51. [Google Scholar]
- Sicińska, E. Przeciwutleniacze w Żywności. Przemysł Spożywczy 2008, 62, 36–38. [Google Scholar]
- Gramza-Michałowska, A.; Sidor, A.; Hes, M. Herb Extract Influence on the Oxidative Stability of Selected Lipids. J. Food Biochem. 2011, 35, 1723–1736. [Google Scholar] [CrossRef]
- Polish Pharmacopoeia VIII; PTFarm; Polish Pharmaceutical Society: Warsaw, Poland, 2008.
- PN-EN ISO 12966-1:2015-01; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. International Organization for Standardization: Geneva, Switzerland, 2015.




| Fatty Acid | Concentration (g/100 g) |
|---|---|
| Palmitic acid 16:0 | 7.40 ± 0.23 |
| Stearic acid 18:0 | 2.67 ± 0.16 |
| Oleic acid 18:1 cis | 22.18 ± 0.84 |
| Linoleic acid 18:2 | 57.91 ± 0.43 |
| α-Linolenic acid 18:3 α | 0.45 ± 0.03 |
| Arachidic acid 20:0 | 0.20 ± 0.04 |
| cis-11-Eicosenoic acid 20:1 | 0.24 ± 0.04 |
| Σ | 91.05 ± 1.05 |
| Compound | RI | RILit | Percentage |
|---|---|---|---|
| α-Pinene | 938 | 932 | 0.52% |
| Camphene | 955 | 946 | tr. |
| Sabinene | 976 | 969 | 0.28% |
| β-Pinene | 982 | 974 | 0.67% |
| Myrcene | 993 | 988 | 0.14% |
| 2-Octanol | 1004 | 994 | tr. |
| p-Cymene | 1028 | 1020 | tr. |
| Limonene | 1031 | 1024 | 0.49% |
| 1,8-Cineole | 1034 | 1026 | 3.73% |
| (Z)-β-Ocimene | 1037 | 1032 | tr. |
| γ-Terpinene | 1060 | 1054 | tr. |
| cis-Sabinene hydrate | 1073 | 1065 | 0.05% |
| Linalool | 1101 | 1095 | 0.17% |
| 3-Octanol acetate | 1118 | 1120 | tr. |
| cis-Sabinol | 1144 | 1135 | 0.07% |
| trans-Sabinol | 1146 | 1137 | 0.12% |
| trans-Verbenol | 1150 | 1140 | 0.10% |
| neo-Isopulegol | 1152 | 1144 | 0.17% |
| Menthone | 1160 | 1148 | 31.26% |
| iso-Menthone | 1170 | 1158 | 8.44% |
| neo-Menthol | 1174 | 1161 | 2.56% |
| Menthol | 1183 | 1167 | 25.71% |
| iso-Menthol | 1194 | 1179 | 0.48% |
| neo-iso-Menthol | 1198 | 1184 | 0.12% |
| Pulegone | 1249 | 1233 | 0.71% |
| Piperitone | 1266 | 1249 | 1.18% |
| neo-Menthyl acetate | 1279 | 1271 | 0.22% |
| Menthyl acetate | 1297 | 1294 | 10.50% |
| iso-Menthyl acetate | 1314 | 1304 | 0.36% |
| α-Copaene | 1382 | 1374 | 0.06% |
| β-Bourbonene | 1390 | 1387 | 0.47% |
| β-Elemene | 1394 | 1389 | 0.61% |
| E-Caryophyllene | 1429 | 1417 | 3.33% |
| β-Copaene | 1440 | 1430 | 0.10% |
| cis-Muurola-3,5-diene | 1457 | 1448 | 0.12% |
| (E)-β-Farnesene | 1461 | 1454 | 0.22% |
| α-Humulene | 1469 | 1452 | 0.17% |
| cis-Muurola-4(14),5-diene | 1475 | 1465 | 0.23% |
| γ-Muurolene | 1488 | 1478 | 0.06% |
| Germacrene D | 1496 | 1484 | 3.38% |
| β-Selinene | 1505 | 1489 | tr. |
| Bicyclogermacrene | 1511 | 1500 | 0.30% |
| Germacrene A | 1522 | 1508 | 0.12% |
| γ-Cadinene | 1527 | 1513 | tr. |
| δ-Cadinene | 1530 | 1522 | 0.14% |
| cis-Calamenene | 1534 | 1528 | 0.05% |
| α-Cadinene | 1549 | 1537 | tr. |
| Spathulenol (-) | 1588 | 1577 | 0.15% |
| Caryophyllene oxide | 1593 | 1582 | 0.39% |
| Viridiflorol | 1605 | 1592 | 0.47% |
| 1,10-di-epi-Cubenol | 1625 | 1618 | 0.06% |
| τ-Muurolol | 1658 | 1642 | 0.20% |
| Compound | RI | RILit | Percentage |
|---|---|---|---|
| Heptane | 702 | 700 | tr. |
| 3-metyhyl-3-Buten-1-ol | 729 | 723 | 0.07% |
| Hexanal | 805 | 801 | tr. |
| 3E-Hexenol | 854 | 844 | tr. |
| N-Hexanol | 867 | 863 | tr. |
| α-Thujene | 927 | 924 | 0.92% |
| α-Pinene | 935 | 932 | 0.98% |
| Camphene | 952 | 946 | 0.42% |
| Sabinene | 974 | 969 | tr. |
| β-Pinene | 982 | 974 | 0.48% |
| 1-Octen-3-ol | 983 | 974 | 0.38% |
| Myrcene | 992 | 988 | 2.21% |
| 2-Octanol | 1001 | 994 | 0.11% |
| α-Phellandrene | 1010 | 1002 | 0.25% |
| δ-3-Carene | 1015 | 1008 | 2.38% |
| α-Terpinene | 1016 | 1014 | 0.10% |
| p-Cymene | 1032 | 1020 | 14.68% |
| Limonene | 1034 | 1024 | 0.32% |
| β-Phellandrene | 1035 | 1025 | 0.14% |
| 1,8-Cineole | 1037 | 1026 | 0.27% |
| γ-Terpinene | 1063 | 1054 | 11.15% |
| cis-Sabinene hydrate | 1075 | 1065 | 0.77% |
| Terpinolene | 1088 | 1086 | 0.14% |
| p-Cymenene | 1095 | 1089 | tr. |
| Linalool | 1105 | 1095 | 2.09% |
| trans-Sabinene hydrate | 1108 | 1065 | tr. |
| Camphor | 1153 | 1141 | tr. |
| Borneol | 1179 | 1165 | 0.17% |
| Terpinen-4-ol | 1185 | 1174 | 0.40% |
| γ-Terpineol | 1213 | 1199 | 0.16% |
| Thymol, methyl ether | 1234 | 1232 | 0.74% |
| Carvacrol, methyl ether | 1242 | 1241 | 0.52% |
| Geranial | 1269 | 1264 | tr. |
| Thymol | 1311 | 1289 | 52.91% |
| Carvacrol | 1316 | 1298 | 2.34% |
| Thymol acetate | 1351 | 1349 | 0.07% |
| Eugenol | 1359 | 1356 | 0.07% |
| α-Copaene | 1378 | 1374 | tr. |
| Isobornyl propanoate | 1380 | 1383 | tr. |
| β-Bourbonene | 1386 | 1387 | tr. |
| Methyl eugenol | 1407 | 1401 | 0.12% |
| E-Caryophyllene | 1423 | 1417 | 1.38% |
| β-Copaene | 1432 | 1430 | tr. |
| Aromadendrene | 1440 | 1439 | tr. |
| α-Humulene | 1457 | 1452 | 0.09% |
| Geranyl propanoate | 1473 | 1476 | 0.18% |
| γ-Muurolene | 1477 | 1478 | 0.12% |
| Germacrene D | 1483 | 1484 | tr. |
| γ-Amorphene | 1494 | 1495 | tr. |
| α-Muurolene | 1500 | 1500 | tr. |
| β-Bisabolene | 1510 | 1505 | 0.08% |
| γ-Cadinene | 1516 | 1513 | 0.22% |
| δ-Cadinene | 1520 | 1522 | 0.27% |
| α-Cadinene | 1539 | 1537 | tr. |
| α-Calacorene | 1544 | 1544 | tr. |
| Elemicin | 1553 | 1555 | 0.15% |
| Geranyl butanoate | 1559 | 1562 | tr. |
| Spathulenol | 1580 | 1577 | tr. |
| Caryophyllene oxide | 1585 | 1582 | 0.25% |
| Humulene epoxide II | 1613 | 1608 | tr. |
| 1,10-di-epi-Cubenol | 1618 | 1618 | 0.06% |
| 10-epi-γ-Eudesmol | 1626 | 1622 | 0.13% |
| 1-epi-Cubenol | 1631 | 1627 | tr. |
| τ-Cadinol | 1646 | 1640 | 0.42% |
| α-Cadinol | 1659 | 1652 | 0.09% |
| 7-epi-α-Eudesmol | 1665 | 1662 | tr. |
| 14-hydroxy-9-epi-(E)-Caryophyllene | 1675 | 1668 | tr. |
| 2Z,6Z-Farnesol | 1698 | 1698 | 0.14% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, R.; Kowalska, G.; Mitura, P.; Rowiński, R.; Pankiewicz, U.; Hawlena, J. The Effect of Peppermint and Thyme Oils on Stabilizing the Fatty Acid Profile of Sunflower Oil. Molecules 2024, 29, 292. https://doi.org/10.3390/molecules29020292
Kowalski R, Kowalska G, Mitura P, Rowiński R, Pankiewicz U, Hawlena J. The Effect of Peppermint and Thyme Oils on Stabilizing the Fatty Acid Profile of Sunflower Oil. Molecules. 2024; 29(2):292. https://doi.org/10.3390/molecules29020292
Chicago/Turabian StyleKowalski, Radosław, Grażyna Kowalska, Przemysław Mitura, Rafał Rowiński, Urszula Pankiewicz, and Joanna Hawlena. 2024. "The Effect of Peppermint and Thyme Oils on Stabilizing the Fatty Acid Profile of Sunflower Oil" Molecules 29, no. 2: 292. https://doi.org/10.3390/molecules29020292
APA StyleKowalski, R., Kowalska, G., Mitura, P., Rowiński, R., Pankiewicz, U., & Hawlena, J. (2024). The Effect of Peppermint and Thyme Oils on Stabilizing the Fatty Acid Profile of Sunflower Oil. Molecules, 29(2), 292. https://doi.org/10.3390/molecules29020292








