Biofortification of Mushrooms: A Promising Approach
Abstract
1. Introduction
2. Search Methodology
3. Nutritional Composition of Mushrooms
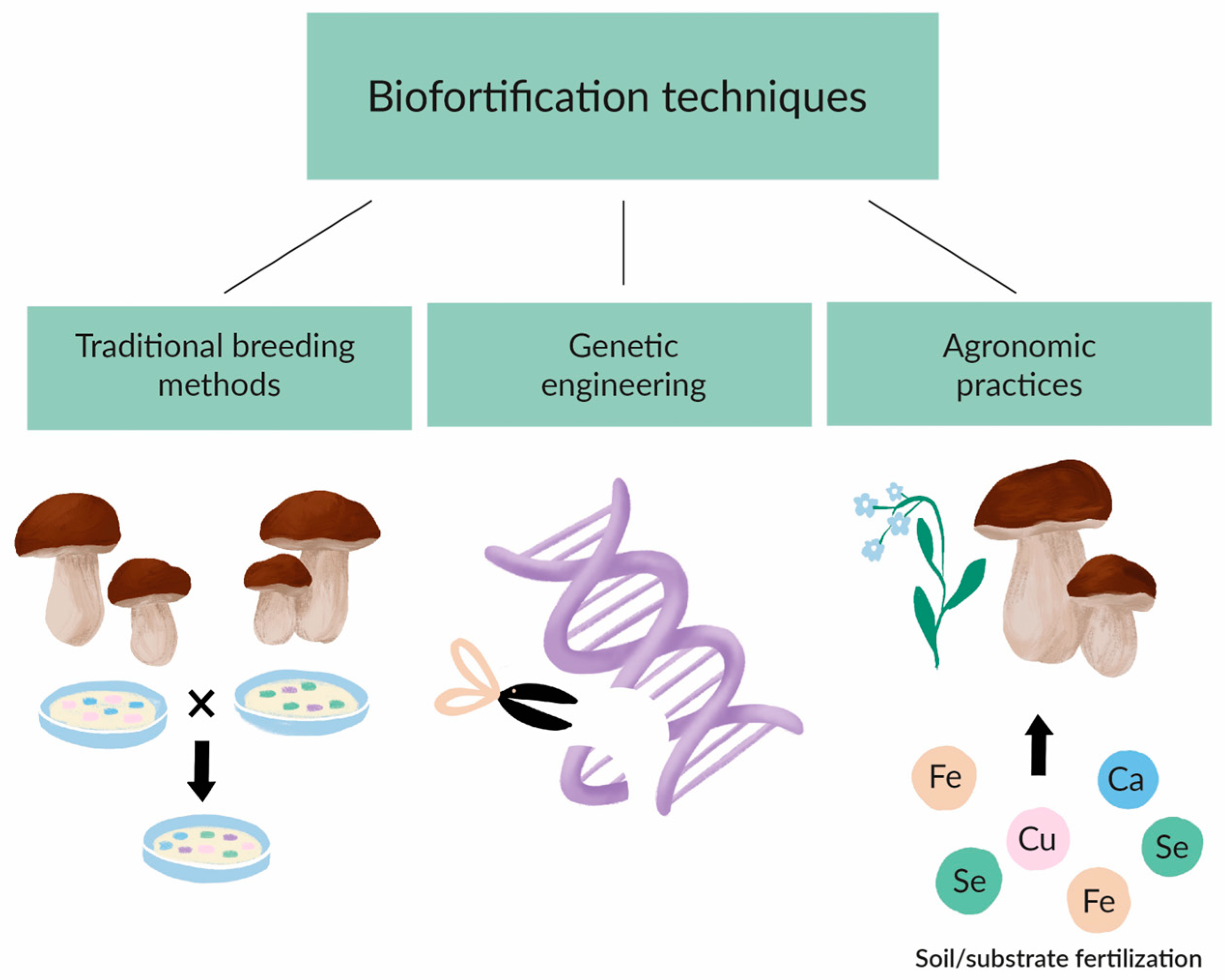
4. Biofortification Techniques
5. Species of Biofortified Mushrooms
6. Bioavailability of Nutrients in Biofortified Mushrooms
6.1. Selenium and Zinc
6.2. Iron
6.3. Calcium
6.4. Lithium
6.5. Copper
7. Agronomic Considerations
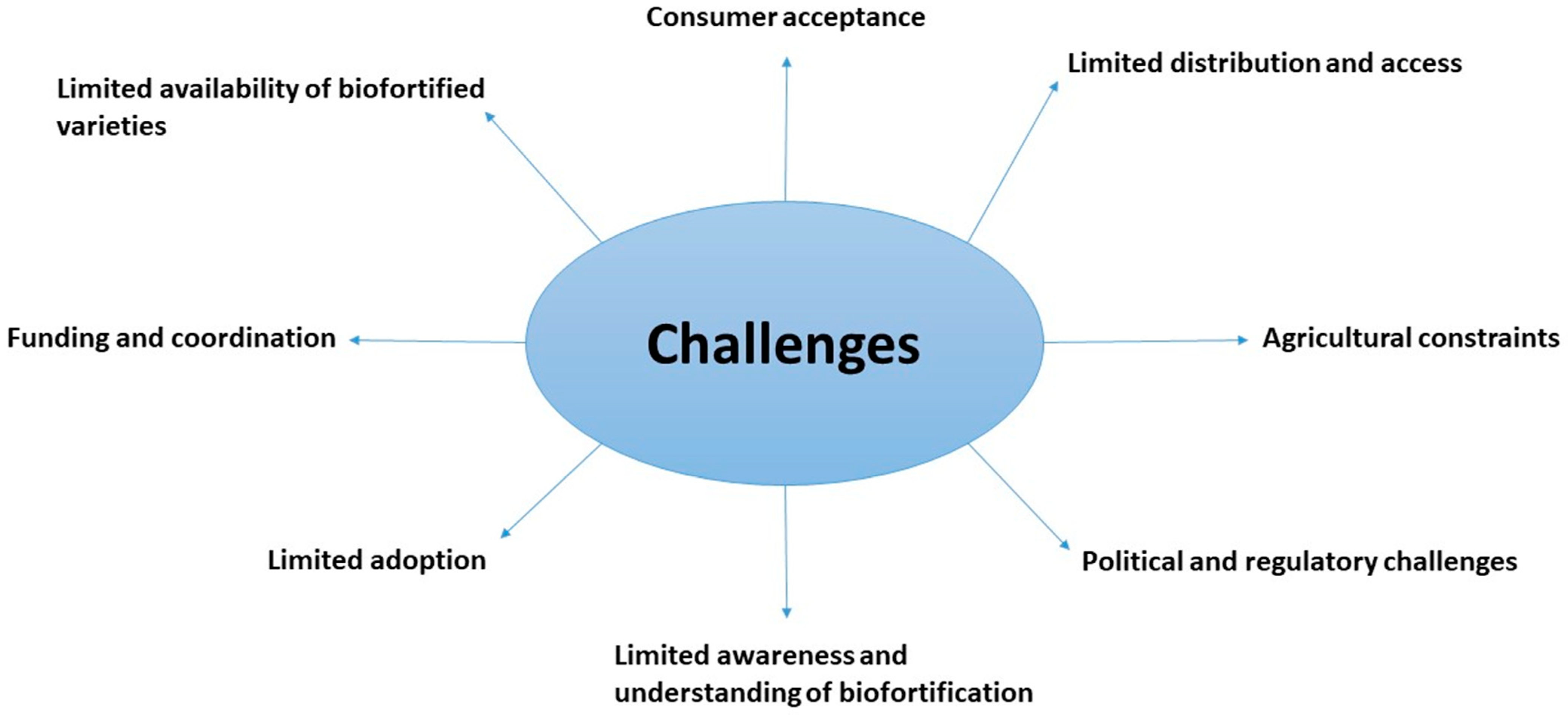
8. Challenges and Future Directions
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Díaz-Gómez, J.; Twyman, R.M.; Zhu, C.; Farré, G.; Serrano, J.C.; Portero-Otin, M.; Muñoz, P.; Sandmann, G.; Capell, T.; Christou, P. Biofortification of crops with nutrients: Factors affecting utilization and storage. Curr. Opin. Biotechnol. 2017, 44, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Praharaj, C.S.; Singh, S.S.; Singh, N.P. Biofortification: Introduction, Approaches, Limitations, and Challenges. In Biofortification of Food Crops; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–490. [Google Scholar] [CrossRef]
- Jha, A.B.; Warkentin, T.D. Biofortification of pulse crops: Status and future perspectives. Plants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; West, K.P.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Rawat, N.; Neelam, K.; Tiwari, V.K.; Dhaliwal, H.S. Biofortification of cereals to overcome hidden hunger. Plant Breed. 2013, 132, 437–445. [Google Scholar] [CrossRef]
- Budzyńska, S.; Siwulski, M.; Gąsecka, M.; Magdziak, Z.; Kalač, P.; Niedzielski, P.; Mleczek, M. Biofortification of Three Cultivated Mushroom Species with Three Iron Salts—Potential for a New Iron-Rich Superfood. Molecules 2022, 27, 2328. [Google Scholar] [CrossRef] [PubMed]
- Oyetayo, V.O. Mineral Element Enrichment of Mushrooms for the Production of More Effective Functional Foods. Asian J. Biol. Sci. 2023, 16, 18–29. [Google Scholar] [CrossRef]
- Singh, D.P.; Gupta, V.K.; Prabha, R. Microbial Interventions in Agriculture and Environment: Volume 1: Research Trends, Priorities and Prospects; Springer: Singapore, 2019; Volume 1, ISBN 9789811383915. [Google Scholar]
- Kora, A.J. Nutritional and antioxidant significance of selenium-enriched mushrooms. Bull. Natl. Res. Cent. 2020, 44, 34. [Google Scholar] [CrossRef]
- De Assunão, L.S.; Da Luz, J.M.R.; Da Silva, M.D.C.S.; Vieira, P.A.F.; Bazzolli, D.M.S.; Vanetti, M.C.D.; Kasuya, M.C.M. Enrichment of mushrooms: An interesting strategy for the acquisition of lithium. Food Chem. 2012, 134, 1123–1127. [Google Scholar] [CrossRef]
- Hyde, K.D.; Jeewon, R.; Chen, Y.J.; Bhunjun, C.S.; Calabon, M.S.; Jiang, H.B.; Lin, C.G.; Norphanphoun, C.; Sysouphanthong, P.; Pem, D.; et al. The Numbers of Fungi: Is the Descriptive Curve Flattening; Springer: Dordrecht, The Netherlands, 2020; Volume 103, ISBN 0123456789. [Google Scholar]
- Siwulski, M.; Sobieralski, K.; Sas-Golak, I. Wartość odżywcza i prozdrowotna grzybów. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2014, 21, 16–28. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef] [PubMed]
- Luque, C.; Cepero, A.; Perazzoli, G.; Mesas, C.; Quiñonero, F.; Cabeza, L.; Prados, J.; Melguizo, C. In Vitro Efficacy of Extracts and Isolated Bioactive Compounds from Ascomycota Fungi in the Treatment of Colorectal Cancer: A Systematic Review. Pharmaceuticals 2023, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal mushrooms: Bioactive compounds, use, and clinical trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Golak-Siwulska, I.; Kałużewicz, A.; Spiżewski, T.; Sobieralski, K. Zawartość związków biologicznie aktywnych w grzybach jadalnych dziko rosnących. Sylwan 2018, 162, 238–247. [Google Scholar]
- Guindon, M.F.; Cazzola, F.; Palacios, T.; Gatti, I.; Bermejo, C.; Cointry, E. Biofortification of pea (Pisum sativum L.): A review. J. Sci. Food Agric. 2021, 101, 3551–3563. [Google Scholar] [CrossRef]
- Malik, K.A.; Maqbool, A. Transgenic Crops for Biofortification. Front. Sustain. Food Syst. 2020, 4, 571402. [Google Scholar] [CrossRef]
- Avnee; Sood, S.; Chaudhary, D.R.; Jhorar, P.; Rana, R.S. Biofortification: An approach to eradicate micronutrient deficiency. Front. Nutr. 2023, 10, 1233070. [Google Scholar] [CrossRef]
- Shahzad, R.; Jamil, S.; Ahmad, S.; Nisar, A.; Khan, S.; Amina, Z.; Kanwal, S.; Aslam, H.M.U.; Gill, R.A.; Zhou, W. Biofortification of Cereals and Pulses Using New Breeding Techniques: Current and Future Perspectives. Front. Nutr. 2021, 8, 721728. [Google Scholar] [CrossRef]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 2011, 32, 31–40. [Google Scholar] [CrossRef]
- Hu, T.; Liang, Y.; Zhao, G.; Wu, W.; Li, H.; Guo, Y. Selenium Biofortification and Antioxidant Activity in Cordyceps militaris Supplied with Selenate, Selenite, or Selenomethionine. Biol. Trace Elem. Res. 2019, 187, 553–561. [Google Scholar] [CrossRef]
- Meniqueti, A.B.; Ruiz, S.P.; Faria, M.G.I.; do Valle, J.S.; Gonçalves, A.C.; Dragunski, D.C.; Colauto, N.B.; Linde, G.A. Iron Bioaccumulation in Lentinus crinitus Mycelia Cultivated in Agroindustrial Byproducts. Waste Biomass Valorization 2021, 12, 4965–4974. [Google Scholar] [CrossRef]
- Scheid, S.S.; Faria, M.G.I.; Velasquez, L.G.; do Valle, J.S.; Gonçalves, A.C.; Dragunski, D.C.; Colauto, N.B.; Linde, G.A. Iron biofortification and availability in the mycelial biomass of edible and medicinal basidiomycetes cultivated in sugarcane molasses. Sci. Rep. 2020, 10, 12875. [Google Scholar] [CrossRef] [PubMed]
- Miletić, D.; Turło, J.; Podsadni, P.; Pantić, M.; Nedović, V.; Lević, S.; Nikšić, M. Selenium-enriched Coriolus versicolor mushroom biomass: Potential novel food supplement with improved selenium bioavailability. J. Sci. Food Agric. 2019, 99, 5122–5130. [Google Scholar] [CrossRef] [PubMed]
- Fontes Vieira, P.A.; Gontijo, D.C.; Vieira, B.C.; Fontes, E.A.F.; de Assunção, L.S.; Leite, J.P.V.; de Oliveira, M.G.A.; Kasuya, M.C.M. Antioxidant activities, total phenolics and metal contents in Pleurotus ostreatus mushrooms enriched with iron, zinc or lithium. LWT 2013, 54, 421–425. [Google Scholar] [CrossRef]
- Silva, M.d.C.S.d.; da Luz, J.M.R.; Paiva, A.P.S.; Mendes, D.R.; Carvalho, A.A.C.; Naozuka, J.; Kasuya, M.C.M. Growth and Tolerance of Pleurotus ostreatus at Different Selenium Forms. J. Agric. Sci. 2019, 11, 151. [Google Scholar] [CrossRef]
- Meniqueti, A.B.; Ruiz, S.P.; Faria, M.G.I.; do Valle, J.S.; Gonçalves, A.C.; Dragunski, D.C.; Colauto, N.B.; Linde, G.A. Iron-enriched mycelia of edible and medicinal basidiomycetes. Environ. Technol. 2022, 43, 1248–1254. [Google Scholar] [CrossRef]
- Kaur, G.; Kalia, A.; Sodhi, H.S. Selenium biofortification of Pleurotus species and its effect on yield, phytochemical profiles, and protein chemistry of fruiting bodies. J. Food Biochem. 2018, 42, e12467. [Google Scholar] [CrossRef]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur. Food Res. Technol. 2016, 242, 723–732. [Google Scholar] [CrossRef]
- Tang, Z.X.; Shi, L.E.; Jiang, Z.B.; Bai, X.L.; Ying, R.F. Calcium Enrichment in Edible Mushrooms: A Review. J. Fungi 2023, 9, 338. [Google Scholar] [CrossRef]
- Siwulski, M.; Budzyńska, S.; Rzymski, P.; Gąsecka, M.; Niedzielski, P.; Kalač, P.; Mleczek, M. The effects of germanium and selenium on growth, metalloid accumulation and ergosterol content in mushrooms: Experimental study in Pleurotus ostreatus and Ganoderma lucidum. Eur. Food Res. Technol. 2019, 245, 1799–1810. [Google Scholar] [CrossRef]
- Almeida, S.M.; Umeo, S.H.; Marcante, R.C.; Yokota, M.E.; Valle, J.S.; Dragunski, D.C.; Colauto, N.B.; Linde, G.A. Iron bioaccumulation in mycelium of Pleurotus ostreatus. Braz. J. Microbiol. 2015, 46, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Umeo, S.H.; Faria, M.G.I.; Dragunski, D.C.; Do Valle, J.S.; Colauto, N.B.; Linde, G.A. Iron or zinc bioaccumulated in mycelial biomass of edible basidiomycetes. An. Acad. Bras. Cienc. 2020, 92, e20191350. [Google Scholar] [CrossRef]
- Fasoranti, O.F.; Ogidi, C.O.; Oyetayo, V.O. Nutrient contents and antioxidant properties of Pleurotus spp. cultivated on substrate fortified with Selenium. Curr. Res. Environ. Appl. Mycol. 2019, 9, 66–76. [Google Scholar] [CrossRef]
- Milovanović, I.; Brčeski, I.; Stajić, M.; Korać, A.; Vukojević, J.; Knežević, A. Potential of pleurotus ostreatus mycelium for selenium absorption. Sci. World J. 2014, 2014, 681834. [Google Scholar] [CrossRef] [PubMed]
- Figlas, D.; Oddera, M.; Curvetto, N. Bioaccumulation and bioavailability of copper and zinc on mineral-enriched mycelium of Grifola frondosa. J. Med. Food 2010, 13, 469–475. [Google Scholar] [CrossRef]
- Li, Q.; Wang, W.; Zhu, Y.; Chen, Y.; Zhang, W.; Yu, P.; Mao, G.; Zhao, T.; Feng, W.; Yang, L.; et al. Structural elucidation and antioxidant activity a novel Se-polysaccharide from Se-enriched Grifola frondosa. Carbohydr. Polym. 2017, 161, 42–52. [Google Scholar] [CrossRef]
- Budzyńska, S.; Siwulski, M.; Magdziak, Z.; Budka, A.; Gąsecka, M.; Kalač, P.; Rzymski, P.; Niedzielski, P.; Mleczek, M. Influence of iron addition (Alone or with calcium) to elements biofortification and antioxidants in pholiota nameko. Plants 2021, 10, 2275. [Google Scholar] [CrossRef]
- Zięba, P.; Kała, K.; Włodarczyk, A.; Szewczyk, A.; Kunicki, E.; Sękara, A.; Muszyńska, B. Selenium and Zinc Biofortification of Pleurotus eryngii Mycelium and Fruiting Bodies as a Tool for Controlling Their Biological Activity. Molecules 2020, 25, 889. [Google Scholar] [CrossRef]
- Niedzielski, P.; Mleczek, M.; Siwulski, M.; Gąsecka, M.; Kozak, L.; Rissmann, I.; Mikołajczak, P. Efficacy of supplementation of selected medicinal mushrooms with inorganic selenium salts. J. Environ. Sci. Health-Part B Pestic. Food Contam. Agric. Wastes 2014, 49, 929–937. [Google Scholar] [CrossRef]
- Rzymski, P.; Mleczek, M.; Niedzielski, P.; Siwulski, M.; Gasecka, M. Potential of Cultivated Ganoderma lucidum Mushrooms for the Production of Supplements Enriched with Essential Elements. J. Food Sci. 2016, 81, C587–C592. [Google Scholar] [CrossRef]
- Kałucka, M.; Roszczyk, A.; Klimaszewska, M.; Kaleta, B.; Drelich, E.; Błażewicz, A.; Górska-Jakubowska, S.; Malinowska, E.; Król, M.; Prus, A.M.; et al. Optimization of Se- and Zn-Enriched Mycelium of Lentinula edodes (Berk.) Pegler as a Dietary Supplement with Immunostimulatory Activity. Nutrients 2023, 15, 4015. [Google Scholar] [CrossRef]
- Bhatia, P.; Aureli, F.; D’Amato, M.; Prakash, R.; Cameotra, S.S.; Nagaraja, T.P.; Cubadda, F. Selenium bioaccessibility and speciation in biofortified Pleurotus mushrooms grown on selenium-rich agricultural residues. Food Chem. 2013, 140, 225–230. [Google Scholar] [CrossRef]
- Madaan, K.; Sharma, S.; Kalia, A. Effect of selenium and zinc biofortification on the biochemical parameters of Pleurotus spp. under submerged and solid-state fermentation. J. Trace Elem. Med. Biol. 2023, 82, 127365. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Krakowska, A.; Sułkowska-Ziaja, K.; Suchanek, M.; Zięba, P.; Opoka, W.; Muszyńska, B. Pleurotus spp. Mycelia Enriched in Magnesium and Zinc Salts as a Potential Functional Food. Molecules 2021, 26, 162. [Google Scholar] [CrossRef]
- Oyetayo, V.O.; Ogidi, C.O.; Bayode, S.O.; Enikanselu, F.F. Evaluation of biological efficiency, nutrient contents and antioxidant activity of Pleurotus pulmonarius enriched with Zinc and Iron. Indian Phytopathol. 2021, 74, 901–910. [Google Scholar] [CrossRef]
- Ogidi, C.O.; Oyebode, K.O. Assessment of nutrient contents and bio-functional activities of edible fungus bio-fortified with copper, lithium and zinc. World J. Microbiol. Biotechnol. 2023, 39, 56. [Google Scholar] [CrossRef]
- Dong, Z.; Xiao, Y.; Wu, H. Selenium accumulation, speciation, and its effect on nutritive value of Flammulina velutipes (Golden needle mushroom). Food Chem. 2021, 350, 128667. [Google Scholar] [CrossRef]
- Hu, T.; Hui, G.; Li, H.; Guo, Y. Selenium biofortification in Hericium erinaceus (Lion’s Mane mushroom) and its in vitro bioaccessibility. Food Chem. 2020, 331, 127287. [Google Scholar] [CrossRef]
- Egressy-Molnár, O.; Ouerdane, L.; Gyorfi, J.; Dernovics, M. Analogy in selenium enrichment and selenium speciation between selenized yeast Saccharomyces cerevisiae and Hericium erinaceus (lion’s mane mushroom). LWT 2016, 68, 306–312. [Google Scholar] [CrossRef]
- Rzymski, P.; Niedzielski, P.; Siwulski, M.; Mleczek, M.; Budzyńska, S.; Gąsecka, M.; Poniedziałek, B. Lithium biofortification of medicinal mushrooms Agrocybe cylindracea and Hericium erinaceus. J. Food Sci. Technol. 2017, 54, 2387–2393. [Google Scholar] [CrossRef]
- Odiketa, J.K.; Whitehall, S.; Adedokun, O.M. Biofortification of mushroom (Pleurotus floridanus) using calcium based supplements. J. Mushrooms 2020, 18, 287–291. [Google Scholar] [CrossRef]
- Rzymski, P.; Mleczek, M.; Niedzielski, P.; Siwulski, M.; Gąsecka, M. Cultivation of Agaricus bisporus enriched with selenium, zinc and copper. J. Sci. Food Agric. 2017, 97, 923–928. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef]
- Zhang, G.; Liang, Y. Improvement of fruiting body production in Cordyceps militaris by molecular assessment. Arch. Microbiol. 2013, 195, 579–585. [Google Scholar] [CrossRef]
- Bertéli, M.B.D.; Oliveira Filho, O.B.Q.; Freitas, J.D.S.; Bortolucci, W.C.; Silva, G.R.; Gazim, Z.C.; Lívero, F.A.R.; Lovato, E.C.W.; Valle, J.S.; Linde, G.A.; et al. Lentinus crinitus basidiocarp stipe and pileus: Chemical composition, cytotoxicity and antioxidant activity. Eur. Food Res. Technol. 2021, 247, 1355–1366. [Google Scholar] [CrossRef]
- Wu, J.Y.; Siu, K.C.; Geng, P. Bioactive ingredients and medicinal values of grifola frondosa (Maitake). Foods 2021, 10, 95. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Wang, X.; Hu, H.; Zhang, Y.; Liu, T.; Zhao, H. Structure characterization and antioxidant activity of carboxymethylated polysaccharide from Pholiota nameko. J. Food Biochem. 2022, 46, e14121. [Google Scholar] [CrossRef]
- Seweryn, E.; Ziała, A.; Gamian, A. Health-promoting of polysaccharides extracted from ganoderma lucidum. Nutrients 2021, 13, 2725. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef]
- Lelamurni, D.; Razak, A.; Ghani, A.A.; Izwan, M.; Lazim, M.; Khulidin, K.A.; Shahidi, F.; Ismail, A. Schizophyllum commune (Fries) mushroom: A review on its nutritional components, antioxidative, and anti-inflammatory properties. Curr. Opin. Food Sci. 2024, 56, 101129. [Google Scholar] [CrossRef]
- Li, S.; Wang, A.; Liu, L.; Tian, G.; Wei, S.; Xu, F. Evaluation of nutritional values of shiitake mushroom (Lentinus edodes) stipes. J. Food Meas. Charact. 2018, 12, 2012–2019. [Google Scholar] [CrossRef]
- Ramos, M.; Burgos, N.; Barnard, A.; Evans, G.; Preece, J.; Graz, M.; Ruthes, A.C.; Jiménez-Quero, A.; Martínez-Abad, A.; Vilaplana, F.; et al. Agaricus bisporus and its by-products as a source of valuable extracts and bioactive compounds. Food Chem. 2019, 292, 176–187. [Google Scholar] [CrossRef]
- Atila, F.; Owaid, M.N.; Shariati, M.A. The nutritional and medical benefits of Agaricus Bisporus: A review. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 281–286. [Google Scholar] [CrossRef]
- Wisitrassameewong, K.; Karunarathna, S.C.; Thongklang, N.; Zhao, R.; Callac, P.; Moukha, S.; Férandon, C.; Chukeatirote, E.; Hyde, K.D. Agaricus subrufescens: A review. Saudi J. Biol. Sci. 2012, 19, 131–146. [Google Scholar] [CrossRef]
- Dong, Y.R.; Cheng, S.J.; Qi, G.H.; Yang, Z.P.; Yin, S.Y.; Chen, G.T. Antimicrobial and antioxidant activities of Flammulina velutipes polysacchrides and polysacchride-iron(III) complex. Carbohydr. Polym. 2017, 161, 26–32. [Google Scholar] [CrossRef]
- Roda, E.; Priori, E.C.; Ratto, D.; De Luca, F.; Di Iorio, C.; Angelone, P.; Locatelli, C.A.; Desiderio, A.; Goppa, L.; Savino, E.; et al. Neuroprotective metabolites of hericium erinaceus promote neuro-healthy aging. Int. J. Mol. Sci. 2021, 22, 6379. [Google Scholar] [CrossRef]
- Wong, J.H.; Wang, H.X.; Ng, T.B. Marmorin, a new ribosome inactivating protein with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from the mushroom Hypsizigus marmoreus. Appl. Microbiol. Biotechnol. 2008, 81, 669–674. [Google Scholar] [CrossRef]
- Tsai, C.C.; Li, Y.S.; Lin, P.P. Inonotus obliquus extract induces apoptosis in the human colorectal carcinoma’s HCT-116 cell line. Biomed. Pharmacother. 2017, 96, 1119–1126. [Google Scholar] [CrossRef]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; van der Bijl, P. The pharmacological potential and possible molecular mechanisms of action of Inonotus obliquus from preclinical studies. Phyther. Res. 2019, 33, 1966–1980. [Google Scholar] [CrossRef]
- Sun, Y. Biological activities and potential health benefits of polysaccharides from Poria cocos and their derivatives. Int. J. Biol. Macromol. 2014, 68, 131–134. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Niksic, M.; Jakovljevic, D.; Todorovic, N.; Stefanoska, I.; Van Griensven, L.J.L.D. The edible mushroom Laetiporus sulphureus as potential source of natural antioxidants. Int. J. Food Sci. Nutr. 2013, 64, 599–610. [Google Scholar] [CrossRef]
- Adamska, I. The Possibility of Using Sulphur Shelf Fungus (Laetiporus sulphureus) in the Food Industry and in Medicine—A Review. Foods 2023, 12, 1539. [Google Scholar] [CrossRef]
- Li, J.P.; Lee, Y.P.; Ma, J.C.; Liu, B.R.; Hsieh, N.T.; Chen, D.C.; Chu, C.L.; You, R.I. The enhancing effect of fungal immunomodulatory protein-volvariella volvacea (Fip-vvo) on maturation and function of mouse dendritic cells. Life 2021, 11, 471. [Google Scholar] [CrossRef]
- Nataraj, A.; Govindan, S.; Ramani, P.; Subbaiah, K.A.; Sathianarayanan, S.; Venkidasamy, B.; Thiruvengadam, M.; Rebezov, M.; Shariati, M.A.; Lorenzo, J.M.; et al. Antioxidant, Anti-Tumour, and Anticoagulant Activities of Polysaccharide from Calocybe indica (APK2). Antioxidants 2022, 11, 1694. [Google Scholar] [CrossRef]
- Tonin, F.; Tieves, F.; Willot, S.; Van Troost, A.; Van Oosten, R.; Breestraat, S.; Van Pelt, S.; Alcalde, M.; Hollmann, F. Pilot-Scale Production of Peroxygenase from Agrocybe aegerita. Org. Process Res. Dev. 2021, 25, 1414–1418. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, M.; Liu, C. A comprehensive review of secondary metabolites from the genus Agrocybe: Biological activities and pharmacological implications. Mycology 2023, 15, 162–179. [Google Scholar] [CrossRef]
- Grimm, D.; Wösten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef]
- Grimm, D.; Kuenz, A.; Rahmann, G. Integration of mushroom production into circular food chains. Org. Agric. 2021, 11, 309–317. [Google Scholar] [CrossRef]
- Özcan, M.M.; Dursun, N.; Al Juhaimi, F.Y. Heavy metals intake by cultured mushrooms growing in model system. Environ. Monit. Assess. 2013, 185, 8393–8397. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Lin, Z.; Broadley, M. Selenium biofortification. In Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects; Springer: Berlin/Heidelberg, Germany, 2015; p. 145. [Google Scholar] [CrossRef]
- Kushwaha, A.; Singh, O.; Patil, A.; Kushwaha, K. Effect of zinc sulphate nanoparticle on productivity of oyster mushroom (Pleurotus florida). Pharma Innov. J. 2021, 10, 2472–2475. [Google Scholar]
- Rana, S.; Kapoor, S.; Sharma, S.; Kalia, A. Pleurotus florida mediated biosynthesis of nanoparticles and biofortification. Food Sci. Biotechnol. 2023, 32, 2079–2092. [Google Scholar] [CrossRef]
- Weng, X.; Li, H.; Ren, C.; Zhou, Y.; Zhu, W.; Zhang, S.; Liu, L. Calcium Regulates Growth and Nutrient Absorption in Poplar Seedlings. Front. Plant Sci. 2022, 13, 887098. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Rzymski, P.; Budzyńska, S.; Gąsecka, M.; Kalač, P.; Niedzielski, P. Cultivation of mushrooms for production of food biofortified with lithium. Eur. Food Res. Technol. 2017, 243, 1097–1104. [Google Scholar] [CrossRef]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional Composition and Biological Properties of Sixteen Edible Mushroom Species. Appl. Sci. 2022, 12, 8074. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Budka, A.; Mleczek, P.; Budzyńska, S.; Szostek, M.; Kuczyńska-Kippen, N.; Kalač, P.; Niedzielski, P.; Gąsecka, M.; et al. Toxicological risks and nutritional value of wild edible mushroom species -a half-century monitoring study. Chemosphere 2021, 263, 128095. [Google Scholar] [CrossRef]
- Hansen, G.H.; Lübeck, M.; Frisvad, J.C.; Lübeck, P.S.; Andersen, B. Production of cellulolytic enzymes from ascomycetes: Comparison of solid state and submerged fermentation. Process Biochem. 2015, 50, 1327–1341. [Google Scholar] [CrossRef]
- Li, J.; Wen, S.; Zhang, B.; Wang, F. Selenium Enrichment of the Edible Medicinal Mushroom Antrodia camphorata by Submerged Fermentation. Molecules 2023, 28, 3036. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Q.; Huang, Z.; Wang, Y.; Roubik, H.; Yang, K.; Cai, M.; Sun, P. Solid-State Fermentation of Soybean Meal with Edible Mushroom Mycelium to Improve Its Nutritional, Antioxidant Capacities and Physicochemical Properties. Fermentation 2023, 9, 322. [Google Scholar] [CrossRef]
- Li, F.; Vijayasankaran, N.; Shen, A.; Kiss, R.; Amanullah, A. Cell culture processes for monoclonal antibody production. MAbs 2010, 2, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Teklu, D.; Gashu, D.; Joy, E.J.M.; Amede, T.; Broadley, M.R. Effectiveness of Agronomic Biofortification Strategy in Fighting against Hidden Hunger. Agronomy 2023, 13, 2173. [Google Scholar] [CrossRef]
- Yadav, D.N.; Bansal, S.; Tushir, S.; Kaur, J.; Sharma, K. Advantage of Biofortification over Fortification Technologies; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128184448. [Google Scholar]
- Rizzo, G.; Goggi, S.; Giampieri, F.; Baroni, L. A review of mushrooms in human nutrition and health. Trends Food Sci. Technol. 2021, 117, 60–73. [Google Scholar] [CrossRef]


| Chemical Compounds | Agaricus Bisporus | Lentinula Edodes | Pleurotus Eryngii | Pea | Rice | Broccoli | Avocado | Chicken Breasts | Bread | Sweet Potato |
|---|---|---|---|---|---|---|---|---|---|---|
| Protein | 3.09 g | 2.24 g | 2.41 g | 5.42 g | 2.69 g | 2.82 g | 2 g | 23.2 g | 10.7 g | 1.57 g |
| Total dietary fibre | 1 g | 2.5 g | 3 g | 5.7 g | 0.4 g | 2.6 g | 6.7 g | - | 4 g | 3 g |
| Vitamin C | 2.1 mg | - | - | 40 mg | - | 89.2 mg | 10 mg | 1.1 mg | 0.2 mg | 2.4 mg |
| Iron, Fe | 0.5 mg | 0.41 mg | 0.34 mg | 1.47 mg | 1.2 mg | 0.73 mg | 0.55 mg | 0.64 mg | 3.6 mg | 0.61 mg |
| Sodium, Na | 5 mg | 9 mg | 1 mg | 5 mg | 1 mg | 33 mg | 7 mg | 67 mg | 473 mg | 55 mg |
| Calcium, Ca | 3 mg | 2 mg | 2.49 mg | 25 mg | 10 mg | 47 mg | 12 mg | 18 mg | 125 mg | 30 mg |
| Magnesium, Mg | 9 mg | 20 mg | 13.5 mg | 33 mg | 12 mg | 21 mg | 29 mg | - | 41 mg | 25 mg |
| Potassium, K | 318 mg | 304 mg | 294 mg | 244 mg | 35 mg | 316 mg | 485 mg | - | 141 mg | 337 mg |
| Zinc, Zn | 0.52 mg | 1.03 mg | 0.63 mg | 1.24 mg | 0.49 mg | 0.41 mg | 0.64 mg | - | 1.04 mg | 0.3 mg |
| Copper, Cu | 0.32 mg | 0.142 mg | 0.47 mg | 0.176 mg | 0.069 mg | 0.049 mg | 0.19 mg | - | 0.148 mg | 0.151 mg |
| Selenium, Se | 9.3 µg | 5.7 µg | 1.2 µg | 1.8 µg | 7.5 µg | 2.5 µg | 0.4 µg | - | 28.8 µg | 0.6 µg |
| Species of Mushroom | Biofortified Chemical Compound | Literature |
|---|---|---|
| Cordyceps militaris | Selenium (Se) | [23] |
| Lentinus crinitus | Iron (Fe) | [24,25] |
| Coriolus versicolor | Selenium (Se) | [26] |
| Pleurotus ostreatus | Iron (Fe), Zinc (Zn), Lithium (Li), Selenium (Se), Calcium (Ca), Germanium (Ge) | [27] [6] [10,25] [28] [29] [30] [31,32,33] [9,34,35,36,37] |
| Grifola frondosa | Copper (Cu), Zinc (Zn), Selenium (Se) | [38,39] |
| Pholiota nameko | Iron (Fe), Calcium (Ca) | [6,40] |
| Pleurotus eryngii | Iron (Fe), Selenium (Se), Zinc (Zn), Calcium (Ca) | [6,41] [25,29] [9,31,32,35] |
| Ganoderma lucidum | Iron (Fe), Selenium (Se), Calcium (Ca), Copper (Cu), Zinc (Zn), Germanium (Ge) | [25,29] [32,42] [33,43] [9] |
| Schizophyllum commune | Iron (Fe), Zinc (Zn) | [25,29] [35] |
| Lentinula edodes | Iron (Fe), Selenium (Se), Zinc (Zn) | [25] [44] [9,35] |
| Agaricus subrufescens | Iron (Fe) | [25,29] |
| Pleurotus florida | Selenium (Se), Zinc (Zn) | [9,30,45] |
| Pleurotus sajor caju | Selenium (Se), Zinc (Zn) | [9,30,46] |
| Pleurotus djamor | Selenium (Se), Zinc (Zn), Calcium (Ca) | [9,32,46] [47] |
| Pleurotus pulmonarius | Zinc (Zn), Iron (Fe), Copper (Cu), Lithium (Li), Selenium (Se) | [9,36,47] [48] [49] |
| Pleurotus citrinopileatus | Zinc (Zn) | [47] |
| Flammulina velutipes | Selenium (Se), Calcium (Ca) | [32,50] |
| Hericium erinaceus | Selenium (Se), Lithium (Li) | [42,51] [52] [53] |
| Pleurotus floridanus | Calcium (Ca) | [54] |
| Hypsizygus marmoreus | Calcium (Ca) | [32] |
| Inonotus obliquus | Calcium (Ca) | [32] |
| Pleurotus nebrodensis | Calcium (Ca) | [32] |
| Poria cocos | Calcium (Ca) | [32] |
| Laetiporus sulphureus | Calcium (Ca) | [32] |
| Agaricus bisporus | Selenium (Se), Zinc (Zn), Copper (Cu) | [9,55] |
| Agrocybe aegerita | Selenium (Se) | |
| Agrocybe cylindracea | Lithium (Li) | [53] |
| Pleurotus cornucopiae | Selenium (Se) | [9] |
| Pleurotus fossulatus | Selenium (Se) | [9] |
| Pleurotus citrinopielatus | Selenium (Se) | [9] |
| Volvariella volvacea | Selenium (Se) | [9] |
| Calocybe indica | Selenium (Se) | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słyszyk, K.; Siwulski, M.; Wiater, A.; Tomczyk, M.; Waśko, A. Biofortification of Mushrooms: A Promising Approach. Molecules 2024, 29, 4740. https://doi.org/10.3390/molecules29194740
Słyszyk K, Siwulski M, Wiater A, Tomczyk M, Waśko A. Biofortification of Mushrooms: A Promising Approach. Molecules. 2024; 29(19):4740. https://doi.org/10.3390/molecules29194740
Chicago/Turabian StyleSłyszyk, Klaudia, Marek Siwulski, Adrian Wiater, Michał Tomczyk, and Adam Waśko. 2024. "Biofortification of Mushrooms: A Promising Approach" Molecules 29, no. 19: 4740. https://doi.org/10.3390/molecules29194740
APA StyleSłyszyk, K., Siwulski, M., Wiater, A., Tomczyk, M., & Waśko, A. (2024). Biofortification of Mushrooms: A Promising Approach. Molecules, 29(19), 4740. https://doi.org/10.3390/molecules29194740










