Optimization of the Deproteinization Process via Response Surface Methodology, Preliminary Characterization, and the Determination of the Antioxidant Activities of Polysaccharides from Vitis vinifera L. SuoSuo
Abstract
1. Introduction
2. Results
2.1. Single-Factor Experiments
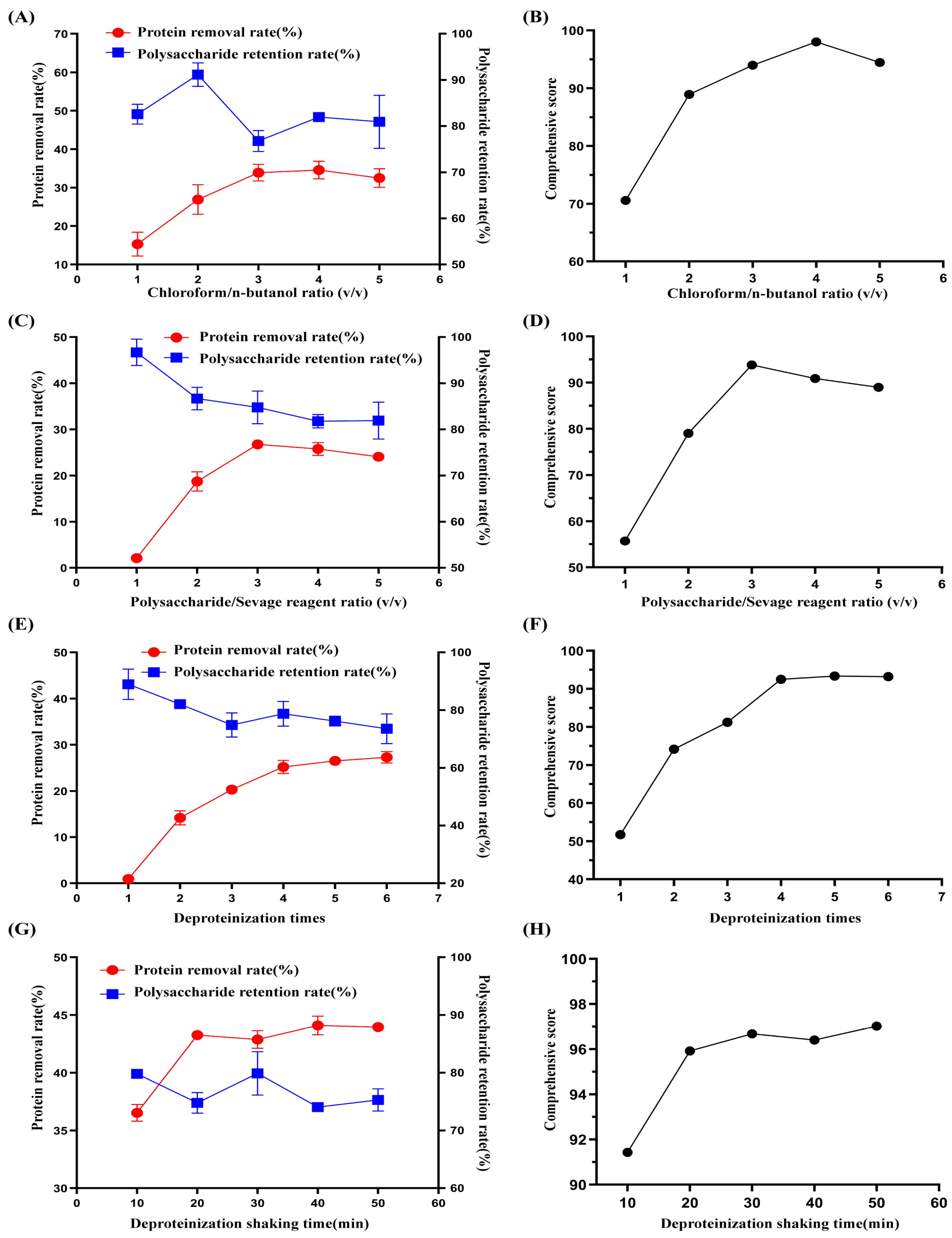
2.1.1. Effects of the Chloroform/n-Butanol Ratio (v/v) on Comprehensive Scores of Deproteination
2.1.2. Effect of the Polysaccharide/Sevage Reagent Ratio on the Comprehensive Deproteinization Score
2.1.3. Effect of the Number of Deproteinizations on the Comprehensive Deproteinization Score
2.1.4. Impact of Shaking Time on the Comprehensive Deproteinization Score
2.2. Response Surface Analysis
2.3. Analysis of Physicochemical Properties and Structural Characterization of Polysaccharides
2.3.1. Content of Uronic Acid in Polysaccharides
2.3.2. Determination of Total Polyphenol Content (TPC)
2.3.3. Determination of Total Flavonoid Content (TFC)
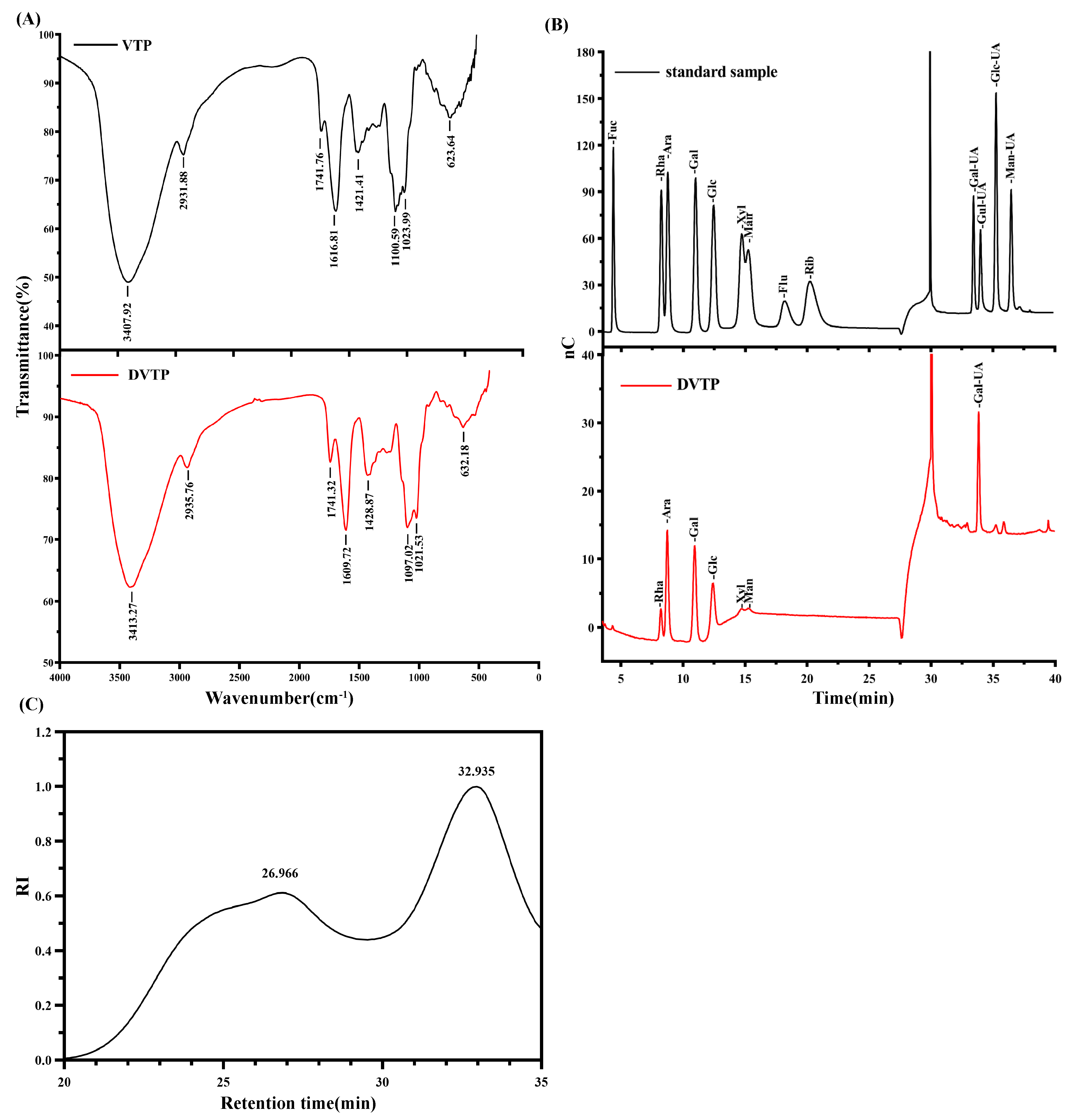
2.3.4. FT-IR Spectra Analysis
2.3.5. Compositional Analysis and Molecular Weight Determination of DVTP Monosaccharides
2.3.6. SEM and AFM Analyses
2.4. In Vitro Antioxidant Activities of the Polysaccharides
2.5. Cell Viability Assay

3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Sample Preparation
4.3. Analytical Methods
4.4. Experimental Design
4.4.1. Single-Factor Experimental Design
4.4.2. Experimental Design through RSM
4.5. Structural Characterization of Polysaccharides
4.5.1. Determination of Uronic Acid Content
4.5.2. Determination of TPC
4.5.3. Determination of TFC
4.5.4. Monosaccharide Composition Determination
4.5.5. FT-IR Analysis
4.5.6. Molecular Weight Determination
4.5.7. SEM Analysis
4.5.8. AFM Analysis
4.6. Antioxidant Activity of VTP and DVTP In Vitro
4.6.1. DPPH Radical Scavenging Test
4.6.2. Hydroxyl Radical Scavenging Test
4.6.3. T-AOC Test
4.7. Cell Culture and Viability Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, G.; Li, J.; Zhang, J.; Liu, H.; Ye, Q.; Wang, Z. Structural characterization and antitumor activity of a polysaccharide from Dendrobium wardianum. Carbohydr. Polym. 2021, 269, 118253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sheng, Y.; Liu, J.; Xu, G.; Yu, W.; Cui, Q.; Lu, X.; Du, P.; An, L. Hair-growth promoting effect and anti-inflammatory mechanism of Ginkgo biloba polysaccharides. Carbohydr. Polym. 2022, 278, 118811. [Google Scholar] [CrossRef] [PubMed]
- Chaiklahan, R.; Srinorasing, T.; Chirasuwan, N.; Tamtin, M.; Bunnag, B. The potential of polysaccharide extracts from Caulerpa lentillifera waste. Int. J. Biol. Macromol. 2020, 161, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Ge, J.C.; Zhang, F.Y.; Zha, X.Q.; Liu, J.; Li, Q.M.; Luo, J.P. Dendrobium officinale polysaccharide promotes M1 polarization of TAMs to inhibit tumor growth by targeting TLR2. Carbohydr. Polym. 2022, 292, 119683. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Z.; Yang, X.; Gao, Y.; Ren, Y.; Li, Q.; Qu, Y.; Chen, G.; Zeng, R. Investigation into the physical properties, antioxidant and antibacterial activity of Bletilla striata polysaccharide/chitosan membranes. Int. J. Biol. Macromol. 2021, 182, 311–320. [Google Scholar] [CrossRef]
- Zhang, B.; Lan, W.; Xie, J. Chemical modifications in the structure of marine polysaccharide as serviceable food processing and preservation assistant: A review. Int. J. Biol. Macromol. 2022, 223 Pt A, 1539–1555. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, B.; Zhang, X.; Xu, M.; Chang, H.; Lu, X.; Ren, X. Purification of a polysaccharide from Boschniakia rossica and its synergistic antitumor effect combined with 5-Fluorouracil. Carbohydr. Polym. 2012, 89, 31–35. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Jeon, Y.J. The potential of sulfated polysaccharides isolated from the Brown Seaweed Ecklonia maxima in cosmetics: Antioxidant, anti-melanogenesis, and photoprotective activities. Antioxidants 2020, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M. Pharmacography of Uyghur; Xinjiang Science & Technology & Hygiene Publishing House: Urumuqi, China, 1999; pp. 496–498. [Google Scholar]
- Department of Health, Xinjiang Uygur Autonomous Region. Uyghur Medicine Standard; Xinjiang Science & Technology & Hygiene Publishing House: Urumuqi, China, 1993; pp. 347–348.
- Abudukerimu, A.; Maimaiti, A. Research progress on chemical constituents of Suosuo grapes. J. Med. Pharm. Chin. 2009, 15, 69–72. [Google Scholar]
- Wang, L.; Zhou, W.; Liu, C.; Chen, P.; Zhou, L. Study on the accumulation pattern of anthocyanins, sugars and organic acids in medicinal Vitis vinifera ‘SuoSuo’ during ripening. Food Chem. 2024, 433, 137294. [Google Scholar] [CrossRef]
- Yuan, F.; Zhang, J.; Ma, L. Protective Effects of Polysaccharides from Vitis vinifera L. on Aβ25-35 Induced Injury in PC12 Cells. Sci. Technol. Rev. 2012, 30, 47–50. [Google Scholar]
- Li, Y.; Ma, L.; Xiao, H.; Tian, Y.; Yuan, Y.; Yuan, F. Effect of polysaccharides extracted from vitis vinifera L. on learning and memory abilities and oxidative stress in rats with Alzheimer’s disease. China Med. 2018, 13, 705–709. [Google Scholar]
- Yuan, F.; Zhou, Y.; Sheng, L.; Hu, H. Effect of Polysaccharides from Vitis vinifera L. on expression of APP in Alzheimer’s Disease Cell Model. Chin. J. Exp. Tradit. 2015, 21, 107–110. [Google Scholar]
- Yuan, F.; Xu, Q.; Sheng, L.; Liu, T. The effects of polysaccharides from Vitis viniferal L. on apoptosis of PC12 cells induced by Aβ25-35. Chin. J. Gerontol. 2015, 35, 5372–5373. [Google Scholar]
- Su, D.; Zhang, F.; Zhao, J.; Liu, T.; Ma, L. Experimental study on the anti-hepatic fibrosis effect of polysaccharides from Vitis viniferal L. J. Toxicol. 2018, 32, 45–48. [Google Scholar]
- Zhang, L.; Ma, L.; Liu, T.; Su, D.; Yu, D.; Zhao, C. Study on the inhibiting capacity of flavonoid and polysaccharides extracted from Suosuo Grapes to HepG2 Cells’ reproductive activity. J. Xinjiang Med. Univ. 2009, 32, 529–532. [Google Scholar]
- Liu, T.; Zhao, J.; Li, H.; Ma, L. Evaluation on anti-hepatitis viral activity of Vitis vinifer L. Molecules 2010, 15, 7415–7422. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.; Li, W.; Zhang, H.; Nie, S.; Wang, Y.; Li, C. An effective method for deproteinization of bioactive polysaccharides extracted from Lingzhi (Ganoderma atrum). Food Sci. Biotechnol. 2012, 21, 191–198. [Google Scholar] [CrossRef]
- Zeng, X.; Li, P.; Chen, X.; Kang, Y.; Xie, Y.; Li, X.; Xie, T.; Zhang, Y. Effects of deproteinization methods on primary structure and antioxidant activity of Ganoderma lucidum polysaccharides. Int. J. Biol. Macromol. 2019, 126, 867–876. [Google Scholar] [CrossRef]
- Ke, L.; Duan, X.; Cui, J.; Song, X.; Ma, W.; Zhang, W.; Liu, Y.; Fan, Y. Research progress on the extraction technology and activity study of epimedium polysaccharides. Carbohydr. Polym. 2023, 306, 120602. [Google Scholar] [CrossRef]
- Peng, X.; Hu, X.; Zhang, Y.; Xu, H.; Tang, J.; Zhang, G.; Deng, J.; Kan, H.; Zhao, P.; Liu, Y. Extraction, characterization, antioxidant and anti-tumor activities of polysaccharides from camellia fascicularis leaves. Int. J. Biol. Macromol. 2022, 222 Pt A, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiang, Q.; Zhao, J.; Mao, G.; Feng, W.; Chen, Y.; Li, Q.; Wu, X.; Yang, L.; Zhao, T. Purification, structural elucidation and physicochemical properties of a polysaccharide from Abelmoschus esculentus L. (okra) flowers. Int. J. Biol. Macromol. 2020, 155, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Zhu, M.; Yang, B.; Wang, X.; Zeng, J.; Yang, Y.; Guo, S.; Jia, X.; Feng, L. Characterization of a novel polysaccharide from red ginseng and its ameliorative effect on oxidative stress injury in myocardial ischemia. Chin. Med. 2022, 17, 111. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Y.; Wang, J.; Guo, X.; Zheng, Y.; Zhao, W.; Mei, X.; Guo, T.; Yang, Z. Physicochemical characteristics and bioactivities of the exopolysaccharide and its sulphated polymer from Streptococcus thermophilus GST-6. Carbohydr. Polym. 2016, 146, 368–375. [Google Scholar] [CrossRef]
- Yang, W.; Huang, G. Preparation and analysis of polysaccharide from Solanum tuberdsm. Ultrason. Sonochem. 2023, 98, 106520. [Google Scholar] [CrossRef]
- Guan, L.; Li, X.; Chen, S.; Tang, X.; Zhao, N.; Li, W. Optimization of deproteinization process of Bletilla Striata polysaccharides by response surface methodology and polysaccharides properties. Sci. Technol. Chem. Ind. 2022, 30, 10–15. [Google Scholar]
- Yang, R.; Meng, D.; Song, Y.; Li, J.; Zhang, Y.; Hu, X.; Ni, Y.; Li, Q. Simultaneous decoloration and deproteinization of crude polysaccharide from pumpkin residues by cross-linked polystyrene macroporous resin. J. Agric. Food Chem. 2012, 60, 8450–8456. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of active ingredients in polysaccharide-protein complex coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, L.; Zhao, Q.; Zhang, L.; Chen, J.; Liu, B.; Zhao, B. Preliminary characterizations, antioxidant and hepatoprotective activity of polysaccharide from Cistanche deserticola. Int. J. Biol. Macromol. 2016, 93 Pt A, 678–685. [Google Scholar] [CrossRef]
- Ji, X.; Yan, Y.; Hou, C.; Shi, M.; Liu, Y. Structural characterization of a galacturonic acid-rich polysaccharide from Ziziphus jujuba cv. Muzao. Int. J. Biol. Macromol. 2020, 147, 844–852. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Fan, L.; Ai, L.; Shan, L. Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus jujuba cv. Jinsixiaozao. Carbohydr. Polym. 2011, 84, 390–394. [Google Scholar] [CrossRef]
- Wu, D.T.; Liu, W.; Han, Q.H.; Du, G.; Li, H.Y.; Yuan, Q.; Fu, Y.; Zhao, L.; Zhang, Q.; Li, S.Q.; et al. Physicochemical characteristics and antioxidant activities of non-starch polysaccharides from different kiwifruits. Int. J. Biol. Macromol. 2019, 136, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Zhang, D.; Huang, B.; Yi, P.; Yan, C. Structural characterization and DPPH· radical scavenging activity of a polysaccharide from Guara fruits. Carbohydr. Polym. 2014, 103, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Jiang, L.; Yang, X.; Huang, L.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Physicochemical and functional properties of a water-soluble polysaccharide extracted from mung bean (Vigna radiate L.) and its antioxidant activity. Int. J. Biol. Macromol. 2019, 138, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, N.; Olas, B. Selected seeds as sources of bioactive compounds with diverse biological activities. Nutrients 2022, 15, 187. [Google Scholar] [CrossRef]
- Cai, L.; Chen, B.; Yi, F.; Zou, S. Optimization of extraction of polysaccharide from dandelion root by response surface methodology: Structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2019, 140, 907–919. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, X.; Ge, Q.; Li, J.; Wang, D.; Wei, Y.; Ouyang, Z. Antioxidant and anti-aging activities of polysaccharides from Cordyceps cicadae. Int. J. Biol. Macromol. 2020, 157, 394–400. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Pan, L.C.; Zhang, L.J.; Yin, Y.; Zhu, Z.Y.; Sun, H.Q.; Liu, C.Y. Chemical structure and antioxidant activity of a polysaccharide from Siraitia grosvenorii. Int. J. Biol. Macromol. 2020, 165 Pt B, 1900–1910. [Google Scholar] [CrossRef]
- Khaskheli, S.G.; Zheng, W.; Sheikh, S.A.; Khaskheli, A.A.; Liu, Y.; Soomro, A.H.; Feng, X.; Sauer, M.B.; Wang, Y.F.; Huang, W. Characterization of Auricularia auricula polysaccharides and its antioxidant properties in fresh and pickled product. Int. J. Biol. Macromol. 2015, 81, 387–395. [Google Scholar] [CrossRef]
- Jin, R.; Guo, Y.; Xu, B.; Wang, H.; Yuan, C. Physicochemical properties of polysaccharides separated from Camellia oleifera Abel seed cake and its hypoglycemic activity on streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2019, 125, 1075–1083. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y.; Cui, Y.; Liu, H.; Dong, C.; Sun, Y. Structural characterization, antioxidant and immunomodulatory activities of a neutral polysaccharide from Cordyceps militaris cultivated on hull-less barley. Carbohydr. Polym. 2020, 235, 115969. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, Y.; Song, H.; Zhou, H.; Zhong, F.; Hu, H.; Feng, Y. Extraction optimization, characterization and antioxidant activity of polysaccharide from Gentiana scabra bge. Int. J. Biol. Macromol. 2016, 93 Pt A, 369–380. [Google Scholar] [CrossRef]
- Cao, C.; Li, C.; Chen, Q.; Huang, Q.; Pérez ME, M.; Fu, X. Physicochemical characterization, potential antioxidant and hypoglycemic activity of polysaccharide from Sargassum pallidum. Int. J. Biol. Macromol. 2019, 139, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Huo, Y.F.; Xu, L.; Xu, Y.Y.; Wang, X.L.; Zhou, T. Purification, characterization and antioxidant activity of polysaccharides from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 165 Pt B, 2116–2125. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef]
- Li, S.F.; Wang, A.J.; Liu, L.N.; Tian, G.R.; Xu, F.F. Effect of deproteinization methods on the antioxidant activity of polysaccharides extracted from Lentinus edodes stipe. J. Food Meas. Charact. 2019, 13, 1382–1389. [Google Scholar] [CrossRef]
- Zheng, S.; Peng, Y.; Chen, Q.; Zheng, M. Biological activity of Tremella polysaccharides with or without deproteinization. Wuyi Sci. J. 2017, 33, 94–100. [Google Scholar]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Gao, J.; Hu, D.; Shen, Y.; Zheng, Y.; Liang, Y. Optimization of ultrasonic-assisted polysaccharide extraction from Hyperici perforati herba using response surface methodology and assessment of its antioxidant activity. Int. J. Biol. Macromol. 2023, 225, 255–265. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, B.; Han, M.; Guo, Y.; Cheng, Y.; Qian, H. Purification, structural characterization and neuroprotective effect of a neutral polysaccharide from Sparassis crispa. Int. J. Biol. Macromol. 2022, 201, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.D.; Wang, B.D.; Qin, D.L.; Su, X.H.; Yu, L.; Wu, J.M.; Law, B.Y.; Guo, M.S.; Yu, C.L.; Zhou, X.G.; et al. Carpesii fructus extract exhibits neuroprotective effects in cellular and Caenorhabditis elegans models of Parkinson’s disease. CNS Neurosci. Ther. 2024, 30, e14515. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, X.; Jiang, Y.; Yao, J.; Jiang, Y.; Li, Z.; Dai, F. Chemical structure and antioxidant activity of a neutral polysaccharide from Asteris radix et rhizoma. Carbohydr. Polym. 2022, 286, 119309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cai, T.; He, X. Characterization, sulfated modification and bioactivity of a novel polysaccharide from Millettia dielsiana. Int. J. Biol. Macromol. 2018, 117, 108–115. [Google Scholar] [CrossRef]
- Liu, M.; Dai, Y.; Song, C.; Wang, J.; Liu, Y.; Wang, Q. Structural characterization of a Pleurotus sajor-caju polysaccharide and its neuroprotection related to the inhibition of oxidative stress. Nutrients 2022, 14, 4047. [Google Scholar] [CrossRef]
- Yuan, L.; Zhong, Z.C.; Liu, Y. Structural characterization and immunomodulatory activity of a neutral polysaccharide from Sambucus adnata Wall. Int. J. Biol. Macromol. 2020, 154, 1400–1407. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhong, W.; Yang, C.; Zhang, Y.; Yang, D. The prebiotic properties of polysaccharides obtained by differentiated deproteinization methods from Flos Sophorae Immaturus on Lactobacillus fermentum. Front. Microbiol. 2022, 13, 1007267. [Google Scholar] [CrossRef]
- Selvendran, R.R.; March, J.F.; Ring, S.G. Determination of aldoses and uronic acid content of vegetable fiber. Anal. Biochem. 1979, 96, 282–292. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Najari, Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int. J. Biol. Macromol. 2019, 125, 621–629. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, R.; Wen, P.; Song, Y.; He, B.; Tan, J.; Hao, H.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohydr. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Shu, Z.P.; Xu, B.Q.; Xing, N.; Jiao, W.J.; Yang, B.Y.; Kuang, H.X. Structural characterization and antioxidant activities of polysaccharides from Citrus aurantium L. Int. J. Biol. Macromol. 2014, 67, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, Q.; Ling, C. Water-soluble yeast β-glucan fractions with different molecular weights: Extraction and separation by acidolysis assisted-size exclusion chromatography and their association with proliferative activity. Int. J. Biol. Macromol. 2019, 123, 269–279. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, W.; Wu, Z.; Liu, R.; Yang, C.; Hui, A.; Huang, X.; Xian, Z. Preparation, characterization, antioxidant and anti-inflammatory activities of acid-soluble pectin from okra (Abelmoschus esculentus L.). Int. J. Biol. Macromol. 2021, 181, 824–834. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Zhong, C.; Pu, Y.; Yang, Z.; Bao, Y. Structure characteristics and immunomodulatory activities of a polysaccharide RGRP-1b from radix ginseng Rubra. Int. J. Biol. Macromol. 2021, 189, 980–992. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.Q.; Zhang, C.H.; Wu, W.L.; Lyu, L.F.; Li, W.L. Growth and physiological characteristics of four blueberry cultivars under different high soil ph treatments. Environ. Exp. Bot. 2022, 197, 104842. [Google Scholar] [CrossRef]
- Liu, M.; Gong, Z.; Liu, H.; Wang, J.; Wang, D.; Yang, Y.; Zhong, S. Structural characterization and anti-tumor activity in vitro of a water-soluble polysaccharide from dark brick tea. Int. J. Biol. Macromol. 2022, 205, 615–625. [Google Scholar] [CrossRef]



| Run | A | B | C | D | Protein Removal Rate (%) | Polysaccharide Retention Rate (%) | Comprehensive Score |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 1 | 0 | 1 | 38.57 ± 0.64 | 71.67 ± 0.84 | 87.70 |
| 2 | 1 | −1 | 0 | 0 | 45.97 ± 0.64 | 65.67 ± 2.34 | 91.46 |
| 3 | −1 | 1 | 0 | 0 | 32.80 ± 0.35 | 71.08 ± 2.03 | 81.28 |
| 4 | 0 | −1 | 0 | 1 | 47.83 ± 0.45 | 66.25 ± 3.41 | 93.79 |
| 5 | −1 | 0 | −1 | 0 | 32.14 ± 0.50 | 73.16 ± 6.45 | 81.96 |
| 6 | 0 | 0 | 0 | 0 | 42.44 ± 0.38 | 67.67 ± 0.68 | 89.10 |
| 7 | 0 | 0 | 0 | 0 | 41.95 ± 0.25 | 72.74 ± 1.58 | 91.94 |
| 8 | −1 | 0 | 1 | 0 | 40.30 ± 0.65 | 67.75 ± 0.91 | 86.92 |
| 9 | 0 | 0 | −1 | −1 | 29.71 ± 0.55 | 73.21 ± 4.13 | 79.45 |
| 10 | 0 | 0 | 1 | 1 | 45.41 ± 0.59 | 67.53 ± 2.59 | 92.11 |
| 11 | 1 | 1 | 0 | 0 | 37.47 ± 2.17 | 69.09 ± 1.47 | 84.84 |
| 12 | 1 | 0 | 0 | −1 | 39.14 ± 1.77 | 68.36 ± 0.80 | 86.11 |
| 13 | −1 | 0 | 0 | 1 | 39.56 ± 0.31 | 70.44 ± 6.88 | 87.92 |
| 14 | 0 | 1 | −1 | 0 | 31.97 ± 0.67 | 75.64 ± 1.05 | 83.42 |
| 15 | 0 | 0 | −1 | 1 | 38.69 ± 0.43 | 68.01 ± 2.81 | 85.40 |
| 16 | 0 | 1 | 0 | −1 | 32.69 ± 0.71 | 70.69 ± 2.79 | 80.90 |
| 17 | 0 | −1 | 1 | 0 | 43.97 ± 0.33 | 69.85 ± 1.40 | 92.13 |
| 18 | −1 | 0 | 0 | −1 | 33.09 ± 0.37 | 66.77 ± 0.87 | 78.73 |
| 19 | 0 | −1 | 0 | −1 | 43.48 ± 1.77 | 66.79 ± 3.52 | 89.60 |
| 20 | −1 | −1 | 0 | 0 | 37.11 ± 0.05 | 68.44 ± 2.95 | 84.04 |
| 21 | 0 | 1 | 1 | 0 | 37.51 ± 0.61 | 70.58 ± 3.08 | 85.87 |
| 22 | 1 | 0 | 1 | 0 | 46.69 ± 0.81 | 63.60 ± 4.60 | 90.85 |
| 23 | 0 | −1 | −1 | 0 | 43.92 ± 0.90 | 64.28 ± 1.85 | 88.41 |
| 24 | 0 | 0 | 1 | −1 | 39.50 ± 0.57 | 68.95 ± 2.53 | 86.87 |
| 25 | 0 | 0 | 0 | 0 | 41.97 ± 0.64 | 67.97 ± 2.05 | 88.81 |
| 26 | 1 | 0 | −1 | 0 | 38.34 ± 1.51 | 67.03 ± 2.38 | 84.39 |
| 27 | 1 | 0 | 0 | 1 | 44.96 ± 1.09 | 66.71 ± 0.91 | 91.10 |
| Origin of Variance | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 419.58 | 14 | 29.97 | 11.75 | <0.0001 |
| A | 64.90 | 1 | 64.90 | 25.44 | 0.0003 |
| B | 104.56 | 1 | 104.56 | 40.99 | <0.0001 |
| C | 83.90 | 1 | 83.90 | 32.89 | <0.0001 |
| D | 110.21 | 1 | 110.21 | 43.21 | <0.0001 |
| AB | 3.72 | 1 | 3.72 | 1.46 | 0.2505 |
| AC | 0.5691 | 1 | 0.5691 | 0.2231 | 0.6452 |
| AD | 4.41 | 1 | 4.41 | 1.73 | 0.2130 |
| BC | 0.4041 | 1 | 0.4041 | 0.1584 | 0.6976 |
| BD | 1.69 | 1 | 1.69 | 0.6636 | 0.4312 |
| CD | 0.1293 | 1 | 0.1293 | 0.0507 | 0.8257 |
| A2 | 40.12 | 1 | 40.12 | 15.73 | 0.0019 |
| B2 | 5.47 | 1 | 5.47 | 2.14 | 0.1689 |
| C2 | 15.77 | 1 | 15.77 | 6.18 | 0.0286 |
| D2 | 11.74 | 1 | 11.74 | 4.60 | 0.0531 |
| Residual | 30.61 | 12 | 2.55 | ||
| Lack of Fit | 24.63 | 10 | 2.46 | 0.8231 | 0.6630 |
| Pure Error | 5.98 | 2 | 2.99 | ||
| Cor Total | 450.19 | 26 |
| Levels | Factors | |||
|---|---|---|---|---|
| A | B | C | D | |
| −1 | 3:1 | 2:1 | 3 | 10 |
| 0 | 4:1 | 3:1 | 4 | 20 |
| 1 | 5:1 | 4:1 | 5 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Wu, Y.; Gao, P.; Zheng, Q.; Lu, Y.; Yuan, F.; Jing, W. Optimization of the Deproteinization Process via Response Surface Methodology, Preliminary Characterization, and the Determination of the Antioxidant Activities of Polysaccharides from Vitis vinifera L. SuoSuo. Molecules 2024, 29, 4734. https://doi.org/10.3390/molecules29194734
Ma X, Wu Y, Gao P, Zheng Q, Lu Y, Yuan F, Jing W. Optimization of the Deproteinization Process via Response Surface Methodology, Preliminary Characterization, and the Determination of the Antioxidant Activities of Polysaccharides from Vitis vinifera L. SuoSuo. Molecules. 2024; 29(19):4734. https://doi.org/10.3390/molecules29194734
Chicago/Turabian StyleMa, Xinnian, Yan Wu, Pei Gao, Qingsong Zheng, Yibo Lu, Fang Yuan, and Weixin Jing. 2024. "Optimization of the Deproteinization Process via Response Surface Methodology, Preliminary Characterization, and the Determination of the Antioxidant Activities of Polysaccharides from Vitis vinifera L. SuoSuo" Molecules 29, no. 19: 4734. https://doi.org/10.3390/molecules29194734
APA StyleMa, X., Wu, Y., Gao, P., Zheng, Q., Lu, Y., Yuan, F., & Jing, W. (2024). Optimization of the Deproteinization Process via Response Surface Methodology, Preliminary Characterization, and the Determination of the Antioxidant Activities of Polysaccharides from Vitis vinifera L. SuoSuo. Molecules, 29(19), 4734. https://doi.org/10.3390/molecules29194734






