Review of the Structural Characteristics and Biological Activities of Tricholoma Secondary Metabolites (2018–2023)
Abstract
1. Introduction
2. Secondary Metabolites of Tricholoma
2.1. Terpenoids
2.1.1. Triterpenes and Sterols
2.1.2. Diterpenoids
2.1.3. Sesterterpenoids and C17 Compounds
2.2. Alkaloids
2.2.1. Diketopiperazine
2.2.2. Indole Derivatives
2.3. Other Compounds
2.3.1. γ-Glutamine Derivative
2.3.2. Amide Derivatives
2.3.3. Acetylene Compounds
2.3.4. Polyketide Compounds
2.3.5. Volatile Compounds
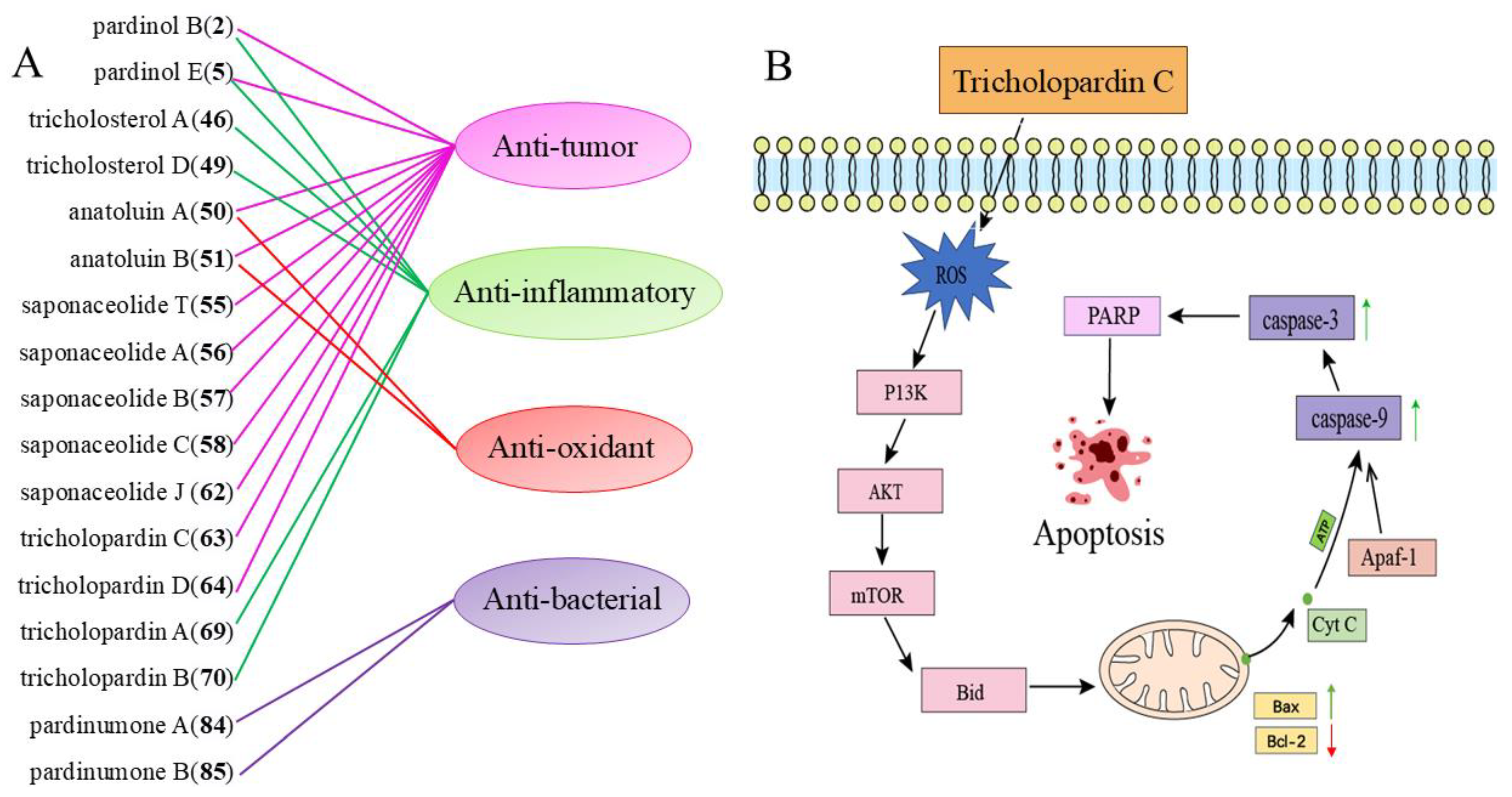
3. Biological Activity
3.1. Antibacterial Activity
3.2. Anti-Cancer Activity
3.3. Anti-Inflammatory Activity
3.4. Antioxidant Activity
| Numbers | Names | Species | Bioactivities | References |
|---|---|---|---|---|
| Lanostane triterpenoids | ||||
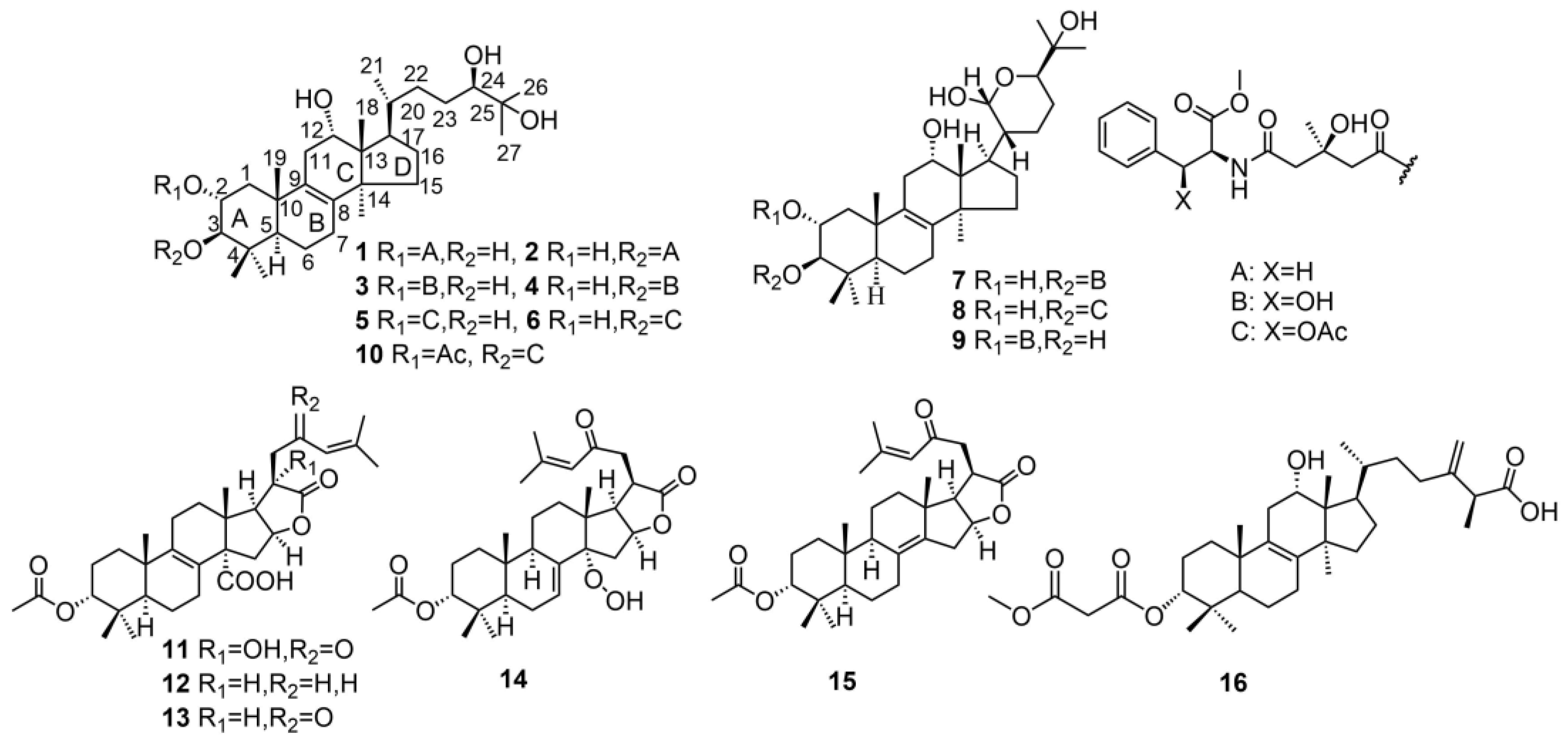
| 1 | pardinol A | T. pardinum | - | [11] |
| 2 | pardinol B | T. pardinum | Anti-inflammatory activity; Anti-cancer activity | [11] |
| 3 | pardinol C | T. pardinum | - | [11] |
| 4 | pardinol D | T. pardinum | - | [11] |
| 5 | pardinol E | T. pardinum | Anti-inflammatory activity; Anti-cancer activity | [11] |
| 6 | pardinol F | T. pardinum | Anti-inflammatory activity; Anti-cancer activity | [11] |
| 7 | pardinol G | T. pardinum | Anti-inflammatory activity; Anti-cancer activity | [11] |
| 8 | pardinol H | T. pardinum | Anti-inflammatory activity; Anti-cancer activity | [11] |
| 9 | saponaceol B | T. pardinum | - | [11] |
| 10 | saponaceol D | T. saponaceum | - | [11] |
| 11 | tricholidic acid B | T. ustaloides | - | [14] |
| 12 | tricholidic acid C | T. ustaloides | - | [14] |
| 13 | tricholidic acid | T. ustaloides | - | [14] |
| 14 | tricholimbrin A | T. imbricatum | - | [15] |
| 15 | Tricholimbrin B | T. imbricatum | - | [15] |
| 16 | (25S)-(+)-12α-hydroxy-3α-methylcarboxyacetate-24-methyllanosta-8,24(31)-diene-26-oic acid | T. imbricatum | Anti-cancer activity | [15] |
| Ergostane triterpenoids | ||||
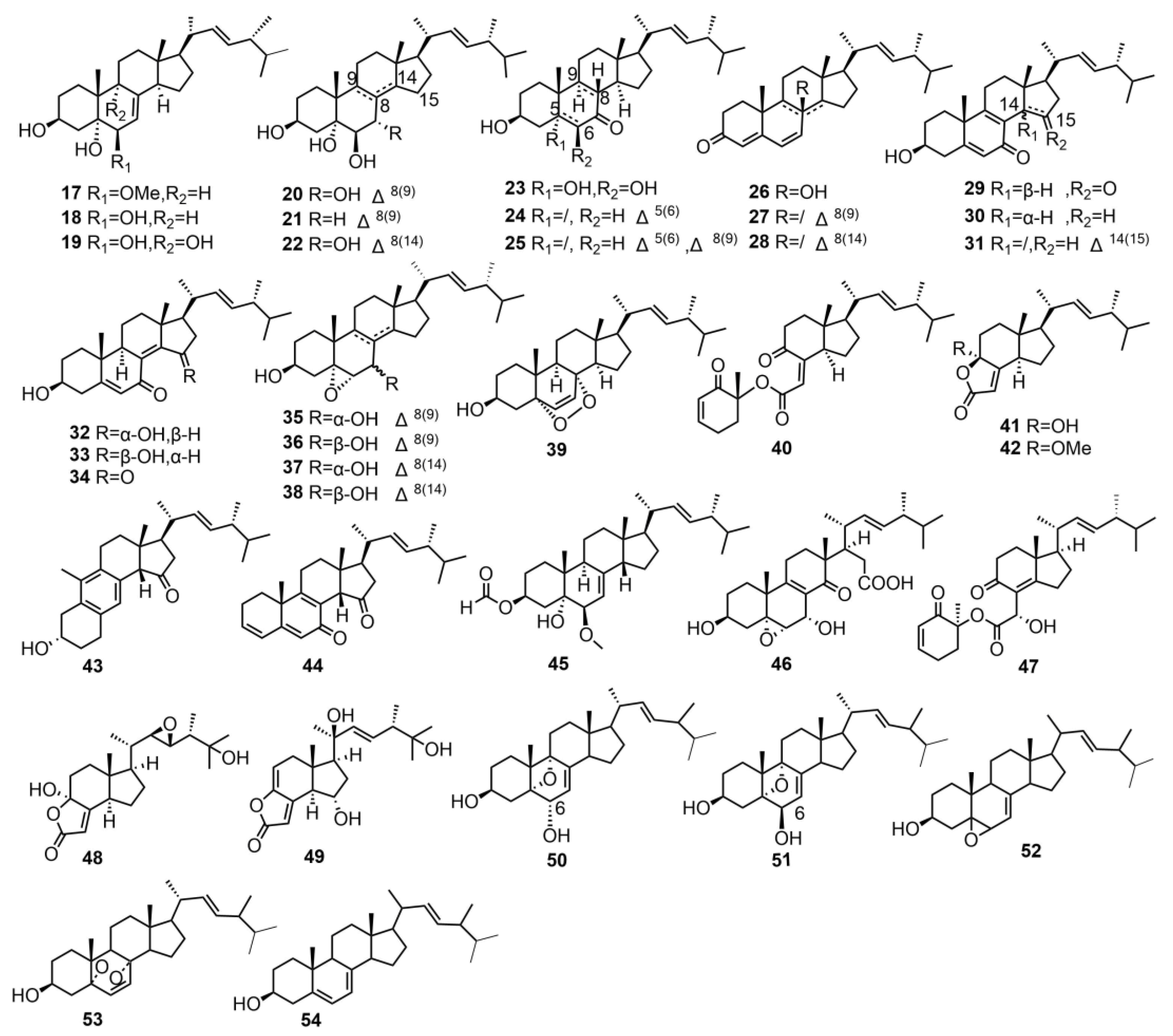
| 17 | 3β,5α-dihydroxy-6β-methoxyergosta-7,22-diene | T. imbricatum | - | [15] |
| 18 | (22E,24R)-5α,6α-epoxyergosta-8,22- | T. imbricatum | - | [15] |
| 19 | dien-3β,7α-diol | T. imbricatum | - | [15] |
| 20 | (22E,24R)-ergosta-7,22-diene-3β,5α,6β,9α-tetraol | T. imbricatum | - | [15] |
| 21 | (22E,24R)-ergosta-8,22-diene-3β,5α,6β,7α-tetrol | T. imbricatum | - | [15] |
| (22E,24R)-ergosta-8,22-diene-3β,5α,6β,7α-tetrol | T. imbricatum | - | [15] | |
| 22 | (22E,24R)-ergosta-8(14),22-diene-3β,5α,6β,7α-tetrol | T. imbricatum | - | [15] |
| 23 | 3β,5α,6β-trihydroxy-(22E,24R)-ergost-22-en-7- one | T. imbricatum | - | [15] |
| 24 | 3β-hydroxy-(22E,24R)-ergosta-5,22- dien-7-one | T. imbricatum | - | [15] |
| 25 | 3β-hydroxy-(22E,24R)-ergosta-5,22- dien-7-one | T. imbricatum | - | [15] |
| 26 | isocyathisterol | T. imbricatum | Anti-cancer activity | [15] |
| 27 | (22E)-ergosta-4,6,8,22-tetraen-3-one | T. imbricatum | - | [15] |
| 28 | (22E,24R)-ergosta-4,6,8(14),22-tetraen-3-one | T. imbricatum | - | [15] |
| 29 | 3β-hydroxyl-(22E,24R)-ergosta-5,8,22-trien-7,15-dione | T. imbricatum | Anti-cancer activity | [15] |
| 30 | 3β-hydroxyl-(22E,24R)-ergosta-5,8,22-trien-7-one | T. imbricatum | Anti-cancer activity | [15] |
| 31 | 3β-hydroxyl-(22E,24R)- ergosta-5,8,14,22-tetraen-7-one | T. imbricatum | - | [15] |
| 32 | 3β,15α-dihydroxyl-(22E,24R)-ergosta-5,8(14),22-trien-7-one | T. imbricatum | Anti-cancer activity | [15] |
| 33 | 3β,15β-dihydroxyl-(22E,24R)-ergosta-5,8(14),22-trien-7-one | T. imbricatum | - | [15] |
| 34 | 3β-hydroxyl-(22E,24R)-ergosta-5,8(14),22-trien-7,15-dione | T. imbricatum | Anti-cancer activity | [15] |
| 35 | 5α,6α-epoxy-(22E,24R)-ergosta-8,22-diene-3β,7β-diol | T. imbricatum | Anti-cancer activity | [15] |
| 36 | 5α,6α-epoxy-(22E,24R)-ergosta-8(14),22-diene-3β,7α-diol | T. imbricatum | - | [15] |
| 37 | 5α,6α-epoxy-(22E,24R)-ergosta-8(14),22-diene-3β,7β-diol | T. imbricatum | - | [15] |
| 38 | 5α,6α-epoxy-(22E,24R)-ergosta-8(14),22-diene-3β,7β-diol | T. imbricatum | - | [15] |
| 39 | 5α,8α-epidioxy-(22E,24R)-ergosta-6,22- dien-3β-ol | T. imbricatum | - | [15] |
| 40 | chaxine C | T. imbricatum | Antibacterial activity; Anti-cancer activity | [15,58] |
| 41 | demethylincisterol A3 | T. imbricatum | Anti-cancer activity | [15] |
| 42 | volemolide | T. imbricatum | Anti-cancer activity | [15] |
| 43 | tricholimbrin C | T. imbricatum | - | [15] |
| 44 | tricholimbrin D | T. imbricatum | - | [15] |
| 45 | tricholimbrin E | T. imbricatum | - | [15] |
| 46 | tricholosterol A | T. terreum | Anti-inflammatory activity | [16] |
| 47 | tricholosterol B | T. terreum | - | [16] |
| 48 | tricholosterol C | T. terreum | - | [16] |
| 49 | tricholosterol D | T. terreum | Anti-inflammatory activity; Cytotoxic against human cancer cell lines | [16] |
| 50 | anatoluin A | T. anatolicum | Antioxidant activity; Cytotoxic against human cancer cell lines | [17] |
| 51 | anatoluin B | T. anatolicum | Antioxidant activity; Cytotoxic against human cancer cell lines | [17] |
| 52 | 5α,6α-epoxy-ergosta-7,22-dien,3β-ol | T. anatolicum | Antioxidant activity; Cytotoxic against human cancer cell lines | [17] |
| 53 | ergosterol-endoperoxide | T. anatolicum | Antioxidant activity; Cytotoxic against human cancer cell lines | [17] |
| 54 | ergosterol,3β-ol | T. anatolicum | - | [17] |
| Triterpenoids | ||||
| 55 | saponaceolide T | T. saponaceum | Cytotoxic against human cancer cell lines | [13] |
| 56 | saponaceolide A | T. saponaceum | Cytotoxic against human cancer cell lines | [13] |
| 57 | saponaceolide B | T. saponaceum | Cytotoxic against human cancer cell lines | [13] |
| 58 | saponaceolide C | T. saponaceum | Cytotoxic against human cancer cell lines | [13] |
| 59 | saponaceolide D | T. saponaceum | - | [13] |
| 60 | saponaceolide F | T. saponaceum | Cytotoxic against human cancer cell lines | [13,14] |
| 61 | saponaceolide H | T. saponaceum | Cytotoxic against human cancer cell lines | [13] |
| 62 | saponaceolide J | T. ustaloides | Cytotoxic against human cancer cell lines | [14] |
| 63 | tricholopardin C | T. pardinum | Cytotoxic against human cancer cell lines | [18] |
| 64 | tricholopardin D | T. pardinum | Cytotoxic against human cancer cell lines | [18] |
| Diterpenoids | ||||
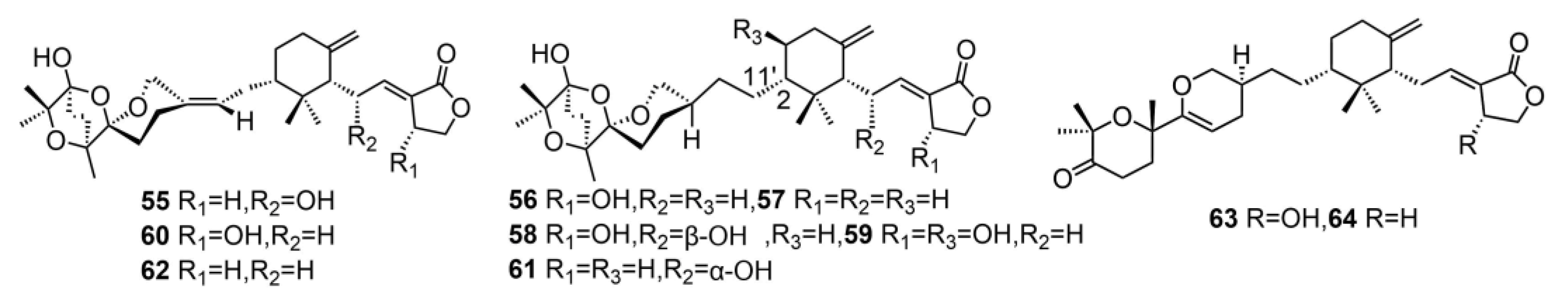
| 65 | tricholomalide D | T. ustaloides | - | [23] |
| 66 | tricholomalide E | T. ustaloides | - | [23] |
| 67 | tricholomalide F | T. ustaloides | - | [23] |
| 68 | tricholomalide G | T. ustaloides | - | [23] |
| Sesterterpenoid | ||||
| 69 | tricholopardin A | T. pardinum | Anti-inflammatory activity | [31] |
| C17 compound | ||||
| 70 | tricholopardin B | T. pardinum | Anti-inflammatory activity | [31] |
| Diketopiperazines | ||||
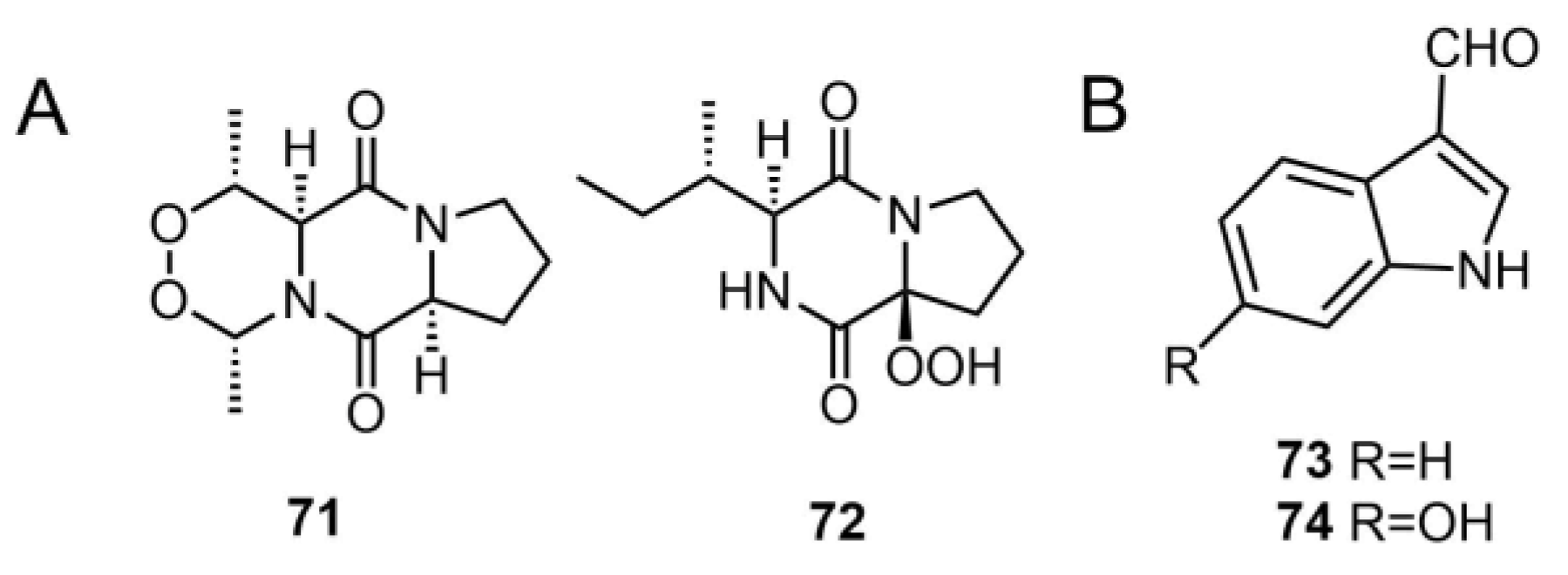
| 71 | matsudipeptide A | T. matsutake | - | [39] |
| 72 | matsudipeptide B | T. matsutake | - | [39] |
| Indole derivatives | ||||
| 73 | 1H-indole-3-carbaldehyde | T. lascivum | - | [45] |
| 74 | 6-hydroxy-1H-indole-3-carbaldehyde | T. pardinum | - | [41] |
| γ-glutamine derivative | ||||
| 75 | lascivol | T. pardinum | - | [41] |
| Amide derivatives | ||||
| 76 | tricholomine A | T. bakamatsutake | - | [47] |
| 77 | tricholomine B | T. bakamatsutake | - | [47] |
| 78 | tricholomine C | T. bakamatsutake | - | [48] |
| Acetylene compounds | ||||
| 79 | tricholomenyn C | T. ustaloides | - | [14] |
| 80 | (Z)-non-7-en-5-yn-1,2,4-triol | T. pardinum | - | [41] |
| 81 | (Z)-non-7-en-5-yn-1,4-diol | T. pardinum | - | [41] |
| 82 | (Z)-1,2-dihydroxynon-7-en-5-yn-4-one | T. pardinum | - | [41] |
| 83 | (Z)-1-hydroxynon-7-en-5-yn-4-one | T. pardinum | - | [41] |
| Polyketide compounds | ||||
| 84 | pardinumone A | T. pardinum | Antibacterial activity | [52] |
| 85 | pardinumone B | T. pardinum | Antibacterial activity | [52] |
| 86 | pardinumone C | T. pardinum | Antibacterial activity | [52] |
| 87 | pardinumone D | T. pardinum | Antibacterial activity | [52] |
| 88 | 3,5-dihydroxyfuran-2(5H)-one | T. anatolicum | - | [17] |
| 89 | 4-chromone derivative | T. imbricatum | - | [15] |
| Volatile compounds | ||||
| 90 | hexanal | T. magnivelare | - | [54] |
| 91 | 1-octen-3-one | T. magnivelare | - | [54] |
| 92 | (E)-oct-2-enal | T. magnivelare | - | [54] |
| 93 | 1-octen-3-ol | T. magnivelare | Antibacterial activity | [55,56] |
| 94 | linalool | T. magnivelare | - | [54] |
| 95 | (2E,4E)-nona- 2,4-dienal | T. magnivelare | - | [54] |
| 96 | ethyl 3-phenylpropanoate | T. magnivelare | - | [54] |
| 97 | 4-methoxybenzaldehyde | T. magnivelare | - | [54] |
| 98 | methyl (E)-3-phenylprop-2-enoate | T. magnivelare | - | [54] |
| 99 | 3,4-dimethoxybenzaldehyde | T. magnivelare | - | [54] |
| 100 | α-pinene | T. magnivelare | - | [54] |
| 101 | linalool | T. magnivelare | - | [54] |
4. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Clericuzio, M.; Mellerio, G.G.; Finzi, P.V.; Vidari, G. Secondary Metabolites Isolated from Tricholoma Species (Basidiomycota, Tricholomatacee): A Review. Nat. Prod. Commun. 2018, 13, 1934578X1801300926. [Google Scholar] [CrossRef]
- Hilber, O. Indol als Hauptkomponente des Geruches einiger Tricholoma-Arten und von Lepiota bucknallii. Z. Pilzkd. 1968, 34, 153–158. [Google Scholar]
- Cheng, H.; Liu, Y.; Liu, X.; Liu, G.; He, C.; Li, L. Separation and purification as well as whitening efficacy of polysaccharide from Tricholoma matsutake Sing. China Surfactant Deterg. Cosmet. 2013, 43, 134–138. [Google Scholar]
- Li, M.; Ge, Q.; Du, H.; Jiang, P.; Bao, Z.; Chen, D.; Lin, S. Potential mechanisms mediating the protective effects of Tricholoma matsutake-derived peptides in mitigating DSS-induced colitis. J. Agric. Food Chem. 2021, 69, 5536–5546. [Google Scholar] [CrossRef]
- Zhao, Z.-Z.; Chen, H.-P.; Wu, B.; Zhang, L.; Li, Z.-H.; Feng, T.; Liu, J.-K. Matsutakone and matsutoic acid, two (nor) steroids with unusual skeletons from the edible mushroom Tricholoma matsutake. J. Org. Chem. 2017, 82, 7974–7979. [Google Scholar] [CrossRef]
- Ma, Y.; Zu, Y.; Huang, S.; Stephanopoulos, G. Engineering a universal and efficient platform for terpenoid synthesis in yeast. Proc. Natl. Acad. Sci. USA 2023, 120, e2207680120. [Google Scholar] [CrossRef]
- Ma, Y.; Shang, Y.; Stephanopoulos, G. Engineering peroxisomal biosynthetic pathways for maximization of triterpene production in Yarrowia lipolytica. Proc. Natl. Acad. Sci. USA 2024, 121, e2314798121. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, G.; Huang, H.; Gao, H.; Yao, X.; Hu, D. Biosynthesis of Fungal Triterpenoids and Steroids. Chin. J. Org. Chem. 2018, 38, 2335–2347. [Google Scholar] [CrossRef]
- Jordá, T.; Puig, S. Regulation of ergosterol biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhu, L.; Wang, X.; Meng, F.; Xia, L.; Zhang, H. Advances in Stigmasterol on its anti-tumor effect and mechanism of action. Front. Oncol. 2022, 12, 1101289. [Google Scholar] [CrossRef]
- Zhang, S.-B.; Li, Z.-H.; Stadler, M.; Chen, H.-P.; Huang, Y.; Gan, X.-Q.; Feng, T.; Liu, J.-K. Lanostane triterpenoids from Tricholoma pardinum with NO production inhibitory and cytotoxic activities. Phytochemistry 2018, 152, 105–112. [Google Scholar] [CrossRef]
- Zhong, S.; Li, L.; Liang, N.; Zhang, L.; Xu, X.; Chen, S.; Yin, H. Acetaldehyde Dehydrogenase 2 regulates HMG-CoA reductase stability and cholesterol synthesis in the liver. Redox Biol. 2021, 41, 101919. [Google Scholar] [CrossRef]
- Gozzini, D.; Mellerio, G.G.; Gilardoni, G.; Clericuzio, M.; Vidari, G. New terpenoids from Tricholoma saponaceum. Nat. Prod. Commun. 2018, 13, 1934578X1801300901. [Google Scholar] [CrossRef]
- Gilardoni, G.; Negri, F.; Vita Finzi, P.; Hussain, F.H.; Vidari, G. New tricholidic acid triterpenoids from the mushroom Tricholoma ustaloides collected in an Italian beech wood. Molecules 2023, 28, 3864. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Yang, H.-X.; Wu, X.; Li, J.-Y.; Wang, S.-Q.; He, J.; Li, Z.-H.; Feng, T.; Liu, J.-K. Chemical constituents and their cytotoxicities from mushroom Tricholoma imbricatum. Phytochemistry 2020, 177, 112431. [Google Scholar] [CrossRef]
- Jin, Y.-X.; Chi, M.-J.; Wei, W.-K.; Zhao, Y.-Q.; Wang, G.-K.; Feng, T. Tricholosterols A–D, four new ergosterol derivatives from the mushroom Tricholoma terreum. Steroids 2023, 191, 109157. [Google Scholar] [CrossRef]
- Kaplaner, E.; Aydoğmuş-Öztürk, F.; Öztürk, M.; Akata, I.; Duru, M.E. Anatoluin A and B isolated from medicinal Tricholoma anatolicum are new cytotoxic ergostanoids against the most common cancers. Nat. Prod. Res. 2023, 37, 3787–3797. [Google Scholar] [CrossRef]
- Shi, C.; Peng, Y.-L.; He, J.; Li, Z.-H.; Liu, J.-K.; Feng, T. Structures, chemical conversions, and cytotoxicity of tricholopardins C and D, two Tricholoma triterpenoids from the wild mushroom Tricholoma pardinum. Nat. Prod. Bioprospecting 2021, 11, 235–241. [Google Scholar] [CrossRef]
- De Bernardi, M.; Garlaschelli, L.; Gattl, G.; Vidari, G.; Finzi, P.V. Fungal metabolites xxii (): The unprecedented structure of saponaceolide a, a cytotoxic c-30 terpenoid from Tricholoma Saponaceum. Tetrahedron 1988, 44, 235–240. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Yin, X.; Zhang, C.-C.; Jia, Q.; Gao, J.-M. Structure diversity, synthesis, and biological activity of cyathane diterpenoids in higher fungi. Curr. Med. Chem. 2015, 22, 2375–2391. [Google Scholar] [CrossRef]
- Qi, J.; Gao, Y.-Q.; Kang, S.-j.; Liu, C.; Gao, J.-M. Secondary metabolites of bird’s nest fungi: Chemical structures and biological activities. J. Agric. Food Chem. 2023, 71, 6513–6524. [Google Scholar] [CrossRef]
- Yiming, Z.; Jianzhao, Q.; Yingce, D.; Min, Z.; Chengwei, L. Research progress of the biosynthesis of diterpenoids in macro-basidiomycetes. Mycosystema 2023, 42, 101–117. [Google Scholar]
- Gilardoni, G.; Negri, F.; Vita Finzi, P.; Hussain, F.H.; Vidari, G. New Tricholomalides D–G from the Mushroom Tricholoma ustaloides Grown in an Italian Beech Wood. Molecules 2023, 28, 7446. [Google Scholar] [CrossRef]
- Schumacher, M.; Juncker, T.; Schnekenburger, M.; Gaascht, F.; Diederich, M. Natural compounds as inflammation inhibitors. Genes. Nutr. 2011, 6, 89–92. [Google Scholar] [CrossRef]
- Baquero, F.; Coque, T.M.; De La Cruz, F. Ecology and evolution as targets: The need for novel eco-evo drugs and strategies to fight antibiotic resistance. Antimicrob. Agents Chemother. 2011, 55, 3649–3660. [Google Scholar] [CrossRef]
- Sithranga Boopathy, N.; Kathiresan, K. Anticancer drugs from marine flora: An overview. J. Oncol. 2010, 2010, 214186. [Google Scholar] [CrossRef]
- Yin, R.; Hong, K. Filamentous fungal sesterterpenoids and their synthases. Sheng Wu Gong. Cheng Xue Bao = Chin. J. Biotechnol. 2016, 32, 1631–1641. [Google Scholar]
- Zhang, P.; Qi, J.; Duan, Y.; Gao, J.-M.; Liu, C. Research progress on fungal sesterterpenoids biosynthesis. J. Fungi 2022, 8, 1080. [Google Scholar] [CrossRef]
- Xu, M.; Xu, H.; Lei, Z.; Xing, B.; Dickschat, J.S.; Yang, D.; Ma, M. Structural Insights Into the Terpene Cyclization Domains of Two Fungal Sesterterpene Synthases and Enzymatic Engineering for Sesterterpene Diversification. Angew. Chem. Int. Ed. 2024, 63, e202405140. [Google Scholar] [CrossRef]
- Wang, L.; Yang, B.; Lin, X.-P.; Zhou, X.-F.; Liu, Y. Sesterterpenoids. Nat. Prod. Rep. 2013, 30, 455–473. [Google Scholar] [CrossRef]
- Feng, T.; Gan, X.-Q.; Zhao, Y.-L.; Zhang, S.-B.; Chen, H.-P.; He, J.; Zheng, Y.-S.; Sun, H.; Huang, R.; Li, Z.-H. Tricholopardins A and B, anti-inflammatory terpenoids from the fruiting bodies of Tricholoma pardinum. J. Nat. Prod. 2019, 82, 45–50. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Li, R.; Wang, Y.-M.; Zhu, X.-Y.; Wang, Y.-Y. Progress on the Method of Classification and Identifying of Alkaloids. J. West. Anhui Univ. 2010, 26, 69–73. [Google Scholar]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Abdulaal, W.H.; Omar, U.M.; Zeyadi, M.; El-Agamy, D.S.; Alhakamy, N.A.; Ibrahim, S.R.; Almalki, N.A.; Asfour, H.Z.; Al-Rabia, M.W.; Mohamed, G.A. Modulation of the crosstalk between Keap1/Nrf2/HO-1 and NF-κB signaling pathways by Tomatidine protects against inflammation/oxidative stress-driven fulminant hepatic failure in mice. Int. Immunopharmacol. 2024, 130, 111732. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, C.-Y.; Zhang, J.; An, Q.; Yi, P.; Yuan, C.-M.; Zhang, Z.-K.; Zhao, L.-H.; Hao, X.-J.; Hu, Z.-X. Quinolizidine Alkaloids and Isoflavones from the Herb of Thermopsis lupinoides and Their Antiviral, Antifungal, and Insecticidal Activities. J. Agric. Food Chem. 2024, 72, 5047–5061. [Google Scholar] [CrossRef]
- Yi, N.; Wang, L.; Jiang, Z.; Xu, G.; Li, L.; Zhang, Y.; Tan, Y. Peiminine triggers ferroptosis to inhibit breast cancer growth through triggering Nrf2 signaling. Tissue Cell 2024, 87, 102323. [Google Scholar] [CrossRef]
- Yang, D.F.; Huang, Y.B.; Pan, L.X. Research Progress of Alkaloids Natural Products from Marine Mangrove Fungi. J. Guangxi Acad. Sci. 2023, 39, 349–362. [Google Scholar]
- Chen, X.L.; Wu, M.; Ti, H.H.; Wei, X.Y.; Li, T.H. Three New 3, 6-Dioxygenated Diketopiperazines from the Basidiomycete Lepista sordida. Helv. Chim. Acta 2011, 94, 1426–1430. [Google Scholar] [CrossRef]
- Zhao, Z.-Z.; Liang, X.-B.; Feng, W.-S.; Xue, G.-M.; Si, Y.-Y.; Chen, H.-P.; Liu, J.-K. Cyclic dipeptides with peroxy groups from the fruiting bodies of the edible mushroom Tricholoma matsutake. Tetrahedron Lett. 2020, 61, 151892. [Google Scholar] [CrossRef]
- Zin, W.W.M.; Buttachon, S.; Dethoup, T.; Pereira, J.A.; Gales, L.; Inácio, Â.; Costa, P.M.; Lee, M.; Sekeroglu, N.; Silva, A.M. Antibacterial and antibiofilm activities of the metabolites isolated from the culture of the mangrove-derived endophytic fungus Eurotium chevalieri KUFA 0006. Phytochemistry 2017, 141, 86–97. [Google Scholar] [CrossRef]
- Clericuzio, M.; Hussain, F.H.; Amin, H.I.M.; Salis, A.; Damonte, G.; Pavela, R.; Vidari, G. New acetylenic metabolites from the toxic mushroom Tricholoma pardinum. Nat. Prod. Res. 2021, 35, 5081–5088. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, M.; Liu, C. A comprehensive review of secondary metabolites from the genus Agrocybe: Biological activities and pharmacological implications. Mycology 2024, 15, 162–179. [Google Scholar] [CrossRef]
- Sterner, O. The isolation and structure determination of sciodole, a new indole derivative from the fruit bodies of Tricholoma sciodes. Nat. Prod. Lett. 1994, 4, 9–14. [Google Scholar] [CrossRef]
- Eizenhöfer, T.; Fugmann, B.; Sheldrick, W.S.; Steffan, B.; Steglich, W. Lascivol, der Bitterstoff des Unverschämten Ritterlings, Tricholoma lascivum (Agaricales). Liebigs Ann. Chem. 1990, 1990, 1115–1118. [Google Scholar] [CrossRef]
- Oba, Y.; Urai, M.; Wu, J.; Tomizawa, M.; Kawagishi, H.; Hashimoto, K. Bitter compounds in two Tricholoma species, T. aestuans and T. virgatum. J. Antibiot. 2020, 73, 697–701. [Google Scholar] [CrossRef]
- Lima, L.M.; da Silva, B.N.M.; Barbosa, G.; Barreiro, E.J. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef]
- Zhang, F.M.; Wang, Y.H.; Yu, F.Q.; Hua, Y. Tricholomines A and B, two new amides from the fruiting bodies of Tricholoma bakamatsutake. Magn. Reson. Chem. 2021, 59, 587–593. [Google Scholar] [CrossRef]
- Zhang, F.M.; Lu, B.; Wang, Y.H.; Yu, F.Q. A new amide from the fruiting bodies of Tricholoma bakamatsutake. Magn. Reson. Chem. 2023, 61, 443–447. [Google Scholar] [CrossRef]
- Qi, J.; Duan, Y.; Li, Z.; Gao, J.; Qi, J.; Liu, C. The alkynyl-containing compounds from mushrooms and their biological activities. Nat. Prod. Bioprospecting 2023, 13, 50. [Google Scholar] [CrossRef]
- Kilimnik, A.; Kuklev, D.; Dembitsky, V. Antitumor Acetylenic Lipids. MJ Phar. 1 (1): 005. MJ Phar. 2016, 1, 005. [Google Scholar]
- Garlaschelli, L.; Vidari, G.; Vita-Finzi, P. Tricholomenyns C, D, and E, novel dimeric dienyne geranyl cyclohexenones from the fruiting bodies of Tricholoma acerbum. Tetrahedron Lett. 1996, 37, 6223–6226. [Google Scholar] [CrossRef]
- Yang, H.-X.; Ma, J.-T.; He, J.; Li, Z.-H.; Huang, R.; Feng, T.; Liu, J.-K. Pardinumones A–D: Antibacterial Polyketide–Amino Acid Derivatives from the Mushroom Tricholoma pardinum. ACS Omega 2021, 6, 25089–25095. [Google Scholar] [CrossRef]
- Diana, E.J.; Kanchana, U.; Mathew, T.V. Current developments in the synthesis of 4-chromanone-derived compounds. Org. Biomol. Chem. 2021, 19, 7995–8008. [Google Scholar] [CrossRef]
- Murray, A.F.; Moore, A.J.; Munafo, J.P., Jr. Key odorants from the american matsutake, Tricholoma magnivelare. J. Agric. Food Chem. 2020, 68, 9768–9775. [Google Scholar] [CrossRef]
- Xiong, C.; Li, Q.; Li, S.; Chen, C.; Chen, Z.; Huang, W. In vitro antimicrobial activities and mechanism of 1-octen-3-ol against food-related bacteria and pathogenic fungi. J. Oleo Sci. 2017, 66, 1041–1049. [Google Scholar] [CrossRef]
- Huang, Z.; Miao, H.; Pei, D.; Tang, X.; Wu, Q.; Huang, Z. Active components and their research progress of Tricholoma matsutake. Mycosystema 2023, 42, 2025–2040. [Google Scholar]
- Karakas, F.P.; Turker, A.U.; Bozat, B.G. Phenolic content, antibacterial and antioxidant potential of several edible Agaricomycetes mushrooms sold in public bazaar in Bolu, Turkey. Int. J. Med. Mushrooms 2023, 25, 45–56. [Google Scholar] [CrossRef]
- Li, G.; Kusari, S.; Kusari, P.; Kayser, O.; Spiteller, M. Endophytic Diaporthe sp. LG23 produces a potent antibacterial tetracyclic triterpenoid. J. Nat. Prod. 2015, 78, 2128–2132. [Google Scholar] [CrossRef]
- Dinçer, E.; Işık, H.; Hepokur, C.; Tutar, U.; Çelik, C. Cytotoxic, Antioxidant, Antibiofilm, and Antimicrobial Activities of Mushroom Species from Turkey. Int. J. Med. Mushrooms 2023, 25, 75–86. [Google Scholar] [CrossRef]
- Du, Y.; Tian, L.; Wang, Y.; Li, Z.; Xu, Z. Chemodiversity, pharmacological activity, and biosynthesis of specialized metabolites from medicinal model fungi Ganoderma lucidum. Chin. Med. 2024, 19, 51. [Google Scholar] [CrossRef]
- Jianzhao, Q.; Jing, W.; Shijie, K.; Jingming, G.; Hirokazu, K.; Hongwei, L.; Chengwei, L. The chemical structures, biosynthesis, and biological activities of secondary metabolites from the culinary-medicinal mushrooms of the genus Hericium: A review. Chin. J. Nat. Med. 2024, 22, 676–698. [Google Scholar]
- Qi, J. Hericium erinaceus: The enchanting medicinal-culinary mushroom of East Asian tradition. Integr. Med. Discov. 2024, 8, e24013. [Google Scholar] [CrossRef]
- Ahmad, I.; Arif, M.; Xu, M.; Zhang, J.; Ding, Y.; Lyu, F. Therapeutic values and nutraceutical properties of shiitake mushroom (Lentinula edodes): A review. Trends Food Sci. Technol. 2023, 134, 123–135. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Yuan, S.; Li, Z.; Liu, C.; Zhang, R. Review of the Structural Characteristics and Biological Activities of Tricholoma Secondary Metabolites (2018–2023). Molecules 2024, 29, 4719. https://doi.org/10.3390/molecules29194719
Zhao M, Yuan S, Li Z, Liu C, Zhang R. Review of the Structural Characteristics and Biological Activities of Tricholoma Secondary Metabolites (2018–2023). Molecules. 2024; 29(19):4719. https://doi.org/10.3390/molecules29194719
Chicago/Turabian StyleZhao, Meili, Shiqin Yuan, Zhiming Li, Chengwei Liu, and Ruiying Zhang. 2024. "Review of the Structural Characteristics and Biological Activities of Tricholoma Secondary Metabolites (2018–2023)" Molecules 29, no. 19: 4719. https://doi.org/10.3390/molecules29194719
APA StyleZhao, M., Yuan, S., Li, Z., Liu, C., & Zhang, R. (2024). Review of the Structural Characteristics and Biological Activities of Tricholoma Secondary Metabolites (2018–2023). Molecules, 29(19), 4719. https://doi.org/10.3390/molecules29194719







