N-Oxide Coordination to Mn(III) Chloride
Abstract
1. Introduction
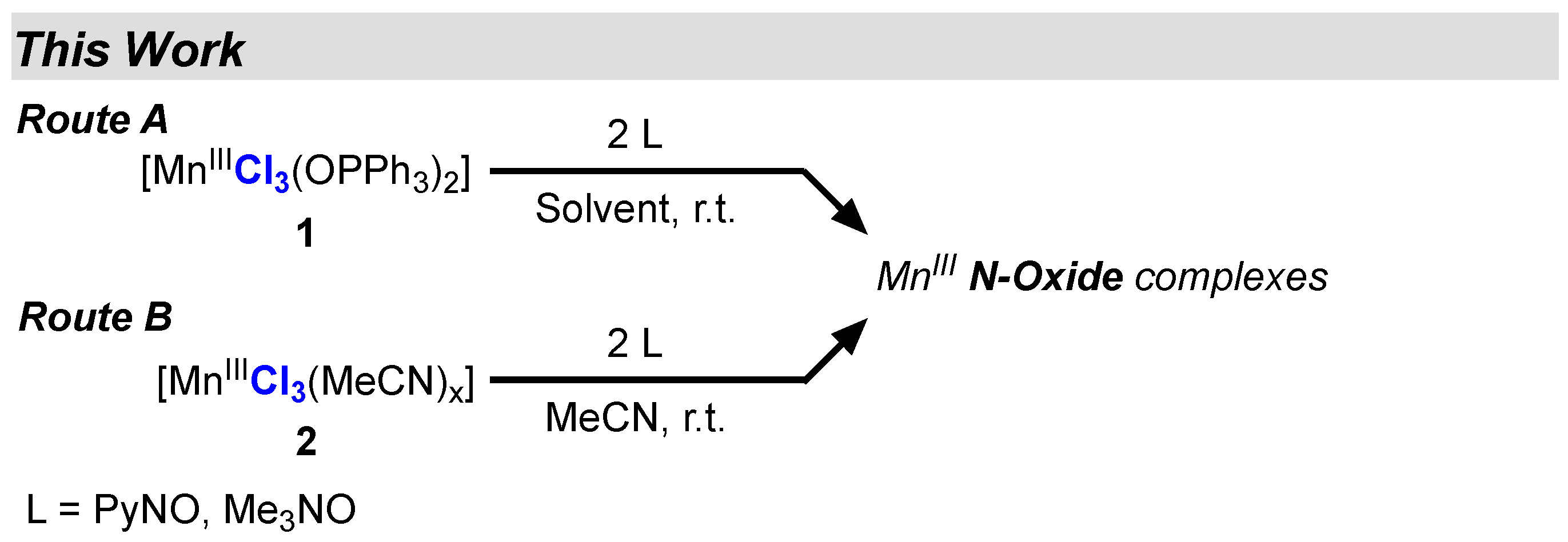
2. Results and Discussion
2.1. Description of Starting Materials
2.2. Synthesis of Mn(III) Trimethylamine-N-Oxide Compounds
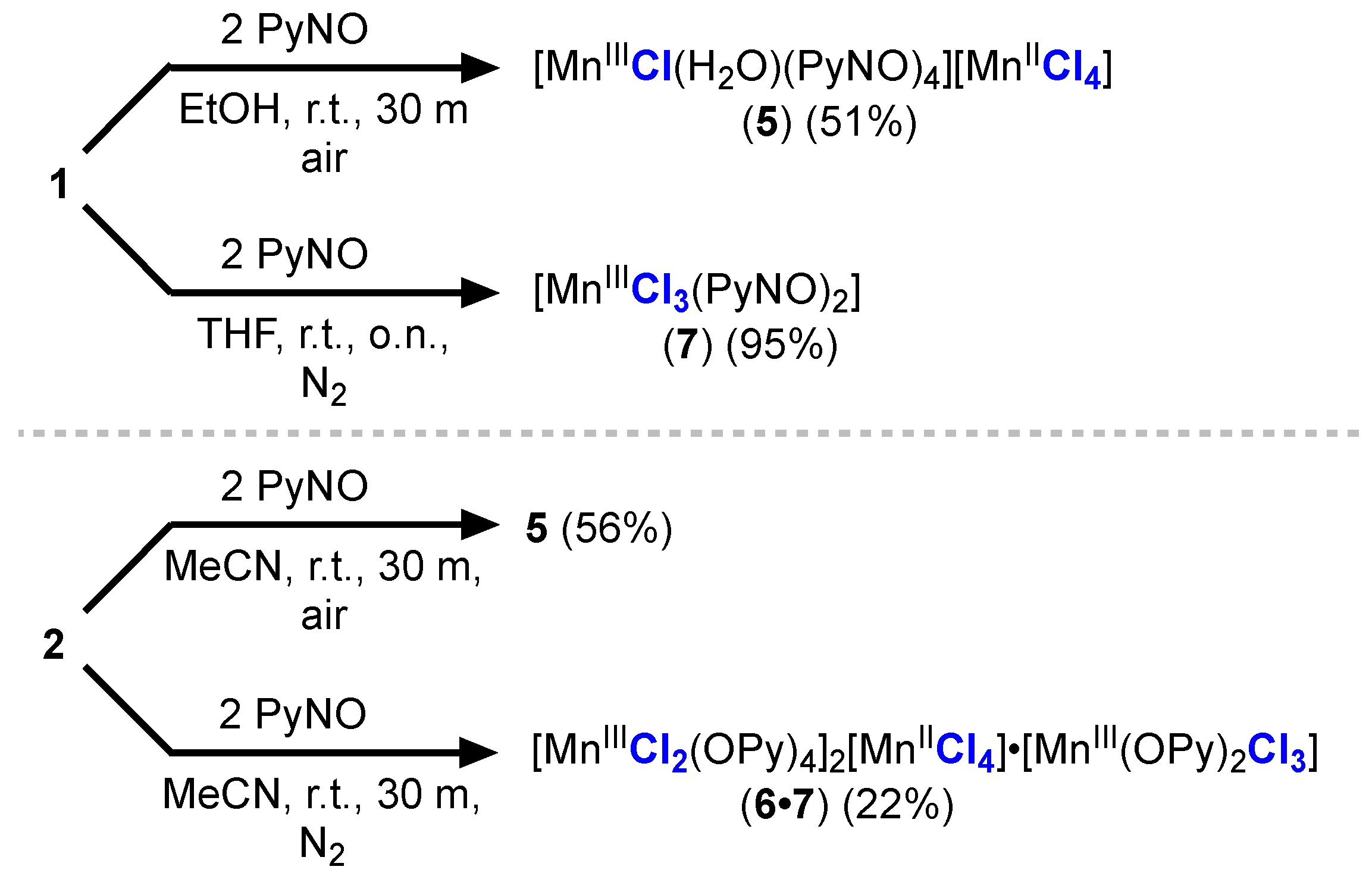
2.3. Synthesis of Mn(III) PyNO Compounds
2.4. Reactivity of Mn(III) Chloride Compounds
2.5. Reaction of 1 with 2,2,6,6-Tetramethyl-1-piperidinyloxy (TEMPO)
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lingappa, U.F.; Monteverde, D.R.; Magyar, J.S.; Valentine, J.S.; Fischer, W.W. How manganese empowered life with dioxygen (and vice versa). Free Radic. Biol. Med. 2019, 140, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Richards, N.G.J. Biological functions controlled by manganese redox changes in mononuclear Mn-dependent enzymes. Essays Biochem. 2017, 61, 259–270. [Google Scholar] [PubMed]
- Li, H.; Santos, F.; Butler, K.; Herndon, E. A critical review on the multiple roles of manganese in stabilizing and destabilizing soil organic matter. Environ. Sci. Technol. 2021, 55, 12136–12152. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; Sauer, G.S.; Saha, A.; Loo, A.; Lin, S. Metal-catalyzed electrochemical diazidation of alkenes. Science 2017, 357, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Sauer, G.S.; Lin, S. An Electrocatalytic approach to the radical difunctionalization of alkenes. ACS Catal. 2018, 8, 5175–5187. [Google Scholar] [CrossRef]
- Dong, X.; Roeckl, J.L.; Waldvogel, S.R.; Morandi, B. Merging shuttle reactions and paired electrolysis for reversible vicinal dihalogenations. Science 2021, 371, 507–514. [Google Scholar] [CrossRef]
- Philip, R.M.; Radhika, S.; Abdulla, C.M.A.; Anilkumar, G. Recent trends and prospects in homogeneous manganese-catalyzed epoxidation. Adv. Synth. Catal. 2021, 363, 1272–1289. [Google Scholar] [CrossRef]
- Eisink, N.N.H.M.; Browne, W.R. Chapter 10. Manganese-catalyzed dihydroxylation and epoxidation of olefins. In Manganese Catalysis in Organic Synthesis; Wiley-VCH: Hoboken, NJ, USA, 2022; pp. 323–343. [Google Scholar]
- Demir, A.S.; Emrullahoglu, M. Manganese(III) acetate: A versatile reagent in organic chemistry. Curr. Org. Synth. 2007, 4, 321–350. [Google Scholar] [CrossRef]
- Mondal, M.; Bora, U. Recent advances in manganese(III) acetate mediated organic synthesis. RSC Adv. 2013, 3, 18716–18754. [Google Scholar] [CrossRef]
- Carney, J.R.; Dillon, B.R.; Thomas, S.P. Recent advances of manganese catalysis for organic synthesis. Eur. J. Org. Chem. 2016, 2016, 3912–3929. [Google Scholar] [CrossRef]
- Snider, B.B. Chapter 9. Manganese(III) acetate-mediated cyclizations. In Manganese Catalysis in Organic Synthesis; Wiley-VCH: Hoboken, NJ, USA, 2022; pp. 293–322. [Google Scholar]
- Goodwin, H.A.; Sylva, R.N. The magnetic properties of some complexes of higher-valent manganese. Aust. J. Chem. 1967, 20, 629–637. [Google Scholar] [CrossRef]
- Funk, H.; Kreis, H. Zur kenntnis des dreiwertigen mangans: Verbindungen des mangan(III)-chlorids mit aminen und einigen Äthern. Z. Anorg. Allg. Chem. 1967, 349, 45–49. [Google Scholar] [CrossRef]
- Davis, T.S.; Fackler, J.P.; Weeks, M.J. spectra of manganese(III) complexes. the origin of the low-energy band. Inorg. Chem. 1968, 7, 1994–2002. [Google Scholar] [CrossRef]
- Nachtigall, O.; Pataki, A.; Molski, M.; Lentz, D.; Spandl, J. solvates of manganese trichloride revisited—Synthesis, isolation, and crystal structure of MnCl3(THF)3. Z. Anorg. Allg. Chem. 2015, 641, 1164–1168. [Google Scholar] [CrossRef]
- Perlepes, S.P.; Blackman, A.G.; Huffman, J.C.; Christou, G. Complete carboxylate removal from [Mn12O12(OAc)16(H2O)4]•2HOAc•4H2O with Me3SiCl: Synthesis and characterization of polymeric [MnCl3(bipy)]n and an improved Synthesis of (NEt4)2MnCl5. Inorg. Chem. 1991, 30, 1665–1668. [Google Scholar] [CrossRef]
- Saju, A.; Griffiths, J.R.; MacMillan, S.N.; Lacy, D.C. Synthesis of a bench-stable manganese(III) chloride compound: Coordination chemistry and alkene dichlorination. J. Am. Chem. Soc. 2022, 144, 16761. [Google Scholar] [CrossRef]
- Kadassery, K.J.; Dey, S.K.; Friedman, A.E.; Lacy, D.C. Exploring the role of carbonate in the formation of an organomanganese tetramer. Inorg. Chem. 2017, 56, 8748–8751. [Google Scholar] [CrossRef]
- Uson, R.; Riera, V.; Ciriano, M.A.; Valderrama, M. Pentacoordinate neutral manganese (III) complexes. Transit. Met. Chem. 1976, 1, 122–126. [Google Scholar]
- Contreras, E.; Riera, V.; Usón, R. Stable complexes of manganese (III) with oxides of pyridine, phosphine and arsine. Inorg. Nucl. Chem. Lett. 1972, 8, 287–291. [Google Scholar] [CrossRef]
- Saju, A.; Crawley, M.R.; MacMillan, S.N.; Lacy, D.C. Manganese(III) nitrate complexes as bench-Stable powerful oxidants. J. Am. Chem. Soc. 2024, 146, 11616–11621. [Google Scholar] [CrossRef]
- Pokhodnya, K.I.; Bonner, M.; DiPasquale, A.G.; Rheingold, A.L.; Her, J.H.; Stephens, P.W.; Park, J.W.; Kennon, B.S.; Arif, A.M.; Miller, J.S. Structural and magnetic properties of MCl2 (M = Fe, Mn, Co): Acetonitrile solvates. Inorg. Chem. 2007, 46, 2471–2477. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Saju, A.; Cohen, C.; Crawley, M.R.; MacMillan, S.N.; Lacy, D.C. Synthesis of Mn(III)X3 (X = Cl, Br, I) Compounds with Phosphine (R3P) Ligands. Inorg. Chem. 2024, 34, 15791–15803. [Google Scholar] [CrossRef] [PubMed]
- Nannenga, B.L.; Gonen, T. The cryo-EM method microcrystal electron diffraction (MicroED). Nat. Methods 2019, 16, 369–379. [Google Scholar]
- Ito, S.; White, F.J.; Okunishi, E.; Aoyama, Y.; Yamano, A.; Hiroyasu, S.; Ferrara, J.D.; Jansnowski, M.; Meyer, M. Structure determination of small molecule compounds by an electron diffractometer for 3D ED/MicroED. CrystEngComm 2021, 23, 8622–8630. [Google Scholar] [CrossRef]
- Caputo, R.E.; Roberts, S.; Willett, R.D.; Gerstein, B.C. Crystal structure and magnetic susceptibility of [(CH3)3NH]3Mn2Cl7. Inorg. Chem. 1976, 15, 820–823. [Google Scholar] [CrossRef]
- Ravindran, M.; Willey, G.R.; Drew, M.G.B. Reactions of trimethylamine with Mn(II) and Cd(II) chlorides: Crystal and molecular structure of [Me3NH][MnCl3]. Inorg. Chim. Acta 1990, 175, 99–103. [Google Scholar] [CrossRef]
- Naito, T.; Inabe, T. Molecular hexagonal perovskite: A new type of organic-inorganic hybrid conductor. J. Solid State Chem. 2003, 176, 243–249. [Google Scholar] [CrossRef]
- Sun, X.-F.; Li, P.-F.; Liao, W.-Q.; Wang, Z.; Gao, J.; Ye, H.-Y.; Zhang, Y. Notable broad dielectric relaxation and highly efficient red photoluminescence in perovskite-type compound: (N-methylpyrrolidinium)MnCl3. Inorg. Chem. 2017, 56, 12193–12198. [Google Scholar] [CrossRef]
- Sun, Q.; Kioussis, N. Prediction of manganese trihalides as two-dimensional Dirac half-metals. Phys. Rev. B 2018, 97, 094408. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z. Theoretical investigation of nonvolatile electrical control behavior by ferroelectric polarization switching in two-dimensional MnCl3/CuInF2S6 van der Waals heterostructures. J. Mater. Chem. C. 2020, 8, 4534. [Google Scholar]
- Guo, T.; Liu, Y.; Sun, Y.; Zhang, S.; Xu, X.; Wang, L.; Zhou, W.; Liu, Y.; Yao, X.; Zhang, X. Insight into tunable electronic and magnetic properties in 2D ferromagnetic/antiferromagnetic van der Waals heterostructure. Appl. Phys. Lett. 2023, 122, 192403. [Google Scholar] [CrossRef]
- Saju, A.; Gunasekera, P.S.; Morgante, P.; MacMillan, S.N.; Autschbach, J.; Lacy, D.C. Experimental and computational determination of a M–Cl homolytic bond dissociation free energy: Mn(III)Cl-mediated C–H cleavage and chlorination. J. Am. Chem. Soc. 2023, 145, 13384–13391. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Pirovano, P.; Das, A.; Farquhar, E.R.; McDonald, A.R. Hydrogen atom transfer by a high-valent nickel-chloride complex. J. Am. Chem. Soc. 2018, 140, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Lovisari, M.; Twamley, B.; McDonald, A.R. Fast hydrocarbon oxidation by a high-valent nickel-fluoride complex. Angew. Chem. Int. Ed. 2020, 59, 13044–13050. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.M.; Lee, Y.; Schmautz, A.K.; Jackson, T.A.; Wang, D. C–H bond activation by a mononuclear nickel(IV)-nitrate complex. J. Am. Chem. Soc. 2022, 144, 12072–12080. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Lee, Y.; Evenson, G.E.; Jackson, T.A.; Wang, D. Crystal structure and C–H bond cleaving reactivity of a mononuclear CoIV-dinitrate complex. J. Am. Chem. Soc. 2020, 142, 13435–13441. [Google Scholar] [CrossRef]
- Bower, J.K.; Reese, M.S.; Mazin, I.M.; Zarnitsa, L.M.; Cypcar, A.D.; Moore, C.E.; Sokolov, A.Y.; Zhang, S. C(Sp3)-H cyanation by a formal copper(III) cyanide complex. Chem. Sci. 2023, 14, 1301–1307. [Google Scholar] [CrossRef]
- Liu, W.; Huang, X.; Cheng, M.; Nielsen, R.J.; Goddard, W.A.; Groves, J.T. Oxidative Aliphatic C-H Fluorination with Fluoride Ion Catalyzed by a Manganese Porphyrin. Science 2012, 337, 1322–1325. [Google Scholar] [CrossRef]
- Yadav, V.; Wen, L.; Yadav, S.; Siegler, M.A.; Goldberg, D.P. Selective radical transfer in a series of nonheme iron(III) complexes. Inorg. Chem. 2023, 62, 17830–17842. [Google Scholar] [CrossRef]
- Kütt, A.; Rodima, T.; Saame, J.; Raamat, E.; Mäemets, V.; Kaljurand, I.; Koppel, I.A.; Garlyauskayte, R.Y.; Yagupolskii, Y.L.; Yagupolskii, L.M.; et al. Equilibrium acidities of superacids. J. Org. Chem. 2011, 76, 391. [Google Scholar] [CrossRef]
- Barman, S.K.; Yang, M.-Y.; Parsell, T.H.; Green, M.T.; Borovik, A.S. Semiemperical method for examining asynchronicity in metal-oxo-mediated C–H bond activation. Proc. Natl. Acad. Sci. USA 2021, 118, e2108648118. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.G.; Coste, S.C.; Groff, B.D.; Heuer, A.M.; Noh, H.; Parada, G.A.; Wise, C.F.; Nichols, E.M.; Warren, J.J.; Mayer, J.M. Free energies of proton-coupled electron transfer reagents and their applications. Chem. Rev. 2022, 122, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Kadassery, K.J.; Sethi, K.; Fanara, P.M.; Lacy, D.C. CO-Photolysis-induced H-atom transfer from MnIO–H Bonds. Inorg. Chem. 2019, 58, 4679–4685. [Google Scholar] [CrossRef] [PubMed]
- Hostmann, T.; Molloy, J.J.; Bussmann, K.; Gilmour, R. Light-enabled enantiodivergence: Stereospecific reduction of activated alkenes using a single organocatalyst enantiomer. Org. Lett. 2019, 21, 10164–10168. [Google Scholar] [CrossRef] [PubMed]
- Eppley, H.J.; Christou, G. Synthesis of dodecaoxohexadecacarboxylatotetraaquo-dodecamanganese [Mn12O12(O2CR)16(H2O)4] (R = Me, Et, Ph, Cr) complexes. Inorg. Syn. 2002, 33, 61. [Google Scholar]
- Rigaku, O.D. CrysAlisPro; Rigaku: The Woodlands, TX, USA, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Cryst. 2008, A64, 112. [Google Scholar] [CrossRef]
- Müller, P. Practical Suggestions for Better Crystal Structures. Crystallogr. Rev. 2009, 15, 57. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]







| Complex | Time | % Yield (a) |
|---|---|---|
| [MnIIICl3(Me3NO)2]n (3a) | 5.0 h | 45 |
| [MnIIICl(H2O)(PyNO)4][MnIICl4] (5) | 6.5 h | 86 |
| [MnIIICl3(PyNO)2] (7) | 4.0 h | 88 |
| [MnIIICl3(MeCN)x] (2) | 1.0 h at r.t. (b) | 78 |
| [MnII(µ-Cl)3MnII(µ-ONMe3)]n[MnII(µ-Cl)3]n·(Me3NO·HCl)3n (4) | 5.0 h | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saju, A.; Crawley, M.R.; MacMillan, S.N.; Le Magueres, P.; Del Campo, M.; Lacy, D.C. N-Oxide Coordination to Mn(III) Chloride. Molecules 2024, 29, 4670. https://doi.org/10.3390/molecules29194670
Saju A, Crawley MR, MacMillan SN, Le Magueres P, Del Campo M, Lacy DC. N-Oxide Coordination to Mn(III) Chloride. Molecules. 2024; 29(19):4670. https://doi.org/10.3390/molecules29194670
Chicago/Turabian StyleSaju, Ananya, Matthew R. Crawley, Samantha N. MacMillan, Pierre Le Magueres, Mark Del Campo, and David C. Lacy. 2024. "N-Oxide Coordination to Mn(III) Chloride" Molecules 29, no. 19: 4670. https://doi.org/10.3390/molecules29194670
APA StyleSaju, A., Crawley, M. R., MacMillan, S. N., Le Magueres, P., Del Campo, M., & Lacy, D. C. (2024). N-Oxide Coordination to Mn(III) Chloride. Molecules, 29(19), 4670. https://doi.org/10.3390/molecules29194670







