Polyphosphoric Acid-Promoted Efficient Synthesis of Cinnamides via Aldol Condensation of Amide
Abstract
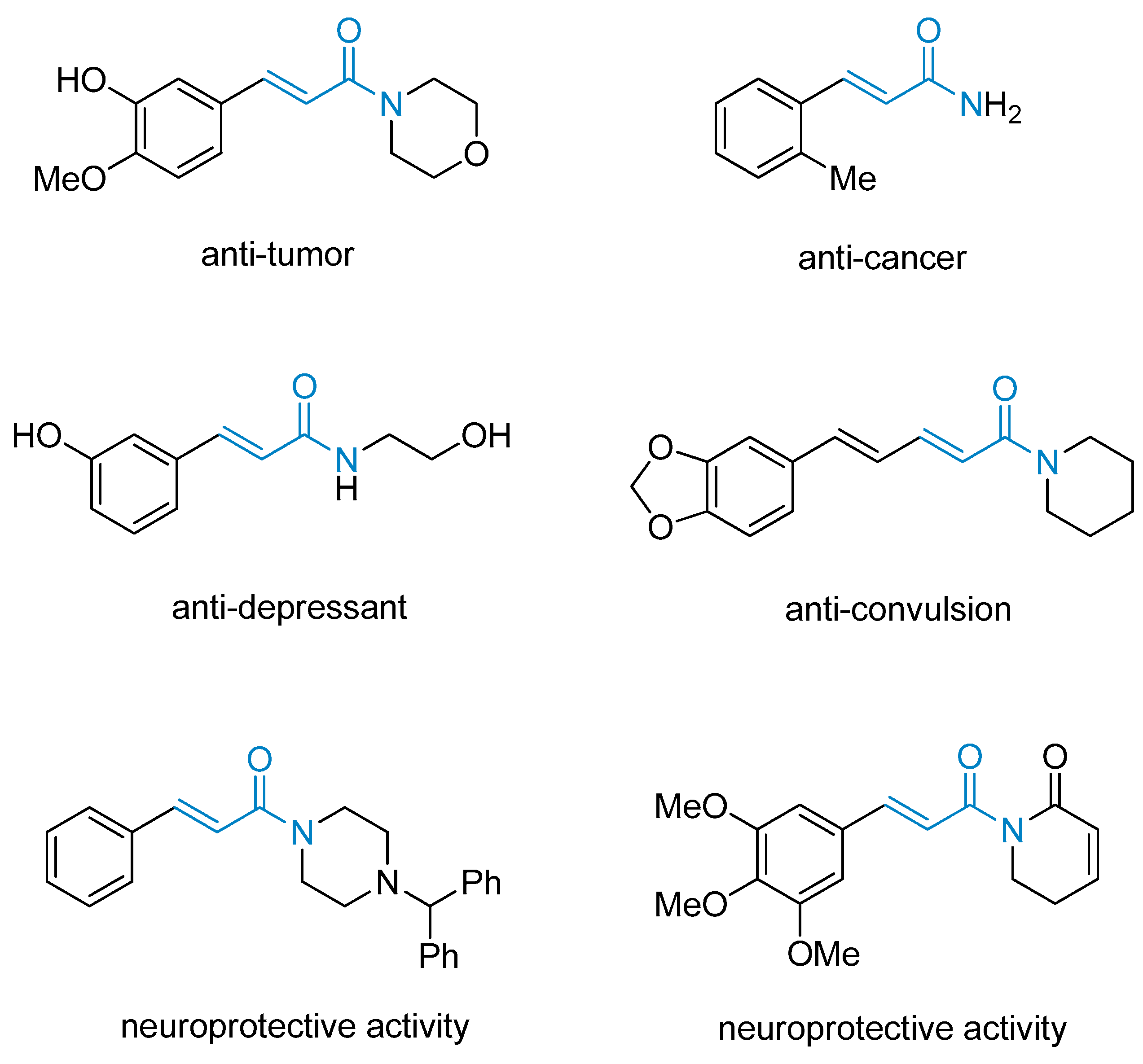
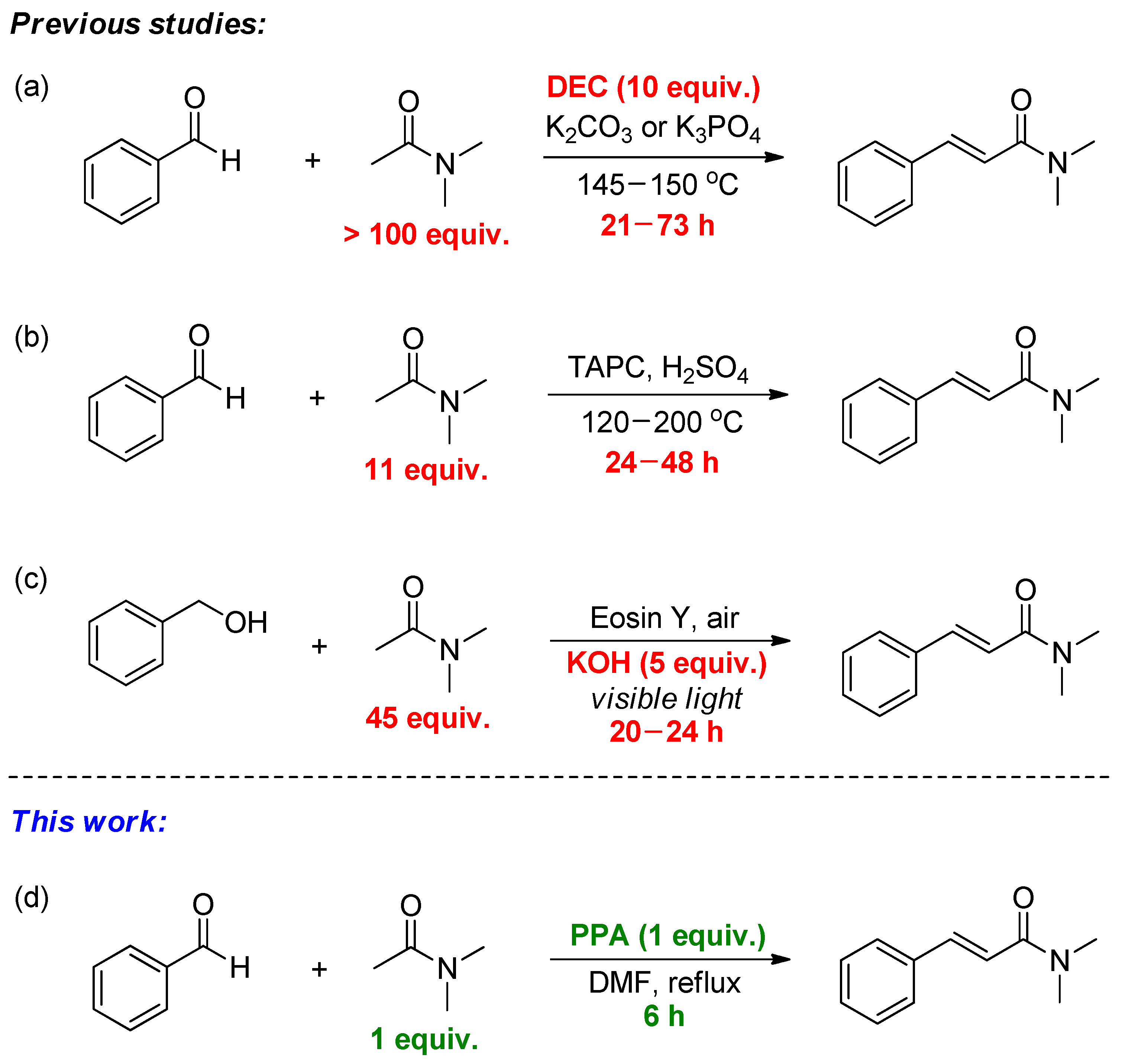
1. Introduction
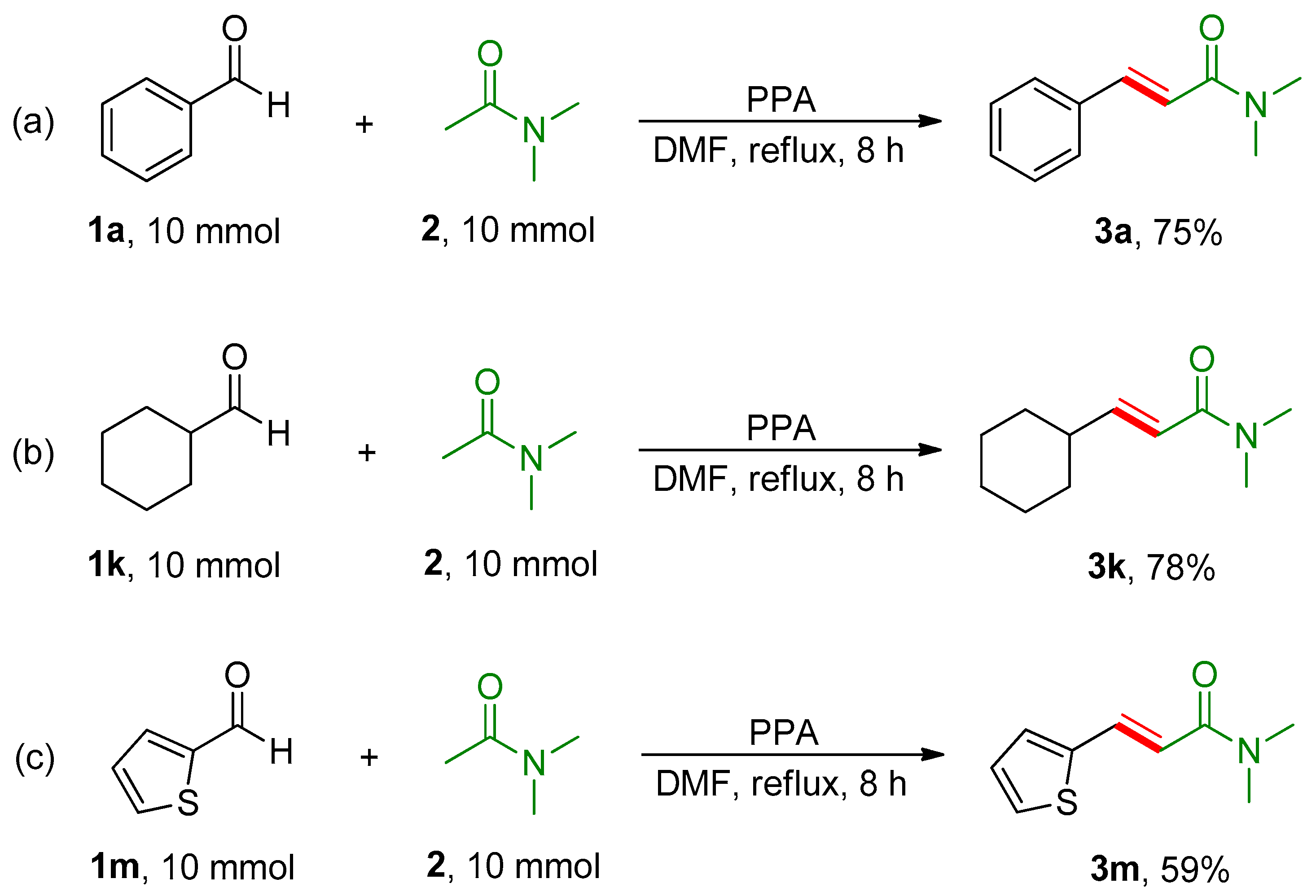
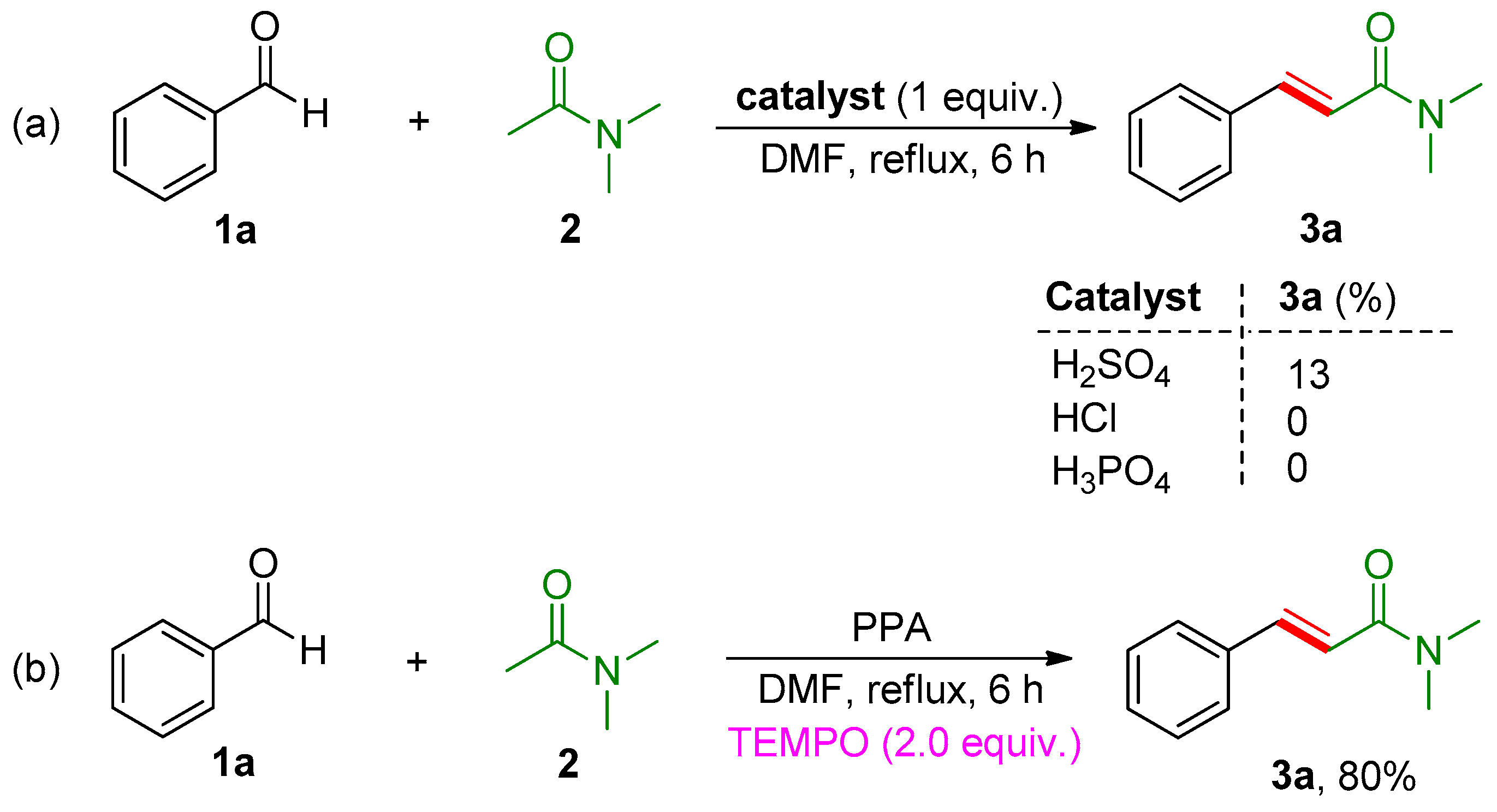
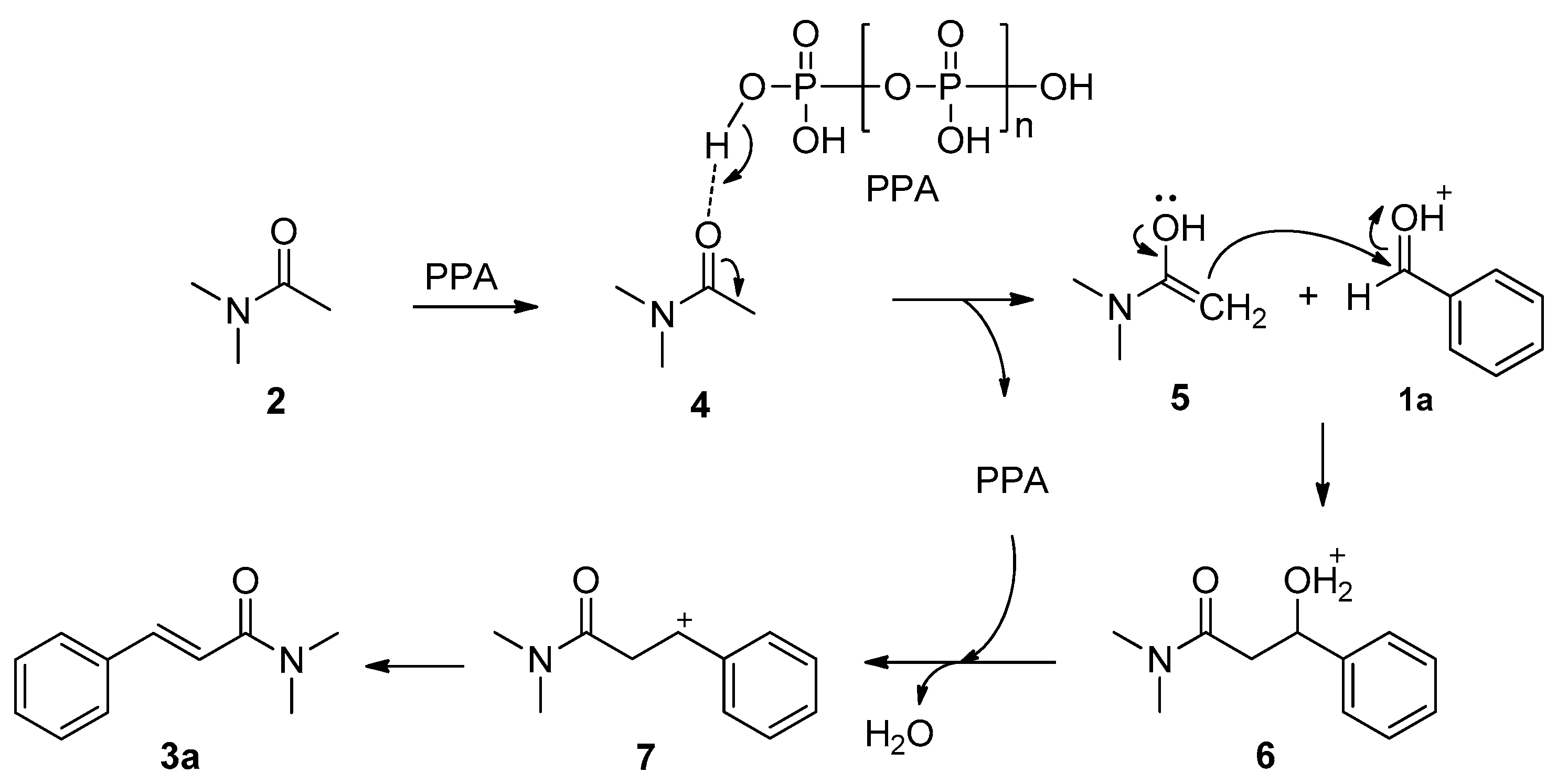
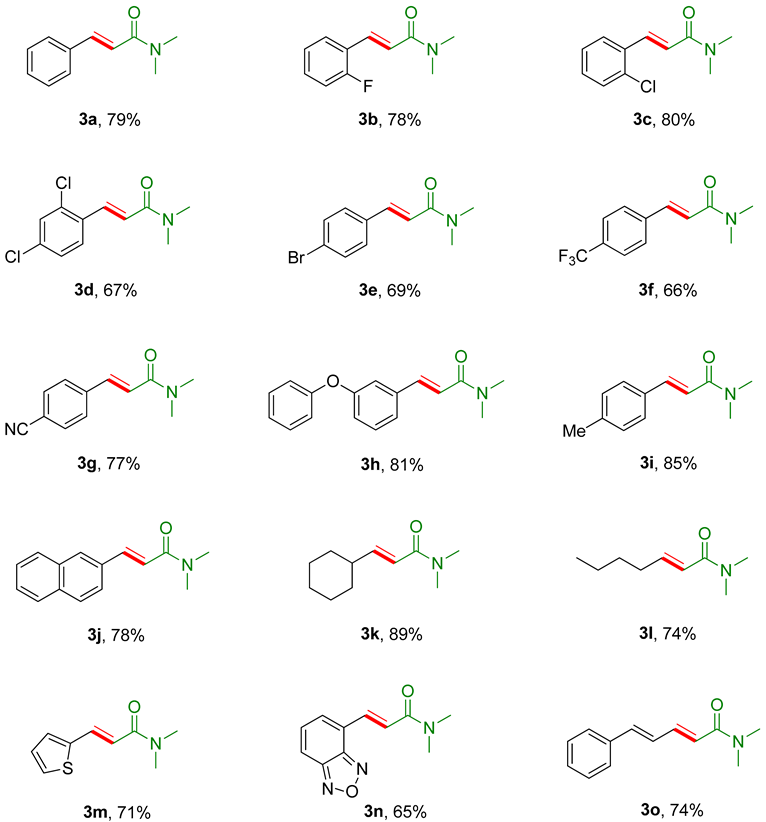
2. Results and Discussion
3. Materials and Methods
3.1. General Information
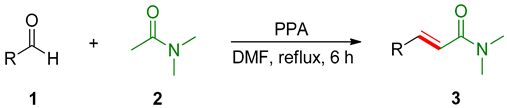
3.2. General Procedure for the Synthesis of Cinnamamides 3a–3o
- (E)-N,N-Dimethylcinnamamide (3a). White solid; yield: 79%; m.p.: 100–104 °C. IR (KBr plate): νmax 1654 (C=O), 1648 (C=C), 1141 (C-N), 766 (Ar-H). 1H NMR (600 MHz, CDCl3) δ 7.67–7.63 (dd, J = 15.4, 2.1 Hz, 1H, Ar-CH=), 7.52–7.49 (m, 2H, Ar-H), 7.36–7.32 (m, 3H, Ar-H), 6.89–6.86 (dd, J = 15.4, 2.1 Hz, 1H, CO-CH=), 3.14 (s, 3H, CH3), 3.04 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 166.7 (C=O), 142.3 (-CH=), 135.4 (Ar-C), 129.6 (Ar-C), 128.8 (Ar-C), 127.8 (Ar-C), 117.4 (-CH=), 37.4 (CH3), 35.9 (CH3). HRMS-ESI (m/z): calcd. for C11H13ONNa [M + Na]+: 198.0889; found: 198.0887.
- (E)-3-(2-Fluorophenyl)-N,N-dimethylacrylamide (3b). White solid; yield: 78%; m.p.: 58–61 °C. IR (KBr plate): νmax 2941 (C-H), 1654 (C=O), 1597 (C=C), 1143 (C-N), 991 (Ar-H), 755 (Ar-H). 1H NMR (600 MHz, CDCl3) δ 7.70 (dd, J = 15.8, 3.1 Hz, 1H, Ar-CH=), 7.49 (q, J = 6.3, 5.5 Hz, 1H, Ar-H), 7.31–7.26 (m, 1H, Ar-H), 7.12 (d, J = 7.3 Hz, 1H, Ar-H), 7.09–6.99 (m, 2H, Ar-H, CO-CH=), 3.14 (s, 3H, CH3), 3.05 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 166.6 (C=O), 161.3 (1JCF = 253.7, Ar-C), 135.3 (-CH=), 130.8 (3JCF = 9.1, Ar-C), 129.8, 124.3 (3JCF = 3.0, Ar-C), 123.3 (2JCF = 12.1, Ar-C), 120.5 (3JCF = 7.6, -CH=), 116.1 (2JCF = 21.1, Ar-C), 37.4 (CH3), 35.9 (CH3). HRMS-ESI (m/z): calcd. for C11H12ONF [M + Na]+: 216.0795; found: 216.0794.
- (E)-3-(2-Chlorophenyl)-N,N-dimethylacrylamide (3c). White solid; yield: 80%; m.p.: 80–84 °C. IR (KBr plate): νmax 1648 (C=O), 1610 (C=C), 1143 (C-N), 759 (Ar-H). 1H NMR (600 MHz, CDCl3) δ 7.99 (d, J = 15.5 Hz, 1H, Ar-CH=), 7.59–7.57 (m, 1H, Ar-H), 7.40–7.36 (m, 1H, Ar-H), 7.26–7.23 (m, 2H, Ar-H), 6.86 (d, J = 15.5 Hz, 1H, CO-CH=), 3.15 (s, 3H, CH3), 3.05 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 166.3 (C=O), 138.2 (-CH=), 134.6 (Ar-C), 133.7 (Ar-C), 130.3 (Ar-C), 130.1 (Ar-C), 127.7 (Ar-C), 126.9 (Ar-C), 120.6 (-CH=), 37.5 (CH3), 35.9 (CH3). HRMS-ESI (m/z): calcd. for C11H12NOClNa [M + Na]+: 232.0500; found: 232.0498.
- (E)-3-(2,4-Dichlorophenyl)-N,N-dimethylacrylamide (3d). White solid; yield: 67%; m.p.: 138–141 °C. IR (KBr plate): νmax 2935 (C-H), 1650 (C=O), 1602 (C=C), 1141 (C-N), 768 (Ar-H). 1H NMR (600 MHz, CDCl3) δ 7.93 (d, J = 15.4 Hz, 1H, Ar-CH=), 7.53 (d, J = 8.4 Hz, 1H, Ar-H), 7.42 (d, J = 2.1 Hz, 1H, Ar-H), 7.24 (dd, J = 8.4, 2.1 Hz, 1H, Ar-H), 6.86 (d, J = 15.4 Hz, 1H, CO-CH=), 3.17 (s, 3H, CH3), 3.07 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 166.1 (C=O), 137.1 (-CH=), 135.4 (Ar-C), 135.2 (Ar-C), 132.4 (Ar-C), 129.9 (Ar-C), 128.4 (Ar-C), 127.4 (Ar-C), 121.0 (-CH=), 37.5 (CH3), 36.0 (CH3). HRMS-ESI (m/z): calcd. for C11H11ONCl2Na [M + Na]+: 266.0110; found: 266.0108.
- (E)-3-(4-Bromophenyl)-N,N-dimethylacrylamide (3e). White solid; yield: 69%; m.p.: 127–130 °C. IR (KBr plate): νmax 1648 (C=O), 1595 (C=C), 1147 (C-N), 751 (Ar-H). 1H NMR (600 MHz, CDCl3) δ 7.59 (d, J = 15.4 Hz, 1H, Ar-CH=), 7.49 (d, J = 8.3 Hz, 2H, Ar-H), 7.38 (d, J = 8.4 Hz, 2H, Ar-H), 6.87 (d, J = 15.4 Hz, 1H, CO-CH=), 3.16 (s, 3H, CH3), 3.06 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 166.4 (C=O), 141.1 (-CH=), 134.3 (Ar-C), 132.0 (Ar-C), 129.2 (Ar-C), 123.6 (Ar-C), 118.1 (-CH=), 37.4 (CH3), 36.0 (CH3). HRMS-ESI (m/z): calcd. for C11H13ONBr [M + H]+: 254.0175; found: 254.0173.
- (E)-N,N-Dimethyl-3-(4-(trifluoromethyl)phenyl)acrylamide (3f). White solid; yield: 66%; m.p.: 110–112 °C. IR (KBr plate): νmax 2935 (C-H), 1656 (C=O), 1607 (C=C), 818 (Ar-H). 1H NMR (600 MHz, CDCl3) δ 7.63–7.57 (m, 1H, Ar-CH=), 7.56–7.50 (m, 4H, Ar-H), 6.92 (dd, J = 15.5, 3.7 Hz, 1H, CO-CH=), 3.13–3.08 (m, 3H, CH3), 3.02–2.97 (m, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 166.0 (C=O), 140.5 (-CH=), 138.7 (Ar-C), 130.9(2JCF = 30.2, Ar-C), 127.9 (Ar-C), 125.6 (Ar-C), 123.9 (1CF = 271.8, CF3), 120.0 (-CH=), 37.3 (CH3), 35.9 (CH3). HRMS-ESI (m/z): calcd. for C12H12ONF3Na [M + Na]+: 266.0763; found: 266.0762.
- (E)-3-(4-Cyanophenyl)-N,N-dimethylacrylamide (3g). White solid; yield: 77%; m.p.: 149–154 °C. IR (KBr plate): νmax 1650 (C=O), 1608 (C=C), 1143 (C-N), 834 (Ar-H), 824 (Ar-H). 1H NMR (600 MHz, CDCl3) δ 7.63 (m, 2H, Ar-CH=, Ar-H), 7.58 (d, J = 8.1 Hz, 3H, Ar-H), 6.96 (d, J = 15.4 Hz, 1H, CO-CH=), 3.16 (d, J = 4.8 Hz, 3H, CH3), 3.05–3.03 (m, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 165.8 (C=O), 140.1 (-CH=), 139.7 (Ar-C), 132.6 (Ar-C), 128.20 (Ar-C), 121.1 (CN), 118.6 (-CH=), 112.6 (Ar-C), 37.5 (CH3), 36.0 (CH3). HRMS-ESI (m/z): calcd. for C12H12ON2Na [M + Na]+: 223.0842; found: 223.0840.
- (E)-N,N-Dimethyl-3-(3-phenoxyphenyl)acrylamide (3h). White solid; yield: 81%; m.p.: 71–73 °C. IR (KBr plate): νmax 3024 (C-H), 2927 (C-H), 1651 (C=O), 1607 (C=C), 1164 (C-N), 754 (Ar-H). 1H NMR (600 MHz, CDCl3) δ 7.62 (d, J = 15.4 Hz, 1H, Ar-CH=), 7.35 (dt, J = 14.8, 7.9 Hz, 3H, Ar-H), 7.27 (d, J = 7.8 Hz, 1H, Ar-H), 7.20 (s, 1H, Ar-H), 7.13 (t, J = 7.4 Hz, 1H, Ar-H), 7.03 (d, J = 8.4 Hz, 2H, Ar-H), 6.99 (dd, J = 8.1, 2.5 Hz, 1H, Ar-H), 6.86 (d, J = 15.4 Hz, 1H, CO-CH=), 3.16 (s, 3H, CH3), 3.07 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 166.5 (C=O), 157.6 (Ar-C), 156.9 (Ar-C), 141.7 (-CH=), 137.2 (Ar-C), 130.1 (Ar-C), 129.9 (Ar-C), 123.5 (Ar-C), 123.0 (Ar-C), 119.8 (Ar-C), 118.9 (Ar-C), 118.2 (-CH=), 117.7 (Ar-C), 37.5 (CH3), 36.0 (CH3). HRMS-ESI (m/z): calcd. for C17H17O2NNa [M + Na]+: 290.1152; found: 290.1148.
- (E)-N,N-Dimethyl-3-(p-tolyl)acrylamide (3i). White solid; yield: 85%; m.p.: 112–117 °C. IR (KBr plate): νmax 2935 (C-H), 1650 (C=O), 1602 (C=C), 1141 (C-N), 768 (Ar-H). 1H NMR (600 MHz, CDCl3) δ 7.64 (d, J = 15.4 Hz, 1H, Ar-CH=), 7.42 (d, J = 8.0 Hz, 2H, Ar-H), 7.17 (d, J = 7.8 Hz, 2H, Ar-H), 6.84 (d, J = 15.4 Hz, 1H, CO-CH=), 3.15 (s, 3H, CH3), 3.05 (s, 3H, CH3), 2.35 (s, 3H, Ar-CH3). 13C NMR (150 MHz, CDCl3) δ 166.9 (C=O), 142.3 (-CH=), 139.8 (Ar-C), 132.6 (Ar-C), 129.5 (Ar-C), 127.8 (Ar-C), 116.3 (-CH=), 37.4 (CH3), 35.9 (CH3), 21.4 (CH3). HRMS-ESI (m/z): calcd. for C12H15ONNa [M + Na]+: 212.1046; found: 212.1044.
- (E)-N,N-Dimethyl-3-(naphthalen-2-yl)acrylamide (3j). Light yellow solid; yield: 78%; m.p.: 158–161 °C. IR (KBr plate): νmax 2934 (C-H), 1648 (C=O), 1601 (C=C), 1138 (C-N), 822 (Ar-H). 1H NMR (600 MHz, CDCl3) δ 7.91 (s, 1H, Ar-H), 7.82 (m, 4H, Ar-CH=, Ar-H), 7.67 (d, J = 8.6 Hz, 1H, Ar-H), 7.48 (dd, J = 6.2, 3.2 Hz, 2H, Ar-H), 6.99 (d, J = 15.4 Hz, 1H, CO-CH=), 3.19 (s, 3H, CH3), 3.08 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 166.6 (C=O), 142.4 (-CH=), 133.9 (Ar-C), 133.4 (Ar-C), 132.8 (Ar-C), 129.2 (Ar-C), 128.5 (Ar-C), 128.5 (Ar-C), 127.7 (Ar-C), 126.9 (Ar-C), 126.6 (Ar-C), 123.7 (Ar-C), 117.6 (-CH=), 37.5 (CH3), 36.0 (CH3). HRMS-ESI (m/z): calcd. for C15H15ONNa [M + Na]+: 248.1046; found: 248.1045.
- (E)-3-Cyclohexyl-N,N-dimethylacrylamide (3k). Light yellow solid; yield: 89%; m.p.: 96–100 °C. IR (KBr plate): νmax 1647 (C=O), 1602 (C=C), 1140 (C-N). 1H NMR (600 MHz, CDCl3) δ 6.80 (dd, J = 15.2, 7.0 Hz, 1H, Ar-CH=), 6.17 (d, J = 15.2 Hz, 1H, CO-CH=), 3.05 (s, 3H, CH3), 2.98 (s, 3H, CH3), 2.14–2.08 (m, 1H, Cy-H), 1.76–1.71 (m, 4H, Cy-H), 1.65 (m, 1H, Cy-H), 1.26 (m, 2H, Cy-H), 1.14 (m, 3H, Cy-H). 13C NMR (150 MHz, CDCl3) δ 167.2 (C=O), 151.3 (-CH=), 117.6 (-CH=), 40.7 (Cy-C), 37.3 (CH3), 35.7 (CH3), 32.0 (Cy-C), 25.9 (Cy-C), 25.7 (Cy-C). HRMS-ESI (m/z): calcd. for C11H20ON [M + H]+: 182.1539; found: 182.1539.
- (E)-N,N-Dimethylhept-2-enamide (3l). White oil; yield: 74%; IR (KBr plate): νmax 2938 (C-H), 1654 (C=O), 1611 (C=C), 1143 (C-N). 1H NMR (600 MHz, CDCl3) δ 6.88 (dt, J = 14.5, 7.0 Hz, 1H, Ar-CH=), 6.25 (d, J = 15.1 Hz, 1H, CO-CH=), 3.09 (s, 3H, CH3), 3.01 (s, 3H, CH3), 2.22 (q, J = 7.4 Hz, 2H, CH2), 1.46 (t, J = 7.7 Hz, 2H, CH2), 1.36 (q, J = 7.4 Hz, 2H, CH2), 0.92 (t, J = 7.3 Hz, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 167.0 (C=O), 146.4 (-CH=), 120.1 (-CH=), 37.4 (CH3), 35.7 (CH3), 32.2 (CH2), 30.5 (CH2), 22.3 (CH2), 13.9 (CH3). HRMS-ESI (m/z): calcd. for C9H17ONNa [M + Na]+: 178.1202; found: 178.1200.
- (E)-N,N-Dimethyl-3-(thiophen-2-yl)acrylamide (3m). Light yellow solid; yield: 71%; m.p.: 98–101 °C. IR (KBr plate): νmax 2930 (C-H), 1643 (C=O), 1601 (C=C), 1140 (C-N), 705 (Th-H). 1H NMR (600 MHz, CDCl3) δ 7.79 (d, J = 15.1 Hz, 1H, Ar-CH=), 7.31 (d, J = 5.1 Hz, 1H, CO-CH=), 7.22 (d, J = 3.6 Hz, 1H, Th-H), 7.03 (dd, J = 5.0, 3.6 Hz, 1H, Th-H), 6.69 (d, J = 15.1 Hz, 1H, Th-H), 3.15 (s, 3H, CH3), 3.06 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 166.4 (C=O), 140.5 (-CH=), 135.2 (Th-C), 130.3 (Th-C), 128.0 (Th-C), 127.2 (Th-C), 116.1 (-CH=), 37.4 (CH3), 36.0 (CH3). HRMS-ESI (m/z): calcd. for C9H11ONNaS [M + Na]+: 204.0454; found: 204.0453.
- (E)-3-(Benzo[c][1,2,5]oxadiazol-4-yl)-N,N-dimethylacrylamide (3n). Light yellow solid; yield: 65%; m.p.: 154–160 °C. IR (KBr plate): νmax 2931 (C-H), 1651 (C=O), 1612 (C=C), 1143 (C-N), 812 (Ar-H), 766 (Ar-H). 1H NMR (600 MHz, CDCl3) δ 7.94 (d, J = 15.3 Hz, 1H, Ar-CH=), 7.82 (d, J = 8.6 Hz, 1H, Ar-H), 7.76 (d, J = 15.2 Hz, 1H, CO-CH=), 7.49–7.44 (m, 2H, Ar-H), 3.28 (s, 3H, CH3), 3.12 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 166.2 (C=O), 149.6 (Ar-C), 147.5 (-CH=), 136.5 (Ar-C), 133.7 (Ar-C), 131.7 (Ar-C), 126.2 (Ar-C), 125.3 (Ar-C), 117.0 (-CH=), 37.5 (CH3), 36.0 (CH3). HRMS-ESI (m/z): calcd. for C11H11O2N3Na [M + Na]+: 240.0743; found: 240.0741.
- (2E, 4E)-N,N-Dimethyl-5-phenylpenta-2,4-dienamide (3o). White solid; yield: 74%; m.p.: 108–113 °C. IR (KBr plate): νmax 1643 (C=O), 1624 (C=C), 1125 (C-N), 812 (Ar-H), 758 (Ar-H). 1H NMR (600 MHz, CDCl3) δ 7.46–7.41 (m, 3H, Ar-CH=, Ar-H, -CH=), 7.33 (t, J = 7.6 Hz, 2H, Ar-H), 7.27 (d, J = 7.3 Hz, 1H, -CH=), 6.92–6.83 (m, 2H, Ar-H), 6.45 (d, J = 14.7 Hz, 1H, CO-CH=), 3.10 (s, 3H, CH3), 3.03 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ 166.8 (C=O), 142.5 (-CH=), 139.0 (-CH=), 136.4 (Ar-C), 128.8 (Ar-C), 128.7 (Ar-C), 127.0 (Ar-C), 126.9 (-CH=), 120.6 (-CH=), 37.4 (CH3), 35.9 (CH3). HRMS-ESI (m/z): calcd. for C13H16ON [M + H]+: 202.1226; found: 202.1224.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrold, M.W.; Wallace, R.A.; Farooqui, T.; Wallace, L.J.; Uretsky, N.; Miller, D.D. Synthesis and D2 dopaminergic activity of pyrrolidinium, tetrahydrothiophenium, and tetrahydrothiophene analogs of sulpiride. J. Med. Chem. 1989, 32, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Pardin, C.; Pelletier, J.N.; Lubell, W.D.; Keillor, J.W. Cinnamoyl inhibitors of tissue transglutaminase. J. Org. Chem. 2008, 73, 5766–5775. [Google Scholar] [CrossRef]
- Kanemasa, S.; Yamamoto, H.; Kobayashi, S. dl-Selective reductive coupling/dieckmann condensation sequence of α,β-unsaturated amides with samarium (II) iodide/HMPA. Synthesis of a new ligand, trans-1,2-cyclopentanediyl-2,2′-biphenol. Tetrahedron Lett. 1996, 37, 8505–8506. [Google Scholar] [CrossRef]
- Nahm, M.R.; Potnick, J.R.; White, P.S.; Johnson, J.S. Metallophosphite-catalyzed asymmetric acylation of α,β-unsaturated amides. J. Am. Chem. Soc. 2006, 128, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, T.; Kakei, H.; Gnanadesikan, V.; Tosaki, S.Y.; Ohshima, T.; Shibasaki, M. Catalytic asymmetric epoxidation of α,β-unsaturated amides: Efficient synthesis of β-aryl α-hydroxy amides using a one-pot tandem catalytic asymmetric epoxidation-pd-catalyzed epoxide opening process. J. Am. Chem. Soc. 2002, 124, 14544–14545. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Yun, J. Catalytic asymmetric boration of acyclic α,β-unsaturated esters and nitriles. Angew. Chem. Int. Ed. 2008, 120, 151–153. [Google Scholar] [CrossRef]
- Jiang, X.F.; Zhen, Y.S. Cinnamamide, an antitumor agent with low cytotoxicity acting on matrix metalloproteinase. Anti-Cancer Drugs 2000, 11, 49–54. [Google Scholar] [CrossRef]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic acid derivatives as anticancer agents-a review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Q.; Wu, D.; Wei, C.X.; Quan, Z.S. Synthesis and antidepressant-like action of N-(2-hydroxyethyl) cinnamamide derivatives in mice. Med. Chem. Res. 2011, 20, 1273–1279. [Google Scholar] [CrossRef]
- Surendran, S.; Babu, M.; Joseph, J.; Padma, U.D. Facilitatory effect of piperine on the anticonvulsant effect of sodium valproate against pentylenetetrazole induced seizures in mice. Res. J. Pharm. Technol. 2020, 13, 651–652. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, M.; Zhong, Y.; Wu, B. Synthesis, crystal structure, hirshfeld surface analyses and biological activity of novel cinnamide derivatives as neuroprotective drugs. Polycycl. Aromat. Compd. 2023, 44, 5138–5149. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, Y.; Tan, X.Z.; Wang, Y.Y.; Ma, S.Y.; Gao, M.J.; Wu, B. Design, synthesis, and biological evaluation of novel cinnamide derivatives as neuroprotective agents for the treatment of cerebral ischemia. Curr. Med. Chem. 2024, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Al Nasr, I.S.; Koko, W.S.; Ma, J.; Eckert, S.; Brehm, L.; Said, R.S.B.; Daoud, I.; Hanachi, R.; Rahali, S.; et al. Evaluation of the antiparasitic and antifungal activities of new synthetic piperlongumine-type cinnamide derivatives: Booster effect by halogen substituents. ChemMedChem 2023, 18, e202300132. [Google Scholar] [CrossRef] [PubMed]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Karuo, Y.; Tsukada, Y.; Kunishima, M. Mild amide-cleavage reaction mediated by electrophilic benzylation. Chem.—Eur. J. 2016, 22, 14042–14047. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Chang, S. Borane-catalyzed reductive α-silylation of conjugated esters and amides leaving carbonyl groups intact. Angew. Chem. Int. Ed. 2016, 55, 218–222. [Google Scholar] [CrossRef]
- Zhang, J.R.; Liao, Y.Y.; Deng, J.C.; Tang, Z.L.; Xu, Y.L.; Xu, L.; Tang, R.Y. DABCO-Promoted decarboxylative acylation: Synthesis of α-keto and α,β-unsaturated amides or esters. Asian J. Org. Chem. 2017, 6, 305–312. [Google Scholar] [CrossRef]
- Fujihara, T.; Katafuchi, Y.; Iwai, T.; Terao, J.; Tsuji, Y. Palladium-catalyzed intermolecular addition of formamides to alkynes. J. Am. Chem. Soc. 2010, 132, 2094–2098. [Google Scholar] [CrossRef]
- Kumar, P.S.; Kumar, G.S.; Kumar, R.A.; Reddy, N.V.; Rajender Reddy, K. Copper-catalyzed oxidative coupling of carboxylic acids with N,N-dialkylformamides: An approach to the synthesis of amides. Eur. J. Org. Chem. 2013, 2013, 1218–1222. [Google Scholar] [CrossRef]
- Priyadarshini, S.; Joseph, P.A.; Kantam, M.L. Copper catalyzed cross-coupling reactions of carboxylic acids: An expedient route to amides, 5-substituted γ-lactams and α-acyloxy esters. RSC Adv. 2013, 3, 18283–18287. [Google Scholar] [CrossRef]
- Woodbury, R.P.; Rathke, M.W. Formation of the lithium enolate of N,N-dimethyl-2-trimethylsilylacetamide. Reaction with carbonyl compounds and epoxides. J. Org. Chem. 1978, 43, 1947–1949. [Google Scholar] [CrossRef]
- Böhm, V.P.; Herrmann, W.A. Nonaqueous Ionic Liquids: Superior reaction media for the catalytic Heck-vinylation of chloroarenes. Chem.—Eur. J. 2000, 6, 1017–1025. [Google Scholar] [CrossRef]
- Saberi, D.; Mahdudi, S.; Cheraghi, S.; Heydari, A. Cu(II)-acetylacetone complex covalently anchored onto magnetic nanoparticles: Synthesis, characterization and catalytic evaluation in amide bond formation via oxidative coupling of carboxylic acids with N,N-dialkylformamides. J. Organomet. Chem. 2014, 772, 222–228. [Google Scholar] [CrossRef]
- Yang, X.H.; Wei, W.T.; Li, H.B.; Song, R.J.; Li, J.H. Oxidative coupling of alkenes with amides using peroxides: Selective amide C(sp3)-H versus C(sp2)-H functionalization. Chem. Commun. 2014, 50, 12867–12869. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Wu, Z.; Su, F.; Yu, Y.; Jing, Y.; Kong, J.; Wang, Z.; Wang, S.; Zhao, M. Synthesis of cinnamides via amidation reaction of cinnamic acids with tetraalkylthiuram disulfides under simple condition. Eur. J. Org. Chem. 2020, 2, 198–208. [Google Scholar] [CrossRef]
- Weidlich, T.; Prokeš, L.; Růžička, A.; Padělková, Z. Condensation of aromatic aldehydes with N,N-dimethylacetamide in presence of dialkyl carbonates as dehydrating agents. Monatshefte Chem.-Chem. Mon. 2010, 141, 205–211. [Google Scholar] [CrossRef]
- Foo, S.W.; Oishi, S.; Saito, S. Aldol condensation of amides using phosphazene-based catalysis. Tetrahedron Lett. 2012, 53, 5445–5448. [Google Scholar] [CrossRef]
- Yang, T.; Lu, M.; Lin, Z.; Huang, M.; Cai, S. Visible-light-promoted oxidation/condensation of benzyl alcohols with dialkylacetamides to cinnamides. Org. Biomol. Chem. 2019, 17, 449–453. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Wang, E.; Li, L.; Luo, Z.; Cao, J.; Chen, J.; Yang, L.; Yang, X. Hydrogen bond assisted three-component tandem reactions to access N-alkyl-4-quinolones. Molecules 2023, 28, 2304. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Yang, L.; Yang, Q.; Yang, F.; Luo, J.; Gan, M.; Wang, X.; Song, S.; Lei, Y.; Yang, X. Polyphosphoric acid-promoted one-pot synthesis and neuroprotective effects of flavanones against NMDA-induced injury in PC12 cells. RSC Adv. 2022, 12, 28098–28103. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, E.; Fan, Y.; Yang, J.; Luo, Z.; Wang, Y.; Peng, M.; Deng, T.; Yang, X. One-pot synthesis of (E)-3-benzylideneflavanones from 2-hydroxyacetophenones and aromatic aldehydes. Tetrahedron Lett. 2020, 61, 151180. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Yang, J.; Wu, Y.; Li, L.; Chen, F.; Wang, E.; Li, L.; Yang, Y.; Yan, Y.; et al. Activation of phenolic oxygen atom using polyphosphoric acid: Synthesis of carbonyl-containing dihydrobenzofurans/dihydrobenzopyrans. Synth. Commun. 2021, 51, 1723–1730. [Google Scholar] [CrossRef]
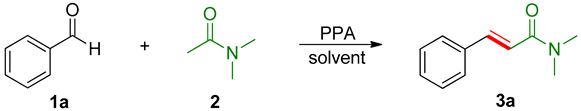 | |||||
|---|---|---|---|---|---|
| Entry | PPA (Equiv.) | T (°C) | t (h) | Solvent | 3a (%) b |
| 1 | 2 | reflux | 4 | DMF | 46 |
| 2 | 1.5 | reflux | 4 | DMF | 45 |
| 3 | 1 | reflux | 4 | DMF | 50 |
| 4 | 0.5 | reflux | 4 | DMF | 22 |
| 5 | 1 | 140 | 4 | DMF | 13 |
| 6 | 1 | reflux | 5 | DMF | 64 |
| 7 | 1 | reflux | 6 | DMF | 79 |
| 8 | 1 | reflux | 7 | DMF | 77 |
| 9 | 1 | reflux | 6 | 1,4-Dioxane | 6 |
| 10 | 1 | reflux | 6 | THF | / |
| 11 | 1 | reflux | 6 | Toluene | 15 |
| 12 | 1 | reflux | 6 | DCE | / |
| 13 | 1 | reflux | 6 | DMSO | / |
| 14 | 1 | reflux | 6 | EtOH | / |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, E.; Yang, L.; He, L.; Yang, Q.; Wang, X.; Liu, Y.; Li, M.; Lei, Y.; Yang, X. Polyphosphoric Acid-Promoted Efficient Synthesis of Cinnamides via Aldol Condensation of Amide. Molecules 2024, 29, 4632. https://doi.org/10.3390/molecules29194632
Wang E, Yang L, He L, Yang Q, Wang X, Liu Y, Li M, Lei Y, Yang X. Polyphosphoric Acid-Promoted Efficient Synthesis of Cinnamides via Aldol Condensation of Amide. Molecules. 2024; 29(19):4632. https://doi.org/10.3390/molecules29194632
Chicago/Turabian StyleWang, Enhua, Lishou Yang, Lanfeng He, Qian Yang, Xue Wang, Yunlu Liu, Manxiang Li, Yang Lei, and Xiaosheng Yang. 2024. "Polyphosphoric Acid-Promoted Efficient Synthesis of Cinnamides via Aldol Condensation of Amide" Molecules 29, no. 19: 4632. https://doi.org/10.3390/molecules29194632
APA StyleWang, E., Yang, L., He, L., Yang, Q., Wang, X., Liu, Y., Li, M., Lei, Y., & Yang, X. (2024). Polyphosphoric Acid-Promoted Efficient Synthesis of Cinnamides via Aldol Condensation of Amide. Molecules, 29(19), 4632. https://doi.org/10.3390/molecules29194632





