The Influence of Rhizobial Nod Factors on the Synthesis of Flavonoids in Common Buckwheat (Fagopyrum esculentum Moench)
Abstract
1. Introduction
2. Results and Discussion
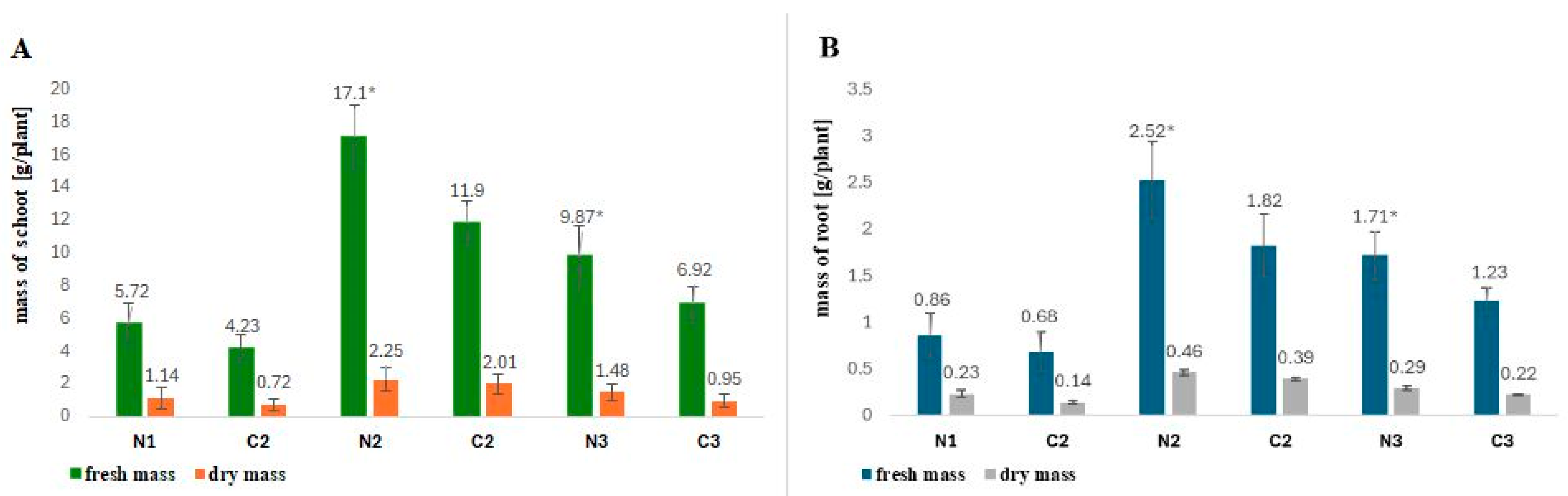
2.1. Influence of Rhizobial Nod Factors on the Fresh and Dry Mass of Fagopyrum esculentum
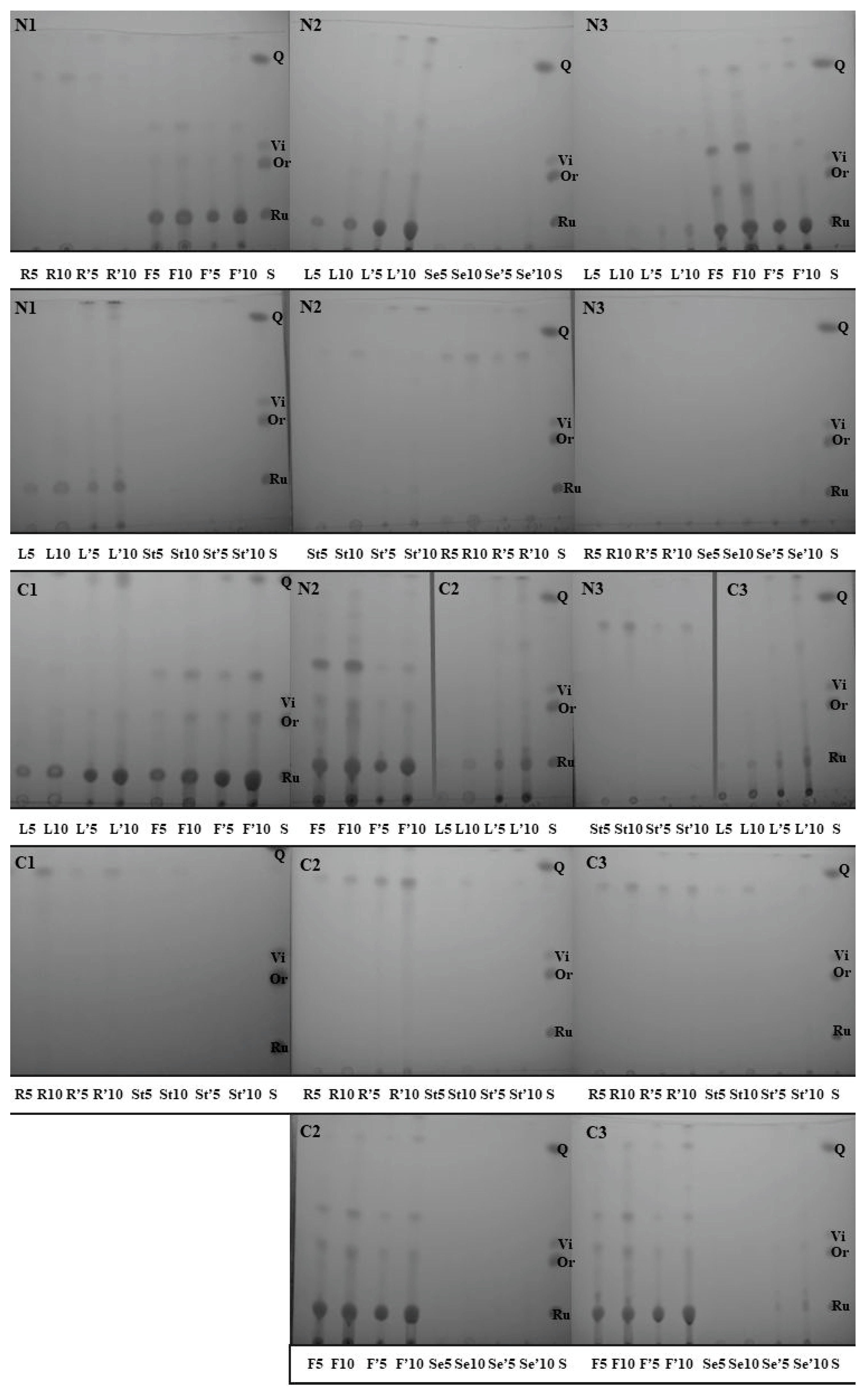
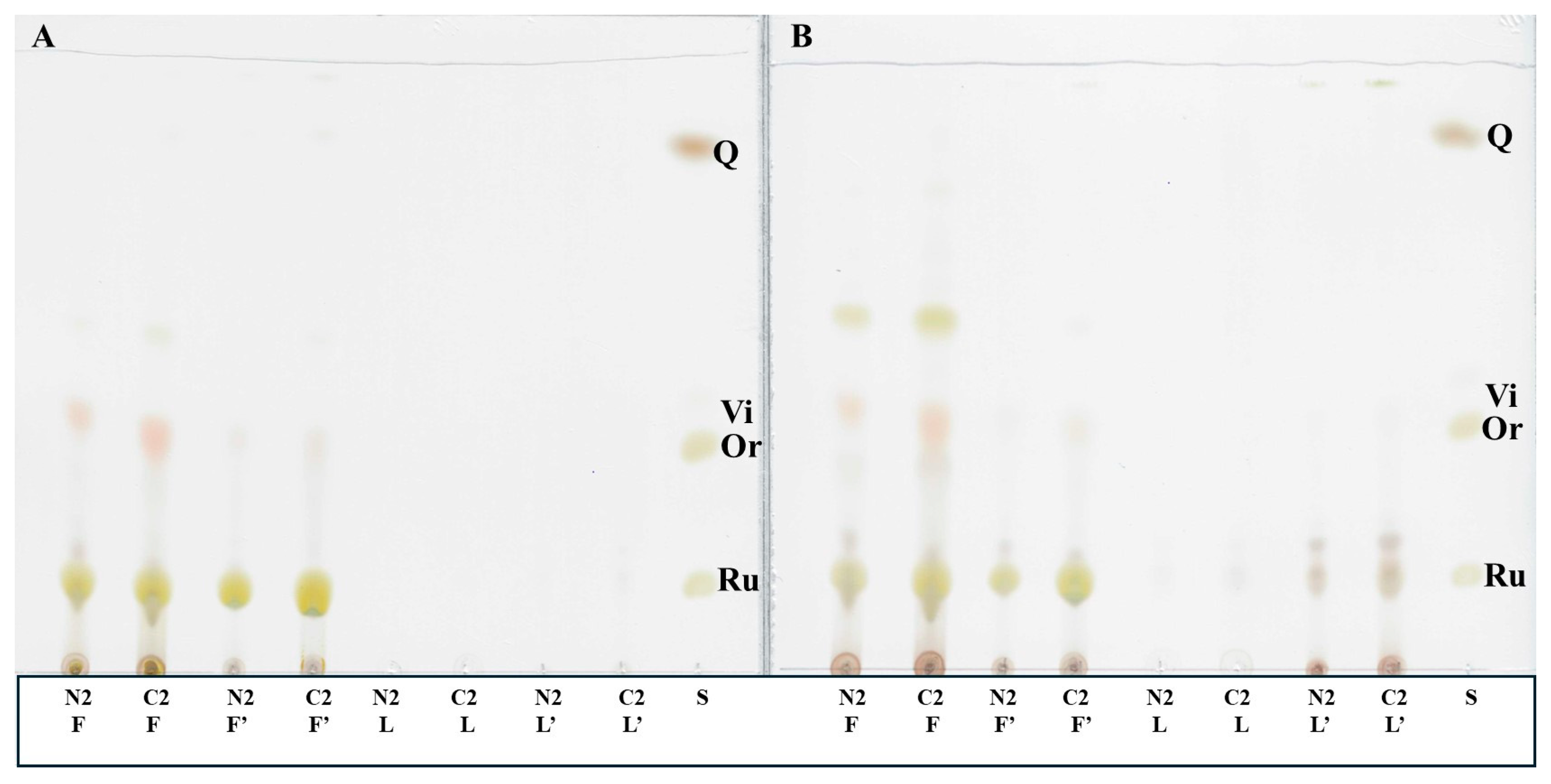
2.2. Extraction and TLC Analysis of Flavonoids from Different Parts of F. esculentum Plants
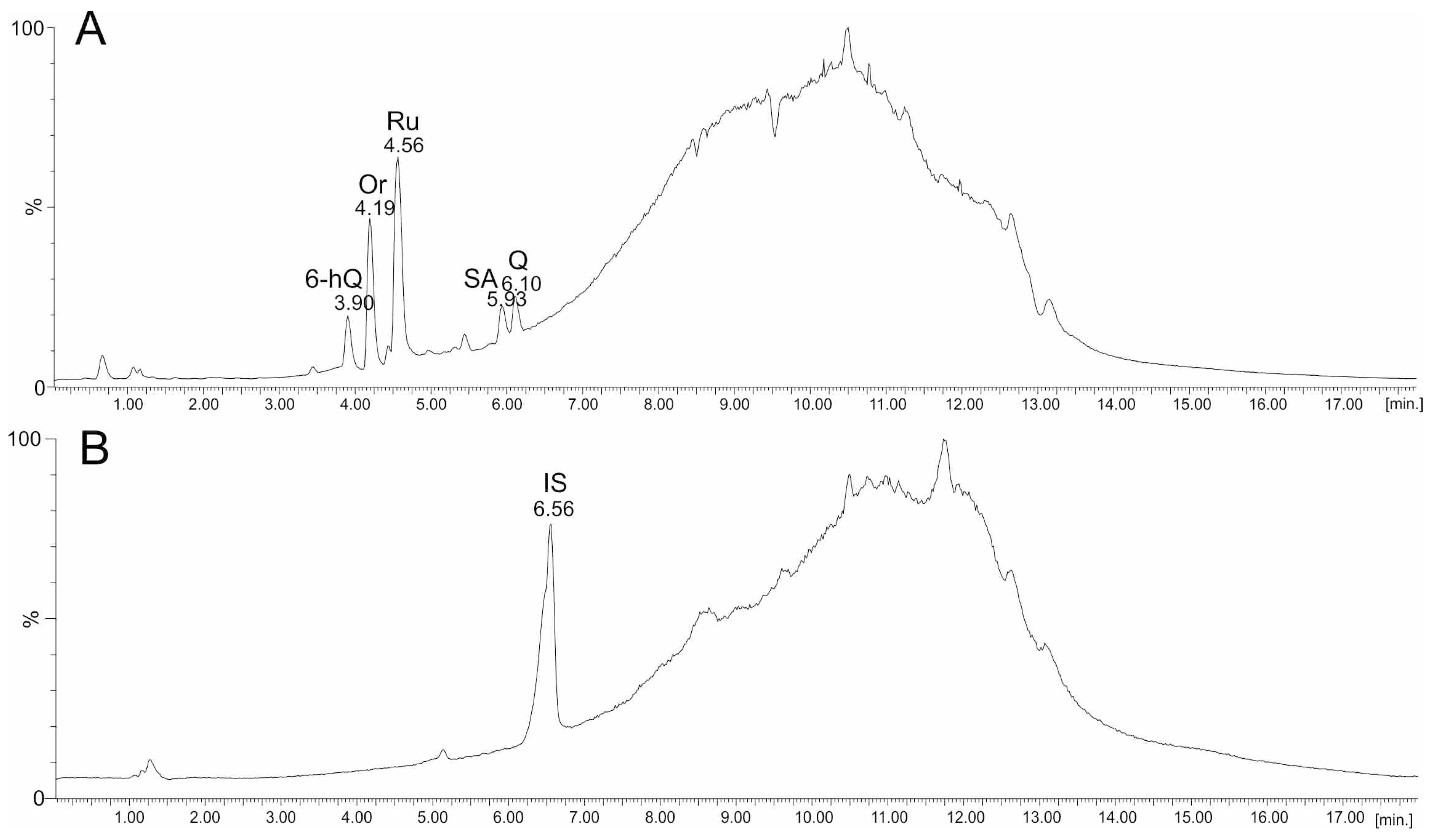
2.3. UPLC-ESI-MS Analyses of Fractions
3. Materials and Methods
3.1. Chemical Reagents
3.2. Isolation of Nod Factors Produced by R. leguminosarum bv. viciae GRO9
3.3. Plant Culture
3.4. Extraction of Flavonoids from Different Parts of Buckwheat Plants
3.5. Thin-Layer Chromatography (TLC)
3.6. UPLC-ESI-MS
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramalingum, N.; Mahomoodally, M.F. The therapeutic potential of medicinal foods. Adv. Pharmacol. Pharm. Sci. 2014, 2014, 354264. [Google Scholar] [CrossRef]
- Watanabe, M.; Ito, M. Changes in antioxidative activity and flavonoid composition of the extracts from aerial parts of buckwheat during growth period. J. Jpn. Soc. Food Sci. Technol. 2002, 49, 119–125. [Google Scholar] [CrossRef]
- Kiprovski, B.; Mikuliv-Petkovsek, M.; Slatnar, A.; Veberic, R.; Stampar, F.; Malencic, D.; Latkovic, D. Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars. Food Chem. 2015, 185, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.G.; Lim, T.-G.; Lee, B.H.; Lim, S.; Kang, H.; Eom, S.H.; Yoo, M.; Jang, H.W.; Kim, D.-O. Comparison of anti-inflammatory effects of flavonoid-rich common and tartary buckwheat sprout extracts in lipopolysaccharide-stimulated RAW264.7 and peritoneal macrophages. Oxid. Med. Cell. Longev. 2017, 2017, 9658030. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; Van Nood, E.L.S.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanism of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Shumilina, J.; Soboleva, A.; Abakumov, E.; Shtark, O.Y.; Zhukov, V.A.; Frolov, A. Signaling in legume–Rhizobia symbiosis. Int. J. Mol. Sci. 2023, 24, 17397. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Park, Y.J.; Morgan, A.M.A.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Influence of indole-3-Acetic acid and gibberellic acid on phenylpropanoid accumulation in common buckwheat (Fagopyrum esculentum Moench) sprouts. Molecules 2017, 22, 374. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Sato, H.; Sakamura, S. Isolation and identification of flavonoids from immature buckwheat seed (Fagopyrum esculentum Moench). Agric. Chem. Soc. Jpn. 1975, 49, 53. [Google Scholar]
- Margna, U.V.; Margna, E.R. Differential nature of quantitative shifts in flavonoid accumulation in buckwheat seedlings of different ages. Sov. Plant Physiol. 1982, 29, 223. [Google Scholar]
- Kim, S.-J.; Zaidul, I.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, J.; Vrchotova, N. Level of catechin, myricetin, quercetin and isoquercitrin in buckwheat (Fagopyrum esculentum Moench), changes of their levels during vegetation and their effect on the growth of selected weeds. J. Agric. Food Chem. 2009, 57, 2719–2725. [Google Scholar] [CrossRef]
- Golisz, A.; Lata, B.; Gawronski, S.W.; Fujii, Y. Specific and total activities of the allelochemicals identified in buckwheat. Weed Biol. Manag. 2007, 7, 164–171. [Google Scholar] [CrossRef]
- Kreft, S.; Knapp, M.; Kreft, I. Extraction of rutin from buckwheat (Fagopyrum esculentum Moench) seeds and determination of capillary electrophoresis. J. Agric. Food Chem. 1999, 41, 4649–4652. [Google Scholar] [CrossRef]
- Kalinova, J.; Triska, J.; Vrchotova, N. Distribution of vitamin E, squalene, epicatechin, and rutin in common buckwheat plants (Fagopyrum esculentum Moench). J. Agric. Food Chem. 2006, 54, 5330–5335. [Google Scholar] [CrossRef]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Kawakami, A.; Inbe, T.; Kayahara, H.; Horii, A. Preparation of Enzymatic Hydrolysates of Buckwheat Globulin and Their Angiotensin I Converting Enzyme Inhibitory Activities. In Advances in Buckwheat Research; Matano, T., Ujihara, A., Eds.; Shinshu University Press: Ina, Japan, 1995; p. 927. [Google Scholar]
- Hirsch, A.M. Developmental biology of legume nodulation. New Phytol. 1992, 122, 211–237. [Google Scholar] [CrossRef]
- Skorupska, A.; Kidaj, D.; Wielbo, J. Flavonoids and Nod Factors: Importance in Legume-Microbe Interactions and Legume Improvement. In Microbes for Legume Improvement; Zaidi, A., Khan, M., Musarrat, J., Eds.; Springer: Ham, Germany, 2017; pp. 75–94. [Google Scholar] [CrossRef]
- Kidaj, D.; Susniak, K.; Matys, J.; Komaniecka, I.; Sroka-Bartnicka, A.; Krysa, M. Biological activity of Nod factors. Acta Biochim. Pol. 2020, 67, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Brewin, N.J. Plant cell wall remodeling in the Rhizobium-legume symbiosis. Crit. Rev. Plant Sci. 2004, 23, 293–316. [Google Scholar] [CrossRef]
- Prithiviraj, B.; Zhou, X.; Souleimanov, A.; Kahn, W.M.; Smith, D.L. A host-specific bacteria-to-plant signal molecule (Nod factor) enhances germination and early growth of diverse crop plants. Planta 2003, 21, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Macchiavelli, R.E.; Brelles-Marino, G. Nod factor-treated Medicago truncatula roots and seeds show an increased number of nodules when inoculated with a limiting population of Sinorhizobium meliloti. J. Exp. Bot. 2004, 55, 2635–2640. [Google Scholar] [CrossRef] [PubMed]
- Kidaj, D.; Wielbo, J.; Skorupska, A. Nod factors stimulate seed germination and promote growth and nodulation of pea and vetch under competitive conditions. Microbiol. Res. 2011, 167, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Podleśny, J.; Wielbo, J.; Podleśna, A.; Kidaj, D. The pleiotropic effects of extract containing rhizobial Nod factors on pea growth and yield. Cent. Eur. J. Biol. 2014, 4, 396–409. [Google Scholar] [CrossRef]
- Siczek, A.; Lipiec, J.; Wielbo, J.; Kidaj, D.; Szarlip, P. Symbiotic activity of pea (Pisum sativum) after application of Nod factors under field conditions. Int. J. Mol. Sci. 2014, 15, 7344–7351. [Google Scholar] [CrossRef]
- Maj, D.; Wielbo, J.; Marek-Kozaczuk, M.; Skorupska, A. Pretreatment of clover seeds with Nod factors improves growth and nodulation of Trifolium pratense. J. Chem. Ecol. 2009, 35, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Souleimanov, A.; Prithiviraj, B.; Smith, D.L. The major Nod factor of Bradyrhizobium japonicum promotes early growth of soybean and corn. J. Exp. Bot. 2002, 376, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; McIver, J.; Yang, Y.; Bai, Y.; Schultz, B.; McIver, A. Foliar application of lipo-chitooligosaccharides (Nod factors) to tomato (Lycopersicon esculentum) enhances flowering and fruit production. Can. J. Plant Sci. 2007, 87, 365–372. [Google Scholar] [CrossRef]
- Susniak, K.; Krysa, M.; Kidaj, D.; Szymańska-Chargot, M.; Komaniecka, I.A.; Zamłynska, K.; Choma, A.; Wielbo, J.; Ilag, L.L.; Sroka-Bartnicka, A. Multimodal spectroscopic imaging of pea root nodules to assess the nitrogen fixation in the presence of biofertilizer based on Nod-factors. Int. J. Mol. Sci. 2021, 22, 12991. [Google Scholar] [CrossRef] [PubMed]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A 2003, 1000, 657–691. [Google Scholar] [CrossRef]
- Matsui, K.; Walker, A.R. Biosynthesis and regulation of flavonoids in buckwheat. Breed. Sci. 2020, 70, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Margna, U.; Margna, E.; Paluteder, A. Localization and distribution of flavonoids in buckwheat seedling cotyledons. J. Plant Physiol. 1990, 136, 166–171. [Google Scholar] [CrossRef]
- Suzuki, T.; Honda, Y.; Funatsuki, W.; Nakatsuka, K. Purification and characterization of flavonol 3-glucosidase, and its activity during ripening in tartary buckwheat seeds. Plant Sci. 2002, 163, 417–423. [Google Scholar] [CrossRef]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef]
- De Rijke, E.; Out, P.; Niessen, W.M.A.; Ariese, F.; Gooijer, C.; Brinkman, U.A.T. Analitycal separation and detection methods for flavonoids. J. Chromatogr. A 2006, 1112, 31–63. [Google Scholar] [CrossRef] [PubMed]
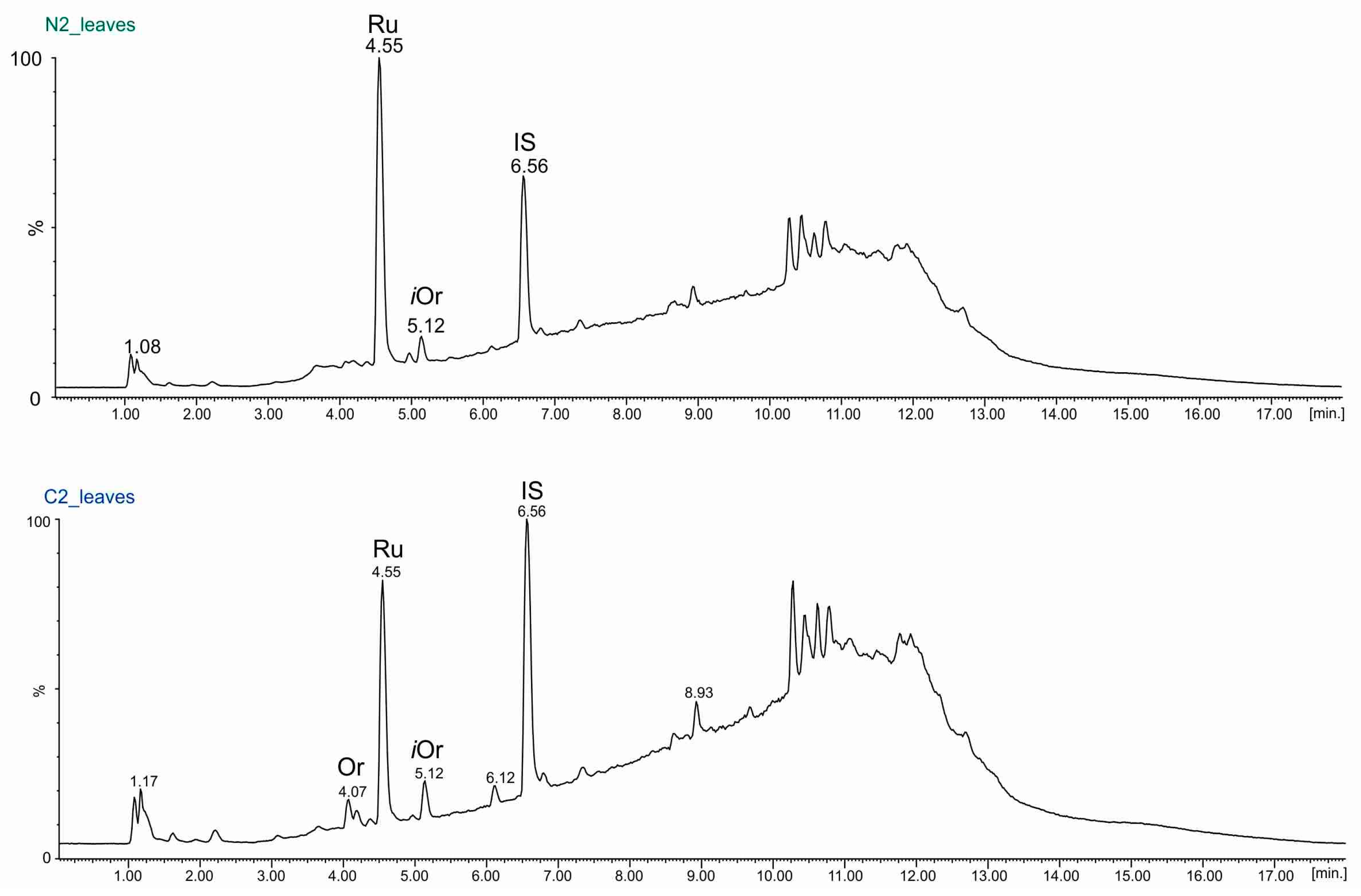
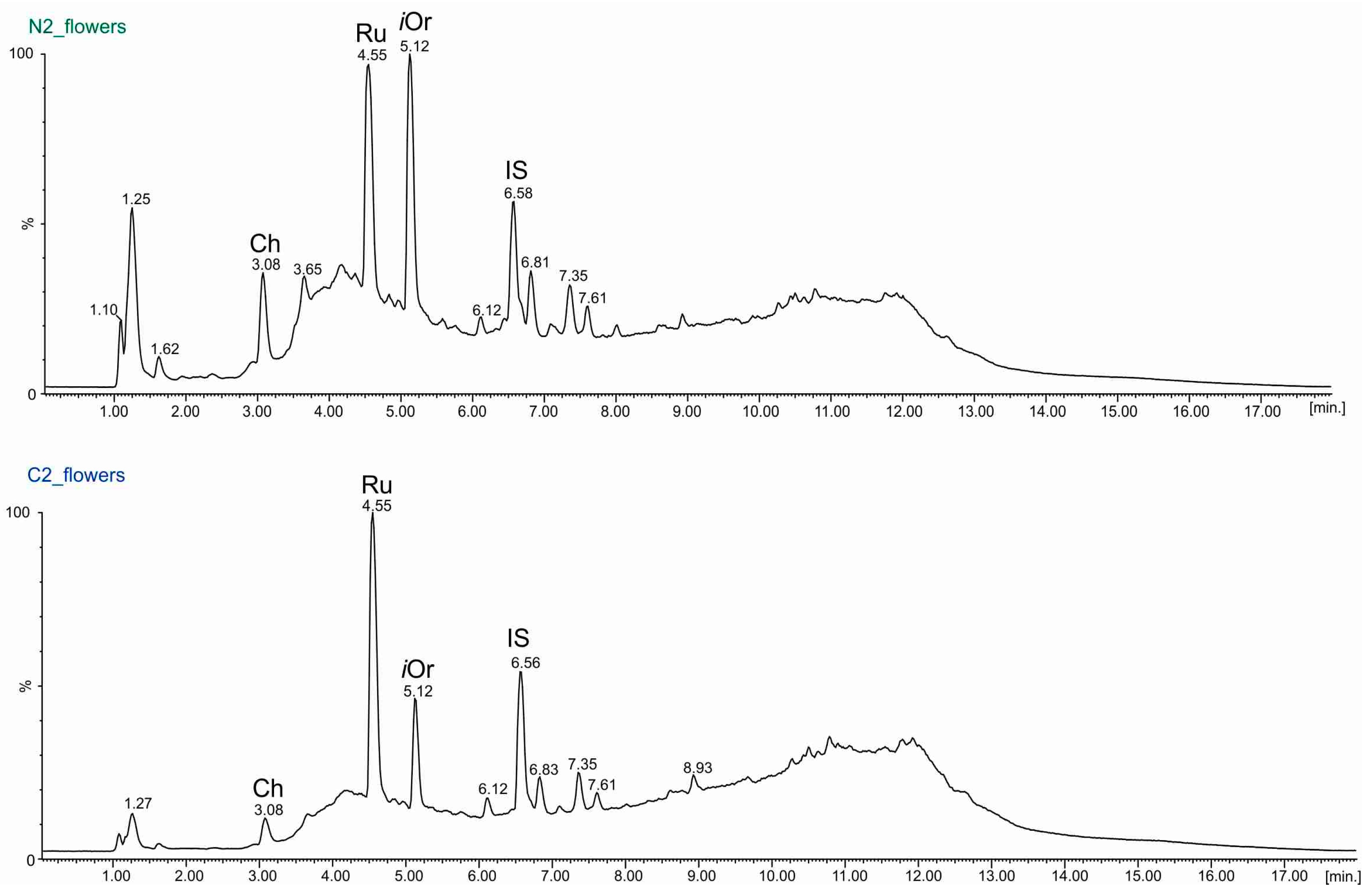
| Rutin (RT = 4.55 min.) (µg/g Dry Mass ± SD) | ||||||
|---|---|---|---|---|---|---|
| Part of Plant | N1 | C1 | N2 | C2 | N3 | C3 |
| leaves | 176.76 ± 0.58 | 230.54 ± 0.34 | 406.67 ± 1.17 | 163.39 ± 0.67 | 139.0 ± 1.68 | 225.84 ± 2.29 |
| flowers | 424.38 ± 1.12 | 144.9 ± 0.7 | 357.94 ± 0.86 | 402.59 ± 0.51 | 351.38 ± 2.66 | 440.57 ± 1.64 |
| seeds | - | - | 11.25 ± 0.51 | 9.72 ± 0.19 | 10.75 ± 0.12 | 44.32 ± 0.3 |
| stem | 2.91 ± 0.14 | 3.77 ± 0.66 | 28.41 ± 0.45 | 9.61 ± 0.19 | ND | 36.02 ± 0.92 |
| root | 2.3 ± 0.13 | ND | 17.97 ± 0.33 | ND | ND | ND |
| Orientin (RT = 4.10 min.) (µg/g Dry Mass ± SD) | ||||||
|---|---|---|---|---|---|---|
| Part of Plant | N1 | C1 | N2 | C2 | N3 | C3 |
| leaves | 32.99 ± 0.2 | 56.81 ± 0.29 | ND | 26.79 ± 0.62 | ND | 44.14 ± 0.02 |
| flowers | ND | ND | ND | ND | ND | ND |
| seeds | - | - | ND | ND | ND | ND |
| stem | ND | ND | ND | ND | ND | ND |
| root | ND | ND | ND | ND | ND | ND |
| Isoorientin (RT = 5.12 min.) (µg/g Dry Mass ± SD) | ||||||
|---|---|---|---|---|---|---|
| Part of Plant | N1 | C1 | N2 | C2 | N3 | C3 |
| leaves | 14.55 ± 0.25 | 22.2 ± 0.06 | 2.73 ± 0.23 | 20.52 ± 0.1 | ND | 9.29 ± 0.36 |
| flowers | 29.96 ± 0.15 | 76.94 ± 1.04 | 245.27 ± 0.26 | 93.86 ± 0.4 | 283.78 ± 1.44 | 119.59 ± 0.43 |
| seeds | - | - | ND | ND | ND | ND |
| stem | ND | ND | ND | ND | ND | ND |
| root | ND | ND | ND | ND | ND | ND |
| Quercetin (RT = 6.10 min.) (µg/g Dry Mass ± SD) | ||||||
|---|---|---|---|---|---|---|
| Part of Plant | N1 | C1 | N2 | C2 | N3 | C3 |
| leaves | 25.93 ± 0.17 | 65.15 ± 0.28 | ND | ND | 19.87 ± 0.26 | 55.63 ± 0.78 |
| flowers | 10.28 ± 0.07. | 7.81 ± 0.11 | ND | ND | 17.49 ± 0.2 | 2.71 ± 0.17 |
| seeds | - | - | ND | ND | ND | ND |
| stem | ND | ND | ND | ND | ND | ND |
| root | ND | ND | ND | ND | ND | ND |
| Chlorogenic Acid (RT = 3.08 min.) (µg/g Dry Mass ± SD) | ||||||
|---|---|---|---|---|---|---|
| Part of Plant | N1 | C1 | N2 | C2 | N3 | C3 |
| leaves | 3.81 ± 0.1 | 12.2 ± 0.06 | ND | ND | ND | ND |
| flowers | 15.39 ± 0.26 | 19.16 ± 0.19 | 93.86 ± 0.79 | 29.8 ± 0.3 | 11.75 ± 0.05 | 31.32 ± 0.24 |
| seeds | - | - | ND | ND | ND | ND |
| stem | ND | ND | ND | ND | ND | ND |
| root | ND | ND | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kidaj, D.; Zamlynska, K.; Swatek, A.; Komaniecka, I. The Influence of Rhizobial Nod Factors on the Synthesis of Flavonoids in Common Buckwheat (Fagopyrum esculentum Moench). Molecules 2024, 29, 4546. https://doi.org/10.3390/molecules29194546
Kidaj D, Zamlynska K, Swatek A, Komaniecka I. The Influence of Rhizobial Nod Factors on the Synthesis of Flavonoids in Common Buckwheat (Fagopyrum esculentum Moench). Molecules. 2024; 29(19):4546. https://doi.org/10.3390/molecules29194546
Chicago/Turabian StyleKidaj, Dominika, Katarzyna Zamlynska, Anita Swatek, and Iwona Komaniecka. 2024. "The Influence of Rhizobial Nod Factors on the Synthesis of Flavonoids in Common Buckwheat (Fagopyrum esculentum Moench)" Molecules 29, no. 19: 4546. https://doi.org/10.3390/molecules29194546
APA StyleKidaj, D., Zamlynska, K., Swatek, A., & Komaniecka, I. (2024). The Influence of Rhizobial Nod Factors on the Synthesis of Flavonoids in Common Buckwheat (Fagopyrum esculentum Moench). Molecules, 29(19), 4546. https://doi.org/10.3390/molecules29194546






