Abstract
Anatase titanium dioxide (TiO2) has emerged as a potential anode material for sodium-ion hybrid capacitors (SICs) in terms of its nontoxicity, high structure stability and cost-effectiveness. However, its inherent poor electrical conductivity and limited reversible capacity greatly hinder its practical application. Here, ultrathin TiO2 nanoplates were synthesized utilizing a hydrothermal technique. The electrochemical kinetics and reversible capacity were significantly improved through sulfur and nitrogen co-doping combined with carbon coating (SN-TiO2/C). Sulfur and nitrogen co-doping generated oxygen vacancies and introduced additional active sites within TiO2, facilitating accelerated Na-ion diffusion and enhancing its reversible capacity. Furthermore, carbon coating provided stable support for electron transfer in SN-TiO2/C during repeated cycling. This synergistic strategy of sulfur and nitrogen co-doping with carbon coating for TiO2 led to a remarkable capacity of 335.3 mAh g−1 at 0.1 A g−1, exceptional rate property of 148.3 mAh g−1 at 15 A g−1 and a robust cycling capacity. Thus, the SN-TiO2/C//AC SIC delivered an impressive energy density of 177.9 W h kg−1. This work proposes an idea for the enhancement of reaction kinetics for energy storage materials through a synergistic strategy.
1. Introduction
Lithium-ion batteries (LIBs) have become the predominant energy storage solution in portable electronics and electric vehicles. However, the escalating demand for renewable energy storage applications is restricted by the growing scarcity of lithium resources, which is making it necessary to explore alternatives. Sodium-based energy storage devices demonstrate significant potential for renewable energy storage grids, primarily attributed to the uniform distribution and low cost of sodium resources [1,2]. Sodium-ion capacitors (SICs), which synergistically combine the high energy density characteristic of Na-ion batteries with the rapid power output of supercapacitors, have garnered considerable attention as potential candidates for advanced energy storage systems [3,4]. The performance of SICs heavily depends on the performance of the anode material. Nevertheless, the increased ionic radius and inherently slower diffusion efficiency of Na+ compared to Li+ lead to substantial challenges, resulting in undesirable electrochemical performance [5]. Various anodes have been explored aimed at improving the Na-ion storage capability of SICs, including carbonaceous materials, [6,7,8] transition metal sulfides/phosphides [9,10,11,12] and alloys [13]. Despite some progress, the development of high-efficiency SICs has so far been limited by the drawbacks of these anode materials. Consequently, an urgent demand exists for anodes with the characteristics of high reversible capacity and extended cycling stability, as well as superior electrochemical kinetics, to advance the performance of SICs.
Among various anode materials, anatase titanium dioxide (TiO2) has attracted great interest in terms of its high structure stability, cost-effectiveness and nontoxicity [14]. Additionally, TiO2 has a favorable insertion potential of approximately 0.7 V vs. Na/Na+, coupled with intercalation pseudocapacitive behavior, which makes it a promising candidate for SICs [15]. Although advantageous, the utilization of TiO2 for Na-ion storage is severely restricted by its intrinsic poor electrical conductivity [16].
To address these challenges, researchers have pursued various strategies to boost the Na-ion storage capability of TiO2. The fabrication of nanostructured TiO2 has demonstrated significant effectiveness in improving Na storage capabilities. Various nanostructures, including nanowires [17], hollow spheres [18], nanoparticles [19], nanotubes [20,21,22], nanoflowers [23], microrods [24,25] and micro-sheets [26] have been studied. Additionally, Shi et al. demonstrated that nanosheet-structured TiO2 significantly enhances electrochemical performance due to its increased surface area, which provides abundant Na-ion adsorption sites and shorter Na-ion diffusion distances [27]. However, its reversible capacity and Na-ion reaction kinetics remain undesirable.
Heteroatom doping has emerged as a promising strategy to overcome these limitations by introducing crystal defects and oxygen vacancies, thereby enhancing conductivity and providing abundant active sites for Na-ion storage. For example, sulfur [28,29], nitrogen [16,30], phosphorus [31], silicon [32] and cobalt [33] doping, as well as co-doping [34], have been proven to significantly boost the Na-ion storage capability of TiO2. Co-doping, for instance, has been shown to reconstruct charge transfer channels as well as reduce the Na-ion transmission energy barrier [33], while S doping has been reported to promote Na-ion diffusion dynamics in TiO2, resulting in an enhanced Na storage capacity and rate performance [28]. Furthermore, nitrogen doping has been substantiated as an effective strategy to enhance the electrical conductivity of TiO2, which, in turn, significantly improves its sodium-ion storage capability [35,36]. Therefore, it is vital to modify TiO2 with heteroatom doping to attain an extraordinary Na-ion storage performance and enhanced cycle capability. Carbon coating not only establishes efficient electron transfer pathways but also significantly enhances the electrical conductivity of electrodes. For instance, Tang et al. reported that the encapsulation of (NiCo)3Se4 within a three-dimensional cross-linked carbon network can substantially improve its sodium-ion storage capabilities [37].
Herein, we present a synergistic strategy integrating sulfur and nitrogen co-doping with carbon coating to augment the Na storage capability of TiO2 nanoplates. The ultrathin TiO2 nanoplates were synthesized utilizing a hydrothermal technique. The synergistic effects of S/N co-doping and carbon coating on the Na-ion storage capabilities of TiO2 nanoplates (SN-TiO2/C) were systematically investigated. S/N co-doping introduces defects and provides additional active sites, thereby promoting faster Na-ion diffusion and enhancing the overall electrochemical kinetics. Simultaneously, the incorporation of carbon markedly boosts the electrical conductivity of the SN-TiO2/C composite, ensuring continuous electron pathways and preventing the agglomeration of SN-TiO2/C nanoplates during the sodiation and desodiation cycles. This study presents a comprehensive modification strategy for titanium dioxide, integrating structural control, heteroatom doping, and carbon coating. These approaches work synergistically through multiple mechanisms to markedly enhance the specific capacity and rate performance of SN-TiO2/C nanoplates. Owing to the significantly enhanced reaction dynamics and capacity of the SN-TiO2/C anode, the fabricated SIC device achieves a high energy density of 177.9 W h kg−1. This work integrates structural design, defect engineering and carbon coating strategies to enhance the performance of titanium dioxide, providing valuable insights for the modification of energy storage electrodes.
2. Results and Discussion
Figure 1a schematically exhibits the synthesis procedure of SN-TiO2/C nanoplates. First, the TiO2 nanoplates were produced using a simple hydrothermal technique via the use of titanium butoxide and hydrofluoric acid. Following this, TiO2@polydopamine (TiO2@PDA) was produced through the self-polymerization of dopamine (DA). The final SN-TiO2/C sample was then obtained by annealing TiO2@PDA in a thiourea vapor atmosphere. The scanning electron microscope (SEM) images in Figure 1b,c illustrate the morphology of the SN-TiO2/C sample. The images reveal a well-defined nanoplate structure. The thin nanoplate structure of SN-TiO2/C, which is crucial to offering plentiful Na-ion adsorption sites and reducing diffusion paths for Na-ions, was further confirmed through transmission electron microscope (TEM) analysis (Figure 1d,e). As can be seen, at the edge of the SN-TiO2/C nanoplates (Figure S1), there is a graphitized structure in the carbon coating layer. The HRTEM image in Figure 1f,g and SAED pattern in Figure 1h exhibit the distinct lattice fringes of SN-TiO2/C, suggesting a high crystallinity. In addition, the lattice spacing of 0.354 nm and 0.193 nm corresponds to the (101) and (200) planes of anatase TiO2, respectively. Figure 1i displays the HAADF-STEM images of SN-TiO2/C, accompanied by EDS elemental mappings, revealing a uniform dispersion of Ti, O, C, N and S elements.
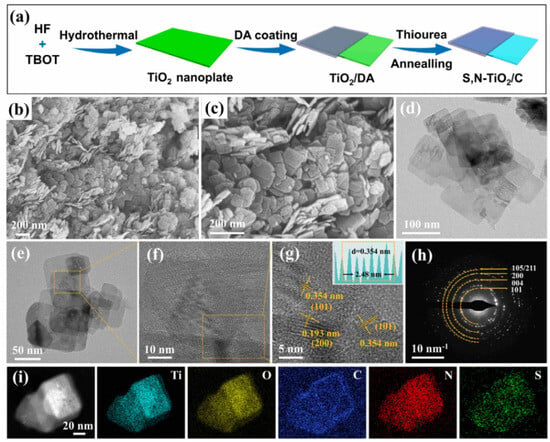
Figure 1.
(a) Diagrammatic image of the fabrication of SN-TiO2/C composite. (b,c) SEM images of SN-TiO2/C. (d,e) TEM image. (f,g) HRTEM image and lattice fringes. (h) SAED pattern of SN-TiO2/C. (i) HAADF-STEM image of SN-TiO2/C and the elements of Ti, O, C, N, and S.
The crystal structures of the TiO2, SN-TiO2 and SN-TiO2/C samples were analyzed using X-ray diffraction (XRD). As depicted in Figure 2a, the sharp diffraction peaks observed for all three samples correspond to the anatase phase of TiO2 (JCPDS: 21–1272) and exhibit high crystallinity and purity, indicating that sulfur and nitrogen doping does not change the anatase structure. The distinct peaks at 25.24, 37.76, 48.00, 53.94, 55.00 and 62.54° are ascribed to the (101), (004), (200), (105), (211) and (204) planes of anatase TiO2, respectively. Figure 2b depicts the Raman spectra of SN-TiO2/C to elucidate their components. The characteristic peaks at 151.6, 391.8, 505.8 and 627.1 cm−1 are attributed to the Eg, B1g, A1g and Eg phonon modes of anatase TiO2, respectively [20]. Additionally, the D-bond (~1360 cm−1) and G-bond (~1577 cm−1) observed in the Raman spectra are indicative of disordered carbon and graphitic carbon, respectively [38], confirming the existence of carbon in the SN-TiO2/C sample. The D-band to G-band intensity ratio (ID/IG) in SN-TiO2/C is 0.91, suggesting a highly disordered carbon structure.
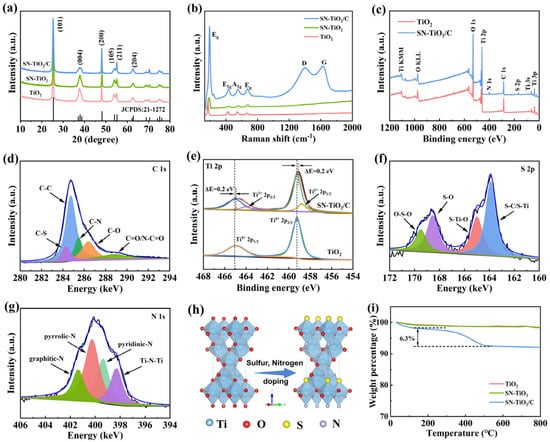
Figure 2.
(a) XRD patterns of TiO2, SN-TiO2 and SN-TiO2/C. (b) Raman profiles of TiO2, SN-TiO2 and SN-TiO2/C. (c) XPS survey spectra of TiO2 and SN-TiO2/C. High-resolution XPS spectra of (d) C1s, (e) Ti 2p, (f) S 2p and (g) N 1s of SN-TiO2/C. (h) Schematic diagram of the structural evolution of sulfur and nitrogen doping into TiO2. (i) TG curves of TiO2, SN-TiO2 and SN-TiO2/C.
X-ray photoelectron spectroscopy (XPS) was performed to study the surface chemical states of TiO2 and SN-TiO2/C. The elements C, N, O, S and Ti were detected in the full survey spectrum of SN-TiO2/C (Figure 2c). The S and N contents of SN-TiO2/C are 0.65 at% and 1.2 at%, respectively. High-resolution XPS spectra of the C component in SN-TiO2/C are shown in Figure 2d. The peaks at 284.3 and 285.5 eV are indicative of the C-S and C-N bonds, revealing the successful incorporation of sulfur and nitrogen atoms into the carbon skeleton [39]. Figure 2e shows the Ti 2p peaks of two samples. In the Ti 2p spectrum for TiO2, the observed peaks at 465.1 and 459.3 eV are indicative of the Ti 2p1/2 and Ti 2p3/2 levels of Ti4+, respectively. In the SN-TiO2/C sample, the Ti 2p spectrum is deconvoluted into four distinct peaks. The peaks at 464.7 and 459.0 eV are indicative of the Ti 2p1/2 and Ti 2p3/2 levels of Ti4+, which shifted to lower binding energies by 0.2 eV related to the oxygen vacancies in TiO2 induced by S and N doping [29,34]. The peaks at 464.1 and 458.8 eV in SN-TiO2/C are indicative of the Ti 2p1/2 and Ti 2p3/2 levels of Ti3+, which arise from the reduction in Ti4+ to balance the charge due to oxygen vacancies [26]. The observed decrease in binding energy, concomitant with the emergence of Ti3+, unequivocally substantiates the emergence of oxygen vacancies. The XPS spectrum of the S component in SN-TiO2/C (Figure 2f) shows peaks at 164.9 and 163.8 eV, corresponding to the S-Ti bonds, implying that S is successfully doped into TiO2 [29]. The high-resolution XPS spectra of the N 1s component in SN-TiO2/C are shown in Figure 2g. The deconvoluted peaks at 401.4, 400.3, and 399.4 eV are indicative of graphitic-N, pyrrolic-N and pyridinic-N, respectively, and the peak at 398.3 eV is associated with Ti-N-Ti bonds, indicating that N-doped TiO2 is obtained through replacing lattice oxygen [16]. These findings verify the effective doping of sulfur and nitrogen into the TiO2 and carbon structures. Figure 2h exhibits the structural evolution following sulfur and nitrogen doping. The thermogravimetric analysis (TGA) curves of the TiO2, SN-TiO2 and SN-TiO2/C samples (Figure 2i) show a significant mass reduction observed between 400 and 450 °C, indicative of the oxidation of carbon to CO2, and the carbon content is 6.3%.
The Na storage capabilities of the SN-TiO2/C electrode were investigated. As revealed in Figure 3a, during the initial cyclic voltammetry (CV) curve, a significantly reduced peak appears around 1.0 V and vanishes in later cycles due to the generation of an SEI film [33]. The prominent cathodic peak that is observed at 0.10 V and appears in all cyclic sweeps is associated with continued electrolyte decomposition [40]. The cathodic peak observed at 0.74 V in the second and subsequent cycles is related to the reduction of Ti4+ to Ti3+. Moreover, the anode peak at 0.83 V corresponds to the oxidation of Ti3+ to Ti4+ [26]. At the second and subsequent cycles in the SN-TiO2/C electrode, there is a cathodic peak at 1.6 V, along with the two anode peaks at 1.75 V and 2.2 V, which are identified as the stepped redox reactions between S and Na. The CV curves for the SN-TiO2/C electrode exhibit sharper and more pronounced redox peaks compared to the TiO2 electrode (Figure S2a), suggesting enhanced electrochemical kinetics due to sulfur and nitrogen co-doping and carbon coating. Figure 3b reveals the initial three galvanostatic charge discharge (GCD) profiles of the SN-TiO2/C electrode. At 0.1 A h g−1, the initial discharge and charge capacities are 1028.4 and 409.1 mA h g−1, with an initial coulomb efficiency (ICE) of 39.8%. However, the TiO2 electrode exhibits significantly lower capacities of 749.9 mA h g−1 and 223.2 mA h g−1, with an ICE of only 29.7% (in Figure S2b). The emergence of irreversible capacities is related to the establishment of the SEI layer and electrolyte decomposition. The higher ICE in the SN-TiO2/C electrode indicates fewer side effects due to S/N co-doping.
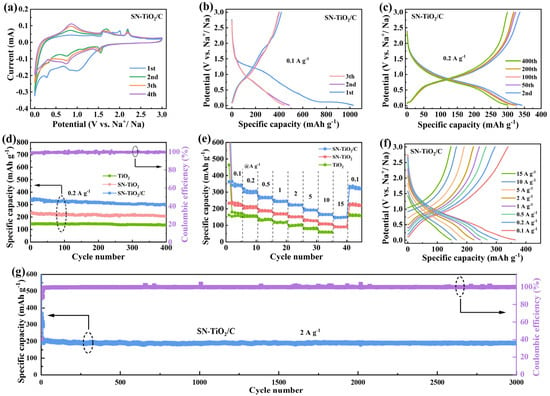
Figure 3.
(a) CV curves of SN-TiO2/C at 0.2 mV s−1 (0.01–3.0 V); (b) initial GCD profiles of SN-TiO2/C at 0.1 A g−1; (c) GCD curves of SN-TiO2/C at 0.2 A g−1; (d) comparison of cycle ability of TiO2, SN-TiO2 and SN-TiO2/C; (e) rate properties of the TiO2, SN-TiO2 and SN-TiO2/C electrodes and (f) the corresponding GCD profiles of SN-TiO2/C; (g) long-term cycle ability of SN-TiO2/C electrode.
Figure 3c displays the GCD profiles of the SN-TiO2/C electrode, and the cycle stability of the three electrodes at 0.2 A g−1 are compared in Figure 3d, which all completed 10 cycles after 0.1 A g−1. The TiO2, SN-TiO2 and SN-TiO2/C electrodes exhibit the capacities of 138.6, 205.1 and 294.1 mA h g−1 at the 400th cycle, leading to a capacity retention of 88.9%, 89.4% and 90.7%, respectively. All three electrodes demonstrate stable cycling performance. The TiO2 electrode exhibits a comparatively lower reversible capacity in contrast to the SN-TiO2/C electrode, which achieves the highest reversible capacity. To further validate the impact of S/N co-doping and carbon coating on Na storage performance, the rate capabilities of the three electrodes were assessed. As depicted in Figure 3e,f and Figure S3a,b, the SN-TiO2/C electrode demonstrates the reversible capacities of 336.3, 295.6, 264.9, 242.8, 221.3, 192.2 and 164.6 mA h g−1 at 0.1 to 0.2, 0.5, 1, 2, 5 and 10 A g−1, respectively. Remarkably, it maintains 148.3 mA h g−1 at 15 A g−1 (~45 C) and even reaches 44% of the initial 0.1 A g−1. Upon reverting to 0.1 A g−1, the capacity is recovered to 330.4 mAh g−1, nearly identical to the initial value, demonstrating the impressive reversibility of the SN-TiO2/C electrode. However, the SN-TiO2 electrode, without carbon coating, exhibits a moderate rate performance, achieving 211.2 mA h g−1 at 0.1 A g−1 and decreases to 80.6 mA h g−1 at 15 A g−1. The TiO2 electrode displays a clearly low average capacity of 156.7 mA h g−1 at 0.1 A g−1, and when increased to 10 A g−1, the capacity drops to 58.2 mA h g−1. These results indicate that the reversible capacity and rate property of the SN-TiO2 electrode were enhanced compared to the TiO2 electrode. This enhancement is due to sulfur and nitrogen co-doping, which introduces oxygen vacancies within the TiO2 lattice, thereby providing additional Na-ion adsorption sites and facilitating faster electron transport, which is pivotal for increasing the reversible capacity and improved electrochemical kinetics. The SN-TiO2/C electrode demonstrates the highest reversible capacity and superior reaction dynamics, attributable to the synergistic effect between sulfur and nitrogen co-doping combined with conductive carbon coating. This indicates that beyond the doping effects, the carbon composite further boosts performance by establishing a highly conductive network that promotes efficient electron transport and additional Na-ion storage capacity. The robust reversible capacity and vigorous reaction dynamics of the SN-TiO2/C electrode confirm the efficacy of combined sulfur and nitrogen co-doping and carbon composite strategies in boosting the electrochemical performance. As depicted in Figure 3g, the extended cyclability of SN-TiO2/C was tested at 2 A g−1 and demonstrates a high capacity of 189.6 mA h g−1 after 3000 cycles, maintaining 94% of its initial capacity, indicating an exceptional cycle stability. To our knowledge, the obtained SN-TiO2/C electrode delivers significant superiority when compared with previously reported TiO2-based materials (Table S1, Supporting Information).
To elucidate the diffusion dynamics of Na-ions within the TiO2, SN-TiO2 and SN-TiO2/C electrodes, electrochemical impedance spectroscopy (EIS), CV and Galvanostatic Intermittent Titration Technique (GITT) tests were performed. As illustrated in Figure 4a, the EIS curves comprise a quasi-semicircle shape and a quasi-linear segment. The quasi-semicircle is indicative of the charge-transfer resistance (Rct), whereas the quasi-linear segment is indicative of the Na+ diffusion process within the electrodes [41]. Clearly, SN-TiO2/C presents a markedly lower Rct value compared to the TiO2 electrode, indicating that sulfur and nitrogen co-doping, coupled with carbon coating, substantially enhances electronic conductivity and facilitates more efficient charge transfer at the interface. Figure 4b depicts the Z′ − ω−1/2 curves, and ω represents the angular frequency (ω = 2πf). The Warburg impedance coefficient (σw) can be calculated based on Equation (1), and a lower σw value signifies faster Na-ion diffusion kinetics within the electrode [42,43].
Z′ = RS + Rct + σwω−1/2
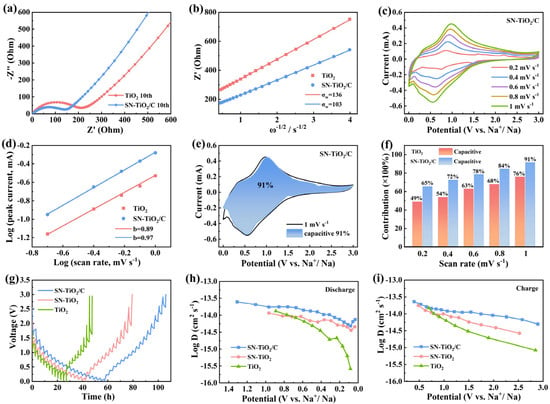
Figure 4.
(a) EIS profiles of TiO2, SN-TiO2 and SN-TiO2/C and (b) correlation between Z′ and ω−1/2. (c) CV curves of SN-TiO2/C electrode. (d) Linear correlation of peak currents and scan rates. (e) Capacitive contribution of SN-TiO2/C electrode at 1.0 mV s−1. (f) Comparison of the capacitive contributions of the two electrodes. (g) GITT profiles and (h,i) diffusion constants of TiO2 and SN-TiO2/C electrodes.
Accordingly, the SN-TiO2/C anode has a clearly lower σw value (103 Ω s−1/2) than that of TiO2 (136 Ω s−1/2), corresponding to superior Na+ diffuse kinetics in the SN-TiO2/C electrode.
Figure 4c, Figure S4a and S5a unravel the CV curves of the three electrodes. The b-values, calculated from the linear relation between the peak current and scan rate, serve as a crucial indicator to distinguish the contribution of pseudocapacitance and diffusion-controlled processes during charge storage. The cathodic b-values (Figure 4d) for the TiO2 and SN-TiO2/C electrodes are 0.89 and 0.97, respectively. The higher b-value of SN-TiO2/C is indicative of fast charge storage kinetics [44]. The pseudocapacitive contribution ratios for the TiO2, SN-TiO2 and SN-TiO2/C electrodes from 0.2 to 1 mV s−1 were calculated using the following formula: i = k1v + k2v1/2 [45], in which k1v and k2v1/2 are indicative of the surface-dominant and diffusion-controlled mechanisms, respectively. As reflected in Figure 4e,f, Figure S4b and S5b, the capacitive contribution increases progressively with higher scan rates, reaching 76%, 85% and 91% of the TiO2, SN-TiO2 and SN-TiO2/C electrodes at 1 mV s−1, respectively. The high capacitive contribution of the SN-TiO2/C electrode unravels a surface-predominant pseudocapacitance process for Na storage, which enables it to exhibit exceptional performance in both its high-rate capability and cycling stability. The GITT technique was employed to assess the Na-ion diffusion coefficients and kinetic properties of the electrodes. The diffusion coefficient (DNa+) can be evaluated in accordance with Fick’s second law [46,47]:
where ΔES represents the steady-state voltage change, ΔEτ denotes the voltage change produced by the continuous current pulse, and τ represents the time of the current pulse. As illustrated in Figure 4h,i, the SN-TiO2 electrode shows a moderate rate of DNa+ values, which is higher than that of the TiO2 electrode, suggesting more efficient Na-ion diffusion, ascribed to the defects introduced by S and N co-doping. The SN-TiO2/C electrode demonstrates larger DNa+ values compared to the SN-TiO2 electrode, implying the further enhanced Na-ion diffusion kinetics resulting from carbon coating. In summary, these results reveal that sulfur and nitrogen co-doping, along with carbon coating, markedly boosts ionic and electronic conductivity and enhances the Na-ion electrochemical kinetics of the SN-TiO2/C electrode. These modifications result in higher specific capacities and robust rate capabilities.
D = 4l (ΔEs/ΔEτ)2/πτ
To assess the practicality of SN-TiO2/C, a sodium-ion capacitor was constructed. Initially, the electrical double-layer energy storage performances of the AC cathode were assessed. The CV profiles of the AC cathode, depicted in Figure S6a, which display an approximately rectangular shape, are indicative of double-layer capacitance behavior. Figure S4 illustrate the rate capability and the corresponding GCD profiles of AC, demonstrating high rates of capacity of 72.1, 66.4, 60.7, 56.5, 50.8, 43.8 and 34.2 mAh g−1 from 0.1 to 10 A g−1, respectively. Furthermore, the AC cathode demonstrated superior cyclability, as shown in Figure S4, preserving 95% of its initial capacity after 150 cycles at 0.1 A g−1.
A sodium-ion capacitor (SIC) was constructed, incorporating SN-TiO2/C as the anode and utilizing commercial activated carbon (AC) as the cathode. The electrochemical performances of the SN-TiO2/C//AC SIC were investigated over 0–4 V. A mass ratio of 1:3 was established between SN-TiO2/C and activated carbon (AC) to ensure capacity matching. The discharge process is depicted in Figure 5a. ClO4− ions desorb from the porous surface of the AC cathode, while Na+ ions desorb and de-intercalate from the SN-TiO2/C anode. As depicted in Figure 5b, the SN-TiO2/C//AC device presents asymmetrical CV curves, which indicate the intercalation/deintercalation of Na+ ions within the SN-TiO2/C anode and the double-layer energy storage mechanism of the AC cathode. Figure 5c,d display the rate performance and GCD profiles of the SN-TiO2/C//AC capacitor, which delivers high discharge capacities of 65, 57.9, 50.9, 45, 40.5, 35, 29.5 and 26 mAh g−1 from 0.1 to 15 A g−1, respectively. As illustrated in Figure 5e, SN-TiO2/C//AC manifests a capacity of 47.6 mAh g−1 after 300 cycles at 0.2 A g−1, retaining 87% of its initial capacity. At a power output of 55.6 W kg−1, the SN-TiO2/C//AC SIC reaches an impressive energy density of 177.9 W h kg−1, maintaining 52.5 Wh kg−1, even at 8333 W kg−1. The device features extremely short charge and discharge times of 26 and 27 s, respectively, indicating its exceptional rate capability. This superiority is clearly illustrated by the Ragone plot in Figure 5f, which implies the energy and power output of the SN-TiO2/C//AC device outperforms many other previously reported sodium-ion capacitors, such as AC//MSC [48], V3S4@CNF//AC [49], MTO@C//AC [50], AC//MNC [51], Fe-MnS/PG//NC [11], Bi2S3-TTAB//AC [5] and so on. Additionally, we investigated the long-cycle performance. As depicted in Figure 5g, the SN-TiO2/C//AC capacitor exhibits exceptional long-term cycle durability at 2 A g−1, maintaining 81% of its capacity after 3000 cycles.
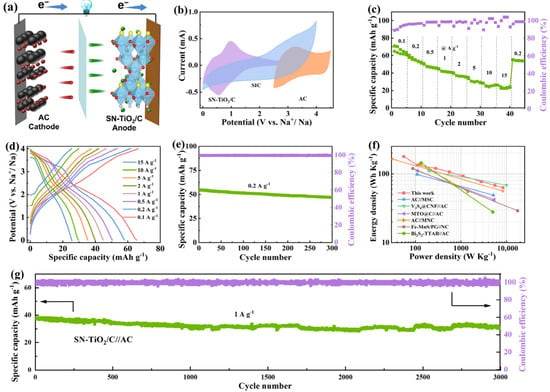
Figure 5.
(a) Schematic representation and electrochemical properties of the SN-TiO2/C//AC SIC. (b) CV curves. (c,d) Rate performance and corresponding GCD curves. (e) Cycling stability. (f) Ragone plot of the SN-TiO2/C//AC device compared to counterparts. (g) Cycling capability of the SIC at 2 A g−1.
3. Materials and Methods
3.1. Synthesis of TiO2 Nanoplates
Initially, 4 mL of 40% HF solution was added to 20 mL of titanium butoxide (TBOT) with agitation for 1 h. The mixture was then placed in a Teflon-lined autoclave and heated at 180 °C for 18 h. After naturally cooling to ambient temperature, the sample was separated by centrifugation. The sample was then immersed in 0.1 M of NaOH aqueous solution for 12 h and subsequently rinsed three times with deionized water. Finally, the TiO2 nanoplate precursor sample was dried at 80 °C for 12 h.
3.2. Synthesis of TiO2/PDA Nanoplates
First, 0.2 g of the TiO2 nanoplate precursor was dispersed in 100 mL of 0.01 M Tris (trimethylol aminomethane) solution. After stirring at ambient temperature for 2 h, 40 mg of dopamine hydrochloride (DA) was introduced into the solution. The solution was then stirred for 24 h at ambient temperature. Finally, the TiO2/PDA nanoplates were separated by centrifugation, rinsed three times with deionized water and dried at 80 °C for 12 h.
3.3. Synthesis of SN-TiO2/C Nanoplates
In the synthesis of SN-TiO2/C nanoplates, 0.2 g of TiO2/PDA nanoplate precursor and thiourea were placed on the downstream side and upstream side in the chamber of the furnace, respectively. Subsequently, the chamber was then heated to 550 °C for 2 h. During this process, the decomposition of thiourea released ammonia and hydrogen sulfide, which acted as nitrogen and sulfur sources for doping titanium dioxide. The PDA was transformed into sulfur- and nitrogen-doped carbon, leading to the formation of a nitrogen and sulfur co-doped and carbon-coated TiO2 sample (SN-TiO2/C). For comparison, the TiO2 nanoplate precursor was annealed at 550 °C for 2 h under an Ar atmosphere to obtain a TiO2 nanoplate sample, denoted as TiO2. The TiO2 nanoplate precursor and thiourea were annealed under the same conditions to obtain a nitrogen- and sulfur-doped TiO2 sample (SN-TiO2).
4. Conclusions
In summary, we constructed a unique sodium-ion capacitor that delivers an impressive energy density of 177.9 W h kg−1, utilizing sulfur and nitrogen co-doped titanium dioxide/carbon (SN-TiO2/C) as the anode and activated carbon (AC) as the cathode. The SN-TiO2/C anode, characterized by an ultrathin structure, is engineered through an integration of structural refinement and heteroatom doping, with carbon coating. The ultrathin nanoplates structure maximizes the available sodium-ion adsorption sites, while sulfur- and nitrogen-doping-induced defects enhance electrical conductivity and provide additional Na-ion adsorption sites. These synergistic modifications markedly enhance the reversible capacity (336.3 mA h g−1 at 0.1 A g−1) and improve the electrochemical kinetics of Na-ion storage in SN-TiO2/C, thereby facilitating their application in sodium-ion capacitors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29184507/s1, Figure S1: SEM images of SN-TiO2/C. Figure S2: (a) CV curves of TiO2 electrode at 0.2 mV s−1. (b) Initial GCD profiles of TiO2 at 0.1 A g−1. Figure S3: (a) The GCD profiles of TiO2 and SN-TiO2 electrodes. Figure S4: (a) CV curves at various sweep rates of TiO2 electrode. (b) Capacitive contribution of TiO2 electrode at a sweep rate of 1.0 mV s−1. Figure S5: (a) CV curves at various sweep rates of SN-TiO2 electrode. (b) Capacitive contribution of SN-TiO2 electrode at a sweep rate of 1.0 mV s−1. Figure S6: The electrochemical properties of the AC cathode in half cell: (a) CV curves of the AC cathode at scan rate from 0.5 to 10 mV s−1, within a voltage range of 2.5–4.5 V (vs Na/Na+). (b) Rate capabilities and (c) the corresponding GCD profiles of AC cathode. (d) Cycle performances of AC cathode at 0.1 A g−1. Table S1: Electrochemical performances of TiO2-based materials for SIBs anodes comparison with the previously reported in the literature.
Author Contributions
Data curation, D.Z. and G.L.; Methodology, Y.L. and Q.L.; Resources, Y.G. and J.C.; Writing—original draft, Y.L.; Writing—review and editing, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Key Science Research Project of Colleges and Universities in Henan Province (No. 24B430015), 2023 Henan Province Key Research and Development and Promotion Special (Grant No. 232102230029) and Nanyang Normal University (No. 2020ZX014 and No. 2023QN001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to institutional restriction.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, H.; He, Y.; Wang, Q.; Gu, S.; Wang, L.; Yu, J.; Zhou, G.; Xu, L. SnSe2/NiSe2@N-doped carbon yolk-shell heterostructure construction and selenium vacancies engineering for ultrastable sodium-ion storage. Adv. Energy Mater. 2023, 13, 2302901. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Dai, Y.; Zhang, H.; Zhang, Y.; Gu, S.; Wang, X.; Gao, T.; Zhou, G.; Xu, L. Heterostructure interface construction of cobalt/molybdenum selenides toward ultra-stable sodium-ion half/full batteries. Adv. Funct. Mater. 2024, 2406915. [Google Scholar] [CrossRef]
- Yuan, J.; Qiu, M.; Hu, X.; Liu, Y.; Zhong, G.; Zhan, H.; Wen, Z. Pseudocapacitive vanadium nitride quantum dots modified one-dimensional carbon cages enable highly kinetics-compatible sodium ion capacitors. ACS Nano 2022, 16, 14807–14818. [Google Scholar] [CrossRef]
- Cai, J.; Wang, L.; Tao, S.; Liu, Y.; Cao, Z.; Song, Z.; Xiao, X.; Zhu, Y.; Deng, W.; Hou, H.; et al. Electrochemistry enabled heterostructure with high tap density for ultrahigh power Na-ion capacitors. Adv. Energy Mater. 2023, 13, 2302426. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, H.; Zhang, K.; Kong, Y.; Zhang, S.; Wang, H.; Yuan, G.; Su, D.; Zhou, J.; Wang, X.; et al. Strain engineering of Bi2S3 microspheres via organic intercalation enabled high performance sodium storage. Chem. Eng. J. 2024, 492, 152274. [Google Scholar] [CrossRef]
- He, Y.; Liu, D.; Jiao, J.; Liu, Y.; He, S.; Zhang, Y.; Cheng, Q.; Fang, Y.; Mo, X.; Pan, H.; et al. Pyridinic N-dominated hard carbon with accessible carbonyl groups enabling 98% initial coulombic efficiency for sodium-ion batteries. Adv. Funct. Mater. 2024, 2403144. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Ma, X.; Cao, T.; Xu, J.; Feng, H.; Diao, R.; Qi, F.; Huang, H.; Ma, P. Efficient preparation of biomass-based ultra-thin 2D porous carbon materials by in situ template-activation and its application in sodium ion capacitors. Adv. Funct. Mater. 2024, 34, 2310717. [Google Scholar] [CrossRef]
- Li, C.; Song, Z.; Liu, M.; Lepre, E.; Antonietti, M.; Zhu, J.; Liu, J.; Fu, Y.; López-Salas, N. Template-induced graphitic nanodomains in nitrogen-doped carbons enable high-performance sodium-ion capacitors. Energy Storage Mater. 2024, 7, e12695. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, R.; Dong, J.; Yu, R.; Tan, S.; Xiong, F.; Wei, Q.; Wang, J.; Cui, L.; Tian, H.; et al. Uncovering the origin of surface-redox pseudocapacitance of molybdenum phosphides enables high-performance flexible sodium-ion capacitors. Chem. Eng. J. 2023, 475, 145962. [Google Scholar] [CrossRef]
- Cai, J.; Zhou, Y.; Tao, S.; Liu, Y.; Deng, W.; Hou, H.; Zou, G.; Ji, X. Nanocrystalline heterostructure with low voltage hysteresis for ultrahigh-power sodium-ion capacitors. Energy Storage Mater. 2024, 71, 103582. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Chen, J.; Gao, L.; Guo, P.; Wei, C.; Fu, J.; Xu, Q. Sulfur-bridged bonds enabled structure modulation and space confinement of MnS for superior sodium-ion capacitors. J. Colloid Interf. Sci. 2024, 664, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, D.; Du, B.; Yin, Q.; Li, Y.; Xue, X.; Wei, F.; Qi, J.; Sui, Y. Defect-induced electron rich nanodomains in CoSe0.5S1.5/GA realize fast ion migration kinetics as sodium-ion capacitor anode. J. Energy Chem. 2023, 87, 583–593. [Google Scholar] [CrossRef]
- Li, Z.; Wu, R.; Zhu, Z.; Zhu, Y.; Wang, Y.; Xu, S.; Kong, Q.; Chen, J.S. Improving the electronegativity of N-doped carbon by encapsulating CoFe alloy clusters with a chainmail-like structure for high-energy sodium-ion capacitors. J. Mater. Chem. A 2024, 12, 20999–21007. [Google Scholar] [CrossRef]
- Han, M.; Zou, Z.; Liu, J.; Deng, C.; Chu, Y.; Mu, Y.; Zheng, K.; Yu, F.; Wei, L.; Zeng, L.; et al. Pressure-induced defects and reduced size endow TiO2 with high capacity over 20 000 cycles and excellent fast-charging performance in sodium ion batteries. Small 2024, 20, 2312119. [Google Scholar] [CrossRef]
- Wei, Q.; Chang, X.; Butts, D.; DeBlock, R.; Lan, K.; Li, J.; Chao, D.; Peng, D.L.; Dunn, B. Surface-redox sodium-ion storage in anatase titanium oxide. Nat. Commun. 2023, 14, 7. [Google Scholar] [CrossRef]
- Li, T.; Kong, L.Y.; Bai, X.; Wang, Y.X.; Qi, Y.X. Promoting amorphization of commercial TiO2 upon sodiation to boost the sodium storage performance. J. Energy Chem. 2023, 81, 379–388. [Google Scholar] [CrossRef]
- Kang, M.; Ruan, Y.; Lu, Y.; Luo, L.; Huang, J.; Zhang, J.M.; Hong, Z. An interlayer defect promoting the doping of the phosphate group into TiO2(B) nanowires with unusual structure properties towards ultra-fast and ultra-stable sodium storage. J. Mater. Chem. A 2019, 7, 16937–16946. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Q.; He, H.; Tang, Y.; Wang, H.; Xie, H. Dual carbon coating engineering endows hollow structured TiO2 with superior sodium storage performance. J. Power Sources 2021, 489, 229516. [Google Scholar] [CrossRef]
- Diao, Z.; Wang, Y.; Zhao, D.; Zhang, X.; Mao, S.S.; Shen, S. Ultra-small TiO2 nanoparticles embedded in carbon nanosheets for high-performance sodium storage. Chem. Eng. J. 2021, 417, 127928. [Google Scholar] [CrossRef]
- Li, B.; Anwer, S.; Huang, X.; Luo, S.; Fu, J.; Liao, K. Nitrogen-doped carbon encapsulated in mesoporous TiO2 nanotubes for fast capacitive sodium storage. J. Energy Chem. 2021, 55, 202–210. [Google Scholar] [CrossRef]
- Lin, D.; Wang, M.; Weng, Q.; Qin, X.; An, L.; Chen, G.; Liu, Q. Three dimensional titanium dioxide nanotube arrays induced nanoporous structures and stable solid electrolyte interphase layer for excellent sodium storage in ether-based electrolyte. J. Power Sources 2023, 587, 233696. [Google Scholar] [CrossRef]
- Chen, J.; Fu, Y.; Sun, F.; Hu, Z.; Wang, X.; Zhang, T.; Zhang, F.; Wu, X.; Chen, H.; Cheng, G.; et al. Oxygen vacancies and phase tuning of self-supported black TiO2-X nanotube arrays for enhanced sodium storage. Chem. Eng. J. 2020, 400, 125784. [Google Scholar] [CrossRef]
- Yang, J.; Huang, M.; Xu, L.; Xia, X.; Peng, C. Self-assembled titanium-deficient undoped anatase TiO2 nanoflowers for ultralong-life and high-rate Li+/Na+ storage. Chem. Eng. J. 2022, 445, 136638. [Google Scholar] [CrossRef]
- Luo, B.; Wang, W.; Wang, Q.; Ji, W.; Yu, G.; Liu, Z.; Zhao, Z.; Wang, X.; Wang, S.; Zhang, J. Facilitating ionic conductivity and interfacial stability via oxygen vacancies-enriched TiO2 microrods for composite polymer electrolytes. Chem. Eng. J. 2023, 460, 141329. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Wang, X.; Lin, C.; Zhao, X.S. Hollow rutile cuboid arrays grown on carbon fiber cloth as a flexible electrode for sodium-ion batteries. Adv. Funct. Mater. 2020, 30, 2002629. [Google Scholar] [CrossRef]
- Wang, C.; Yao, Q.; Wang, M.; Zheng, C.; Wang, N.; Bai, Z.; Yang, J.; Dou, S.; Liu, H. Highly conductive hierarchical TiO2 micro-sheet enables thick electrodes in sodium storage. Adv. Funct. Mater. 2023, 34, 2301996. [Google Scholar] [CrossRef]
- Guan, S.; Fan, Q.; Shen, Z.; Zhao, Y.; Sun, Y.; Shi, Z. Heterojunction TiO2@TiOF2 nanosheets as superior anode materials for sodium-ion batteries. J. Mater. Chem. A 2021, 9, 5720–5729. [Google Scholar] [CrossRef]
- He, T.; An, Q.; Zhang, M.; Kang, N.; Kong, D.; Song, H.; Wu, S.; Wang, Y.; Hu, J.; Zhang, D.; et al. Multiscale interface engineering of sulfur-doped TiO2 anode for ultrafast and robust sodium storage. ACS Nano 2024, 18, 5672–5683. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, K.; Liang, P.; Rao, Y.; Li, X.; Zheng, H.; Yan, K.; Wang, J.; Liu, J. Metal-organic framework derived S-doped anatase TiO2@C to store Na+ with high-rate and long-cycle life. J. Alloy. Compd. 2023, 969, 172395. [Google Scholar] [CrossRef]
- Cai, Q.; Li, X.; Hu, E.; Wang, Z.; Lv, P.; Zheng, J.; Yu, K.; Wei, W.; Ostrikov, K. Overcoming ion transport barrier by plasma heterointerface engineering: Epitaxial titanium carbonitride on nitrogen-doped TiO2 for high-performance sodium-ion batteries. Small 2022, 18, 2200694. [Google Scholar] [CrossRef]
- Chen, D.; Wu, Y.; Huang, Z.; Chen, J. A novel hybrid point defect of oxygen vacancy and phosphorus doping in TiO2 anode for high-performance sodium ion capacitor. Nano-Micro Lett. 2022, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Wang, H.; Ji, X.; Wang, D.; Zhang, Q.; Meng, L.; Shi, J.W.; Han, X.; Cheng, Y. Introducing hybrid defects of silicon doping and oxygen vacancies into MOF-derived TiO2–X@carbon nanotablets toward high-performance sodium-ion storage. Small 2023, 19, 2302831. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Meng, C.; Guo, X.; Wu, B.; Sui, X.; Wang, Z. Defect-driven reconstruction of Na-ion diffusion channels enabling high-performance Co-doped TiO2 anodes for Na-ion hybrid capacitors. Adv. Energy Mater. 2024, 14, 2400558. [Google Scholar] [CrossRef]
- Fan, M.; Lin, Z.; Zhang, P.; Ma, X.; Wu, K.; Liu, M.; Xiong, X. Synergistic effect of nitrogen and sulfur dual-doping endows TiO2 with exceptional sodium storage performance. Adv. Energy Mater. 2020, 11, 2003037. [Google Scholar] [CrossRef]
- Wang, Q.; He, H.; Luan, J.; Tang, Y.; Huang, D.; Peng, Z.; Wang, H. Synergistic effect of N-doping and rich oxygen vacancies induced by nitrogen plasma endows TiO2 superior sodium storage performance. Electrochim. Acta 2019, 309, 242–252. [Google Scholar] [CrossRef]
- Qu, Y.; Zhu, S.; Dong, X.; Huang, H.; Qi, M. Nitrogen-doped TiO2 nanotube anode enabling improvement of electronic conductivity for fast and long-term sodium storage. J. Alloy. Compd. 2021, 889, 161612. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, X.; He, S.; Lu, Y.; Shen, X.; Tang, S. In situ construction of (NiCo)3Se4 nanobeads embedded in n-doped carbon 3D interconnected networks for enhanced sodium storage. Inorg. Chem. 2024, 63, 15081–15089. [Google Scholar] [CrossRef]
- He, H.; He, J.; Yu, H.; Zeng, L.; Luo, D.; Zhang, C. Dual-interfering chemistry for soft-hard carbon translation toward fast and durable sodium storage. Adv. Energy Mater. 2023, 13, 2300357. [Google Scholar] [CrossRef]
- Du, Y.; Fan, H.; Bai, L.; Song, J.; Jin, Y.; Liu, S.; Li, M.; Xie, X.; Liu, W. Molten salt-assisted construction of hollow carbon spheres with outer-order and inner-disorder heterostructure for ultra-stable potassium ion storage. ACS Appl. Mater. Interfaces 2023, 15, 4081–4091. [Google Scholar] [CrossRef]
- Zhao, H.; Zhong, J.; Qi, Y.; Liang, K.; Li, J.; Huang, X.; Chen, W.; Ren, Y. 90 C fast-charge Na-ion batteries for pseudocapacitive faceted TiO2 anodes based on robust interface chemistry. Chem. Eng. J. 2023, 465, 143032. [Google Scholar] [CrossRef]
- Li, J.; Hu, G.; Yu, R.; Liao, X.; Zhao, K.; Li, T.; Zhu, J.; Chen, Q.; Su, D.; Ren, Y.; et al. Revolutionizing lithium storage capabilities in TiO2 by expanding the redox range. ACS Nano 2023, 17, 21604–21613. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Gao, S.; Bai, T.; Li, H.; Zhang, X.; Mu, Y.; Guo, W.; Cui, Z.; Wang, N.; Zhao, Y. Heterogeneous MoS2 nanosheets on porous TiO2 nanofibers toward fast and reversible sodium-ion storage. Small 2024, 2402774. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Jiang, Y.; Zhang, M.; Min, H.; Yang, H.; Wang, J. A 3D crinkled MXene/TiO2 heterostructure with interfacial coupling for ultra-fast and reversible potassium storage. J. Mater. Chem. A 2024, 12, 7598–7604. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, Z.; Wang, L.; Huang, Y.; Huang, H.; Xia, Y.; Zeng, S.; Xu, R.; Yang, Y.; He, S.; et al. Oxygen defect engineering toward zero-strain V2O2.8@porous reticular carbon for ultrastable potassium storage. ACS Nano 2023, 17, 16478–16490. [Google Scholar] [CrossRef]
- Pan, Z.; Qian, Y.; Li, Y.; Xie, X.; Lin, N.; Qian, Y. Novel bilayer-shelled N, O-doped hollow porous carbon microspheres as high performance anode for potassium-ion hybrid capacitors. Nano-Micro Lett. 2023, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xiong, Y.; Liu, Y.; Wei, X.; Wei, F.; Wang, M.; Cao, Y.; Tao, G.; Zhang, Q.; Wan, Q.; et al. Regulation of the surface activity of carbon anodes for rationalization of potassium storage. Chem. Commun. 2023, 59, 10173–10176. [Google Scholar] [CrossRef]
- Pei, Y.R.; Zhou, H.Y.; Zhao, M.; Li, J.C.; Ge, X.; Zhang, W.; Yang, C.C.; Jiang, Q. High-efficiency sodium storage of Co0.85Se/WSe2 encapsulated in N-doped carbon polyhedron via vacancy and heterojunction engineering. Carbon Energy 2023, 6, 374. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, B.; Lu, Z.; Hu, J.; Xie, J.; Hao, A.; Cao, Y. Sulfur-bridged bonds heightened Na-storage properties in MnS nanocubes encapsulated by S-doped carbon matrix synthesized via solvent-free tactics for high-performance hybrid sodium ion capacitors. Small 2023, 19, e2207214. [Google Scholar] [CrossRef]
- Mao, Z.F.; Shi, X.J.; Zhang, T.Q.; Liang, P.J.; Wang, R.; Jin, J.; He, B.B.; Gong, Y.S.; Wang, Q.; Tong, X.L.; et al. Mechanically flexible V3S4@carbon composite fiber as a high-capacity and fast-charging anode for sodium-ion capacitors. Rare Met. 2023, 42, 2633–2642. [Google Scholar] [CrossRef]
- Chen, C.; Li, N.W.; Zhang, X.Y.; Zhang, C.H.; Qiu, J.; Yu, L. Interlayer-expanded titanate hierarchical hollow spheres embedded in carbon nanofibers for enhanced Na storage. Small 2022, 18, 2107890. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, B.; Wang, S.; Yuan, C.; Lu, Z.; Hu, J.; Xie, J.; Cao, Y. 2D heterostructural Mn2O3 quantum dots embedded N-doped carbon nanosheets with strongly stable interface enabling high-performance sodium-ion hybrid capacitors. J. Colloid Interf. Sci. 2024, 656, 545–555. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).