Abstract
Selective catalytic reduction of nitrogen oxides (NOx) with ammonia (NH3-SCR) has been implemented in response to the regulation of NOx emissions from stationary and mobile sources above 300 °C. However, the development of NH3-SCR catalysts active at low temperatures below 200 °C is still needed to improve the energy efficiency and to cope with various fuels. In this review article, recent reports on low-temperature NH3-SCR catalysts are systematically summarized. The redox property as well as the surface acidity are two main factors that affect the catalytic activity. The strong redox property is beneficial for the low-temperature NH3-SCR activity but is responsible for N2O formation. The multiple electron transfer system is more plausible for controlling redox properties. H2O and SOx, which are often found with NOx in flue gas, have a detrimental effect on NH3-SCR activity, especially at low temperatures. The competitive adsorption of H2O can be minimized by enhancing the hydrophobic property of the catalyst. Various strategies to improve the resistance to SOx poisoning are also discussed.
1. Introduction
Anthropogenic pollutant emissions increased with human activity until a few decades ago, posing a threat to human well-being. However, over the past few decades, regulations on the emission of these pollutants and the development of technologies to control emissions have had some success in preventing significant pollution. Sulfur oxides (SOx), nitrogen oxides (NOx and N2O), CO, and volatile organic compounds (VOCs) are representative gaseous pollutants that are now strictly regulated [1]. In addition, greenhouse gases, including CO2 and methane, are currently or will be regulated in the near future, depending on the country.
NOx, such as nitrogen monoxide (NO) and nitrogen dioxide (NO2), is formed from a variety of stationary and mobile sources. Fossil fuel-based power plants and internal combustion engine-based transportation vehicles are prime examples of stationary and mobile sources of NOx emissions, respectively. In any case, thermal NOx, formed at high temperatures when nitrogen oxidizes with oxygen in the air, is the primary pathway for NOx emissions. As conventional power plants and internal combustion engine-based vehicles are replaced with renewable energy and electric vehicles, respectively, to cope with the CO2 emissions problem, the NOx emissions are expected to continue to decline. Meanwhile, with ammonia gaining much attention as a non-carbon fuel, fuel NOx, which is formed through the partial oxidation of ammonia, can be another pathway for NOx emissions in the stationary sources [2,3,4].
The selective catalytic reduction of NOx with ammonia (NH3-SCR) is the most widely adopted method for controlling NOx emissions from stationary sources among the various methods developed to date [5,6]. The following reactions occur during NH3-SCR in the absence of oxygen.
6NO + 4NH3 → 5N2 + 6H2O
6NO2 + 8NH3 → 7N2 + 12H2O
NO + NO2 + 2NH3 → 2N2 + 3H2O
In practice, because most flue gases contain varying concentrations of oxygen, the following reactions take place.
4NO + 4NH3 + O2 → 4N2 + 6H2O
2NO + 4NH3 + 2O2 →3N2 + 6H2O
2NO2 + 4NH3 + O2 → 3N2 + 6H2O
In addition, ammonia oxidations, shown below, can occur in the presence of oxygen, which is not favorable for NH3-SCR.
4NH3 + 3O2 → 2N2 + 6H2O
4NH3 + 5O2 → 4NO + 6H2O
4NH3 + 7O2 → 4NO2 + 6H2O
2NH3 + 2O2 → N2O + 3H2O
N2O can be additionally formed via the following reaction in the presence of the catalyst.
4NO + 4NH3 + 3O2 → 4N2O + 6H2O
Metal (W and/or Mo) oxides-promoted V2O5/TiO2 is a representative commercial catalyst for NH3-SCR, achieving high NOx conversion over a wide operating temperature range of 300–400 °C [7,8,9]. Because flue gas composition varies depending on the fuel (e.g., coal, biomass, organic wastes, oil, natural gas, etc.), the NH3-SCR unit is placed in different locations in the flue gas treatment process depending on the process characteristics [10]. Generally, since the flue gas contains fly ash, impurities, and SO2, the dust removal device (e.g., electrostatic precipitator) and the flue gas desulfurization device can be installed to remove the fly ash and SO2 in the flue gas, respectively [11]. After these units, additional preheaters are required to raise the flue gas temperature to the proper operating temperature of vanadia-based commercial catalysts because the flue gas temperature is reduced below 200 °C. Therefore, low-temperature NH3-SCR catalysts need to be developed to eliminate the increased operating cost and an extra capital cost due to these additional preheating units.
To date, a variety of NH3-SCR catalysts [12,13,14,15,16,17,18,19,20,21,22,23], including Cu-based [24], Ce-based [25,26,27,28], Mn-based [29,30,31], Ba-based [32], and carbon materials-supported catalysts [33,34], have been studied that are active below 200 °C. Compared with NH3-SCR catalysts operating at medium and high temperatures, low-temperature NH3-SCR catalysts are susceptible to poisoning by water vapor and SO2 in flue gas [19,21]. In particular, SO2 in flue gas can be catalytically oxidized to form SO3, which reacts with ammonia and then converts to ammonium salts (NH4HSO4 (ABS) and (NH4)2SO4 (AS)), which can block the active sites of NH3-SCR [11,35]. Furthermore, additional side reactions forming N2O have been observed over these catalysts. Low-temperature NH3-SCR catalysts capable of solving the above problems have not yet been reported. This review article summarizes recent progress on low-temperature NH3-SCR catalysis and briefly discusses research directions to address the current obstacles.
2. Low-Temperature NH3-SCR Catalysts
Vanadia-based catalysts active at moderate temperatures can provide meaningful information about low-temperature NH3-SCR catalysis. Arnarson et al. [36] proposed a reaction mechanism of ‘Standard NH3-SCR’ combining with ‘Fast NH3-SCR’ on a VOx/TiO2(001) catalyst model (Figure 1). The two cycles shared the same reduction part (A B D E F P R A in Figure 1) but used NO + O2 (F I M N P in Figure 1) and NO2 (F G′ H′ P in Figure 1) for the re-oxidation process, respectively. They noted that the rate of formation and desorption of H2O is a decisive factor in the ‘Standard NH3-SCR’ reaction at low temperatures and that the reaction of NO2 with the reduction sites is responsible for accelerating the ‘Fast NH3-SCR’ reaction at low temperatures [36].
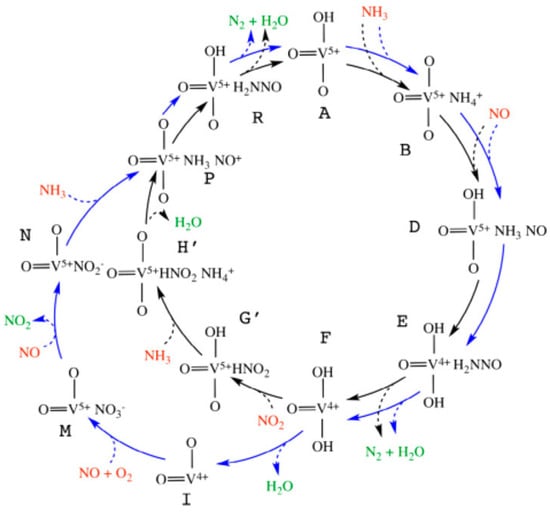
Figure 1.
Proposed reaction mechanism over the VOx/TiO2(001) model. The black circle represents the ‘Fast NH3-SCR’ reaction, and the blue circle represents the NO-activation cycle; the ‘Standard NH3-SCR’ reaction is the sum of the black and blue cycles. This schematic diagram is reprinted with permission from ref [36]. Copyright 2017 Elsevier.
The reaction mechanisms [36,37,38] over V2O5/TiO2 catalyst reveal that NH3-SCR activity depends on a number of factors, including the redox property of vanadium species between V+5 and V+4, Brønsted and Lewis acid sites on the catalyst surface, dispersion of vanadium species, acidic and basic properties of the support, contribution of a support to the redox property of vanadium species, the surface hydrophobicity of the support, the hydrothermal stability of the support, and adsorption property of SOx. Therefore, these factors should also be considered when designing low-temperature NH3-SCR catalysts. First of all, the redox properties of the active metal oxides are crucial for this reaction, so Mn, Fe, Co, Cu, and Ce can be selected as promising candidates for the active metal because they have a variety of metal oxides with different oxidation states and are interconvertible under reaction conditions. However, cobalt oxide can be excluded as a promising candidate for this reaction because of its very high activity towards complete oxidation [39]. Therefore, Cu-, Fe-, Mn-, and Ce-based low-temperature NH3-SCR catalysts are covered in the following sections.
2.1. Cu-Based Catalysts
Cu-containing metal oxides and Cu-based small-pore zeolites have been reported to be active for low- and medium-temperature NH3-SCR. Various factors, including support, Cu precursor, promoter, crystal structure, preparation method, and interface engineering between Cu and support, have been considered [24]. Research on Cu-based catalysts has been motivated by their high NH3-SCR activity, especially at low temperatures, but their low SO2 tolerance is recognized as a significant barrier to field application. Moreover, due to the low hydrothermal stability of Cu-containing metal oxides, recent research has focused on Cu-based small pore zeolites, as shown in Table 1 [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76]. Among them, low-temperature NH3-SCR activity was reported over Cu-zeolites such as Cu-LTA [41], Cu-ZSM-5 [42,43,44], Cu-SSZ-13 [47], Cu-SSZ-16 [49], Cu-SSZ-52 [51], Cu-SAPO-34 [52], Cu-UZM-35 [56], and Cu-ZJM-7 [57], metal-promoted Cu-zeolites such as Ce-Cu-SAPO-18 [59], Fe/Cu-SSZ-13 [62], CuY-SAPO-34 [63], CuNd/SAPO-34, Cu-Ce-La-SSZ-13 [65], and Cu-Ce-USY [68], and other Cu-based oxides such as Cu/ZrO2 [70] and CuAl layered double oxide (LDO) supported on carbon nanotubes (CNTs) [72].

Table 1.
NH3-SCR activity over some Cu-based catalysts.
Only a few reports can be found on Cu-containing metal oxides in the last few years. Chen et al. [69] examined the crystal-plane effects of CuO over CuO/TiO2 catalysts on NH3-SCR and reported that the proportion of Cu+ and surface-adsorbed oxygen (Oα) in CuO(111)/TiO2 catalyst was higher than that of CuO(001)/TiO2 catalyst, which could facilitate the NH3-SCR reactions. Liu et al. [71] reported that LaCuO3-x/meso-Al2O3 enriched with Cu3+ species exhibited significantly higher catalytic activity for NH3-SCR than CuO/meso-Al2O3 counterpart in the low temperature range (100–280 °C), which was attributed to the unique nature of Cu3+ species, which had more acid sites and higher redox properties to promote the adsorption and activation of ammonia and NOx.
Cu-containing zeolites have been intensively studied for NH3-SCR and/or urea-SCR for their applications to stationary and mobile sources [57,77,78]. The NH3-SCR performance of Cu-exchanged zeolite catalysts depends on the amount of isolated copper ion sites, SiO2/Al2O3 ratios, and topological structures. The isolated Cu2+ species serve as redox sources, the zeolite supplies acidic sites, and the channel structure affects the diffusion of reactants, intermediates, and products. A variety of zeolite catalysts for Fe or Cu ion exchange have been developed, all of which exhibit a wide temperature window, including ZSM-5, SAPO-18, SAPO-34, SSZ-13, SSZ-16, SSZ-39, AFX, BEA, ERI, KFI, LTA, Nu-3, PST-7, RHO, RTH, Sigma-1, UZM-35, etc. [78,79,80,81,82,83]. In terms of the hydrothermal stability, ion-exchanged small-pore zeolites such as Cu-SSZ-13, Cu-SSZ 16, Cu-SSZ-39, Cu-SAPO-18, Cu-SAPO-34, and Cu-KFI are superior to the medium-pore ZSM-5 zeolite catalysts, and finally, Cu-SSZ-13 catalysts have been commercialized for NOx control in diesel-powered vehicles owing to their outstanding NOx removal efficiency and hydrothermal stability [77,79,84]. A comparison of the NH3-SCR activity of Cu- and Fe-zeolites showed that at low temperatures (below 350 °C), the former was more active than the latter, but above 350 °C, the latter was more active than the former, which was ascribed to the fact that Cu-zeolites had higher adsorption sites for NH3 than Fe-zeolites [85].
The reaction mechanism over Cu-SSZ-13 is shown in Figure 2 [86]. A mobile NH3-complex, monomeric Cu ion was identified to be active in the NOx reduction cycle in the redox mechanism. The Cu+ ion complexes thus formed subsequently migrate between the zeolite cages to form dimeric species that are important for O2 activation, which is the rate-limiting step essential for the re-oxidation of Cu+ to complete the catalytic cycle [87]. Density functional theory (DFT) calculations on a reaction mechanism for low-temperature NH3-SCR over Cu-CHA reveal that ammonia-solvated Cu cations, Cu(NH3)2+, are responsible for O2 activation as well as the formation of the key intermediates HO-NO and H2N-NO and that Brønsted acid site is related to decomposition of HO-NO and H2N-NO to N2 and H2O [88]. Oxygen activation requires pairs of Cu(NH3)2+ complexes, but HO-NO and H3N-NO coupling may occur on single complexes [88].
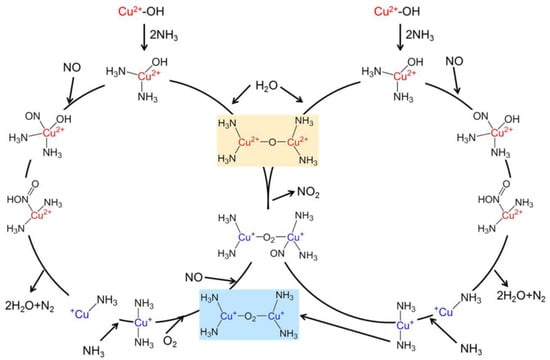
Figure 2.
Complete redox cycling mechanism for low-temperature standard NH3-SCR derived from DFT calculations that involves two Cu(I) centers in the initiation of the oxidation half-cycle [87]. Adapted from permission from [86]. Copyright 2017, American Chemical Society.
Catalyst deactivation in the presence of SOx in the flue gas can be classified into two categories. One is irreversible deactivation due to the formation of inactive Cu sulfate species through interaction between SOx and active Cu species [89,90,91,92,93,94,95]. The other is reversible deactivation resulting from the deposition of sulfate species (e.g., ABS and AS) [96]. SO2 poisoning inhibits the oxidation of NO to NO2, resulting in a lower NH3-SCR activity below 350 °C [79]. Above this temperature, sulfate compounds are unstable so that the available Cu sites can be sufficient for NH3-SCR activity [79]. Therefore, sulfur poisoning beyond 350 °C is insignificant. Table 2 summarizes the effects of H2O and SO2 on the NH3-SCR over some Cu-based catalysts [97,98,99,100,101,102,103,104,105,106,107,108,109]. Among them, CuO@Cu-metal organic frameworks (MOFs) core-shell catalyst [101] and Cu-doped phosphomolybdic acid catalyst [106] showed a relatively stable catalytic performance even in the presence of H2O and SO2 at low temperatures.

Table 2.
NH3-SCR activity in the presence of H2O and/or SO2 over some Cu-based catalysts.
Hammershøi et al. [89] reported that the NH3-SCR activity over Cu-SSZ-13 catalysts was significantly inhibited at 160–350 °C after exposure to SO2, but preserved above 350 °C. Jangjou et al. [110] compared SO2-induced catalyst deactivation at [CuIIOH]+ and Cu2+ sites in Cu-SSZ-13. For Cu2+ sites, the NH3-SCR activity was inhibited due to the formation of ammonium sulfate, which could be fully recovered by regeneration at >380 °C. This implies that Cu2+ sites do not adsorb sulfur during the exposure to SO2 [110]. On the other hand, [CuIIOH]+ sites could adsorb sulfur directly to form Cu bisulfite, causing the irreversible deactivation [110]. Therefore, the sulfur poisoning of [CuIIOH]+ was much more severe than that of Cu2+. H2SO4 formed in the presence of SO2 and H2O plays an important role in the formation of ammonium sulfate [111,112]. Another route to the formation of Cu bisulfate is the interaction between SO2 and CuOx nanoclusters [113].
The effect of SO2 on Cu-SAPO-34 deactivation was also investigated [91,114]. The NH3-SCR activity of sulfur-poisoned Cu-SAPO-34 was decreased significantly at 100–500 °C, though sulfur accumulation and zeolite structure collapse were not observed [114]. The deactivation was attributed to the decreased amount of isolated Cu2+ ions [91,114]. It was speculated that sulfur poisoning could hinder the copper redox transformation (between CuII and CuI) in Cu-SAPO-34, which led to the sulfation of Cu sites [115]. In addition, Cu-SAPO-34 favored the formation of stable Al2(SO4)3 species compared with Cu-SSZ-13, resulting in an irrecoverable loss in NH3-SCR activity [111].
When screening Cu-containing zeolite catalysts, hydrothermal stability has become an important selection criterion in addition to high NOx conversion over a wide range of reaction temperatures for application in transport vehicles, but this is not as important for low-temperature NH3-SCR catalysts for application in stationary sources. Rather, resistance to water and SO2 at low temperatures is an additional factor to be considered in the development of NH3-SCR catalysts. In order to improve the low-temperature NH3-SCR activity of Cu-containing zeolite catalysts, the Cu active sites can be modified by introducing the heteroatoms to facilitate the redox property of Cu and metal oxides to accelerate NO oxidation for ‘Fast NH3-SCR’ [116]. Other zeolites with different structures, including AEI and LTA, the small-pore intergrown zeolites, including AFX/CHA and CHA/AEI, and the zeolite morphology can be further examined even though they were excluded because of their poor hydrothermal stability at high temperatures [116]. Additional doping of Y [63] and lanthanides [59,60,61,62,63,64] to Cu-zeolites was reported to be effective for NH3-SCR. The composite catalysts such as MnO2-CeO2/Cu/SSZ-13 showed excellent NH3-SCR activity and N2 selectivity in the temperature ranges of 125 to 450 °C because more active monodentate nitrate was formed on the surface of the composite catalyst compared with Cu/SSZ-13 alone [99].
Chen et al. [107] synthesized a core–shell structure Cu–Ce–La/SSZ-13@ZSM-5 catalyst by a self-assembly method and applied it to NH3-SCR. They observed that Cu–Ce–La/SSZ-13@ZSM-5 with an appropriate shell thickness presented better NH3-SCR activity and hydrothermal stability than Cu–Ce–La/SSZ-13 because some metal ions were transferred and redistributed during the assembly of the ZSM-5 shell, resulting in the conversion of [Cu(OH)]+–Z to Cu2+–2Z species and the functionalization of the shell phase, which was beneficial for the adsorption and activation of NH3. Chen et al. [109] synthesized a multifunctional core-shell catalyst with Cu-SSZ-13 as the core phase and Ce-MnOx supported mesoporous silica as the shell phase via self-assembly and impregnation. The core-shell catalyst exhibited excellent low-temperature activity, SO2 tolerance, and hydrothermal stability compared with the Cu-SSZ-13 [109]. The Ce-MnOx species dispersed in the shell can rapidly activate NO and oxidize it to NO2, which allows the NH3-SCR reaction on the core-shell catalyst to be initiated in the shell phase [109]. Meanwhile, Ce-MnOx species can react preferentially with SO2 as sacrifice components, effectively avoiding the sulfur inactivation of the copper active sites [109]. This catalyst showed relatively stable NO conversion in the presence of 10% H2O and 100 ppm SO2 at 250 °C [109]. It is noteworthy that few low-temperature Cu-based catalysts with high resistance to H2O and SO2 poisoning have been reported.
2.2. Fe-Based Catalysts
Various Fe-based catalysts, including single iron oxides, Fe-containing mixed metal oxides, supported iron oxides, supported Fe-containing multicomponent metal oxides, and Fe-containing zeolites, have been reported for NH3-SCR due to their excellent redox properties and low cost. Another advantage of Fe-based catalysts is their relatively good resistance to water and SO2, although this has only been reported at temperatures above 200 °C [11].
γ-Fe2O3 has been mainly reported for NH3-SCR among various single-phase Fe2O3 with different crystalline structures (e.g., α, β, γ, and ε) [11]. Yu et al. [117] proposed two different reaction mechanisms over γ-Fe2O3 (Figure 3). One is the Langmuir–Hinshelwood (L–H) mechanism in which NH3 and NO are adsorbed on the active sites, Fe3+-OH and Fe3+=O, respectively, and reacted to form N2 and H2O, which is prevalent at temperatures below 300 °C [117]. The other is the Eley–Rideal (E–R) mechanism in which NH3 is first chemisorbed on Fe3+-OH to form Fe3+-O−H-N+H3, which can be further reacted with gaseous NO to produce N2 and H2O, which is the main pathway at high temperatures above 300 °C [117]. In any case, this reaction requires the cooperation of acidic sites and redox properties. They also confirmed that the NH3-SCR activity decreased firstly and then increased slowly after the introduction of SO2 at temperatures ranging from 225 to 275 °C [117]. This was ascribed to the formation of iron sulfate species inhibiting the adsorption of NOx, thus interfering with the L–H reaction pathway [117]. On the other hand, the iron sulfate species formed enhanced the surface acidity, which promoted the E–R reaction pathway and further promoted NH3-SCR activity [117].
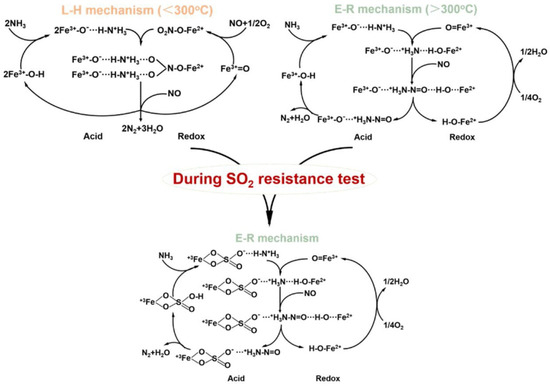
Figure 3.
The proposed reaction mechanism before and during the SO2 resistance test on the γ-Fe2O3 catalyst [117]. Adapted from permission from ref [117]. Copyright 2021 Elsevier.
Recent work on single Fe oxides has focused on how to increase the low-temperature NH3-SCR activity. Qin et al. [118] prepared various Fe3O4 nanostructures exposed with different crystal planes from MIL-100(Fe) as the Fe precursor and found that the catalysts with more (1 1 1) had better NH3-SCR performance than those with (1 0 0) exposure, which they attributed to the preferential exposure of the Fe3O4 (1 1 1) crystal faces leading to higher adsorbed oxygen concentration and surface acidity. Yang et al. [119] found through DFT calculations that the ‘Fast NH3-SCR’ reaction was the dominant pathway for the NH3-SCR reaction on Fe2-N6 catalysts, with the energy barrier of the rate-determining step (HONO formation) being 1.00 eV, much lower than that of other NH3-SCR catalysts, enabling excellent low-temperature activity in the temperature window of 300–500 K. Zhang et al. [120] prepared a highly defective α-Fe2O3 with enhanced acid (Lewis and Brønsted) and redox properties on homoatomic dinuclear sites comprising more positively charged Fe3+ and oxygen vacancy-coupled Fe2+ ions. The catalyst showed enhanced NH3-SCR activity at low temperatures without the addition of other acid transition metals and showed resistance to poisoning of H2O and SO2 due to the large amount of Brønsted acid [120].
Various metal oxide-promoted iron oxides have been investigated to enhance the cooperation of surface acidity and redox property to facilitate the low-temperature NH3-SCR. The positive effects of Mn [121], Nb [122], Mo [123], Ce [124], Sm [125], and W [123,126,127,128] in promoted iron oxides on the catalytic activity have been reported. Table 3 summarizes the effects of H2O and SO2 on the NH3-SCR over some Fe-based catalysts [120,121,124,125,128,129,130,131,132,133,134,135,136,137]. Among them, a relatively stable catalytic performance was observed over Mn-Fe oxides [121] even in the presence of H2O and SO2 at low temperatures.

Table 3.
NH3-SCR activity in the presence of H2O and/or SO2 over some Fe-based catalysts.
It is noteworthy that, except for catalysts containing additional Mn, few Fe-based catalysts are active for NH3-SCR at low temperatures (<200 °C) while being resistant to H2O and SO2 poisoning. Jiang et al. [138] prepared a phosphotungstic acid (HPW)-promoted Fe-based catalyst from MIL-100(Fe) as the Fe precursor by hydrothermal method and reported that active oxygen species-rich γ-Fe2O3 was the main Fe phase, which enhanced NO adsorption and activation, leading to faster NH3-SCR. The role of HPW was to increase the total acidic sites along with promoting the reactivity of NH3 adsorbed on Lewis acidic sites at low temperatures. Notably, WO3-promoted Fe2O3 was reported to have a wide temperature window and excellent water and sulfur resistance, showing relatively stable NO conversion in the presence of 100 ppm SO2 at 300 °C [128]. The roles of WO3 are known to inhibit the crystallization of the Fe2O3 phase and formation of inactive nitrate, instead increasing Lewis acid sites and appropriate redox properties [127,128]. A kind of composite catalyst, HPW-decorated ring-like Fe2O3 synthesized via mechanical-chemistry grinding of HPW and Fe2O3 nanorings prepared by a microwave-assisted hydrothermal method, showed good NH3-SCR performance over a wide temperature window of 250–500 °C [139] and an outstanding resistance against SO2, showing relatively stable NO conversion in the presence of 10% H2O and 200 ppm SO2 at 280 °C [132]. Sun et al. [125] reported that Sm modification could weaken the sulfation of active Fe sites in Sm-doped Fe2O3, which was also supported by the DFT calculation results that SO2 could be more easily adsorbed on the Sm/Fe2O3 catalyst with the adsorption sites located at the Sm atom and its neighboring Fe atoms. They observed that more reactive nitrate species were formed on the sulfated Sm/Fe2O3 catalyst due to the presence of more un-sulfated Fe sites, which they explained as making the Sm/Fe2O3 catalyst resistant to SO2 poisoning, showing relatively stable NO conversion in the presence of 5% H2O and 100 ppm SO2 at 275 °C [125]. Tan et al. [140] reported that the low-temperature (<250 °C) NH3-SCR activity of FeTiOx catalyst could be dramatically enhanced by CeO2 doping, which can be attributed to the presence of a unique Ce-O-Fe structure that contributes to the improvement of redox properties. Chen et al. [124] prepared a single-atom Ce-modified α-Fe2O3 catalyst by a citric acid-assisted sol–gel method and reported that a high NO conversion was maintained in the presence of 5% H2O and 200 ppm SO2 at 250 °C. They claimed that the atomic dispersion of the Ce species to maximize the amounts of Fe–O–Ce sites in the Ce-doped FeOx catalyst was critical because the formation of oxygen vacancies in the Fe–O–Ce sites, which could promote the oxidation of NO to NO2 and decomposition of ABS, was more favorable than that in the Fe–O–Fe sites in the Ce-free α-Fe2O3 catalyst [124]. Ma et al. [141] compared a serial of Cu0.02Fe0.2CeyTi1-yOx catalysts prepared by the sol–gel method and found that Cu0.02Fe0.2Ce0.2Ti0.8Ox exhibited superior low-temperature NH3-SCR performance in the presence and absence of water, which they attributed to the optimal distribution of surface acidity, enhanced surface oxygen content, and surface redox cycle (Ce4+ + Fe2+ Ce3+ + Fe3+). Yao et al. [133] prepared Mn-W-Sb modified siderite catalysts by impregnation method and found that the Mn-doping enhanced adsorbed NO2 formation by synergistic catalysis with Fe3+ and that the addition of Sb inhibited sulfate formation on the surface of the catalyst in the presence of SO2 and H2O, showing relatively stable NO conversion in the presence of 5% H2O and 100 ppm SO2 at 210 °C. Xu et al. [121] prepared various MnFeOx catalysts with different molar ratios and observed high low-temperature NH3-SCR activity. The Mn–Fe-0.2 (Mn/Fe = 0.2) catalyst presented excellent SO2/H2O tolerance, showing a rather stable NOx conversion even in the presence of 5% H2O and 50 ppm SO2 at 100 °C [121]. Doping Mn not only inhibited the phase transformation of iron oxide (Fe2O3) but also strengthened the interaction between MnOx and Fe2O3 due to the electron transfer between them, which led to the formation of Mn–O–Fe [121]. Bai et al. [142] prepared an amorphous metal oxide (FeOx-Mn0.1Oy) with a large surface area, sufficient oxygen vacancies, and excellent redox properties and observed high adsorption and activation capacities for O2 and NO, which further enhanced the catalytic activity at low temperatures (90–240 °C). The incorporation of Mn into the FeOx species suppressed the crystallization of hematite, further increasing the surface area and surface acid sites [142]. In addition, the incorporation of manganese increased the number of oxygen vacancies, which decreased the apparent activation energy of hematite and enhanced the redox properties of the amorphous FeOx-MnOy catalyst [142].
The mesoporous Fe-doped CeO2 catalyst after modifying organic sulfate functional groups showed excellent activity in a temperature range of 250–450 °C, which was ascribed to the strong electron interaction between Fe3+-O-Ce4+ species and sulfate groups, which modifies the acidity and redox properties of the catalyst [143]. Wang et al. [144] prepared a series of sulfated modified Fe–Ce composite oxide Fe1–xCexOδ-S catalysts and reported that the Fe0.79Ce0.21Oδ-S catalyst achieved the low-temperature NH3-SCR activity at temperatures of 175–375 °C. They claimed that sulfation formed a large amount of sulfate on the catalyst surface and provided abundant Brønsted acid sites, which enhances NH3 adsorption capacity and improves overall NOx conversion efficiency [144]. The introduction of Ce was the main determinant to control the low-temperature activity of the catalyst by modulating its redox ability, and they found that there was a strong interaction between Fe and Ce in the Fe0.79Ce0.21Oδ-S catalyst, which changed the electron density around the Fe ions, which weakened the strength of the Fe–O bond and improved the lattice oxygen mobility of the catalyst [144]. In addition, during the reaction, the Fe-Ce composite oxide catalyst showed higher surface lattice oxygen activity and a faster bulk lattice oxygen replenishment rate [144].
The support of the supported iron oxide can increase the dispersion of the iron oxide, providing high surface active sites per mass of iron oxide, provide additional surface acid sites on the support itself and at the interface between the support and the iron oxide, and improve the redox properties of the iron oxide. Different crystal planes of the support (e.g., TiO2 [145] and CeO2 [146]) of supported iron oxides were compared. Monolayer Fe2O3 supported on TiO2 nanosheets exhibited a better low-temperature NH3-SCR activity than that supported on TiO2 nanospindles because the former had more acidic sites, oxygen defects, and reactive oxygen species [145]. The iron oxides supported on CeO2 nanorods achieved higher catalytic activity for NH3-SCR than those supported on CeO2 nanopolyhedra, which was explained by the DFT calculation results that the Fe2O3/CeO2 {110} catalyst was more reactive to NO and NH3 than the Fe2O3/CeO2 {110} [146]. The Fe2O3{1 1 3}-TiO2 exhibited superior NOx removal capacity and a broader temperature operating range than Fe2O3{0 1 2}-TiO2 and Fe2O3{0 1 4}-TiO2, which can be attributed to the improved redox properties, as well as the presence of additional active oxygen species, surface acid sites, and adsorbed nitrate species on the Fe2O3{1 1 3}-TiO2 catalyst [147].
2.3. Mn-Based Catalysts
Mn-based catalysts, including MnOx, Mn-containing mixed metal oxides, supported MnOx, and supported Mn-containing multicomponent metal oxides, have been regarded as the promising low-temperature NH3-SCR catalysts [30,148,149,150]. A comparison of the low-temperature NH3-SCR activity between various transition metal oxides supported on TiO2 showed that it decreased in the following order: Mn > Cu ≥ Cr >> Co. > Fe >> V >> Ni [151]. First-principles calculations also revealed that the superior oxidative dehydrogenation performance of Mn-based catalysts to NH3 lowered the energy barrier for the activation of NH3 and reduced the formation of the key intermediate NH2NO, the rate-determining step in NH3-SCR, over Mn-, Fe-, and Ce-based oxide catalysts [152].
Among various single-phase manganese oxides, including MnO2, Mn5O8, Mn2O3, Mn3O4, and MnO, MnO2, and Mn2O3 were reported to have the highest low-temperature activity per unit surface area and N2 selectivity, respectively [153]. The crystallinity and valence state of MnOx affected by the preparation method and pretreatment conditions have been reported to influence the NH3-SCR performance [154]. Higher low-temperature NH3-SCR activity was obtained for the less crystalline MnOx [155,156]. A comparison of β-MnO2 and α-Mn2O3 showed that the former had a higher NOx conversion and N2O production rate per unit surface area than the latter, which was explained by the lower Mn–O bond energy of β-MnO2, which promoted the activation of NH3 [157]. The birnessite-type MnO2 catalyst, prepared from Mn(NO3)2·4H2O, having the fewest OH groups, was reported to exhibit the best catalytic activity and excellent SO2 resistance among the same-type catalysts prepared from different Mn precursors [158].
A novel synthetic method was applied to prepare high-surface-area manganese oxide with low crystallinity for this reaction. Mesoporous α-MnO2 nanosheets prepared by a solvent-free synthetic method had a high surface area and a mesopore size of 4 nm with large oxygen vacancies and showed 100% NOx conversion under a gas hourly space velocity (GHSV) of 700,000 h−1 at 100 °C [159]. Xu et al. [160] prepared MnOx catalysts with 3D structure by the hard-template method using KIT-6 as a template possessing high reducibility with abundant surface oxygen species and Mn4+ species and reported that more Lewis acid sites and Brønsted acid sites on the surface were beneficial for the adsorption and activation of NH3, leading to the higher NH3-SCR activity. Chen et al. [161] synthesized a novel MnOx catalyst with a large surface area, small particle size, and more crystalline defects from MOF-Mn3(BTC)2(H2O)6 using different amounts of polyvinyl pyrrolidone (PVP) and found that this catalyst had abundant acid sites, Mn4+, and surface chemical oxygen, promoting the NH3-SCR reaction. Moreover, the high SO2 tolerance was also observed because an irreversible sulfurization rate was reduced and an adsorption of active bidentate nitrates and NH4+ was promoted even in the coexistence of sulfates, showing relatively stable NO conversion in the presence of 50 ppm SO2 at 175 °C. Zhang et al. [162] also prepared various MnOx catalysts from MOF-74 under different pretreatment conditions and reported that their redox capability could be controlled by changing the ratio of Mn4+/Mnn+ and Oα/(Oα + Oβ), resulting in the promotion of the adsorption and oxidation of NO to facilitate the ‘Fast NH3-SCR’ reaction. Zhou et al. [163] prepared hydrothermally stable MnOx/Al2O3 catalysts with highly dispersed low-coordinated Mn active sites that were originally created with triethanolamine and observed excellent low-temperature NH3-SCR activity because of active low-coordinated Mn species possessing reactive redox sites and Lewis acid sites.
Li et al. [164] employed DFT calculations for the reaction mechanism of NH3-SCR over Mn/γ-Al2O3 catalyst. NH3 is mainly adsorbed on the Lewis acid sites and forms coordinated NH3. Subsequently, an N–H bond in the adsorbed NH3 can be dissociated to form NH2*, which can react with the gaseous NO and generate NH2NO*. NH2NO* was finally decomposed into N2* and H2O* (Figure 4). On the surface of the Mn/γ-Al2O3 catalyst, the adsorbed O2 was decomposed into the active oxygen atoms, which could oxidize NO into NO2. Among three possible routes for N2O formation, such as NO decomposition, deep dehydrogenation of NH3, and two-step dehydrogenation of NH2NO, the deep dehydrogenation of NH3 appears to be mainly responsible over the Mn/γ-Al2O3 catalyst in both the L–H and E–R reaction mechanisms.
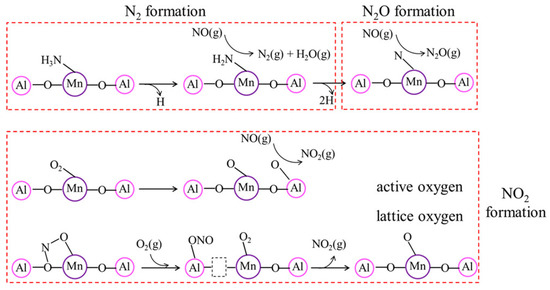
Figure 4.
The reaction mechanism of NH3-SCR on the Mn/γ-Al2O3 catalyst calculated by Li et al. [164] Reproduced from ref [164]. Copyright 2019 American Chemical Society.
The formation of N2O over Mn-based catalysts was systematically examined over MnOx-TiO2 catalysts with different Mn/Ti ratios [165]. As the Mn/Ti ratio increased, the MnOx species layer expanded on TiO2 support, leading to an increase of redox sites but a decrease of surface acid sites, contributing to the non-selective oxidation of NH3 on MnOx species and the over-activation of NH3 at the Mn-Ti interface and resulting in the formation of N2O. Yang et al. [166] proposed the scheme for N2O formation according to the L–H and E–R mechanisms, in which monodentate –NO3− (and NH4NO3*) and –NH are key intermediates for N2O formation, respectively (Figure 5). DFT calculations and thermodynamic/kinetic analysis of NH3-SCR over α-MnO2 with specific (100), (110) and (310) exposure planes showed that the α-MnO2 catalyst exposed with the (310) plane exhibited the best NH3-SCR catalytic performance and the highest N2 selectivity due to the low energy barrier of NH3 dehydrogenation and NH2NO generation and the difficulty of NH2 dissociation [167].
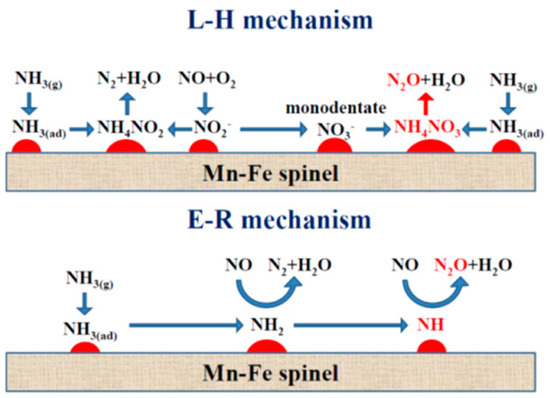
Figure 5.
Proposed N2O formation mechanisms over Mn–Fe spinel. This schematic diagram is reprinted with permission from ref [166]. Copyright 2014 American Chemical Society.
Although manganese oxides exhibit high NH3-SCR activity at low temperatures, their application in practical processes is limited due to their sensitivity to SO2 and low N2 selectivity [21,168]. Therefore, Mn-containing mixed metal oxides and supported Mn-containing metal oxides have been adopted to address these issues. For Mn-containing mixed metal oxides, various second metals including Al [169], Si [170], Ti [171], V [172], Cr [173], Fe [174,175,176,177,178], Co [179,180,181,182], Ni [183], Cu [184,185], Y [186], Zr [187], Nb [188], Mo [189,190,191], Ag [192,193], Sn [194], Sb [195], La [196,197], Ce [197,198,199,200,201,202,203,204], Pr [205], Nd [206], Sm [207,208,209], Eu [210], Gd [211], Dy [212,213], Ho [214], Er [215], Tm [216], Ta [217], W [218,219], and Bi [220] have been examined. The effects of H2O and SO2 on the NH3-SCR activity over unsupported Mn-based catalysts, supported Mn-based catalysts, and core-shell Mn-based catalysts are summarized in Table 4 [161,173,194,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262], Table 5 [171,187,189,195,212,215,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292], and Table 6 [293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308], respectively. Among them, a relatively stable catalytic performance was observed over unsupported Mn-based oxides such as Mn oxides [161], Mn-Cr oxides [173,223], Mn-Fe oxides [224], Mn-Fe-Co oxides [225], Mn-Fe-Mg oxides [226], Mn-Fe-Al oxides [227], Mn-Co oxides [230,232,233,234], Mn-Co-Ce oxides [235], Mn-Co-V oxides [236], Mn-Ni oxides [235,238,239], Mn-Ni-Al oxides [240], Mn-Ni-Fe oxides [244], Mn-Ni-Ce oxides [235], Mn-Zr-Ti oxides [246], Mn-Ce oxides [248,249], Mn-Ce-Ti oxides [250,251,252], Mn-Ce-Sn oxides [248], Mn-Sm-Ti oxides [255,256], Mn-Sm-Fe oxides [257], Mn-Sm-Zr-Ti oxides [258], Mn-Sm-Ce-Ti oxides [259], Mn-Nd oxides [206], Mn-Gd oxides [261], Mn-W-Ce oxides [262], and Bi-Mn oxides [220], and supported Mn-based catalysts such as Mn/Fe-Ti spinel [263], Mn/CeO2-ZrO2 [266], Mn/CeO2-ZrO2-Al2O3 [267], TiO2-MnOx/CeO2-ZrO2 [171], Fe-Mn/TiO2 [270], Zr-Mn/attapulgite [273], Ce-Mn-V-W/TiO2 [277], Nd-Mn/TiO2 [278], Ho-Fe-Mn/TiO2 [215], Gd-MnOx/ZSM-5 [212], NiMnOx/activated coke [283], La-Mn-Fe/activated coke [284], Mn/biochar (BC) [285], Zr-Mn/BC [286], MnCe/Granular activated carbon (AC)-carbon nanotubes (CNTs) [291], and MnOx-CeO2/graphene [292], and Mn-based core-shell catalysts such as MnOx@Eu-CeOx [300], Mn-titanium nanotubes@Ce [303], and Fe2O3@MnOx@carbon nanotube (CNT) [306] even in the presence of H2O and SO2 at low temperatures.

Table 4.
NH3-SCR activity in the presence of H2O and/or SO2 over some unsupported Mn-based catalysts.

Table 5.
NH3-SCR activity in the presence of H2O and/or SO2 over some supported Mn-based catalysts.

Table 6.
NH3-SCR activity in the presence of H2O and/or SO2 over some Mn-based core-shell catalysts.
Liang et al. [221] reported that the MnOx-SiO2 mixed oxide catalyst synthesized by the co-precipitation method showed high NH3-SCR activity with high N2 selectivity and sulfur resistance because doping SiO2 increased the specific surface area of the catalyst, surface acidity, and NH3 adsorption. Doping with SiO2 inhibited the adsorption of SO2 for surface deposition of sulfate, effectively protecting the redox properties of the catalyst so that the MnOx-SiO2 catalyst showed relatively stable NO conversion in the presence of 100 ppm SO2 at 225 °C [221]. Zhu et al. [222] synthesized mesoporous Mn-TiOx with a three-dimensional structure by a solvent-free self-assembling strategy and observed an outstanding sulfur poisoning tolerance. The hierarchical cross-linked structure with a large specific surface area appeared to be favorable for enhancing the synergy between Mn and Ti, exposing more active sites, and dispersing the poisoning species [222]. Wang et al. [309] synthesized a series of MnxCeyTiz catalysts with three-dimensionally ordered macroporous (3DOM) structure by a soft template method and reported that after adding Ti, the redox capacity of the 3DOM-Mn3Ce1Ti1 catalyst decreased and the N2 selectivity was enhanced due to the strong adsorption of NH3 on the acid sites, widening the temperature window. Yao et al. [171] found that the TiO2-modified MnOx/CeO2-ZrO2 nanorod exhibited higher catalytic activity than the MnOx/CeO2-ZrO2 nanorod due to the larger amount of oxygen vacancy, the higher ratio of Mn4+ among Mn species, the higher proportion of surface adsorbed oxygen species among oxygen species, and the improvement of surface acidity. Furthermore, the TiO2 modification appropriately weakened the redox properties of the MnOx/CeO2-ZrO2 nanorods, effectively inhibiting the nonselective oxidation of NH3 to N2O [171]. Finally, TiO2-modified MnOx/CeO2-ZrO2 nanorod exhibited excellent H2O + SO2 tolerance, showing relatively stable NO conversion in the presence of 5% H2O and 100 ppm SO2 at 200 °C [171]. Xu et al. [256] reported that over a wide operating temperature range (60–225 °C), Ti-doped Sm–Mn mixed oxide showed a superior NH3-SCR performance, especially excellent SO2/H2O resistance. Stable NOx conversion was achieved even in the presence of 5% H2O and 100 ppm SO2 at 100 °C [256]. The inclusion of Ti can inhibit MnOx crystallization to increase the high specific surface area and the amount of acid sites, while Sm doping can modulate the redox properties to promote NO oxidation and inhibit NH3 nonselective oxidation [256].
Jiang et al. [282] reported that doping V2O5 to Mn-Ce/AC enhanced the NO conversion, N2 selectivity, and SO2 tolerance than Mn-Ce/AC because doping with V2O5 enhanced surface acidity and enriched surface chemisorbed oxygen. The stronger surface acidity of Mn-Ce-V/AC inhibited the competitive adsorption of SO2 and suppressed the reaction between adsorbed SO2 and NH3 species [282]. In addition, the cluster of vanadium oxide species partially prevented the dispersed Mn-Ce solid solution from being sulfated by SO2 [282]. This catalyst showed relatively stable NO conversion in the presence of 10% H2O and 100 ppm SO2 at 200 °C [282]. Li et al. [236] reported that the Mn0.50Co0.49V0.01Ox catalyst exhibited superior catalytic performance over a wide temperature window (162–508 °C) among ternary MnCoVOx catalysts and also displayed enhanced SO2 tolerance, showing relatively stable NO conversion in the presence of 5% H2O and 100 ppm SO2 at 200 °C.
Gao et al. [173] prepared Cr-Mn mixed-oxide catalysts by citric acid method and observed good low-temperature NH3-SCR performance at 100–225 °C owing to their high specific surface area, more active sites (Mn3+ and Mn4+), and effective electron transfer (Cr5+ + 2Mn3+ Cr3+ + 2Mn4+). The stronger Mn–O binding energy and weaker dehydrogenation ability in CrMn2O4 spinel were the main reasons for the lower N2O by-product than Mn3O4 [173]. The CrMn2O4 spinel showed relatively stable NO conversion in the presence of 10% H2O and 150 ppm SO2 at 200 °C [173]. The formation of Cr(III) sulfate could protect Mn active sites from sulfating. Furthermore, the transformation of -HSO3 and SO42− to (H⋯SO42−) can provide new Brønsted acid sites for ionic NH4+, enhancing the NH3-SCR activity via ‘Fast-NH3-SCR’ (NO2 + NH4+ NH4NO2 N2 + 2H2O) [173].
The DFT calculation results indicated that Fe doping promoted the adsorption of NH3 on the Mn sites over the β-MnO2 (110) surface, weakened SO2 adsorption capacity, and limited the oxidation of SO2, which promoted the decomposition of ammonium sulfate and relieved the catalytic site blockage on the catalyst surface [310]. DFT calculation demonstrated that the thermodynamically most favored structure after Fe doping was MnO2-x, and the structure remained stable as the doping amount increased [311]. The adsorption behavior of gas molecules on MnO2-x showed that NH3 and H2O preferred to adsorb on the Mn Lewis acid sites, while NO and SO2 preferred to adsorb on the O sites. MnO2-x with enhanced acidity after Fe doping showed good SO2 tolerance [311]. Wu et al. [312] prepared various Mn/TiO2 catalysts promoted with different transition metals, including Fe, Cu, Ni, and Cr, by the sol–gel method and found that more NO could be oxidized to NO2 and nitrate and then reacted with NH3, thus leading to the enhanced NH3-SCR activity at low temperatures. The promoting effect of Fe on Mn-Fe composite oxide catalysts was explained by the fact that Fe promoted NO oxidation and generated more monodentate and bidentate nitrate adsorption species on the catalyst surface [312]. It was also reported that the addition of Fe enhanced the amount and intensity of Brønsted and Lewis acid sites, which promoted the absorption of NH3 to form active intermediates, thereby improving the low-temperature NH3-SCR performance [313].
Jiang et al. [232] prepared MnO2–CoOx catalysts with different Mn/Co ratios using PVP as an assistant to control the morphology and exposed crystal faces of composite oxides. The best Mn–Co oxide showed stable NOx conversions in the presence of 5% H2O and 100 ppm SO2 at 160 °C [232]. Feng et al. [314] prepared a series of Mn-Co mixed metal oxides, MnyCo3–yOx, by an ultrasonic technology followed by a hydrothermal treatment and found that they exhibited much better NH3-SCR performance than single metal oxides (MnOx or CoOx). This can be attributed to the strong interaction between MnOx and CoOx, which reduces the crystallinity of the MnyCo3–yOx catalyst, increases the specific surface area, establishes the redox cycle of Co3+ + Mn3+ Co2+ + Mn4+, improves the reducibility, enhances the surface acid sites, and accelerates the reaction between adsorbed NOx species and coordinated NH3 bound to Lewis acid sites [314]. Chen et al. [233] prepared a MnCoOx catalyst using glucose as the forming pore agent and urea as the structural direction reagent and found that it exhibited higher low-temperature activity and SO2 resistance than pure MnOx catalysts. They revealed that the high surface area, abundant chemisorbed oxygen species, and the redox cycle (Co3+ + Mn3+ Mn4+ + Co2+) collectively account for the enhanced catalytic activity of MnCoOx catalysts [233]. The Mn(5)Co(5)Ox catalyst showed stable NOx conversions even in the presence of 5% H2O and 50 ppm SO2 at 125 °C [233]. Zhang et al. [281] prepared a highly active Ce/CoMnAl from layered double hydroxides (LDHs), which showed low-temperature NH3-SCR with good thermal stability and SO2/H2O resistance. The Ce0.5/Co1Mn0.5Al0.5Ox-layered double oxide (LDO) showed relatively stable NOx conversion at 200 °C for 10 h with 100 ppm SO2 in the feed stream [281].
Gao et al. [238] reported that the Mn-Ni spinel nanosheet (NiMn2O4) prepared by the urea hydrolysis hydrothermal synthesis exhibited excellent resistance to H2O and SO2 in the low temperature range at 150–300 °C. This catalyst showed stable NOx conversions even in the presence of 150 ppm SO2 at 175 °C [238]. Ni-doped MnOx catalysts prepared by the solvent-free doping method maintained excellent low temperature (100–200 °C) NH3-SCR activity, which was attributed to the fact that the appropriate nickel loading could effectively adjust the surface lattice oxygen activity and acidity of the catalyst, ensuring its ability to activate NH3 at low temperatures [315]. Liu et al. [316] reported that NiMn2O4 catalysts prepared via the solvothermal method exhibited excellent NH3-SCR performance in the temperature range of 85 to 285 °C due to their large specific surface area, high Mn4+/Mnn+ ratio, sufficient Oα, appropriate acidity, and redox ability. Yan et al. [240] reported that the Ni1Mn0.5Al0.5Ox showed the highest NH3-SCR activity at 100–250 °C among LDHs-derived NiMnAlOx catalysts because of more active species, abundant surface oxygen, moderate acidic sites, and redox properties. This catalyst also exhibited better resistance to SO2 with fewer sulfate species deposited on the surface, showing relatively stable NO conversion in the presence of 5% H2O and 100 ppm SO2 at 200 °C [240]. Hou et al. [241] prepared a series of highly dispersed Ni4-xMnxAlOy catalysts derived from LDHs and reported that the optimal Ni4-xMnxAlOy catalyst exhibited high NH3-SCR performance at a low temperature range of 120~210 °C but was deactivated in the presence of 5% H2O and 100 ppm SO2 at 210 °C. Yan et al. [244] prepared NiMnFeOx catalysts by the LDH-derived oxide method and reported that the optimized Ni0.5Mn0.5Fe0.5Ox catalyst had the best NH3-SCR activity, excellent N2 selectivity, a wider active temperature range (100–250 °C), higher thermal stability, and better H2O and/or SO2 resistance. This catalyst showed relatively stable NO conversion even in the presence of 5% H2O and 100 ppm SO2 at 200 °C [244]. Wang et al. [283] reported that NiMnOx supported on active coke prepared by deposition-precipitation method showed the high NH3-SCR activity because of the higher content of (Mn3+ + Mn4+), the high ratio of Mn3+/Mn4+, and Ni3+/Ni2+. This catalyst showed a stable NO conversion even in the presence of 200 ppm SO2 at 200 °C [283].
Doping MnOx and CeMnOx with Y did not improve NO conversion, but it broadened the range of high N2 selectivity compared with the undoped case [186]. The addition of Zr to Fe-Mn-Ti oxide catalysts was reported to decrease the deactivation rate in the presence of 100 ppm SO2 at 150 °C [187]. Chen et al. [285] prepared several metal (Zr, Ni, and Co) oxide-doped BC-supported Mn oxide (MnOx) catalysts by the impregnation method and found that the Zr-Mn/BC catalyst exhibited the best NH3-SCR activity among them because of the high concentration of Mn4+, more surface oxygen (Oα), excellent redox property, and more Lewis acid sites and Brønsted acid sites. Furthermore, the Zr-Mn/BC catalyst showed stable NO conversion in the presence of 5 vol% H2O and 100 ppm SO2 at 200 °C [285]. Che et al. [317] reported that the highly dispersed active MnOx species supported on the amorphous structure of ZrTiOx had a uniquely bridged Mn3+ bonded with the support through oxygen linked to Ti4+ and Zr4+, respectively, which regulated the optimal oxidizability of the MnOx species, leading to high NH3-SCR activity and N2 selectivity.
Zhou et al. [188] found that doping Nb2O5 could effectively enhance the NH3-SCR performance and N2 selectivity of the Zn-Mn-Ce/AC catalyst. Meanwhile, Nb2O5 could significantly improve the SO2 poisoning resistance of the Zn-Mn-Ce/AC catalyst, showing relatively stable NO conversion in the presence of 100 ppm SO2 at 225 °C because Nb2O5 could react with SO2 in a preferential way to restrain the sulfuration of manganese and cerium oxides on the catalyst [188]. More importantly, Nb2O5 reacted with SO2 to form Nb sulfate and then formed a new acidic site on the Zn-Mn-Ce/AC catalyst surface, which promoted the adsorption of NH3 and inhibited the adsorption of SO2, then restricted the reaction between NH3 and SO2 and hindered the formation of ammonium sulfate channels [188]. The promotional effect of Mo in Mo-MnOx catalyst was reported to be due to its higher Mn4+ content, abundant surface adsorbed oxygen (Oα), and more acid sites [189]. Li et al. [318] employed Mo and V to simultaneously fine-tune the acid and redox sites of MnOx and found that the adjusted Mn0.90Mo0.09V0.01Ox catalyst demonstrated excellent low-temperature activity and a significantly broader active temperature window.
Chang et al. [194] reported that SnO2–MnOx–CeO2 catalysts prepared by a co-precipitation method showed remarkably high activity, N2 selectivity, and SO2 resistance at temperatures in the range of 200–500 °C, which can be due to the significant enhancement of Lewis acid sites generated by surface sulfation during the SO2-containing NH3-SCR reaction. They also explained that the high SO2 tolerance can be ascribed to the inhibited formation of MnSO4 [194]. This catalyst showed stable NOx conversions in the presence of 12% H2O and 100 ppm SO2 at 110 °C [194]. Wang et al. [268] synthesized Sn-doped modified CoAl-LDH by a hydrothermal method and used it as a support for the preparation of the active hexagonal sheet structure of the MnOx(0.25)/CoSn3Al-LDO catalyst, which also showed relatively stable NO conversion at 200 °C after the introduction of 150 ppm SO2. Sn doping increased the ratios of Mn4+/Mn3+ and Co3+/Co2+ as well as the adsorbed oxygen amount on the catalyst surface, improved the redox capacity of the catalyst, and increased the number of acid sites, resulting in the excellent low-temperature NH3-SCR performance [268].
Xie et al. [195] prepared a series of xSb-4Ce-10Mn/TiO2 (x = 0, 2, 3, 4, 5, 6) catalysts by a reverse co-precipitation method and observed that the addition of Sb5+/Sb3+ slightly decreased the catalytic activity but helped improve the H2O/SO2 resistance. They found that the Sb5+/Sb3+ addition decreased the Mn4+ and Ti4+ content but increased the Ce3+, surface adsorbed oxygen content, and the surface area, which promoted the decomposition of AS and inhibited the formation of MnSO4 [195]. However, stable NOx conversion was not achieved in the presence of 5% H2O and 100 ppm SO2 at 200 °C [195].
Li et al. [319] reported that the addition of Mn to ceria greatly promotes the formation of surface oxygen vacancies that readily capture O2 from the air and form surface reactive oxygen species (superoxide and peroxide), which efficiently oxidize NO to NO2 and then promote the ‘Fast NH3-SCR’ reaction. Rong et al. [206] prepared the MnXOx catalysts (i.e., MnSmOx, MnNdOx, and MnCeOx) by the reverse co-precipitation method and found that the MnCeOx catalyst showed the best low-temperature catalytic activity and excellent H2O + SO2 resistance because of the largest amount of acid sites and the best reducibility among these MnXOx catalysts. However, stable NOx conversion was not achieved over these catalysts in the presence of 5% H2O and 100 ppm SO2 at 175 °C [206]. Fang et al. [320] reported enhanced NH3-SCR activity and SO2 tolerance of Ce-modified birnessite-MnO2 and attributed it to the preferential adsorption and oxidation of SO2 onto Ce species to form a new adsorption site for NH4+, Ce2(SO4)3, which protects the Mn active site from sulfidation and deactivation.
It is generally accepted that homogeneous metal doping into the MnOx matrix is beneficial for NH3-SCR activity. However, Cheng et al. [321] claimed that the presence of crystalline Mn3O4 and its interfacial interaction with CeO2 played a significant role in boosting the NH3-SCR performance, suggesting the great synergy between CeO2 and the crystalline phase of Mn3O4, which were responsible for NOx adsorption and the formation of NH2 species through the activation of NH3, respectively. Zhu et al. [322] prepared a series of Cex-Mn-Tiy catalysts by the co-precipitation method and found that the best NH3-SCR activity was obtained over the Ce0.1-Mn-Ti0.1 catalyst with the proper redox ability, abundant acid sites, high content of Mn4+ and Ce3+, and surface-adsorbed oxygen. Wei et al. [251] prepared Mn–Ce–Ti–O composite aerogels with large mesopore size and reported that the Mn–Ce–Ti–O catalyst calcined at 600 °C showed stable NO conversion in the presence of 5 vol% H2O and 100 ppm SO2 at 200 °C, which they attributed to its large pore size, averaging 32 nm, and abundant Lewis acid sites, as the former promoted the decomposition of ABS and the latter reduced the vapor pressure of NH3. Teng et al. [323] prepared a series of rare-earth (Ce, La, Nd, and Y) oxide-doped Mn/Al2O3 catalysts by the impregnation method and found that rare earth oxide addition significantly enhanced the NH3-SCR catalytic activity of Mn/Al2O3, with CeMn/Al2O3 obtaining the highest activity at 125–300 °C. Except for Y2O3, the rare earth oxide additives promoted the surface distribution of Mn elements and enhanced the ratio of chemisorbed oxygen and Mn4+ on the surface of Mn/Al2O3 [323]. In addition, the introduction of rare earth oxides enhanced the reducibility of MnO2 species and obtained a larger amount of weak acid sites [323]. Contrary to some reports on the promotional effect of Ce in Ce-MnOx catalysts, Gevers et al. [324] reported that the intrinsic NH3-SCR activity of the Mn active sites is not positively affected by Ce species at low temperatures based on the fact that the surface-area normalized activity did not increase by Ce addition. They concluded that Ce decreased the average oxidation state and activity of Mn active species and was just a structural promotor, increasing catalyst surface area, and that addition of Ce suppressed second-step oxidation reactions and thus N2O formation by structurally diluting MnOx [324]. The diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) analysis showed that N2O was mainly generated by the reaction between NO and excessive hydrogen abstraction of NH3 (i.e., forming NH species) and that Ca addition inhibited the formation of NH species on the catalyst surface, thereby reducing the formation of N2O [325]. Ca addition could also lead to a decreased formation of NO2, which could react with NH3 to form N2O, contributing to the promoted selectivity of N2 over Ca-modified Ce-Mn/TiO2 [325].
Wu et al. [205] reported that the Pr modification could boost the MnCeOx catalysts’ NH3-SCR activity and SO2 resistance because of the expanded specific surface area, the improved dispersion of active components, the enhanced surface acidity and redox ability, and the generated more chemisorbed oxygen (Oads) species. The MnCePrOx-0.3 catalyst exhibited stable NO conversion even in the presence of 5 vol% H2O and 100 ppm SO2 at 250 °C [205]. Wang et al. [259] reported that the MnCeSmTiOx catalyst preserved higher catalytic performance after introducing H2O and SO2 compared with the catalysts without adding Sm. They claimed that the synergistic effect of the Lewis acid sites and oxidation catalytic sites of mixed oxides was responsible for NH3-SCR by following the L–H mechanism [261]. Doping Sm into MnCeSmTiOx was reported to be able to increase oxygen vacancies and transfer electrons to Mn4+ and Ce4+, promoting the formation of active adsorbed NO2, bidentate nitrate, and bridging nitrate intermediates and suppressing SO2 poisoning by inhibiting the oxidation of SO2 by Mn4+ and Ce4+ [259]. The MnCeSmTiOx catalyst exhibited a rather stable NO conversion even in the presence of 5% H2O and 200 ppm SO2 at 200 °C [259]. Kim et al. [326] reported that the addition of Sm to MnOx/TiO2 increased the redox properties and the proportion of Mn4+ in MnOx catalysts, and the addition of Ce further improved the redox properties of Sm-MnOx/TiO2 catalysts. They also reported that when exposed to SO2/O2, Sm and Ce-modified MnOx/TiO2 not only inhibited the degradation but also increased the N2 selectivity by inhibiting the formation of N2O4 intermediate species, thereby inhibiting the formation of N2O [326].
The addition of Eu to MnOx/TiO2 was reported to be effective against the resistance of SO2 (i.e., NOx conversion gradually decreased but reached a steady-state value in the presence of 100 ppm SO2 at 150 °C) [279]. Wang et al. [210] prepared a series of MEuMnOx ternary oxides (M = Ce, Ni, Co, Sb, Sn, Mo) by one-pot co-precipitation method and observed that CeEuMnOx ternary oxide catalysts showed a high NH3-SCR activity at 100–250 °C and stable NOx conversions in the presence of 10 vol% H2O and 50 ppm SO2 at 230 °C. The facile electron transfer through the redox cycle of Mn3+ + Ce4+ Mn4+ + Ce3+ and enhanced oxygen mobility can promote the formation of more Mn species in high oxidation states and chemisorbed oxygen, accelerating oxidation of NO, and the adsorbed NO2 formed can facilitate the ‘Fast NH3-SCR’ reaction to improve low-temperature activity [210]. The addition of Ce to the EuMnOx catalyst boosts adsorption of NH3 and NOx species. NH3 species are activated as crucial intermediates (NH2) to promote the NH3-SCR reaction [210]. Guan et al. [211] synthesized a series of Gd-modified MnOx/ZSM-5 catalysts via a citric acid–ethanol dispersion method and found that the Gd-modified MnOx/ZSM-5 catalyst presented the higher catalytic activity and better SO2 resistance than MnOx/ZSM-5 in the presence of 100 ppm SO2. The high catalytic performance was mainly owing to the large surface area, enriched Mn4+ and surface chemisorbed oxygen species, strong redox properties, and the proper acidity of the catalyst [211]. The addition of Gd inhibited the reaction between the SO2 and MnOx active sites to form inactive bulk manganese sulfate, resulting in high SO2 resistance [211]. The GdMn/Z-0.3 catalyst showed a rather stable NO conversion in the presence of 10% H2O and 100 ppm SO2 at 180 °C [211]. Gao et al. [212] synthesized Dy-doped MnFe oxides with the morphology of one-dimensional nanowire by using the electrospinning method and found that the doping of Dy with an appropriate amount enhanced the surface Mn4+ concentration and surface chemical adsorption of oxygen and increased adsorption capacity for NH3 and NO. Zhuang et al. [214] investigated the effect of SO2 on the low-temperature NH3-SCR activity for Ho-modified Fe-Mn/TiO2 catalyst. The Fe0.3Ho0.1Mn0.4/TiO2 catalyst showed excellent SO2 durability at 120 °C when the concentration of SO2 was lower than 400 ppm, and the catalytic activity could recover considerably after the SO2 supply was interrupted [214]. When the concentration of SO2 was increased to 1000 ppm, the deactivation behavior was irreversible, but the deactivated catalyst could be regenerated after thermal reduction (350 °C) with 5% NH3 [214]. Zhao et al. [304] synthesized the Ho-modified titanium nanotubes (TNTs)@MnOx, where the manganese oxide active species was confined in a Ho-modified TNTs, and observed the improved SO2 resistance and N2 selectivity during NH3-SCR at low temperatures, showing NO conversion, which decreased slowly over time in the presence of 100 ppm SO2 at 180 °C. They claimed that the pore confinement effect of Ho-TNTs on Mn increased the dispersion of Mn, thereby promoting the interfacial effect between Mn and Ho, and the electron synergy between Mn and Ho inhibited the electron transfer between SO2 and Mn, thus preventing the poisoning of SO2 [302]. They also found that the interaction between Ho and Mn contributed to the proper redox ability to induce electron transfer to inhibit the generation of Mn4+, thereby achieving high N2 selectivity [302].
Zha et al. [218] found that the addition of tungsten created more Brønsted acid sites and reduced the energy barrier for NOx species adsorbed on the surface. They also claimed that the formed NH3 species and NOx species of MnCeW/m-TiO2 were more reactive due to the promotional effects of W than those of MnCe/m-TiO2 [218]. Liu et al. [327] reported the promotional effect of W-doping on the CoMn2O4/TiO2 catalyst on the resistance to H2O at low temperature. The superior performance of the CoMn2O4/W-TiO2 catalyst was ascribed to its unique spinel structure, mesoporous structure, highly dispersed tungsten species, and larger surface acidity [327]. The electron cycle among Mn, Co, W, and Ti helped to keep the Mn3+/Mn4+ and Co3+ at high concentrations and improve lattice oxygen mobility [327]. Both Lewis and Brønsted acid sites were generated by the incorporation of tungsten in the presence of H2O, which enhanced NH3 adsorption and thus promoted the E–R reaction pathway [327]. In addition, the N2 selectivity was significantly enhanced by H2O, which could be attributed to the decreased redox activity [327].
Yan et al. [305] assembled a confined MnCeOx catalyst using a mesoporous zeolite (ZSM-5) as the shell and Mn-Ce oxides as the active core (MnCeOx@ ZSM-5) with a simple one-pot method and found that the novel MnCeOx@ ZSM-5 catalyst displayed enhanced water and SO2 resistance as compared with the MnCeOx supported on ZSM-5 (MnCeOx/ZSM-5) and its precursor (MnCeOx@Al-SiO2), which was ascribed to the zeolite shell’s shielding effect and the synergy between the alumina-silica zeolite shell’s acidic properties and the mixed oxide cores’ redox properties. This catalyst showed relatively stable NO conversion in the presence of 5% H2O and 100 ppm SO2 at 300 °C [305]. Cai et al. [328] fabricated MnFe@CeOx@TiOx nanocage with a yolk-shell structure, where the CeO2 shell could effectively increase the oxygen vacancy defect sites and the TiO2 shell could remarkably improve the surface acid sites, which exhibited excellent NH3-SCR performance in the temperature range of 120–240 °C, which was ascribed to the increased proportion of active species (Mn4+, Fe3+, Ce3+, and Oads) and enhanced interaction between metal oxides. Huang et al. [304] prepared a series of yolk-shell-structured Ce@Mn@TiOx, where the TiO2 shell could provide more surface acid sites to promote the adsorption and activation of ammonia (Figure 6). The Ce@Mn@TiOx exhibited excellent NH3-SCR performance at low temperature and H2O and SO2 tolerance, showing relatively stable NO conversion in the presence of 100 ppm SO2 at 200 °C [304]. Qiao et al. [329] prepared various MnOx-ZSM-5 catalysts with different MnOx locations and found that MnOx clusters dispersed on the outside of ZSM-5 exhibited higher catalytic activity at low temperature than MnOx nanoparticles encapsulated in the channels of the support because the excellent redox ability and the dominant Mn3+ species over the former one improved the oxidation of NO to NO2 and the formation of unstable NOx intermediates, enhancing the low-temperature NH3-SCR activity. Ran et al. [330] synthesized a series of MnOx-CeOx confined in the mesopores of SBA-15 and found that they displayed enhanced resistance to SO2. Guo et al. [331] reported that the catalyst with larger mesopores exhibited much improved sulfur resistance. Zhang et al. [307] synthesized a core-shell structural catalyst, carbon nanotube (CNT)-supported MnOx and CeOx nanoparticles coated with mesoporous TiO2 sheaths (mesoTiO2@MnCe/CNTs), in which the meso–TiO2 sheaths could prevent the generation of ammonium/manganese sulfate species from blocking the active sites, resulting in a higher SO2-tolerance during NH3-SCR. The reversible deactivation was observed over this catalyst due to the presence of 200 ppm SO2 at 300 °C [307]. Yan et al. [305] prepared the core-shell MnCeOx@mesorporus ZSM-5 catalysts, in which a mesoporous zeolite (ZSM-5) covers the active core Mn-Ce oxides as the shell, and observed enhanced water and SO2 resistance during NH3-SCR owing to the zeolite shell’s shielding effect, which hinders the formation of sulfate species, and the synergy between the alumina-silica zeolite shell’s acidic properties and the mixed oxide cores’ redox properties. Zhao et al. [303] constructed a Mn-TNTs@Ce catalyst, where MnOx was confined in TNT via in situ introduction following Ce ion exchange into the skeleton of TNTs. The nanotube-confined structure improved the surface acidity to restrain SO2 adsorption, and Ce species acted as a protective site protecting Mn from SO2 attacking [303]. Furthermore, the short-range structure Ce-O-Ti promoted the electron transformation between Mn and Ce to suppress the formation of -NH and NH4NO3, resulting in inhibiting the generation of N2O [303]. It is remarkably that the Mn-TNTs@Ce catalyst showed a relatively stable NO conversion even in the presence of 5 vol% H2O and 100 ppm SO2 at 200 °C [303].
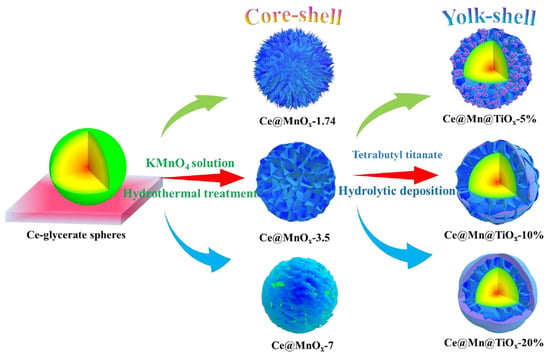
Figure 6.
The schematic illustration of the construction for yolk-shell Ce@Mn@TiOx [306]. Adapted from permission from ref [306]. Copyright 2021 Elsevier.
It is noteworthy that several promising Mn-based catalysts capable of performing NH3-SCR at low temperatures, even in the presence of H2O and SO2, have been reported; however, most of them have only been shown to be stable for short reaction times, and catalyst analysis of post-reaction samples has been limited. For previously reported promising catalysts, long-term lifetime tests should be performed at low temperatures using flue gases containing H2O and SO2 to observe deactivation phenomena, and real-time analysis should be performed to identify and validate detailed mechanisms of resistance to H2O and SO2.
2.4. Ce-Based Catalysts
Since cerium-exchanged sodium-type mordenite (CeNa-MOR) was first reported to be active for NH3-SCR over a wide temperature range of 250–560 °C [332], unsupported and supported ceria, metal-doped ceria, and multicomponent Ce-containing mixed metal oxides [333,334,335] have been applied to NH3-SCR [336]. While NH3-SCR activity was reported to be dependent on ceria morphology (i.e., nanosphere is better than nanocube), unsupported ceria showed relatively low catalytic activity at low temperatures, even after sulfation to provide surface acidic sites [337]. Therefore, metal-doped ceria and multicomponent Ce-containing mixed metal oxide catalysts have received much attention for application in low-temperature NH3-SCR. Since Cu-, Fe-, and Mn-containing Ce-based catalysts have already been covered in the previous sections, this section mainly introduces Ce-based catalysts with other metals. The effects of H2O and SO2 on the NH3-SCR activity over Ce-based catalysts are summarized in Table 7 [338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365,366,367,368,369,370,371,372,373,374,375,376]. Among them, a relatively stable catalytic performance was observed over CO-pretreated CeO2 [338], F-Ce-Ti oxides [345], P-CeO2/TiO2 [346], Ti-Sn-Ce-Ox [352], and CeBi/TiO2 [353] even in the presence of H2O and SO2 at low temperatures.

Table 7.
NH3-SCR activity in the presence of H2O and/or SO2 over some Ce-based catalysts.
Zhou et al. [344] utilized bimetallic MOFs to prepare CeMOx (M=Ti, Cu) catalysts, allowing the homogeneous distribution of promoters. The CeTiOx catalyst with high acidity and good redox properties with abundant Ce3+ and Ti4+ showed high NH3-SCR activity from 180 to 300 °C and maintained stable performance in SO2/H2O at 225 °C [344]. Meanwhile, the aliovalent substitution of ceria by Cu2+ in CeCuOx formed oxygen vacancies and enhanced its redox properties but led to poor N2 selectivity due to NH3 over-oxidation [344]. Guo et al. [353] used Bi as the modifier to boost the performance of the Ce/TiO2 catalyst for NH3-SCR. The CeBi/TiO2 catalyst with a proper Bi content showed a rather stable NO conversion in the presence of 5% H2O and 100 ppm SO2 at 150 °C because the addition of Bi could generate more Ce3+ and chemisorbed surface oxygen species, along with enhanced redox capability and surface acidity [353].
Jin et al. [371] investigated the role of each metal component in Ce-Sn-W-Ba-Ox/TiO2 and found that TiO2 provided sufficient acid sites, CeO2 enhanced redox properties, weakened acid strength, and increased chemisorbed oxygen concentration, which helped to promote the activation and desorption of NH3. They also reported that SnO2 increased chemisorbed oxygen concentration, enhanced redox properties, and improved the activation rate of NH3, WO3 increased the amount of acid, and BaO enhanced resistance to water vapor and SO2 [371]. A rather stable NO conversion was achieved at 350 °C in the presence of 5% H2O and 200 ppm SO2 [371].
Zeng et al. [346] prepared a P-doped CeO2/TiO2 catalyst by dispersing CeO2 on strongly acidic and highly stable mesoporous P-doped TiO2. The P-doped CeO2/TiO2 catalyst exhibited much higher catalytic activity than the CeO2/TiO2 catalyst at temperatures of 200–450 °C and a rather stable NO conversion even in the presence of 5% H2O and 500 ppm SO2 at 200 °C [346]. Mu et al. [354] synthesized an ordered mesoporous CeSnOx/TiO2 catalyst through a classical evaporation-induced self-assembly (EISA) strategy and ascribed the low-temperature NH3-SCR activity to abundant reactive oxygen species (Oα), improving the surface acidity and redox capacity of the catalyst. This catalyst also showed a rather stable NO conversion even in the presence of 5% H2O and 100 ppm SO2 at 220 °C [354]. Mu et al. [356] prepared an ordered mesoporous catalyst, CeSnTiOx, modified with copper sulfate and revealed that the copper sulfate-modified one increased the reaction sites (redox site and acid site). They reported that Cu species can interfere with the electron cycle of the catalyst and reduce the strong redox performance of the Ce active site, which can effectively inhibit the adsorption of SO2, while S-species (from SO42−) can change the distribution of acid sites on the catalyst surface and the total acid content to realize a ‘Fast NH3-SCR’ reaction [356]. This catalyst also showed a rather stable NO conversion even in the presence of 5% H2O and 100 ppm SO2 at 240 °C [356]. Liu et al. [377] prepared a series of W-modified Ce-Sn catalysts by the co-precipitation method and reported that the W species regulated the structure by promoting the formation of the Ce-doped tetragonal SnO2 (t-Sn(Ce)O2) active phase while preventing the generation of the Sn-doped cubic CeO2 (c-Ce(Sn)O2) phase. In addition, the highly dispersed W species on the surface of the Ce1W0.24Sn2Ox catalyst also coupled with Ce species to form new Ce-O-W active sites [377]. The W modification also promoted the ability of the catalysts to oxidize NO to NO2 at 150–300 °C [377]. Liu et al. [378] reported the promotional effect of Ti in Ti-doped Ce– Sn mixed oxide (Ce–Sn–Ti) catalysts on the low-temperature NH3-SCR. The appropriate doping Ti formed Sn–O-Ti, Sn–O-Ce and Ti–O-Ce structures, which could increase the content of Ce3+ through electron transfer from Sn or Ti to Ce (Ce3+ + Ti4+ Ce4+ + Ti3+ and 2Ce4+ + Sn2+ 2Ce3+ + Sn4+) [378]. The solid solution structure increased specific surface areas, active sites (Ce3+), and Lewis acid sites over the Ce0.6Sn2.4Ti2 catalysts [378].
Yang et al. [379] reported that the emergence of Ce3+–O–Ce3+ structural units induced by Mo doping in Mo-doped CeO2 catalysts led to much better NH3-SCR performance by achieving low-energy barrier activation of NH3 molecules, thereby changing the dominant reaction mechanism in the catalytic reaction. They also observed the same trend in (W)-doped CeO2 catalysts, further confirming the pivotal role of Ce3+–O–Ce3+ structural units [379]. The addition of MoO3 improved the activity of the CeO2/TiO2 catalyst for NH3-SCR irrespective of the presence of H2O and SO2 because the introduction of Mo to the CeO2/TiO2 catalyst can suppress the adsorption of H2O and SO2 and the formation of surface sulfate species [365]. The MoO3-promoted CeO2/TiO2 exhibited higher activity than CeO2/TiO2 even in the co-presence of H2O and SO2, showing relatively stable NO conversion in the presence of 5% H2O and 50 ppm SO2 at 300 °C [365]. Liu et al. [380] reported the enhanced NH3-SCR performance of MoO3/CeO2 catalyst by introducing Cu to induce the generation of active Mo=O structure because the adding of Cu into MoO3/CeO2 catalyst could create unsaturated sites on CeO2 for Mo anchor, and the enhanced electron transfer from Mo to Cu would cause the formation of a new terminal Mo=O with highly distorted octahedral geometry, which is a new Lewis acid site for coordinated NH3 production. Meanwhile, the added Cu creates the adsorbed site for gaseous NO, and the formed Mo-O-Cu pair center facilitates the transformation of ionic NO2– generated from NO adsorption to NO2, which is conducive to the ‘Fast NH3-SCR reaction’ process [380]. Mo doping to CeO2-SiO2 mixed-oxide NH3-SCR catalyst exhibited high low-temperature NH3-SCR activity and superior N2 selectivity and resistance to SO2/H2O poisoning [366]. The Mo-doped CeO2-SiO2 catalyst showed a rather stable NO conversion even in the presence of 5% H2O and 200 ppm SO2 at 250 °C [366]. Two elements (Mo and Cu) could be selected for doping into CeO2 to promote the NH3 adsorption of CeO2-based materials by means of DFT calculations [381].
A comparison of NH3-SCR activity among CeOx supported on WO3 nanorods (WO3-NR) and WO3 microspheres self-assembled by nanorods (WO3-MP), and WO3 nanoparticles (WO3-NP) revealed that WO3-NP enclosed with (0 0 1) facets of the hexagonal WO3 showed the best redox ability due to the largest molar ratio of W5+/(W5+ + W6+), the highest concentration of Ce4+ on the surface of Ce/WO3-NP, the strongest surface redox properties, and the largest molar ratio of Oα/(Oα + Oβ), which was beneficial for NH3-SCR activity in the temperature range of 250–400 °C [382]. Hao et al. [103] designed a novel WOx/Cu-CeO2 oxide catalyst in which the WOx species are highly dispersed on the {111}/{100}-terminated surface of Cu-doped CeO2 nanospheres, which exhibits excellent NH3-SCR performance over a wide operating temperature window (200–400 °C), as well as good sulfur resistance and N2 selectivity. The stable NO conversion was obtained in the presence of 5% H2O and 100 ppm SO2 at 250 °C [103]. They found that the Cu introduction into the lattice of CeO2 not only increased the surface Ce3+ and oxygen vacancy concentration but also provided more sites for capture and dispersion of WOx species, thus mediating and improving the Lewis and Brønsted acid sites and reducibility of catalyst [103]. They also found that electronic interaction between WOx and Cu-doped CeO2 was enhanced due to the existence of interactions of short-range Ce-O-Cu and Ce-O-W [103]. Fang et al. [383] designed a Ce1–W1/TiO2 model catalyst by anchoring Ce1–W1 atom pairs on anatase TiO2(001) to investigate the synergy between Ce and W in NH3-SCR and found that the Ce1–W1 synergy not only shifted down the lowest unoccupied states of Ce1 near the Fermi level, thus enhancing the abilities in adsorbing and oxidizing NH3 but also makes the frontier orbital electrons of W1 delocalized, thus accelerating the activation of O2.
The promotional effect of Nb doping to CeO2/TiO2 in terms of the resistance to high GHSV, H2O, and SO2 was ascribed to the better dispersion of Ce, more chemisorbed oxygen species, and Ce3+/Ce4+ redox pairs on the catalyst surface [361]. The Ce20Nb20Ti catalyst was slowly deactivated in the presence of 10% H2O and 200 ppm SO2 at 300 °C [361]. Zhu et al. [360] prepared a series of porous CexNb1-x (x = 0, 0.2, 0.4, 0.6, 1) hollow nanospheres by a modified seed-mediated growth approach and found that a strong interaction between Nb2O5 and CeO2 affected the oxygen defects, valence, reducibility, and the number of acid sites. The porous Ce0.4Nb0.6 nanospheres with more acid sites and excellent reducibility exhibited superior catalytic activity in the temperature range of 250–450 °C and high catalytic stability for H2O and SO2, showing reversible deactivation in the presence of 200 ppm SO2 at 300 °C, which can be attributed to the porous double-shell structure and the strong interaction of Nb2O5 and CeO2 species [360]. Jiang et al. [362] synthesized Ce-Nb-Ti metal oxide catalysts (CeNbTi) and found that CeNbTi prepared with the impregnation method possessed superior NH3-SCR performance in the temperature range of 250–450 °C and better resistance to SO2 and potassium, showing irreversible deactivation in the presence of 500 ppm SO2. The best catalyst had relatively high concentrations of Ce3+ and chemisorbed oxygen, a strong synergistic effect among different components, and well-dispersed active species [362]. More importantly, the catalyst had stronger reduction capacity and a large number of Lewis acid sites, which contributed to its excellent catalytic performance [362]. Zhang et al. [359] synthesized CeO2, Ce–Nb–Ox and Nb2O5 catalysts by the citric acid method and found that the mixed oxide Ce–Nb–Ox presented a higher NH3-SCR activity than the single oxide CeO2 or Nb2O5 catalyst. In addition, the Ce–Nb–Ox catalyst showed high resistance towards H2O and SO2 at 280 °C [359]. The incorporation of Nb provides abundant oxygen vacancies for capturing more surface adsorbed oxygen, which provides a superior redox capability and accelerates the renewal of active sites. Niobium pentoxide shows high surface acidity, which is partly retained in the Ce–Nb–Ox catalyst possessing a high content of Lewis and Brønsted acid sites [359]. Ding et al. [363] synthesized a set of catalysts for the Nb/CeSi2 with different loadings of niobium and found that a catalyst for 20Nb/CeSi2 exhibited the best low-temperature NH3-SCR performance while maintaining excellent SO2/H2O resistance, showing relatively stable NO conversion in the presence of 5% H2O and 200 ppm SO2 at 250 °C.
Since the redox properties of ceria can operate at medium and high temperatures, in order to achieve low-temperature NH3-SCR activity, catalysts containing ceria should include Cu, Fe, and Mn together in the catalyst composition. Among them, Mn is the most promising metal component for this purpose according to the literature.
2.5. Other Catalysts
Generally, the surface concentration of vanadium is an important factor to determine the surface vanadium species such as monomeric vanadyl without V–O–V bonds, oligomeric vanadyl with V–O–V bridge, and crystallized V2O5 nanoparticles, which can be found at a low surface concentration (<2 V atoms/nm2), moderate surface concentration (2–8 V atoms/nm2), and high surface density (>8 V atoms/nm2), respectively [7]. The polymeric vanadyl species have been reported to have a higher NH3-SCR activity than the monomeric vanadyl species because the coupling effect of the polymeric structure shortens the reaction pathway for the regeneration of redox sites [38]. Inomata et al. [384] reported that defective bulk vanadium oxide (V(V) + V(IV)) catalysts, synthesized by the calcination of vanadium(IV)-oxalate at 270 °C, showed NH3-SCR activity at a low temperature below 150 °C. The transformation of crystalline V2O5 on low-vanadium-loading catalysts into an active polymeric vanadyl species under NH3-SCR reaction conditions was reported to explain a remarkably enhanced catalytic performance of the vanadia-based catalyst at low temperatures [385]. Highly active supported oligomeric surface vanadia sites, which can provide exclusive centers for adsorbed bridging and bidentate nitrates and assist in NH3 activation to generate amide intermediates, could be fabricated by transformation from monomeric surface VOx sites from a classic supported V2O5–WO3/TiO2 catalyst via a H2 plasma treatment [386]. Lin et al. [387] prepared vanadia supported on defective TiO2 nanosheets, which demonstrated excellent NH3-SCR performance over a wide temperature range of 140–380 °C. This catalyst was also resistant to H2O and SO2 poisoning, maintaining an NO conversion at 180 °C [387]. Hwang et al. [388] mechanochemically localized vanadia on the surface of WO3-TiO2 by physically grinding high-vanadia-loading V2O5/WO3-TiO2 with WO3-TiO2. They found that clustered vanadia sites exhibited enhanced activity for low-temperature (<250 °C) NH3-SCR [388]. This catalyst also exhibited superior sulfur resistance at 220 °C because the exposed TiO2 sites absorbed ABS from the clustered vanadia sites, preventing the blockage of the catalytic active sites [388].
A comparison of NH3-SCR activity among V2O5, Na0.33V2O5, and Na1.2V3O8 synthesized by the oxalate method revealed that the reaction rate of Na0.33V2O5 was 6.1-times higher than that of V2O5, indicating that Na intercalation accelerates NH3-SCR cycles [389]. Bulk W-substituted vanadium oxide catalysts with the high redox ability and reactivity of Brønsted acid sites were reported to be active for NH3-SCR at a low temperature (100–150 °C) and in the presence of water (~20 %). Lewis acid sites of W-substituted vanadium oxide are converted to Brønsted acid sites in the presence of water vapor, and NH4+ species adsorbed on Brønsted acid sites react with NO with the reduction of V5+ sites at 150 °C [390]. Zr-doped CeVO4 solid solution catalysts showed excellent NH3-SCR performance from 150 to 375 °C because of the increased Brønsted/Lewis acid sites and facilitated electron transfer among Ce, Zr, and V [391]. The Ce0.85Zr0.15VO4 catalyst was slowly deactivated at 190 °C in the presence of 8% H2O and 200 ppm SO2 [391]. Furthermore, TiO2 nanosheets promoted the activity and H2O/SO2 tolerance compared with TiO2 nanoparticles for Zr–CeVO4/TiO2 catalysts [392]. However, TiO2 nanosheets were slowly deactivated at 225 °C in the presence of 8% H2O and 200 ppm SO2 [392]. FeVO4/TiO2 was reported to show stable NO conversion in the presence of 200 ppm SO2 at 190 °C and reversible deactivation due to 5% H2O at 240 °C [393]. Kim et al. [394] prepared various Ce0.5RM0.5V1O4 catalysts in which half of Ce in TiO2-supported Ce1V1O4 was replaced by RM (Tb, Er, or Yb) and observed the promotive effect anticipated by RM substitution for Ce0.5RM0.5V1O4 only at high temperatures. Ce0.5Er0.5V1O4 possessed the greatest Lewis acidity/redox feature, thus revealing the best performance at elevated temperatures [394].
Liu et al. [395] prepared a serial of WaCo0.4TiOx catalysts (a = 0.04, 0.06, 0.08, 0.10) by the sol–gel method, and the W0.08Co0.4TiOx catalyst achieved the best NH3-SCR activity at 190–430 °C and good resistance to H2O/SO2. However, the W0.08Co0.4TiOx catalyst was slowly deactivated at 280 °C in the presence of 100 ppm SO2 [395]. The doping of W enhanced the acidity and weakened the oxidation capacity of WaCo0.4TiOx, but the high surface acidity, especially the large number of Brønsted acid sites, can compensate for the effect of the reduced oxidation capacity on the low-temperature activity [395].
Phosphates are used in a variety of catalytic reactions due to their excellent thermal stability, proton conductivity, ion exchange, and acidity, and in P-doped CeO2/TiO2 catalysts, the amorphous CePO4 species has been demonstrated to promote NH3-SCR activity by activating the NH4+ species at the Brønsted acid site of the phosphate radical while generating NH2 species that react with gaseous NO via the E–R mechanism [12]. The phosphorus is the acid site for NH3 adsorption, and CeO2 is the redox site. The NH3-SCR proceeded according to the L–H mechanism, and the excellent redox properties promoted the ‘Fast NH3-SCR’ reaction after the introduction of 3% H2O + 100 ppm SO2 at 240 °C [396]. The Ce-O-P catalysts prepared with a sol–gel method exhibited enhanced NH3-SCR activity due to increased surface acidity and redox capacity and improved SO2 tolerance in the presence of 5% H2O and 100 ppm SO2 at 250 °C due to the effect of SO2 capture by abundant reactive oxygen species [397]. Zhao et al. [398] synthesized a series of cerium phosphate catalysts with different crystal phases by hydrothermal method and co-precipitation method. Hexagonal cerium phosphate (CePO4-H) showed better NH3-SCR activity in the temperature range of 300–500 °C than monoclinic cerium phosphate (CePO4-M) and mixed phases of CePO4-H and CePO4-M because CePO4-H had much stronger surface acidity and more surface adsorbed oxygen species [398].
Fe- and Cu-based catalysts have been demonstrated to have enhanced high-temperature NH3-SCR activity in the presence of SO2 due to the increased acidity derived from the sulfate formed. However, supported sulfate catalysts have the disadvantage of exhibiting low-temperature NH3-SCR activity due to their poor redox properties. Therefore, it is necessary to enhance the redox ability of the catalyst to improve its low-temperature NH3-SCR activity. The active ingredient, sulfate, can prevent the formation of metal sulfates, but the deposition of AS/ABS with decreasing temperature is the main cause of catalyst deactivation. Therefore, future research on sulfate-based catalysts should focus on improving their redox capacity while promoting the degradation of AS/ABS [399]. NOx reduction conversions and N2 selectivity of sulfated CeO2 cubes prepared by the impregnation of CeO2 cubes by ammonium sulfates could be significantly improved compared with pure CeO2 cubes. Sulfation treatment could create and strengthen Brønsted acid sites originated from the protons on surface sulfates, further facilitating ammonia adsorption and activation [400]. Yu et al. [401] prepared CuSO4/TiO2, Fe2(SO4)3/TiO2, MnSO4/TiO2, Ce(SO4)2/TiO2, and CoSO4/TiO2 catalysts via a sol–gel protocol. The presence of SO2 had little influence on the activity of all metal sulfate catalyst samples [401]. CuSO4/TiO2 showed the highest NH3-SCR activity and apparent activation energy among the metal sulfate catalysts following the sequence of CuSO4/TiO2 < Fe2(SO4)3/TiO2 < MnSO4/TiO2 < Ce(SO4)2/TiO2 < CoSO4/TiO2 [401]. The amount of acid sites was the main factor that influenced the catalytic activity of the metal sulfate catalysts [401].
3. Strategies to Improve the Low-Temperature NH3-SCR Activity
The reaction mechanism for NH3-SCR indicates that both surface acid site and redox property are required to complete the catalytic cycle. Two representative reaction mechanisms have been proposed for this reaction: The L–H and E–R mechanisms, which predominate at low and medium/high temperatures, respectively. In any case, adsorption of NH3 on the surface acid sites followed by oxidative dehydrogenation is an important step in this reaction. Therefore, the NH3-SCR catalyst surfaces must have specific acid sites that are required for the adsorption of NH3 onto the catalyst surface. Lewis and Brønsted acidic sites enable the adsorption of NH3 and conversion of NH3 to NH4+, respectively, which is a prerequisite for the generation of the -NH2 active species, and its subsequent interaction with NO2 and NO species is an essential component of this reaction. The key to broadening the active temperature window of NH3-SCR catalysts, including the low-temperature region, is to activate the N-H bond in the adsorbed NH3. The oxidative activation of adsorbed NH3 is very important. Dehydrogenation of NH3 produces the active NH2 species, and -NH2 can react with gaseous NO or adsorbed NO/NO2/nitrate, nitrite, and other species to form N2. The oxidation capability of the catalyst surface determines the efficiency of NH3 dehydrogenation; therefore, the surface redox sites of the catalyst play an important role. At low temperatures, the adsorption of NH3 is not a significant obstacle; rather, the oxidation capacity of the catalyst itself becomes particularly important at low temperatures. However, it is important to note that the surface oxidation of the catalyst should not be too strong, as too much surface oxidation will result in the over-oxidation of NH3 to NH or -N, resulting in the consumption of NH3 and the production of significant amounts of N2O (Figure 7). The over-oxidation of NO2 to NO3– also adversely affects NH3-SCR activity and N2 selectivity, since the formation of N2O is usually due to the reaction of adsorbed -NH with gas-phase NO or surface-bound NH4+ with NO3– (Figure 7). Therefore, the oxidizing power of the catalyst should be ‘just right,’ meaning that it should have enough oxidizing power to remove the first H from NH3, but not over-oxidize NH3 to -NH.
Figure 7.
The schematic illustration of the plausible surface reactions during NH3-SCR over multicomponent mixed metal oxide catalysts with multiple redox circles to avoid over-oxidation of NH3 [12,166].
Many researchers have observed that low-temperature NH3-SCR reactions occur primarily through the L–H mechanism. NO2, nitrite, and nitrate species are important intermediates in the L–H mechanism, and the activation of NO to NO2 (gas phase or adsorption) is very important. The importance of NO2 generation by NO oxidation can also be seen from the fact that NO2 contributes to the occurrence of the ‘Fast NH3-SCR’ pathway. It is widely recognized that when a certain amount of NO2 coexists with NO, the reaction rate increases significantly, resulting in ‘Fast NH3-SCR’, which significantly improves NH3-SCR performance. Koebel et al. [402,403] first discovered that partial conversion of NO to NO2 with the help of an oxidizing catalyst greatly improved the catalytic performance of the NH3-SCR, especially at low temperatures. The optimal NO2:NO ratio is 1:1, which allows all NOx to react through a ‘Fast NH3-SCR’ reaction. The evolution of NO2 into nitrate and nitrite after adsorption is also important. Nitrite/nitrate bonding can occur to form monodentate nitrate, linear nitrite, bridging nitrate, and bidentate nitrate. These nitrates/nitrites have specific reactivity at certain temperatures; the level of reactivity depends on the catalyst system. In general, low-stability nitrates/nitrites react with NH4+ at low temperatures and contribute to enhanced low-temperature activity, whereas high-stability nitrates/nitrites are detrimental to low-temperature activity because they are too stable to react with NHx species and thus poison the active site.
Some promising strategies to improve the acidity and redox properties of metal oxide catalysts have been proposed, such as modification or doping of transition and rare earth metals, optimization of preparation methods, generation of new nanostructures, morphology modification, exposure of specific crystal faces, etc. [12]. As shown in the previous sections, MnOx-based catalysts exhibit very good low-temperature NH3-SCR activity but always produce a large amount of by-product N2O. In order to regulate the redox property and surface acidity of MnOx catalysts, multicomponent Mn-based composite oxides have been reported. They generally contain VOx, Fe2O3, or CuO as the main active metal oxides and Al2O3, SiO2, TiO2, CrOx, CoOx, NiO, Y2O3, ZrO2, NbOx, MoOx, AgOx, SnO2, SbOx, LaOx, CeOx, PrOx, NdOx, SmOx, EuOx, GdOx, DyOx, HoOx, ErOx, TmOx, TaOx, and WO3 as promoters. The creation of a multi redox cycle over a multicomponent oxide catalyst effectively promotes electron transfer and facilitates the adsorption/activation of NO/NH3 (Figure 7).
Highly dispersed metal oxide composites can be prepared by utilizing MOF [375,404,405,406] or LDH [216,407,408,409] as precursors and constructing solid solution catalysts. Designing specific nanostructures such as nanoneedles, nanospheres, nanotubes, or core-shell structures can increase the amount of acid sites/reactive oxygen species and promote the formation of active species. Selective exposure of certain facets of the active ingredient, such as MnOx and Fe2O3, as well as TiO2 and CeO2 support, can improve redox/acidic properties and metal-support interactions. Additionally, acidification or functionalization of the support with specific acids or oxygen/nitrogen-containing groups can significantly improve acidity and the interaction between the active ingredient and support.
4. Strategies to Improve the H2O/SO2 Tolerance at Low Temperatures
Since water vapor is an unavoidable part of real flue gas, its effect on NH3-SCR activity has been an important issue. Positive or negative effects have been reported depending on the reaction conditions (e.g., reaction temperature), catalyst, and reducing agent [410,411]. Although the negative effect of water vapor on NH3-SCR activity over low-temperature catalysts has been frequently reported due to its competitive adsorption of NH3 and/or NO onto the active sites, regardless of the L–H and E–R mechanisms, irreversible deactivation was seldom reported and reversible deactivation was commonly observed, where initial catalytic activity could be recovered after stopping the introduction of water vapor into the feed [412]. Controlling the surface hydrophobicity of the catalyst by incorporating hydrophobic metal oxide shells into the active site [293] or using hydrophobic supports [33,34] can be effective in minimizing the adverse effects of water at low temperatures.
In contrast to the effect of water vapor, SO2 has been reported to have a negative effect on NH3-SCR catalytic activity and is known to cause irreversible deactivation, especially for low-temperature catalysts [19,31,412,413,414,415], which limits the practical application of low-temperature catalysts.
There are several strategies to improve the SO2 tolerance of catalysts: (1) reducing the adsorption of SO2 and its subsequent oxidation; (2) improving the adsorption of NH3 and NO to form active intermediates in the presence of surface sulfate species; (3) building sacrificial sites to protect active sites; (4) promoting the decomposition of sulfate; and (5) searching for SO2-tolerant compounds with good NH3-SCR activity [12].
- (1)
- Reducing the adsorption of SO2 and its subsequent oxidation. Inhibiting the adsorption and oxidation of SO2 can significantly reduce the deposition of sulfates. Increasing the acidity of the catalyst can reduce SO2 adsorption to some extent. However, on the other hand, more NH3 can also be adsorbed on the surface acidic sites, promoting the formation of ammonium sulfate. Therefore, it is important to fine-tune the surface acidity to reduce the adsorption of SO2 and NH3 simultaneously. The introduction of SiO2, Al2O3, and TiO2 to the low-temperature NH3-SCR catalysts might be effective for this purpose. Inactive metal sulfates and ammonium sulfates (i.e., AS and ABS) are derived from the reaction of SO3 with metal oxides and NH3, respectively, so it is necessary to inhibit the oxidation of SO2 to SO3 by decreasing the oxidation ability of active sites via adding electron-donating promoters [282,310]. Since NO2, which can form under ‘Fast NH3-SCR’ conditions at low temperatures, can promote the oxidation of SO2 to SO3, designing a catalyst that can follow the ‘Fast NH3-SCR’ while inhibiting the oxidation of SO2 to SO3 is challenging but necessary to achieve high low-temperature NH3-SCR activity as well as SO2 tolerance (Figure 8).
 Figure 8. The schematic illustration of the plausible surface reactions during low-temperature NH3-SCR in the presence of SO2.
Figure 8. The schematic illustration of the plausible surface reactions during low-temperature NH3-SCR in the presence of SO2. - (2)
- Improving the adsorption of NH3 and NO to form active intermediates in the presence of surface sulfate species. When the active sites begin to be sulfated by SOx, the redox capacity of the active sites is reduced, and this leads to a decrease in NH3-SCR activity, especially at low temperatures. Active NOx species are adsorbed competitively with SOx, but the adsorption of NH4+ species is generally enhanced by new Brønsted acid sites derived from the deposited sulfate. Therefore, improving the adsorption of active nitrite/nitrate and gaseous NO2 species in the presence of sulfate species is important to achieve SO2 durability in NH3-SCR reactions that follow the L–H mechanism. Compared with un-doped catalysts, more NH4+, nitrates, and NO2 were formed on Eu-modified Mn/TiO2 [278] and Zr-modified Fe–Mn/TiO2 [187] catalysts following the L–H mechanism, leading to improved SO2 tolerance. If the catalyst follows the E–R mechanism, where the active NH3 and NH4+ species react directly with gaseous NO, this can lead to high SO2 tolerance as NO does not need to be competitively adsorbed onto the catalyst surface as nitrate or nitrite. However, the E–R mechanism is not prevalent at low temperatures.
- (3)
- Building sacrificial sites to protect active sites. A rather simple strategy in which H-Y zeolite was physically mixed with V2O5/TiO2 to trap ABS was reported to be effective to protect vanadium active sites, maintaining stable performance in the presence of 10% H2O and 30 ppm SO2 at a low temperature of 220 °C [416]. Ceria is well known to interact with SO2 to form CeSO4 or Ce2(SO4)3, which can be used as a sacrificial site to protect the main active phase from sulfation, thereby improving the SO2 tolerance of the catalyst [417]. Cr [173] and Nb [188] were also reported to react with SO2 preferentially to prevent the sulfuration of active metal oxides (Figure 8). Designing a core–shell structure with outer sacrificial sites for SOx adsorption and ammonium sulfate deposition and inner active sites for NH3-SCR can effectively protect active sites from sulfate deposition [17,22,23,216,418,419].
- (4)
- Promoting the decomposition of sulfates. Ammonium sulfate and metal sulfate are two common sulfates observed during NH3-SCR in the presence of SO2. The former can be deposited on the catalyst and block surface active sites, but can be removed by water washing and heat treatment. However, the thermally stable metal sulfates formed through sulfation of active sites irreversibly deactivate the catalysts. Compared with metal sulfates, ammonium sulfates (AS and ABS) can decompose at lower temperatures. Therefore, reducing the decomposition temperature of ammonium sulfates can improve the SO2 tolerance at lower temperatures. It was found that ABS decomposed more easily on SBA-15 with larger pores [330]. Therefore, it can be said that the decomposition of ammonia sulfate can be facilitated via constructing some mesoporous structure. Since metal sulfates are very difficult to decompose at low temperatures, it is desirable to interfere with their formation by adding suitable electrophilic or nucleophilic additives that can interact strongly with SO42– and metal cations [12]. Chen et al. [420] proposed to place a single Mo atom on TiO2 to form a Mo-Ti acid-base double site on Mo and Ti that can adsorb NH4+ and HSO4− from ABS, respectively. When NH4+ is oxidized by surface lattice oxygen on the Mo site, the electrons left on the dual site will transfer to the adsorbed HSO4− on the Ti site, releasing SO2 at low temperatures. Fe doping [310] and Sb addition [195] were also reported to promote the decomposition of ammonium sulfate and inhibit the formation of MnSO4.
- (5)
- Searching for SO2-resistant compounds. Some metal sulfates, including CuSO4 and FeSO4, are known to have improved SO2 tolerance, but due to their poor redox properties, they can exhibit NH3-SCR activity only at moderate and/or high temperatures. Therefore, it is necessary to improve the redox properties of the catalyst to enhance its low-temperature activity. Nevertheless, the removal of deposited ammonia sulfate is not easy at low temperatures, and the resulting catalyst deactivation is inevitable. Therefore, future research should focus on preventing the deposition of ammonia sulfate at low temperatures or promoting its decomposition even if it is deposited while enhancing its redox ability.
The strategies mentioned above may seem different, but they are closely related. Considering all the above strategies, a promising low-temperature NH3-SCR catalyst could have the following properties:
- (1)
- The active site must be composed of a multi-component metal oxide with moderate oxidation capacity not to oxidize SO2 to SO3, but still retain the oxidation capacity to oxidize NO to NO2 and follow the ‘Fast NH3-SCR’ pathway. Different catalyst compositions can be screened by DFT calculations that allow for the comparison of competing oxidation reactions, including SO2 oxidation and NO oxidation, on the proposed catalyst surface [421,422,423]. The other type of active site is the promoted metal sulfate, which has both a high oxidation capacity to activate adsorbed NO species to follow the L–H reaction mechanism and the ability to decompose ammonium sulfate at low temperatures.
- (2)
- The active site mentioned above should be protected from the adsorption of SOx and/or the ammonium sulfate produced by introducing some kind of sacrificial and/or protective component on or near the surface of the active site. The surface acidity, pore structure, and surface composition of the protective layer must be fine-tuned to take into account the adsorption property of SOx and the decomposition of ammonium sulfate.
5. Summary
The redox properties and surface acidity are important factors affecting the catalytic activity of NH3-SCR. Ammonia is adsorbed on the Lewis and Brønsted acid sites in the form of NH3 and NH4+, respectively, and is oxidatively dehydrogenated to form active intermediates. NO can react directly with adsorbed intermediates derived from NH3 in the E–R mechanism, or in the L–H mechanism prevalent at low temperatures, NO can be adsorbed as is or oxidized to NO2 and then adsorbed and react with adsorbed ammonia or ammonia derivatives. Moderate redox properties are very important for low-temperature NH3-SCR catalysts, as strong redox properties can reduce N2 selectivity due to the formation of unwanted N2O, and weak redox properties can reduce the overall denitrification rate.
Water vapor and SOx are often found in flue gas along with NOx, and their effect on NH3-SCR activity is particularly important at low temperatures. Water vapor in flue gas can cause reversible deactivation because it adsorbs competitively with NOx and ammonia for active sites. SO2 in flue gas can adsorb strongly to the active site or oxidize to SO3 and further react with ammonia in the presence of water vapor to form ammonium bisulfate and/or ammonium sulfate, which irreversibly blocks the active site. SO3 can react directly with active metal oxides to form metal sulfates with weak redox properties, resulting in low NH3-SCR activity at low temperatures.
Among Cu-, Fe-, Mn-, and Ce-based catalysts, Mn-containing multivalent metal oxides can be promising candidates for low-temperature NH3-SCR. The redox properties of MnOx can be tuned by the incorporation of other multivalent metal oxides. The electronic properties of the active metal can be fine-tuned by adding appropriate promoters. The reduction of NH3-SCR activity by water vapor can be overcome by increasing the surface hydrophobicity, which can be accomplished by using a hydrophobic support or by applying a hydrophobic sheath to the outside of the active metal oxide. When selecting the active metal oxide, DFT calculations should be performed to compare SO2 adsorption, NO oxidation, and SO2 oxidation to ensure that the oxidation of NO occurs without the adsorption of SO2 and the oxidation of SO2, allowing ‘Fast NH3-SCR’ to proceed. The mesoporous shell will not only help to remove ammonium sulfate at the reaction temperature but also prevent SO2 poisoning. Metal sulfate catalysts with strong redox properties and V-based catalysts containing oligomeric vanadyl species with sulfur-resistant properties still need to be further investigated for application in NH3-SCR at low temperatures.
Funding
This research was supported by the Ministry of Trade, Industry and Energy (MOTIE), and the Korea Evaluation Institute of Industrial Technology (KEIT) (No. 20026357).
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
E.D.P. would like to thank Seohyeon Park, Eunyeong Yang, Jung Hun Maeng, and Bohyeon Hwang for their editorial assistance.
Conflicts of Interest
The author declare no conflicts of interest.
References
- Asghar, U.; Rafiq, S.; Anwar, A.; Iqbal, T.; Ahmed, A.; Jamil, F.; Khurram, M.S.; Albar, M.M.; Farooq, A.; Shah, N.S.; et al. Review on the progress in emission control technologies for the abatement of CO2, SOx and NOx from fuel combustion. J. Environ. Chem. Eng. 2021, 9, 106064. [Google Scholar] [CrossRef]
- Zhu, X.; Du, J.; Yu, Z.; Cheng, Y.B.; Wang, Y. NOx Emission and control in ammonia combustion: State-of-the-art review and future perspectives. Energy Fuels 2023, 38, 43–60. [Google Scholar] [CrossRef]
- Yao, N.; Pan, W.; Zhang, J.; Wei, L. The advancement on carbon-free ammonia fuels for gas turbine: A review. Energy Convers Manag. 2024, 315, 118745. [Google Scholar] [CrossRef]
- Park, Y.K.; Kim, B.S. Catalytic removal of nitrogen oxides (NO, NO2, N2O) from ammonia-fueled combustion exhaust: A review of applicable technologies. Chem. Eng. J. 2023, 461, 141958. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Chen, W. Denitrification technology and the catalysts: A review and recent advances. ChemCatChem 2024, 16, e202301662. [Google Scholar] [CrossRef]
- Elkaee, S.; Phule, A.D.; Yang, J.H. Advancements in selective catalytic reduction (SCR) technologies for NOx reduction: A comprehensive review of reducing agents. Process Saf. Environ. Prot. 2024, 184, 854–880. [Google Scholar] [CrossRef]
- Lai, J.K.; Wachs, I.E. A perspective on the selective catalytic reduction (SCR) of NO with NH3 by supported V2O5–WO3/TiO2 catalysts. ACS Catal. 2018, 8, 6537–6551. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Niu, X.; Zhu, Y. Vanadium-based catalysts for selective catalytic reduction of NOx with ammonia: Synthesis, poisoning mechanism, regeneration methods and research prospects. Fuel 2024, 365, 131184. [Google Scholar] [CrossRef]
- Ye, B.; Jeong, B.; Lee, M.J.; Kim, T.H.; Park, S.S.; Jung, J.; Lee, S.; Kim, H.D. Recent trends in vanadium-based SCR catalysts for NOx reduction in industrial applications: Stationary sources. Nano Converg. 2022, 9, 51. [Google Scholar] [CrossRef]
- Schreifels, J.J.; Wang, S.; Hao, J. Design and operational considerations for selective catalytic reduction technologies at coal-fired boilers. Front. Energy 2012, 6, 98–105. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, L.; Liu, Z.; Wang, B.; Han, Y. Progress of selective catalytic reduction denitrification catalysts at wide temperature in carbon neutralization. Front. Chem. 2022, 10, 946133. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zou, R.; Wang, X. Toward an atomic-level understanding of the catalytic mechanism of selective catalytic reduction of NOx with NH3. ACS Catal. 2022, 12, 14347–14375. [Google Scholar] [CrossRef]
- Chen, J.; Guan, B.; Liu, Z.; Wu, X.; Guo, J.; Zheng, C.; Zhou, J.; Su, T.; Han, P.; Yang, C.; et al. Review on advances in structure–activity relationship, reaction & deactivation mechanism and rational improving design of selective catalytic reduction deNOx catalysts: Challenges and opportunities. Fuel 2023, 343, 127924. [Google Scholar] [CrossRef]
- Shi, Z.; Peng, Q.; Jiaqiang, E.; Xie, B.; Wei, J.; Yin, R.; Fu, G. Mechanism, performance and modification methods for NH3-SCR catalysts: A review. Fuel 2023, 331, 125885. [Google Scholar] [CrossRef]
- Raja, S.A.M.S.; Alphin, M.S.; Sivachandiran, L. Promotional effects of modified TiO2-and carbon-supported V2O5-and MnOx-based catalysts for the selective catalytic reduction of NOx: A review. Catal. Sci. Technol. 2020, 10, 7795–7813. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, Z.; Qiao, Y.; Zhou, S.; Chen, G.; Guo, R.; Pan, W.; Wu, J.; Li, F.; Ren, J. The impact of catalyst structure and morphology on the catalytic performance in NH3-SCR reaction: A review. Fuel 2024, 361, 130541. [Google Scholar] [CrossRef]
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A review of low temperature NH3-SCR for removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef]
- Wang, H.; Huang, B.; Yu, C.; Lu, M.; Huang, H.; Zhou, Y. Research progress, challenges and perspectives on the sulfur and water resistance of catalysts for low temperature selective catalytic reduction of NOx by NH3. Appl. Catal. A Gen. 2019, 588, 117207. [Google Scholar] [CrossRef]
- Zhu, H.; Song, L.; Li, K.; Wu, R.; Qiu, W.; He, H. Low-temperature SCR catalyst development and industrial applications in China. Catalysts 2022, 12, 341. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Zhang, Z.; Liu, Q. The latest research progress of NH3-SCR in the SO2 resistance of the catalyst in low temperatures for selective catalytic reduction of NOx. Catalysts 2020, 10, 1034. [Google Scholar] [CrossRef]
- Wu, T.; Guo, R.T.; Li, C.F.; You, Y.H.; Pan, W.G. Recent advances in core-shell structured catalysts for low-temperature NH3-SCR of NOx. Chemosphere 2023, 333, 138942. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Schill, L.; Fehrmann, R.; Riisager, A. Recent developments of core–shell structured catalysts for the selective catalytic reduction of NOx with ammonia. Inorg. Chem. Front. 2023, 10, 727–755. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, H.; Li, Y.; Wang, P.; Zhan, S. Selective catalytic reduction of NOx with NH3 over copper-based catalysts: Recent advances and future prospects. EES Catal. 2024, 2, 231–252. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, N.; Huang, Z.; Zhao, G.; Chu, F.; Yang, R.; Tang, X.; Wang, G.; Gao, F.; Huang, X. Recent advances in low-temperature NH3-SCR of NOx over Ce-based catalysts: Performance optimizations, reaction mechanisms and anti-poisoning countermeasures. Chem. Eng. J. 2023, 476, 146889. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, R.T.; Zhang, X.F.; Liu, Y.Z.; Duan, C.P.; Wu, G.L.; Pan, W.G. Cerium oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Energy Fuels 2021, 35, 2981–2998. [Google Scholar] [CrossRef]
- Yao, X.J.; Gong, Y.T.; Li, H.L.; Yang, F.M. Research progress of ceria-based catalysts in the selective catalytic reduction of NOx by NH3. Acta Phys. -Chim. Sin. 2015, 31, 817–828. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Zou, X.; Tang, T.; Zhang, Q.; Guo, F.; Liu, H. Recent advances and perspectives in the resistance of SO2 and H2O of cerium-based catalysts for NOx selective catalytic reduction with ammonia. New J. Chem. 2022, 46, 2053–2067. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Tian, J.; Zhong, Y.; Zou, Z.; Dong, R.; Gao, S.; Xu, W.; Tan, D. The effects of Mn-based catalysts on the selective catalytic reduction of NOx with NH3 at low temperature: A review. Fuel Process. Technol. 2022, 230, 107213. [Google Scholar] [CrossRef]
- Xu, G.; Guo, X.; Cheng, X.; Yu, J.; Fang, B. A review of Mn-based catalysts for low-temperature NH3-SCR: NO x removal and H2O/SO2 resistance. Nanoscale 2021, 13, 7052–7080. [Google Scholar] [CrossRef]
- Wei, L.G.; Guo, R.T.; Zhou, J.; Qin, B.; Chen, X.; Bi, Z.X.; Pan, W.G. Chemical deactivation and resistance of Mn-based SCR catalysts for NOx removal from stationary sources. Fuel 2022, 316, 123438. [Google Scholar] [CrossRef]
- Ogugua, P.C.; Wang, E.; Jinyang, Z.; Wang, Q.; Su, H. Advancements in low-temperature NH3-SCR of NOx using Ba-based catalysts: A critical review of preparation, mechanisms, and challenges. Environ. Sci. Pollut. Res. 2023, 30, 84972–84998. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, M.; Ren, S.; Li, X.; Chen, Z.; Wang, M.; Chen, T.; Yang, J. Recent advance for NOx removal with carbonaceous material for low-temperature NH3-SCR reaction. Catal. Today 2023, 418, 114053. [Google Scholar] [CrossRef]
- Huang, G.; Yang, J.; Lv, C.; Li, D.; Fang, D. Research progress of NH3-SCR over carbon-based catalysts for NOx removal. J. Environ. Chem. Eng. 2023, 11, 110966. [Google Scholar] [CrossRef]
- Guo, K.; Ji, J.; Song, W.; Sun, J.; Tang, C.; Dong, L. Conquering ammonium bisulfate poison over low-temperature NH3-SCR catalysts: A critical review. Appl. Catal. B 2021, 297, 120388. [Google Scholar] [CrossRef]
- Arnarson, L.; Falsig, H.; Rasmussen, S.B.; Lauritsen, J.V.; Moses, P.G. A complete reaction mechanism for standard and fast selective catalytic reduction of nitrogen oxides on low coverage VOx/TiO2 (0 0 1) catalysts. J. Catal. 2017, 346, 188–197. [Google Scholar] [CrossRef]
- Topsoe, N.Y.; Dumesic, J.A.; Topsoe, H. Vanadia-titania catalysts for selective catalytic reduction of nitric-oxide by ammonia: II Studies of active sites and formulation of catalytic cycles. J. Catal. 1995, 151, 241–252. [Google Scholar] [CrossRef]
- He, G.; Lian, Z.; Yu, Y.; Yang, Y.; Liu, K.; Shi, X.; Yan, Z.; Shan, W.; He, H. Polymeric vanadyl species determine the low-temperature activity of V-based catalysts for the SCR of NOx with NH3. Sci. Adv. 2018, 4, eaau4637. [Google Scholar] [CrossRef]
- Chen, X.; Yu, S.; Liu, W.; Zhang, S.; Liu, S.; Feng, Y.; Zhang, X. Recent advance on cobalt-based oxide catalyst for the catalytic removal of volatile organic compounds: A review. Resour. Chem. Mater. 2022, 1, 27–46. [Google Scholar] [CrossRef]
- Jo, D.; Ryu, T.; Park, G.T.; Kim, P.S.; Kim, C.H.; Nam, I.S.; Hong, S.B. Synthesis of high-silica LTA and UFI zeolites and NH3–SCR performance of their copper-exchanged form. ACS Catal. 2016, 6, 2443–2447. [Google Scholar] [CrossRef]
- Ryu, T.; Kim, H.; Hong, S.B. Nature of active sites in Cu-LTA NH3-SCR catalysts: A comparative study with Cu-SSZ-13. Appl. Catal. B 2019, 245, 513–521. [Google Scholar] [CrossRef]
- Tarach, K.A.; Jabłońska, M.; Pyra, K.; Liebau, M.; Reiprich, B.; Gläser, R.; Góra-Marek, K. Effect of zeolite topology on NH3-SCR activity and stability of Cu-exchanged zeolites. Appl. Catal. B 2021, 284, 119752. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, J.; Liu, J.; Wang, D.; Zhao, Z.; Cheng, K.; Li, J. Enhanced hydrothermal stability of Cu-ZSM-5 catalyst via surface modification in the selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2016, 375, 186–195. [Google Scholar] [CrossRef]
- Park, J.H.; Park, H.J.; Baik, J.H.; Nam, I.S.; Shin, C.H.; Lee, J.H.; CHO, B.K.; Oh, S.H. Hydrothermal stability of CuZSM5 catalyst in reducing NO by NH3 for the urea selective catalytic reduction process. J. Catal. 2006, 240, 47–57. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Jin, Y.; Zhang, R. Zeolite structure effects on Cu active center, SCR performance and stability of Cu-zeolite catalysts. Catal. Today 2019, 327, 295–307. [Google Scholar] [CrossRef]
- Wilken, N.; Nedyalkova, R.; Kamasamudram, K.; Li, J.; Currier, N.W.; Vedaiyan, R.; Yezerets, A.; Olsson, L. Investigation of the effect of accelerated hydrothermal aging on the Cu sites in a Cu-BEA catalyst for NH3-SCR applications. Top. Catal. 2013, 56, 317–322. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, R.; Zhao, R.; Shi, C.; Gies, H.; Xiao, F.S.; Vos, D.D.; Yokoi, T.; Bao, X.; Kolb, U.; et al. Cu-exchanged Al-rich SSZ-13 zeolite from organotemplate-free synthesis as NH3-SCR catalyst: Effects of Na+ ions on the activity and hydrothermal stability. Appl. Catal. B 2017, 217, 421–428. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Ren, L.; Shi, X.; Xiao, F.S.; He, H. Excellent performance of one-pot synthesized Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx with NH3. Environ. Sci. Technol. 2014, 48, 566–572. [Google Scholar] [CrossRef]
- Fickel, D.W.; D’Addio, E.; Lauterbach, J.A.; Lobo, R.F. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Shan, Y.; Shan, W.; Shi, X.; Du, J.; Yu, Y.; He, H. A comparative study of the activity and hydrothermal stability of Al-rich Cu-SSZ-39 and Cu-SSZ-13. Appl. Catal. B 2020, 264, 118511. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Y.; Zhang, Z.; Zhang, C.; Fu, G.; Yi, X.; Huang, Q.; Yang, F.; Liang, W.; Zheng, A.; et al. Remarkable performance of selective catalytic reduction of NOx by ammonia over copper-exchanged SSZ-52 catalysts. Appl. Catal. B 2021, 283, 119641. [Google Scholar] [CrossRef]
- Cao, Y.; Fan, D.; Tian, P.; Cao, L.; Sun, T.; Xu, S.; Yang, M.; Liu, Z. The influence of low-temperature hydration methods on the stability of Cu-SAPO-34 SCR catalyst. Chem. Eng. J. 2018, 354, 85–92. [Google Scholar] [CrossRef]
- Jo, D.; Lim, J.B.; Ryu, T.; Nam, I.S.; Camblor, M.A.; Hong, S.B. Unseeded hydroxide-mediated synthesis and CO2 adsorption properties of an aluminosilicate zeolite with the RTH topology. J. Mater. Chem. A Mater. 2015, 3, 19322–19329. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Z.; Xu, L.; Ohnishi, T.; Yanaba, Y.; Ogura, M.; Wakihara, T.; Okubo, T. Understanding the high hydrothermal stability and NH3-SCR activity of the fast-synthesized ERI zeolite. J. Catal. 2020, 391, 346–356. [Google Scholar] [CrossRef]
- Wei, X.; Ke, Q.; Cheng, H.; Guo, Y.; Yuan, Z.; Zhao, S.; Sun, T.; Wang, S. Seed-assisted synthesis of Cu-(Mn)-UZM-9 zeolite as excellent NO removal and N2O inhibition catalysts in wider temperature window. Chem. Eng. J. 2020, 391, 123491. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.J.; Ryu, T.; Kim, P.S.; Kim, C.H.; Hong, S.B. Synthesis of zeolite UZM-35 and catalytic properties of copper-exchanged UZM-35 for ammonia selective catalytic reduction. Appl. Catal. B 2017, 200, 428–438. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Zhao, Z.; Liao, J.; Chen, C.; Li, Q. Recent progress of metal-exchanged zeolites for selective catalytic reduction of NOx with NH3 in diesel exhaust. Fuel 2021, 305, 121482. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, C.; Pang, L.; Ming, S.; Guo, W.; Liu, P.; Chen, H.; Li, T. One-pot synthesis of high performance Cu-SAPO-18 catalyst for NO reduction by NH3-SCR: Influence of silicon content on the catalytic properties of Cu-SAPO-18. Chem. Eng. J. 2018, 348, 608–617. [Google Scholar] [CrossRef]
- Gao, Q.; Han, S.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Effects of lanthanide doping on the catalytic activity and hydrothermal stability of Cu-SAPO-18 for the catalytic removal of NOx (NH3-SCR) from diesel engines. Catalysts 2020, 10, 336. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, Q.; Pan, X.; Bao, X. Growth of Cu/SSZ-13 on SiC for selective catalytic reduction of NO with NH3. Chin. J. Catal. 2018, 39, 71–78. [Google Scholar] [CrossRef]
- Lin, Q.; Feng, X.; Zhang, H.; Lin, C.; Liu, S.; Xu, H.; Chen, Y. Hydrothermal deactivation over CuFe/BEA for NH3-SCR. J. Ind. Eng. Chem. 2018, 65, 40–50. [Google Scholar] [CrossRef]
- Wan, J.; Chen, J.; Zhao, R.; Zhou, R. One-pot synthesis of Fe/Cu-SSZ-13 catalyst and its highly efficient performance for the selective catalytic reduction of nitrogen oxide with ammonia. J. Environ. Sci. 2021, 100, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Feng, X.; Xu, H.; Lan, L.; Gong, M.; Chen, Y. Novel promotional effect of yttrium on Cu–SAPO-34 monolith catalyst for selective catalytic reduction of NOx by NH3 (NH3-SCR). Catal. Commun. 2016, 76, 33–36. [Google Scholar] [CrossRef]
- Feng, X.; Lin, Q.; Cao, Y.; Zhang, H.; Li, Y.; Xu, H.; Lin, C.; Chen, Y. Neodymium promotion on the low-temperature hydrothermal stability of a Cu/SAPO-34 NH3-SCR monolith catalyst. J. Taiwan. Inst. Chem. Eng. 2017, 80, 805–812. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, L.; Qu, H.; Liu, L.; Xie, H.; Zhong, Q. Controllable positions of Cu2+ to enhance low-temperature SCR activity on novel Cu-Ce-La-SSZ-13 by a simple one-pot method. Chem. Commun. 2020, 56, 2360–2363. [Google Scholar] [CrossRef]
- Han, S.; Cheng, J.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Ce doping to Cu-SAPO-18: Enhanced catalytic performance for the NH3-SCR of NO in simulated diesel exhaust. Microporous Mesoporous Mater. 2019, 276, 133–146. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Z.; Zhao, N.; Teng, Y. One-pot synthesis of Cu–Ce co-doped SAPO-5/34 hybrid crystal structure catalysts for NH3-SCR reaction with SO2 resistance. J. Rare Earth 2021, 39, 1217–1223. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, C.; Lin, J.; Yang, H.; Zhou, R. Promotional effects of cerium modification of Cu-USY catalysts on the low-temperature activity of NH3-SCR. Catal. Commun. 2018, 114, 60–64. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Teng, W.; Liu, W.; Ren, S.; Yang, J.; Liu, Q. Revealing the crystal-plane effects of CuO during the NH3-SCR over CuO/TiO2 catalysts. J. Environ. Chem. Eng. 2023, 11, 110787. [Google Scholar] [CrossRef]
- Bjørkedal, O.H.; Regli, S.K.; Nuguid, R.J.G.; Vullum, P.E.; Kröcher, O.; Ferri, D.; Rønning, M. One-pot synthesis of highly dispersed mesoporous Cu/ZrO2 catalysts for NH3-SCR. Catal. Today 2022, 384, 113–121. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, S.; Cheng, H.; Li, H.; Zhu, W.; Liu, J. Unveiling the role of high-valent copper cations in the selective catalytic reduction of NOx with NH3 at low temperature. Fuel 2022, 318, 123607. [Google Scholar] [CrossRef]
- Wu, X.; Meng, H.; Du, Y.; Liu, J.; Hou, B.; Xie, X. Insight into Cu2O/CuO collaboration in the selective catalytic reduction of NO with NH3: Enhanced activity and synergistic mechanism. J. Catal. 2020, 384, 72–87. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Li, J. Design and synthesis of core-shell structured meso-Cu-SSZ-13@ mesoporous aluminosilicate catalyst for SCR of NOx with NH3: Enhancement of activity, hydrothermal stability and propene poisoning resistance. Appl. Catal. B 2016, 195, 48–58. [Google Scholar] [CrossRef]
- Ting-Ting, X.; Gang-Gang, L.; Kai-Hua, Z.; Xin-Yan, Z.; Xin, Z.; Shao-Qing, Z. Effective reduction of nitric oxide over a core–shell Cu-SAPO-34@ Fe-MOR zeolite catalyst. RSC Adv. 2023, 13, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, J.; Huang, D.; Zhao, J.; Zhang, J.; Li, K.; Li, Z.; Zhu, W.; Zhao, Z.; Liu, J. Interface engineering of a bifunctional Cu-SSZ-13@ CZO core–shell catalyst for boosting potassium ion and SO2 tolerance. ACS Catal. 2022, 12, 11281–11293. [Google Scholar] [CrossRef]
- Deng, S.; Wang, Z.; Deng, D.; Chen, Z.; He, D.; He, H.; Ji, Y.; Hou, G.; Sun, W.; Liu, L. One-pot synthesis of hollow single crystal SSZ-13 zeolite by creating aluminum gradients with excellent activity for NH3-SCR. Microporous Mesoporous Mater. 2021, 314, 110865. [Google Scholar] [CrossRef]
- Jabłońska, M. Review of the application of Cu-containing SSZ-13 in NH3-SCR-DeNOx and NH3-SCO. RSC Adv. 2022, 12, 25240–25261. [Google Scholar] [CrossRef]
- Chen, J.; Huang, W.; Bao, S.; Zhang, W.; Liang, T.; Zheng, S.; Yi, L.; Guo, L.; Wu, X. A review on the characterization of metal active sites over Cu-based and Fe-based zeolites for NH3-SCR. RSC Adv. 2022, 12, 27746–27765. [Google Scholar] [CrossRef]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, S.; Zhang, X.; Chen, S.; Wang, L.; Zhang, C.; Gao, S.; Yu, D.; Fan, X.; Cheng, Y.; et al. Research progress on the preparation of transition metal-modified zeolite catalysts and their catalytic performance for the purification of engine exhausts. J. Mater. Chem. A Mater. 2024, 12, 16293–16328. [Google Scholar] [CrossRef]
- Guo, A.; Liu, H.; Li, Y.; Luo, Y.; Ye, D.; Jiang, J.; Chen, P. Recent progress in novel zeolite catalysts for selective catalytic reduction of nitrogen oxides. Catal. Today 2023, 422, 114212. [Google Scholar] [CrossRef]
- Gao, F. Fe-exchanged small-pore zeolites as ammonia selective catalytic reduction (NH3-SCR) catalysts. Catalysts 2020, 10, 1324. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Liu, Z. Selective catalytic reduction of NOx by NH3 over Cu-AEI zeolite catalyst: Current status and future perspective. Appl. Catal. B 2023, 343, 123479. [Google Scholar] [CrossRef]
- Leistner, K.; Mihai, O.; Wijayanti, K.; Kumar, A.; Kamasamudram, K.; Currier, N.W.; Yezerts, A.; Olsson, L. Comparison of Cu/BEA, Cu/SSZ-13 and Cu/SAPO-34 for ammonia-SCR reactions. Catal. Today 2015, 258, 49–55. [Google Scholar] [CrossRef]
- Kamasamudram, K.; Currier, N.W.; Chen, X.; Yezerets, A. Overview of the practically important behaviors of zeolite-based urea-SCR catalysts, using compact experimental protocol. Catal. Today 2010, 151, 212–222. [Google Scholar] [CrossRef]
- Gao, F.; Mei, D.; Wang, Y.; Szanyi, J.; Peden, C.H. Selective catalytic reduction over Cu/SSZ-13: Linking homo-and heterogeneous catalysis. J. Am. Chem. Soc. 2017, 139, 4935–4942. [Google Scholar] [CrossRef]
- Gao, F.; Peden, C.H. Recent progress in atomic-level understanding of Cu/SSZ-13 selective catalytic reduction catalysts. Catalysts 2018, 8, 140. [Google Scholar] [CrossRef]
- Chen, L.; Janssens, T.V.; Vennestrøm, P.N.; Jansson, J.; Skoglundh, M.; Gronbeck, H. A complete multisite reaction mechanism for low-temperature NH3-SCR over Cu-CHA. ACS Catal. 2020, 10, 5646–5656. [Google Scholar] [CrossRef]
- Hammershøi, P.S.; Jensen, A.D.; Janssens, T.V. Impact of SO2-poisoning over the lifetime of a Cu-CHA catalyst for NH3-SCR. Appl. Catal. B 2018, 238, 104–110. [Google Scholar] [CrossRef]
- Hammershøi, P.S.; Jangjou, Y.; Epling, W.S.; Jensen, A.D.; Janssens, T.V. Reversible and irreversible deactivation of Cu-CHA NH3-SCRcatalysts by SO2 and SO3. Appl. Catal. B 2018, 226, 38–45. [Google Scholar] [CrossRef]
- Hammershøi, P.S.; Vennestrøm, P.N.; Falsig, H.; Jensen, A.D.; Janssens, T.V. Importance of the Cu oxidation state for the SO2-poisoning of a Cu-SAPO-34 catalyst in the NH3-SCR reaction. Appl. Catal. B 2018, 236, 377–383. [Google Scholar] [CrossRef]
- Shan, Y.; Shi, X.; Yan, Z.; Liu, J.; Yu, Y.; He, H. Deactivation of Cu-SSZ-13 in the presence of SO2 during hydrothermal aging. Catal. Today 2019, 320, 84–90. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Wang, J.; Wang, C.; Wang, J. Nature of SO3 poisoning on Cu/SAPO-34 SCR catalysts. J. Catal. 2018, 358, 277–286. [Google Scholar] [CrossRef]
- Wijayanti, K.; Andonova, S.; Kumar, A.; Li, J.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.; Olsson, L. Impact of sulfur oxide on NH3-SCR over Cu-SAPO-34. Appl. Catal. B 2015, 166, 568–579. [Google Scholar] [CrossRef]
- Wijayanti, K.; Xie, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Effect of gas compositions on SO2 poisoning over Cu/SSZ-13 used for NH3-SCR. Appl. Catal. B 2017, 219, 142–154. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Fan, X. Deactivation of Cu SCR catalysts based on small-pore SSZ-13 zeolites: A review. Chem. Phys. 2023, 6, 100207. [Google Scholar] [CrossRef]
- Han, S.; Ye, Q.; Gao, Q.; Dai, H. Improved SO2 tolerance of Cu-SAPO-18 by Ce-doping in the selective catalytic reduction of NO with NH3. Catalysts 2020, 10, 783. [Google Scholar] [CrossRef]
- Yang, J.; Li, Z.; Cui, J.; Ma, Y.; Li, Y.; Zhang, Q.; Song, K.; Yang, C. Fabrication of wide temperature lanthanum and cerium doped Cu/TNU-9 catalyst with excellent NH3-SCR performance and outstanding SO2+ H2O tolerance. J. Rare Earth 2023, 41, 1195–1202. [Google Scholar] [CrossRef]
- Liu, Q.; Fu, Z.; Ma, L.; Niu, H.; Liu, C.; Li, J.; Zhang, Z. MnOx-CeO2 supported on Cu-SSZ-13: A novel SCR catalyst in a wide temperature range. Appl. Catal. A Gen. 2017, 547, 146–154. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Z.; Yu, Y.; Li, Y. Reasonably designed CuSbTiOx series catalysts with high NH3-SCR activity and obviously improved SO2 tolerance. New J. Chem. 2024, 48, 4335–4345. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.; He, C.; Miao, J.; Chen, J. In situ growth synthesis of CuO@Cu-MOFs core-shell materials as novel low-temperature NH3-SCR catalysts. ChemCatChem 2019, 11, 979–984. [Google Scholar] [CrossRef]
- Shi, J.W.; Wang, Y.; Duan, R.; Gao, C.; Wang, B.; He, C.; Niu, C. The synergistic effects between Ce and Cu in CuyCe1− yW5Ox catalysts for enhanced NH3-SCR of NOx and SO2 tolerance. Catal. Sci. Technol. 2019, 9, 718–730. [Google Scholar] [CrossRef]
- Hao, Z.; Liu, G.; Ma, N.; Zhang, H.; Li, Y.; Xia, Y.; Zhang, D.; Zhan, S. Oxygen-vacancy mediated acidity and redox properties on WOx/Cu-doped CeO2 for the removal of NOx. J. Environ. Chem. Eng. 2021, 9, 106024. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Ying, Q.; Yao, W.; Wu, Z. The superior performance of Nb-modified Cu-Ce-Ti mixed oxides for the selective catalytic reduction of NO with NH3 at low temperature. Appl. Catal. A Gen. 2018, 562, 19–27. [Google Scholar] [CrossRef]
- Qin, B.; Guo, R.T.; Zhou, J.; Wei, L.G.; Yin, T.Y.; Pan, W.G. Promotional role of Nb modification on CuCeOx catalyst for low temperature selective catalytic reduction of NO with NH3: A mechanism investigation. Fuel 2022, 329, 125390. [Google Scholar] [CrossRef]
- Jiang, J.; Zheng, R.; Jia, Y.; Guo, L.; Huang, M.; Hu, J.; Xia, Y. Investigation of SO2 and H2O poisoning over Cu-HPMo/TiO2 catalyst for Low temperature SCR: An experimental and DFT study. Mol. Catal. 2020, 493, 111044. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Qu, H.; Zhou, B.; Xie, H.; Zhong, Q. Migration of cations and shell functionalization for Cu-Ce-La/SSZ-13@ ZSM-5: The contribution to activity and hydrothermal stability in the selective catalytic reduction reaction. J. Catal. 2020, 392, 217–230. [Google Scholar] [CrossRef]
- Di, Z.; Wang, H.; Zhang, R.; Chen, H.; Wei, Y.; Jia, J. ZSM-5 core–shell structured catalyst for enhancing low-temperature NH3-SCR efficiency and poisoning resistance. Appl. Catal. A Gen. 2022, 630, 118438. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Zhang, X.; Wu, M.; Qu, H. Construction of multifunctional interface engineering on Cu-SSZ-13@ Ce-MnOx/Mesoporous-silica catalyst for boosting activity, SO2 tolerance and hydrothermal stability. J. Hazard. Mater. 2024, 477, 135268. [Google Scholar] [CrossRef]
- Jangjou, Y.; Do, Q.; Gu, Y.; Lim, L.G.; Sun, H.; Wang, D.; Kumar, A.; Li, J.; Grabow, L.C.; Epling, W.S. Nature of Cu active centers in Cu-SSZ-13 and their responses to SO2 exposure. ACS Catal. 2018, 8, 1325–1337. [Google Scholar] [CrossRef]
- Su, W.; Li, Z.; Zhang, Y.; Meng, C.; Li, J. Identification of sulfate species and their influence on SCR performance of Cu/CHA catalyst. Catal. Sci. Technol. 2017, 7, 1523–1528. [Google Scholar] [CrossRef]
- Wijayanti, K.; Leistner, K.; Chand, S.; Kumar, A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.; Olsson, L. Deactivation of Cu-SSZ-13 by SO2 exposure under SCR conditions. Catal. Sci. Technol. 2016, 6, 2565–2579. [Google Scholar] [CrossRef]
- Bergman, S.L.; Dahlin, S.; Mesilov, V.V.; Xiao, Y.; Englund, J.; Xi, S.; Tang, C.; Skoglundh, M.; Pettersson, L.J.; Bernasek, S.L. In-situ studies of oxidation/reduction of copper in Cu-CHA SCR catalysts: Comparison of fresh and SO2-poisoned catalysts. Appl. Catal. B 2020, 269, 118722. [Google Scholar] [CrossRef]
- Shen, M.; Wen, H.; Hao, T.; Yu, T.; Fan, D.; Wang, J.; Li, W.; Wang, J. Deactivation mechanism of SO2 on Cu/SAPO-34 NH3-SCR catalysts: Structure and active Cu2+. Catal. Sci. Technol. 2015, 5, 1741–1749. [Google Scholar] [CrossRef]
- He, J.; Impeng, S.; Zhang, J.; Zhang, J.; Wang, P.; Zhang, D. SO2-tolerant NOx reduction over SO42--coordinated Cu-SAPO-34 catalysts via protecting the reduction and re-oxidation of Cu sites. Chem. Eng. J. 2022, 448, 137720. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Zhang, H.; Yang, F.; Tang, A.; Han, D.; Jia, J.; Wang, J.; Li, Z.; Zhang, Z. Recent progress in performance optimization of Cu-SSZ-13 catalyst for selective catalytic reduction of NOx. Front. Chem. 2022, 10, 1033255. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, W.; An, D.; Wang, X.; Liu, A.; Zou, W.; Tang, C.; Ge, C.; Tong, Q.; Sun, J.; et al. Insight into the SO2 resistance mechanism on γ-Fe2O3 catalyst in NH3-SCR reaction: A collaborated experimental and DFT study. Appl. Catal. B 2021, 281, 119544. [Google Scholar] [CrossRef]
- Qin, G.; Zheng, J.; Li, Y.; Yang, Y.; Liu, X.; Han, X.; Huang, Z. Tailor the crystal planes of MIL-101 (Fe) derivatives to enhance the activity of SCR reaction at medium and low temperature. J. Colloid Interface Sci. 2022, 615, 432–444. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, B.; Zhang, Y.; Ren, J.; Wu, C.; Gates, I.D.; Liu, Y.; Gao, Z. A novel low-temperature Fe-Fe double-atom catalyst for a “fast SCR” reaction. Mol. Catal. 2022, 533, 112769. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Li, Q.; Xin, Y.; Zheng, L.; Wang, Y.; Zhang, Z. Enhanced selective catalytic reduction of NOx with NH3 over homoatomic dinuclear sites in defective α-Fe2O3. Chem. Eng. J. 2021, 426, 131845. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Z.; Wang, L.; Zhan, W.; Guo, Y.; Guo, Y. Understanding the role of redox properties and NO adsorption over MnFeOx for NH3-SCR. Catal. Sci. Technol. 2022, 12, 2030–2041. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, X.; Yan, Z.; Shan, Y.; Zhu, Y.; Yu, Y.; He, H. Design of high-performance iron–niobium composite oxide catalysts for NH3-SCR: Insights into the interaction between Fe and Nb. ACS Catal. 2021, 11, 9825–9836. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, N.; Li, Q.; Zhang, Z.; Cao, X.; Zheng, L.; Zeng, Y.; Anderson, J.A. Active site identification and modification of electronic states by atomic-scale doping to enhance oxide catalyst innovation. ACS Catal. 2018, 8, 1399–1404. [Google Scholar] [CrossRef]
- Chen, W.; Yang, S.; Liu, H.; Huang, F.; Shao, Q.; Liu, L.; Sun, J.; Sun, C.; Chen, D.; Dong, L. Single-atom Ce-modified α-Fe2O3 for selective catalytic reduction of NO with NH3. Environ. Sci. Technol. 2022, 56, 10442–10453. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Jin, S.; Wang, J.; Wu, J.; Cai, L.; Zhou, Y.; Wang, X.; Zhang, R.; Ling, L.; Jin, M. Deep insights into the promotion role of Sm doping in the sulfur resistance of Fe2O3 catalyst for NH3-SCR: A combined experimental and DFT study. J. Phys. Chem. Solids 2024, 184, 111666. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, N.; Li, Q.; Zhang, Z.; Cao, X.; Zheng, L.; Zeng, Y.; Anderson, J.A. Selective catalytic reduction of NOx with NH3 over short-range ordered WOFe structures with high thermal stability. Appl. Catal. B 2018, 229, 81–87. [Google Scholar] [CrossRef]
- Liu, Z.; Su, H.; Chen, B.; Li, J.; Woo, S.I. Activity enhancement of WO3 modified Fe2O3 catalyst for the selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2016, 299, 255–262. [Google Scholar] [CrossRef]
- Liu, F.; Shan, W.; Lian, Z.; Liu, J.; He, H. The smart surface modification of Fe2O3 by WOx for significantly promoting the selective catalytic reduction of NOx with NH3. Appl. Catal. B 2018, 230, 165–176. [Google Scholar] [CrossRef]
- Shu, Y.; Sun, H.; Quan, X.; Chen, S. Enhancement of catalytic activity over the iron-modified Ce/TiO2 catalyst for selective catalytic reduction of NOx with ammonia. J. Phys. Chem. C 2012, 116, 25319–25327. [Google Scholar] [CrossRef]
- Tang, X.; Shi, Y.; Gao, F.; Zhao, S.; Yi, H.; Xie, Z. Promotional role of Mo on Ce0. 3FeOx catalyst towards enhanced NH3-SCR catalytic performance and SO2 resistance. Chem. Eng. J. 2020, 398, 125619. [Google Scholar] [CrossRef]
- Stahl, A.; Wang, Z.; Schwämmle, T.; Ke, J.; Li, X. Novel Fe-W-Ce mixed oxide for the selective catalytic reduction of NOx with NH3 at low temperatures. Catalysts 2017, 7, 71. [Google Scholar] [CrossRef]
- Ren, Z.; Fan, H.; Wang, R. A novel ring-like Fe2O3-based catalyst: Tungstophosphoric acid modification, NH3-SCR activity and tolerance to H2O and SO2. Catal. Commun. 2017, 100, 71–75. [Google Scholar] [CrossRef]
- Yao, G.; Wei, Y.; Gui, K.; Ling, X. Catalytic performance and reaction mechanisms of NO removal with NH3 at low and medium temperatures on Mn-W-Sb modified siderite catalysts. J. Environ. Sci. 2022, 115, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Han, L.; He, J.; Li, H.; Yan, T.; Chen, G.; Zhang, J.; Shi, L.; Zhang, D. Improved NOx reduction in the presence of SO2 by using Fe2O3-promoted halloysite-supported CeO2–WO3 catalysts. Environ. Sci. Technol. 2018, 53, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Gao, M.; Feng, C.; Shi, L.; Zhang, D. Fe2O3–CeO2@Al2O3 nanoarrays on Al-mesh as SO2-tolerant monolith catalysts for NOx reduction by NH3. Environ. Sci. Technol. 2019, 53, 5946–5956. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Cong, Q.; Ma, H.; Li, S.; Li, W. Design of a hierarchical Fe-ZSM-5@ CeO2 catalyst and the enhanced performances for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2019, 369, 957–967. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Zhao, Z.; Wei, Y.; Song, W. Fe-Beta@ CeO2 core-shell catalyst with tunable shell thickness for selective catalytic reduction of NOx with NH3. AIChE J. 2017, 63, 4430–4441. [Google Scholar] [CrossRef]
- Jiang, M.; Yan, Z.; Zhang, Y.; Zhang, C.; Chang, C.; Xiao, M.; Ruan, L.; Yan, Y.; Yu, Y.; He, H. Simultaneous modification of redox and acidic properties of FeOx catalysts derived from MIL-100 (Fe) via HPW incorporation for NH3-SCR. Appl. Catal. B 2024, 358, 124416. [Google Scholar] [CrossRef]
- Ren, Z.; Teng, Y.; Zhao, L.; Wang, R. Keggin-tungstophosphoric acid decorated Fe2O3 nanoring as a new catalyst for selective catalytic reduction of NOx with ammonia. Catal. Today 2017, 297, 36–45. [Google Scholar] [CrossRef]
- Tan, W.; Xie, S.; Shan, W.; Lian, Z.; Xie, L.; Liu, A.; Gao, F.; Dong, L.; He, H.; Liu, F. CeO2 doping boosted low-temperature NH3-SCR activity of FeTiOx catalyst: A microstructure analysis and reaction mechanistic study. Front. Environ. Sci. Eng. 2022, 16, 60. [Google Scholar] [CrossRef]
- Ma, S.; Tan, H.; Li, Y.; Wang, P.; Zhao, C.; Niu, X.; Zhu, Y. Excellent low-temperature NH3-SCR NO removal performance and enhanced H2O resistance by Ce addition over the Cu0. 02Fe0. 2CeyTi1-yOx (y= 0.1, 0.2, 0.3) catalysts. Chemosphere 2020, 243, 125309. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Hou, Y.; Li, Q.; Han, X.; Wang, H.; Wu, Z.; Huang, Z. Amorphous FeOx–Mn0. 1Oy catalyst with rich oxygen vacancies for ammonia selective catalytic reduction of nitrogen oxide at low temperatures. Fuel 2023, 349, 128644. [Google Scholar] [CrossRef]
- Wang, H.; Qu, Z.; Liu, L.; Dong, S.; Qiao, Y. Promotion of NH3-SCR activity by sulfate-modification over mesoporous Fe doped CeO2 catalyst: Structure and mechanism. J. Hazard. Mater. 2021, 414, 125565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Wang, W.; Wang, X.; Li, B.; Zhang, S.; Li, W.; Li, S. Revealing the excellent low-temperature activity of the Fe1–x CexOδ-S catalyst for NH3-SCR: Improvement of the lattice oxygen mobility. ACS Appl. Mater. Interfaces 2023, 15, 17834–17847. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meeprasert, J.; Namuangruk, S.; Zha, K.; Li, H.; Huang, L.; Maitarad, P.; Shi, L.; Zhang, D. Facet–activity relationship of TiO2 in Fe2O3/TiO2 nanocatalysts for selective catalytic reduction of NO with NH3: In situ DRIFTs and DFT studies. J. Phys. Chem. C 2017, 121, 4970–4979. [Google Scholar] [CrossRef]
- Han, J.; Meeprasert, J.; Maitarad, P.; Nammuangruk, S.; Shi, L.; Zhang, D. Investigation of the facet-dependent catalytic performance of Fe2O3/CeO2 for the selective catalytic reduction of NO with NH3. J. Phys. Chem C 2016, 120, 1523–1533. [Google Scholar] [CrossRef]
- Teng, W.; Li, J.; Dai, X.; Chen, Y.; Wu, H.; Liu, W.; Ren, S.; Yang, J.; Liu, Q. Promoting the NH3-SCR performance of Fe2O3-TiO2 catalyst through regulation of the exposed crystal facets of Fe2O3. Appl. Surf. Sci. 2024, 647, 158938. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Liu, B.; Sun, S. A review of Mn-containing oxide catalysts for low temperature selective catalytic reduction of NOx with NH3: Reaction mechanism and catalyst deactivation. RSC Adv. 2017, 7, 26226–26242. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Li, C.; Li, J.; Shi, Y.; Meng, X. A review on selective catalytic reduction of NOx by NH3 over Mn–based catalysts at low temperatures: Catalysts, mechanisms, kinetics and DFT calculations. Catalysts 2017, 7, 199. [Google Scholar] [CrossRef]
- Guo, R.T.; Qin, B.; Wei, L.G.; Yin, T.Y.; Zhou, J.; Pan, W.G. Recent progress of low-temperature selective catalytic reduction of NOx with NH3 over manganese oxide-based catalysts. Phys. Chem. Chem. Phys. 2022, 24, 6363–6382. [Google Scholar] [CrossRef]
- Peña, D.A.; Uphade, B.S.; Smirniotis, P.G. TiO2-supported metal oxide catalysts for low-temperature selective catalytic reduction of NO with NH3: I. Evaluation and characterization of first row transition metals. J. Catal. 2004, 221, 421–431. [Google Scholar] [CrossRef]
- He, G.; Gao, M.; Peng, Y.; Yu, Y.; Shan, W.; He, H. Superior oxidative dehydrogenation performance toward NH3 determines the excellent low-temperature NH3-SCR activity of Mn-based catalysts. Environ. Sci. Technol. 2021, 55, 6995–7003. [Google Scholar] [CrossRef] [PubMed]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Tang, X.; Hao, J.; Xu, W.; Li, J. Low temperature selective catalytic reduction of NOx with NH3 over amorphous MnOx catalysts prepared by three methods. Catal. Commun. 2007, 8, 329–334. [Google Scholar] [CrossRef]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl. Catal. A Gen. 2007, 327, 261–269. [Google Scholar] [CrossRef]
- Kang, M.; Yeon, T.H.; Park, E.D.; Yie, J.E.; Kim, J.M. Novel MnOx catalysts for NO reduction at low temperature with ammonia. Catal. Lett. 2006, 106, 77–80. [Google Scholar] [CrossRef]
- Tang, X.; Li, J.; Sun, L.; Hao, J. Origination of N2O from NO reduction by NH3 over β-MnO2 and α-Mn2O3. Appl. Catal. B 2010, 99, 156–162. [Google Scholar] [CrossRef]
- Fang, X.; Liu, Y.; Chen, L.; Cheng, Y. Influence of surface active groups on SO2 resistance of birnessite for low-temperature NH3-SCR. Chem. Eng. J. 2020, 399, 125798. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Y.; Li, P.Z.; Zhang, P.; Su, W.; Sun, Y.; Zou, R.; Zhao, Y. Experimental and theoretical investigation of mesoporous MnO2 nanosheets with oxygen vacancies for high-efficiency catalytic DeNOx. ACS Catal. 2018, 8, 3865–3874. [Google Scholar] [CrossRef]
- Xu, S.; Chen, J.; Li, Z.; Liu, Z. Highly ordered mesoporous MnOx catalyst for the NH3-SCR of NOx at low temperatures. Appl. Catal. A Gen. 2023, 649, 118966. [Google Scholar] [CrossRef]
- Chen, R.; Fang, X.; Li, Z.; Liu, Z. Selective catalytic reduction of NOx with NH3 over a novel MOF-derived MnOx catalyst. Appl. Catal. A Gen. 2022, 643, 118754. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Chen, G.; Leng, Z.; Xia, D.; Wang, Z. Enabling wide-temperature selective catalytic reduction of NOX via modulating redox ability of Mn-based catalysts. Energy Fuels 2024, 38, 8960–8967. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, P.; Shen, Z.; Chen, S.; Wang, Q.; Cheng, D.; Zhang, D. Low-temperature NOx reduction over hydrothermally stable SCR catalysts by engineering low-coordinated Mn active sites. Chem. Eng. J. 2022, 442, 136182. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Zhong, L.; Song, Z.; Yu, S.; Zhang, C.; Fang, Q.; Chen, G. DFT analysis of the reaction mechanism for NH3-SCR of NOx over Mn/γ-Al2O3 catalyst. J. Phys. Chem. C 2019, 123, 25185–25196. [Google Scholar] [CrossRef]
- Zeng, Y.; Lyu, F.; Wang, Y.; Zhang, S.; Zhong, Q.; Zhong, Z. New insight on N2O formation over MnOx/TiO2 catalysts for selective catalytic reduction of NOx with NH3. Mol. Catal. 2022, 525, 112356. [Google Scholar] [CrossRef]
- Yang, S.; Xiong, S.; Liao, Y.; Xiao, X.; Qi, F.; Peng, Y.; Fu, Y.; Shan, W.; Li, J. Mechanism of N2O formation during the low-temperature selective catalytic reduction of NO with NH3 over Mn–Fe spinel. Environ. Sci. Technol. 2014, 48, 10354–10362. [Google Scholar] [CrossRef]
- Guo, J.; Gan, F.; Zhao, Y.; He, J.; Wang, B.; Gao, T.; Jiang, X.; Ma, S. Revealing the crystal facet effect on N2O formation during the NH3-SCR over α-MnO2 catalysts. RSC Adv. 2023, 13, 4032–4039. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, B.; Zheng, C.; Zhou, J.; Su, T.; Guo, J.; Chen, J.; Chen, Y.; Zhang, J.; Dang, H.; et al. Research on the resistance of catalysts for selective catalytic reduction: Current progresses and future perspectives. J. Clean. Prod. 2023, 434, 139920. [Google Scholar] [CrossRef]
- Hou, Q.; Liu, Y.; Hou, Y.; Han, X.; Huang, Z. Ordered mesoporous MnAlOx oxides dominated by calcination temperature for the selective catalytic reduction of NOx with NH3 at low temperature. Catalysts 2022, 12, 637. [Google Scholar] [CrossRef]
- Li, Y.; Wan, Y.; Sun, R.; Qian, G.; Liu, Z.; Dan, J.; Yu, F. Novel 2D layered manganese silicate nanosheets with excellent performance for selective catalytic reduction of NO with ammonia. ChemCatChem 2022, 14, e202200909. [Google Scholar] [CrossRef]
- Yao, X.; Chen, L.; Cao, J.; Chen, Y.; Tian, M.; Yang, F.; Sun, J.; Tang, C.; Dong, L. Enhancing the deNOx performance of MnOx/CeO2-ZrO2 nanorod catalyst for low-temperature NH3-SCR by TiO2 modification. Chem. Eng. J. 2019, 369, 46–56. [Google Scholar] [CrossRef]
- Yang, R.; Gao, Z.; Sun, M.; Fu, G.; Cheng, G.; Liu, W.; Yang, X.; Zhao, X.; Yu, L. A highly active VOx-MnOx/CeO2 for selective catalytic reduction of NO: The balance between redox property and surface acidity. J. Rare Earth 2021, 39, 1370–1381. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Wang, J.; Gu, T. Improvement of activity, selectivity and H2O & SO2-tolerance of micro-mesoporous CrMn2O4 spinel catalyst for low-temperature NH3-SCR of NOx. Appl. Surf. Sci. 2019, 466, 411–424. [Google Scholar] [CrossRef]
- Fan, H.; Fan, J.; Chang, T.; Wang, X.; Wang, X.; Huang, Y.; Zhang, Y.; Shen, Z. Low-temperature Fe–MnO2 nanotube catalysts for the selective catalytic reduction of NOx with NH3. Catal. Sci. Technol. 2021, 11, 6553–6563. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, Y.; Li, Z.; Li, K.; Ren, X.; Jia, L.; Li, H.; Cheng, H.; Liu, J.; Liu, J. Modulating the acidity and reducibility of FeMn/TiO2 to boost the low-temperature selective catalytic reduction of NOx. Fuel 2024, 366, 131316. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, S.; Xing, X.; Li, X.; Chen, L.; Wang, M. Unveiling the inductive strategy of different precipitants on MnFeOx catalyst for low-temperature NH3-SCR reaction. Fuel 2023, 335, 126986. [Google Scholar] [CrossRef]
- Wei, L.; Li, X.; Mu, J.; Wang, X.; Fan, S.; Yin, Z.; Tadé, M.O.; Liu, S. Rationally tailored redox properties of a mesoporous Mn–Fe spinel nanostructure for boosting low-temperature selective catalytic reduction of NOx with NH3. ACS Sustain. Chem. Eng. 2020, 8, 17727–17739. [Google Scholar] [CrossRef]
- Gu, J.; Zhu, B.; Duan, R.; Chen, Y.; Wang, S.; Liu, L.; Wang, X. Highly dispersed MnOx–FeOx supported by silicalite-1 for the selective catalytic reduction of NOx with NH3 at low temperatures. Catal. Sci. Technol. 2020, 10, 5525–5534. [Google Scholar] [CrossRef]
- Chen, C.; Feng, C.; Wang, Y.; Li, J.; Liu, Z.; Wang, W.; Pan, Y.; Liu, Y. Design of robust Co-doped Mn3O4 spinel catalysts for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Surf. Sci. 2022, 602, 154384. [Google Scholar] [CrossRef]
- Wang, G.; Liang, Y.; Song, J.; Xu, K.; Pan, Y.; Xu, X.; Zhao, Y. Co-doped MnCeOx/ZrO2 catalysts for low temperature selective catalytic reduction of NO. Res. Chem. Intermed. 2022, 48, 2627–2640. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, X.; Wang, J.; Ma, C.; Jia, X.; Qiao, W.; Ling, L. Enhanced activity and water resistance of hierarchical flower-like Mn-Co binary oxides for ammonia-SCR reaction at low temperature. Appl. Surf. Sci. 2021, 569, 150989. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, B.; Li, J.; Wang, X.; Crocker, M.; Shi, C. Insights into the structure-activity relationships of highly efficient CoMn oxides for the low temperature NH3-SCR of NOx. Appl. Catal. B 2020, 277, 119215. [Google Scholar] [CrossRef]
- Perumal, S.K.; Yu, H.; Aghalayam, P.; Kim, H.S. Unravelling the role of Zr–Laponite supports in enhancing the activity of a MnNi catalyst for the low-temperature NH3-selective catalytic reduction reaction. Energy Fuels 2024, 38, 4516–4525. [Google Scholar] [CrossRef]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Cu–Mn mixed oxides for low temperature NO reduction with NH3. Catal. Today 2006, 111, 236–241. [Google Scholar] [CrossRef]
- Luo, W.; Yang, L.; Zhang, Z.; Cao, G.; Li, J.; Liu, B. Flexible Ti mesh-supported MnOx–CuOx/TiO2 nanosheet monolithic catalysts for low-temperature selective catalytic reduction of NOx with NH3. ACS Appl. Nano Mater. 2024, 7, 6262–6272. [Google Scholar] [CrossRef]
- La Greca, E.; Kharlamova, T.S.; Grabchenko, M.V.; Svetlichnyi, V.A.; Pantaleo, G.; Consentino, L.; Stonkus, O.A.; Vodyankina, O.V.; Liotta, L.F. Influence of Y doping on catalytic activity of CeO2, MnOx, and CeMnOx catalysts for selective catalytic reduction of NO by NH3. Catalysts 2023, 13, 901. [Google Scholar] [CrossRef]
- Jiang, B.; Deng, B.; Zhang, Z.; Wu, Z.; Tang, X.; Yao, S.; Lu, H. Effect of Zr addition on the low-temperature SCR activity and SO2 tolerance of Fe–Mn/Ti catalysts. J. Phys. Chem. C 2014, 118, 14866–14875. [Google Scholar] [CrossRef]
- Zhou, Y.; Su, B.; Ren, S.; Chen, Z.; Su, Z.; Yang, J.; Chen, L.; Wang, M. Nb2O5-modified Mn-Ce/AC catalyst with high ZnCl2 and SO2 tolerance for low-temperature NH3-SCR of NO. J. Environ. Chem. Eng. 2021, 9, 106323. [Google Scholar] [CrossRef]
- Hu, X.; Shi, Q.; Zhang, H.; Wang, P.; Zhan, S.; Li, Y. NH3-SCR performance improvement over Mo modified Mo (x)-MnOx nanorods at low temperatures. Catal. Today 2017, 297, 17–26. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Hu, X.; Huang, Y. A Novel MnOx–MoOx Codoped Iron-Based Catalyst for NH3-SCR with Superior Catalytic Activity over a Wide Temperature Range. Ind. Eng. Chem. Res. 2024, 63, 8163–8174. [Google Scholar] [CrossRef]
- Dong, Y.; Ran, M.; Zhang, X.; Lin, S.; Li, W.; Yang, Y.; Song, H.; Wu, W.; Liu, S.; Zheng, C.; et al. Promoting effect of polyoxometallic acid modification on the NH3-SCR performance of Mn-based catalysts. J. Environ. Chem. Eng. 2024, 12, 112823. [Google Scholar] [CrossRef]
- Cao, F.; Xiang, J.; Su, S.; Wang, P.; Hu, S.; Sun, L. Ag modified Mn–Ce/γ-Al2O3 catalyst for selective catalytic reduction of NO with NH3 at low-temperature. Fuel Process Technol. 2015, 135, 66–72. [Google Scholar] [CrossRef]
- Wang, H.; Lin, M.; Murayama, T.; Feng, S.; Haruta, M.; Miura, H.; Shishido, T. Ag size/structure-dependent effect on low-temperature selective catalytic oxidation of NH3 over Ag/MnO2. ACS Catal. 2021, 11, 8576–8584. [Google Scholar] [CrossRef]
- Chang, H.; Chen, X.; Li, J.; Ma, L.; Wang, C.; Liu, C.; Schwank, J.W.; Hao, J. Improvement of activity and SO2 tolerance of Sn-modified MnOx–CeO2 catalysts for NH3-SCR at low temperatures. Environ. Sci. Technol. 2013, 47, 5294–5301. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, C.; He, P.; Mu, G.; Wang, K.; Yang, C.; Chai, S.; Wang, N.; Ge, C. Promoting H2O/SO2 resistance of Ce-Mn/TiO2 nanostructures by Sb5+/Sb3+ addition for Selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2022, 600, 154146. [Google Scholar] [CrossRef]
- Li, X.; Yin, Y.; Yao, C.; Zuo, S.; Lu, X.; Luo, S.; Ni, C. La1− xCexMnO3/attapulgite nanocomposites as catalysts for NO reduction with NH3 at low temperature. Particuology 2016, 26, 66–72. [Google Scholar] [CrossRef]
- Fan, X.; Hao, L.; Gu, X.; Li, S. Low-temperature selective catalytic reduction of NO with NH3 over a biochar-supported perovskite oxide catalyst. Energy Fuels 2023, 37, 7339–7352. [Google Scholar] [CrossRef]
- Yao, X.; Ma, K.; Zou, W.; He, S.; An, J.; Yang, F.; Dong, L. Influence of preparation methods on the physicochemical properties and catalytic performance of MnOx-CeO2 catalysts for NH3-SCR at low temperature. Chin. J. Catal. 2017, 38, 146–159. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.; Fang, P.; Ren, T.; Liu, Y.; Cen, C.; Wang, H.; Wu, Z. Tuning the property of Mn-Ce composite oxides by titanate nanotubes to improve the activity, selectivity and SO2/H2O tolerance in middle temperature NH3-SCR reaction. Fuel Process Technol. 2017, 167, 221–228. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, L.; Sun, N.; Wang, H.; Zhong, L.; He, C.; Wei, W.; Sun, Y. Hollow MnOx-CeO2 mixed oxides as highly efficient catalysts in NO oxidation. Chem. Eng. J. 2017, 322, 46–55. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Z.; Zhao, Z.; Wang, Y.; Yao, Y.; Liu, Y.; Zhang, J.; Zhang, Z. CeO2 Nanoparticle-loaded MnO2 nanoflowers for selective catalytic reduction of NOx with NH3 at low temperatures. Molecules 2022, 27, 4863. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Shi, J.W.; Niu, C.; Wang, B.; He, C.; Liu, W.; Xiao, L.; Ma, D.; Wang, H.; Cheng, Y. FeVO4-supported Mn–Ce oxides for the low-temperature selective catalytic reduction of NOx by NH3. Catal. Sci. Technol. 2021, 11, 6770–6781. [Google Scholar] [CrossRef]
- Huang, X.; Dong, F.; Zhang, G.; Tang, Z. Constructing TiO2@CeMnOx nanocages by self-sacrificial hydrolytic etching MIL-125 for efficient wide-temperature selective catalytic reduction of nitrogen oxides. Chem. Eng. J. 2022, 432, 134236. [Google Scholar] [CrossRef]
- Wu, T.; Ren, S.; Guo, R.T.; Li, C.F.; You, Y.H.; Guo, S.Y.; Pan, W.G. The promotion effect of Pr doping on the catalytic performance of MnCeOx catalysts for low-temperature NH3-SCR. Fuel 2024, 357, 129917. [Google Scholar] [CrossRef]
- Rong, J.; Zhao, W.; Luo, W.; Kang, K.; Long, L.; Chen, Y.; Yao, X. Doping effect of rare earth metal ions Sm3+, Nd3+ and Ce4+ on denitration performance of MnOx catalyst in low temperature NH3-SCR reaction. J. Rare Earth 2023, 41, 1323–1335. [Google Scholar] [CrossRef]
- Ji, X.; Cai, Y.; Zhang, B.; Yu, H.; Liu, Q.; Wang, X.; Liu, A.; Qian, Q.; Tong, Q.; Tan, W.; et al. Optimization of robust FeMnSmOx catalyst for low-temperature (<150 °C) NH3–SCR of NOx. Ind. Eng. Chem. Res. 2024, 63, 2705–2716. [Google Scholar] [CrossRef]
- He, X.; Zhu, F.; Dong, L.; Guo, H.; Liu, X.; Ren, G.; Ma, X. Sm-MnOx/TiO2-{001} with preferentially exposed anatase {001} facet for selective catalytic reduction of NO with NH3. Appl. Catal. A Gen. 2023, 664, 119353. [Google Scholar] [CrossRef]
- Wang, F.; Wang, P.; Lan, T.; Shen, Y.; Ren, W.; Zhang, D. Ultralow-temperature NOx reduction over SmMn2O5 Mullite catalysts via modulating the superficial dual-functional active sites. ACS Catal. 2022, 12, 7622–7632. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Wei, L.; Wang, K.; Liu, C.; Ma, D.; Liu, Q. Promotional mechanism of activity of CeEuMnOx ternary oxide for low temperature SCR of NOx. J. Rare Earth 2023, 41, 965–974. [Google Scholar] [CrossRef]
- Guan, J.; Zhou, L.; Li, W.; Hu, D.; Wen, J.; Huang, B. Improving the performance of Gd addition on catalytic activity and SO2 resistance over MnOx/ZSM-5 catalysts for low-temperature NH3-SCR. Catalysts 2021, 11, 324. [Google Scholar] [CrossRef]
- Gao, C.; Xiao, B.; Shi, J.W.; He, C.; Wang, B.; Ma, D.; Cheng, Y.; Niu, C. Comprehensive understanding the promoting effect of Dy-doping on MnFeOx nanowires for the low-temperature NH3-SCR of NOx: An experimental and theoretical study. J. Catal. 2019, 380, 55–67. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Z.; Hu, J.; Zhang, L.; Zhang, Z.; Liang, H.; Zhang, Y.; Fan, G. Dy-modified Mn/TiO2 catalyst used for the selective catalytic reduction of NO in ammonia at low temperatures. Molecules 2024, 29, 277. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, K.; ZHANG, Y.P.; HUANG, T.J.; Bin, L.U.; Kai, S.H.E.N. Sulfur-poisoning and thermal reduction regeneration of holmium-modified Fe-Mn/TiO2 catalyst for low-temperature SCR. J. Fuel Chem. Technol. 2017, 45, 1356–1364. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, S.; Wang, A.; Guo, Y.; Guo, Y.; Wang, L.; Zhan, W. Er-modified MnOx for selective catalytic reduction of NOx with NH3 at low temperature: Promoting effect of erbium on catalytic performance. J. Rare Earth 2023, 41, 917–925. [Google Scholar] [CrossRef]
- Niu, C.; Wang, B.; Xing, Y.; Su, W.; He, C.; Xiao, L.; Xu, Y.; Zhao, S.; Cheng, Y.; Shi, J.W. Thulium modified MnOx/TiO2 catalyst for the low-temperature selective catalytic reduction of NO with ammonia. J. Clean. Prod. 2021, 290, 125858. [Google Scholar] [CrossRef]
- Liu, J.; Lv, D.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, G. Influence of catalyst structural remodelling on the performance of NH3-SCR reactions: A Mini Review. ChemCatChem, 2024; e202401076, Early View. [Google Scholar] [CrossRef]
- Zha, K.; Cai, S.; Hu, H.; Li, H.; Yan, T.; Shi, L.; Zhang, D. In situ DRIFTs investigation of promotional effects of tungsten on MnOx-CeO2/meso-TiO2 catalysts for NOx reduction. J. Phys. Chem. C 2017, 121, 25243–25254. [Google Scholar] [CrossRef]
- Xue, H.; Guo, X.; Mao, D.; Meng, T.; Yu, J.; Ma, Z. Phosphotungstic acid-modified MnOx for selective catalytic reduction of NOx with NH3. Catalysts 2022, 12, 1248. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Wang, J.; Chen, Q.; Zhang, Z.; Pan, X.; Miao, Z.; Zhan, B.; Wu, Z.; Yang, X. BiMnO3 perovskite catalyst for selective catalytic reduction of NO with NH3 at low temperature. Chin. J. Catal. 2012, 33, 1448–1454. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, W.; Wu, H.; Liu, Q.; Yao, L. Promoting effect of Si on MnOx catalysts for low-temperature NH3-SCR of NO: Enhanced N2 selectivity and SO2 resistance. Fuel 2024, 355, 129478. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Ma, C.; Zhang, Y.; Qiao, W.; Ling, L. Development of highly active three-dimensional cross-linked structural manganese-titanium composite oxides for NOx abatement: Insight into the resistance to sulfur and alkali metal poisoning mechanism. Chem. Eng. J. 2023, 474, 145573. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Li, H.; Li, X.; Wang, L.; Tsang, S.C. Cr–MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3 at low temperature. J. Catal. 2010, 276, 56–65. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, F.; Li, H.; Yang, Q.; Wang, L.; Li, X. Low-temperature selective catalytic reduction of NOx with NH3 over Fe–Mn mixed-oxide catalysts containing Fe3Mn3O8 phase. Ind. Eng. Chem. Res. 2012, 51, 202–212. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, F.; Sun, R.; Tian, J.; Wang, Q.; Dai, B.; Dan, J.; Pfeiffer, H. Two-dimensional MnFeCo layered double oxide as catalyst for enhanced selective catalytic reduction of NOx with NH3 at low temperature (25–150 °C). Appl. Catal. A Gen. 2020, 592, 117432. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, L.; Niu, S.; Lu, C.; Wang, D.; Zhang, Q.; Li, J. Iron-manganese-magnesium mixed oxides catalysts for selective catalytic reduction of NOx with NH3. Korean J. Chem. Eng. 2017, 34, 1858–1866. [Google Scholar] [CrossRef]
- Chen, S.; Yan, Q.; Zhang, C.; Wang, Q. A novel highly active and sulfur resistant catalyst from Mn-Fe-Al layered double hydroxide for low temperature NH3-SCR. Catal. Today 2019, 327, 81–89. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, S.; Wang, M.; Yang, J.; Chen, Z.; Chen, L. Mn and Fe oxides co-effect on nanopolyhedron CeO2 catalyst for NH3-SCR of NO. J. Energy Inst. 2021, 99, 97–104. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.; Zhu, J.; Liu, Y.; Su, F.; Gao, S.; Xu, G. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with NH3. Appl. Catal. B 2010, 95, 160–168. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, N.; Wang, Z.; Sun, W.; Sun, K. Porous bimetallic Mn2Co1Ox catalysts prepared by a one-step combustion method for the low temperature selective catalytic reduction of NOx with NH3. Catal. Commun. 2015, 72, 111–115. [Google Scholar] [CrossRef]
- Hu, X.; Huang, L.; Zhang, J.; Li, H.; Zha, K.; Shi, L.; Zhang, D. Facile and template-free fabrication of mesoporous 3D nanosphere-like MnxCo3–xO4 as highly effective catalysts for low temperature SCR of NOx with NH3. J. Mater. Chem. A 2018, 6, 2952–2963. [Google Scholar] [CrossRef]
- Jiang, H.; Kong, D.; Niu, Y.; Wang, S. Synthesis of Co-doped MnO2 catalysts with the assistance of PVP for low-temperature SCR. Catal. Sci. Technol. 2020, 10, 8086–8093. [Google Scholar] [CrossRef]
- Chen, R.; Fang, X.; Li, J.; Zhang, Y.; Liu, Z. Mechanistic investigation of the enhanced SO2 resistance of Co-modified MnOx catalyst for the selective catalytic reduction of NOx by NH3. Chem. Eng. J 2023, 452, 139207. [Google Scholar] [CrossRef]
- Qiu, M.; Zhan, S.; Yu, H.; Zhu, D. Low-temperature selective catalytic reduction of NO with NH3 over ordered mesoporous MnxCo3−xO4 catalyst. Catal. Commun. 2015, 62, 107–111. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Li, J.; Zhao, S.; Wang, J.; Chu, C.; Li, C. Promotional mechanisms of activity and SO2 tolerance of Co-or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Chen, L.; Zhang, Y.; Mi, Y.; Liao, M.; Liu, W.; Wu, D.; Li, Z.; Peng, H. Ternary MnCoVOx catalysts with remarkable deNOx performance: Dual acid-redox sites control strategy. Appl. Catal. B 2022, 318, 121779. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, W.; Tang, Y.; Li, L.; Wang, H.; Cui, Y.; Gu, J.; Li, Y.; Shi, J. Ni-Mn bi-metal oxide catalysts for the low temperature SCR removal of NO with NH3. Appl. Catal. B 2014, 148, 114–122. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Sani, Z.; Yi, H.; Zhao, S.; Yu, Q.; Zhou, Y.; Shi, Y.; Ni, S. Spinel-structured Mn–Ni nanosheets for NH3-SCR of NO with good H2O and SO2 resistance at low temperature. Catal. Sci. Technol. 2020, 10, 7486–7501. [Google Scholar] [CrossRef]
- Han, Y.; Mu, J.; Li, X.; Gao, J.; Fan, S.; Tan, F.; Zhao, Q. Triple-shelled NiMn2O4 hollow spheres as an efficient catalyst for low-temperature selective catalytic reduction of NOx with NH3. Chem. Commun. 2018, 54, 9797–9800. [Google Scholar] [CrossRef]
- Yan, Q.; Xiao, J.; Gui, R.; Chen, Z.; Wang, Y.; Li, Y.; Zhu, T.; Wang, Q.; Xin, Y. Insights into enhancement of NH3-SCR activity and N2 selectivity of LDHs-derived NiMnAlOx catalysts: Combination of experiments and DFT calculations. Appl. Catal. B 2024, 343, 123489. [Google Scholar] [CrossRef]
- Hou, Q.; Liu, Y.; Hou, Y.; Han, X.; Huang, Z. Tunable highly dispersed Nia-xMnxAlOy catalysts derived from layered double oxide for low temperature NOx removal. Catal. Lett. 2024, 154, 4389–4402. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Li, R.; Zhao, Q.; Ke, J.; Xiao, H.; Wang, L.; Liu, S.; Tade, M.; Wang, S. Facile synthesis of tube-shaped Mn-Ni-Ti solid solution and preferable Langmuir-Hinshelwood mechanism for selective catalytic reduction of NOx by NH3. Appl. Catal. A Gen. 2018, 549, 289–301. [Google Scholar] [CrossRef]
- Chen, L.; Li, R.; Li, Z.; Yuan, F.; Niu, X.; Zhu, Y. Effect of Ni doping in NixMn1− xTi10 (x = 0.1–0.5) on activity and SO2 resistance for NH3-SCR of NO studied with in situ DRIFTS. Catal. Sci. Technol. 2017, 7, 3243–3257. [Google Scholar] [CrossRef]
- Yan, Q.; Xiao, J.; Gui, R.; Chen, Z.; Li, Y.; Zhu, T.; Wang, Q.; Xin, Y. Mechanistic insight into the promotion of the low-temperature NH3–SCR activity over NiMnFeOx LDO catalysts: A Combined Experimental and DFT Study. Environ. Sci. Technol. 2023, 57, 20708–20717. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Chen, Z.; Wang, F.; Yu, Y.; Wang, L.; Li, X. Low-temperature selective catalytic reduction of NOx with NH3 over novel Mn–Zr mixed oxide catalysts. Ind. Eng. Chem. Res. 2014, 53, 2647–2655. [Google Scholar] [CrossRef]
- Zhang, B.; Liebau, M.; Liu, B.; Li, L.; Zhang, S.; Gläser, R. Selective catalytic reduction of NOx with NH3 over Mn–Zr–Ti mixed oxide catalysts. J. Mater. Sci. 2019, 54, 6943–6960. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, F.; He, H.; Shi, X.; Mo, J.; Wu, Z. Manganese–niobium mixed oxide catalyst for the selective catalytic reduction of NOx with NH3 at low temperatures. Chem. Eng. J. 2014, 250, 390–398. [Google Scholar] [CrossRef]
- Chang, H.; Li, J.; Chen, X.; Ma, L.; Yang, S.; Schwank, J.W.; Hao, J. Effect of Sn on MnOx–CeO2 catalyst for SCR of NOx by ammonia: Enhancement of activity and remarkable resistance to SO2. Catal. Commun. 2012, 27, 54–57. [Google Scholar] [CrossRef]
- Wang, C.; Gao, F.; Ko, S.; Liu, H.; Yi, H.; Tang, X. Structural control for inhibiting SO2 adsorption in porous MnCe nanowire aerogel catalysts for low-temperature NH3-SCR. Chem. Eng. J. 2022, 434, 134729. [Google Scholar] [CrossRef]
- Song, J.; Liu, S.; Ji, Y.; Xu, W.; Yu, J.; Liu, B.; Chen, W.; Zhang, J.; Jia, L.; Zhu, T.; et al. Dual single-atom Ce-Ti/MnO2 catalyst enhances low-temperature NH3-SCR performance with high H2O and SO2 resistance. Nano Res. 2023, 16, 299–308. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, S.; Zhang, R.; Li, W.; Wang, J.; Yang, S.; Wang, H.; Yang, M.; Liu, Y.; Qiao, W.; et al. Preparation of mesoporous Mn–Ce–Ti–O aerogels by a one-pot sol–gel method for selective catalytic reduction of NO with NH3. Materials 2020, 13, 475. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, J.; Li, J.; Ma, L.; Woo, S.I. Novel Mn–Ce–Ti mixed-oxide catalyst for the selective catalytic reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2014, 6, 14500–14508. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yao, X.; Cao, J.; Yang, F.; Tang, C.; Dong, L. Effect of Ti4+ and Sn4+ co-incorporation on the catalytic performance of CeO2-MnOx catalyst for low temperature NH3-SCR. Appl. Surf. Sci. 2019, 476, 283–292. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G. A highly effective catalyst of Sm-MnOx for the NH3-SCR of NOx at low temperature: Promotional role of Sm and its catalytic performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.W.; Fan, Z.; Wang, B.; Wang, Y.; He, C.; Wang, X.; Li, J.; Niu, C. “Fast SCR” reaction over Sm-modified MnOx-TiO2 for promoting reduction of NOx with NH3. Appl. Catal. A Gen. 2018, 564, 102–112. [Google Scholar] [CrossRef]
- Xu, Q.; Fang, Z.; Chen, Y.; Guo, Y.; Guo, Y.; Wang, L.; Wang, Y.; Zhang, J.; Zhan, W. Titania–samarium–manganese composite oxide for the low-temperature selective catalytic reduction of NO with NH3. Environ. Sci. Technol. 2020, 54, 2530–2538. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, S.; Wang, M.; Yang, J.; Chen, L.; Liu, W.; Liu, Q.; Su, B. Insights into samarium doping effects on catalytic activity and SO2 tolerance of MnFeOx catalyst for low-temperature NH3-SCR reaction. Fuel 2022, 321, 124113. [Google Scholar] [CrossRef]
- Sun, C.; Liu, H.; Chen, W.; Chen, D.; Yu, S.; Liu, A.; Dong, L.; Feng, S. Insights into the Sm/Zr co-doping effects on N2 selectivity and SO2 resistance of a MnOx-TiO2 catalyst for the NH3-SCR reaction. Chem. Eng. J. 2018, 347, 27–40. [Google Scholar] [CrossRef]
- Wang, B.; Wang, M.; Han, L.; Hou, Y.; Bao, W.; Zhang, C.; Feng, G.; Chang, L.; Huang, Z.; Wang, J. Improved activity and SO2 resistance by Sm-modulated redox of MnCeSmTiOx mesoporous amorphous oxides for low-temperature NH3-SCR of NO. ACS Catal. 2020, 10, 9034–9045. [Google Scholar] [CrossRef]
- Sun, P.; Guo, R.T.; Liu, S.M.; Wang, S.X.; Pan, W.G.; Li, M.Y. The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu. Appl. Catal. A Gen. 2017, 531, 129–138. [Google Scholar] [CrossRef]
- Fan, Z.; Shi, J.W.; Gao, C.; Gao, G.; Wang, B.; Wang, Y.; He, C.; Niu, C. Gd-modified MnOx for the selective catalytic reduction of NO by NH3: The promoting effect of Gd on the catalytic performance and sulfur resistance. Chem. Eng. J. 2018, 348, 820–830. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Feng, Y.; Si, Z.; Weng, D. Effects of WO3 doping on stability and N2O escape of MnOx–CeO2 mixed oxides as a low-temperature SCR catalyst. Catal. Commun. 2015, 69, 188–192. [Google Scholar] [CrossRef]
- Yang, S.; Qi, F.; Xiong, S.; Dang, H.; Liao, Y.; Wong, P.K.; Li, J. MnOx supported on Fe–Ti spinel: A novel Mn based low temperature SCR catalyst with a high N2 selectivity. Appl. Catal. B 2016, 181, 570–580. [Google Scholar] [CrossRef]
- Jia, B.; Guo, J.; Shu, S.; Fang, N.; Li, J.; Chu, Y. Effects of different Zr/Ti ratios on NH3–SCR over MnOx/ZryTi1-yO2: Characterization and reaction mechanism. Mol. Catal. 2017, 443, 25–37. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, K.H.; Hong, S.C. MnOx/CeO2–TiO2 mixed oxide catalysts for the selective catalytic reduction of NO with NH3 at low temperature. Chem. Eng. J. 2012, 195, 323–331. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, X.; Ma, H.; Yao, Y.; Liu, T. A comparative study of Mn/CeO2, Mn/ZrO2 and Mn/Ce-ZrO2 for low temperature selective catalytic reduction of NO with NH3 in the presence of SO2 and H2O. J. Environ. Sci. 2013, 25, 791–800. [Google Scholar] [CrossRef]
- Zhang, Q.; Qiu, C.; Xu, H.; Lin, T.; Lin, Z.; Gong, M.; Chen, Y. Low-temperature selective catalytic reduction of NO with NH3 over monolith catalyst of MnOx/CeO2–ZrO2–Al2O3. Catal. Today 2011, 175, 171–176. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Jin, W.; Liu, Y. Mn mixed oxide catalysts supported on Sn-doped CoAl-LDO for low-temperature NH3-SCR. Catal. Sci. Technol. 2023, 13, 3147–3157. [Google Scholar] [CrossRef]
- Xie, A.; Tang, Y.; Huang, X.; Jin, X.; Gu, P.; Luo, S.; Yao, C.; Li, X. Three-dimensional nanoflower MnCrOx/Sepiolite catalyst with increased SO2 resistance for NH3-SCR at low temperature. Chem. Eng. J. 2019, 370, 897–905. [Google Scholar] [CrossRef]
- Mu, J.; Li, X.; Sun, W.; Fan, S.; Wang, X.; Wang, L.; Qin, M.; Gan, G.; Yin, Z.; Zhang, D. Enhancement of low-temperature catalytic activity over a highly dispersed Fe–Mn/Ti catalyst for selective catalytic reduction of NOx with NH3. Ind. Eng. Chem. Res. 2018, 57, 10159–10169. [Google Scholar] [CrossRef]
- Shen, B.; Liu, T.; Zhao, N.; Yang, X.; Deng, L. Iron-doped Mn-Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO with NH3. J. Environ. Sci. 2010, 22, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, B.; Smirniotis, P.G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- Xiao, F.; Gu, Y.; Tang, Z.; Han, F.; Shao, J.; Xu, Q.; Zhu, H. ZrO2 modified MnOx/attapulgite catalysts for NH3-SCR of NO at low temperature. J. Chem. Eng. Jpn. 2015, 48, 481–487. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, X.; Zhao, T.; Cui, R.; Zhang, J.; Tang, Z. Rationally designed confined structure Ce-Mn-TNTs catalyst for low-temperature NH3-SCR reaction with superior activity and H2O/SO2 tolerance. ACS Sustain. Chem. Eng. 2024, 12, 9987–10001. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wang, Y.; Cen, W.; Wu, Z.; Wang, H.; Weng, X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B 2014, 148, 582–588. [Google Scholar] [CrossRef]
- Li, F.; Xie, J.; Fang, D.; He, F.; Qi, K.; Gong, P. Mechanistic study of Ce-modified MnOx/TiO2 catalysts with high NH3-SCR performance and SO2 resistance at low temperatures. Res. Chem. Intermediat. 2017, 43, 5413–5432. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Dong, G. Mn-Ce-V-WOx/TiO2 SCR catalysts: Catalytic activity, stability and interaction among catalytic oxides. Catalysts 2018, 8, 76. [Google Scholar] [CrossRef]
- Huang, J.; Huang, H.; Jiang, H.; Liu, L. The promotional role of Nd on Mn/TiO2 catalyst for the low-temperature NH3-SCR of NOx. Catal. Today 2019, 332, 49–58. [Google Scholar] [CrossRef]
- Liu, J.; Guo, R.T.; Li, M.Y.; Sun, P.; Liu, S.M.; Pan, W.G.; Liu, S.W.; Sun, X. Enhancement of the SO2 resistance of Mn/TiO2 SCR catalyst by Eu modification: A mechanism study. Fuel 2018, 223, 385–393. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Li, X.; Tan, P.; Zhou, A.; Fang, Q.; Chen, G. Ho-modified Mn-Ce/TiO2 for low-temperature SCR of NOx with NH3: Evaluation and characterization. Chin. J. Catal. 2018, 39, 1653–1663. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Q.; Wang, Q. Design of practical Ce/CoMnAl-LDO catalyst for low-temperature NH3-SCR. Catal. Commun. 2020, 142, 106037. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Q.; Ran, G.; Kong, M.; Ren, S.; Yang, J.; Li, J. V2O5-modified Mn-Ce/AC catalyst with high SO2 tolerance for low-temperature NH3-SCR of NO. Chem. Eng. J. 2019, 370, 810–821. [Google Scholar] [CrossRef]
- Wang, C.; Sani, Z.; Tang, X.; Wang, Y.; Yi, H.; Gao, F. Novel Ni-Mn bi-oxides doped active coke catalysts for NH3-SCR De-NOx at low temperature. ChemistrySelect 2020, 5, 6494–6503. [Google Scholar] [CrossRef]
- Li, R.; Yue, T.; Zheng, Y.; Li, G.; Gao, J.; Tong, Y.; Wang, J.; Ma, M.; Su, W. Promotional mechanism of La-Mn-Fe modification on activated coke for NH3-SCR of NOx at low temperatures. Fuel 2024, 371, 132016. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, J.; Tong, W.; Zhang, T.; Kong, M.; Ren, S. Low-temperature flue gas denitration with transition metal oxides supported on biomass char. J. Energy Inst. 2019, 92, 1158–1166. [Google Scholar] [CrossRef]
- Chen, L.; Ren, S.; Liu, W.; Yang, J.; Chen, Z.; Wang, M.; Liu, Q. Low-temperature NH3-SCR activity of M (M= Zr, Ni and Co) doped MnOx supported biochar catalysts. J. Environ. Chem. Eng. 2021, 9, 106504. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, D.; Cai, S.; Zhang, L.; Huang, L.; Li, H.; Maitarad, P.; Shi, L.; Gao, R.; Zhang, J. Low-temperature selective catalytic reduction of NO with NH3 over nanoflaky MnOx on carbon nanotubes in situ prepared via a chemical bath deposition route. Nanoscale 2013, 5, 9199–9207. [Google Scholar] [CrossRef]
- Pourkhalil, M.; Moghaddam, A.Z.; Rashidi, A.; Towfighi, J.; Mortazavi, Y. Preparation of highly active manganese oxides supported on functionalized MWNTs for low temperature NOx reduction with NH3. Appl. Surf. Sci. 2013, 279, 250–259. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Fang, C.; Gao, R.; Qian, Y.; Shi, L.; Zhang, J. MnOx–CeOx/CNTs pyridine-thermally prepared via a novel in situ deposition strategy for selective catalytic reduction of NO with NH3. RSC Adv. 2013, 3, 8811–8819. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Shi, L.; Fang, C.; Li, H.; Gao, R.; Huang, L.; Zhang, J. In situ supported MnOx–CeOx on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2013, 5, 1127–1136. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, P.; Pu, Y.; Jiang, L.; Yang, L.; Jiang, W.; Yao, L. MnCe/GAC-CNTs catalyst with high activity, SO2 and H2O tolerance for low-temperature NH3-SCR. Sep. Purif. Technol. 2023, 305, 122498. [Google Scholar] [CrossRef]
- You, X.; Sheng, Z.; Yu, D.; Yang, L.; Xiao, X.; Wang, S. Influence of Mn/Ce ratio on the physicochemical properties and catalytic performance of graphene supported MnOx-CeO2 oxides for NH3-SCR at low temperature. Appl. Surf. Sci. 2017, 423, 845–854. [Google Scholar] [CrossRef]
- Sheng, Z.; Ma, D.; Yu, D.; Xiao, X.; Huang, B.; Yang, L.; Wang, S. Synthesis of novel MnOx@ TiO2 core-shell nanorod catalyst for low-temperature NH3-selective catalytic reduction of NOx with enhanced SO2 tolerance. Chin. J. Catal. 2018, 39, 821–830. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, G.; Han, W.; Tang, Z. The water resistance enhanced strategy of Mn based SCR catalyst by construction of TiO2 shell and superhydrophobic coating. Chem. Eng. J. 2021, 426, 131334. [Google Scholar] [CrossRef]
- Ma, D.; Yang, L.; Huang, B.; Wang, L.; Wang, X.; Sheng, Z.; Dong, F. MnOx–CeO2@TiO2 core–shell composites for low temperature SCR of NOx. New J. Chem. 2019, 43, 15161–15168. [Google Scholar] [CrossRef]
- Cheng, S.; Shao, J.; Huang, B.; Guan, J.; Zhou, L. Promotion effect of urchin-like MnOx@PrOx hollow core–shell structure catalysts for the low-temperature selective catalytic reduction of NO with NH3. RSC Adv. 2020, 10, 13855–13865. [Google Scholar] [CrossRef]
- Li, S.; Huang, B.; Yu, C. A CeO2-MnOx core-shell catalyst for low-temperature NH3-SCR of NO. Catal. Commun. 2017, 98, 47–51. [Google Scholar] [CrossRef]
- Gan, L.; Li, K.; Yang, W.; Chen, J.; Peng, Y.; Li, J. Core-shell-like structured α-MnO2@CeO2 catalyst for selective catalytic reduction of NO: Promoted activity and SO2 tolerance. Chem. Eng. J. 2020, 391, 123473. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, G.; Tang, Z.; Zhang, J. MnFe@ CeOx core–shell nanocages for the selective catalytic reduction of NO with NH3 at low temperature. ACS Appl. Nano Mater. 2022, 5, 3619–3631. [Google Scholar] [CrossRef]
- Yu, C.; Hou, D.; Huang, B.; Lu, M.; Peng, R.; Zhong, Z. A MnOx@Eu-CeOx nanorod catalyst with multiple protective effects: Strong SO2-tolerance for low temperature DeNOx processes. J. Hazard. Mater. 2020, 399, 123011. [Google Scholar] [CrossRef]
- Li, Y.; Hou, Y.; Zhang, Y.; Yang, Y.; Huang, Z. Confinement of MnOx@Fe2O3 core-shell catalyst with titania nanotubes: Enhanced N2 selectivity and SO2 tolerance in NH3-SCR process. J. Colloid Interface Sci. 2022, 608, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Huang, X.; Cui, R.; Zhang, G.; Tang, Z. Unveiling a remarkable enhancement role by designing a confined structure Ho-TNTs@Mn catalyst for low-temperature NH3-SCR reaction. Nanoscale 2023, 15, 12540–12557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Huang, X.; Cui, R.; Song, X.; Tang, Z. Design the confinement structure Mn-TNTs@Ce catalyst for selective catalytic reduction of nitrogen oxides with superior SO2 resistance over a wider operation window. Appl. Catal. B 2024, 358, 124353. [Google Scholar] [CrossRef]
- Huang, X.; Dong, F.; Zhang, G.; Guo, Y.; Tang, Z. A strategy for constructing highly efficient yolk-shell Ce@Mn@TiOx catalyst with dual active sites for low-temperature selective catalytic reduction of NO with NH3. Chem. Eng. J. 2021, 419, 129572. [Google Scholar] [CrossRef]
- Yan, R.; Lin, S.; Li, Y.; Liu, W.; Mi, Y.; Tang, C.; Wang, L.; Peng, H. Novel shielding and synergy effects of Mn-Ce oxides confined in mesoporous zeolite for low temperature selective catalytic reduction of NOx with enhanced SO2/H2O tolerance. J. Hazard Mater. 2020, 396, 122592. [Google Scholar] [CrossRef]
- Cai, S.; Hu, H.; Li, H.; Shi, L.; Zhang, D. Design of multi-shell Fe2O3@ MnOx@CNTs for the selective catalytic reduction of NO with NH3: Improvement of catalytic activity and SO2 tolerance. Nanoscale 2016, 8, 3588–3598. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, D.; Zhang, J.; Cai, S.; Fang, C.; Huang, L.; Li, H.; Gao, R.; Shi, L. Design of meso-TiO2@MnOx–CeOx/CNTs with a core–shell structure as DeNOx catalysts: Promotion of activity, stability and SO2-tolerance. Nanoscale 2013, 5, 9821–9829. [Google Scholar] [CrossRef]
- Qi, Z.; Gao, F.; Ko, S.; Tang, X.; Yi, H.; Liu, H.; Luo, N.; Du, Y. Synthesis of novel Co(3-x) MnxO4@TiO2 core-shell catalyst for low-temperature NH3-SCR of NOx with enhanced SO2 tolerance. Chem. Phys. 2022, 5, 100120. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Xu, Y.; Li, Q. Promoting effect of Ti addition on three-dimensionally ordered macroporous Mn-Ce catalysts for NH3-SCR reaction: Enhanced N2 selectivity and remarkable water resistance. Appl. Surf. Sci. 2021, 569, 151047. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, B.; Chen, J.; Sun, Y.; Xu, M. Effect of Fe doping on NH3 adsorption and resistance to sulfur poisoning on the surface of β-MnO2 (110): A DFT-D study. J. Mater. Sci. 2022, 57, 18468–18485. [Google Scholar] [CrossRef]
- Tian, B.; Ma, S.; Guo, J.; Zhao, Y.; Gao, T.; Jiang, X. Superior indicative and regulative function of Fe doping amount for MnO2 catalyst with an oxygen vacancy in NH3-SCR reaction: A DFT+ U study. Appl. Surf. Sci. 2022, 601, 154162. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, B.; Liu, Y. Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B 2008, 79, 347–355. [Google Scholar] [CrossRef]
- Lin, Q.; Li, J.; Ma, L.; Hao, J. Selective catalytic reduction of NO with NH3 over Mn–Fe/USY under lean burn conditions. Catal. Today 2010, 151, 251–256. [Google Scholar] [CrossRef]
- Feng, X.; Wang, B.; Gao, G.; Gao, S.; Xie, C.; Shi, J.W. MnyCo3−yOx bimetallic oxide prepared by ultrasonic technology for significantly improved catalytic performance in the reduction of NOx with NH3. Fuel 2023, 352, 129159. [Google Scholar] [CrossRef]
- Kang, H.; Wang, J.; Zheng, J.; Chu, W.; Tang, C.; Ji, J.; Ren, R.; Wu, M.; Jing, F. Solvent-free elaboration of Ni-doped MnOx catalysts with high performance for NH3-SCR in low and medium temperature zones. Mol. Catal. 2021, 501, 111376. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.; Hou, B.; Du, Y.; Liu, L.; Yang, B. NiMn2O4 sphere catalyst for the selective catalytic reduction of NO by NH3: Insight into the enhanced activity via solvothermal method. J. Environ. Chem. Eng. 2021, 9, 105152. [Google Scholar] [CrossRef]
- Che, Y.; Liu, X.; Shen, Z.; Zhang, K.; Hu, X.; Chen, A.; Zhang, D. Improved N2 selectivity of MnOx catalysts for NOx reduction by engineering bridged Mn3+ sites. Langmuir 2023, 39, 7434–7443. [Google Scholar] [CrossRef]
- Li, Y.; Li, G.; Zou, Y.; Liu, W.; Zhang, H.; Lu, S.; Li, Z.; Zhang, S.; Peng, H. Unveiling the remarkable deNOx performance of MnMoVOx catalysts via dual regulation of the redox and acid sites. Appl. Catal. B 2024, 344, 123612. [Google Scholar] [CrossRef]
- Li, M.; Gao, M.; He, G.; Yu, Y.; He, H. Mechanistic insight into the promotion of the low-temperature NH3-selective catalytic reduction activity over MnxCe1–xOy catalysts: A combined experimental and density functional theory study. Environ. Sci. Technol. 2023, 57, 3875–3882. [Google Scholar] [CrossRef]
- Fang, X.; Liu, Y.; Cheng, Y.; Cen, W. Mechanism of Ce-modified birnessite-MnO2 in promoting SO2 poisoning resistance for low-temperature NH3-SCR. ACS Catal. 2021, 11, 4125–4135. [Google Scholar] [CrossRef]
- Cheng, L.; Sin, S.; Ji, J.; Yang, S.; Tan, C.; Gu, Z.; Song, W.; Huang, C.; Sun, C.; Tang, C.; et al. Selective catalytic reduction of NO with NH3 over MnOx-CeO2 catalysts: The great synergy between CeO2 and crystalline phase of Mn3O4. Fuel 2023, 342, 127772. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, A.; Zhang, J.; Guo, Y.; Guo, Y.; Wang, L.; Zhan, W. Low-temperature NH3-SCR on Cex-Mn-Tiy mixed oxide catalysts: Improved performance by the mutual effect between Ce and Ti. Catalysts 2022, 12, 471. [Google Scholar] [CrossRef]
- Teng, Y.; Guo, X.; Xue, H.; Meng, T.; Han, L. Activity improvement of Mn/Al2O3 for NH3-SCR reaction via the rare-earth (Ce, La, Nd and Y) oxides modification. Catal. Lett. 2024, 154, 3645–3653. [Google Scholar] [CrossRef]
- Gevers, L.E.; Enakonda, L.R.; Shahid, A.; Ould-Chikh, S.; Silva, C.I.; Paalanen, P.P.; Aguilar-Tapia, A.; Jean-Louis, H.; Nejib Hedhili, M.; Wen, F.; et al. Unraveling the structure and role of Mn and Ce for NOx reduction in application-relevant catalysts. Nat. Commun. 2022, 13, 2960. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, T.; Weng, X.; Wang, Y.; Wu, Z.; Wang, H. DRIFT studies on the selectivity promotion mechanism of Ca-modified Ce-Mn/TiO2 catalysts for low-temperature NO reduction with NH3. J. Phys. Chem. C 2012, 116, 16582–16592. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, Y.J.; Lee, K.Y.; Ha, H.P.; Kwon, D.W. Surface insights into MnOx-based catalysts containing metal oxides for the selective catalytic reduction of NOx with NH3. Appl. Catal. A Gen. 2022, 643, 118770. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, F.; Ko, S.; Wang, C.; Liu, H.; Tang, X.; Yi, H.; Zhou, Y. Superior catalytic performance within H2O vapor of W-modified CoMn2O4/TiO2 catalyst for selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2022, 434, 134770. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, G.; Tang, Z.; Zhang, J. Engineering yolk–shell MnFe@CeOx@TiOx nanocages as a highly efficient catalyst for selective catalytic reduction of NO with NH3 at low temperatures. Nanoscale 2022, 14, 12281–12296. [Google Scholar] [CrossRef]
- Qiao, T.; Liu, Z.; Liu, C.; Meng, W.; Sun, H.; Lu, Y. MnOx location on MnOx-ZSM-5 to influence the catalytic activity for selective catalytic reduction of NOx by NH3. Appl. Catal. A Gen. 2021, 617, 118128. [Google Scholar] [CrossRef]
- Ran, X.; Li, M.; Wang, K.; Qian, X.; Fan, J.; Sun, Y.; Luo, W.; Teng, W.; Zhang, W.; Yang, J. Spatially confined tuning the interfacial synergistic catalysis in mesochannels toward selective catalytic reduction. ACS Appl. Mater. Interfaces 2019, 11, 19242–19251. [Google Scholar] [CrossRef]
- Guo, K.; Fan, G.; Gu, D.; Yu, S.; Ma, K.; Liu, A.; Tan, W.; Wang, J.; Du, X.; Zou, W.; et al. Pore size expansion accelerates ammonium bisulfate decomposition for improved sulfur resistance in low-temperature NH3-SCR. ACS Appl. Mater. Interfaces 2019, 11, 4900–4907. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.; Hultermans, R.J.; Lugt, P.M.; Burgers, M.H.W.; Rigutto, M.S.; Van Bekkum, H.; Van den Bleek, C.M. Selective reduction of NOx with ammonia over cerium-exchanged mordenite. Appl. Catal. B 1994, 4, 95–104. [Google Scholar] [CrossRef]
- Liu, J.; Shi, X.; Lv, Z.; Yu, Y.; He, H. Ceria–tungsten–tin oxide catalysts with superior regeneration capacity after sulfur poisoning for NH3-SCR process. Catal. Sci. Technol. 2022, 12, 2471–2481. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Li, Q.; Wang, Z.; Zhang, Z.; Zheng, L. Ce–Ti amorphous oxides for selective catalytic reduction of NO with NH3: Confirmation of Ce–O–Ti active sites. Environ. Sci. Technol. 2012, 46, 9600–9605. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.; Xin, L.; Zhang, T.; Xu, Y.; Jiang, F.; Liu, X. Unraveling reactivity descriptors and structure sensitivity in low-temperature NH3-SCR reaction over CeTiOx catalysts: A combined computational and experimental study. ACS Catal. 2021, 11, 7613–7636. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, H.; Dong, L. Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2016, 6, 1248–1264. [Google Scholar] [CrossRef]
- Ma, L.; Seo, C.Y.; Nahata, M.; Chen, X.; Li, J.; Schwank, J.W. Shape dependence and sulfate promotion of CeO2 for selective catalytic reduction of NOx with NH3. Appl. Catal. B 2018, 232, 246–259. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, R.T.; Wu, L.J.; Pan, W.G. The enhancement of NH3-SCR performance for CeO2 catalyst by CO pretreatment. Environ. Sci. Pollut. Res. 2020, 27, 13617–13636. [Google Scholar] [CrossRef]
- Yao, X.; Wang, Z.; Yu, S.; Yang, F.; Dong, L. Acid pretreatment effect on the physicochemical property and catalytic performance of CeO2 for NH3-SCR. Appl. Catal. A Gen. 2017, 542, 282–288. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Wang, X.; Cong, Q.; Ma, H.; Guo, T.; Li, S.; Li, W. High-performance CeO2/halloysite hierarchical catalysts with promotional redox property and acidity for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2020, 390, 124251. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Cao, Y.; Yao, X.; Ge, C.; Gao, F.; Deng, Y.; Tang, C.; Dong, L. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B 2015, 165, 589–598. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, R.; Wang, M.; Li, Y.; Tong, Y.; Yang, P.; Zhu, Y. Two steps synthesis of CeTiOx oxides nanotube catalyst: Enhanced activity, resistance of SO2 and H2O for low temperature NH3-SCR of NOx. Appl. Catal. B 2021, 282, 119542. [Google Scholar] [CrossRef]
- Yao, X.; Kang, K.; Cao, J.; Chen, L.; Luo, W.; Zhao, W.; Rong, J.; Chen, Y. Enhancing the denitration performance and anti-K poisoning ability of CeO2-TiO2/P25 catalyst by H2SO4 pretreatment: Structure-activity relationship and mechanism study. Appl. Catal. B 2020, 269, 118808. [Google Scholar] [CrossRef]
- Zhou, Q.; He, K.; Wang, X.; Lim, K.H.; Liu, P.; Wang, W.; Wang, Q. Investigation on the redox/acidic features of bimetallic MOF-derived CeMOx catalysts for low-temperature NH3-SCR of NOx. Appl. Catal. A Gen. 2022, 643, 118796. [Google Scholar] [CrossRef]
- Zhang, R.; Zhong, Q.; Zhao, W.; Yu, L.; Qu, H. Promotional effect of fluorine on the selective catalytic reduction of NO with NH3 over CeO2-TiO2 catalyst at low temperature. Appl. Surf. Sci. 2014, 289, 237–244. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Hongmanorom, P.; Wang, Z.; Zhang, S.; Chen, J.; Zhong, Q.; Kawi, S. Active sites adjustable phosphorus promoted CeO2/TiO2 catalysts for selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2021, 409, 128242. [Google Scholar] [CrossRef]
- Zhao, W.; Tang, Y.; Wan, Y.; Li, L.; Yao, S.; Li, X.; Gu, J.; Li, Y.; Shi, J. Promotion effects of SiO2 or/and Al2O3 doped CeO2/TiO2 catalysts for selective catalytic reduction of NO by NH3. J. Hazard. Mater. 2014, 278, 350–359. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Li, J.; Ma, L.; Arandiyan, H.; Du, Y.; Xu, J.; Hao, J. Enhancement of activity and sulfur resistance of CeO2 supported on TiO2–SiO2 for the selective catalytic reduction of NO by NH3. Environ. Sci. Technol. 2012, 46, 6182–6189. [Google Scholar] [CrossRef]
- Li, L.; Tan, W.; Wei, X.; Fan, Z.; Liu, A.; Guo, K.; Ma, K.; Yu, S.; Ge, C.; Tang, C.; et al. Mo doping as an effective strategy to boost low temperature NH3-SCR performance of CeO2/TiO2 catalysts. Catal. Commun. 2018, 114, 10–14. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Y.; Yang, Q.; Hu, F.; Li, J.; Crittenden, J. NH3-SCR performance of WO3 blanketed CeO2 with different morphology: Balance of surface reducibility and acidity. Catal. Today 2019, 332, 42–48. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Zhao, K.; Shan, Y.; Ma, Y.; Wang, C.; Li, Z.; Sun, X.; Li, K.; He, H.; et al. The design of the highly active NH3-SCR catalyst Ce-W/UiO-66: Close coupling of active sites and acidic sites. Sep. Purif. Technol. 2022, 300, 121864. [Google Scholar] [CrossRef]
- Yang, B.; Shen, Y.; Zeng, Y.; Li, B.; Shen, S.; Zhu, S. Effects of tin doping level on structure, acidity and catalytic performance of Ti-Ce-Ox catalyst for selective catalytic reduction of NO by ammonia. J. Mol. Catal. A Chem. 2016, 418, 138–145. [Google Scholar] [CrossRef]
- Guo, R.T.; Li, M.Y.; Sun, P.; Pan, W.G.; Liu, S.M.; Liu, J.; Sun, X.; Liu, S.W. Mechanistic investigation of the promotion effect of bi modification on the NH3–SCR performance of Ce/TiO2 catalyst. J. Phys. Chem. C 2017, 121, 27535–27545. [Google Scholar] [CrossRef]
- Mu, Y.; Huang, X.; Tang, Z.; Wang, Q. Ordered mesoporous TiO2 framework confined CeSn catalyst exhibiting excellent high activity for selective catalytic reduction of NO with NH3 at low temperature. Chem. Eng. J. 2023, 454, 140181. [Google Scholar] [CrossRef]
- Zhang, G.; Han, W.; Zhao, H.; Zong, L.; Tang, Z. Solvothermal synthesis of well-designed ceria-tin-titanium catalysts with enhanced catalytic performance for wide temperature NH3-SCR reaction. Appl. Catal. B 2018, 226, 117–126. [Google Scholar] [CrossRef]
- Mu, Y.; Huang, X.; Tang, Z.; Wang, Q. Facile strategy to promote SO2-resistance ability for NH3-SCR catalyst CeSnTiOx via copper sulfate-modified: Boost from a double reaction site. Sep. Purif. Technol. 2025, 353, 128490. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhao, Q.; Ke, J.; Xiao, H.; Lv, X.; Liu, S.; Tade, M.; Wang, S. Mechanistic investigation of the enhanced NH3-SCR on cobalt-decorated Ce-Ti mixed oxide: In situ FTIR analysis for structure-activity correlation. Appl. Catal. B 2017, 200, 297–308. [Google Scholar] [CrossRef]
- Chang, H.; Li, J.; Yuan, J.; Chen, L.; Dai, Y.; Arandiyan, H.; Xu, J.; Hao, J. Ge, Mn-doped CeO2–WO3 catalysts for NH3–SCR of NOx: Effects of SO2 and H2 regeneration. Catal. Today 2013, 201, 139–144. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, L.; Liebau, M.; Ren, Y.; Luo, C.; Liu, B.; Zhang, S.; Gläser, R. Promotion effect of niobium on ceria catalyst for selective catalytic reduction of NO with NH3. J. Rare Earth 2022, 40, 1535–1545. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, R. Rational design of porous CexNb1− x oxide hollow nanospheres as a novel NH3-SCR catalyst. J. Mater. Chem. A Mater. 2022, 10, 12269–12277. [Google Scholar] [CrossRef]
- Ma, S.; Gao, W.; Yang, Z.; Lin, R.; Wang, X.; Zhu, X.; Jiang, Y. Superior Ce–Nb–Ti oxide catalysts for selective catalytic reduction of NO with NH3. J. Energy Inst. 2021, 94, 73–84. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, W.; Bao, C.; Yang, Z.; Lin, R.; Wang, X. Comparative study of Ce-Nb-Ti oxide catalysts prepared by different methods for selective catalytic reduction of NO with NH3. Mol. Catal. 2020, 496, 111161. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, S.; Liu, Q.; Yang, P.; Cai, Y.; Tan, W.; Song, W.; Gao, F.; Dong, L. The enhancement effect of Nb over CeSi2 catalyst for the low-temperature NH3-SCR performance. Chem. Phys. 2023, 6, 100205. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, H.; Du, T.; Ma, W.; Zhong, Q. H2O and SO2 tolerance, activity and reaction mechanism of sulfated Ni–Ce–La composite oxide nanocrystals in NH3-SCR. Chem. Eng. J. 2016, 296, 122–131. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, J.; Zhang, S.; Ma, L.; Woo, S.I. Selective catalytic reduction of NOx by NH3 over MoO3-promoted CeO2/TiO2 catalyst. Catal. Commun. 2014, 46, 90–93. [Google Scholar] [CrossRef]
- Tan, W.; Wang, J.; Cai, Y.; Li, L.; Xie, S.; Gao, F.; Liu, F.; Dong, L. Molybdenum oxide as an efficient promoter to enhance the NH3-SCR performance of CeO2-SiO2 catalyst for NOx removal. Catal. Today 2022, 397, 475–483. [Google Scholar] [CrossRef]
- Liu, W.; Gao, Z.; Zhao, X.; Gao, J.; Yang, R.; Yu, L. Promotion effect of chromium on the activity and SO2 resistance of CeO2–TiO2 catalysts for the NH3-SCR reaction. Ind. Eng. Chem. Res. 2021, 60, 11676–11688. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Guo, R.T.; Guan, Z.Z.; Shi, X.; Pan, W.G.; Fu, Z.G.; Qin, H.; Liu, X.Y. The promotion effect of Cr additive on CeZr2Ox catalyst for the low-temperature selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2019, 485, 133–140. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, R.; Chu, B.; Xie, S.; Chen, K.; Li, L.; Zhao, S.; Li, B.; Dong, L. Significantly enhanced activity and SO2 resistance of Zr-modified CeTiOx catalyst for low-temperature NH3-SCR by H2 reduction treatment. Mol. Catal. 2022, 518, 112069. [Google Scholar] [CrossRef]
- Gong, P.; Xie, J.; Fang, D.; He, F.; Li, F.; Qi, K. Enhancement of the NH3-SCR property of Ce-Zr-Ti by surface and structure modification with P. Appl. Surf. Sci. 2020, 505, 144641. [Google Scholar] [CrossRef]
- Jin, Q.; Chen, M.; Tao, X.; Lu, B.; Shen, J.; Shen, Y.; Zeng, Y. Component synergistic catalysis of Ce-Sn-W-Ba-Ox/TiO2 in selective catalytic reduction of NO with ammonia. Appl. Surf. Sci. 2020, 512, 145757. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, G.; Mu, B.; Tang, Z.; Zhang, J. The promoting effect of palygorskite on CeO2-WO3-TiO2 catalyst for the selective catalytic reduction of NOx with NH3. Appl. Clay Sci. 2020, 192, 105641. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, W.; Wu, Q.; Mi, J.; Wang, X.; Ma, L.; Jiang, L.; Au, C.; Li, J. Effects of anaerobic SO2 treatment on nano-CeO2 of different morphologies for selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2020, 382, 122910. [Google Scholar] [CrossRef]
- Tan, W.; Wang, J.; Li, L.; Liu, A.; Song, G.; Guo, K.; Luo, Y.; Liu, F.; Gao, F.; Dong, L. Gas phase sulfation of ceria-zirconia solid solutions for generating highly efficient and SO2 resistant NH3-SCR catalysts for NO removal. J. Hazard. Mater. 2020, 388, 121729. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yu, D.; Sheng, Z.; Yang, L. Novel CeO2@TiO2 core–shell nanostructure catalyst for selective catalytic reduction of NOx with NH3. J. Environ. Sci. 2017, 55, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Luo, J.; Tang, W.; Li, B.; Li, A.; Zhou, D.; Ou, Y.; Hou, C. A superior CeO2@TiO2 catalyst with high dispersion of CeO2 for selective catalytic reduction of NOx with NH3. Appl. Catal. A Gen. 2023, 661, 119168. [Google Scholar] [CrossRef]
- Liu, J.; Huo, Y.; Shi, X.; Liu, Z.; Shan, Y.; Yu, Y.; Shan, W.; He, H. Insight into the remarkable enhancement of NH3-SCR performance of Ce-Sn oxide catalyst by tungsten modification. Catal. Today 2023, 410, 36–44. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Y.; Wang, H.; Zhang, Z.; Wang, J.; Guo, M.; Liu, Q. Promotional effects on NH3-SCR performance of CeO2–SnO2 catalysts doped by TiO2: A mechanism study. Cataly. Surv. Asia 2021, 25, 48–57. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, X.; Chen, S.; Zhu, X.; Liu, H.; Chen, J.; Chen, D.; Sun, C.; Li, J. Hexavalent metal cations doped into ceria inducing the formation of binuclear sites Ce3+–O–Ce3+ to boost the NH3-SCR reaction. ACS Catal. 2024, 14, 7277–7288. [Google Scholar] [CrossRef]
- Liu, H.; Gao, C.; Chen, J.; Mi, J.; Yang, S.; Chen, D.; Si, W.; Peng, Y.; Sun, C.; Li, J. Optimized local geometry and electronic structure of MoO3/CeO2 catalyst by adding copper cations for boosted nitrogen oxide reduction performance. Appl. Catal. B 2023, 332, 122742. [Google Scholar] [CrossRef]
- Chen, W.; Gu, J.; He, B.; Duan, R.; Liu, L.; Wang, X. Computational screening and synthesis of M(M= Mo and Cu)-doped CeO2/silicalite-1 for medium-/low-temperature NH3–SCR. Ind. Eng. Chem. Res. 2022, 61, 10091–10105. [Google Scholar] [CrossRef]
- He, J.F.; Xiong, Z.B.; Du, Y.P.; Lu, W. Morphology effect of tungsten oxide on Ce/W catalyst for selective catalytic reduction of NO with NH3: Influence of structure-directing agents. J. Energy Inst. 2021, 94, 85–95. [Google Scholar] [CrossRef]
- Fang, X.; Qu, W.; Qin, T.; Hu, X.; Chen, L.; Ma, Z.; Liu, X.; Tang, X. Abatement of nitrogen oxides via selective catalytic reduction over Ce1–W1 atom-pair sites. Environ. Sci. Technol. 2022, 56, 6631–6638. [Google Scholar] [CrossRef] [PubMed]
- Inomata, Y.; Hata, S.; Mino, M.; Kiyonaga, E.; Morita, K.; Hikino, K.; Yoshida, K.; Kubota, H.; Toyao, T.; Shimizu, K.; et al. Bulk vanadium oxide versus conventional V2O5/TiO2: NH3–SCR catalysts working at a low temperature below 150 °C. ACS Catal. 2019, 9, 9327–9331. [Google Scholar] [CrossRef]
- Lian, Z.; Wei, J.; Shan, W.; Yu, Y.; Radjenovic, P.M.; Zhang, H.; He, G.; Liu, F.; Li, J.-F.; Tian, Z.-Q.; et al. Adsorption-induced active vanadium species facilitate excellent performance in low-temperature catalytic NOx abatement. J. Am. Chem. Soc. 2021, 143, 10454–10461. [Google Scholar] [CrossRef]
- Yin, Y.; Luo, B.; Li, K.; Moskowitz, B.M.; Mosevizky Lis, B.; Wachs, I.E.; Zhu, M.; Sun, Y.; Zhu, T.; Li, X. Plasma-assisted manipulation of vanadia nanoclusters for efficient selective catalytic reduction of NOx. Nat. Commun. 2024, 15, 3592. [Google Scholar] [CrossRef]
- Lin, L.Y.; Wang, Y.C.; Liu, Z.L. Highly active and stable VOx/TiO2 nanosheets for low-temperature NH3-SCR of NO: Structure-directing role of support. Chem. Eng. J. 2024, 484, 149637. [Google Scholar] [CrossRef]
- Hwang, K.H.; Park, N.; Lee, H.; Lee, K.M.; Jeon, S.W.; Kim, H.S.; Lee, Y.; Kim, T.J.; Lee, W.B.; Kim, D.H. Mechanochemical localization of vanadia on titania to prepare a highly sulfur-resistant catalyst for low-temperature NH3-SCR. Appl. Catal. B 2023, 324, 122290. [Google Scholar] [CrossRef]
- Inomata, Y.; Kubota, H.; Honmatsu, Y.; Takamitsu, H.; Sakotani, S.; Yoshida, K.; Toyao, T.; Shimizu, K.; Murayama, T. Sodium ion intercalation in vanadium oxide promotes low-temperature NH3-SCR activity: Sodium vanadium bronzes (Na0.33V2O5) for NOx removal. Appl. Catal. B 2023, 328, 122536. [Google Scholar] [CrossRef]
- Inomata, Y.; Kubota, H.; Hata, S.; Kiyonaga, E.; Morita, K.; Yoshida, K.; Sakaguchi, N.; Toyao, T.; Shimizu, K.; Ishikawa, S.; et al. Bulk tungsten-substituted vanadium oxide for low-temperature NOx removal in the presence of water. Nat. Commun. 2021, 12, 557. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Li, H.; Hu, H.; Hu, X.; Shi, L.; Zhang, D. Promotional effects of zirconium doped CeVO4 for the low-temperature selective catalytic reduction of NOx with NH3. Appl. Catal. B 2016, 183, 269–281. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Namuangruk, S.; Hu, H.; Hu, X.; Shi, L.; Zhang, D. Morphology-dependent performance of Zr–CeVO4/TiO2 for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2016, 6, 5543–5553. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Lian, Z.; Shan, W.; Xie, L.; Asakura, K.; Yang, W.; Deng, H. Highly dispersed iron vanadate catalyst supported on TiO2 for the selective catalytic reduction of NOx with NH3. J. Catal. 2013, 307, 340. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.H.; Kwon, D.W.; Lee, K.Y.; Ha, H.P. Unveiling the traits of rare earth metal (RM)-substituted bimetallic Ce0.5RM0.5V1O4 phases to activate selective NH3 oxidation and NOx reduction. Appl. Surf. Sci. 2020, 518, 146238. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, X.; Li, Y.; Niu, X.; Zhu, Y. Improving NH3-SCR denitrification performance over WaC0.4TiOx catalysts: Effect of surface acidity due to W addition on low-temperature and high-temperature activity. Appl. Catal. A Gen. 2022, 643, 118705. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Zhang, S.; Zhong, Q.; Rong, W.; Li, X. One-pot synthesis of ceria and cerium phosphate (CeO2-CePO4) nanorod composites for selective catalytic reduction of NO with NH3: Active sites and reaction mechanism. J. Colloid Interface Sci. 2018, 524, 8–15. [Google Scholar] [CrossRef]
- Yao, W.; Liu, Y.; Wang, X.; Weng, X.; Wang, H.; Wu, Z. The superior performance of sol–gel made Ce–O–P catalyst for selective catalytic reduction of NO with NH3. J. Phys. Chem. C 2016, 120, 221–229. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Zhang, Z.; Tan, H.; Yuan, F.; Zhu, Y. Influence of CePO4 with different crystalline phase on selective catalytic reduction of NOx with ammonia. J. Rare Earth 2022, 40, 1219–1231. [Google Scholar] [CrossRef]
- Lin, C.H.; Qin, R.C.; Cao, N.; Wang, D.; Liu, C.G. Synergistic effects of Keggin-type phosphotungstic acid-supported single-atom catalysts in a fast NH3-SCR reaction. Inorg. Chem. 2022, 61, 19156–19171. [Google Scholar] [CrossRef]
- Xie, R.; Ma, L.; Sun, K.; Zhou, G.; Qu, Z.; Yan, N. Catalytic performance and mechanistic evaluation of sulfated CeO2 cubes for selective catalytic reduction of NOx with ammonia. J. Hazard. Mater. 2021, 420, 126545. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Chen, C.; Ma, M.; He, C.; Miao, J.; Li, H.; Chen, J. Selective catalytic reduction of NOx with NH3 over TiO2 supported metal sulfate catalysts prepared via a sol–gel protocol. New J. Chem. 2020, 44, 13598–13605. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Elsener, M. Selective catalytic reduction of NO and NO2 at low temperatures. Catal. Today 2002, 73, 239–247. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Madia, G. Reaction pathways in the selective catalytic reduction process with NO and NO2 at low temperatures. Ind. Eng. Chem. Res. 2001, 40, 52–59. [Google Scholar] [CrossRef]
- Wei, Z.; Kang, X.; Yunhao, T.; Chuan, Q.; Shan, C.; Ying, M. Application of transition metal based MOF materials in selective catalytic reduction of nitrogen oxides. Prog. Chem. 2022, 34, 2638. [Google Scholar] [CrossRef]
- Adil, K.; Świrk, K.; Zaki, A.; Assen, A.H.; Delahay, G.; Belmabkhout, Y.; Cadiau, A. Perspectives in adsorptive and catalytic mitigations of NOx using metal–organic frameworks. Energy Fuels 2022, 36, 3347–3371. [Google Scholar] [CrossRef]
- Gao, Y.; Gong, S.Y.; Chen, B.; Xing, W.H.; Fei, Y.F.; Hu, Z.T.; Pan, Z. Progress in metal-organic framework catalysts for selective catalytic reduction of NOx: A mini-review. Atmosphere 2022, 13, 793. [Google Scholar] [CrossRef]
- Szymaszek, A.; Motak, M.; Samojeden, B. The application of modified layered double hydroxides in selective catalytic reduction of nitrogen oxides by ammonia (NH-SCR). Pol. J. Chem. Technol. 2020, 22, 61–67. [Google Scholar] [CrossRef]
- Yan, Q.; Hou, X.; Liu, G.; Li, Y.; Zhu, T.; Xin, Y.; Wang, Q. Recent advances in layered double hydroxides (LDHs) derived catalysts for selective catalytic reduction of NOx with NH3. J. Hazard. Mater. 2020, 400, 123260. [Google Scholar] [CrossRef]
- Tan, Y.; Yi, H.; Tang, X.; Yu, Q.; Gao, F.; Liu, J.; Wang, Y.; Zhou, Y.; Kang, D.; Zhao, S. Layered double hydroxides for air pollution control: Applications, mechanisms and trends. J. Clean. Prod. 2024, 436, 140635. [Google Scholar] [CrossRef]
- Gui, R.; Yan, Q.; Xue, T.; Gao, Y.; Li, Y.; Zhu, T.; Wang, Q. The promoting/inhibiting effect of water vapor on the selective catalytic reduction of NOx. J. Hazard. Mater. 2022, 439, 129665. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Tan, D.; Ye, Y.; Zhang, B.; Huang, B.; Zhong, W.; Hu, J. Overview of mechanisms of promotion and inhibition by H2O for selective catalytic reduction denitrification. Fuel Process. Technol. 2023, 252, 107956. [Google Scholar] [CrossRef]
- Zhang, N.; He, H.; Wang, D.; Li, Y. Challenges and opportunities for manganese oxides in low-temperature selective catalytic reduction of NOx with NH3: H2O resistance ability. J. Solid State Chem. 2020, 289, 121464. [Google Scholar] [CrossRef]
- Shen, Z.; Ren, S.; Zhang, B.; Bian, W.; Xing, X.; Zheng, Z. Sulfur and water resistance of carbon-based catalysts for low-temperature selective catalytic reduction of NOx: A review. Catalysts 2023, 13, 1434. [Google Scholar] [CrossRef]
- He, Z.; Wang, Y.; Liu, Y.; Lian, L.; Kong, D.; Zhao, Y. Recent advances in sulfur poisoning of selective catalytic reduction (SCR) denitration catalysts. Fuel 2024, 365, 131126. [Google Scholar] [CrossRef]
- Szymaszek, A.; Samojeden, B.; Motak, M. The deactivation of industrial SCR catalysts—A short review. Energies 2020, 13, 3870. [Google Scholar] [CrossRef]
- Song, I.; Lee, H.; Jeon, S.W.; Ibrahim, I.A.; Kim, J.; Byun, Y.; Jun, D.K.; Han, J.W.; Kim, D.H. Simple physical mixing of zeolite prevents sulfur deactivation of vanadia catalysts for NOx removal. Nat. Commun. 2021, 12, 901. [Google Scholar] [CrossRef]
- Song, J.; Sun, X.; Zhang, G.; Cheng, S.; Xu, Y.; Jiang, Y. Recent advances in improving SO2 resistance of Ce-based catalysts for NH3-SCR: Mechanisms and strategies. Mol. Catal. 2024, 564, 114347. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, F.; Hou, Y.; Jia, Y.; Liao, B.; Shen, B.; Zhang, D. Core-shell materials for selective catalytic reducing of NOx with ammonia: Synthesis, anti-poisoning performance, and remaining challenges. Fuel Process. Technol. 2023, 243, 107675. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Yang, J. Spatially nanoconfined architectures: A promising design for selective catalytic reduction of NOx. ChemCatChem 2020, 12, 5599–5610. [Google Scholar] [CrossRef]
- Chen, J.; Fang, X.; Ren, Z.; Qu, W.; Hu, X.; Ma, Z.; Chen, L.; Liu, X.; Chen, Y.; Tang, X. Single Mo atoms paired with neighbouring Ti atoms catalytically decompose ammonium bisulfate formed in low-temperature SCR. J. Mater. Chem. A Mater. 2022, 10, 6065–6072. [Google Scholar] [CrossRef]
- Guan, B.; Jiang, H.; Wei, Y.; Liu, Z.; Wu, X.; Lin, H.; Huang, Z. Density functional theory researches for atomic structure, properties prediction, and rational design of selective catalytic reduction catalysts: Current progresses and future perspectives. Mol. Catal. 2021, 510, 111704. [Google Scholar] [CrossRef]
- Dong, Y.; Ran, M.; Zhang, X.; Lin, S.; Zhao, H.; Yang, Y.; Liu, S.; Zheng, C.; Gao, X. Machine learning-accelerated disentanglement of activity–selectivity trade-off of multielement oxide denitration catalysts. ACS ES T Eng. 2024, 4, 1312–1320. [Google Scholar] [CrossRef]
- Lu, M.; Gao, F.; Tan, Y.; Yi, H.; Gui, Y.; Xu, Y.; Wang, Y.; Zhou, Y.; Tang, X.; Chen, L. Knowledge-driven experimental discovery of Ce-based metal oxide composites for selective catalytic reduction of NOx with NH3 through interpretable machine learning. ACS Appl. Mater. Interfaces 2024, 16, 3593–3604. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).