Strength of London Dispersion Forces in Organic Structure Directing Agent—Zeolite Assemblies
Abstract
1. Introduction
2. Results
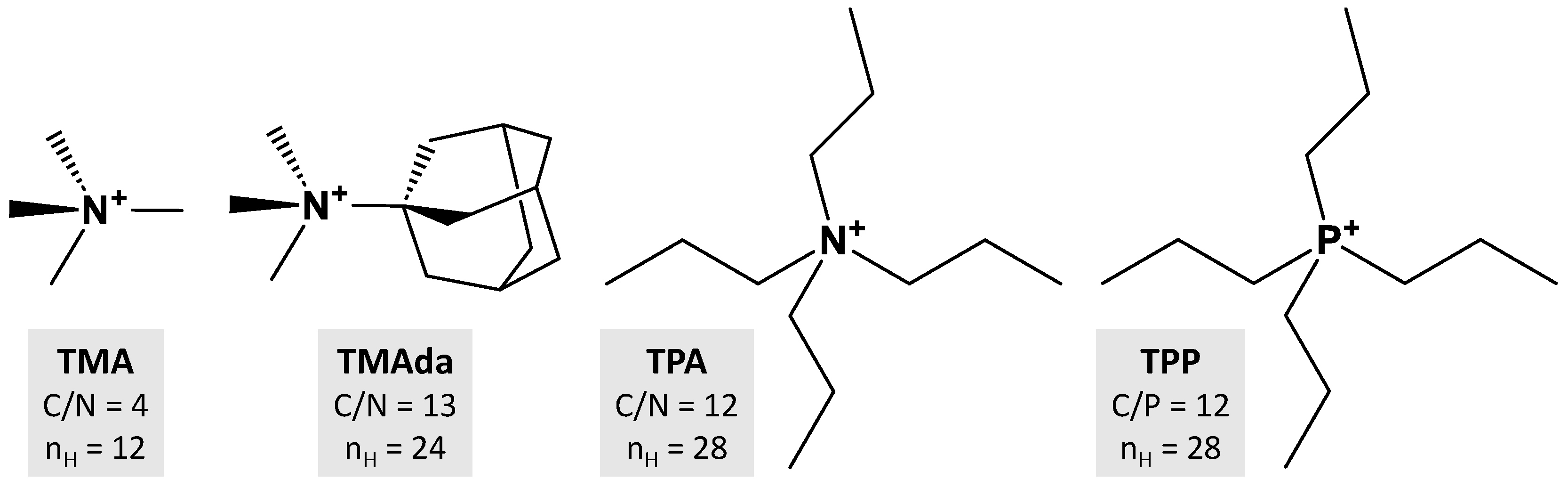
2.1. DFT-D3 Calculations
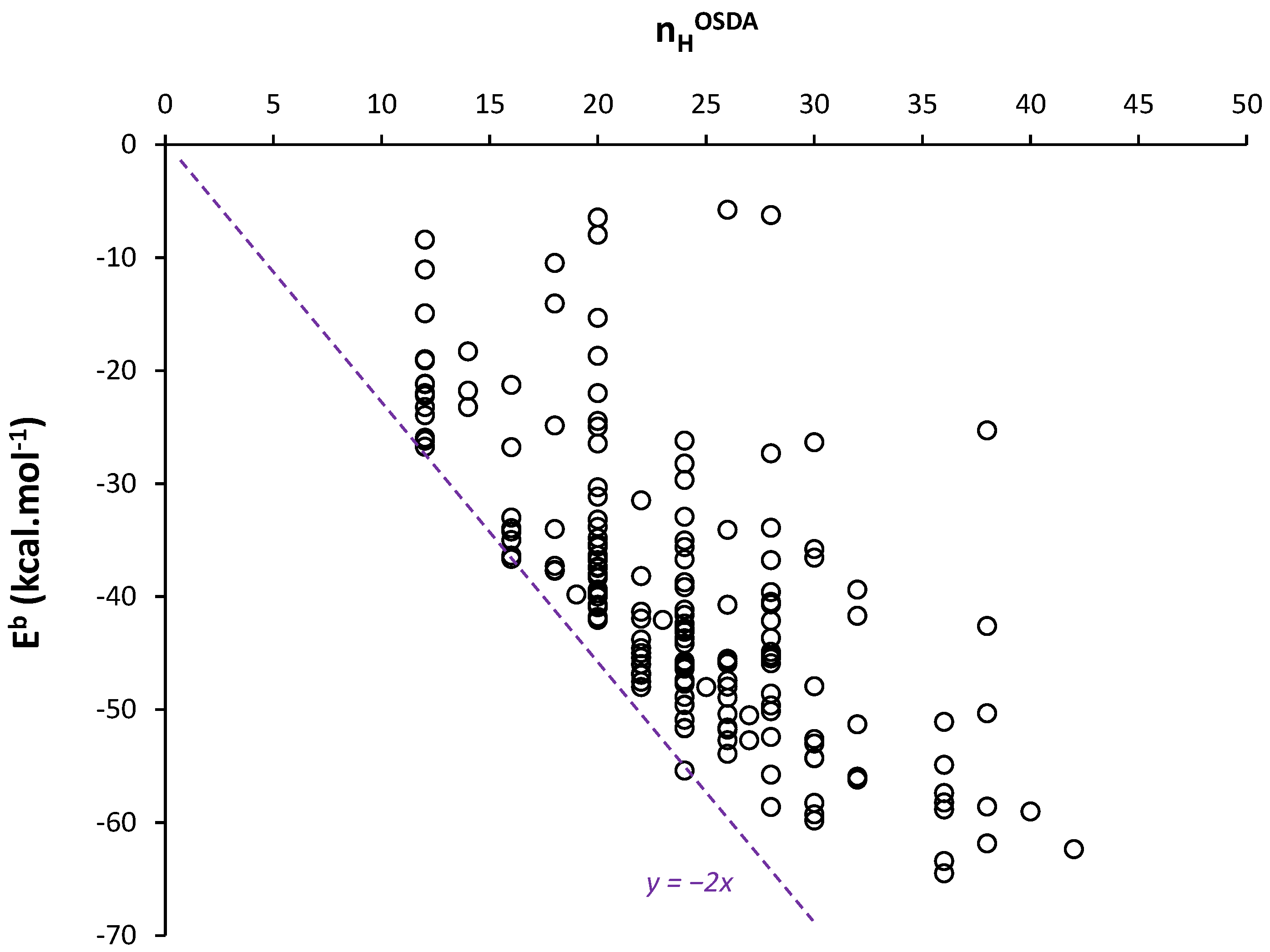
2.2. Comparisons with n-Alkanes
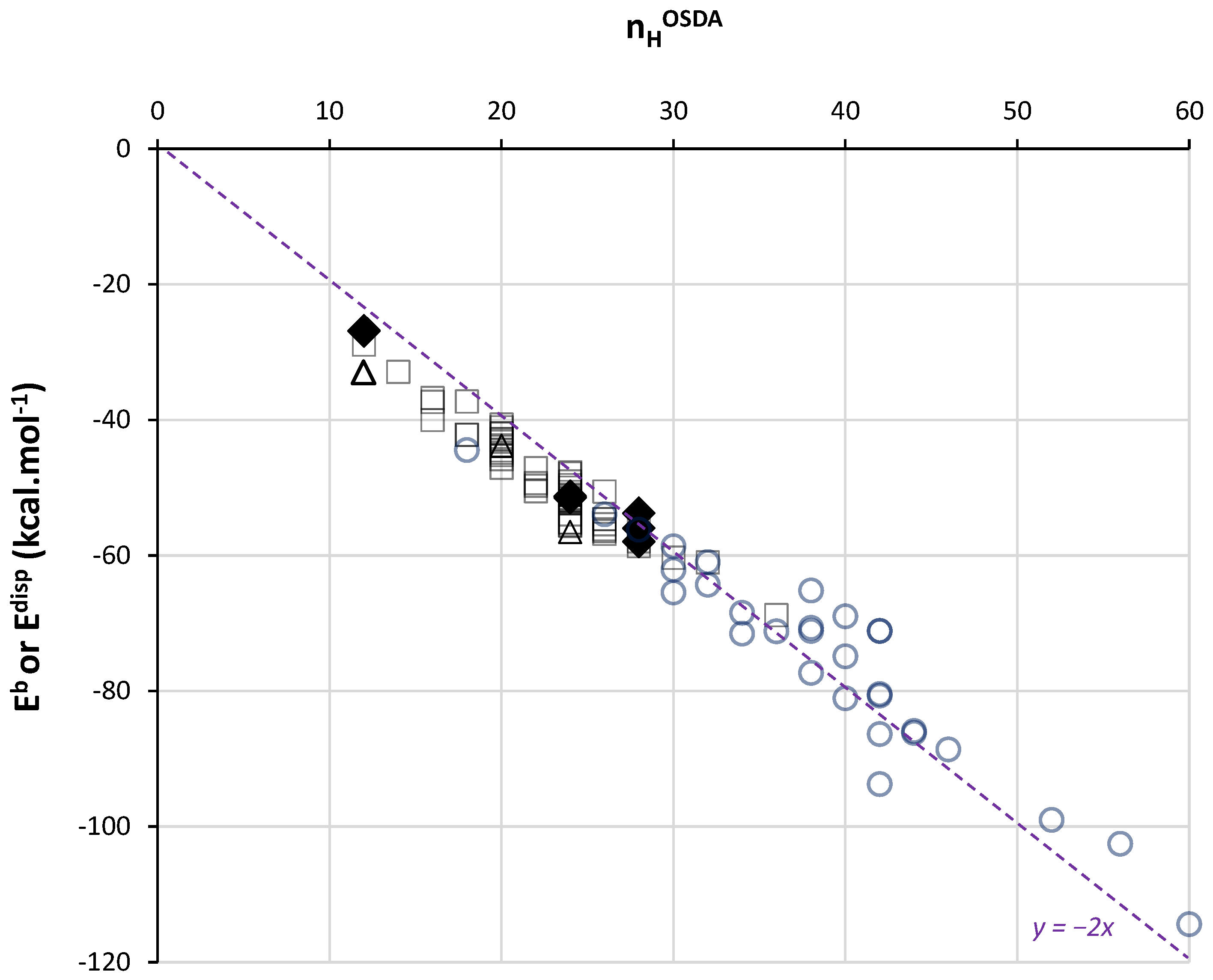
2.3. Effects of Framework, Composition, Charges and Motions
2.4. Comparison with MM Calculations
3. Methods
3.1. Geometry Optimizations
| System | Space Group | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | V (Å3) | F Location | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| TMA@AST | I4/m a | 9.0680 | 9.0680 | 13.4379 | 90 | 90 | 90 | 1104.98 | [46] | [56] |
| TMAda@CHA | R-32/m a | 13.4902 | 13.4902 | 14.7578 | 90 | 90 | 120 | 2325.89 | [4662] | [57] |
| TMAda@STT | P121/n1 | 12.9594 | 21.7919 | 13.5980 | 90 | 101.9 | 90 | 3758.30 | [5443] | [58] |
| TPA@MFI | Pn21a b | 19.7271 | 19.8624 | 13.3155 | 90 | 90 | 90 | 5217.38 | [415262] | [59] |
| TPA@MFI | Pnma | 20.0026 | 19.9934 | 13.3923 | 90 | 90 | 90 | 5355.85 | [415262] | [60] |
| TPP@MFI | Pnma | 20.0706 | 19.9733 | 13.4017 | 90 | 90 | 90 | 5372.42 | [415262] | [60] c |
3.2. Dispersion Corrections
3.3. Interactions Energies
3.4. Dynamics Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrer, R.M.; Denny, P.J. Hydrothermal chemistry of the silicates. Part IX. Nitrogenous aluminosilicates. J. Chem. Soc. 1961, 971–982. [Google Scholar] [CrossRef]
- Kerr, G.T.; Kokotailo, G.T. Sodium zeolite ZK-4, a new synthetic crystalline aluminosilicate. J. Am. Chem. Soc. 1961, 83, 4675. [Google Scholar] [CrossRef]
- Kerr, G.T. Chemistry of crystalline aluminosilicates. II. The synthesis and properties of zeolite ZK-4. Inorg. Chem. 1966, 5, 1537–1539. [Google Scholar] [CrossRef]
- Gies, H.; Marker, B. The structure-controlling role of organic templates for the synthesis of porosils in the systems SiO2/template/H2O. Zeolites 1992, 12, 42–49. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Burton, A.W.; Zones, S.I.; Elomari, S. The chemistry of phase selectivity in the synthesis of high-silica zeolites. Curr. Opin. Colloid Interf. Sci. 2005, 10, 211–219. [Google Scholar] [CrossRef]
- Moliner, M.; Rey, F.; Corma, A. Towards the rational design of efficient organic structure-directing agents for zeolite synthesis. Angew. Chem. Int. Ed. 2013, 52, 13880–13889. [Google Scholar] [CrossRef]
- Burkett, S.L.; Davis, M.E. Mechanisms of structure direction in the synthesis of pure-silica zeolites. 1. Synthesis of Tpa/Si-Zsm-5. Chem. Mater. 1995, 7, 920–928. [Google Scholar] [CrossRef]
- Lobo, R.F.; Zones, S.I.; Davis, M.E. Structure-direction in zeolite synthesis. J. Incl. Phenom. Mol. Recognit. Chem. 1995, 21, 47–78. [Google Scholar] [CrossRef]
- Behrens, P.; van de Goor, G.; Freyhardt, C.C. Structure-determining C-H…O-Si hydrogen bonds in cobaltocenium fluoride nonasil. Angew. Chem. Int. Ed. 1996, 34, 2680–2682. [Google Scholar] [CrossRef]
- Zicovich-Wilson, C.M.; Gándara, F.; Monge, A.; Camblor, M.A. In situ transformation of TON silica zeolite into the less dense ITW: Structure-direction overcoming framework instability in the synthesis of SiO2 zeolites. J. Am. Chem. Soc. 2010, 10, 3461–3471. [Google Scholar] [CrossRef] [PubMed]
- Dib, E.; Freire, M.; Pralong, V.; Mineva, T.; Alonso, B. Intermolecular interactions in AST zeolites through 14N NMR and DFT calculations. Acta Cryst. C 2017, 73, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Mineva, T.; Dib, E.; Gaje, A.; Petitjean, H.; Bantignies, J.-L.; Alonso, B. Zeolite structure direction: Identification, strength and involvement of weak CH···O hydrogen bonds. ChemPhysChem 2020, 21, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Al-Nahari, S.; Ata, K.; Mineva, T.; Alonso, B. Ubiquitous presence of intermolecular CH…O hydrogen bonds in as-synthesized host-guest zeolite materials. ChemistrySelect 2021, 6, 9728–9734. [Google Scholar] [CrossRef]
- Lok, B.M.; Cannan, T.R.; Messina, C.A. The role of organic molecules in molecular sieve synthesis. Zeolites 1983, 3, 282–291. [Google Scholar] [CrossRef]
- Davis, M.E.; Lobo, R.F. Zeolite and molecular sieve synthesis. Chem. Mater. 1992, 4, 756–768. [Google Scholar] [CrossRef]
- Schmitt, K.D.; Kennedy, G.J. Toward the rational design of zeolite synthesis: The synthesis of zeolite ZSM-18. Zeolites 1994, 14, 635–642. [Google Scholar] [CrossRef]
- Nakagawa, Y. Use of Diels-Alder derived templates to prepare zeolites with multidimensional pore systems. Stud. Surf. Sci. Catal. 1994, 84, 323–330. [Google Scholar] [CrossRef]
- Harris, T.V.; Zones, S.I. A Study of guest/host energetics for the synthesis of cage structures NON and CHA. Stud. Surf. Sci. Catal. 1994, 84, 29–36. [Google Scholar] [CrossRef]
- Paik, W.C.; Shin, C.H.; Lee, J.M.; Ahn, B.J.; Hong, S.B. Host−guest interactions in P1, SUZ-4, and ZSM-57 zeolites containing N,N,N,N‘,N‘,N‘-Hexaethylpentanediammonium ion as a guest Molecule. J. Phys. Chem. B 2001, 105, 9994–10000. [Google Scholar] [CrossRef]
- Catlow, C.R.A.; Coombes, D.S.; Lewis, D.W.; Pereira, J.C.G. Computer modeling of nucleation, growth, and templating in hydrothermal synthesis. Chem. Mater. 1998, 10, 3249–3265. [Google Scholar] [CrossRef]
- Jorge, M.; Auerbach, S.M.; Monson, P.A. Modeling spontaneous formation of precursor nanoparticles in clear-solution zeolite synthesis. J. Am. Chem. Soc. 2005, 127, 14388–14400. [Google Scholar] [CrossRef] [PubMed]
- Lemishko, T.; Simancas, J.; Hernández-Rodríguez, M.; Jiménez-Ruiz, M.; Sastre, G.; Rey, F. An INS study of entrapped organic cations within the micropores of zeolite RTH. Phys. Chem. Chem. Phys. 2016, 18, 17244–17252. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hortigüela, L.; Camblor, M.A. Introduction to the zeolite structure-directing phenomenon by organic species: General aspects. In Insights into the Chemistry of Organic Structure-Directing Agents in the Synthesis of Zeolitic Materials; Gómez-Hortigüela, L., Camblor, M.A., Eds.; Structure and Bonding 175; Springer: Cham, Switzerland, 2017; pp. 1–41. [Google Scholar]
- Leon, S.; Sastre, G. Zeolite phase selectivity using the same organic structure-directing agent in fluoride and hydroxide media. J. Phys. Chem. C 2022, 126, 2078–2087. [Google Scholar] [CrossRef]
- Corma, A.; Rey, F.; Rius, J.; Sabater, M.J.; Valencia, S. Supramolecular self-assembled molecules as organic directing agent for synthesis of zeolites. Nature 2004, 431, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hortigüela, L.; Corà, F.; Catlow, C.R.A.; Pérez-Pariente, J. Computational study of the structure-directing effect of benzylpyrrolidine and its fluorinated derivatives in the synthesis of the aluminophosphate AlPO-5. J. Am. Chem. Soc. 2004, 126, 12097–12102. [Google Scholar] [CrossRef]
- Ikuno, T.; Chaikittisilp, W.; Liu, Z.; Iida, T.; Yanaba, Y.; Yoshikawa, T.; Kohara, S.; Wakihara, T.; Okubo, T. Structure-directing behaviors of tetraethylammonium cations toward zeolite Beta revealed by the evolution of aluminosilicate species formed during the crystallization process. J. Am. Chem. Soc. 2015, 137, 14533–14544. [Google Scholar] [CrossRef]
- Lewis, D.W.; Freeman, C.M.; Catlow, C.R.A. Predicting the templating ability of organic additives for the synthesis of microporous materials. J. Phys. Chem. 1995, 99, 11194–11202. [Google Scholar] [CrossRef]
- Lewis, D.W.; Willock, D.J.; Catlow, C.R.A.; Thomas, J.M.; Hutchings, G.J. De novo design of structure-directing agents for the synthesis of microporous solids. Nature 1996, 382, 604–606. [Google Scholar] [CrossRef]
- Stevens, A.P.; Gorman, A.M.; Freeman, C.M.; Cox, P.A. Prediction of template location via a combined Monte Carlo–simulated annealing approach. J. Chem. Soc. Faraday Trans. 1996, 92, 2065–2073. [Google Scholar] [CrossRef]
- Zones, S.I.; Nakagawa, Y.; Yuen, L.T.; Harris, T.V. Guest/host interactions in high silica zeolite synthesis: [5.2.1.02.6 tricyclodecanes as template molecule. J. Am. Chem. Soc. 1996, 118, 7558–7567. [Google Scholar] [CrossRef]
- Lewis, D.W.; Sankar, G.; Wyles, J.K.; Thomas, J.M.; Catlow, C.R.A.; Willock, D.J. Synthesis of a small-pore microporous material using a computationally designed template. Angew. Chem. Int. Ed. 1997, 36, 2675–2677. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Lee, G.S.; Harris, T.V.; Yuen, L.T.; Zones, S.I. Guest/host relationships in zeolite synthesis: Ring-substituted piperidines and the remarkable adamantane mimicry by 1-azonio spiro [5.5] undecanes. Microporous Mesoporous Mater. 1998, 22, 69–85. [Google Scholar] [CrossRef]
- Burton, A.W.; Lee, G.S.; Zones, S.I. Phase selectivity in the syntheses of cage-based zeolite structures: An investigation of thermodynamic interactions between zeolite hosts and structure directing agents by molecular modeling. Microporous Mesoporous Mater. 2006, 90, 129–144. [Google Scholar] [CrossRef]
- Pophale, R.; Daeyaert, F.; Deem, M.W. Computational prediction of chemically synthesizable organic structure directing agents for zeolites. J. Mater. Chem. A 2013, 1, 6750–6760. [Google Scholar] [CrossRef]
- Gálvez-Llompart, M.; Cantín, A.; Rey, F.; Sastre, G. Computational screening of structure directing agents for the synthesis of zeolites. A simplified model. Z. Fur Krist. Mater. 2019, 234, 451–460. [Google Scholar] [CrossRef]
- Daeyaert, F.; Ye, F.; Deem, M.W. Machine-learning approach to the design of OSDAs for zeolite beta. Proc. Nat. Acad. Sci. USA 2019, 116, 3413–3418. [Google Scholar] [CrossRef]
- Muraoka, K.; Chaikittisilp, W.; Okubo, T. Multi-objective de novo molecular design of organic structure-directing agents for zeolites using nature-inspired ant colony optimization. Chem. Sci. 2020, 11, 8214–8223. [Google Scholar] [CrossRef]
- Jensen, Z.; Kwon, S.; Schwalbe-Koda, D.; Paris, C.; Gómez-Bombarelli, R.; Román-Leshkov, Y.; Corma, A.; Moliner, M.; Olivetti, E.A. Discovering relationships between OSDAs and zeolites through data mining and generative neural networks. ACS Cent. Sci. 2021, 7, 858–867. [Google Scholar] [CrossRef]
- Schwalbe-Koda, D.; Kwon, S.; Paris, C.; Bello-Jurado, E.; Jensen, Z.; Olivetti, E.; Willhammar, T.; Corma, A.; Román-Leshkov, Y.; Moliner, M. A priori control of zeolite phase competition and intergrowth with high-throughput simulations. Science 2021, 374, 308–315. [Google Scholar] [CrossRef]
- van Speybroeck, V.; Hemelsoet, K.; Joos, L.; Waroquier, M.; Bell, R.G.; Catlow, C.R.A. Advances in theory and their application within the field of zeolite chemistry. Chem. Soc. Rev. 2015, 44, 7044–7111. [Google Scholar] [CrossRef] [PubMed]
- Turrina, A.; Cox, P.A. Molecular modelling of structure direction phenomena. Struct. Bond. 2017, 175, 75–102. [Google Scholar] [CrossRef]
- Sauer, J. Ab initio calculations for molecule–surface interactions with chemical accuracy. Acc. Chem. Res. 2019, 52, 3502–3510. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. Intermolecular & Surface Forces, 2nd ed.; Academic Press: London, UK, 1992. [Google Scholar]
- Heßelmann, A.; Meitei, O.R. Intermolecular dispersion energies from coupled exact-exchange Kohn-Sham excitation energies and vectors. Comput. Theor. Chem. 2018, 1129, 57–69. [Google Scholar] [CrossRef]
- London, F. The general theory of molecular forces. Trans. Faraday Soc. 1937, 33, 8–26. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Savitz, S.; Siperstein, F.; Gorte, R.J.; Myers, A.L. Calorimetric study of adsorption of alkanes in high-silica zeolites. J. Phys. Chem. B 1998, 102, 6865–6872. [Google Scholar] [CrossRef]
- OSDB. A Database for Organic Structure-Directing Agents for Zeolites. Available online: https://zeodb.mit.edu (accessed on 10 September 2024).
- León, S.; Sastre, G. Computational screening of structure-directing agents for the synthesis of pure silica ITE zeolite. J. Phys. Chem. Lett. 2020, 11, 6164–6167. [Google Scholar] [CrossRef]
- Schwalbe-Koda, D.; Gómez-Bombarelli, R. Benchmarking binding energy calculations for organic structure-directing agents in pure-silica zeolites. J. Chem. Phys. 2021, 154, 174109. [Google Scholar] [CrossRef]
- Pulido, A.; Moliner, M.; Corma, A. Rigid/flexible organic structure directing agents for directing the synthesis of multipore zeolites: A computational approach. J. Phys. Chem. C 2015, 119, 7711–7720. [Google Scholar] [CrossRef]
- Altundal, O.F.; Leon, S.; Sastre, G. Different zeolite phases obtained with the same organic structure directing agent in the presence and absence of aluminum: The directing role of aluminum in the synthesis of zeolites. J. Phys. Chem. C 2023, 127, 10797–10805. [Google Scholar] [CrossRef]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. WIREs Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Yang, X. Synthesis and crystal structure of tetramethylammonium fluoride octadecasil. Mater. Res. Bull. 2006, 41, 54–66. [Google Scholar] [CrossRef]
- Villaescusa, L.A.; Bull, I.; Wheatley, P.S.; Lightfoot, P.; Morris, R.E. The location of fluoride and organic guests in ‘as-made’ pure silica zeolites FER and CHA. J. Mater. Chem. 2003, 13, 1978–1982. [Google Scholar] [CrossRef]
- Camblor, M.A.; Díaz-Cabañas, M.-J.; Perez-Pariente, J.; Teat, S.J.; Clegg, W.; Shannon, I.J.; Lightfoot, P.; Wright, P.A.; Morris, R.E. SSZ-23: An odd zeolite with pore openings of seven and nine tetrahedral atoms. Angew. Chem. Int. Ed. 1998, 37, 2122–2126. [Google Scholar] [CrossRef]
- Fabbiani, M.; Al-Nahari, S.; Piveteau, L.; Dib, E.; Veremeienko, V.; Gaje, A.; Dumitrescu, D.G.; Gaveau, P.; Mineva, T.; Massiot, D.; et al. Host–guest silicalite-1 zeolites: Correlated disorder and phase transition inhibition by a small guest modification. Chem. Mater. 2022, 34, 366–387. [Google Scholar] [CrossRef]
- Aubert, E.; Porcher, F.; Souhassou, M.; Petricek, V.; Lecomte, C. Location of fluoride counterion in as-synthesized silicalite-1 by single crystal X-ray diffraction. J. Phys. Chem. B 2002, 106, 1110–1117. [Google Scholar] [CrossRef]
- Mathisen, H.; Norman, N.; Pedersen, B.F.; Haug, A.; Theorell, H.; Blinc, R.; Paušak, S.; Ehrenberg, L.; Dumanović, J. The crystal structure of lower paraffins. IV. Refinement of the crystal structures of pentane and octane. Acta Chem. Scand. 1967, 21, 127–135. [Google Scholar] [CrossRef]
- Nyburg, S.C.; Gerson, A.R. Crystallography of the even n-alkanes: Structure of C20H42. Acta Cryst. B 1992, 48, 103–106. [Google Scholar] [CrossRef]
- Nyburg, S.C.; Lüth, H. n-Octadecane: A correction and refinement of the structure given by Hayashida. Acta Cryst. B 1972, 28, 2992–2995. [Google Scholar] [CrossRef]
- Gerson, A.R.; Nyburg, S.C. A twinned structure for n-tetracosane. Acta Cryst. B 1992, 48, 737–741. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Density functional theory with London dispersion corrections. WIREs Comput. Mol. Sci. 2011, 1, 211–228. [Google Scholar] [CrossRef]
- Erba, A.; Desmarais, J.K.; Casassa, S.; Civalleri, B.; Donà, L.; Bush, I.J.; Searle, B.; Maschio, L.; Edith-Daga, L.; Cossard, A.; et al. CRYSTAL23: A program for computational solid state physics and chemistry. J. Chem. Theory Comput. 2023, 19, 6891–6932. [Google Scholar] [CrossRef] [PubMed]
- Peintinger, M.F.; Oliveira, D.V.; Bredow, T. Consistent Gaussian basis sets of triple-zeta valence with polarization quality for solid-state calculations. J. Comput. Chem. 2013, 34, 451–459. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Wagner, J.P.; Schreiner, P.R. London dispersion in molecular chemistry—Reconsidering steric effects. Angew. Chem. Int. Ed. 2015, 54, 12274–12296. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, C.J.; Bryenton, K.R.; Price, A.J.A.; Johnson, E.R. Comparison of density-functional theory dispersion corrections for the DES15K database. J. Phys. Chem. A 2023, 127, 8712–8722. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-C.; Vayssilov, G.N.; Genest, A.; Borgna, A.; Rösch, N. Predicting adsorption enthalpies on silicalite and HZSM-5: A benchmark study on DFT strategies addressing dispersion interactions. J. Comput. Chem. 2014, 35, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Awati, R.; Boulfelfel, S.E.; Ravikovitch, P.I.; Sholl, D.S. First-principles-derived Force Fields for CH4 adsorption and diffusion in siliceous zeolites. J. Phys. Chem. C 2018, 122, 12880–12891. [Google Scholar] [CrossRef]
- Rehak, F.R.; Piccini, G.M.; Alessio, M.; Sauer, J. Including dispersion in density functional theory for adsorption on flat oxide surfaces, in metal–organic frameworks and in acidic zeolites. Phys. Chem. Chem. Phys. 2020, 22, 7577–7585. [Google Scholar] [CrossRef]
- Fischer, M.; Brauer, J. Studying the adsorption of emerging organic contaminants in zeolites with dispersion-corrected density functional theory calculations: From numbers to recommendations. ChemistryOpen 2024, 13, e202300273. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Laun, J.; Bredow, T. BSSE-corrected consistent Gaussian basis sets of triple-zeta valence with polarization quality of the fifth period for solid-state calculations. J. Comput. Chem. 2022, 43, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Gatti, C. TOPOND a Program for the Topological Analysis of the Electron Density, CNR-ISTM Milano. Available online: https://www.crystal.unito.it/topond/topond.php (accessed on 10 September 2024).
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter. 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter. 2017, 29, 465901. [Google Scholar] [CrossRef]
- Quantum Espresso Pseudopotentials. Available online: https://www.quantum-espresso.org/pseudopotentials// (accessed on 10 September 2024).
- Verlet, L. Computer “experiments” on classical fluids. I. Thermodynamical properties of Lennard-Jones molecules. Phys. Rev. 1967, 159, 98–103. [Google Scholar] [CrossRef]
- Swope, W.C.; Andersen, H.C.; Berens, P.H.; Wilson, K.R.A. A computer simulation method for the calculation of equilibrium constants for the formation of physical clusters of molecules: Application to small water clusters. J. Chem. Phys. 1982, 76, 637–649. [Google Scholar] [CrossRef]

| Etot/Si | Edisp/Si | Etot/OSDA | Edisp/OSDA | EHB/OSDA | Eother/OSDA | Etot/H | Edisp/H | |
|---|---|---|---|---|---|---|---|---|
| TMA@AST | −17.7 | −2.7 | −177.2 | −26.9 | −6.6 | −143.8 | −14.77 | −2.24 |
| TMAda@CHA | −13.1 | −4.3 | −156.9 | −51.2 | −20.0 | −85.6 | −6.54 | −2.13 |
| TMAda@STT | −11.6 | −3.2 | −185.1 | −51.5 | −10.9 | −122.7 | −7.71 | −2.14 |
| TPA@MFI (Pn21a) | −9.0 | −2.3 | −217.0 | −56.0 | −8.8 | −152.2 | −7.75 | −2.00 |
| TPA@MFI (Pnma) | −8.9 | −2.2 | −214.2 | −53.7 | −11.2 | −149.3 | −7.65 | −1.92 |
| TPP@MFI (Pnma) | −8.9 | −2.4 | −213.9 | −58.0 | −12.8 | −143.1 | −7.64 | −2.07 |
| TMA*@AST a | −3.1 | −2.7 | −31.1 | −26.9 | −7.0 | 2.8 | −2.59 | −2.24 |
| Zeolite FW Type | AST | STT | MFI |
|---|---|---|---|
| OSDA | TMA | TMAda | TPP |
| Initial DFT-D3 optimization | |||
| Space group | P1 | P121/n1 | Pn21a |
| Edisp/OSDA (Edisp/H) | −26.9 (−2.24) | −51.5 (−2.14) | −56.0 (−2.00) |
| BOMD simulation T = 300 K | |||
| Space group | P1 | P1 | P1 |
| Edisp/OSDA (Edisp/H) at t = 2 ps | −27.2 (−2.27) | −51.7 (−2.15) | −56.8 (−2.03) |
| Edisp/OSDA (Edisp/H) at t = 3 ps | −27.0 (−2.25) | −51.6 (−2.15) | −56.4 (−2.01) |
| Edisp/OSDA (Edisp/H) at t = 4 ps | −27.2 (−2.27) | −51.7 (−2.16) | −56.6 (−2.02) |
| Edisp/OSDA (Edisp/H) at t = 5 ps | −27.1 (−2.26) | −51.6 (−2.15) | −56.2 (−2.01) |
| Interaction Energies (in kcal.mol−1) | ||||
|---|---|---|---|---|
| Etot/OSDA | Edisp/OSDA | Etot/H | Edisp/H | |
| TMA@AST (D3) | −177.2 | −26.9 | −14.77 | −2.24 |
| TMA@AST (D2) | −175.2 | −25.8 | −14.60 | −2.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ata, K.; Mineva, T.; Alonso, B. Strength of London Dispersion Forces in Organic Structure Directing Agent—Zeolite Assemblies. Molecules 2024, 29, 4489. https://doi.org/10.3390/molecules29184489
Ata K, Mineva T, Alonso B. Strength of London Dispersion Forces in Organic Structure Directing Agent—Zeolite Assemblies. Molecules. 2024; 29(18):4489. https://doi.org/10.3390/molecules29184489
Chicago/Turabian StyleAta, Karima, Tzonka Mineva, and Bruno Alonso. 2024. "Strength of London Dispersion Forces in Organic Structure Directing Agent—Zeolite Assemblies" Molecules 29, no. 18: 4489. https://doi.org/10.3390/molecules29184489
APA StyleAta, K., Mineva, T., & Alonso, B. (2024). Strength of London Dispersion Forces in Organic Structure Directing Agent—Zeolite Assemblies. Molecules, 29(18), 4489. https://doi.org/10.3390/molecules29184489






