Driving Forces in the Formation of Paracetamol Cocrystals and Solvate with Naphthalene, Quinoline and Acridine
Abstract
1. Introduction
2. Results and Discussion
2.1. Cocrystal Preparation and Thermal Analysis
2.2. IR Spectroscopy
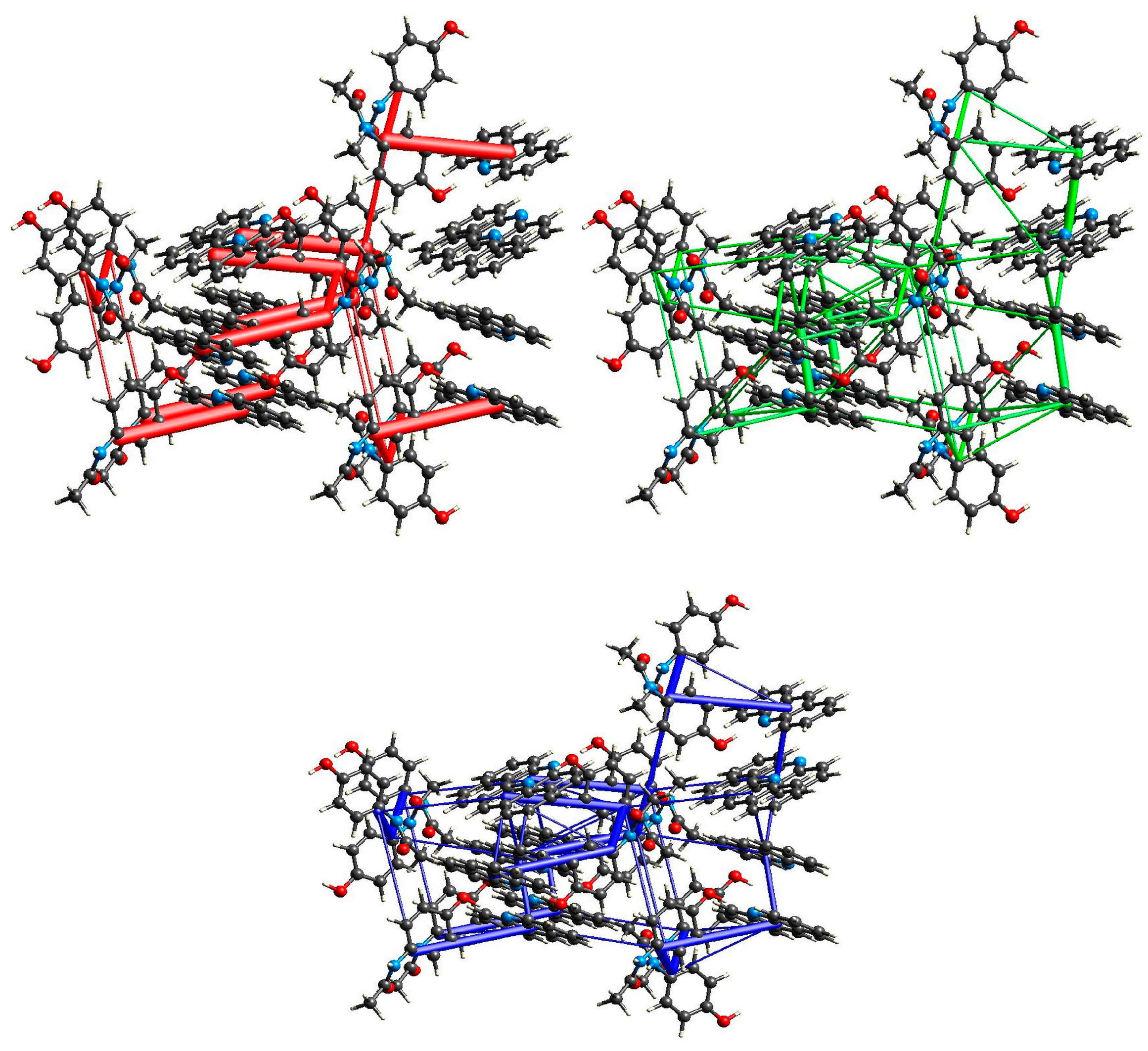
2.3. Structural Studies
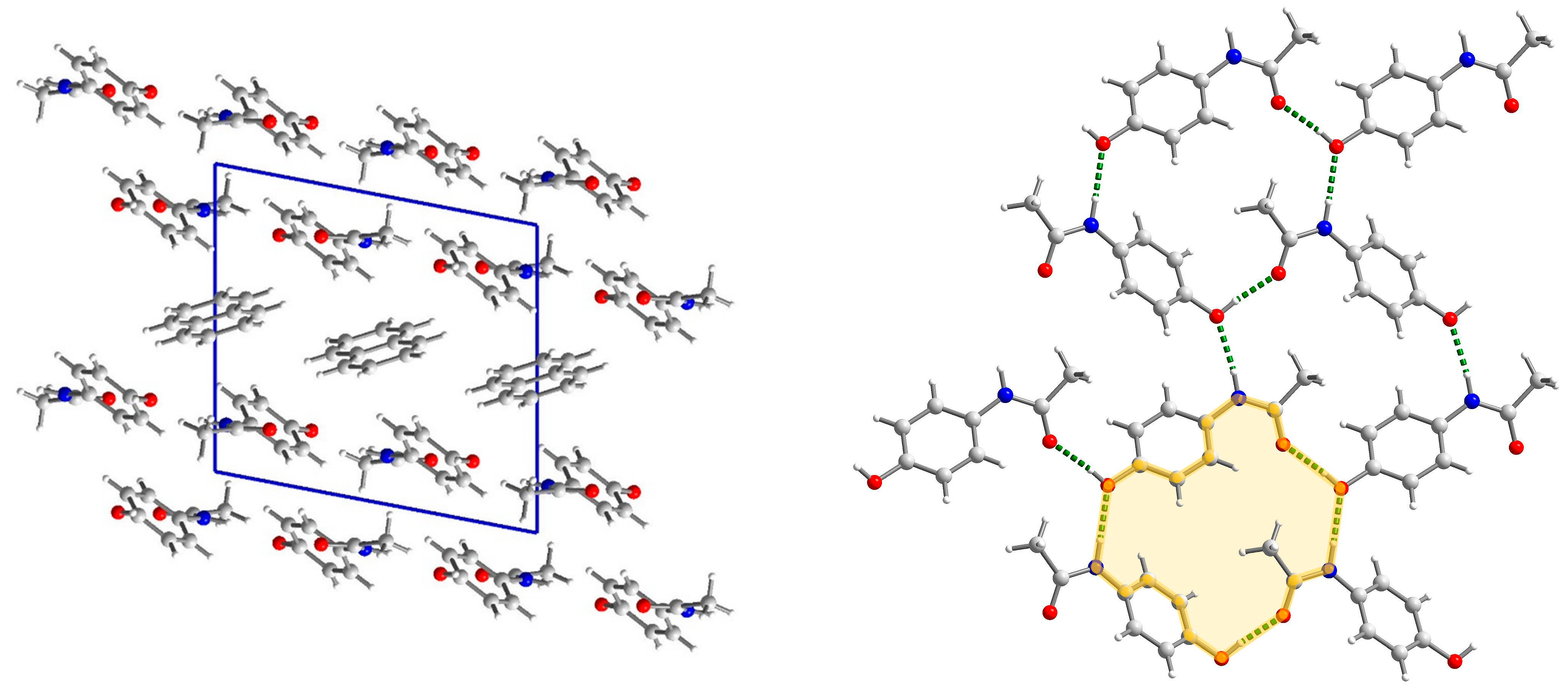
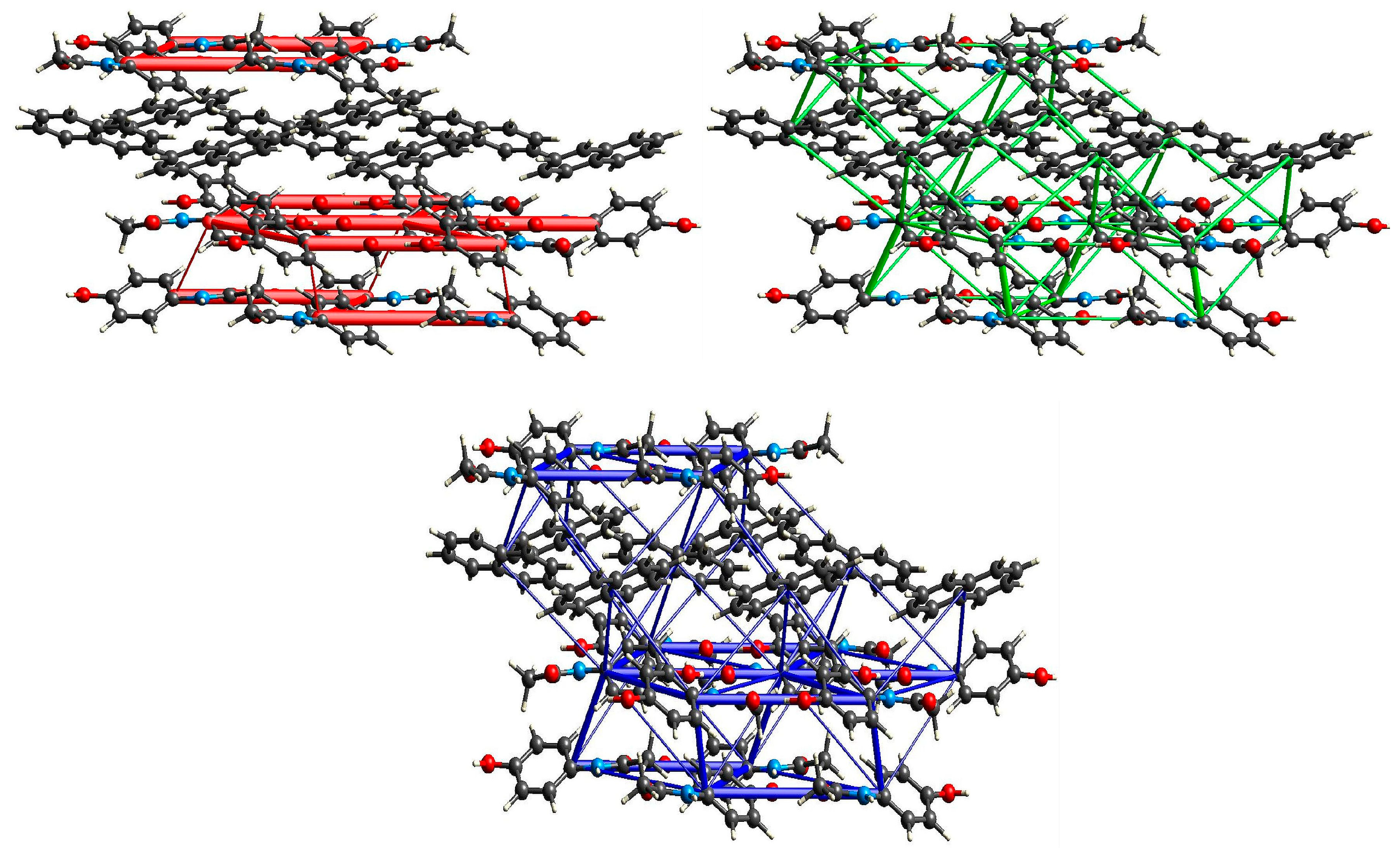
2.3.1. Structure of (par)2∙(nap) (1)
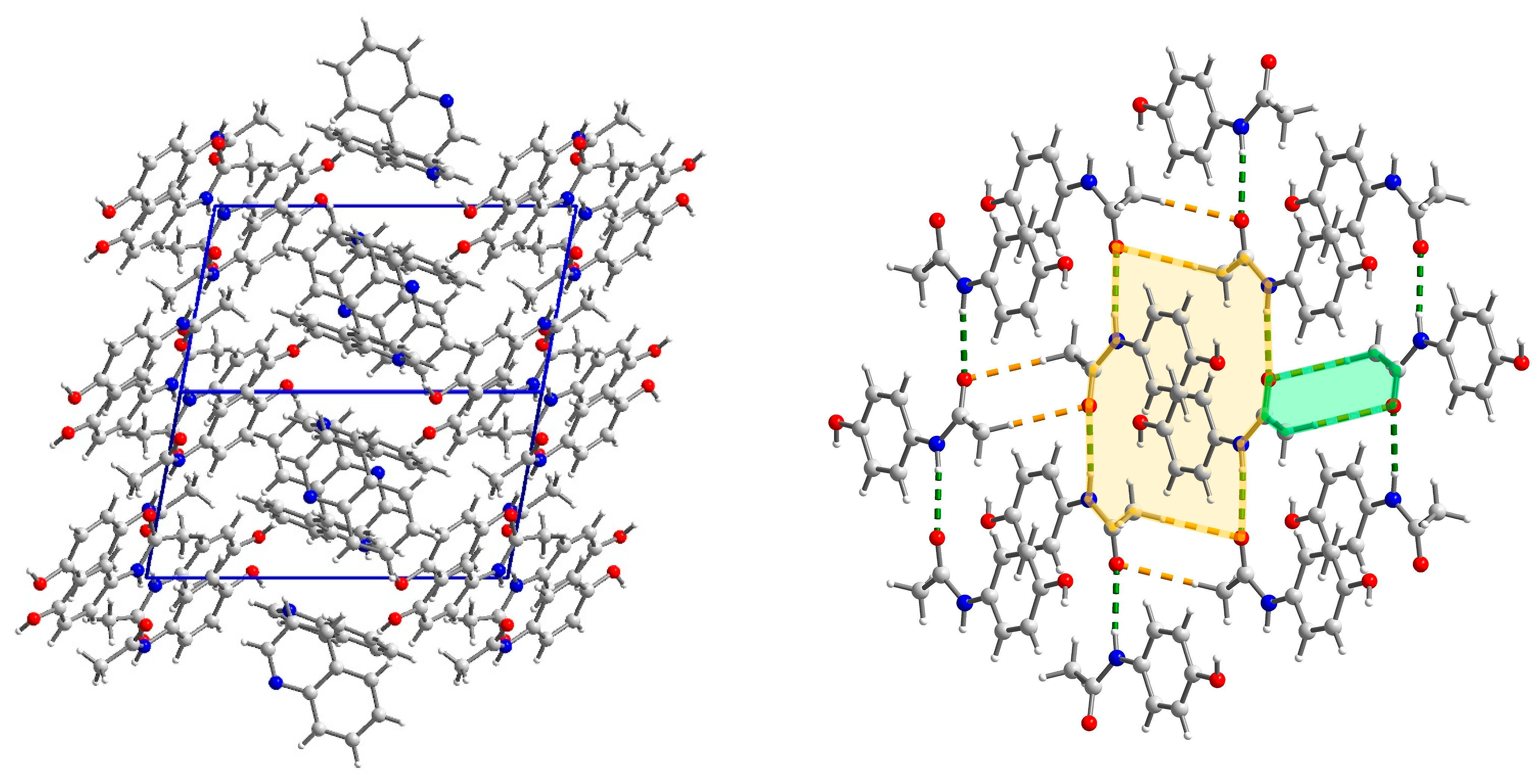
2.3.2. Structure of (par)∙(quin) (2)
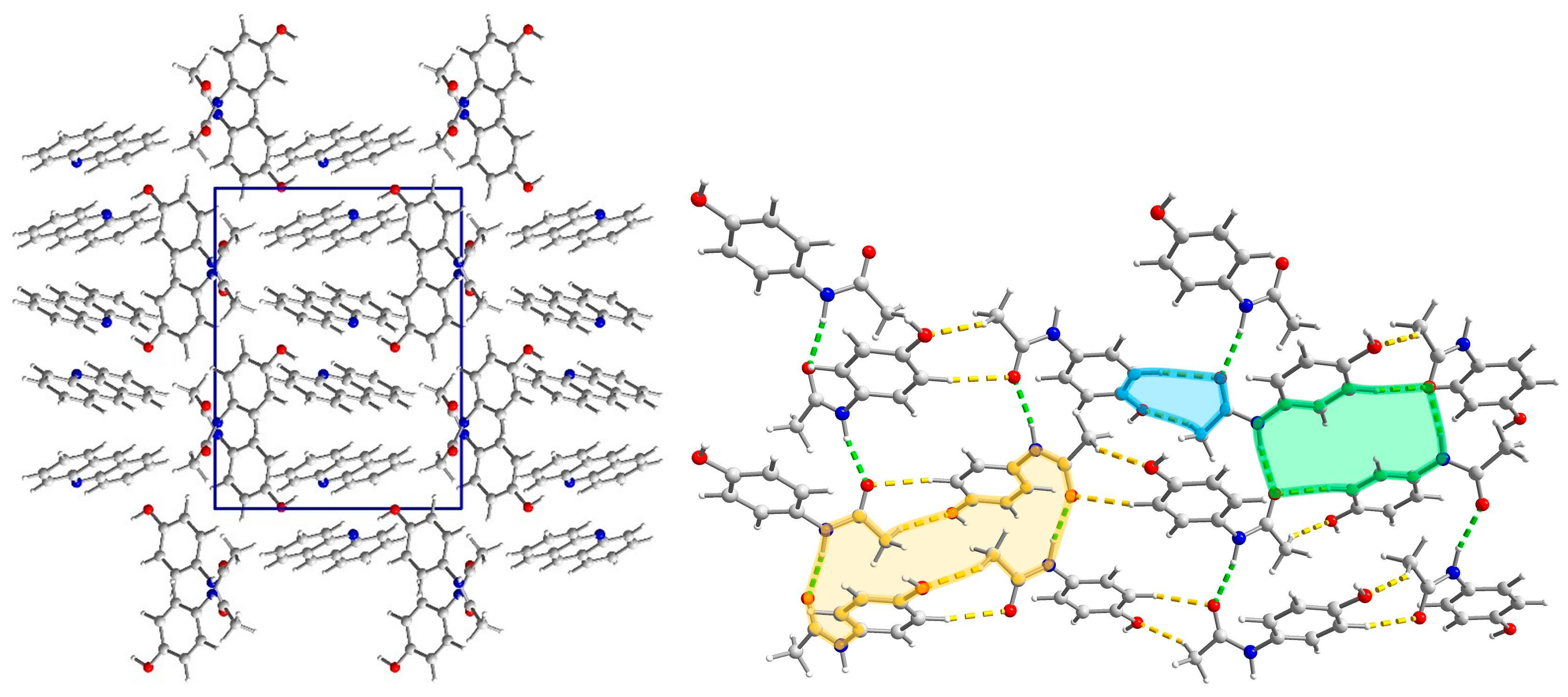
2.3.3. Structure of (par)∙(acr) (3)
2.3.4. Structural Studies of Selected Coformers
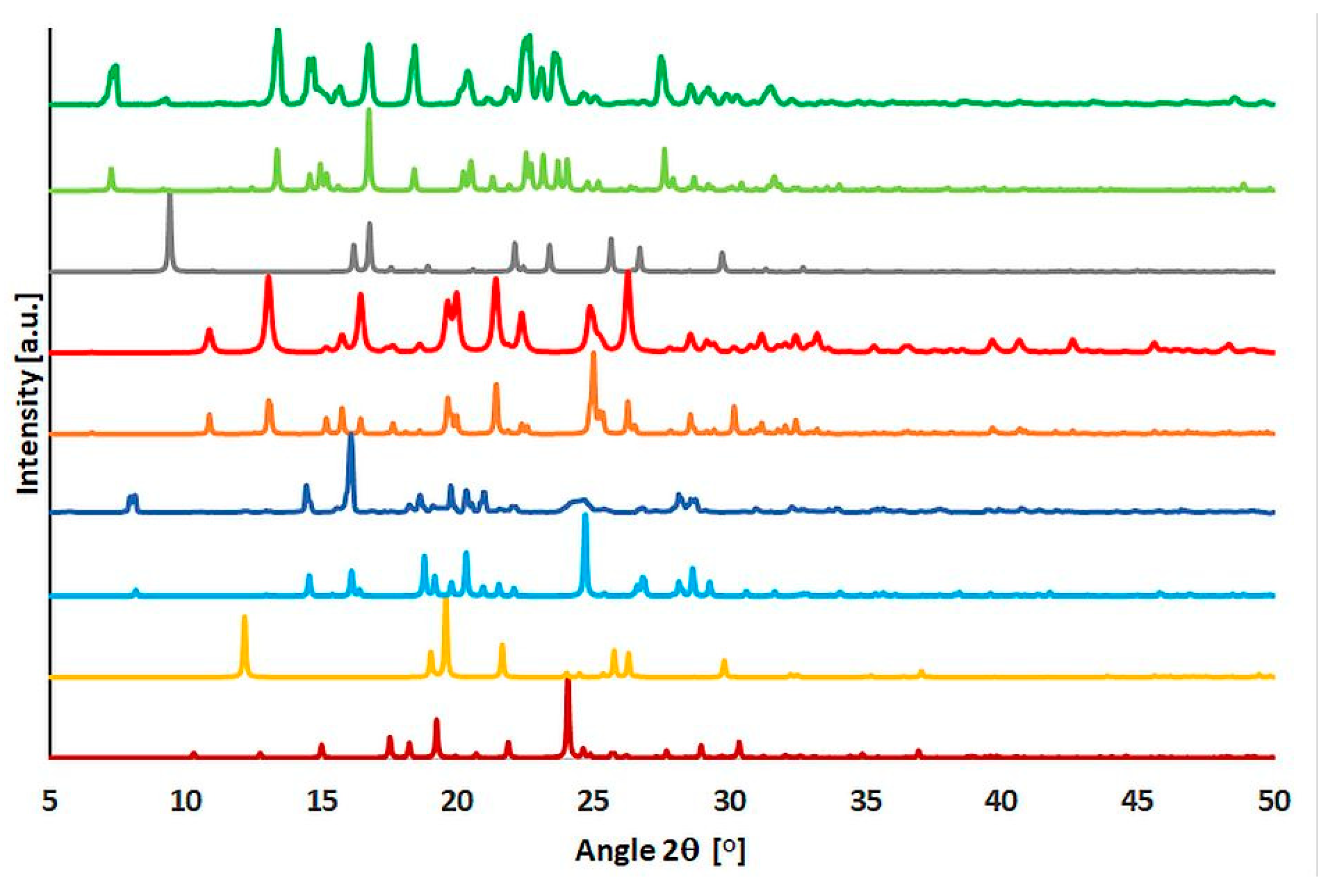
2.4. Powder Diffraction Experiments
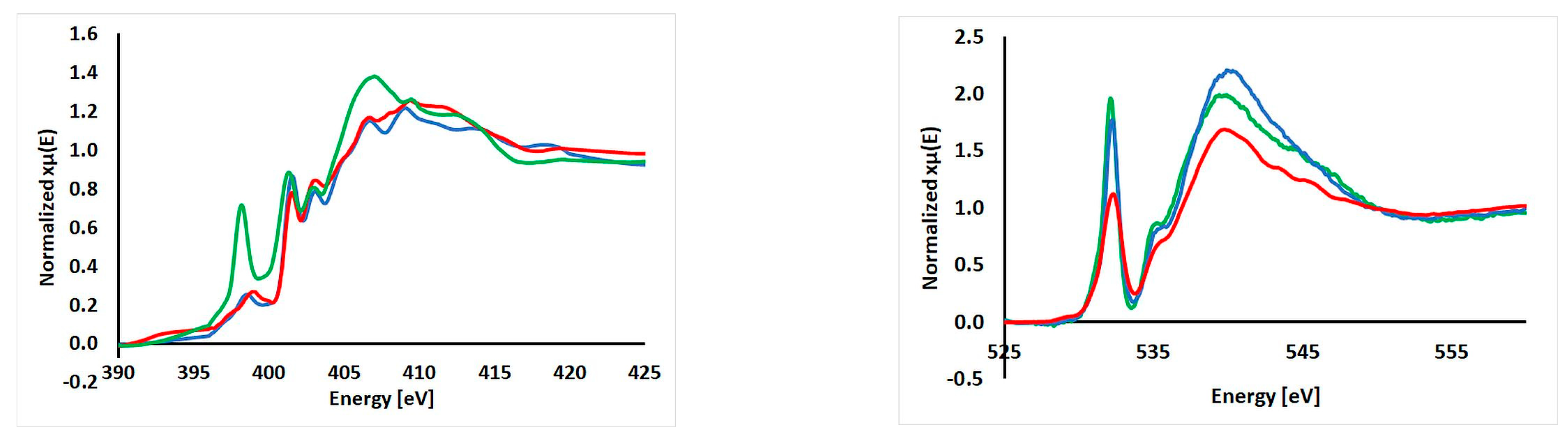
2.5. XAS Results
3. Materials and Methods
3.1. Materials and General Procedure
3.2. Preparation of (par)2∙(nap)
3.3. Preparation of (par)∙(quin)
3.4. Preparation of (par)∙(acr)
3.5. Single Crystal X-ray Diffraction Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, K.; Shimpi, M.R.; Srivastava, A.; Tandon, P.; Sinha, K.; Velaga, S.P. Vibrational Analysis and Chemical Activity of Paracetamol-Oxalic Acid Cocrystal Based on Monomer and Dimer Calculations: DFT and AIM Approach. RSC Adv. 2016, 6, 10024–10037. [Google Scholar] [CrossRef]
- Wichianphong, N.; Charoenchaitrakool, M. Application of Box-Behnken Design for Processing of Mefenamic Acid-Paracetamol Cocrystals Using Gas Anti-Solvent (GAS) Process. J. CO2 Util. 2018, 26, 212–220. [Google Scholar] [CrossRef]
- Srirambhatla, V.K.; Kraft, A.; Watt, S.; Powell, A.V. Crystal Design Approaches for the Synthesis of Paracetamol Co-Crystals. Cryst. Growth Des. 2012, 12, 4870–4879. [Google Scholar] [CrossRef]
- Bučar, D.K.; Elliott, J.A.; Eddleston, M.D.; Cockcroft, J.K.; Jones, W. Sonocrystallization Yields Monoclinic Paracetamol with Significantly Improved Compaction Behavior. Angew. Chem. Int. Ed. 2015, 54, 249–253. [Google Scholar] [CrossRef]
- Lee, W.M. Acetaminophen and the U.S. Acute Liver Failure Study Group: Lowering the Risks of Hepatic Failure. Hepatology 2004, 40, 6–9. [Google Scholar] [CrossRef]
- Maeno, Y.; Fukami, T.; Kawahata, M.; Yamaguchi, K.; Tagami, T.; Ozeki, T.; Suzuki, T.; Tomono, K. Novel Pharmaceutical Cocrystal Consisting of Paracetamol and Trimethylglycine, a New Promising Cocrystal Former. Int. J. Pharm. 2014, 473, 179–186. [Google Scholar] [CrossRef]
- Suzuki, N.; Kanazawa, T.; Takatori, K.; Suzuki, T.; Fukami, T. Crystal Structure Analysis and Pharmaceutical Properties of Amide Salts Consisting of Paracetamol/Sulfonic Acids as Solid Forms Prepared by Grinding. Cryst. Growth Des. 2020, 20, 590–599. [Google Scholar] [CrossRef]
- Trzybiński, D.; Domagała, S.; Kubsik, M.; Woźniak, K. Solid-State Analysis of Monohydrated Halide Salts of Paracetamol. Cryst. Growth Des. 2016, 16, 1156–1161. [Google Scholar] [CrossRef]
- Chadha, R.; Kuhad, A.; Arora, P.; Kishor, S. Characterisation and Evaluation of Pharmaceutical Solvates of Atorvastatin Calcium by Thermoanalytical and Spectroscopic Studies. Chem. Cent. J. 2012, 6, 114. [Google Scholar] [CrossRef]
- Elder, D.P.; Holm, R.; De Diego, H.L. Use of Pharmaceutical Salts and Cocrystals to Address the Issue of Poor Solubility. Int. J. Pharm. 2013, 453, 88–100. [Google Scholar] [CrossRef]
- Fael, H.; Demirel, A.L. Indomethacin Co-Amorphous Drug-Drug Systems with Improved Solubility, Supersaturation, Dissolution Rate and Physical Stability. Int. J. Pharm. 2021, 600, 120448. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Jin, Y.; Li, S.; Yang, Z.; Shi, B.; Chang, C.; Abramov, Y.A. Virtual Coformer Screening by Crystal Structure Predictions: Crucial Role of Crystallinity in Pharmaceutical Cocrystallization. J. Phys. Chem. Lett. 2020, 11, 8832–8838. [Google Scholar] [CrossRef] [PubMed]
- Elbagerma, M.A.; Edwards, H.G.M.; Munshi, T.; Scowen, I.J. Identification of a New Cocrystal of Citric Acid and Paracetamol of Pharmaceutical Relevance. CrystEngComm 2011, 13, 1877–1884. [Google Scholar] [CrossRef]
- Srivastava, K.; Shukla, A.; Karthick, T.; Velaga, S.P.; Tandon, P.; Sinha, K.; Shimpi, M.R. Molecular Structure, Spectroscopic Signature and Reactivity Analyses of Paracetamol Hydrochloride Monohydrate Salt Using Density Functional Theory Calculations. CrystEngComm 2019, 21, 857–865. [Google Scholar] [CrossRef]
- Islam, N.U.; Khan, E.; Umar, M.N.; Shah, A.; Zahoor, M.; Ullah, R.; Bari, A. Enhancing Dissolution Rate and Antibacterial Efficiency of Azithromycin through Drug-Drug Cocrystals with Paracetamol. Antibiotics 2021, 10, 939. [Google Scholar] [CrossRef]
- Suzuki, N.; Kawahata, M.; Yamaguchi, K.; Suzuki, T.; Tomono, K.; Fukami, T. Comparison of the Relative Stability of Pharmaceutical Cocrystals Consisting of Paracetamol and Dicarboxylic Acids. Drug Dev. Ind. Pharm. 2018, 44, 582–589. [Google Scholar] [CrossRef]
- Al-Ani, A.J.; Sugden, P.; Wilson, C.C.; Castro-Dominguez, B. Elusive Seed Formation via Electrical Confinement: Control of a Novel Cocrystal in Cooling Crystallization. Cryst. Growth Des. 2021, 21, 3310–3315. [Google Scholar] [CrossRef]
- Ahmed, H.; Shimpi, M.R.; Velaga, S.P. Relationship between Mechanical Properties and Crystal Structure in Cocrystals and Salt of Paracetamol. Drug Dev. Ind. Pharm. 2017, 43, 89–97. [Google Scholar] [CrossRef]
- Kennedy, A.R.; King, N.L.C.; Oswald, I.D.H.; Rollo, D.G.; Spiteri, R.; Walls, A. Structural Study of Salt Forms of Amides; Paracetamol, Benzamide and Piperine. J. Mol. Struct. 2018, 1154, 196–203. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid-Base Crystalline Complexes and the PKa Rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Fabián, L.; Laity, P.R.; Day, G.M.; Jones, W. Improving Mechanical Properties of Crystalline Solids by Cocrystal Formation: New Compressible Forms of Paracetamol. Adv. Mater. 2009, 21, 3905–3909. [Google Scholar] [CrossRef]
- Zhang, C.; Xiong, Y.; Jiao, F.; Wang, M.; Li, H. Redefining the Term of “Cocrystal” and Broadening Its Intention. Cryst. Growth Des. 2019, 19, 1471–1478. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Zakharov, B.A.; Ogienko, A.G.; Yunoshev, A.S.; Ancharov, A.I.; Boldyreva, E.V. Bis(Paracetamol) Pyridine-a New Elusive Paracetamol Solvate: From Modeling the Phase Diagram to Successful Single-Crystal Growth and Structure-Property Relations. CrystEngComm 2015, 17, 7543–7550. [Google Scholar] [CrossRef]
- Latif, S.; Abbas, N.; Hussain, A.; Arshad, M.S.; Bukhari, N.I.; Afzal, H.; Riffat, S.; Ahmad, Z. Development of Paracetamol-Caffeine Co-Crystals to Improve Compressional, Formulation and in Vivo Performance. Drug Dev. Ind. Pharm. 2018, 44, 1099–1108. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Ye, X.; Yao, C.; Song, S.; Qu, Y.; Jiang, J.; Wang, H.; Han, P.; Liu, Y.; et al. Efficient Screening of Pharmaceutical Cocrystals by Microspacing In-Air Sublimation. J. Am. Chem. Soc. 2024, 146, 11592–11598. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S.V. Cocrystallization of an Antiretroviral Drug Nevirapine: An Eutectic, a Cocrystal Solvate, and a Cocrystal Hydrate. Cryst. Growth Des. 2021, 21, 2076–2092. [Google Scholar] [CrossRef]
- Roy, S.; Gaur, R.; Paul, M.; Rajkumar, M.; Desiraju, G.R. Synthetic Strategies toward Higher Cocrystals of Some Resorcinols. Cryst. Growth Des. 2022, 22, 7578–7589. [Google Scholar] [CrossRef]
- Paul, M.; Desiraju, G.R. From a Binary to a Quaternary Cocrystal: An Unusual Supramolecular Synthon. Angew. Chem. 2019, 131, 12155–12159. [Google Scholar] [CrossRef]
- Mir, N.A.; Dubey, R.; Desiraju, G.R. Strategy and Methodology in the Synthesis of Multicomponent Molecular Solids: The Quest for Higher Cocrystals. Acc. Chem. Res. 2019, 52, 2210–2220. [Google Scholar] [CrossRef]
- Saha, S.; Desiraju, G.R. Acid⋯Amide Supramolecular Synthon in Cocrystals: From Spectroscopic Detection to Property Engineering. J. Am. Chem. Soc. 2018, 140, 6361–6373. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.; Sutradhar, D.; Roy, S.; Desiraju, G.R. Synthetic Approaches to Halogen Bonded Ternary Cocrystals. Angew. Chem. Int. Ed. 2021, 60, 12841–12846. [Google Scholar] [CrossRef]
- Hiendrawan, S.; Veriansyah, B.; Widjojokusumo, E.; Soewandhi, S.N.; Wikarsa, S.; Tjandrawinata, R.R. Physicochemical and Mechanical Properties of Paracetamol Cocrystal with 5-Nitroisophthalic Acid. Int. J. Pharm. 2016, 497, 106–113. [Google Scholar] [CrossRef]
- Srivastava, K.; Khan, E.; Shimpi, M.R.; Tandon, P.; Sinha, K.; Velaga, S.P. Molecular Structure and Hydrogen Bond Interactions of a Paracetamol-4,4′-Bipyridine Cocrystal Studied Using a Vibrational Spectroscopic and Quantum Chemical Approach. CrystEngComm 2018, 20, 213–222. [Google Scholar] [CrossRef]
- Manonmani, M.; Balakrishnan, C.; Ahamed, S.R.; Vinitha, G.; Meenakshisundaram, S.P.; Sockalingam, R.M. Cocrystallization of Paracetamol-Picric Acid: Hirshfeld Surface Analysis, Supramolecular Architecture and Third-Order Nonlinear Optical Properties. J. Mol. Struct. 2019, 1190, 1–10. [Google Scholar] [CrossRef]
- Ayudhya, T.; Raymond, C.; Dingra, N. Synthesis and Structure of a 1:1 Co-Crystal of Hexamethylenetetramine Carboxyborane and Acetaminophen. Acta Crystallogr. E Crystallogr. Commun. 2020, 76, 1854–1858. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Patil, S.; Shettigar, H.; Jana, S. Effect of Biofield Treatment on Spectral Properties of Paracetamol and Piroxicam. Chem. Sci. J. 2015, 6, 100098. [Google Scholar] [CrossRef]
- Pauzat, F.; Talbi, D.; Miller, M.D.; DeFrees, D.J.; Ellinger, Y. Theoretical IR Spectra of Ionized Naphthalene. J. Phys. Chem. 1992, 96, 7882–7886. [Google Scholar] [CrossRef]
- Ricks, A.M.; Douberly, G.E.; Duncan, M.A. The Infrared Spectrum of Protonated Naphthalene and Its Relevance for the Unidentified Infrared Bands. Astrophys. J. 2009, 702, 301–306. [Google Scholar] [CrossRef]
- Lagutschenkov, A.; Dopfer, O. Infrared Spectrum of a Protonated Fluorescence Dye: Acridine Orange. J. Mol. Spectrosc. 2011, 268, 66–77. [Google Scholar] [CrossRef]
- Prochorow, J.; Deperasinska, I.; Morawskí, O. Fluorescence Excitation and Fluorescence Spectra of Jet-Cooled Acridine Molecules: Acridine Dimer Formation and Structure. Chem. Phys. Lett. 2000, 316, 24–30. [Google Scholar] [CrossRef]
- Özel, A.E.; Büyükmurat, Y.; Akyüz, S. Infrared-Spectra and Normal-Coordinate Analysis of Quinoline and Quinoline Complexes. J. Mol. Struct. 2001, 565–566, 455–462. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting Intermolecular Interactions in Molecular Crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer Model Energies and Energy Frameworks: Extension to Metal Coordination Compounds, Organic Salts, Solvates and Open-Shell Systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef]
- Stone, K.H.; Lapidus, S.H.; Stephens, P.W. Implementation and Use of Robust Refinement in Powder Diffraction in the Presence of Impurities. J. Appl. Crystallogr. 2009, 42, 385–391. [Google Scholar] [CrossRef]
- Fioretti, A.N.; Schwartz, C.P.; Vinson, J.; Nordlund, D.; Prendergast, D.; Tamboli, A.C.; Caskey, C.M.; Tuomisto, F.; Linez, F.; Christensen, S.T.; et al. Understanding and Control of Bipolar Self-Doping in Copper Nitride. J. Appl. Phys. 2016, 119, 181508. [Google Scholar] [CrossRef]
- Shimada, H.; Fukao, T.; Minami, H.; Ukai, M.; Fujii, K.; Yokoya, A.; Fukuda, Y.; Saitoh, Y. Nitrogen K-Edge X-ray Absorption near Edge Structure (XANES) Spectra of Purine-Containing Nucleotides in Aqueous Solution. J. Chem. Phys. 2014, 141, 055102. [Google Scholar] [CrossRef]
- Shimada, H.; Minami, H.; Okuizumi, N.; Sakuma, I.; Ukai, M.; Fujii, K.; Yokoya, A.; Fukuda, Y.; Saitoh, Y. Nitrogen K-Edge x-Ray Absorption near Edge Structure of Pyrimidine-Containing Nucleotides in Aqueous Solution. J. Chem. Phys. 2015, 142, 175102. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.; Schlichting, A.; Siemens, J.; Regier, T.; Leinweber, P. Pyrolysis-Field Ionization Mass Spectrometry and Nitrogen K-Edge XANES Spectroscopy Applied to Bulk Soil Leachates-a Case Study. Sci. Total Environ. 2010, 408, 4910–4915. [Google Scholar] [CrossRef] [PubMed]
- Leinweber, P.; Kruse, J.; Walley, F.L.; Gillespie, A.; Eckhardt, K.U.; Blyth, R.I.R.; Regier, T. Nitrogen K-Edge XANES—An Overview of Reference Compounds Used to Identify “unknown” Organic Nitrogen in Environmental Samples. J. Synchrotron Radiat. 2007, 14, 500–511. [Google Scholar] [CrossRef]
- Turner, J.A.; Thomas, K.M.; Russell, A.E. The Identification of Oxygen Functional Groups in Carbonaceous Materials by Oxygen K-edge XANES. Carbon 1997, 35, 983–992. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data Analysis for X-ray Absorption Spectroscopy Using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Fnnctional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colic-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Oxford Diffraction Ltd. CrysAlis Red and CrysAlis CCD; Oxford Diffraction Ltd.: Abingdon, UK, 2000. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Diamond—Crystal and Molecular Structure Visualization, Crystal Impact—Dr. H. Putz & Dr. K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn, Germany. Available online: https://www.crystalimpact.de/diamond (accessed on 15 September 2024).
- Lanzilotto, V.; Silva, J.L.; Zhang, T.; Stredansky, M.; Grazioli, C.; Simonov, K.; Giangrisostomi, E.; Ovsyannikov, R.; De Simone, M.; Coreno, M.; et al. Spectroscopic Fingerprints of Intermolecular H-Bonding Interactions in Carbon Nitride Model Compounds. Chem. A Eur. J. 2018, 24, 14198–14206. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzioł, T.M.; Bronikowska, E. Driving Forces in the Formation of Paracetamol Cocrystals and Solvate with Naphthalene, Quinoline and Acridine. Molecules 2024, 29, 4437. https://doi.org/10.3390/molecules29184437
Muzioł TM, Bronikowska E. Driving Forces in the Formation of Paracetamol Cocrystals and Solvate with Naphthalene, Quinoline and Acridine. Molecules. 2024; 29(18):4437. https://doi.org/10.3390/molecules29184437
Chicago/Turabian StyleMuzioł, Tadeusz M., and Emilia Bronikowska. 2024. "Driving Forces in the Formation of Paracetamol Cocrystals and Solvate with Naphthalene, Quinoline and Acridine" Molecules 29, no. 18: 4437. https://doi.org/10.3390/molecules29184437
APA StyleMuzioł, T. M., & Bronikowska, E. (2024). Driving Forces in the Formation of Paracetamol Cocrystals and Solvate with Naphthalene, Quinoline and Acridine. Molecules, 29(18), 4437. https://doi.org/10.3390/molecules29184437






