Packing Incubation and Addition of Rot Fungi Extracts Improve BTEX Elimination from Air in Biotrickling Filters
Abstract
1. Introduction
| Compound | Solubility in Water, mg dm−3 (25 °C) | Henry’s law Constant, mol m−3 Pa−1 [15] ** | Po/w (20 °C) | Boiling Point, °C (101,325 Pa) |
|---|---|---|---|---|
| Benzene | 1770 | 0.0014–0.0018 | 2.13 | 90.1 |
| Toluene | 580 | 0.0013–0.0017 | 2.73 | 110.0 |
| Ethylbenzene | 200 | 0.0011–0.0013 | 3.60 | 136.1 |
| Xylene (mixture of xylenes) | 156 | 0.0012–0.0023 | 3.15 | 139.1 |
| Compound/s | Packing Material/Volume of Packing | Inoculum | Inlet Loading, g m−3 h−1 | Removal Efficiency, % | EBRT, s | Trickling Liquid Pattern | Reference |
|---|---|---|---|---|---|---|---|
| Benzene | Zeolite-contained polyethylene media/7.5 dm3 | Defined microorganism consortium with Bacillus cereus 1 | 100 | 70 | 40 | Continuous, 4 m3 per 1 m3 of packing | [16] |
| Polypropylene spheres and fibre balls/16 dm3 | No information | 67 | 65 | 30 | Intermittent, 3.5 dm3 of mineral medium once per hour | [17] | |
| Pelletized diatomaceous earth (Celite)/2.7 dm3 | Activated sludge/packing with developed microorganisms from other biofilter | 48 | 90 | 120 | 2 dm3 per day (buffered mineral medium) | [18] | |
| Toluene | Ceramsite/ 4 dm3 | Burkholderia sp. strain T3 isolated from WWTP activated sludge | 474 | 98 | 32 | Intermittent spraying, 0.2 mL/s | [19] |
| Polyurethane foam cubes/ 5 dm3 | Acclimated activated sludge | 600 | 70 | 30 | sulphate-free mineral salt medium (MSM); 0.00048 m3/h | [20] | |
| Slags/144 dm3 | Dried yeast powder | 55 | 85 | 76 | Spraying 32 m/h | [21] | |
| Pall rings and pumice/2 dm3 | Ralstonia eutropha | 200 | 85 | 45 | Spraying 20 mL/min | [22] | |
| Glass beads/ 3.9 dm3 | Inoculum taken from previously working BTF | 360 | 97 | 28 | Differential biotrickling filter | [23] | |
| Ceramic pellets/ 4.27 cm3 | Fungi BTF: Fusarium, Paramicrosporidium saccamoebae (from activated sludge and cultivation fungi-oriented) | 70 | 82 | 77 | Intermittent trickling pattern (inorganic mineral medium) | [24] | |
| Ethylbenzene | Open-pore reticulated polyurethane sponge | Activated sludge from WWTP | 189 | 69 | 40 | Spraying for 3 s every 3 min; 4.5 L/day, modification of trickling liquid with surfactant and Zn(II) | [25] |
| Polyurethane sponge | Fresh biological sludge from WWTP | 264 | 50 | 30 | 0.2 L/h; Biosurfactant addition to liquid phase | [26] | |
| Polyurethane sponge | Activated sludge from WWTP | 180 | 80 | 30 | 4.8 L/day; Addition of saponins to liquid phase | [27] | |
| Xylene (isomers) | Ceramic particles/4.7 dm3 | Bacillus firmus | 1450 | 98 | 84.8 | Continuous trickling | [28] |
| Packing material with porosity of 0.95/1.7 dm3 | Enriched mixed culture from activated sludge of pharmaceutical plant | 80 | 87.5 | 90 | Silicone-oil addition (5% v/v), continuous trickling | [29] | |
| Diatomaceous earth pellets/ 2.4 dm3 | Enriched activated sludge from wastewater treatment plant | 150 | 73 | 25 | Intermittent spraying, 100 mL once each 3 h | [30] | |
| BTEX | Waste blue mussel shells/6 m3 | Effluent from refinery wastewater treatment plant | 2 | 76 | 60 | Continuous trickling, 0.9 m3 h−1 | [31] |
| Polyurethane foam/1 dm3 | Microbial consortium enriched from petroleum polluted soil | 100 | 60 | 30 | Continuous trickling with M9 medium and vitamins | [32] | |
| Kaldnes rings/ 2 dm3 | Activated sludge from denitrification-nitrification wastewater treatment plant | 5.7 | 30–60 | 1800 | Mineral salt medium, 2 m h−1 | [33] |
2. Results
2.1. Design of Experiment
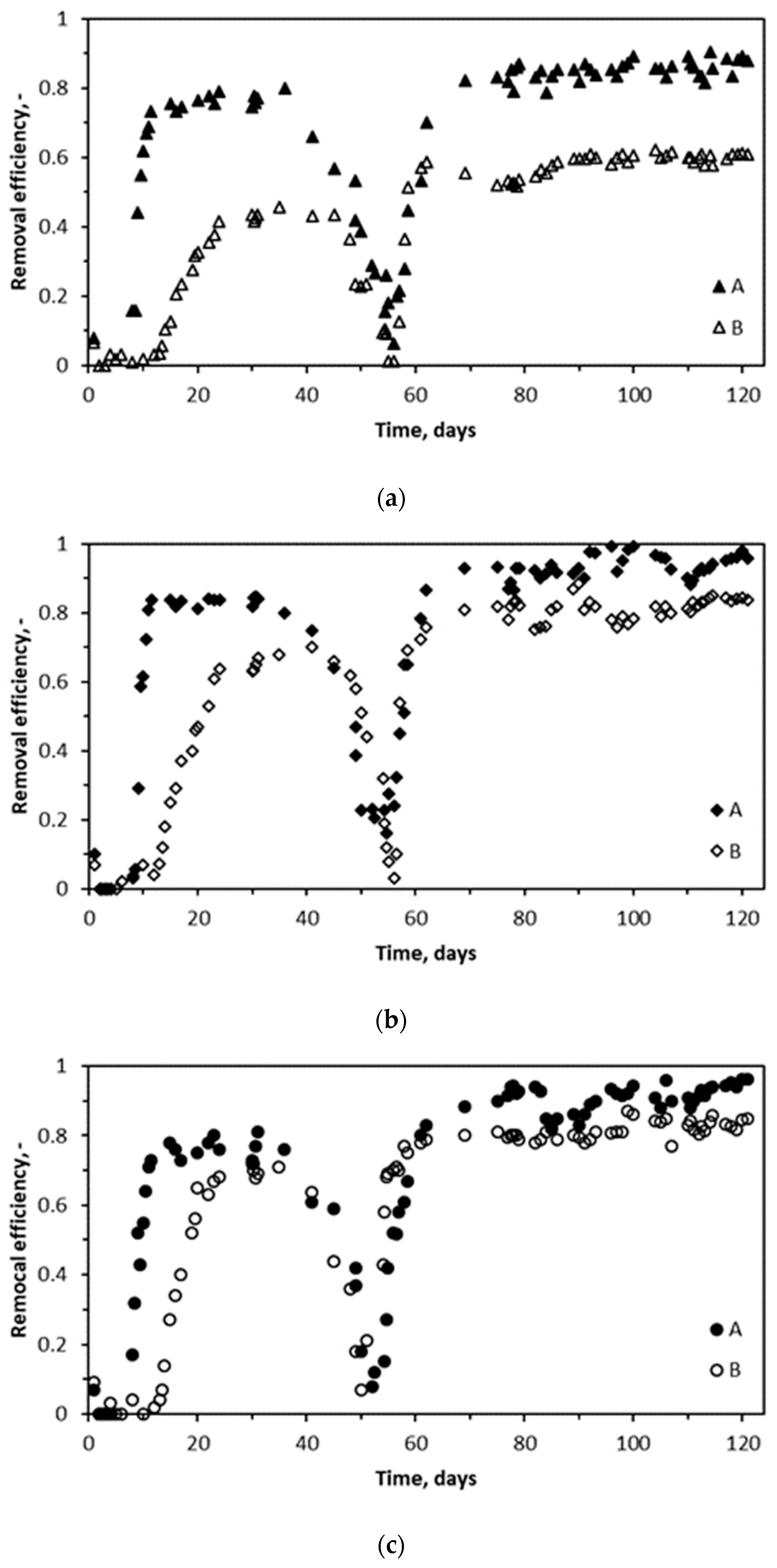
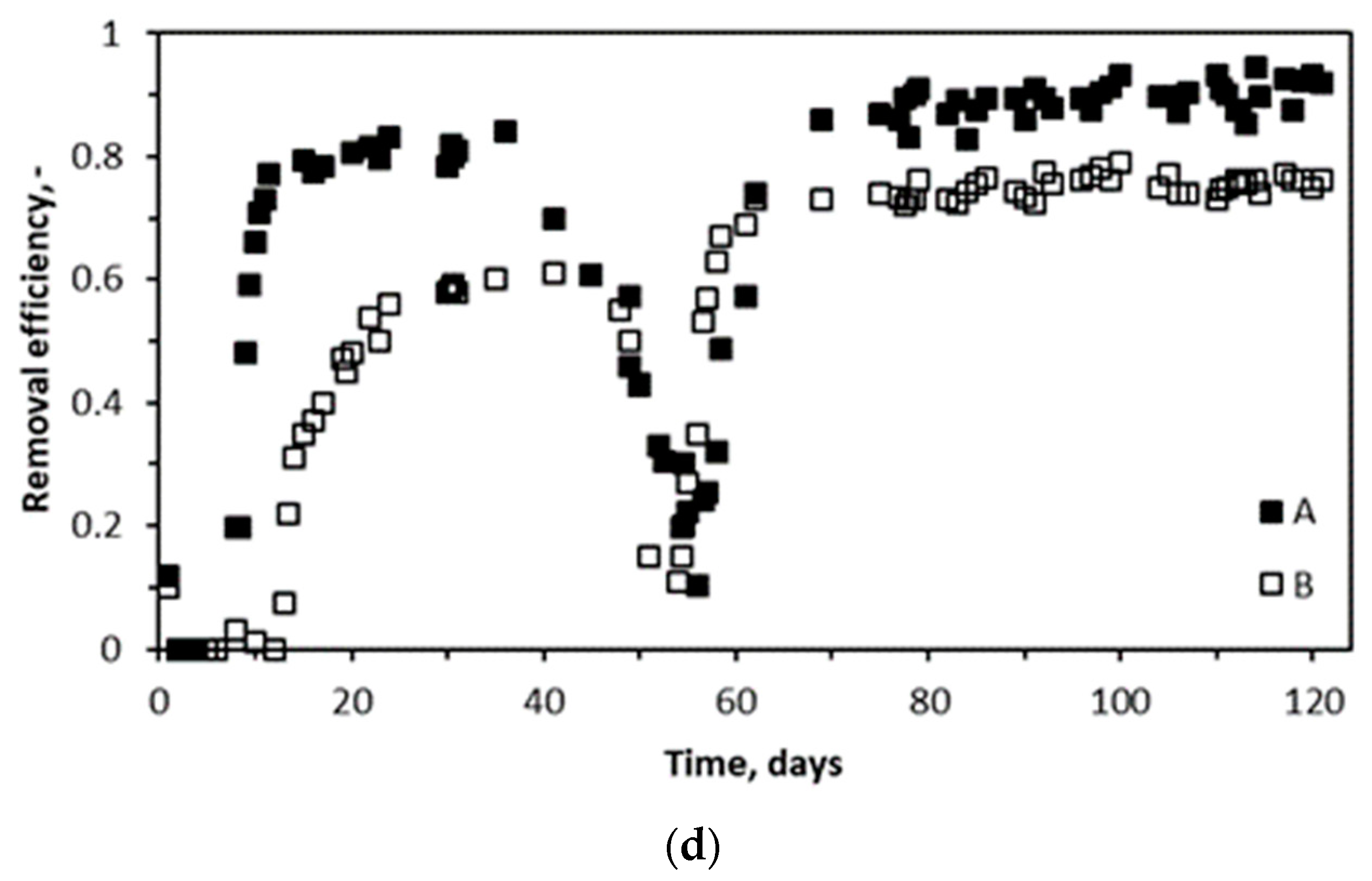
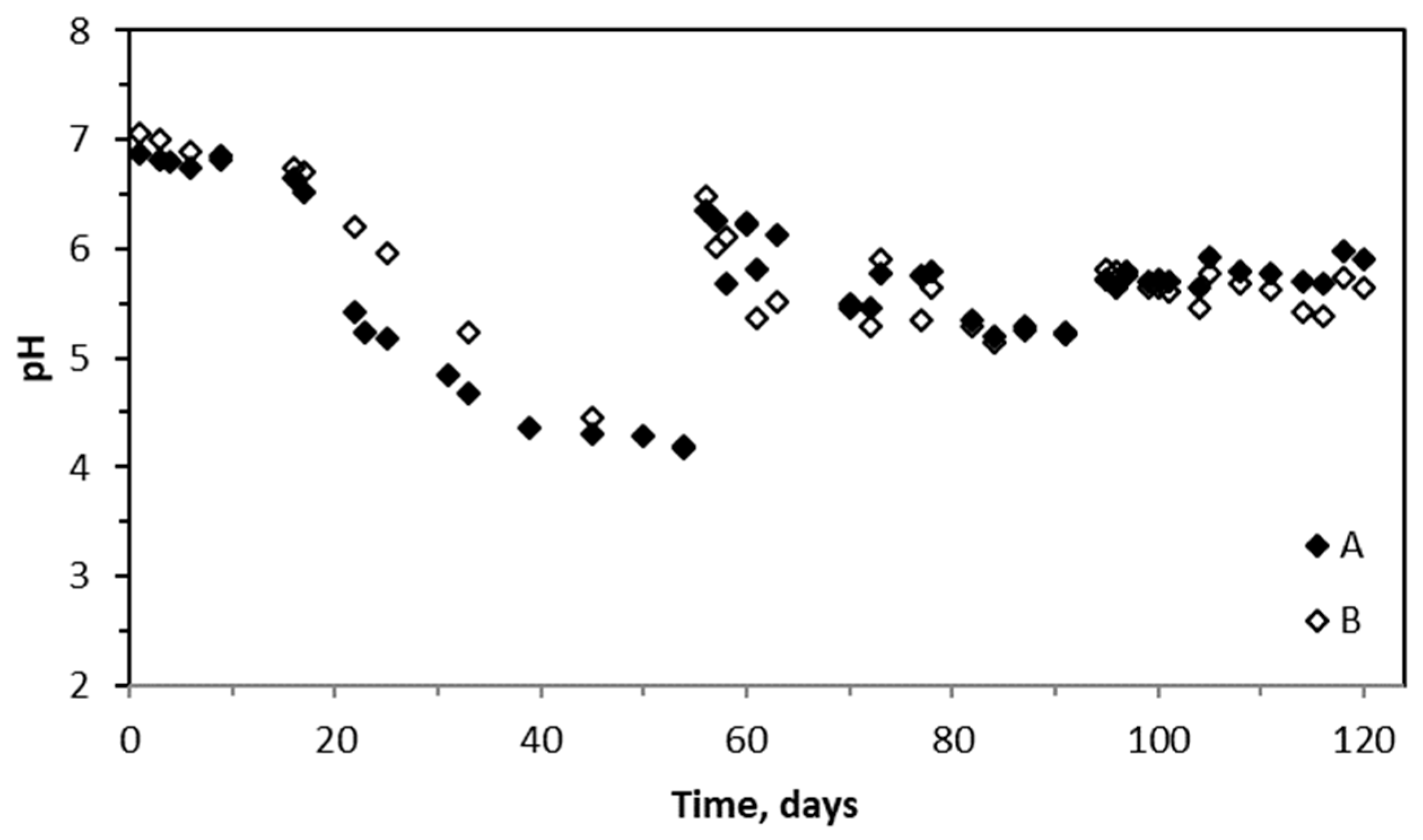
2.2. Performance of Biotrickling Filters and pH Variations
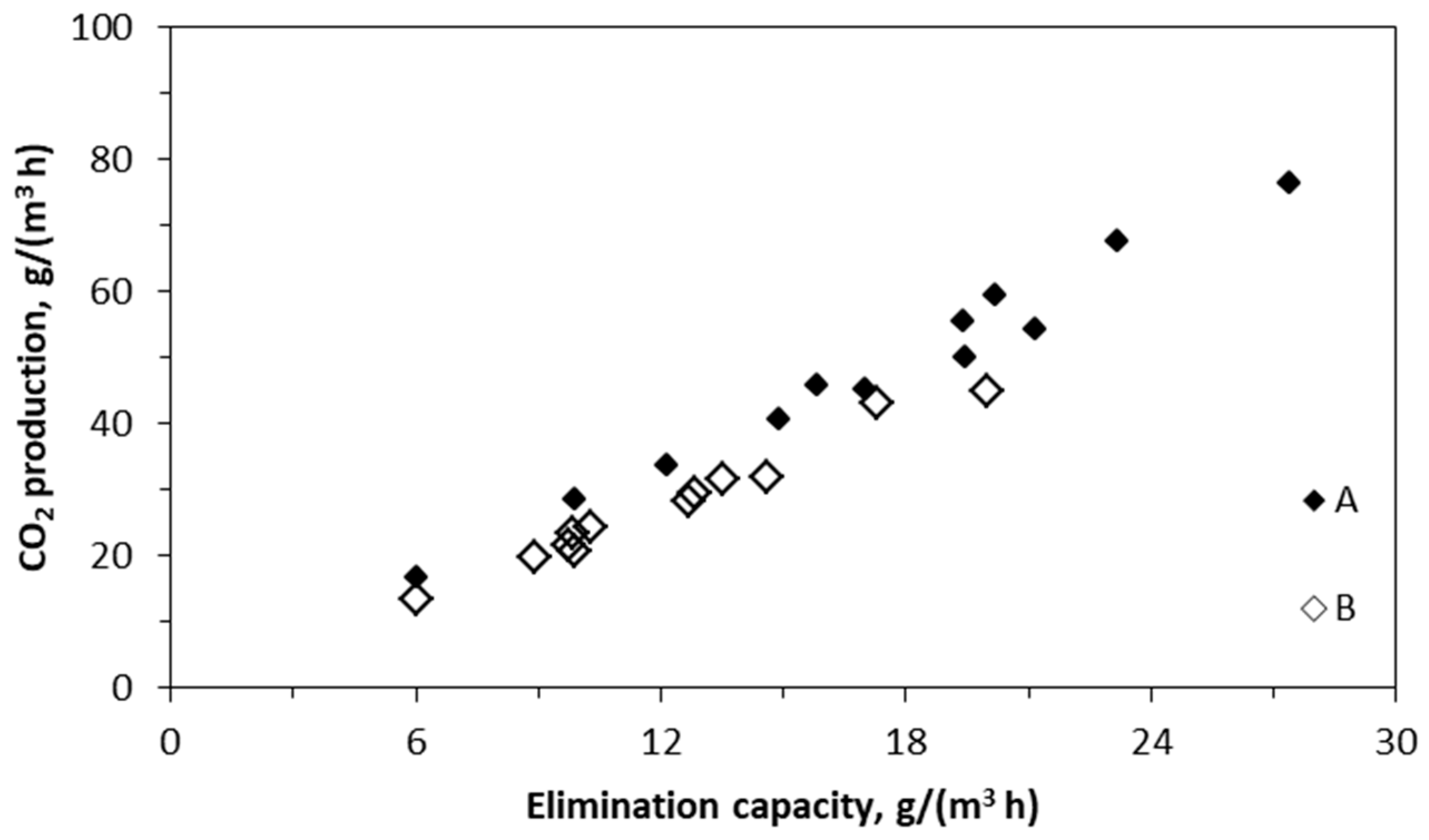
2.3. Production of CO2
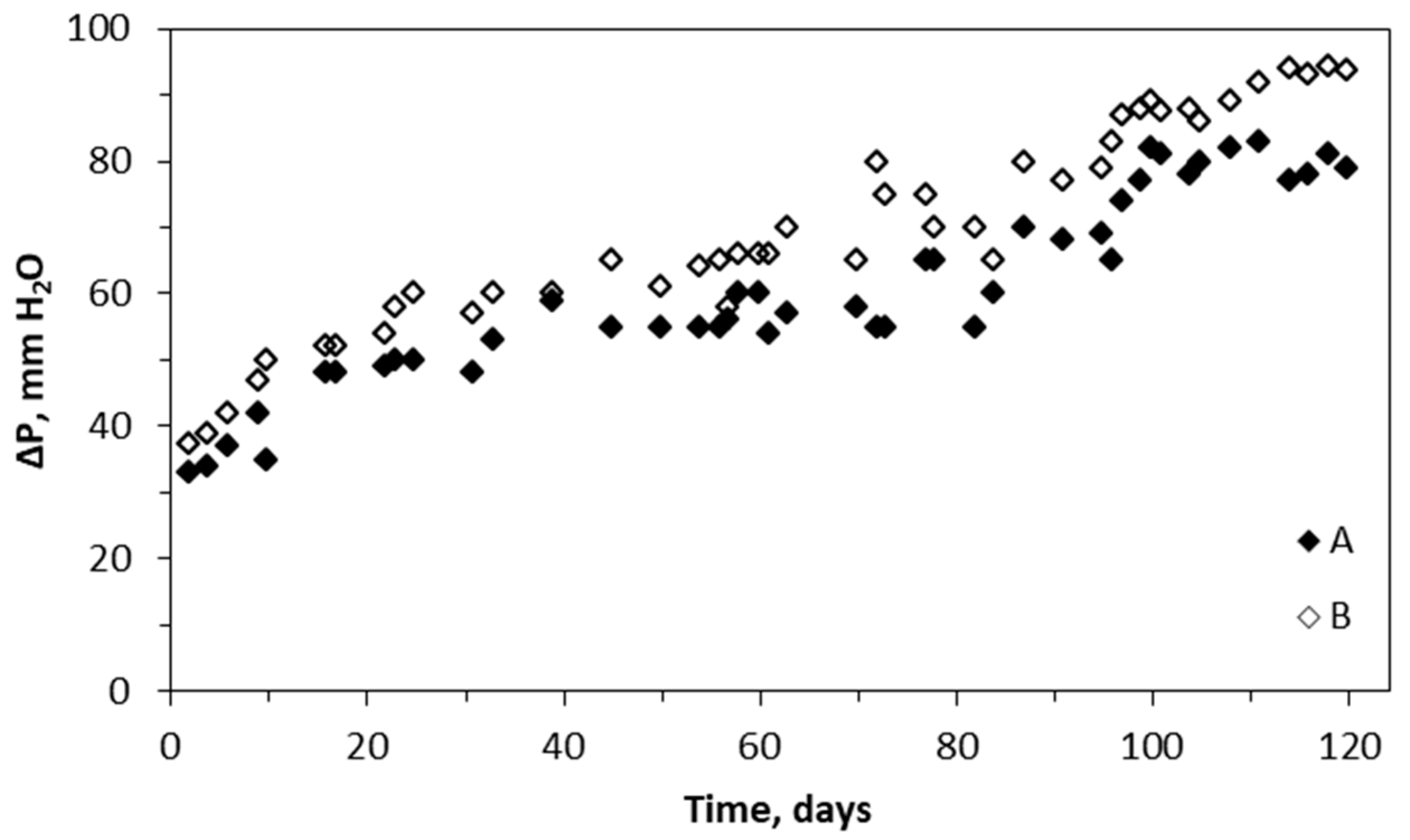
2.4. Pressure Drop across the Biotrickling Filter Packing
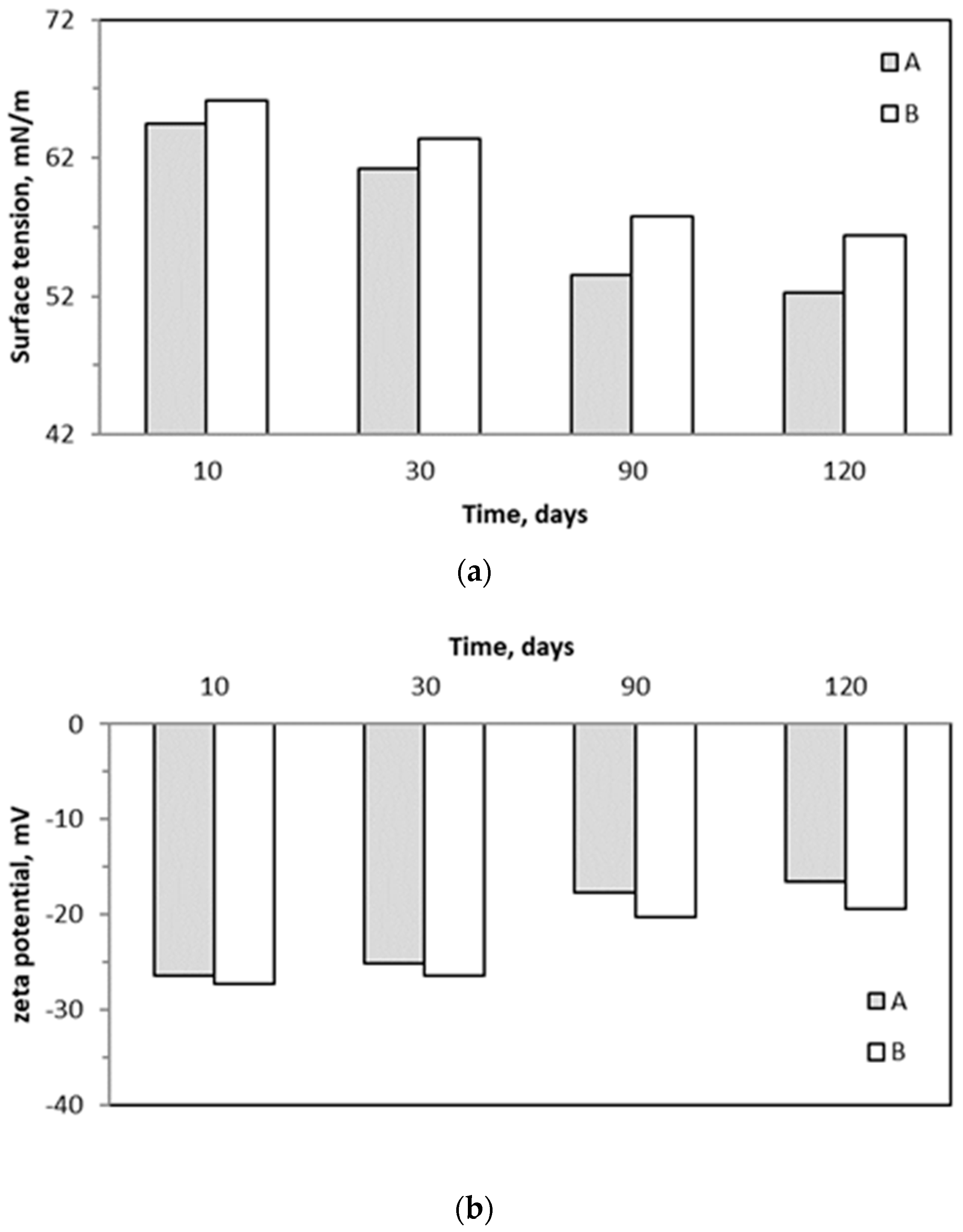
2.5. Surface Tension and Zeta Potential of Trickling Liquid
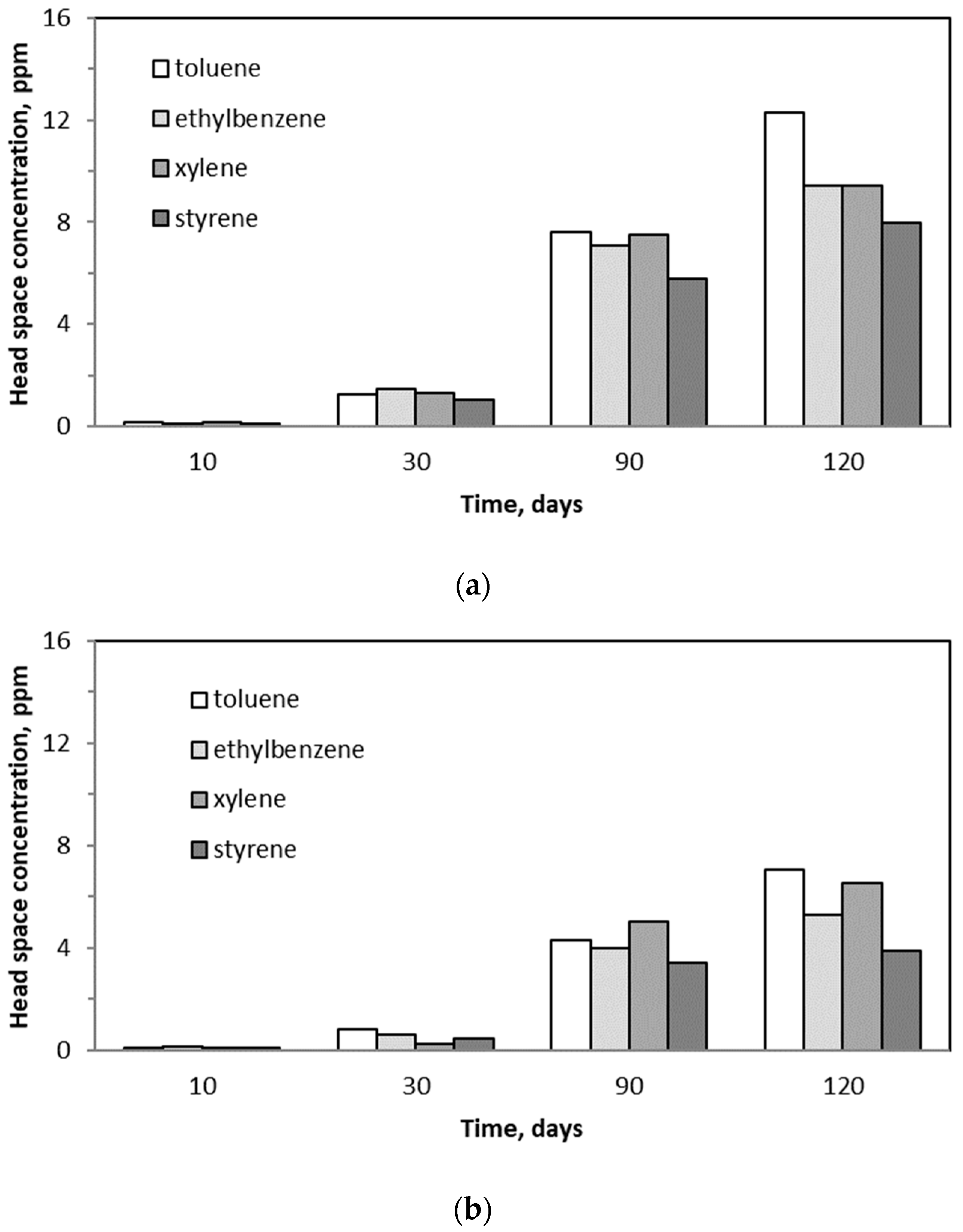
2.6. Changes in BTEX Concentrations in the Trickling Liquid
2.7. Biofilm Layer Development
3. Discussion
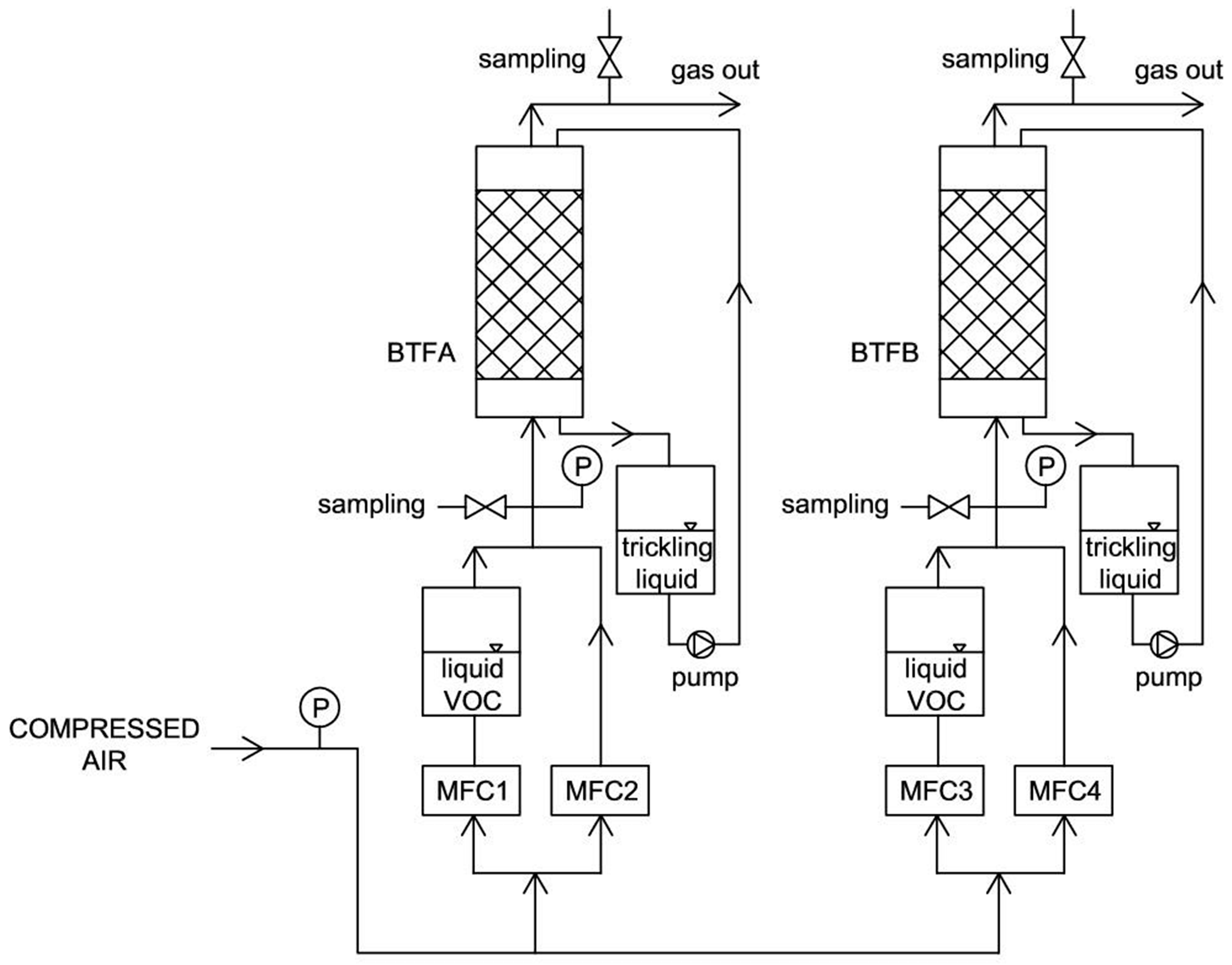
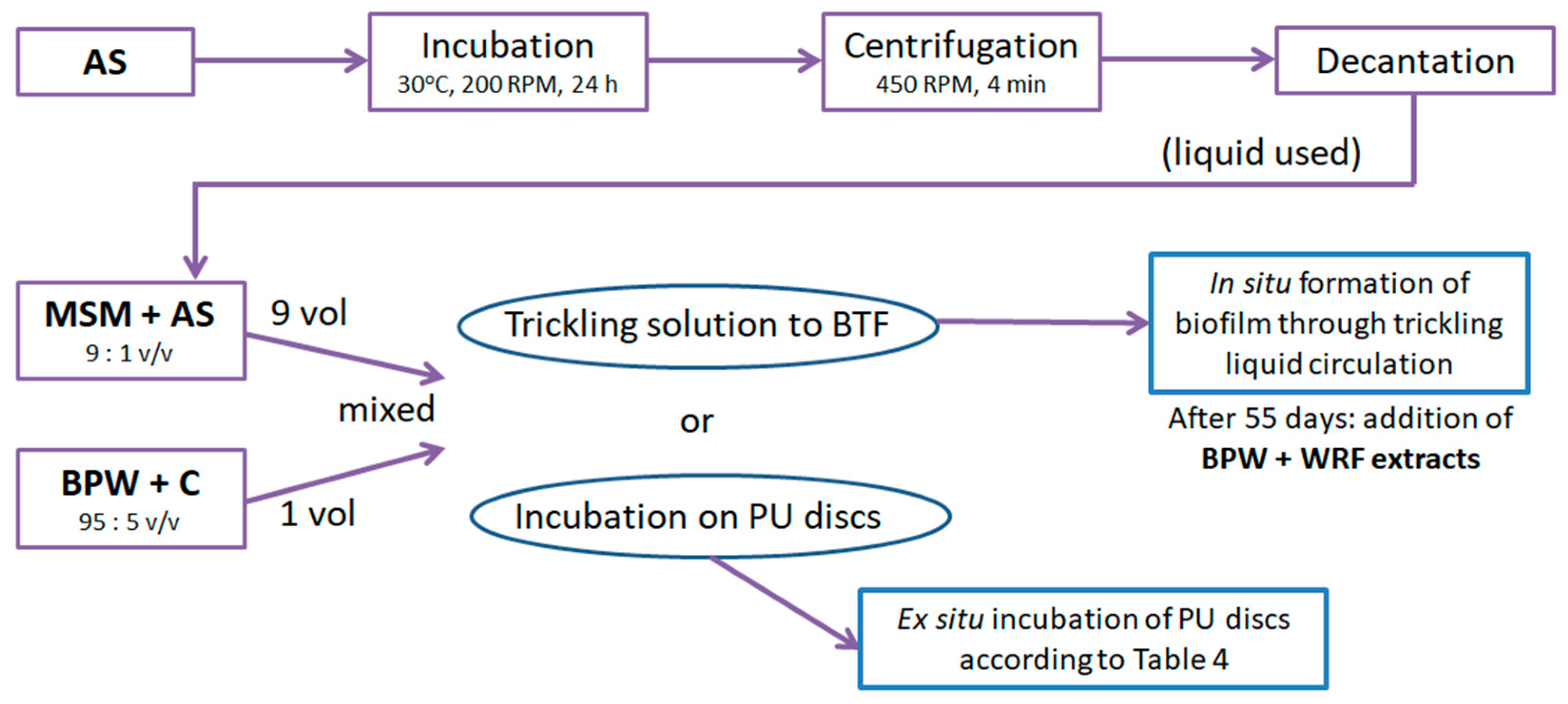
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
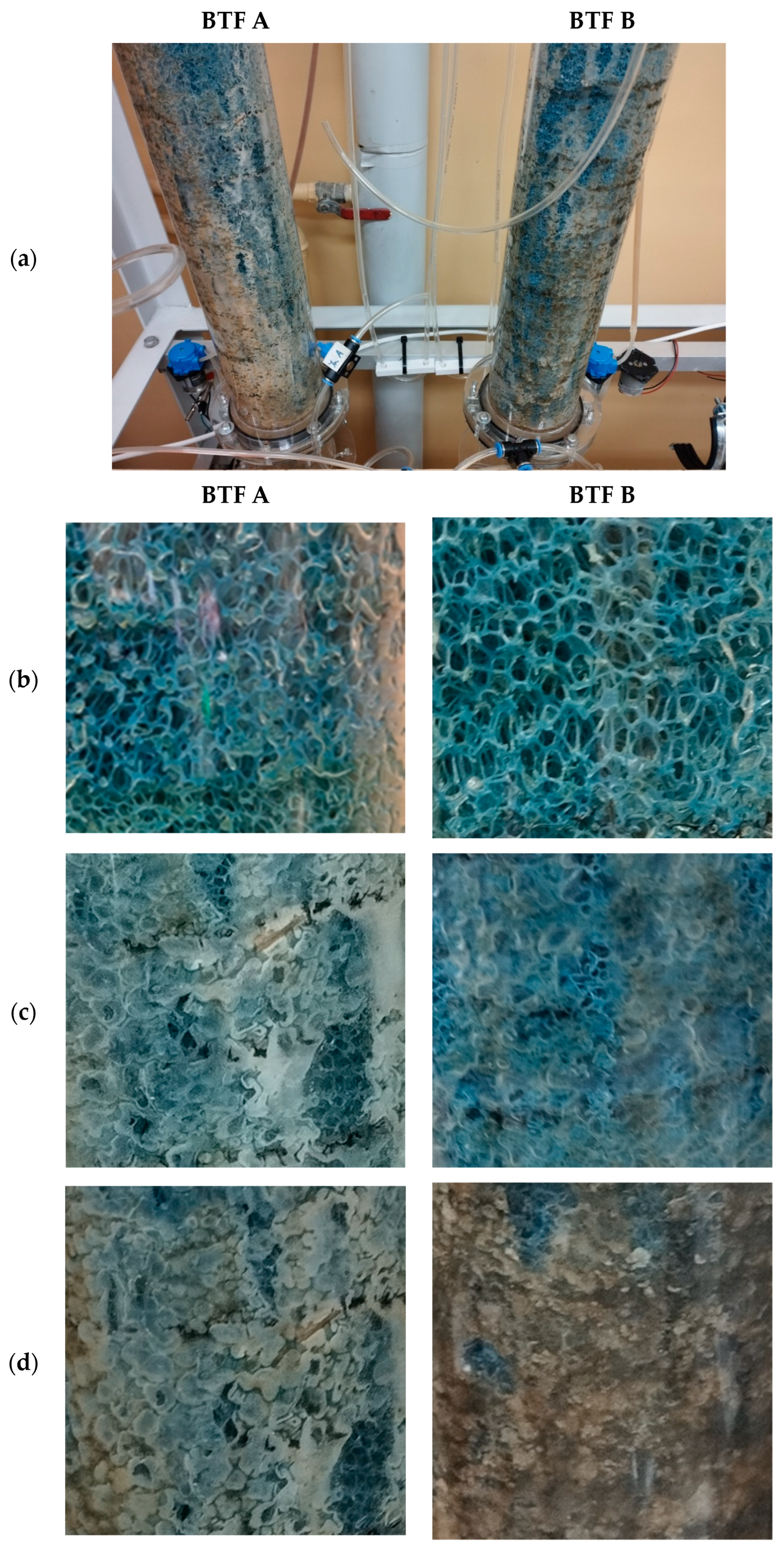
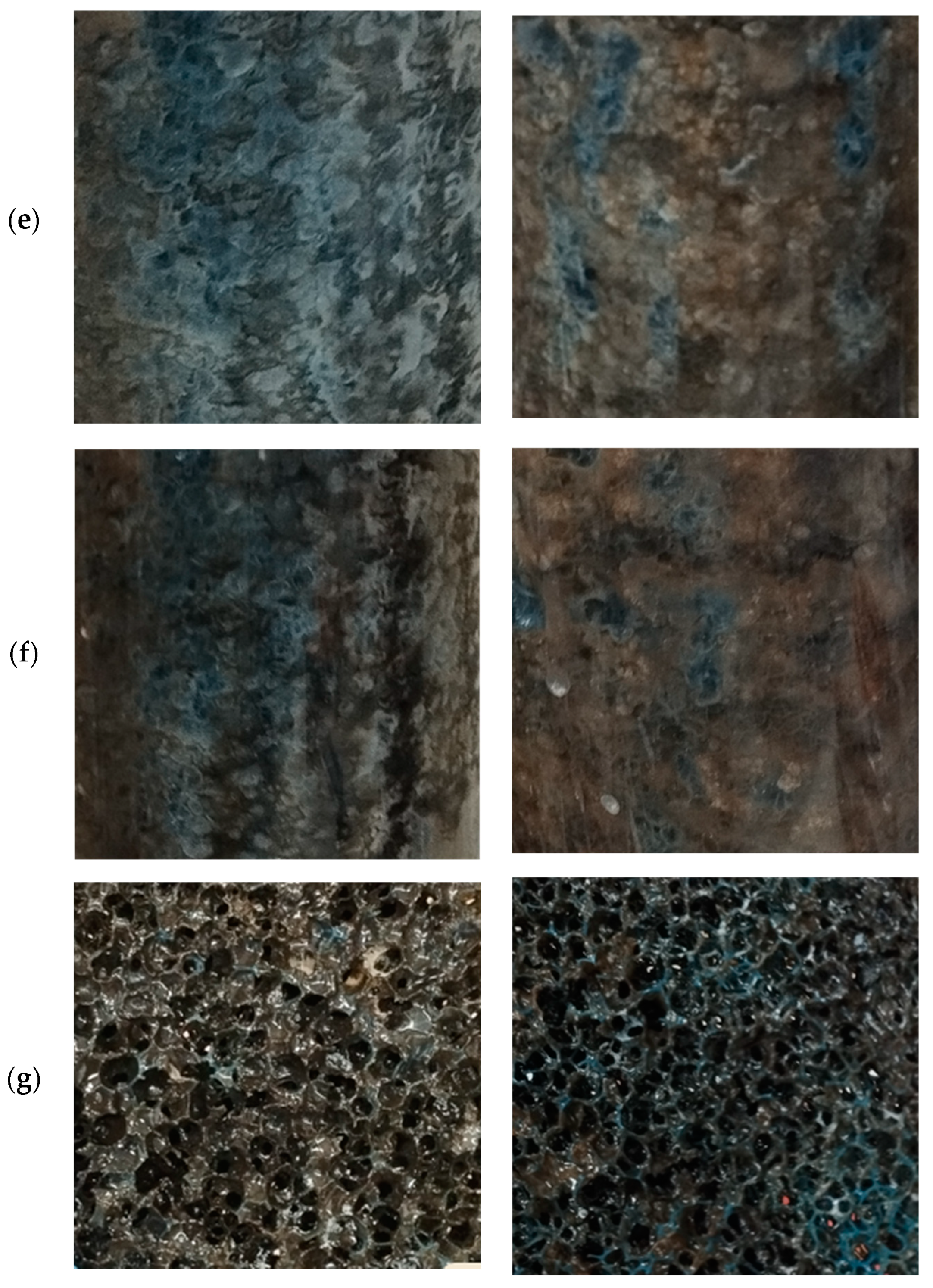
References
- Wu, X.; Lin, Y.; Wang, Y.; Wu, S.; Li, X.; Yang, C. Enhanced Removal of Hydrophobic Short-Chain n-Alkanes from Gas Streams in Biotrickling Filters in Presence of Surfactant. Environ. Sci. Technol. 2022, 56, 10349–10360. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, Y.; Wang, Y.; Wu, S.; Yang, C. Volatile organic compound removal via biofiltration: Influences, challenges, and strategies. Chem. Eng. J. 2023, 471, 144420. [Google Scholar] [CrossRef]
- Wu, X.; Lin, Y.; Wang, Y.; Dai, M.; Wu, S.; Li, X.; Yang, C. Chemical structure of hydrocarbons significantly affects removal performance and microbial responses in gas biotrickling filters. Bioresour. Technol. 2024, 398, 130480. [Google Scholar] [CrossRef] [PubMed]
- Spigno, G.; Pagella, C.; Fumi, M.D.; Molteni, R.; De Faveri, D.M. VOCs removal from waste gases: Gas-phase bioreactor for the abatement of hexane by Aspergillus niger. Chem. Eng. Sci. 2003, 58, 739–746. [Google Scholar] [CrossRef]
- McNevin, D.; Barford, J. Biofiltration as an odour abatement strategy. Biochem. Eng. J. 2000, 5, 231–242. [Google Scholar] [CrossRef]
- Barbusinski, K.; Kalemba, K.; Kasperczyk, D.; Urbaniec, K.; Kozik, V. Biological methods for odor treatment—A review. J. Clean. Prod. 2017, 152, 223–241. [Google Scholar] [CrossRef]
- Barbusiński, K.; Urbaniec, K.; Kasperczyk, D.; Thomas, M. Biofilters versus bioscrubbers and biotrickling filters: State-of-the-art biological air treatment. In From Biofiltration to Promising Options in Gaseous Fluxes Biotreatment: Recent Developments, New Trends, Advances, and Opportunities; Soreanu, G., Dumont, É., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–51. ISBN 9780128190647. [Google Scholar]
- Rybarczyk, P.; Szulczyński, B.; Gębicki, J.; Hupka, J. Treatment of malodorous air in biotrickling filters: A review. Biochem. Eng. J. 2019, 141, 146–162. [Google Scholar] [CrossRef]
- Rybarczyk, P. Removal of Volatile Organic Compounds (VOCs) from Air: Focus on Biotrickling Filtration and Process Modeling. Processes 2022, 10, 2531. [Google Scholar] [CrossRef]
- Cheng, Y.; He, H.; Yang, C.; Zeng, G.; Li, X.; Chen, H.; Yu, G. Challenges and solutions for biofiltration of hydrophobic volatile organic compounds. Biotechnol. Adv. 2016, 34, 1091–1102. [Google Scholar] [CrossRef]
- Yang, C.; Qian, H.; Li, X.; Cheng, Y.; He, H.; Zeng, G.; Xi, J. Simultaneous Removal of Multicomponent VOCs in Biofilters. Trends Biotechnol. 2018, 36, 673–685. [Google Scholar] [CrossRef]
- Wu, X.; Lin, Y.; Wang, Y.; Yang, C. Interactive effects of dual short-chain n-alkanes on removal performances and microbial responses of biotrickling filters. Chem. Eng. J. 2023, 461, 141747. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, B.; Wang, L.; Ding, C.; Li, Z. Toluene-styrene secondary acclimation improved the styrene removal ability of biotrickling filter. Chem. Speciat. Bioavailab. 2017, 29, 54–59. [Google Scholar] [CrossRef]
- Ferdowsi, M.; Khabiri, B.; Buelna, G.; Jones, J.P.; Heitz, M. Air biofilters for a mixture of organic gaseous pollutants: An approach for industrial applications. Crit. Rev. Biotechnol. 2023, 43, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Sander, R. Compilation of Henry’s law constants (version 5.0.0) for water as solvent. Atmos. Chem. Phys. 2023, 23, 10901–12440. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, J.K. Removal of benzene from benzene-laden air in a biotrickling filter. Stud. Surf. Sci. Catal. 2006, 159, 585–588. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, Y.; Zhang, H.; Yang, T. Pilot study on removal of benzene by using high efficient biotrickling filter. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010. [Google Scholar] [CrossRef]
- Aly Hassan, A.; Sorial, G. Biological treatment of benzene in a controlled trickle bed air biofilter. Chemosphere 2009, 75, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, J.; Xu, M.; An, T.; Sun, G.; Guo, J. Toluene removal efficiency, process robustness, and bacterial diversity of a biotrickling filter inoculated with Burkholderia sp. Strain T3. Biotechnol. Bioprocess Eng. 2013, 18, 125–134. [Google Scholar] [CrossRef]
- Lebrero, R.; Estrada, J.M.; Muñoz, R.; Quijano, G. Toluene mass transfer characterization in a biotrickling filter. Biochem. Eng. J. 2012, 60, 44–49. [Google Scholar] [CrossRef]
- Chou, M.-S.; Wu, F.-L. Treatment of Toluene in an Air Stream by a Biotrickling Filter Packed with Slags. J. Air Waste Manage. Assoc. 1999, 49, 386–398. [Google Scholar] [CrossRef]
- Alinejad, A.; Zamir, S.M.; Shojaosadati, S.A. Different strategies for transient-state operation of a biotrickling filter treating toluene vapor. Appl. Microbiol. Biotechnol. 2017, 101, 3451–3462. [Google Scholar] [CrossRef]
- De Vela, R.J.L.; Gostomski, P.A. Design and Performance of a Toluene-Degrading Differential Biotrickling Filter as an Alternative Research Tool to Column Reactors. J. Environ. Eng. 2020, 147, 04020159. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Qin, Y.; Yang, Z.; Cao, J.; Xing, Y.; Li, J. Performance and microbial community evolution of toluene degradation using a fungi-based bio-trickling filter. J. Hazard. Mater. 2019, 365, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, C.; Cheng, Y.; Huang, J.; Yang, H.; Zeng, G.; Lu, L.; He, S. Enhanced removal of ethylbenzene from gas streams in biotrickling filters by Tween-20 and Zn(II). J. Environ. Sci. 2014, 26, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gao, Q. Enhancement of ethylbenzene removal from contaminated gas and corresponding mechanisms in biotrickling filters by a biosurfactant from piggery wastewater. J. Environ. Manage. 2021, 277, 111411. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Cheng, Y.; Yang, C.; Wu, S.; Zeng, G.; Xi, J. Performance and biofilm characteristics of biotrickling filters for ethylbenzene removal in the presence of saponins. Environ. Sci. Pollut. Res. 2017, 25, 30021–30030. [Google Scholar] [CrossRef]
- Liu, Q.; Babajide, A.E.; Zhu, P.; Zou, L. Removal of Xylene from Waste Gases using Biotrickling Filters. Chem. Eng. Technol. 2006, 29, 320–325. [Google Scholar] [CrossRef]
- Wu, C.; Xu, P.; Xu, B.; Li, W.; Li, S.; Wang, X. o-Xylene removal using one- and two-phase partitioning biotrickling filters: Steady/transient-state performance and microbial community. Environ. Technol. 2018, 39, 109–119. [Google Scholar] [CrossRef]
- Dou, X.; Liu, J.; Qi, H.; Li, P.; Lu, S.; Li, J. Synergistic removal of m-xylene and its corresponding mechanism in a biotrickling filter. Process Biochem. 2022, 118, 404–412. [Google Scholar] [CrossRef]
- Raboni, M.; Torretta, V.; Viotti, P. Treatment of airborne BTEX by a two-stage biotrickling filter and biofilter, exploiting selected bacterial and fungal consortia. Int. J. Environ. Sci. Technol. 2017, 14, 19–28. [Google Scholar] [CrossRef]
- Liao, D.; Li, E.; Li, J.; Zeng, P.; Feng, R.; Xu, M.; Sun, G. Removal of benzene, toluene, xylene and styrene by biotrickling filters and identification of their interactions. PLoS ONE 2018, 13, e0189927. [Google Scholar] [CrossRef]
- Akmirza, I.; Pascual, C.; Carvajal, A.; Pérez, R.; Muñoz, R.; Lebrero, R. Anoxic biodegradation of BTEX in a biotrickling filter. Sci. Total Environ. 2017, 587–588, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Shi, P.; Wang, Z.; Jiang, H.; Long, C. A comparative study of bacterial and fungal-bacterial steady-state stages of a biofilter in gaseous toluene removal: Performance and microbial community. J. Chem. Technol. Biotechnol. 2017, 92, 2853–2861. [Google Scholar] [CrossRef]
- Jorio, H.; Jin, Y.; Elmrini, H.; Nikiema, J.; Brzezinski, R.; Heitz, M. Treatment of VOCs in biofilters inoculated with fungi and microbial consortium. Environ. Technol. 2009, 30, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Fernandez, A.; Scott, F.; Carreno-Lopez, F.; Aroca, G.; Moreno-Casas, P.; Gonzalez-Sanchez, A.; Munoz, R. A comparative assessment of the performance of fungal-bacterial and fungal biofilters for methane abatement. J. Environ. Chem. Eng. 2020, 8, 104421. [Google Scholar] [CrossRef]
- Marycz, M.; Brillowska-Dąbrowska, A.; Cantera, S.; Gębicki, J.; Muñoz, R. Fungal co-culture improves the biodegradation of hydrophobic VOCs gas mixtures in conventional biofilters and biotrickling filters. Chemosphere 2023, 313, 137609. [Google Scholar] [CrossRef]
- Marycz, M.; Brillowska-Dąbrowska, A.; Muñoz, R.; Gębicki, J. A state of the art review on the use of fungi in biofiltration to remove volatile hydrophobic pollutants. Rev. Environ. Sci. Biotechnol. 2022, 21, 225–246. [Google Scholar] [CrossRef]
- Nagarajan, A.; Anandakumar, S. Mini review on Corncob biomass: A potential resource for value-added metabolites. Eur. J. Exp. Biol. 2016, 6, 9–13. [Google Scholar]
- Doddapaneni, T.R.K.C.; Jain, R.; Praveenkumar, R.; Rintala, J.; Romar, H.; Konttinen, J. Adsorption of furfural from torrefaction condensate using torrefied biomass. Chem. Eng. J. 2018, 334, 558–568. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Dutra, E.D.; Santos, F.A.; Alencar, B.R.A.; Reis, A.L.S.; de F.R. de Souza, R.; da S. Aquino, K.A.; Morais, M.A., Jr.; Menezes, R.S.C. Alkaline hydrogen peroxide pretreatment of lignocellulosic biomass: Status and perspectives. Biomass Convers. Biorefinery 2018, 8, 225–234. [Google Scholar] [CrossRef]
- Mir-Tutusaus, J.A.; Masís-Mora, M.; Corcellas, C.; Eljarrat, E.; Barceló, D.; Sarrà, M.; Caminal, G.; Vicent, T.; Rodríguez-Rodríguez, C.E. Degradation of selected agrochemicals by the white rot fungus Trametes versicolor. Sci. Total Environ. 2014, 500–501, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Gusse, A.C.; Miller, P.D.; Volk, T.J. White-rot fungi demonstrate first biodegradation of phenolic resin. Environ. Sci. Technol. 2006, 40, 4196–4199. [Google Scholar] [CrossRef] [PubMed]
- Lazim, Z.M.; Hadibarata, T. Ligninolytic fungus Polyporus sp. S133 mediated metabolic degradation of fluorene. Braz. J. Microbiol. 2016, 47, 610–616. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; Pérez-Trujillo, M.; Vicent, T.; Caminal, G. Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere 2009, 74, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itävaara, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Lamprea-Pineda, P.A.; Carmona, F.J.; Demeestere, K.; González-Cortés, J.J.; Van Langenhove, H.; Walgraeve, C.; Lebrero, R. Effect of surfactant type and concentration on the gas-liquid mass transfer in biotrickling filters used for air pollution control. J. Environ. Manage. 2024, 367, 121968. [Google Scholar] [CrossRef] [PubMed]
- Sakhaei, A.; Zamir, S.M.; Rene, E.R.; Veiga, M.C.; Kennes, C. Neural network-based performance assessment of one- and two-liquid phase biotrickling filters for the removal of a waste-gas mixture containing methanol, α-pinene, and hydrogen sulfide. Environ. Res. 2023, 237, 116978. [Google Scholar] [CrossRef]
- Ghasemi, R.; Golbabaei, F.; Rezaei, S.; Pourmand, M.R.; Nabizadeh, R.; Jafari, M.J.; Masoorian, E. A comparison of biofiltration performance based on bacteria and fungi for treating toluene vapors from airflow. AMB Express 2020, 10, 8. [Google Scholar] [CrossRef]
- Sun, Z.; Cheng, Z.; Luo, P.; Chen, J.; Yu, J.; Chen, D.; Zhao, P. Cyclohexane removal and UV post-control of bioaerosols in a combination of UV pretreatment and biotrickling filtration. Front. Environ. Sci. 2022, 10, 1010980. [Google Scholar] [CrossRef]
- Torretta, V.; Collivignarelli, M.C.; Raboni, M.; Viotti, P. Experimental treatment of a refinery waste air stream, for BTEX removal, by water scrubbing and biotrickling on a bed of Mitilus edulis shells. Environ. Technol. 2015, 36, 2300–2307. [Google Scholar] [CrossRef]
- Bonilla-Blancas, W.; Garduño-Montero, A.; Salazar-Pereyra, M.; Gonzalez-Sanchez, A. Automatic control of water content in a polyurethane foam packed bed used in biotrickling filters for polluted air treatment. J. Environ. Manage. 2024, 349, 119554. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.; De Oca, M.; Danila, V.; Zagorskis, A.; Januševičius, T. Effects of Water Content and Irrigation of Packing Materials on the Performance of Biofilters and Biotrickling Filters: A Review. Processes 2022, 10, 1304. [Google Scholar] [CrossRef]
- Lee, S.H.; Kurade, M.B.; Jeon, B.H.; Kim, J.; Zheng, Y.; Salama, E.S. Water condition in biotrickling filtration for the efficient removal of gaseous contaminants. Crit. Rev. Biotechnol. 2021, 41, 1279–1296. [Google Scholar] [CrossRef] [PubMed]
- Lamprea Pineda, P.A.; Demeestere, K.; Toledo, M.; Van Langenhove, H.; Walgraeve, C. Enhanced removal of hydrophobic volatile organic compounds in biofilters and biotrickling filters: A review on the use of surfactants and the addition of hydrophilic compounds. Chemosphere 2021, 279, 130757. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, J.; Li, J.; He, H.; Peng, S.; Li, C.; Chen, Y. Removal of H2S by co-immobilized bacteria and fungi biocatalysts in a bio-trickling filter. Process Saf. Environ. Prot. 2013, 91, 145–152. [Google Scholar] [CrossRef]
- Marycz, M.; Brillowska-Dabrowska, A.; Gebicki, J. Evaluation of Immobilization of Selected Peat-Isolated Yeast Strains of the Species Candida albicans and Candida subhashii on the Surface of Artificial Support Materials Used for Biotrickling Filtration. Processes 2020, 8, 801. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Xing, H.; Li, J. Effect of liquid supply on the performance of a fungal bio-trickling filter treating hydrophobic VOC. Biochem. Eng. J. 2020, 161, 107658. [Google Scholar] [CrossRef]
- Hong, T.; Wei, L.; Cui, K.; Dong, Y.; Luo, L.; Zhang, T.; Li, R.; Li, Z.; Tang, Y. Response Surface Methodology and Artificial Neural Network Modeling for the Removal of Volatile Organic Compounds in Biotrickling Filters. Water Air Soil Pollut. 2023, 234, 624. [Google Scholar] [CrossRef]
- Muñoz, R.; Daugulis, A.J.; Hernández, M.; Quijano, G. Recent advances in two-phase partitioning bioreactors for the treatment of volatile organic compounds. Biotechnol. Adv. 2012, 30, 1707–1720. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, X.; Liu, H.; Yang, C.; Wu, S.; Du, C.; Nie, L.; Zhong, Y. Effect of presence of hydrophilic volatile organic compounds on removal of hydrophobic n-hexane in biotrickling filters. Chemosphere 2020, 252, 126490. [Google Scholar] [CrossRef]
- Salamanca, D.; Dobslaw, D.; Engesser, K.-H. Removal of cyclohexane gaseous emissions using a biotrickling filter system. Chemosphere 2017, 176, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yang, G.; Lens, P.N.L.; He, Y.; Qie, L.; Shen, X.; Chen, J.; Cheng, Z.; Chen, D. Enhanced removal of mixed VOCs with different hydrophobicities by Tween 20 in a biotrickling filter: Kinetic analysis and biofilm characteristics. J. Hazard. Mater. 2023, 450, 131063. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, J.; Zhang, Y.; Wu, H. Effect of commutation on pressure drop and microbial diversity in a horizontal biotrickling filter for toluene removal. Arch. Microbiol. 2024, 206, 109. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, Y.; Li, Y.; Sun, Y.; Song, C.; Huang, Z.; Yang, Z.; Han, Y. Shift of microbial diversity and function in high-efficiency performance biotrickling filter for gaseous xylene treatment. J. Air Waste Manage. Assoc. 2019, 69, 1059–1069. [Google Scholar] [CrossRef]
- Avalos Ramirez, A.; Jones, J.P.; Heitz, M. Biotrickling filtration of air contaminated with ethanol. J. Chem. Technol. Biotechnol. 2007, 82, 149–157. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, S.; Luo, Y.; Ma, H.; Wang, Y. A biofilter for treating toluene vapors: Performance evaluation and microbial counts behavior. PeerJ 2016, 2016, e2045. [Google Scholar] [CrossRef]
- Gallastegui, G.; Barona, A.; Rojo, N.; Gurtubay, L.; Elías, A. Comparative response of two organic biofilters treating ethylbenzene and toluene after prolonged exposure. Process Saf. Environ. Prot. 2013, 91, 112–122. [Google Scholar] [CrossRef]
- Dewidar, A.A.; Sorial, G.A. Effect of rhamnolipids on the fungal elimination of toluene vapor in a biotrickling filter under stressed operational conditions. Environ. Res. 2022, 204, 111973. [Google Scholar] [CrossRef]
- Soreanu, G.; Diaconu, M.; Maier, S.S.; Volf, I.; Igor, C. Enhancing the environmental performance of biotrickling filters treating volatile organic compounds in air. IOP Conf. Ser. Earth Environ. Sci. 2021, 906, 012124. [Google Scholar] [CrossRef]
- Soreanu, G.; Diaconu, M.; Maier, S.S.; Volf, I.; Cretescu, I. Moving forward sustainable solutions for VOCs biotrickling filtration through co-immobilised microorganisms. Rom. J. Ecol. Environ. Chem. 2021, 3, 54–60. [Google Scholar] [CrossRef]
- Sempere, F.; Gabaldón, C.; Martínez-Soria, V.; Marzal, P.; Penya-roja, J.M.; Javier Álvarez-Hornos, F. Performance evaluation of a biotrickling filter treating a mixture of oxygenated VOCs during intermittent loading. Chemosphere 2008, 73, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Den, W.; Huang, C.; Li, C.H. Effects of cross-substrate interaction on biotrickling filtration for the control of VOC emissions. Chemosphere 2004, 57, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Marycz, M.; Rodríguez, Y.; Gębicki, J.; Muñoz, R. Systematic comparison of a biotrickling filter and a conventional filter for the removal of a mixture of hydrophobic VOCs by Candida subhashii. Chemosphere 2022, 306, 135608. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, X.; Zhu, S.; Chen, J.; He, Y.; Shi, Y.; Liu, H.; Qin, L. Co-treatment with single and ternary mixture gas of dimethyl sulfide, propanethiol, and toluene by a macrokinetic analysis in a biotrickling filter seeded with alcaligenes sp. Sy1 and pseudomonas putida S1. Fermentation 2021, 7, 309. [Google Scholar] [CrossRef]
- Schiavon, M.; Ragazzi, M.; Rada, E.C.; Torretta, V. Air pollution control through biotrickling filters: A review considering operational aspects and expected performance. Crit. Rev. Biotechnol. 2016, 36, 1143–1155. [Google Scholar] [CrossRef]
- Rybarczyk, P.; Szulczyński, B.; Dobrzyniewski, D.; Kucharska, K.; Gębicki, J. Removal of cyclohexane vapors from air in biotrickling filters: Effects of gas mixture composition and circular economy approach. Chem. Process Eng. New Front. 2023, 44, e40. [Google Scholar] [CrossRef]
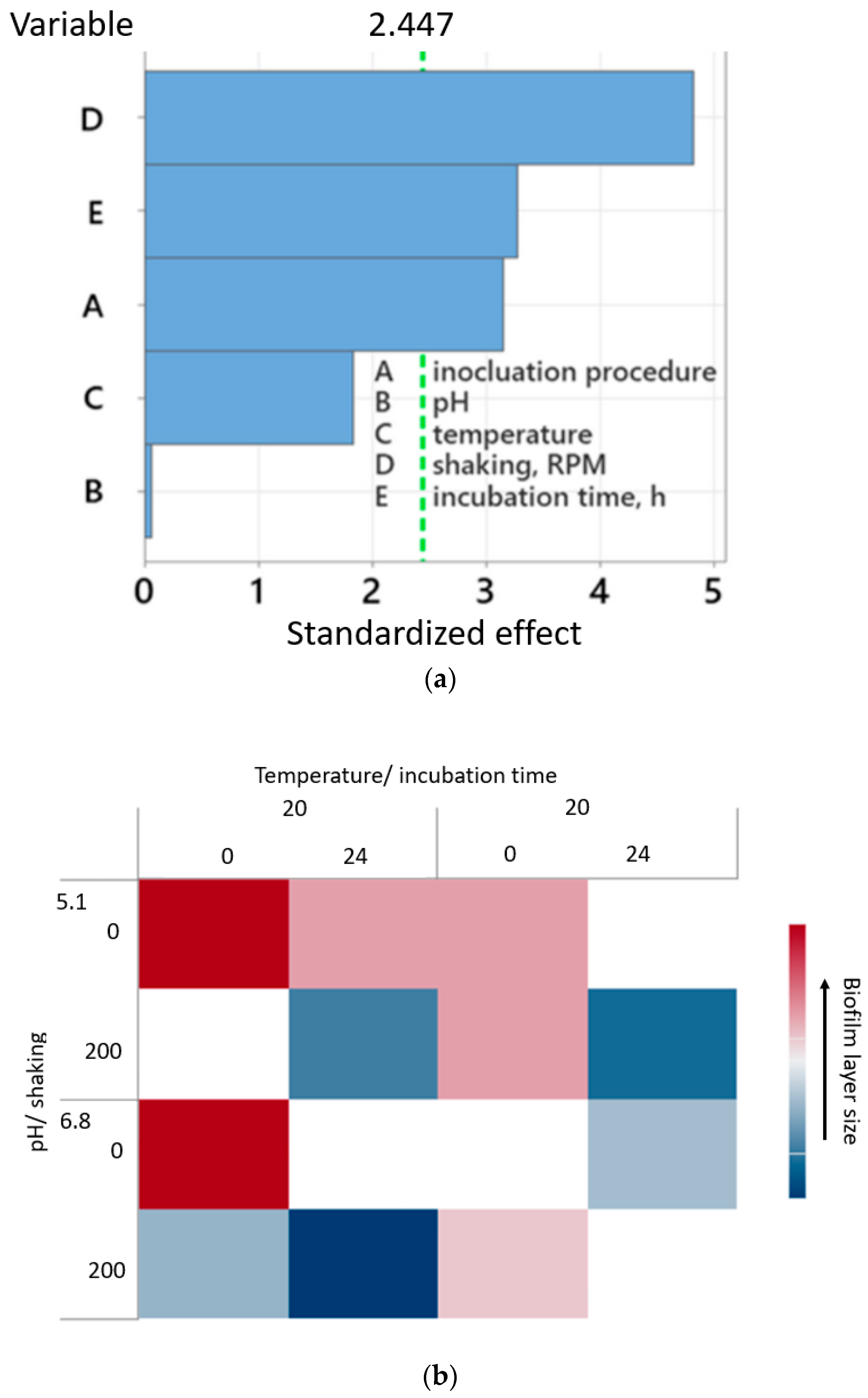

| No. | Variable | Lower Value (−1) | Upper Value (1) |
|---|---|---|---|
| A | Inoculation procedure | −1 (without incubation) | 1 (with incubation) |
| B | pH | 5.1 | 6.8 |
| C | Temperature, °C | 20 | 30 |
| D | Shaking, RPM | 0 | 200 |
| E | Incubation time, h | 0 | 24 |
| Run | Inoculation Procedure | pH | Temperature, °C | Shaking, RPM | Incubation Time, h |
|---|---|---|---|---|---|
| 1. | 1 | 6.8 | 30 | 0 | 24 |
| 2. | 1 | 6.8 | 20 | 200 | 0 |
| 3. | −1 | 6.8 | 30 | 0 | 24 |
| 4. | 1 | 6.8 | 20 | 200 | 24 |
| 5. | 1 | 5.1 | 20 | 0 | 24 |
| 6. | −1 | 6.8 | 20 | 0 | 0 |
| 7. | 1 | 5.1 | 30 | 0 | 0 |
| 8. | −1 | 5.1 | 20 | 200 | 24 |
| 9. | −1 | 5.1 | 20 | 0 | 0 |
| 10. | −1 | 5.1 | 30 | 200 | 24 |
| 11. | −1 | 6.8 | 30 | 200 | 0 |
| 12. | 1 | 5.1 | 30 | 200 | 0 |
| Biofilter | Inoculation Procedure | pH | Temperature, °C | Shaking, RPM | Incubation Time, h |
|---|---|---|---|---|---|
| A | applied | 6.8 | 20 | 200 | 24 |
| B | not applied | 6.8 | 20 | 0 | 0 |
| Parameter | Value |
|---|---|
| Packing volume, dm3 | 2.5 |
| Gas flow rate, dm3 min−1 | 2.5 |
| Empty bed residence time (EBRT), min | 1 |
| Inlet loading (sum of BTEX), g m−3 h−1: Days 1–40 Days 41–80 Days 81–120 | 10 ± 0.4 20 ± 0.4 30 ± 0.5 |
| Trickling for BTF A | 5.76 dm3 h−1 (5 s each 10 min) |
| Trickling for BTF B | Days 1–40: 1.44 dm3 h−1 (continuous trickling) Days 41–120: 5.76 dm3 h−1 (5 s each 10 min) |
| Trickling liquid | Days 1–20: MSM; days 21–55: tap water; days 56–120: MSM |
| Temperature, °C | 22–25 (room temperature) |
| Target Compound(s) | (MCO2/EC) | Inoculum | Reference |
|---|---|---|---|
| Toluene | 1.45 | Activated sludge from WWTP | [68] |
| Toluene | 2.84 | Aerobic activated sludge | [69] |
| Toluene | 1.99 | Fungal consortium (Trichoderma asperellum and Fusarium solani) with rhamnolipids addition | [70] |
| Ethanol | 1.02 | Compost-derived microorganisms with microalgae | [71] |
| 1.31 | Compost-derived microorganisms | ||
| Ethanol | 0.44 | Compost-derived microorganisms with microalgae on alginate beads | [72] |
| Ethylbenzene | 1.36 | Aerobic activated sludge | [69] |
| Xylene | 2.86 | [66] | |
| Ethanol, ethyl acetate, MEK | 0.30 | Activated sewage sludge | [73] |
| Acetone, toluene, trichloroethylene | 0.54 | Microbial seeds from WWTP on granular activated carbon | [74] |
| Mixed VOCs (n-hexane, trichloroethylene, toluene, α-pinene) | 0.41 | Candida subhashii | [75] |
| Dimehtyl sulfide, propanethiol, toluene | 2.26 | Alcaligenes sp. SY1, Pseudomonas putida S1, and microorganisms from activated sludge | [76] |
| Mixed VOCs (BTEX) | 2.37–2.71 | Activated sewage sludge, composting starter, and white rot fungi extract | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybarczyk, P.; Cichon, K.; Kucharska, K.; Dobrzyniewski, D.; Szulczyński, B.; Gębicki, J. Packing Incubation and Addition of Rot Fungi Extracts Improve BTEX Elimination from Air in Biotrickling Filters. Molecules 2024, 29, 4431. https://doi.org/10.3390/molecules29184431
Rybarczyk P, Cichon K, Kucharska K, Dobrzyniewski D, Szulczyński B, Gębicki J. Packing Incubation and Addition of Rot Fungi Extracts Improve BTEX Elimination from Air in Biotrickling Filters. Molecules. 2024; 29(18):4431. https://doi.org/10.3390/molecules29184431
Chicago/Turabian StyleRybarczyk, Piotr, Krzysztof Cichon, Karolina Kucharska, Dominik Dobrzyniewski, Bartosz Szulczyński, and Jacek Gębicki. 2024. "Packing Incubation and Addition of Rot Fungi Extracts Improve BTEX Elimination from Air in Biotrickling Filters" Molecules 29, no. 18: 4431. https://doi.org/10.3390/molecules29184431
APA StyleRybarczyk, P., Cichon, K., Kucharska, K., Dobrzyniewski, D., Szulczyński, B., & Gębicki, J. (2024). Packing Incubation and Addition of Rot Fungi Extracts Improve BTEX Elimination from Air in Biotrickling Filters. Molecules, 29(18), 4431. https://doi.org/10.3390/molecules29184431








