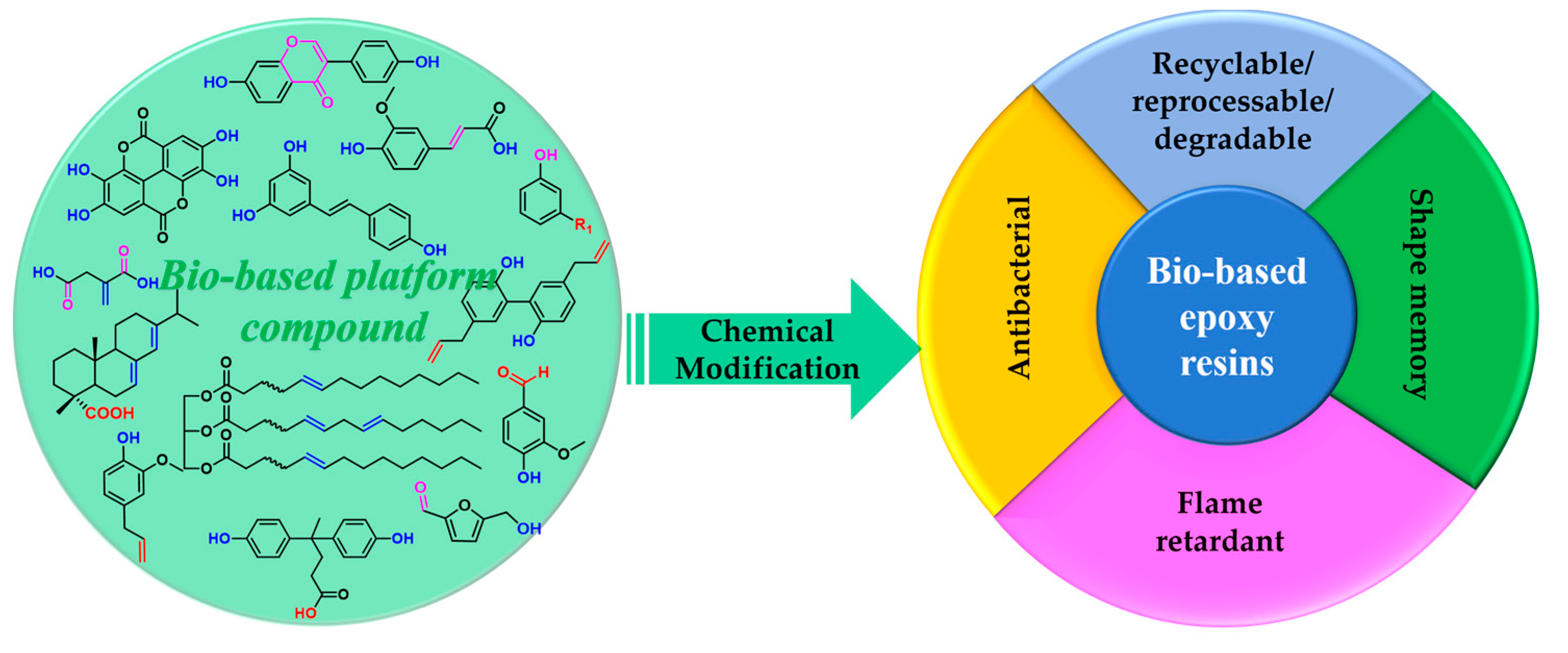
Recent Development of Functional Bio-Based Epoxy Resins
Abstract
1. Introduction
2. Synthesis Methods of Various Functionalized Bio-Based Epoxy Resins
3. Functional Bio-Based Epoxy Resins
3.1. Flame Retardant
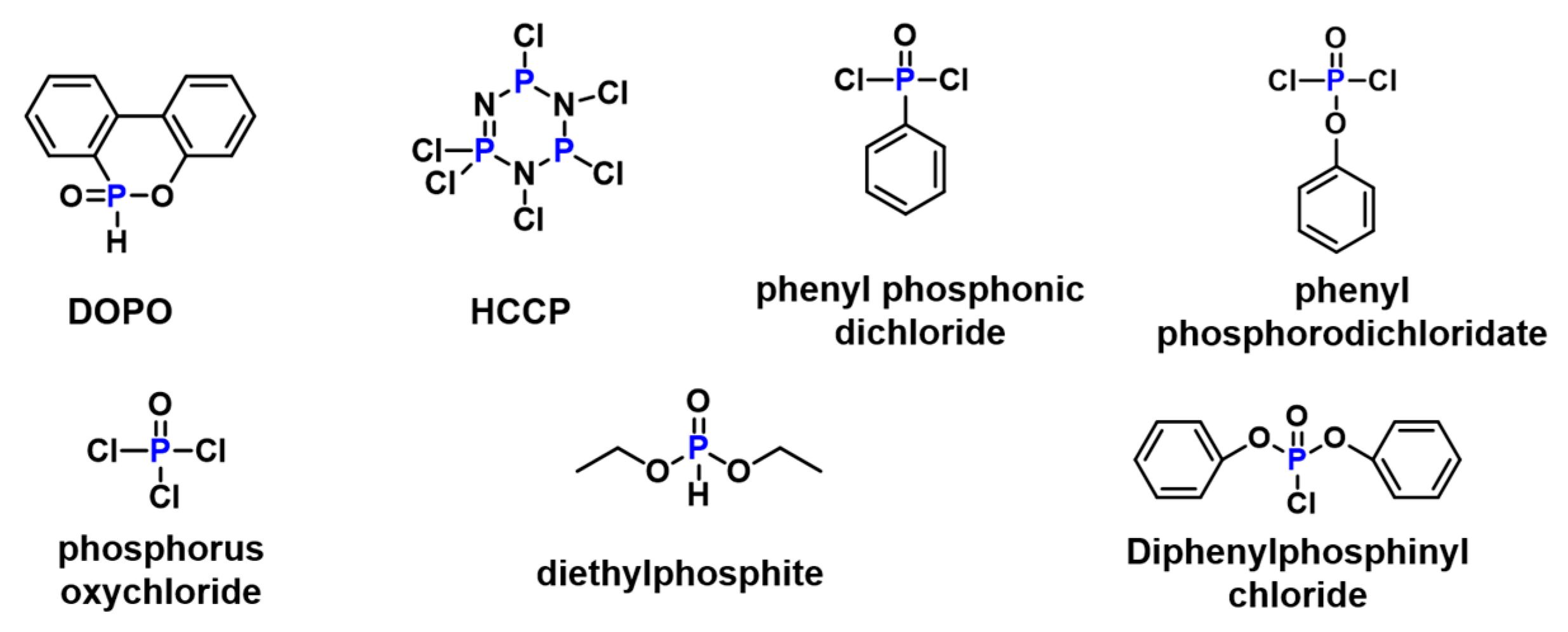
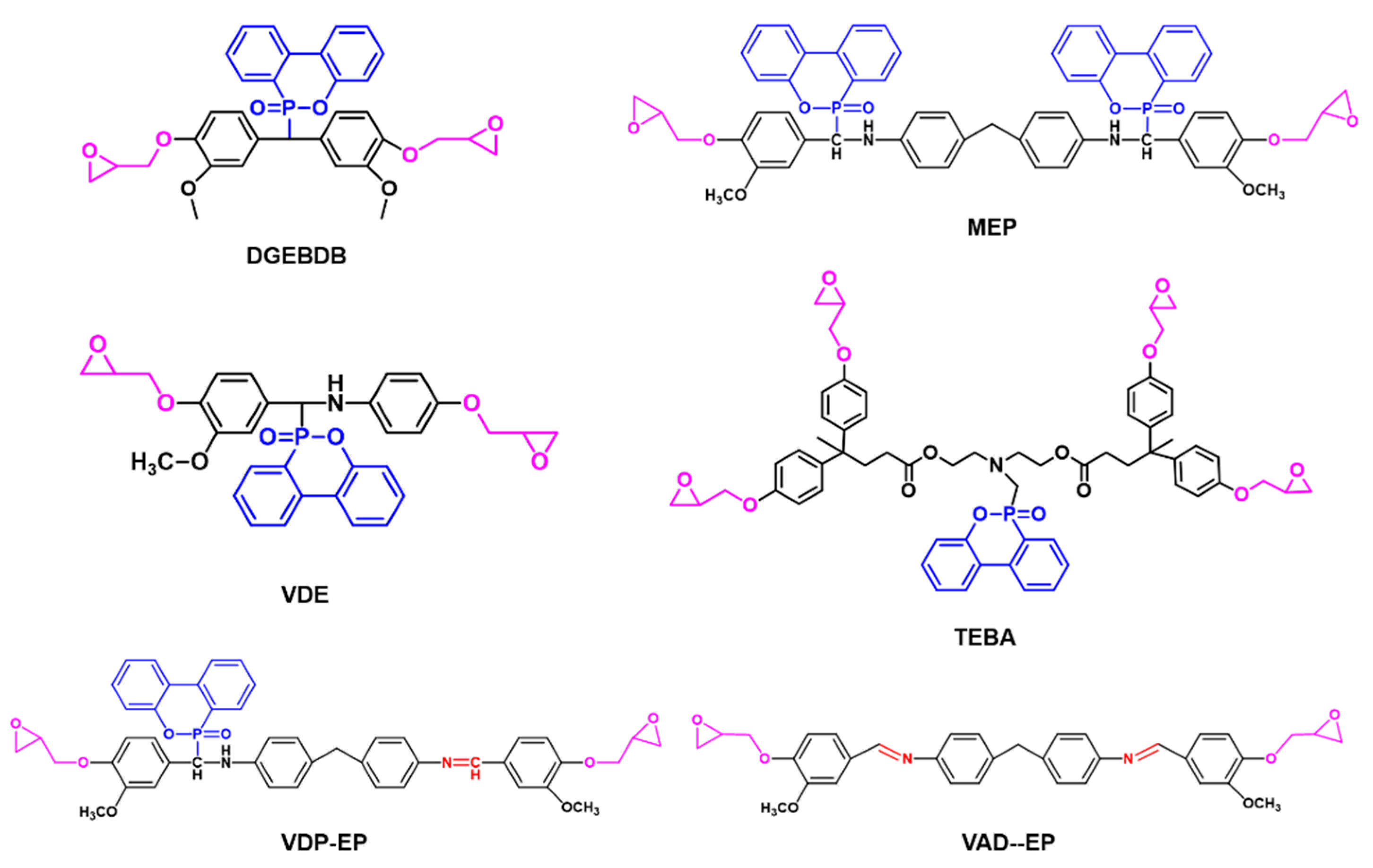
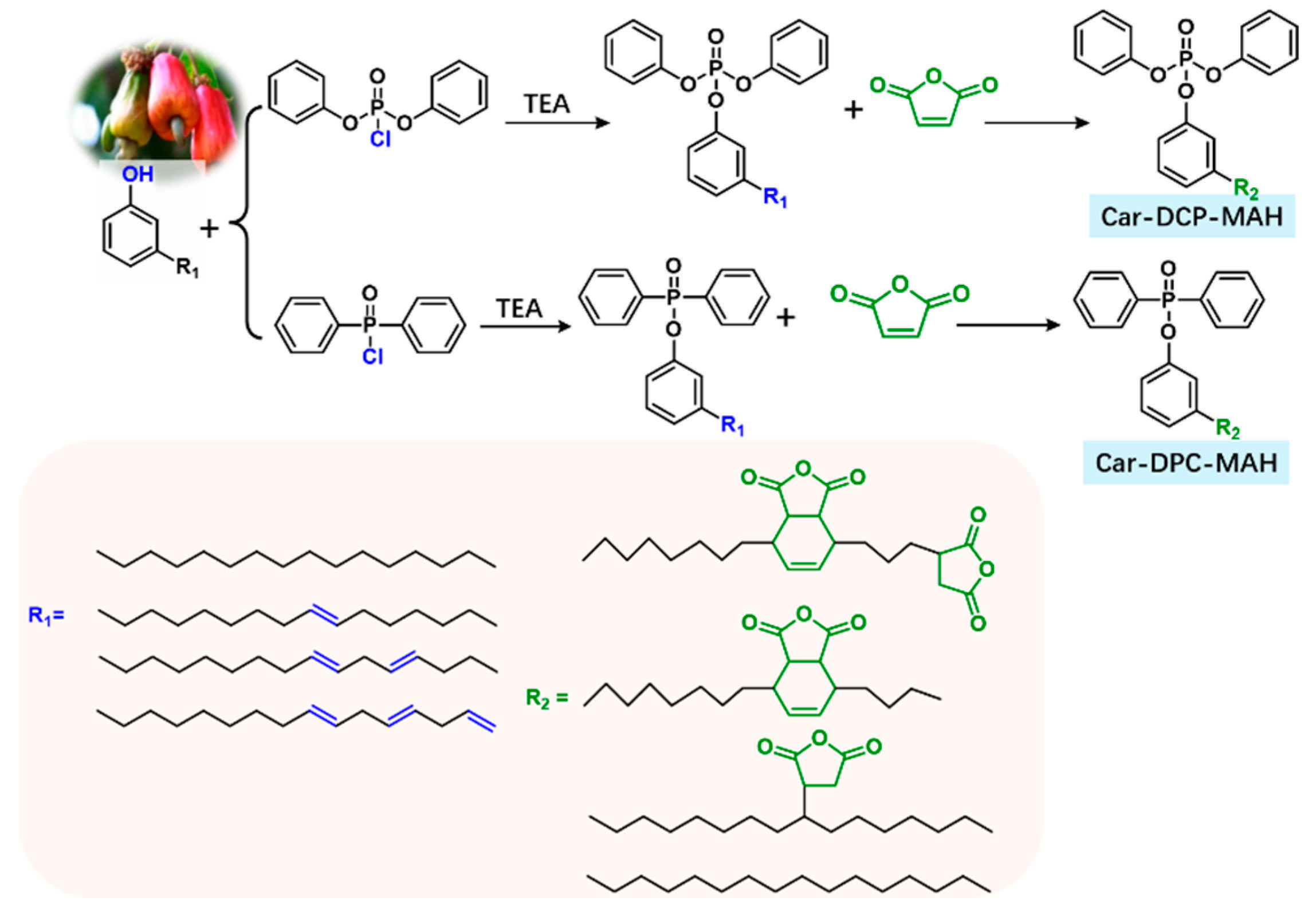
3.1.1. Phosphorus-Containing Flame-Retardant Bio-Based Epoxy Thermosets
3.1.2. Phosphorus-Free Flame-Retardant Bio-Based Epoxy Thermosets
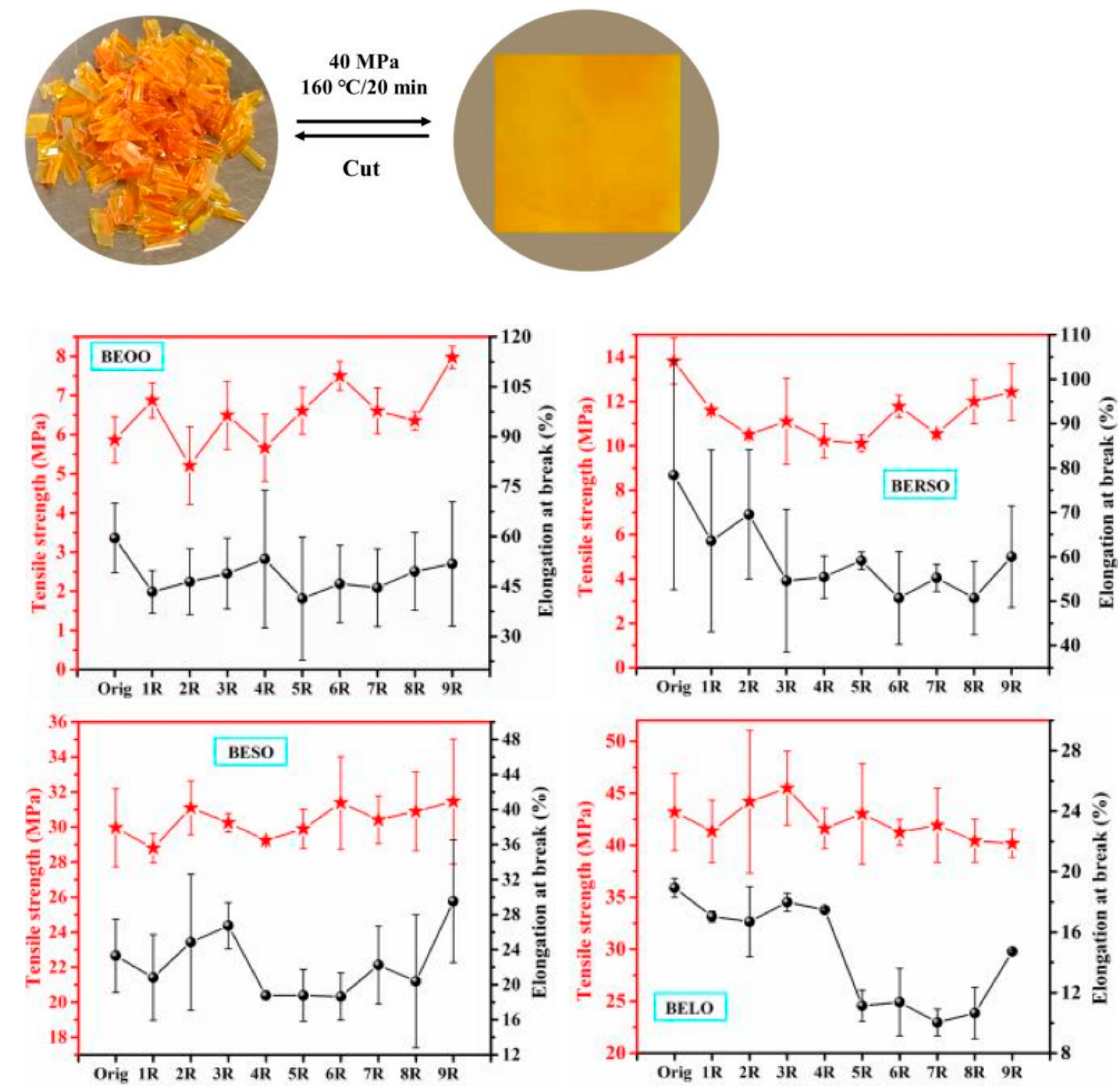
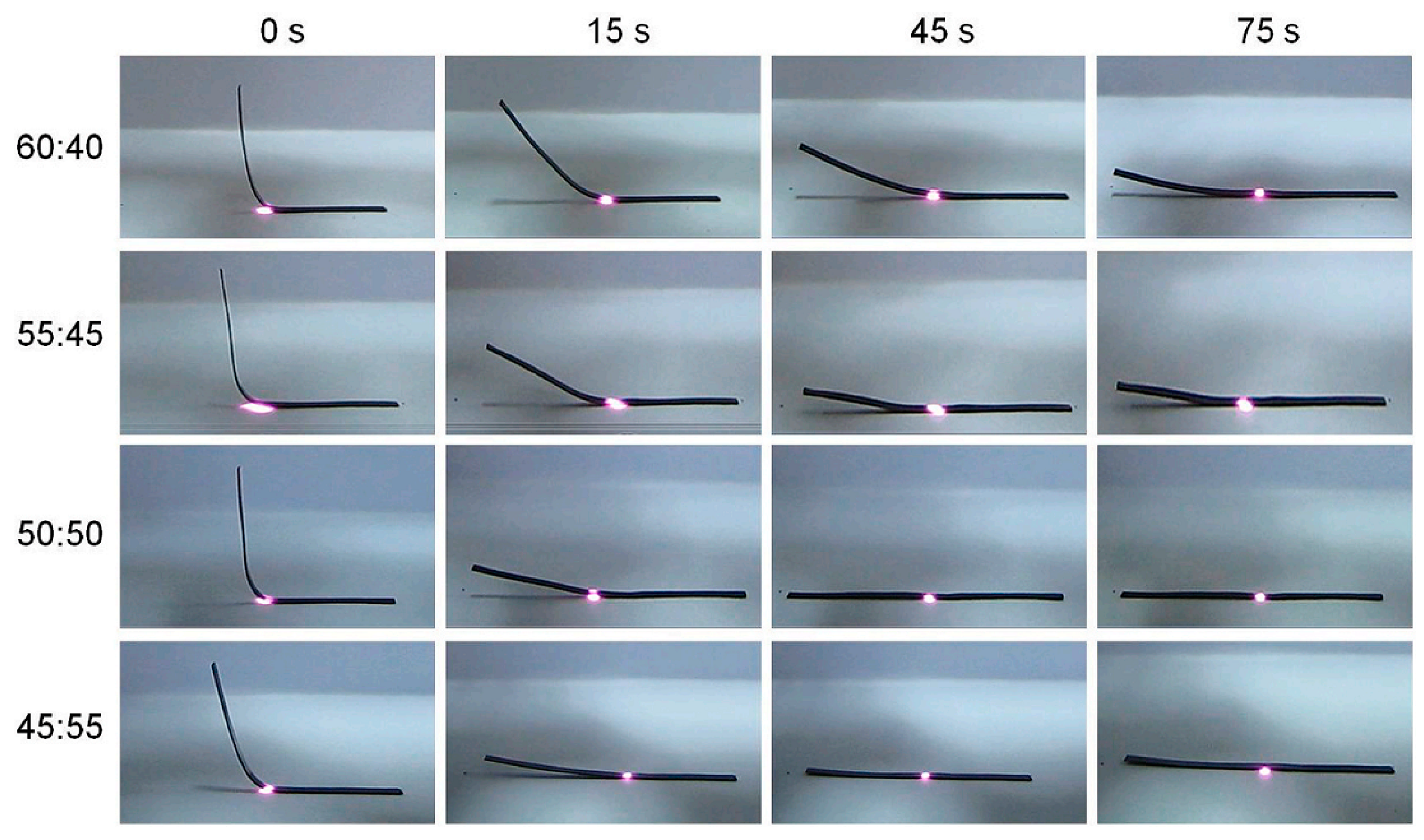
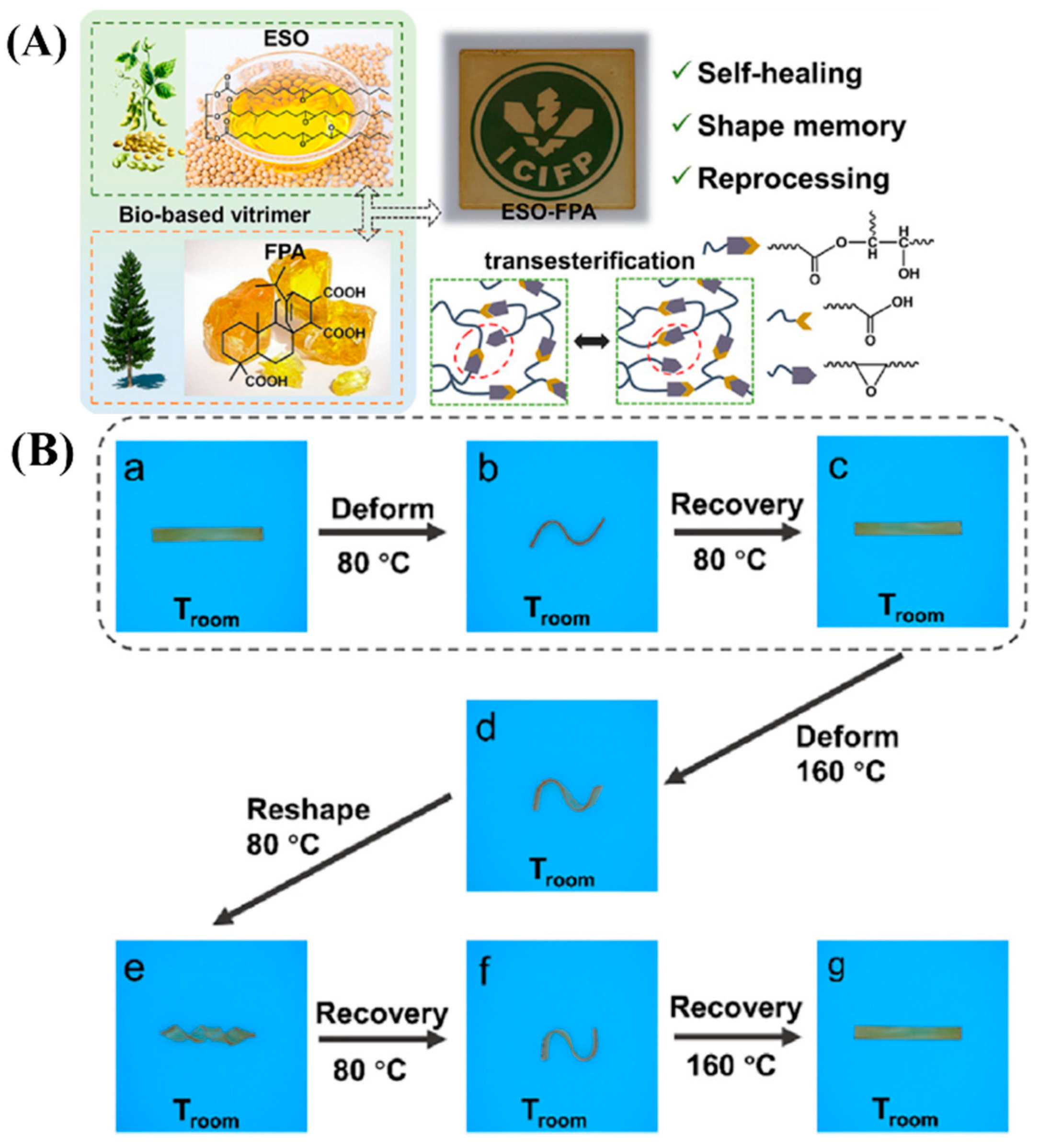
3.2. Recyclable/Reprocessable/Degradable Bio-Based Epoxy Thermosets
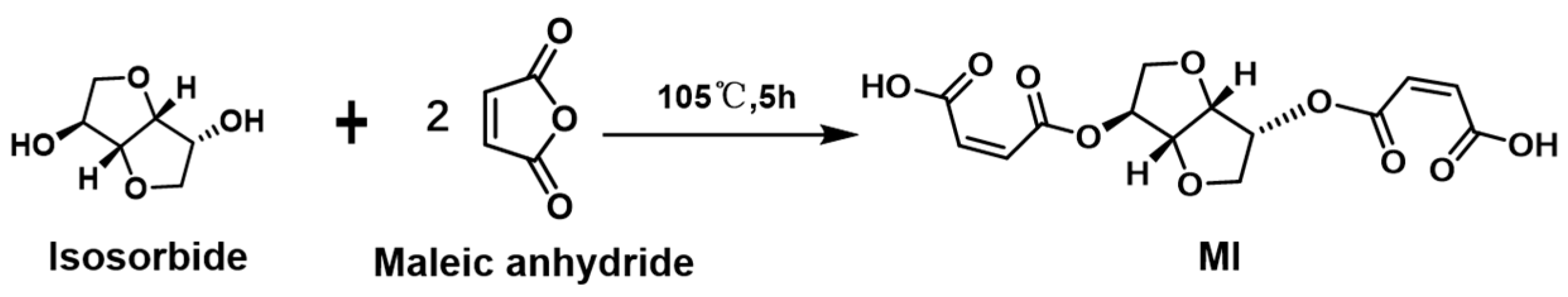
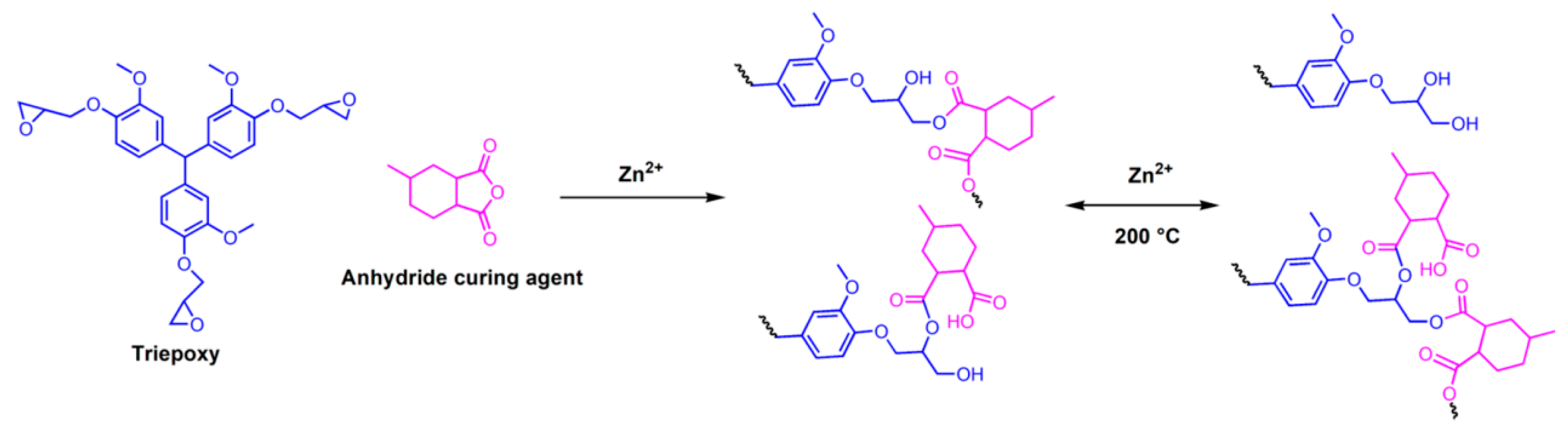
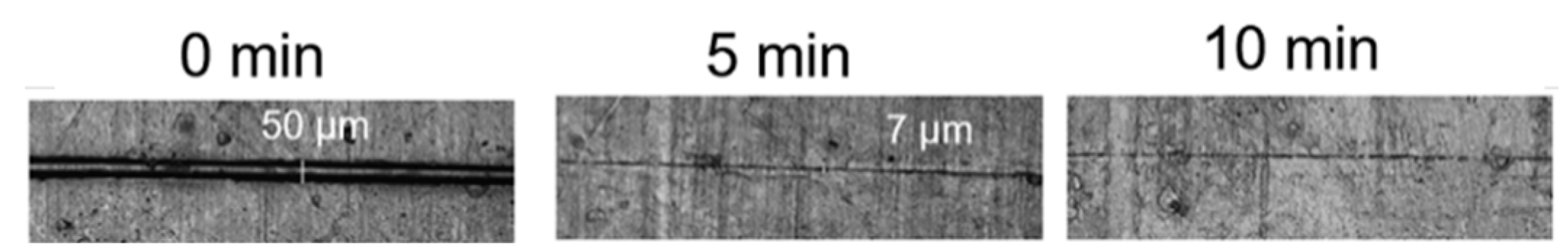
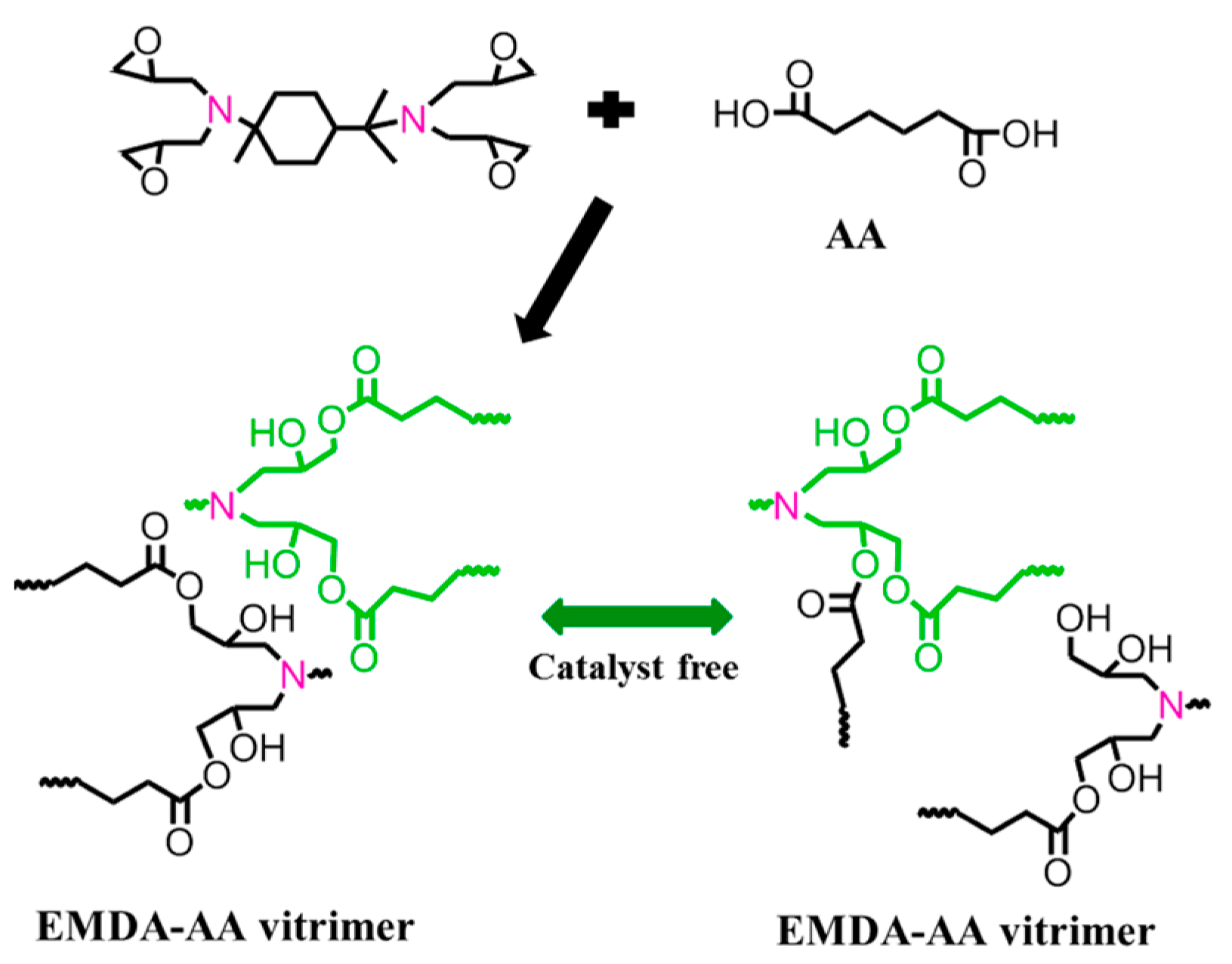
3.2.1. Ester Bonds and Similar Structures
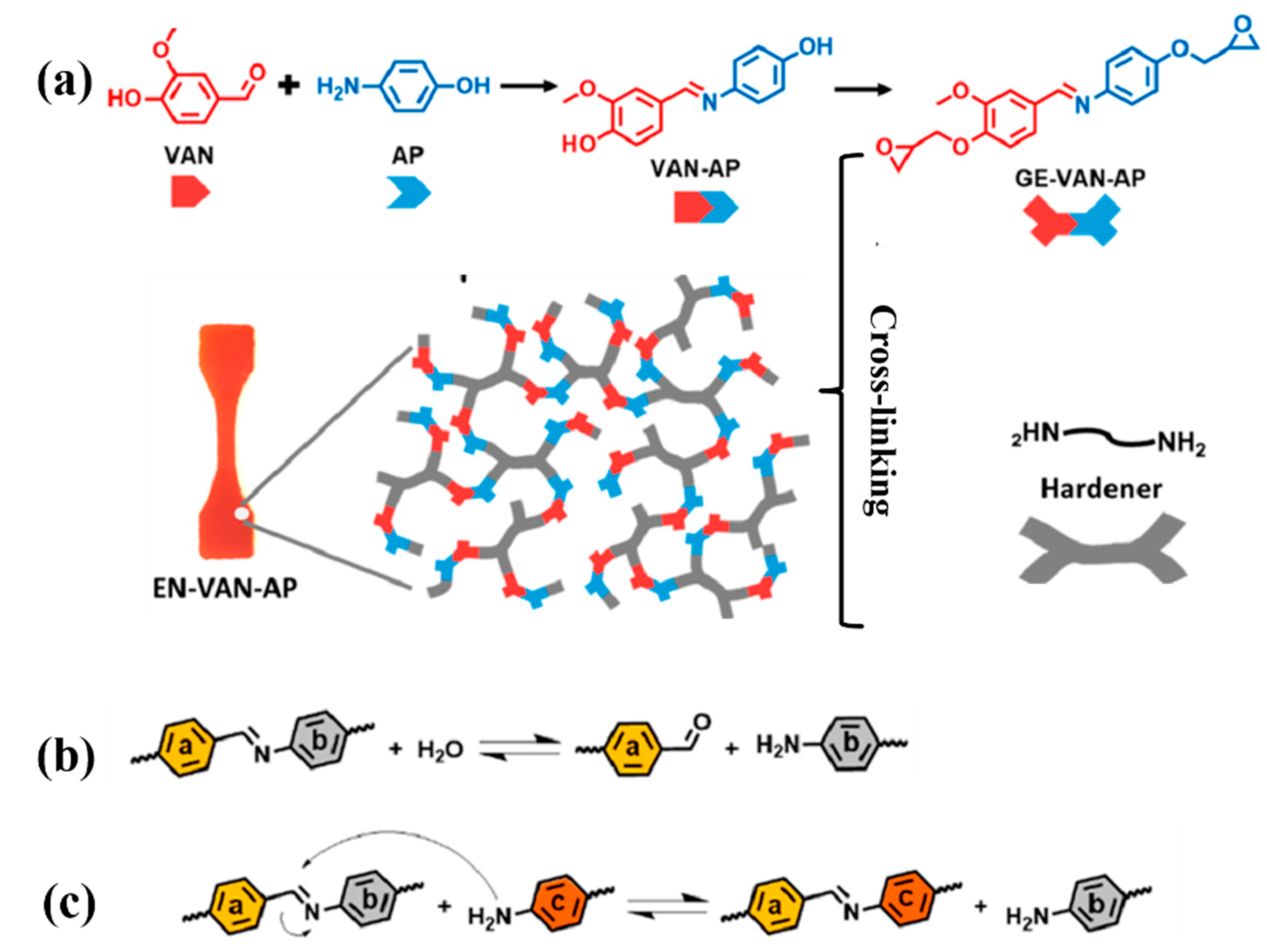
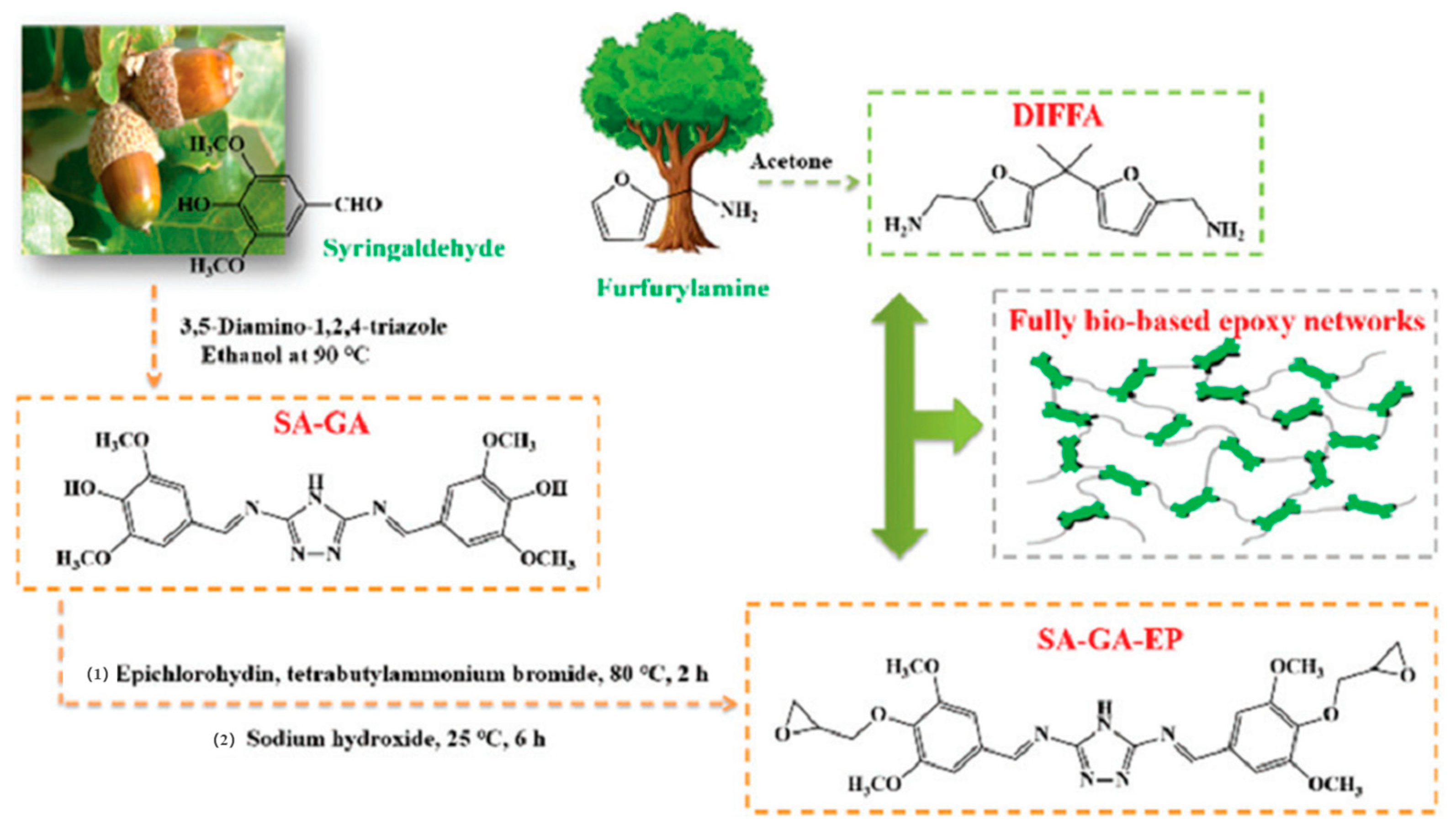
3.2.2. Schiff Bases
3.2.3. Acetal Structure
3.2.4. Diels–Alder Addition Structures
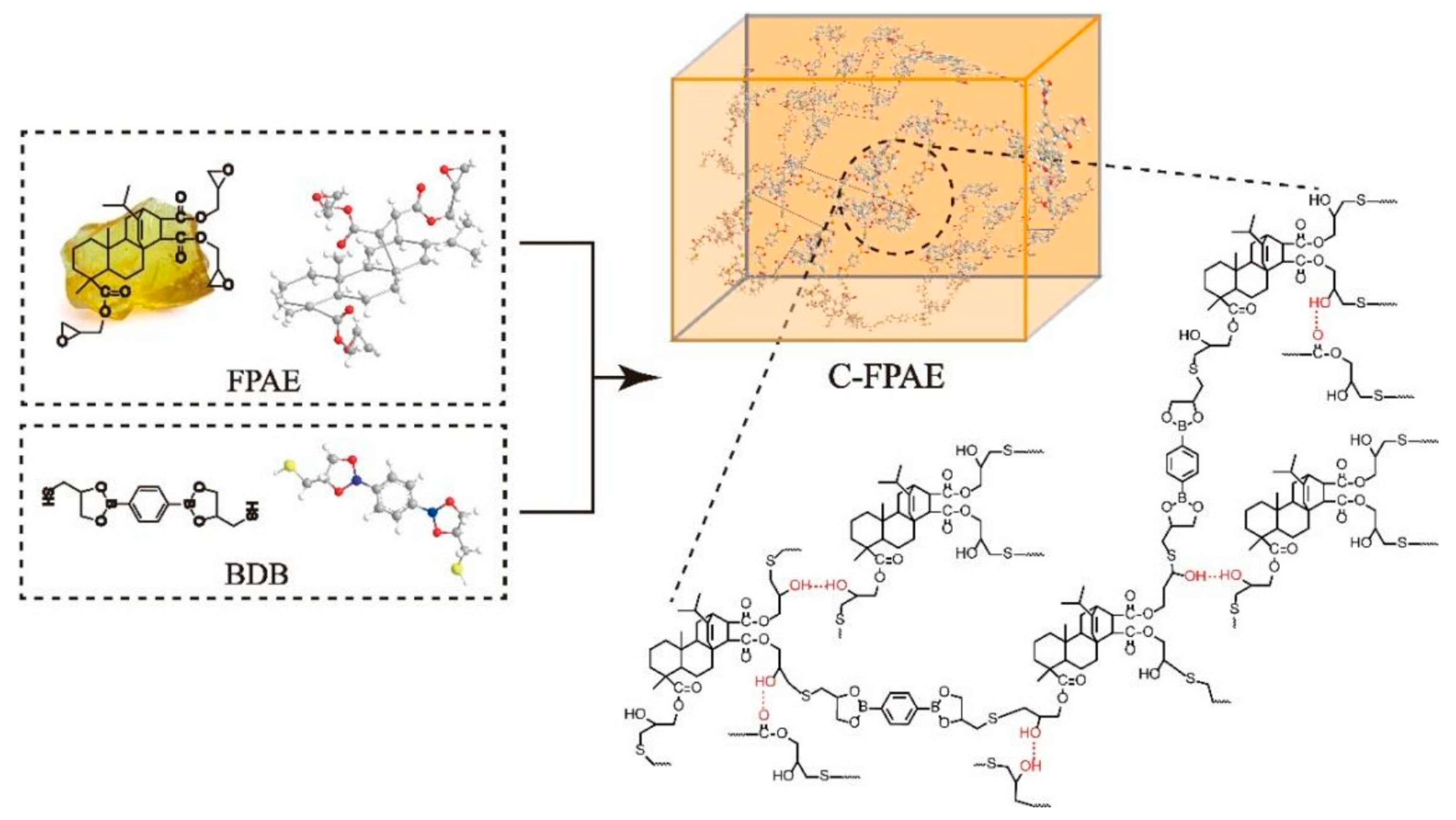
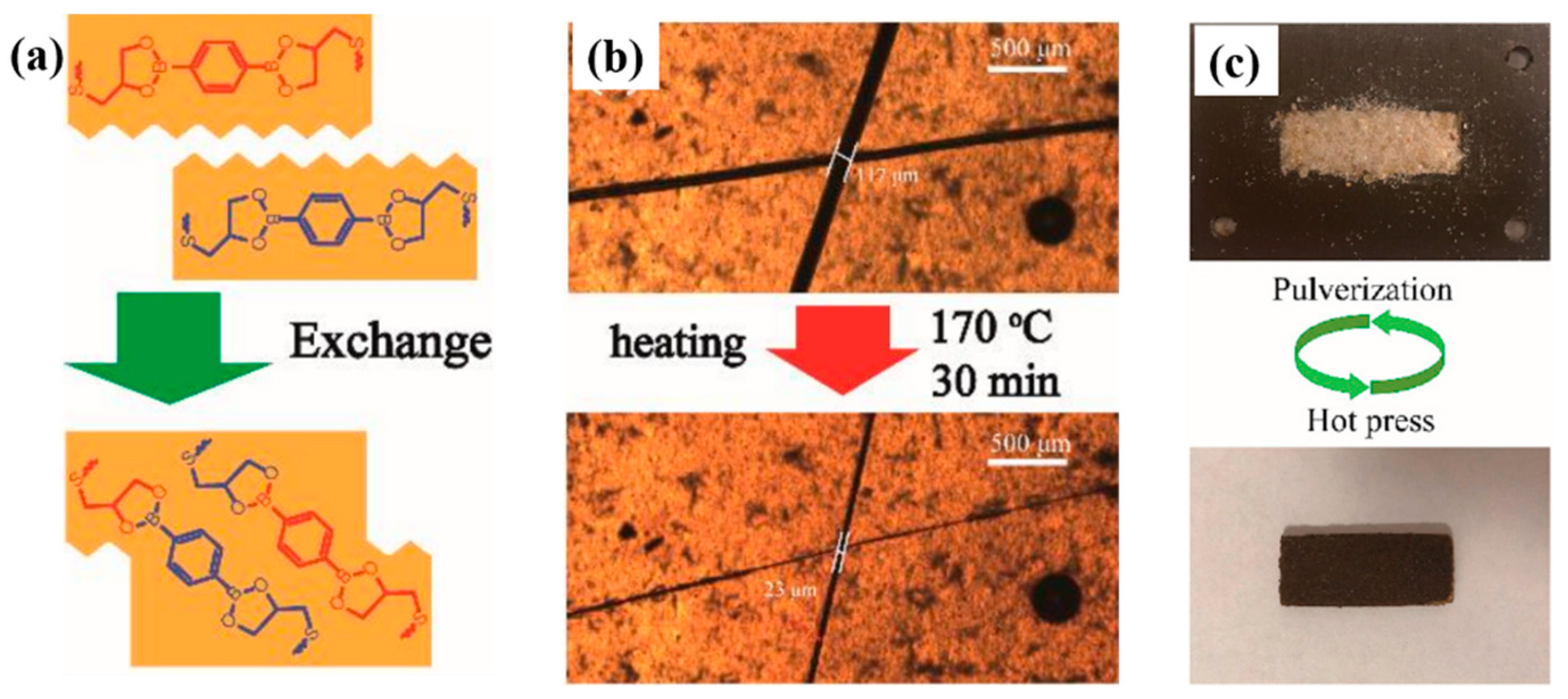
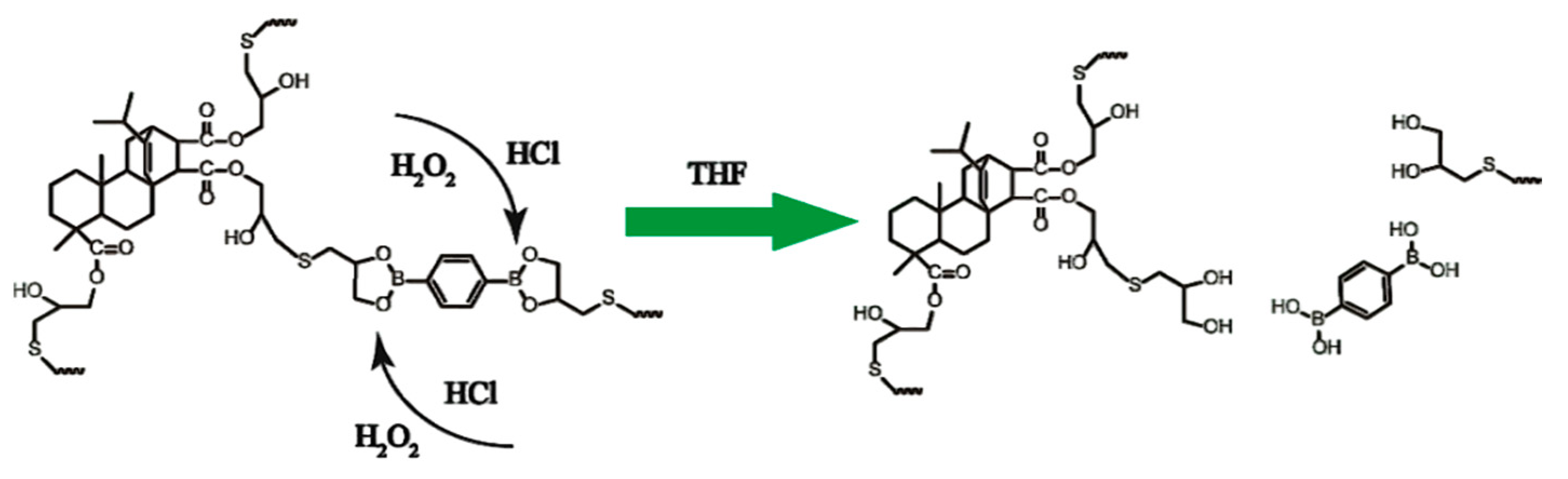
3.2.5. Disulfide Bonds
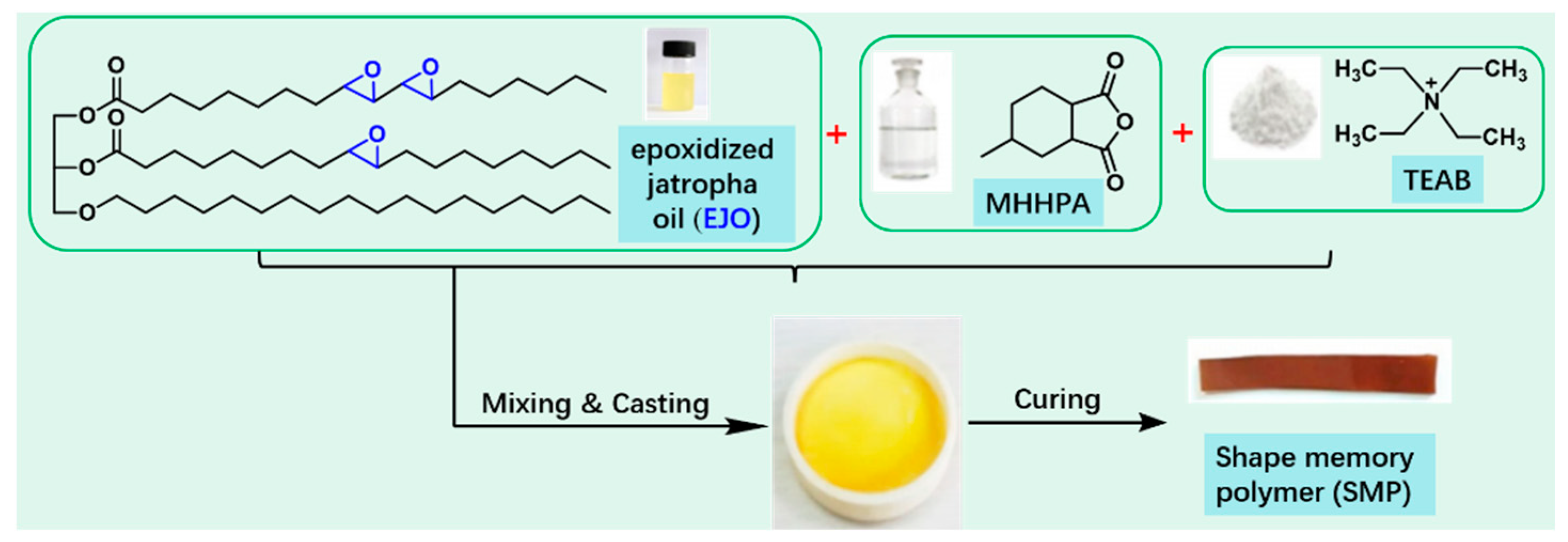
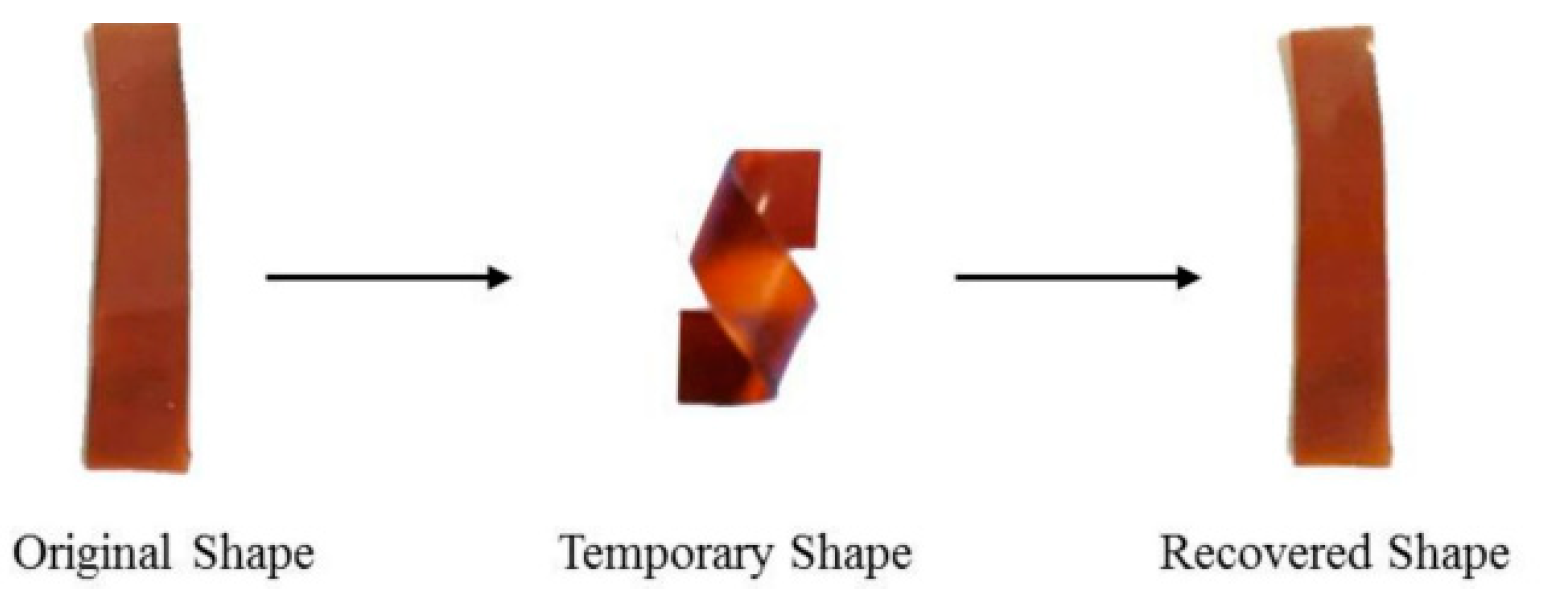
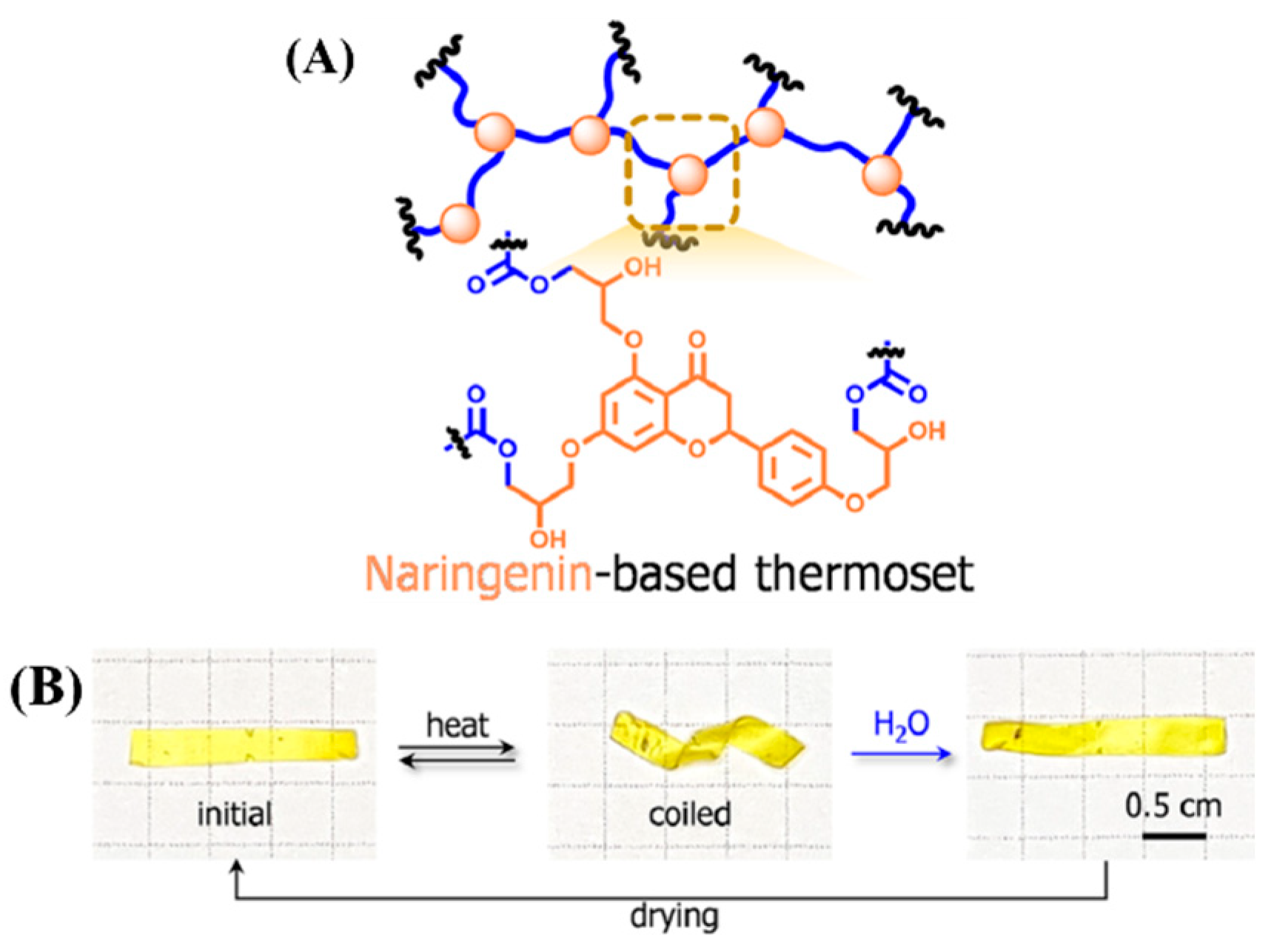
3.3. Shape Memory
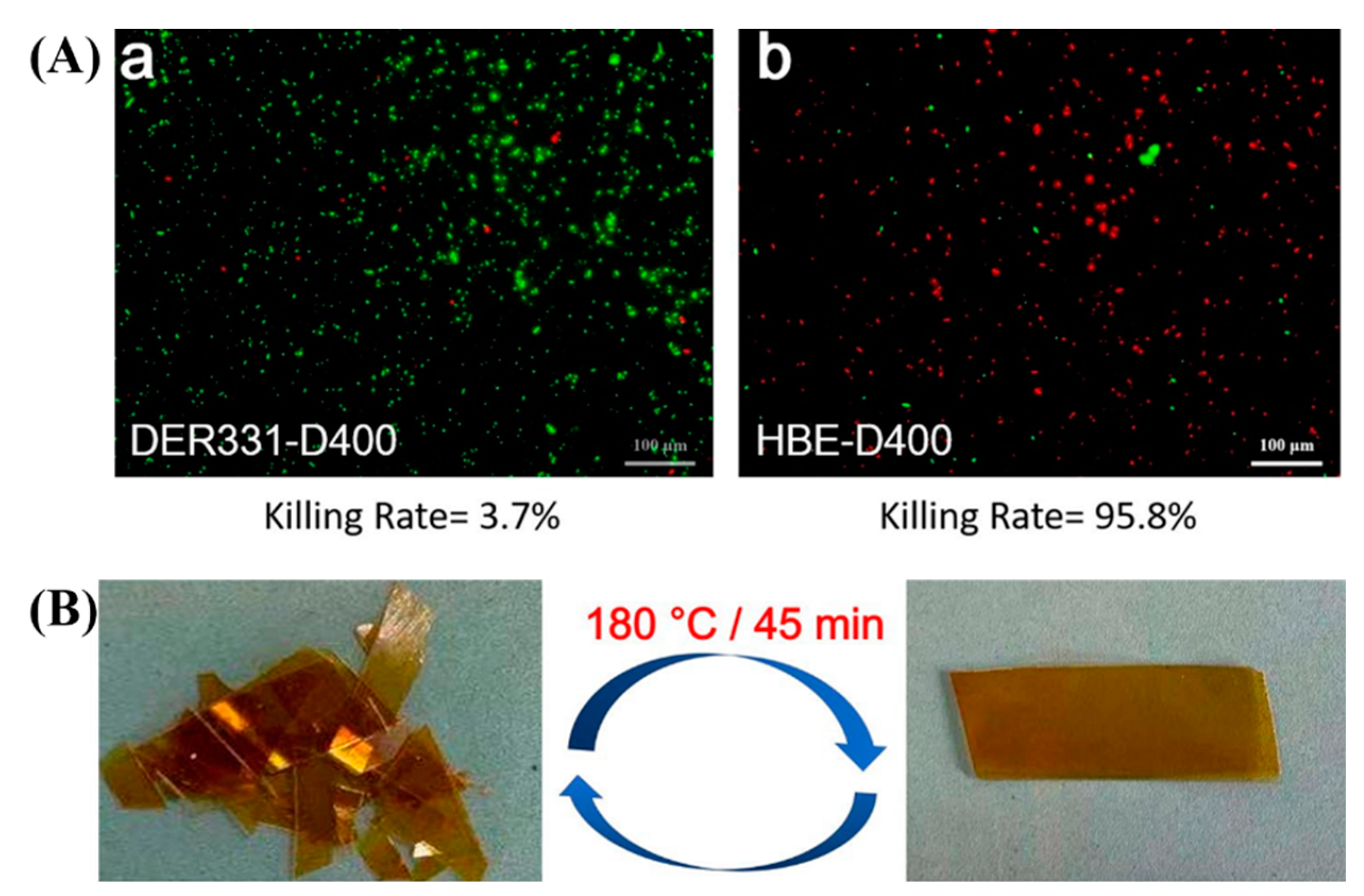
3.4. Antibacterial
4. Conclusions, Challenges, and Opportunities
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| Abbreviations | Full names or Interpretation of abbreviations |
| DGEBA | Diglycidyl ether bisphenol A |
| BPA | Bisphenol A |
| ECH | Epichlorohydrin |
| TEIA | A trifunctional epoxy resin from itaconic acid |
| Tg | Glass transition temperature |
| m-CPBA | m-chloroperbenzoic acid |
| DOPO | 9,10-dihydro-9-oxo-10-phosphaphenanthrene-10-oxide |
| HCCP | Hexachlorocyclotriphosphazene |
| DGEBDB | A bio-based epoxy monomer containing DOPO units from vanillin and guaiacol |
| DDM | 4,4-diaminodiphenyl methane |
| D230 | Poly(propylene glycol) bis(2-aminopropyl ether) (D230, Mn 230 g/mol) |
| E51 | commercial epoxy resin |
| PPDEG-EP | A novel epoxy monomer from eugenol and phenylphosphonic dichloride |
| Ea | Activation energy |
| EP1 and EP2 | Two epoxy monomers from vanillin, two diamines and diethyl phosphate. |
| DGEM | A fully bio-based epoxy resin precursor (DGEM) was synthesized from a naturally occurring magnolol through a highly efficient one step process |
| DDS | 4, 4′-diaminodiphenyl sulfone |
| MTEP | A bio-based tetra-functional epoxy resin from a sustainable biomass feedstock, magnolol |
| DGEA | An apigenin-based epoxy monomer |
| LOI | Limiting oxygen index |
| OmbFdE | A bis-furan diepoxide |
| TEGA | 2,2′-(ethane-1,2-diylbis(oxy)) bis(ethan-1-amine) |
| MI | A dicarboxylic acid oligomer from isosorbate and maleic anhydride |
| ESS | epoxidized sucrose soybean meal |
| TEP | A bio-based triepoxy from vanillin and guaiacol |
| FPAE | Rosin derivative |
| C-FPAE | A bio-based epoxy vitrimer containing dynamic reversible boronic ester bonds from FPAE |
| BDB | 2,2′-(1,4-phenylene)-bis(4-mercaptan-1,3,2-dioxaborolane) |
| EHCPP | A cardanol-derived epoxy monomer |
| DMA | Dynamic mechanical analysis |
| VAN-AP | An imine-embedded bisphenol intermediate |
| GE-VAN-AP | An epoxy monomer from vanillin and aminophenol used VAN-AP |
| Get | Glycerol triglycidyl ether |
| CF | Carbon fiber |
| DADE | An epoxy monomer containing aldehyde group from vanillin |
| DADE-D230 | a polymer network that cured DADE with D230 |
| HMDO | A bisphenol of HMDO containing acetal from natural resources of vanillin and glycerol |
| D-A reaction | Diels-Alder reaction |
| FRP | Fiber reinforced plastic |
| MDS-EPO | The epoxy resin containing dynamic disulfide bond |
| MDA-EPO | The epoxy resin cured by traditional curing agent |
| ESO | Epoxidized soybean oil |
| ELO | Epoxidized linseed oil |
| ERSO | Epoxidized rubber seed oil |
| EOO | Epoxidized olive oi |
| SMPs | Shape memory polymers |
| Car-DCP-MAH | A cardanol-derived curing agent |
| Car-DPC-MAH | A cardanol-derived curing agent |
| PCM | Phase change material |
| EETS | An epoxy monomer (EETS) from eugenol and 1,1,3,3-tetramethyldisiloxane |
| APDS | 4-aminophenyl disulfide |
| FPA | Fumarhippoic acid |
| MGOL-EP | A bio-based epoxy monomer from natural magnolol |
| MGOL-EP-SC | A fully bio-based and high-performance epoxy thermosetting resin |
| DIFFA | A furan-derived amine |
| SA-GA-EP/DIFFA | A fully bio-based epoxy thermosetting resin from syringaldehyde-derived Schiff base epoxy monomer that cured with DIFFA |
| GDE | The bio-based epoxy of glycerol diglycidyl ether |
| HVPA | A novel vanillin-derived curing agent containing Schiff base |
| HBE | Dihydrazone-containing epoxide monomers by condensation of vanillin and hydrazine hydrate |
References
- Liu, J.; Zhang, L.; Shun, W.; Dai, J.; Peng, Y.; Liu, X. Recent development on bio-based thermosetting resins. J. Polym. Sci. 2021, 59, 1474–1490. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Zhao, W.; Jiang, Y.; Liu, X. Research progress of bio-based thermal Resin. Thermosetting Resin 2020, 35, 61–70. [Google Scholar]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent development of biobased epoxy resins: A Review. Polym.-Plast. Technol. 2016, 57, 133–155. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of petroleum and biobased epoxy resins: A review. Polym. Int. 2018, 67, 815–839. [Google Scholar] [CrossRef]
- Mustapha, R.; Rahmat, A.R.; Abdul Majid, R.; Mustapha, S.N.H. Vegetable oil-based epoxy resins and their composites with bio-based hardener: A short review. Polym.-Plast. Technol. Mater. 2019, 58, 1311–1326. [Google Scholar] [CrossRef]
- Fei, X.; Jia, Y.; Yang, R.; Lu, G.; Ma, Y. Research progress of bio-based phenolic synthetic resins. Polym. Bull. 2020, 12, 9–17. [Google Scholar]
- Zhao, X.; Hou, G.; Yu, S. Progress on research of plant phenolic bio-based epoxy resin. Polym. Mat. Sci. Eng. 2022, 38, 167–175. [Google Scholar]
- Santacesaria, E.; Tesser, R.; Di Serio, M.; Casale, L.; Verde, D. New process for producing epichlorohydrin via glycerol chlorination. Ind. Eng. Chem. Res. 2010, 49, 964–970. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, J.; Yang, X.; Ke, Y.; Ou, R.; Wang, Y.; Madbouly, S.A.; Wang, Q. From plant phenols to novel bio-based polymers. Prog. Polym. Sci. 2022, 125, 101473. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Peng, Y.; Zhu, J.; Zhao, W.; Liu, X. Advances in sustainable thermosetting resins: From renewable feedstock to high performance and recyclability. Prog. Polym. Sci. 2021, 113, 101353. [Google Scholar] [CrossRef]
- Wan, J.; Zhao, J.; Zhang, X.; Fan, H.; Zhang, J.; Hu, D.; Jin, P.; Wang, D.-Y. Epoxy thermosets and materials derived from bio-based monomeric phenols: Transformations and performances. Prog. Polym. Sci. 2020, 108, 101287. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Gonçalves, F.A.M.M.; Santos, M.; Cernadas, T.; Ferreira, P.; Alves, P. Advances in the development of biobased epoxy resins: Insight into more sustainable materials and future applications. Int. Mater. Rev. 2021, 67, 119–149. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkita, T. Fully biobased epoxy resin systems composed of a vanillin-derived epoxy resin and renewable phenolic hardeners. Eur. Polym. J. 2017, 92, 165–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, M.; Ma, F.; Li, Y.; Lyu, B.; Liu, T.; Gao, Z.; Wang, L.; Vincent, D.; Kessler, M.R. Fully eugenol-based epoxy thermosets: Synthesis, curing, and properties. Macromol. Mater. Eng. 2021, 307, 2100833. [Google Scholar] [CrossRef]
- Brocas, A.-L.; Llevot, A.; Mantzaridis, C.; Cendejas, G.; Auvergne, R.; Caillol, S.; Carlotti, S.; Cramail, H. Epoxidized rosin acids as co-precursors for epoxy resins. Des. Monomers Polym. 2013, 17, 301–310. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Fan, L.; Jiang, Y.; Cao, L.; Tang, Z.; Zhu, J. Synthesis and properties of a bio-based epoxy resin with high epoxy value and low viscosity. ChemSusChem 2014, 7, 555–562. [Google Scholar] [CrossRef]
- Gonçalves, F.A.M.M.; Ferreira, P.; Alves, P. Synthesis and characterization of itaconic-based epoxy resin: Chemical and thermal properties of partially biobased epoxy resins. Polymer 2021, 235, 124285. [Google Scholar] [CrossRef]
- Janvier, M.; Hollande, L.; Jaufurally, A.S.; Pernes, M.; Menard, R.; Grimaldi, M.; Beaugrand, J.; Balaguer, P.; Ducrot, P.H.; Allais, F. Syringaresinol: A renewable and safer alternative to bisphenol A for epoxy-amine resins. ChemSusChem 2017, 10, 738–746. [Google Scholar] [CrossRef]
- Wei, J.; Duan, Y.; Wang, H.; Hui, J.; Qi, J. Bio-based trifunctional diphenolic acid epoxy resin with high Tg and low expansion coefficient: Synthesis and properties. Polym. Bull. 2022, 80, 10457–10471. [Google Scholar] [CrossRef]
- Savonnet, E.; Grau, E.; Grelier, S.; Defoort, B.; Cramail, H. Divanillin-based epoxy precursors as DGEBA substitutes for biobased epoxy thermosets. ACS Sustain. Chem. Eng. 2018, 6, 11008–11017. [Google Scholar] [CrossRef]
- Ye, J.; Ma, S.; Wang, B.; Chen, Q.; Huang, K.; Xu, X.; Li, Q.; Wang, S.; Lu, N.; Zhu, J. High-performance bio-based epoxies from ferulic acid and furfuryl alcohol: Synthesis and properties. Green Chem. 2021, 23, 1772–1781. [Google Scholar] [CrossRef]
- Garrison, M.D.; Savolainen, M.A.; Chafin, A.P.; Baca, J.E.; Bons, A.M.; Harvey, B.G. Synthesis and characterization of high-performance, bio-based epoxy-amine networks derived from resveratrol. ACS Sustain. Chem. Eng. 2020, 8, 14137–14149. [Google Scholar] [CrossRef]
- Bu, M.; Zhang, X.; Zhou, T.; Lei, C. Fully bio-based epoxy resins derived from magnolol and varying furan amines: Cure kinetics, superior mechanical and thermal properties. Eur. Polym. J. 2022, 180, 111595. [Google Scholar] [CrossRef]
- Mora, A.S.; Decostanzi, M.; David, G.; Caillol, S. Cardanol-based epoxy monomers for high thermal properties thermosets. Eur. J. Lipid Sci. Tech. 2019, 121, 1800421. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Fu, S. Bio-based epoxy resin from gallic acid and its thermosets toughened with renewable tannic acid derivatives. J. Mater. Sci. 2022, 57, 9493–9507. [Google Scholar] [CrossRef]
- Meng, J.; Zeng, Y.; Chen, P.; Zhang, J.; Yao, C.; Fang, Z.; Guo, K. New ultrastiff bio-furan epoxy networks with high Tg: Facile synthesis to excellent properties. Eur. Polym. J. 2019, 121, 109292. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Song, L.; Hu, Y. Intrinsically flame retardant bio-based epoxy thermosets: A review. Compos. Part B-Eng. 2019, 179, 107487. [Google Scholar] [CrossRef]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, X.; Fan, R.; Yue, S.; Zheng, L.; Liu, Q.; He, Y. A comprehensive review of reactive flame-retardant epoxy resin: Fundamentals, recent developments, and perspectives. Polym. Degrad. Stab. 2022, 201, 109976. [Google Scholar] [CrossRef]
- Niu, H.; Wang, X.; Song, L.; Hu, Y. Progress on intrinsically flame-retardant bio-based epoxy thermosets. Acta Polym. Sinica. 2022, 53, 894–905. [Google Scholar]
- Rashid, M.A.; Liu, W.; Wei, Y.; Jiang, Q. Review of intrinsically recyclable biobased epoxy thermosets enabled by dynamic chemical bonds. Polym.-Plast. Tech. Mat. 2022, 61, 1740–1782. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Wang, B.; Li, P.; Zhu, J.; Ma, S. Closed-loop chemical recycling of thermosetting polymers and their applications: A review. Green Chem. 2022, 24, 5691–5708. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Li, K.; Lin, H.; Wang, M.; Zheng, L.; Wu, C.; Zhang, X. Recycling of epoxy resins with degradable structures or dynamic cross-linking networks: A review. Ind. Eng. Chem. Res. 2024, 63, 5005–5027. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, J.; Wang, S.; Dai, J.; Liu, X. Bio-based thermosetting resins: From molecular engineering to intrinsically multifunctional customization. Adv. Mater. 2024, 2311242. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Xie, R.; Yuan, Y.; Sun, P.; Liu, Z.; Ma, J.; Yang, G.; Wang, K.; Li, M.; Shang, L.; Ao, Y. Design of epoxy resin with sustainability, high adhesion and excellent flame retardancy based on bio-based molecules. J. Mater. Sci. 2022, 57, 13078–13096. [Google Scholar] [CrossRef]
- Liu, J.; Dai, J.; Wang, S.; Peng, Y.; Cao, L.; Liu, X. Facile synthesis of bio-based reactive flame retardant from vanillin and guaiacol for epoxy resin. Compos. Part B-Eng. 2020, 190, 107926. [Google Scholar] [CrossRef]
- Zhou, J.; Heng, Z.; Zhang, H.; Chen, Y.; Zou, H.; Liang, M. High residue bio-based structural-functional integration epoxy and intrinsic flame retardant mechanism study. RSC Adv. 2019, 9, 41603–41615. [Google Scholar] [CrossRef]
- Chi, Z.; Guo, Z.; Xu, Z.; Zhang, M.; Li, M.; Shang, L.; Ao, Y. A DOPO-based phosphorus-nitrogen flame retardant bio-based epoxy resin from diphenolic acid: Synthesis, flame-retardant behavior and mechanism. Polym. Degrad. Stab. 2020, 176, 109151. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Liu, T.; Ou, R.; Lin, J.; Puglia, D.; Xu, P.; Wang, Q.; Dong, W.; Du, M.; et al. Design of intrinsically flame-retardant vanillin-based epoxy resin for thermal-conductive epoxy/graphene aerogel composites. ACS Appl. Mater. Interfaces 2021, 13, 59341–59351. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Zhou, W.; Liu, T.; Xu, P.; Puglia, D.; Kenny, J.M.; Ma, P. Design of inherent fire retarding and degradable bio-based epoxy vitrimer with excellent self-healing and mechanical reprocessability. Compos. Sci. Technol. 2022, 230, 109776. [Google Scholar] [CrossRef]
- Liu, J.; He, Z.; Wu, G.; Zhang, X.; Zhao, C.; Lei, C. Synthesis of a novel nonflammable eugenol-based phosphazene epoxy resin with unique burned intumescent char. Chem. Eng. J. 2020, 390, 124620. [Google Scholar] [CrossRef]
- Huang, J.; Guo, W.; Wang, X.; Song, L.; Hu, Y. Intrinsically flame retardant cardanol-based epoxy monomer for high-performance thermosets. Polym. Degrad. Stab. 2021, 186, 109519. [Google Scholar] [CrossRef]
- Zhou, W.; Lv, D.; Ding, H.; Xu, P.; Zhang, C.; Ren, Y.; Yang, W.; Ma, P. Synthesis of eugenol-based phosphorus-containing epoxy for enhancing the flame-retardancy and mechanical performance of DGEBA epoxy resin. React. Funct. Polym. 2022, 180, 105383. [Google Scholar] [CrossRef]
- Ma, C.; Qian, L.; Li, J. Effect of functional groups of magnolol-based cyclic phosphonate on structure and properties of flame retardant epoxy resin. Polym. Degrad. Stab. 2021, 190, 109630. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, Y.; Chen, Y.; Li, L.; Guo, C. Synthesis of eugenol-modified epoxy resin and application on wood flame retardant coating. Ind. Crop. Prod. 2022, 183, 114979. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; He, F.; Ying, J.; Li, S.N.; Peng, L.; Wu, Q.; Fan, Z.; Jiang, B. Facile synthesis of intrinsically flame-retardant epoxy thermosets with high mechanical properties from lignin derivatives. J. Appl. Polym. Sci. 2023, 140, 53636. [Google Scholar] [CrossRef]
- Xie, W.; Tang, D.; Liu, S.; Zhao, J. Facile synthesis of bio-based phosphorus-containing epoxy resins with excellent flame resistance. Polym. Test. 2020, 86, 106466. [Google Scholar] [CrossRef]
- Miao, J.T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Biobased epoxy resin derived from eugenol with excellent integrated performance and high renewable carbon content. Polym. Int. 2018, 67, 1194–1202. [Google Scholar] [CrossRef]
- Faye, I.; Decostanzi, M.; Ecochard, Y.; Caillol, S. Eugenol bio-based epoxy thermosets: From cloves to applied materials. Green Chem. 2017, 19, 5236–5242. [Google Scholar] [CrossRef]
- Park, H.-W.; Toan, M.; Kim, H.-J.; Lee, J.-H.; Shin, S. Renewable epoxy thermosets with extremely high biomass content from furan derivatives and their flame retardancy. J. Ind. Eng. Chem. 2020, 92, 184–190. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J.; et al. Vanillin-derived high-performance flame retardant epoxy resins: Facile synthesis and properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Kumar, B.; Roy, S.; Agumba, D.O.; Pham, D.H.; Kim, J. Effect of bio-based derived epoxy resin on interfacial adhesion of cellulose film and applicability towards natural jute fiber-reinforced composites. Int. J. Biol. Macromol. 2022, 222 Pt A, 1304–1313. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Huang, Y.; Hu, M.; Li, L. Facile preparation of magnolol-based epoxy resin with intrinsic flame retardancy, high rigidity and hydrophobicity. Ind. Crop. Prod. 2023, 192, 116124. [Google Scholar] [CrossRef]
- Wang, X.; Niu, H.; Guo, W.; Song, L.; Hu, Y. Cardanol as a versatile platform for fabrication of bio-based flame-retardant epoxy thermosets as DGEBA substitutes. Chem. Eng. J. 2021, 421, 129738. [Google Scholar] [CrossRef]
- Qi, Y.; Weng, Z.; Zhang, K.; Wang, J.; Zhang, S.; Liu, C.; Jian, X. Magnolol-based bio-epoxy resin with acceptable glass transition temperature, processability and flame retardancy. Chem. Eng. J. 2020, 387, 124115. [Google Scholar] [CrossRef]
- Qi, Y.; Weng, Z.; Kou, Y.; Li, J.; Cao, Q.; Wang, J.; Zhang, S.; Jian, X. Facile synthesis of bio-based tetra-functional epoxy resin and its potential application as high-performance composite resin matrix. Compos. Part B-Eng. 2021, 214, 108749. [Google Scholar] [CrossRef]
- Lu, C.; Bian, S.; Hu, K.; Li, C.; Zheng, K.; Sun, Q. Biomass-based epoxy resin derived from resveratrol with high temperature resistance and intrinsic flame retardant properties. Ind. Crop. Prod. 2022, 187, 115500. [Google Scholar] [CrossRef]
- Song, X.; Deng, Z.-P.; You, C.-W.; Sun, R.-Y.; Song, F.; Wang, X.-L.; Chen, L.; Wang, Y.-Z. High-performance and fire-resistant epoxy thermosets derived from plant-derived ferulic acid. Ind. Crop. Prod. 2022, 187, 115445. [Google Scholar] [CrossRef]
- Song, X.; Deng, Z.-P.; Li, C.-B.; Song, F.; Wang, X.-L.; Chen, L.; Guo, D.-M.; Wang, Y.-Z. A bio-based epoxy resin derived from p-hydroxycinnamic acid with high mechanical properties and flame retardancy. Chin. Chem. Lett. 2022, 33, 4912–4917. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Wang, P.; Liu, Y.; Wan, M.; Zhang, K. Phosphorus-free curcumin-derived epoxy resin with exceptional flame retardancy, glass transition temperature and mechanical properties. Polym. Degrad. Stab. 2023, 215, 110440. [Google Scholar] [CrossRef]
- Li, J.; Weng, Z.; Cao, Q.; Qi, Y.; Lu, B.; Zhang, S.; Wang, J.; Jian, X. Synthesis of an aromatic amine derived from biomass and its use as a feedstock for versatile epoxy thermoset. Chem. Eng. J. 2022, 433, 134512. [Google Scholar] [CrossRef]
- Niu, H.-X.; Yang, T.-M.; Wang, X.; Zhang, P.; Guo, W.; Song, L.; Hu, Y. High biomass content, anti-flammable and degradable epoxy thermosets by curing a tyramine-derived epoxy monomer with a furan-derived diamine for non-destructively recyclable carbon fiber composite application. Green Chem. 2024, 26, 5519–5530. [Google Scholar] [CrossRef]
- Xie, W.; Huang, S.; Tang, D.; Liu, S.; Zhao, J. Biomass-derived Schiff base compound enabled fire-safe epoxy thermoset with excellent mechanical properties and high glass transition temperature. Chem. Eng. J. 2020, 394, 123667. [Google Scholar] [CrossRef]
- Dai, J.; Peng, Y.; Teng, N.; Liu, Y.; Liu, C.; Shen, X.; Mahmud, S.; Zhu, J.; Liu, X. High-performing and fire-resistant biobased epoxy resin from renewable sources. ACS Sustain. Chem. Eng. 2018, 6, 7589–7599. [Google Scholar] [CrossRef]
- Gao, T.-Y.; Wang, F.-D.; Xu, Y.; Wei, C.-X.; Zhu, S.-E.; Yang, W.; Lu, H.-D. Luteolin-based epoxy resin with exceptional heat resistance, mechanical and flame retardant properties. Chem. Eng. J. 2022, 428, 131173. [Google Scholar] [CrossRef]
- Dai, J.; Teng, N.; Liu, J.; Feng, J.; Zhu, J.; Liu, X. Synthesis of bio-based fire-resistant epoxy without addition of flame retardant elements. Compos. Part B-Eng. 2019, 179, 107523. [Google Scholar] [CrossRef]
- Nabipour, H.; Qiu, S.; Wang, X.; Song, L.; Hu, Y. Phosphorus-free ellagic acid-derived epoxy thermosets with intrinsic antiflammability and high glass transition temperature. ACS Sustain. Chem. Eng. 2021, 9, 10799–10808. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Synthesis of a bio-based and intrinsically anti-flammable epoxy thermoset and the application of its carbonized foam as an efficient CO2 capture adsorbent. Mater. Today Sustain. 2023, 21, 100265. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, J.; Kou, Y.; Pang, H.; Zhang, S.; Li, N.; Liu, C.; Weng, Z.; Jian, X. Synthesis of an aromatic N-heterocycle derived from biomass and its use as a polymer feedstock. Nat. Commun. 2019, 10, 2107. [Google Scholar] [CrossRef]
- Qi, Y.; Weng, Z.; Kou, Y.; Song, L.; Li, J.; Wang, J.; Zhang, S.; Liu, C.; Jian, X. Synthesize and introduce bio-based aromatic s-triazine in epoxy resin: Enabling extremely high thermal stability, mechanical properties, and flame retardancy to achieve high-performance sustainable polymers. Chem. Eng. J. 2021, 406, 126881. [Google Scholar] [CrossRef]
- Niu, H.; Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Phosphorus-free vanillin-derived intrinsically flame-retardant epoxy thermoset with extremely low heat release rate and smoke emission. ACS Sustain. Chem. Eng. 2021, 9, 5268–5277. [Google Scholar] [CrossRef]
- Liu, S.H.; Zhang, X.Q.; Liu, J.H.; Lei, C.H.; Dong, Z.X. A novel bio-based epoxy resin from oligomer: Excellent processability, high heat resistance, and intrinsic flame retardancy. Express Polym. Lett. 2021, 15, 1189–1205. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Bu, M.; Lei, C. Properties tailoring of biobased epoxy resins by regulating the degree of polymerization of oligomers. Eur. Polym. J. 2022, 173, 111253. [Google Scholar] [CrossRef]
- Meng, J.; Chen, P.; Yang, R.; Dai, L.; Yao, C.; Fang, Z.; Guo, K. Thermal stable honokiol-derived epoxy resin with reinforced thermal conductivity, dielectric properties and flame resistance. Chem. Eng. J. 2021, 412, 128647. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Q.; Shen, L.; Cui, Z.; Kou, L.; Cheng, J.; Zhang, J. A renewable resveratrol-based epoxy resin with high Tg, excellent mechanical properties and low flammability. Chem. Eng. J. 2020, 383, 123124. [Google Scholar] [CrossRef]
- Tian, Y.; Ke, M.; Wang, X.; Wu, G.; Zhang, J.; Cheng, J. A resveratrol-based epoxy resin with ultrahigh Tg and good processability. Eur. Polym. J. 2021, 147, 110282. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Cai, J.; Xie, J. Plant-derived p-hydroxyphenylacrylic acid-derived epoxy resins exhibit excellent flame retardancy, hydrophobicity, degradability, and low dielectric loss after curing with bio-based fluorinated Schiff bases. Polym. Degrad. Stab. 2023, 209, 110270. [Google Scholar] [CrossRef]
- Wan, J.; Gan, B.; Li, C.; Molina-Aldareguia, J.; Kalali, E.N.; Wang, X.; Wang, D.-Y. A sustainable, eugenol-derived epoxy resin with high biobased content, modulus, hardness and low flammability: Synthesis, curing kinetics and structure–property relationship. Chem. Eng. J. 2016, 284, 1080–1093. [Google Scholar] [CrossRef]
- Wan, J.; Gan, B.; Li, C.; Molina-Aldareguia, J.; Li, Z.; Wang, X.; Wang, D.-Y. A novel biobased epoxy resin with high mechanical stiffness and low flammability: Synthesis, characterization and properties. J. Mater. Chem. A 2015, 3, 21907–21921. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, S.; Wan, J.; Wang, J.; Li, Y.; Jin, P.; Hu, D. A dieugenol-based epoxy monomer with high bio-based content, low viscosity and low flammability. Mater. Today Commun. 2021, 29, 102846. [Google Scholar] [CrossRef]
- Wang, X.; Nabipour, H.; Kan, Y.-C.; Song, L.; Hu, Y. A fully bio-based, anti-flammable and non-toxic epoxy thermosetting network for flame-retardant coating applications. Prog. Org. Coat. 2022, 172, 107095. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Intrinsically anti-flammable apigenin-derived epoxy thermosets with high glass transition temperature and mechanical strength. Chin. J. Polym. Sci. 2022, 40, 1259–1268. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, G.; Shi, G.; Wang, Y.; Li, W.; Ren, S. Effect of crosslink structure on mechanical properties, thermal stability and flame retardancy of natural flavonoid based epoxy resins. Eur. Polym. J. 2022, 162, 110898. [Google Scholar] [CrossRef]
- Meng, J.; Zeng, Y.; Zhu, G.; Zhang, J.; Chen, P.; Cheng, Y.; Fang, Z.; Guo, K. Sustainable bio-based furan epoxy resin with flame retardancy. Polym. Chem. 2019, 10, 2370–2375. [Google Scholar] [CrossRef]
- Miao, J.-T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Biobased heat resistant epoxy resin with extremely high biomass content from 2,5-furandicarboxylic acid and eugenol. ACS Sustain. Chem. Eng. 2017, 5, 7003–7011. [Google Scholar] [CrossRef]
- Meng, J.; Zeng, Y.; Chen, P.; Zhang, J.; Yao, C.; Fang, Z.; Ouyang, P.; Guo, K. Flame retardancy and mechanical properties of bio-based furan epoxy resins with high crosslink density. Macromol. Mater. Eng. 2019, 305, 1900587. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. A furan-derived epoxy thermoset with inherent anti-flammability, degradability, and raw material recycling. Mater. Today Chem. 2023, 27, 101315. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Kandola, B.; Song, L.; Kan, Y.; Chen, J.; Hu, Y. A bio-based intrinsically flame-retardant epoxy vitrimer from furan derivatives and its application in recyclable carbon fiber composites. Polym. Degrad. Stab. 2023, 207, 110206. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Naturally occurring acids as cross-linkers to yield VOC-free, high-performance, fully bio-based, degradable thermosets. Macromolecules 2015, 48, 7127–7137. [Google Scholar] [CrossRef]
- Liu, T.; Guo, X.; Liu, W.; Hao, C.; Wang, L.; Hiscox, W.C.; Liu, C.; Jin, C.; Xin, J.; Zhang, J. Selective cleavage of ester linkages of anhydride-cured epoxy using a benign method and reuse of the decomposed polymer in new epoxy preparation. Green Chem. 2017, 19, 4364–4372. [Google Scholar] [CrossRef]
- Zhong, L.; Hao, Y.; Zhang, J.; Wei, F.; Li, T.; Miao, M.; Zhang, D. Closed-loop recyclable fully bio-based epoxy vitrimers from ferulic acid-derived hyperbranched epoxy resin. Macromolecules 2022, 55, 595–607. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, K.; Xu, X.; Wang, B.; Zhang, W.; Su, Y.; Hu, K.; Zhang, C.; Zhu, J.; Weng, G.; et al. Rigid-and-flexible, degradable, fully biobased thermosets from lignin and soybean oil: Synthesis and properties. ACS Sustain. Chem. Eng. 2023, 11, 3466–3473. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C.; Jabeen, F. Hard and Flexible, Degradable thermosets from renewable bioresources with the assistance of water and ethanol. Macromolecules 2016, 49, 3780–3788. [Google Scholar] [CrossRef]
- Yan, X.; Liu, T.; Hao, C.; Shao, L.; Chang, Y.-C.; Cai, Z.; Shang, S.; Song, Z.; Zhang, J. Rosin derived catalyst-free vitrimer with hydrothermal recyclability and application in high performance fiber composite. Ind. Crop. Prod. 2023, 202, 116976. [Google Scholar] [CrossRef]
- Di Mauro, C.; Genua, A.; Mija, A. Fully bio-based reprocessable thermosetting resins based on epoxidized vegetable oils cured with itaconic acid. Ind. Crop. Prod. 2022, 185, 115116. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Wang, L.; Li, Y.; Liu, W.; Xin, J.; Zhang, J. Eugenol-derived biobased epoxy: Shape memory, repairing, and recyclability. Macromolecules 2017, 50, 8588–8597. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Zhang, S.; Yang, X.; Wang, L.; Han, J.; Li, Y.; Xin, J.; Zhang, J. A self-healable high glass transition temperature bioepoxy material based on vitrimer chemistry. Macromolecules 2018, 51, 5577–5585. [Google Scholar] [CrossRef]
- Feng, X.; Fan, J.; Li, A.; Li, G. Biobased tannic acid cross-linked epoxy thermosets with hierarchical molecular structure and tunable properties: Damping, shape memory, and recyclability. ACS Sustain. Chem. Eng. 2019, 8, 874–883. [Google Scholar] [CrossRef]
- Cao, L.; Fan, J.; Huang, J.; Chen, Y. A robust and stretchable cross-linked rubber network with recyclable and self-healable capabilities based on dynamic covalent bonds. J. Mater. Chem. A 2019, 7, 4922–4933. [Google Scholar] [CrossRef]
- Kadam, A.; Pawar, M.; Yemul, O.; Thamke, V.; Kodam, K. Biodegradable biobased epoxy resin from karanja oil. Polymer 2015, 72, 82–92. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, S.; Hao, C.; Verdi, C.; Liu, W.; Liu, H.; Zhang, J. Glycerol induced catalyst-free curing of epoxy and vitrimer preparation. Macromol. Rapid Commun. 2019, 40, 1800889. [Google Scholar] [CrossRef]
- Xu, Y.-z.; Fu, P.; Dai, S.-l.; Zhang, H.-b.; Bi, L.-w.; Jiang, J.-x.; Chen, Y.-x. Catalyst-free self-healing fully bio-based vitrimers derived from tung oil: Strong mechanical properties, shape memory, and recyclability. Ind. Crop. Prod. 2021, 171, 113678. [Google Scholar] [CrossRef]
- Wu, J.; Yu, X.; Zhang, H.; Guo, J.; Hu, J.; Li, M.-H. Fully biobased vitrimers from glycyrrhizic acid and soybean oil for self-healing, shape memory, weldable, and recyclable materials. ACS Sustain. Chem. Eng. 2020, 8, 6479–6487. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, S.; Bi, L.; Jiang, J.; Zhang, H.; Chen, Y. Catalyst-free self-healing bio-based vitrimer for a recyclable, reprocessable, and self-adhered carbon fiber reinforced composite. Chem. Eng. J. 2022, 429, 132518. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Zhang, S.; Shao, L.; Fei, M.; Yu, H.; Zhang, J. Catalyst-free vitrimer elastomers based on a dimer acid: Robust mechanical performance, adaptability and hydrothermal recyclability. Green Chem. 2020, 22, 870–881. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, S.; Bi, L.; Jiang, J.; Zhang, H.; Chen, Y. Catalyst-free self-healing bio-based polymers: Robust mechanical properties, shape memory, and recyclability. J. Agric. Food Chem. 2021, 69, 9338–9349. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, J.; Liu, S.; Yang, B. Rosin-based epoxy vitrimers with dynamic boronic ester bonds. Polymers 2021, 13, 3386. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Z.; Liu, Y.; Wu, S.; Guo, B. Mechanically robust, self-healable, and reprocessable elastomers enabled by dynamic dual cross-links. Macromolecules 2019, 52, 3805–3812. [Google Scholar] [CrossRef]
- Ke, Y.; Yang, X.; Chen, Q.; Xue, J.; Song, Z.; Zhang, Y.; Madbouly, S.A.; Luo, Y.; Li, M.; Wang, Q.; et al. Recyclable and fluorescent epoxy polymer networks from cardanol via solvent-free epoxy-thiol chemistry. ACS Appl. Polym. Mater. 2021, 3, 3082–3092. [Google Scholar] [CrossRef]
- Ma, J.; Li, G.; Hua, X.; Liu, N.; Liu, Z.; Zhang, F.; Yu, L.; Chen, X.; Shang, L.; Ao, Y. Biodegradable epoxy resin from vanillin with excellent flame-retardant and outstanding mechanical properties. Polym. Degrad. Stab. 2022, 201, 109989. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhou, L.; Wang, L.; Majeed, K.; Zhang, B.; Zhou, F.; Zhang, Q. Preparation of environmentally friendly bio-based vitrimers from vanillin derivatives by introducing two types of dynamic covalent C=N and S-S bonds. Polymer 2020, 197, 122483. [Google Scholar] [CrossRef]
- Song, F.; Li, Z.; Jia, P.; Zhang, M.; Bo, C.; Feng, G.; Hu, L.; Zhou, Y. Tunable “soft and stiff”, self-healing, recyclable, thermadapt shape memory biomass polymers based on multiple hydrogen bonds and dynamic imine bonds. J. Mater. Chem. A 2019, 7, 13400–13410. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Recyclable and malleable epoxy thermoset bearing aromatic imine bonds. Macromolecules 2018, 51, 9816–9824. [Google Scholar] [CrossRef]
- Liu, X.; Liang, L.; Lu, M.; Song, X.; Liu, H.; Chen, G. Water-resistant bio-based vitrimers based on dynamic imine bonds: Self-healability, remodelability and ecofriendly recyclability. Polymer 2020, 210, 123030. [Google Scholar] [CrossRef]
- Yu, Q.; Peng, X.; Wang, Y.; Geng, H.; Xu, A.; Zhang, X.; Xu, W.; Ye, D. Vanillin-based degradable epoxy vitrimers: Reprocessability and mechanical properties study. Eur. Polym. J. 2019, 117, 55–63. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Liu, G.-L.; Li, Y.-D.; Weng, Y.; Zeng, J.-B. Biobased high-performance epoxy vitrimer with UV shielding for recyclable carbon fiber reinforced composites. ACS Sustain. Chem. Eng. 2021, 9, 4638–4647. [Google Scholar] [CrossRef]
- Memon, H.; Liu, H.; Rashid, M.A.; Chen, L.; Jiang, Q.; Zhang, L.; Wei, Y.; Liu, W.; Qiu, Y. Vanillin-based epoxy vitrimer with high performance and closed-loop recyclability. Macromolecules 2020, 53, 621–630. [Google Scholar] [CrossRef]
- Su, X.; Zhou, Z.; Liu, J.; Luo, J.; Liu, R. A recyclable vanillin-based epoxy resin with high-performance that can compete with DGEBA. Eur. Polym. J. 2020, 140, 110053. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Yuan, W.; Wang, B.; Zhu, J. Robust, Fire-Safe, Monomer-recovery, highly malleable thermosets from renewable bioresources. Macromolecules 2018, 51, 8001–8012. [Google Scholar] [CrossRef]
- Xie, W.; Huang, S.; Liu, S.; Zhao, J. Imine-functionalized biomass-derived dynamic covalent thermosets enabled by heat-induced self-crosslinking and reversible structures. Chem. Eng. J. 2021, 404, 126598. [Google Scholar] [CrossRef]
- Xu, X.; Ma, S.; Wu, J.; Yang, J.; Wang, B.; Wang, S.; Li, Q.; Feng, J.; You, S.; Zhu, J. High-performance, command-degradable, antibacterial Schiff base epoxy thermosets: Synthesis and properties. J. Mater. Chem. A 2019, 7, 15420–15431. [Google Scholar] [CrossRef]
- Yuan, W.; Ma, S.; Wang, S.; Li, Q.; Wang, B.; Xu, X.; Huang, K.; Chen, J.; You, S.; Zhu, J. Synthesis of fully bio-based diepoxy monomer with dicyclo diacetal for high-performance, readily degradable thermosets. Eur. Polym. J. 2019, 117, 200–207. [Google Scholar] [CrossRef]
- Ma, S.; Wei, J.; Jia, Z.; Yu, T.; Yuan, W.; Li, Q.; Wang, S.; You, S.; Liu, R.; Zhu, J. Readily recyclable, high-performance thermosetting materials based on a lignin-derived spiro diacetal trigger. J. Mater. Chem. A 2019, 7, 1233–1243. [Google Scholar] [CrossRef]
- Wang, B.; Ma, S.; Li, Q.; Zhang, H.; Liu, J.; Wang, R.; Chen, Z.; Xu, X.; Wang, S.; Lu, N.; et al. Facile synthesis of “digestible”, rigid-and-flexible, bio-based building block for high-performance degradable thermosetting plastics. Green Chem. 2020, 22, 1275–1290. [Google Scholar] [CrossRef]
- Karami, Z.; Zohuriaan-Mehr, M.J.; Rostami, A. Bio-based thermo-healable non-isocyanate polyurethane DA network in comparison with its epoxy counterpart. J. CO2 Util. 2017, 18, 294–302. [Google Scholar] [CrossRef]
- Shen, X.; Liu, X.; Wang, J.; Dai, J.; Zhu, J. Synthesis of an epoxy monomer from bio-based 2,5-furandimethanol and its toughening via Diels-Alder reaction. Ind. Eng. Chem. Res. 2017, 56, 8508–8516. [Google Scholar] [CrossRef]
- Lejeail, M.; Fischer, H.R. Investigations on the replacement of bismaleimide by the bio-based bisitaconimide for recyclable thermoset composites based on thermo-reversible Diels-Alder cross-links. Eur. Polym. J. 2020, 131, 109699. [Google Scholar] [CrossRef]
- Wu, P.; Liu, L.; Wu, Z. Synthesis of Diels-Alder reaction-based remendable epoxy matrix and corresponding self-healing efficiency to fibrous composites. Macromol. Mater. Eng. 2020, 305, 2000359. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, J.; Qu, D.; Wang, H.; Chai, C.; Feng, L. Thermo-adjusted self-healing epoxy resins based on Diels–Alder dynamic chemical reaction. Polym. Eng. Sci. 2021, 61, 2257–2266. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, L.; Shi, X.; Wang, Y.; Liu, Y. Synthesis and healing behavior of thermo-reversible self-healing epoxy resins. Acta Polym. Sin. 2018, 3, 395–401. [Google Scholar]
- Liu, Z.; Zhu, X.; Tian, Y.; Zhou, K.; Cheng, J.; Zhang, J. Bio-based recyclable Form-Stable phase change material based on thermally reversible Diels–Alder reaction for sustainable thermal energy storage. Chem. Eng. J. 2022, 448, 137749. [Google Scholar] [CrossRef]
- Mauro, C.D.; Genua, A.; Mija, A. Building thermally and chemically reversible covalent bonds in vegetable oil based epoxy thermosets. Influence of epoxy–hardener ratio in promoting recyclability. Mater. Adv. 2020, 1, 1788–1798. [Google Scholar] [CrossRef]
- Ocando, C.; Ecochard, Y.; Decostanzi, M.; Caillol, S.; Avérous, L. Dynamic network based on eugenol-derived epoxy as promising sustainable thermoset materials. Eur. Polym. J. 2020, 135, 109860. [Google Scholar] [CrossRef]
- Shan, S.; Mai, D.; Lin, Y.; Zhang, A. Self-Healing, Reprocessable, and Degradable bio-based epoxy elastomer bearing aromatic disulfide bonds and its application in strain sensors. ACS Appl. Polym. Mater. 2021, 3, 5115–5124. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Bio-based epoxy vitrimers: Reprocessibility, controllable shape memory, and degradability. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1790–1799. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Liang, D.; Deng, H.; Lin, Z.; Feng, P.; Wang, Q. Rapid self-healing, multiple recyclability and mechanically robust plant oil-based epoxy resins enabled by incorporating tri-dynamic covalent bonding. J. Mater. Chem. A 2021, 9, 18431–18439. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, B.; Yuan, R.; Luo, Z. Fabrication and properties of rosin-based epoxy vitrimer with dual dynamic covalent bonds. Chem. J. Chin. Univ. 2023, 44, 198–209. [Google Scholar]
- di Mauro, C.; Tran, T.-N.; Graillot, A.; Mija, A. Enhancing the recyclability of a vegetable oil-based epoxy thermoset through initiator influence. ACS Sustain. Chem. Eng. 2020, 8, 7690–7700. [Google Scholar] [CrossRef]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic covalent polymer networks: A molecular platform for designing functions beyond chemical recycling and self-healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef] [PubMed]
- Herath, M.; Epaarachchi, J.; Islam, M.; Fang, L.; Leng, J. Light activated shape memory polymers and composites: A review. Eur. Polym. J. 2020, 136, 109912. [Google Scholar] [CrossRef]
- Rodriguez, J.N.; Zhu, C.; Duoss, E.B.; Wilson, T.S.; Spadaccini, C.M.; Lewicki, J.P. Shape-morphing composites with designed micro-architectures. Sci. Rep. 2016, 6, 27933. [Google Scholar] [CrossRef]
- Taung Mai, L.L.; Aung, M.M.; Muhamad Saidi, S.A.; H’Ng, P.S.; Rayung, M.; Jaafar, A.M. Non edible oil-based epoxy resins from jatropha oil and their shape memory behaviors. Polymers 2021, 13, 2177. [Google Scholar] [CrossRef]
- Huang, J.-L.; Ding, H.-L.; Wang, X.; Song, L.; Hu, Y. Cardanol-derived anhydride cross-linked epoxy thermosets with intrinsic anti-flammability, toughness and shape memory effect. Chem. Eng. J. 2022, 450, 137906. [Google Scholar] [CrossRef]
- Lu, C.; Wang, X.; Shen, Y.; Wang, J.; Yong, Q.; Chu, F. Fabrication of sustainable, toughening epoxy thermosets with rapidly thermal and light-triggered shape memory property. J. Polym. Sci. 2022, 60, 2866–2874. [Google Scholar] [CrossRef]
- Amornkitbamrung, L.; Srisaard, S.; Jubsilp, C.; Bielawski, C.W.; Um, S.H.; Rimdusit, S. Near-infrared light responsive shape memory polymers from bio-based benzoxazine/epoxy copolymers produced without using photothermal filler. Polymer 2020, 209, 122986. [Google Scholar] [CrossRef]
- Lu, C.; Liu, Y.; Wang, C.; Yong, Q.; Wang, J.; Chu, F. An integrated strategy to fabricate bio-based dual-cure and toughened epoxy thermosets with photothermal conversion property. Chem. Eng. J. 2022, 433, 134582. [Google Scholar] [CrossRef]
- Li, W.; Xiao, L.; Wang, Y.; Chen, J.; Nie, X. Self-healing silicon-containing eugenol-based epoxy resin based on disulfide bond exchange: Synthesis and structure-property relationships. Polymer 2021, 229, 123967. [Google Scholar] [CrossRef]
- Yang, X.; Guo, L.; Xu, X.; Shang, S.; Liu, H. A fully bio-based epoxy vitrimer: Self-healing, triple-shape memory and reprocessing triggered by dynamic covalent bond exchange. Mater. Des. 2020, 186, 108248. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, K.M.; Jung, D.; Chae, J.A.; Kim, H.J.; Chang, M.; Park, J.J.; Kim, H. Sustainable, naringenin-based thermosets show reversible macroscopic shape changes and enable modular recycling. ACS Macro. Lett. 2019, 8, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, C.; Malburet, S.; Graillot, A.; Mija, A. Recyclable, repairable, and reshapable (3R) thermoset materials with shape memory properties from bio-based epoxidized vegetable oils. Appl. Bio. Mater. 2020, 3, 8094–8104. [Google Scholar] [CrossRef]
- Podgorski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward stimuli-responsive dynamic thermosets through continuous development and improvements in covalent adaptable networks (CANs). Adv. Mater. 2020, 32, 1906876. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Hu, J.; Jin, D.; Wang, S.; Dai, J.; Liu, X. Bio-based Epoxy Resin: Controllable Degradation, Chemical Recovery and Antimicrobial Property. Acta Polym. Sin. 2022, 53, 1083–1094. [Google Scholar]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. A high performance fully bio-based epoxy thermoset from a syringaldehyde-derived epoxy monomer cured by furan-derived amine. Green Chem. 2021, 23, 501–510. [Google Scholar] [CrossRef]
- Cao, Q.; Weng, Z.; Qi, Y.; Li, J.; Liu, W.; Liu, C.; Zhang, S.; Wei, Z.; Chen, Y.; Jian, X. Achieving higher performances without an external curing agent in natural magnolol-based epoxy resin. Chin. Chem. Lett. 2022, 33, 2195–2199. [Google Scholar] [CrossRef]
- Huang, K.; Fan, X.; Ashby, R.; Ngo, H. Structure-activity relationship of antibacterial bio-based epoxy polymers made from phenolic branched fatty acids. Prog. Org. Coat. 2021, 155, 106228. [Google Scholar] [CrossRef]
- Modjinou, T.; Versace, D.-L.; Abbad-Andaloussi, S.; Langlois, V.; Renard, E. Antibacterial and antioxidant photoinitiated epoxy co-networks of resorcinol and eugenol derivatives. Mater. Today Commun. 2017, 12, 19–28. [Google Scholar] [CrossRef]
- Chen, M.-X.; Dai, J.-Y.; Zhang, L.-Y.; Wang, S.-P.; Liu, J.-K.; Wu, Y.-G.; Ba, X.-W.; Liu, X.-Q. The role of renewable protocatechol acid in epoxy coating modification: Significantly improved antibacterial and adhesive properties. Chin. J. Polym. Sci. 2023, 42, 63–72. [Google Scholar] [CrossRef]
- Lobiuc, A.; Paval, N.-E.; Mangalagiu, I.I.; Gheorghitã, R.; Teliban, G.-C.; Amariucai-Mantu, D.; Stoleru, V. Improving the antimicrobial and mechanical properties of epoxy resins via nanomodification: An Overview. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Huang, S.; Liu, S.; Yuan, Y.; Zhao, J.; Zhang, S. A novel biomass-derived Schiff base waterborne epoxy coating for flame retardation and anti-bacteria. Polym. Degrad. Stab. 2022, 199, 109910. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Lu, G.; Chen, W.; Ye, Z.; He, Y.; Tang, Z.; Zhu, J. Versatile levulinic acid-derived dynamic covalent thermosets enabled by in situ generated imine and multiple hydrogen bonds. Chem. Eng. J. 2023, 451, 139053. [Google Scholar] [CrossRef]
- Peng, J.; Xie, S.; Liu, T.; Wang, D.; Ou, R.; Guo, C.; Wang, Q.; Liu, Z. High-performance epoxy vitrimer with superior self-healing, shape-memory, flame retardancy, and antibacterial properties based on multifunctional curing agent. Compos. Part B-Eng. 2022, 242, 110109. [Google Scholar] [CrossRef]
- Xu, X.; Ma, S.; Wang, S.; Wu, J.; Li, Q.; Lu, N.; Liu, Y.; Yang, J.; Feng, J.; Zhu, J. Dihydrazone-based dynamic covalent epoxy networks with high creep resistance, controlled degradability, and intrinsic antibacterial properties from bioresources. J. Mater. Chem. A 2020, 8, 11261–11274. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A review on the antimicrobial activity of Schiff bases: Data collection and recent studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef]



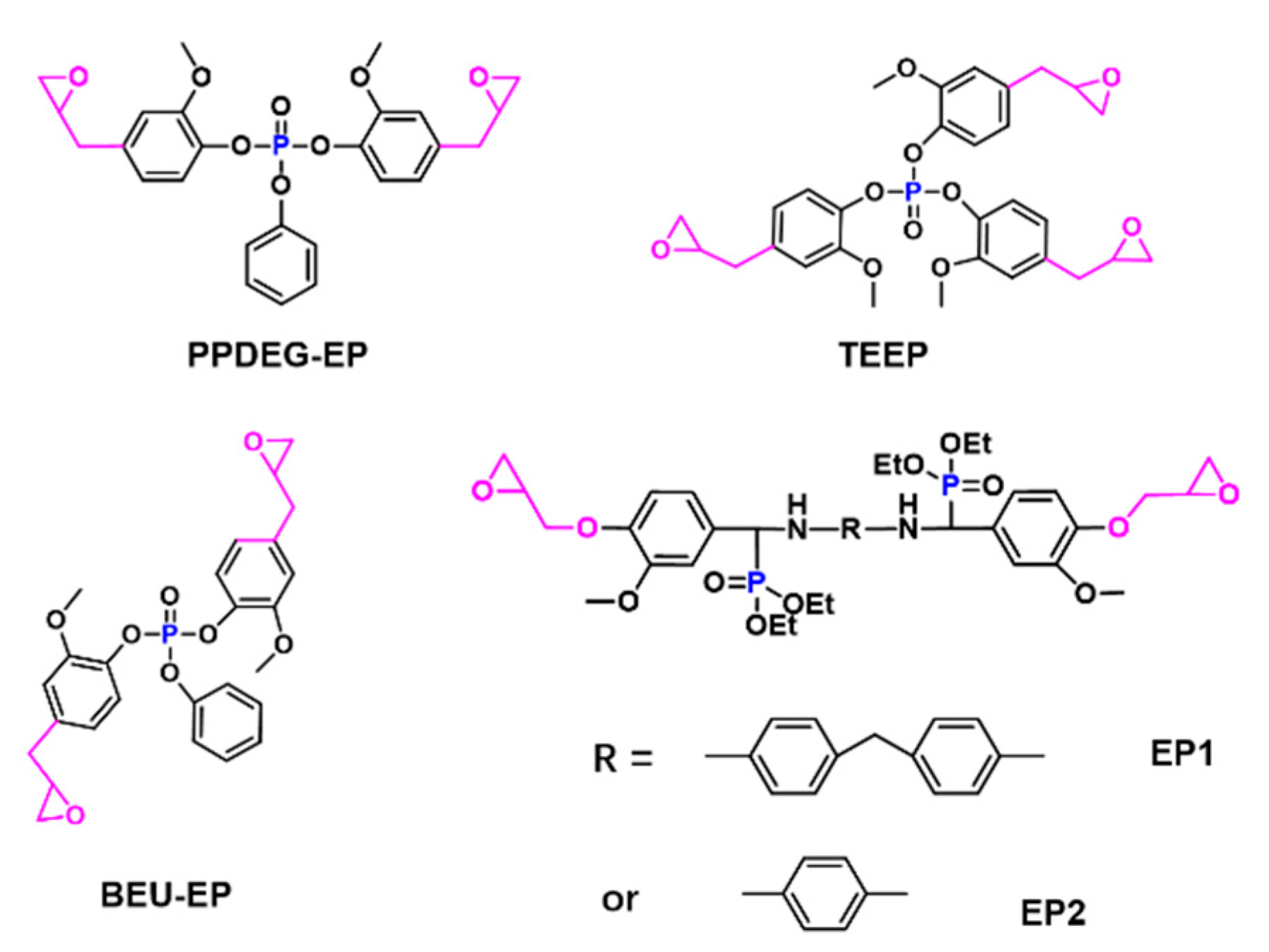

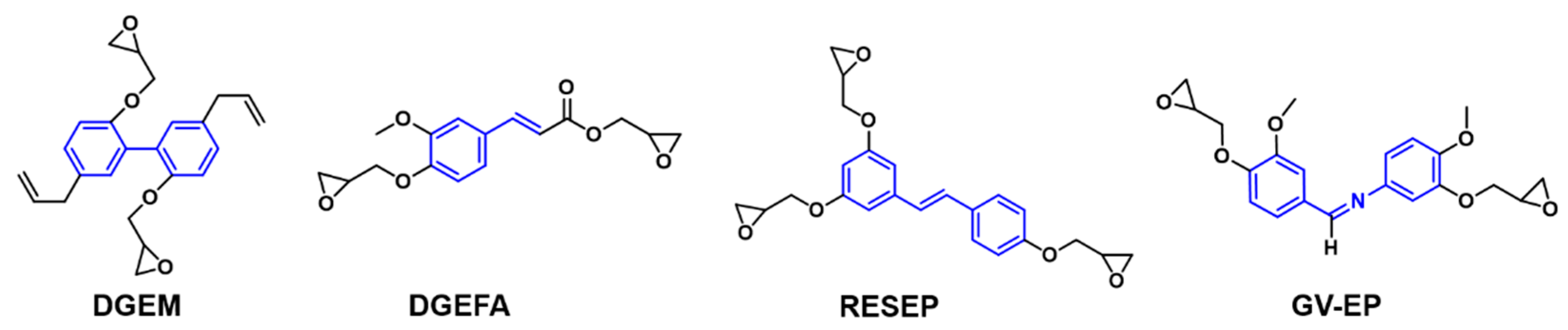
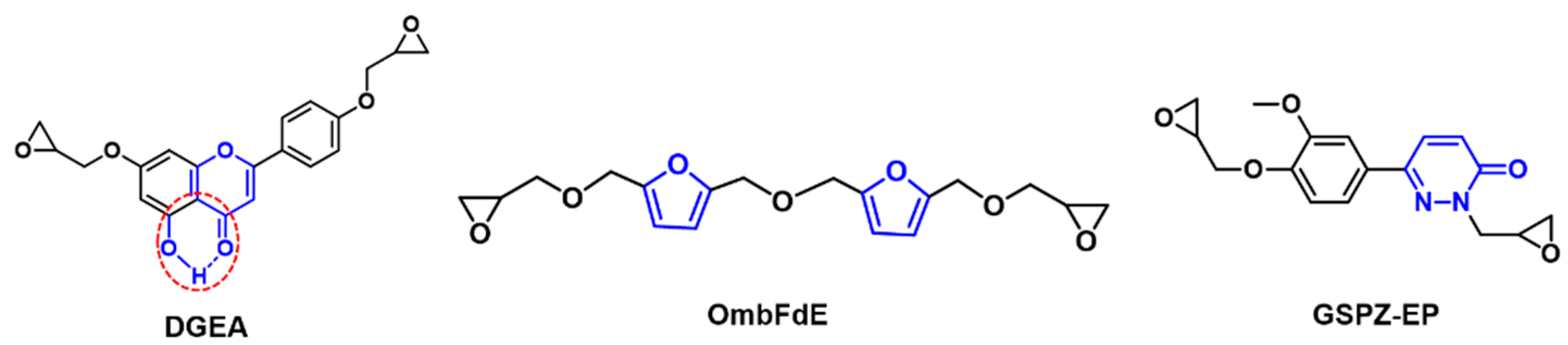



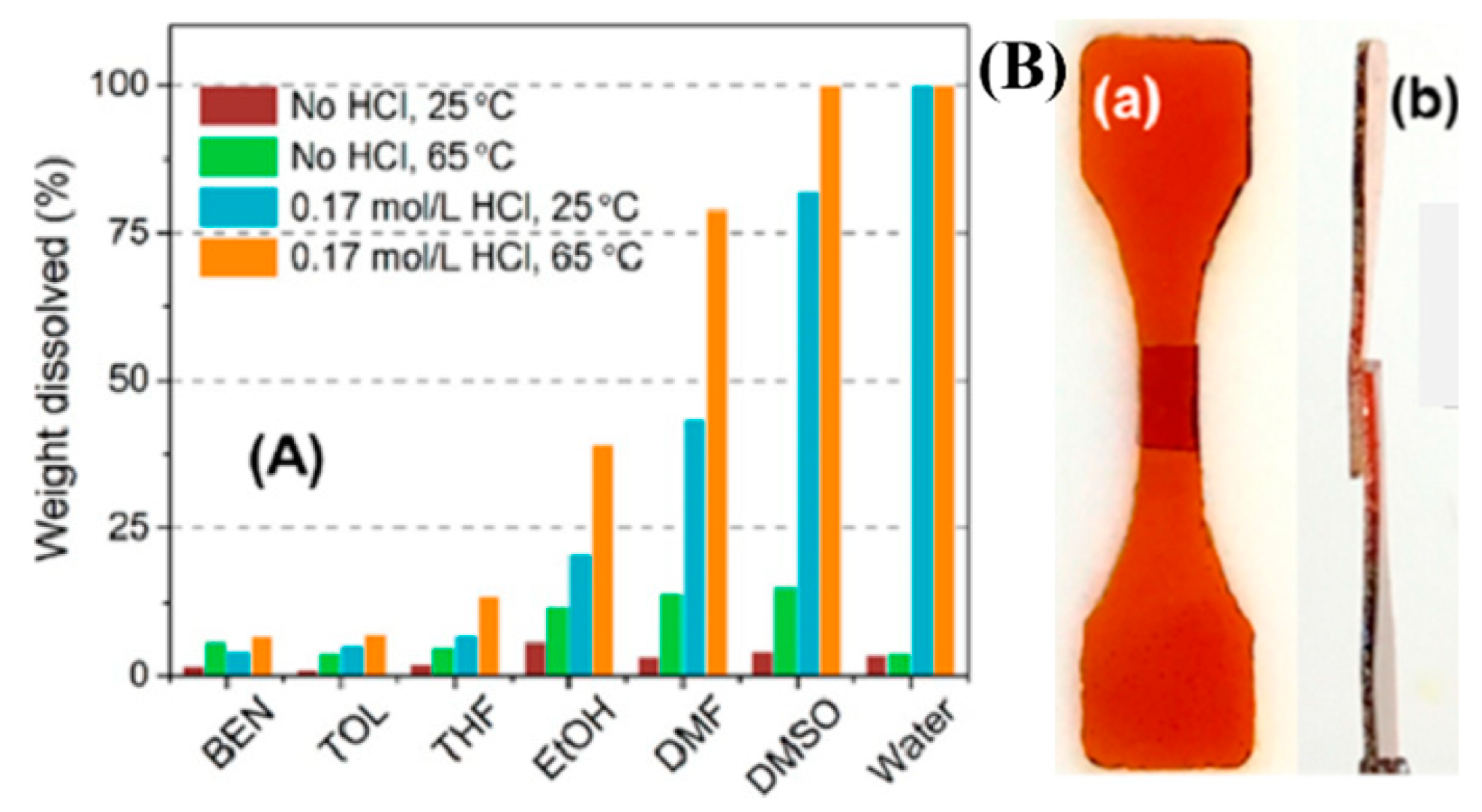
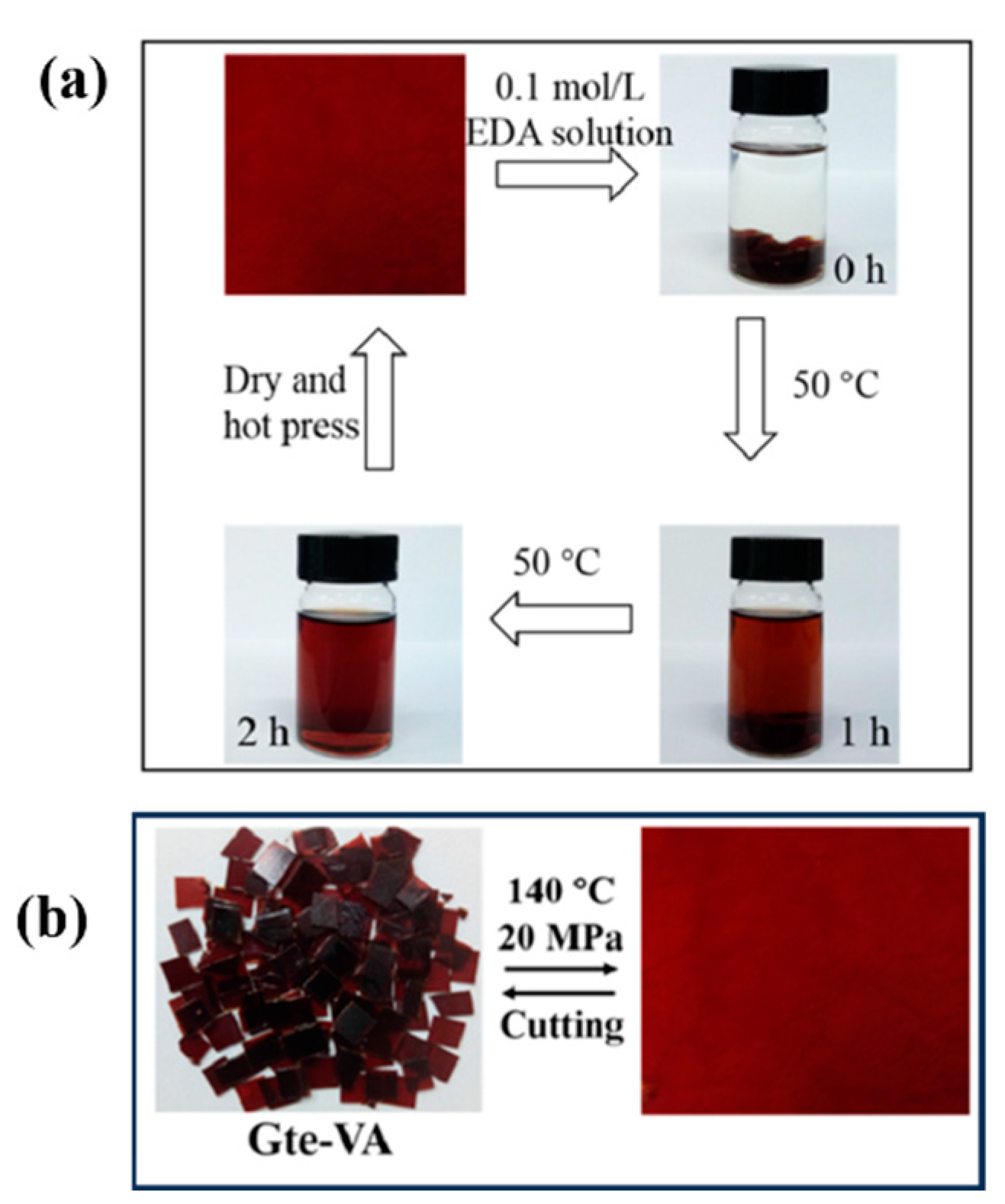
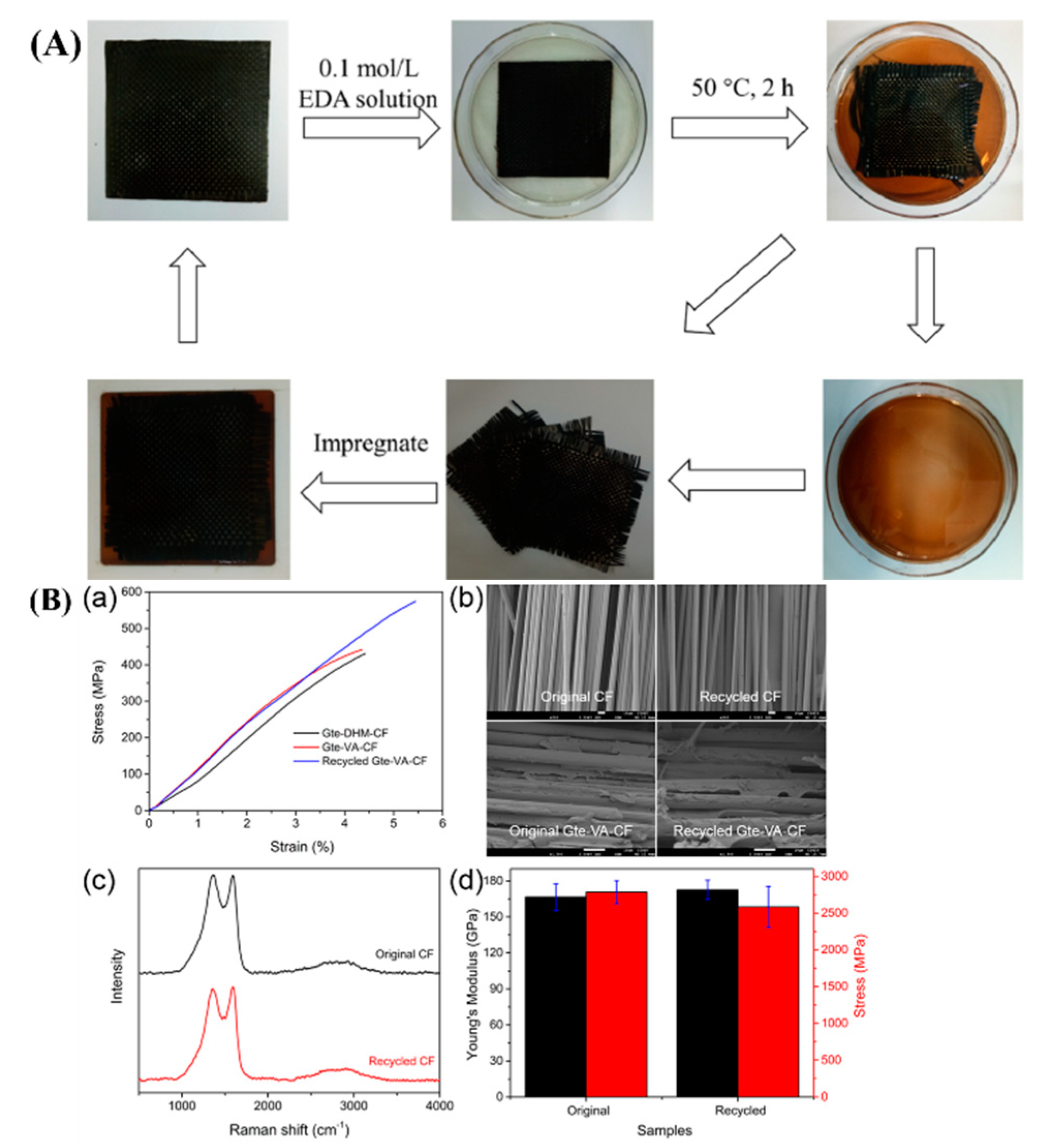
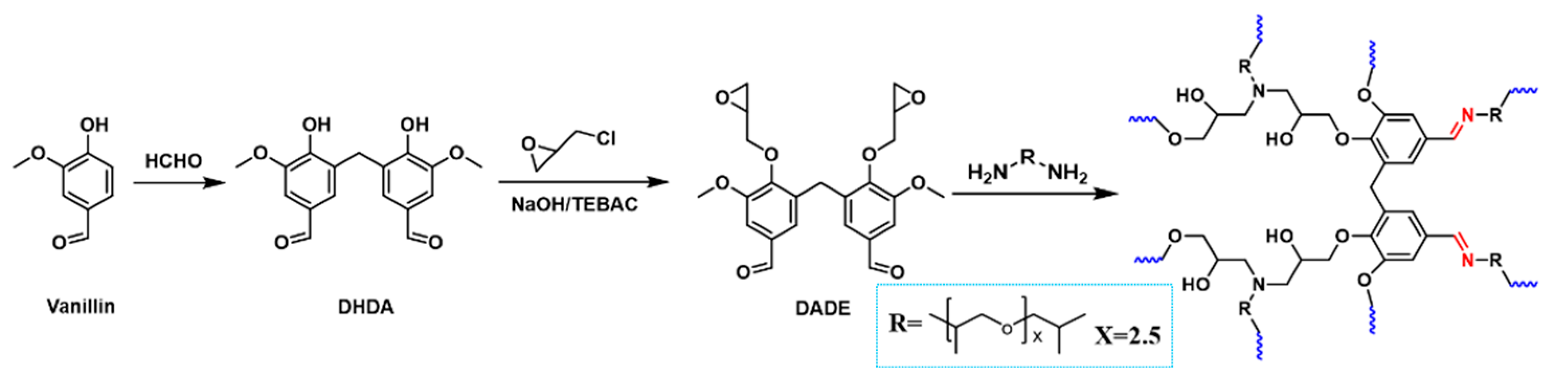

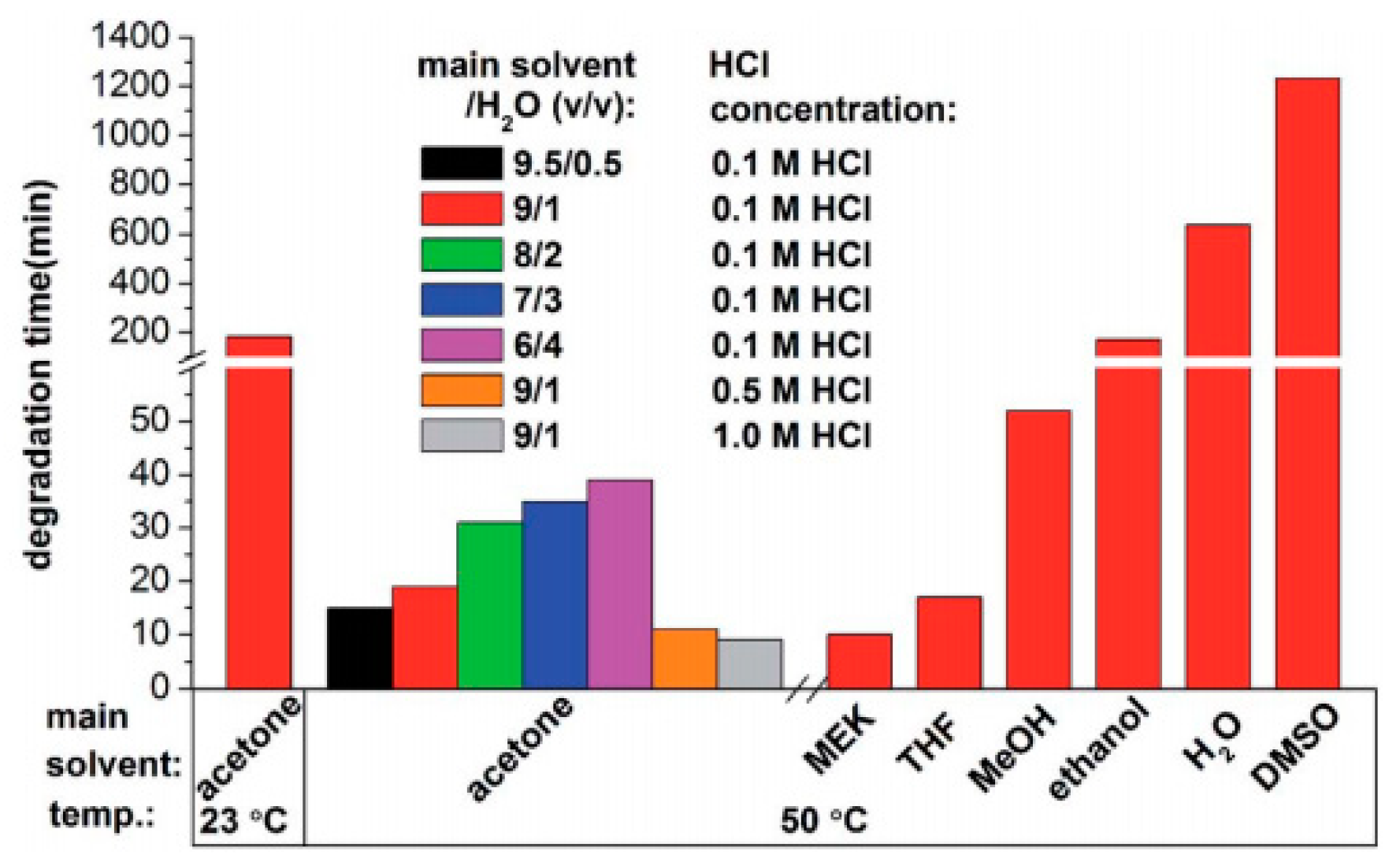
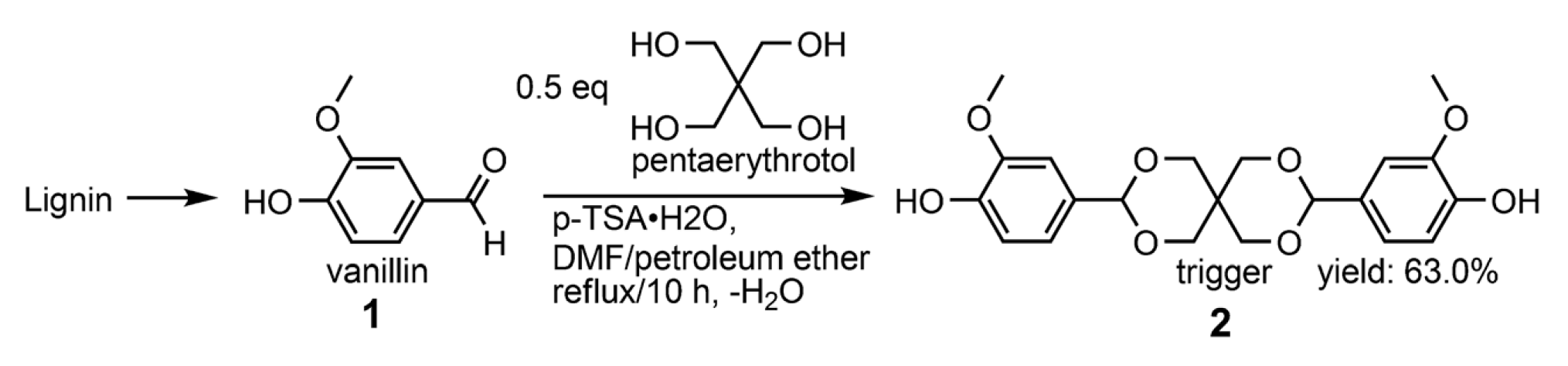
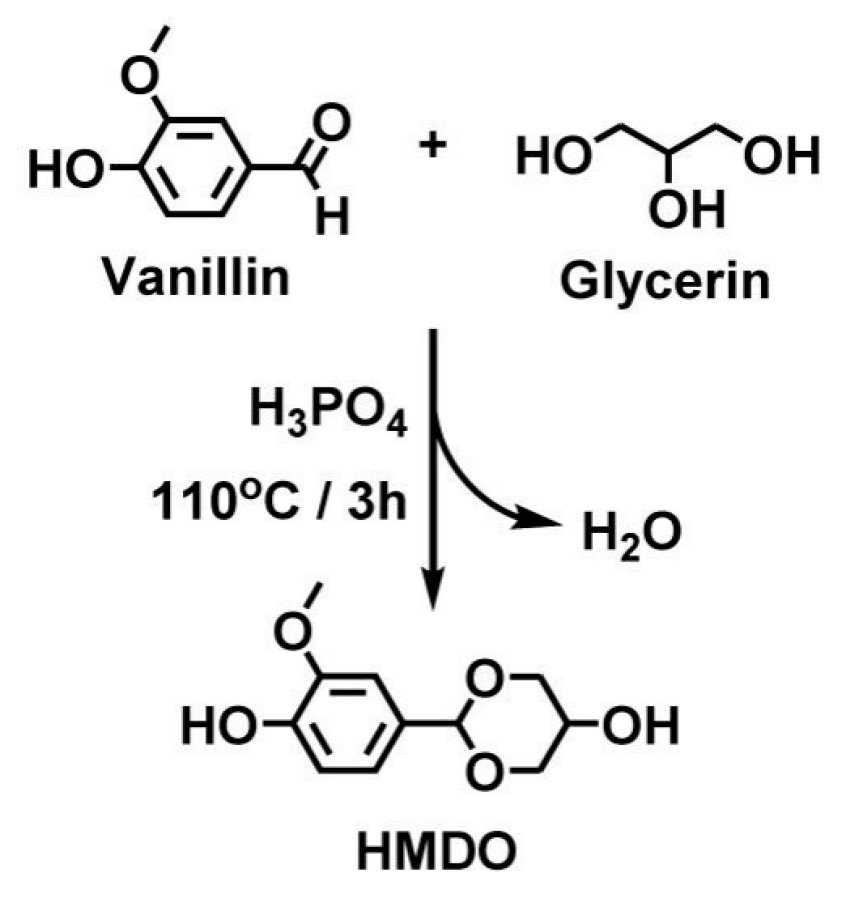
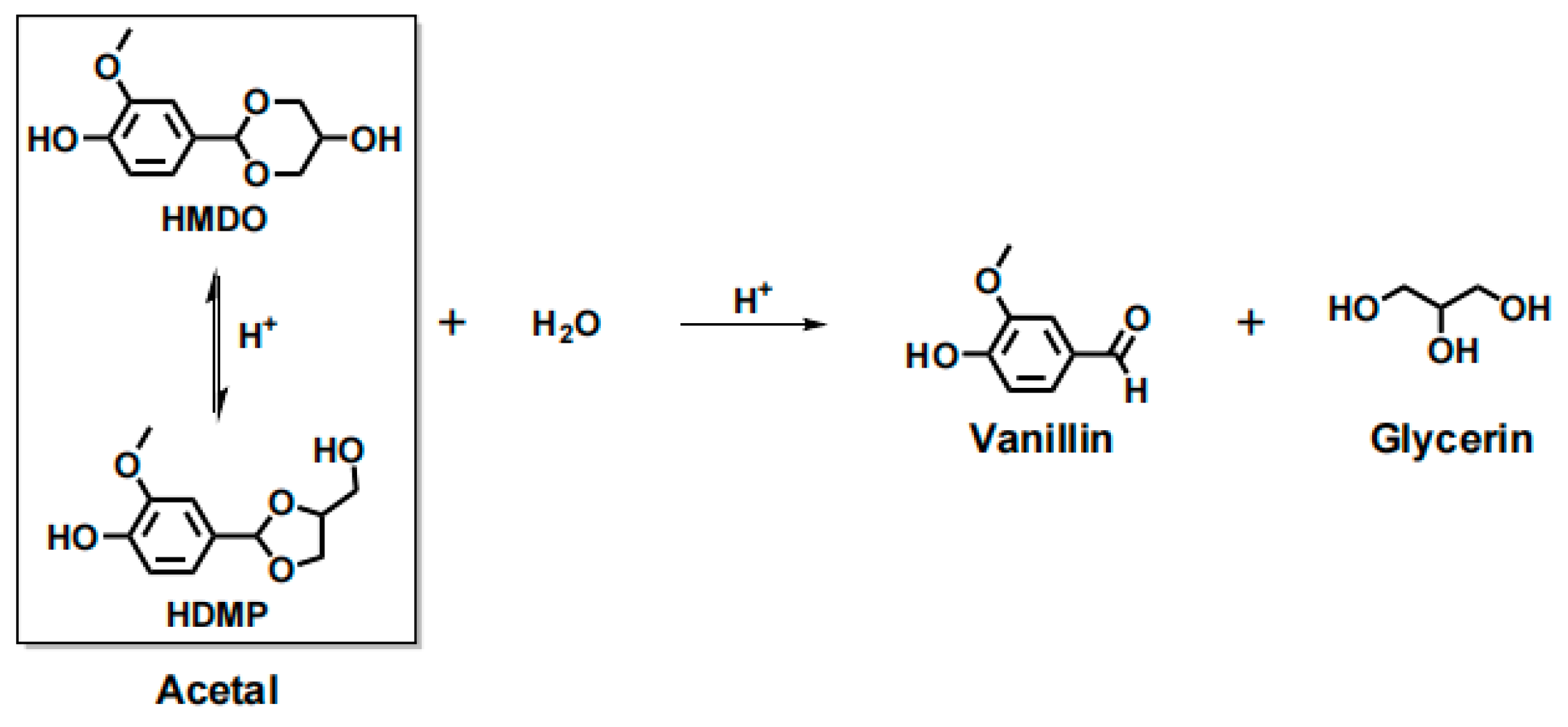

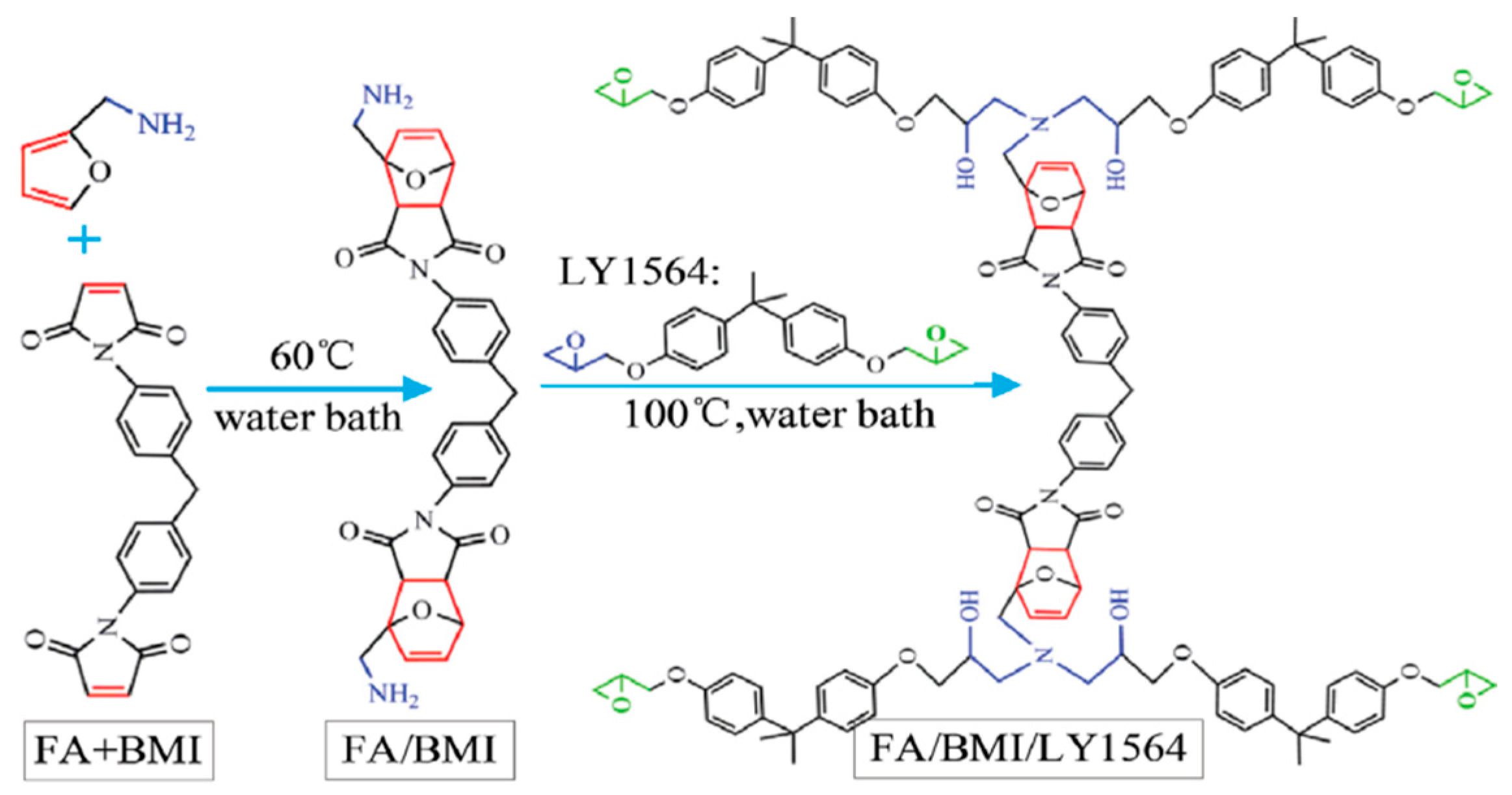
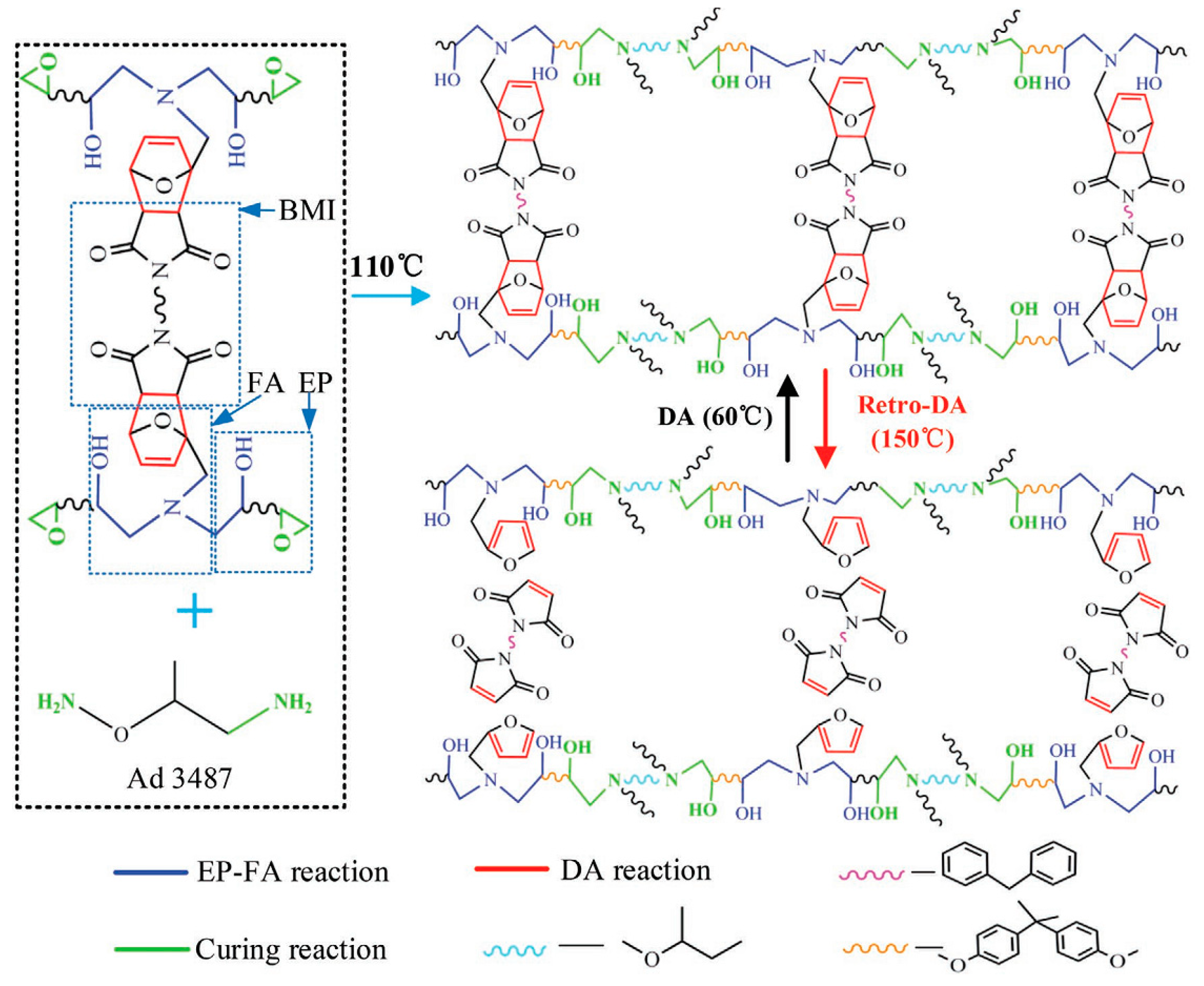
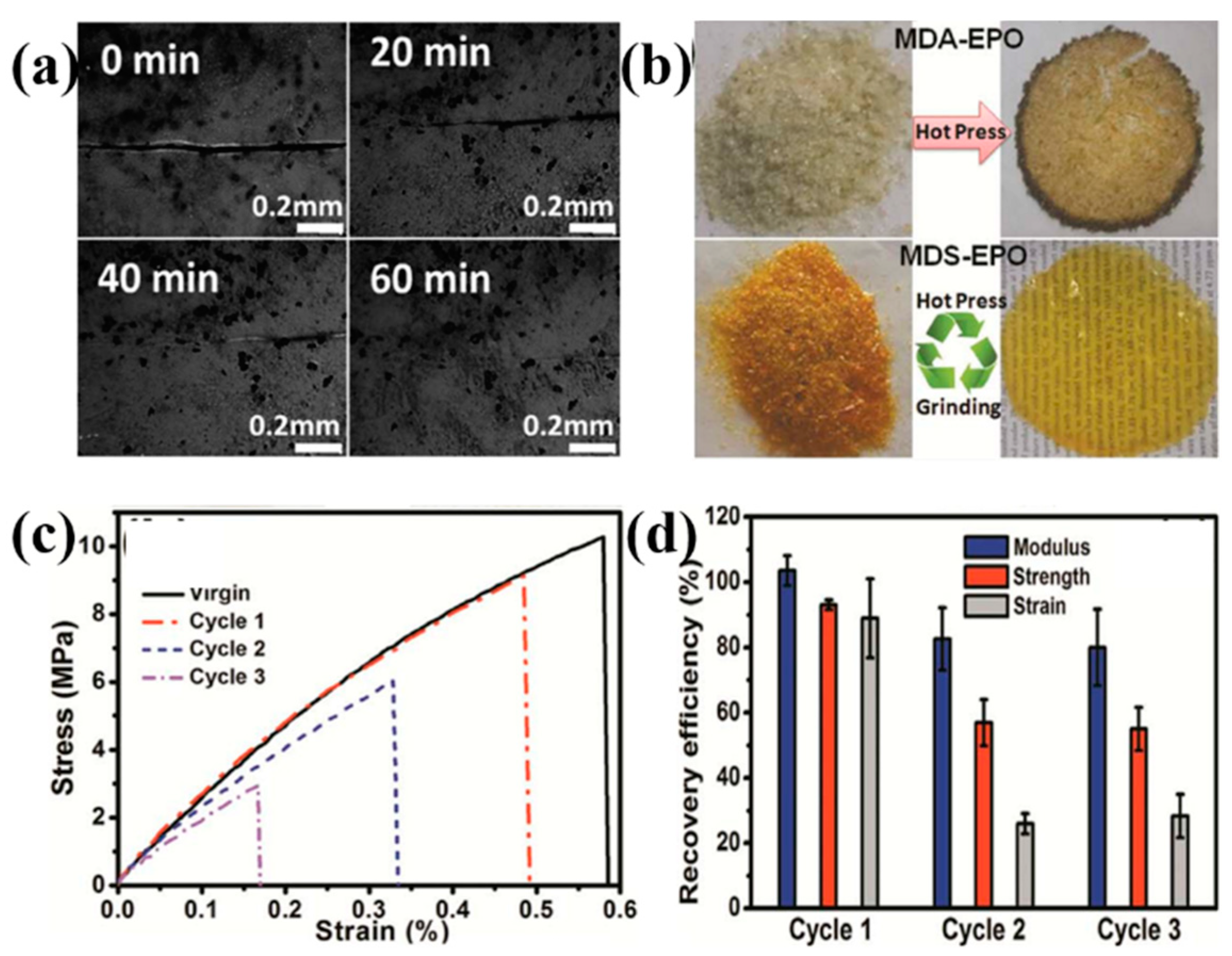
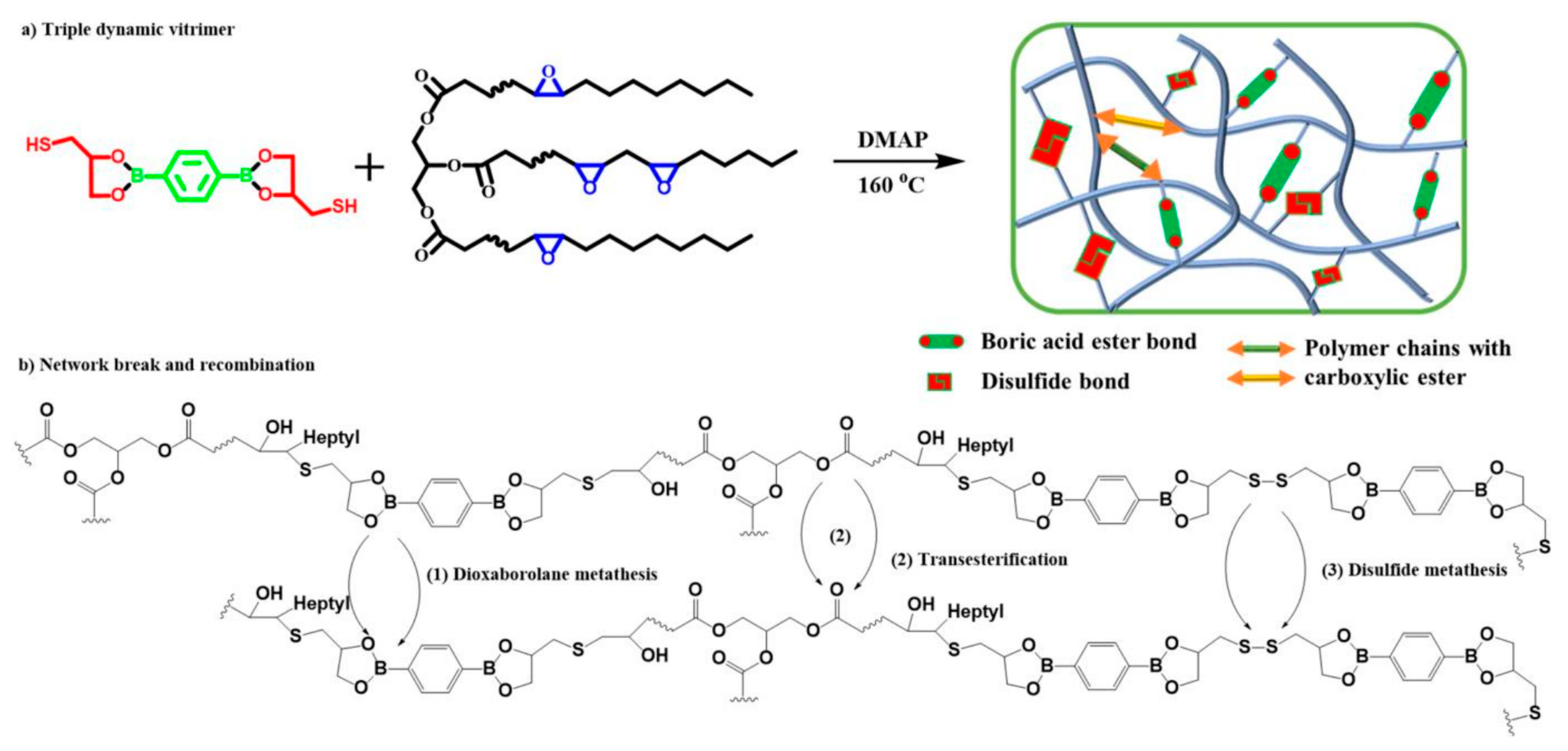

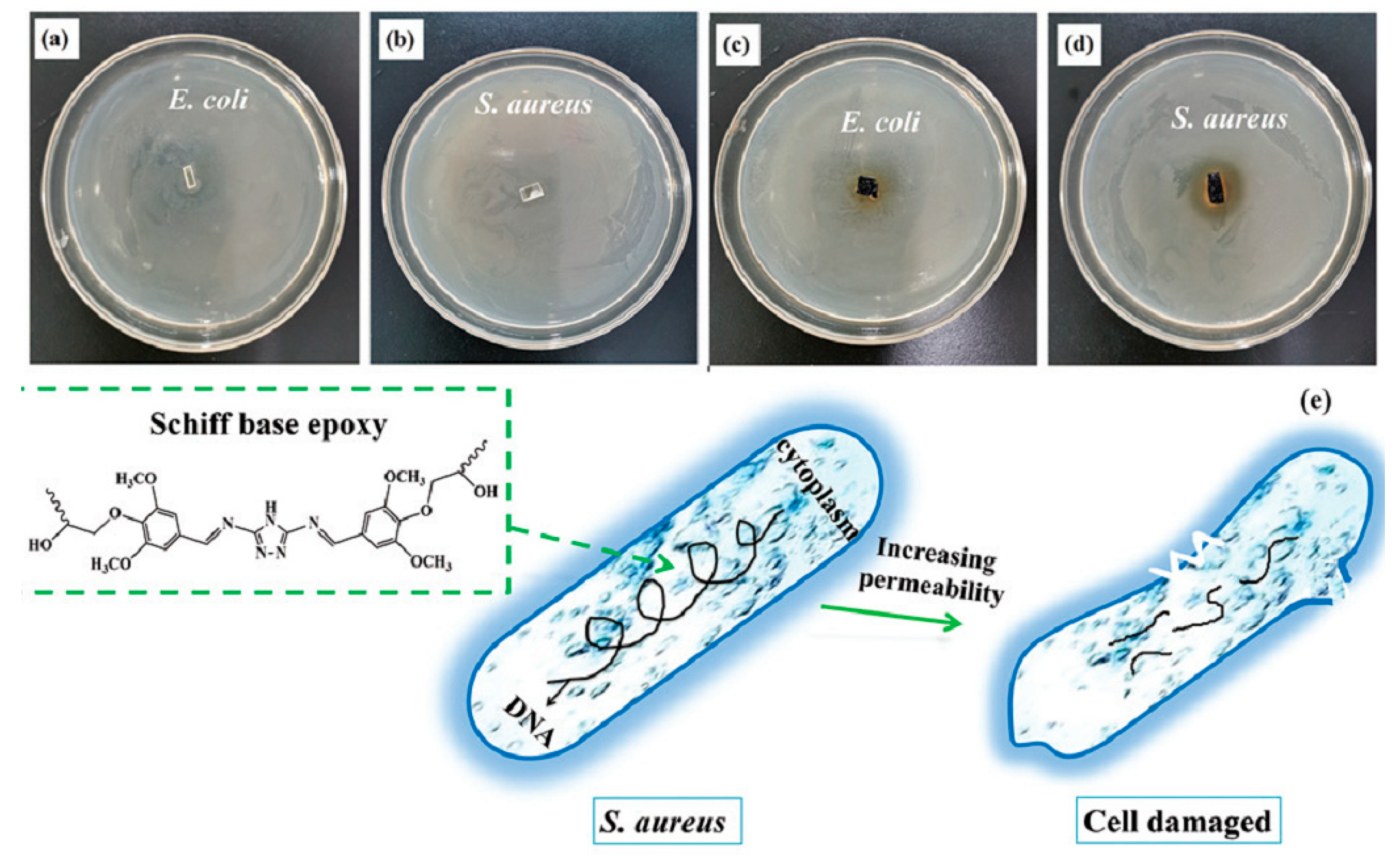
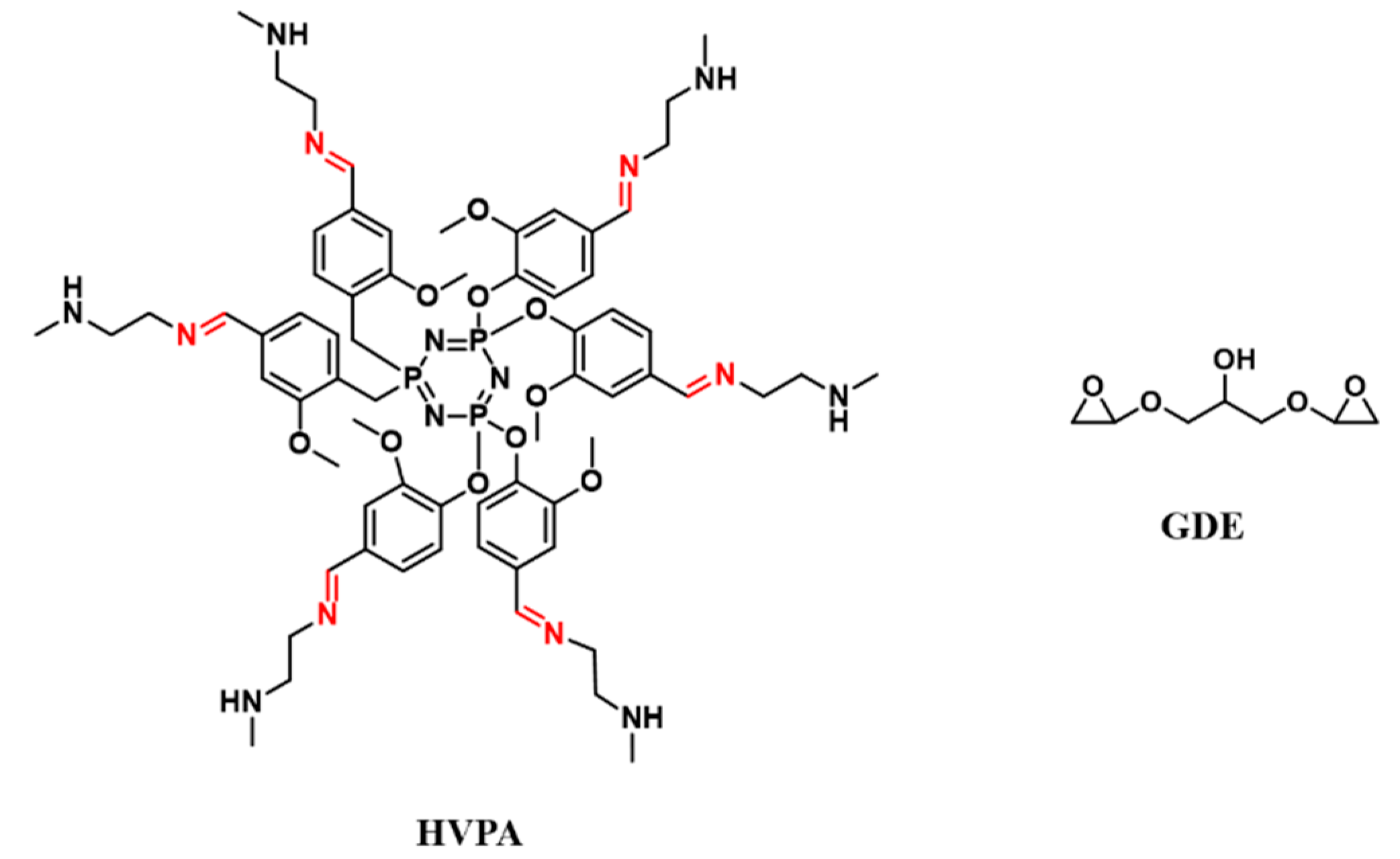
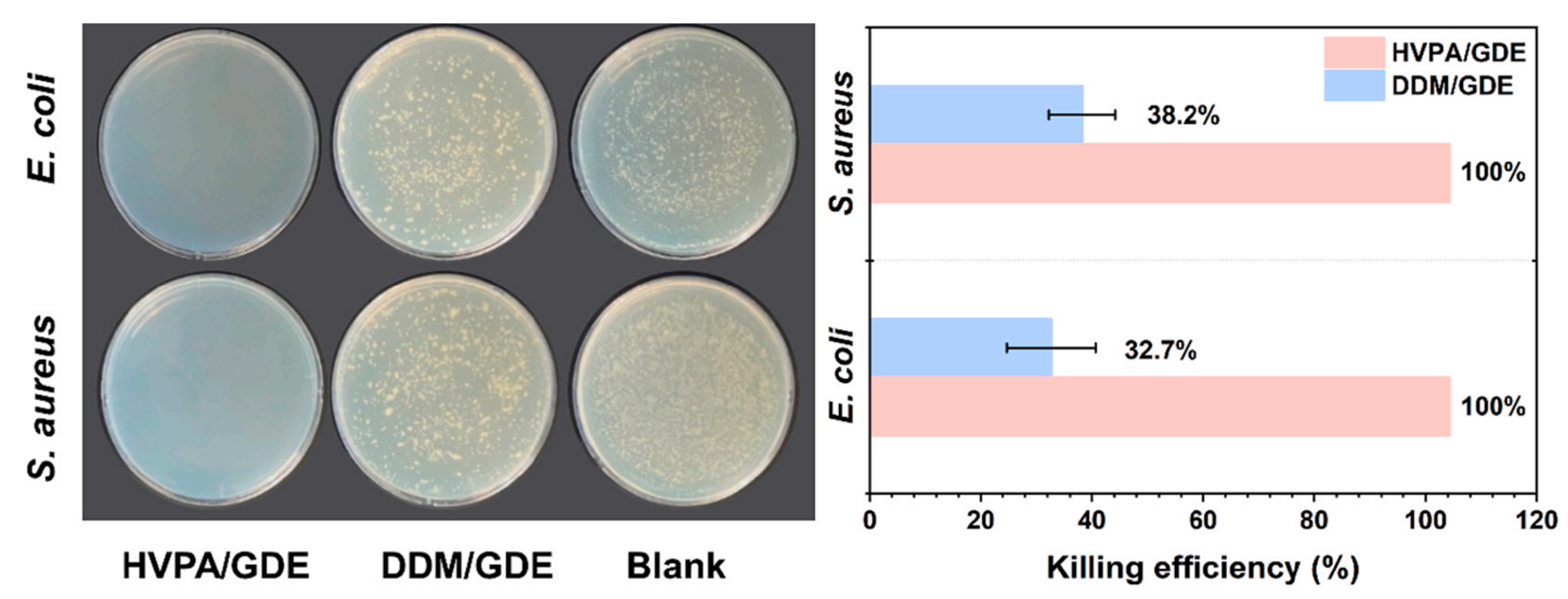
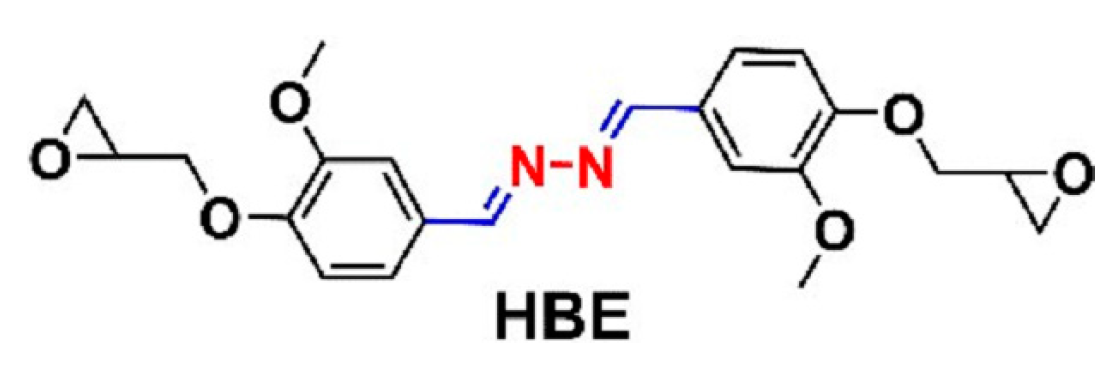
| Epoxy Resin | Curing Agent | Tg (°C) | Td5% (°C) | Char Yield (wt%) | UL-94 | LOI (%) | Combustion Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| TDBE | DDM | 140 | 217 | Y700 = 29.8 | V-0 | 42.0 | pHRR-69.3% THR-53.0% | [38] |
| DGEBDB/ DGEBA = 1/9 | DDM | 176.4 | - | Y800 = 19.1 | V-1 | 30.2 | THR-11.9% | [39] |
| DGEBDB/ DGEBA = 2/8 | DDM | 169.2 | - | Y800 = 21.2 | V-0 | 32.4 | THR-18.3% | [39] |
| VDE | DDM | 129.6 | 248.8 | Y800 = 28 | V-0 | 34.5 | - | [40] |
| VSE | DDM | 176.1 | 254.9 | Y800 = 34.5 | V-0 | 38.7 | - | [40] |
| TEBA | DDM | 136 | 271 | Y700 = 29.9 | V-0 | 42.3 | pHRR-67% THR-27% | [41] |
| MEP/ DGEBA = 8/2 | DDM | 147.1 | 314.3 | - | V-0 | 27.5 | pHRR-34.7% THR-27.0% | [42] |
| VAD-EP/VDP-EP = 8/2 | D230 | 82.3 | 212.7 | Y800 = 32.8 | V-0 | 27.0 | - | [43] |
| VAD-EP/VDP-EP = 7/3 | D230 | 80.3 | 205.3 | Y800 = 30.2 | V-0 | 28.7 | pHRR-47.9% THR-32.0% | [43] |
| EHEP | D230 | 122 | 270 | Y700 = 39.0 | V-0 | 31.0 | pHRR-66% THR-65% | [44] |
| HECarCP | DDM | - | - | Y800 = 14.3 | V-0 | 33.0 | pHRR-63% THR-24% | [45] |
| BEEP/DGEBA = 2/8 | DDM | 138.7 | 332.2 | Y800 = 23.9 | V-0 | 27.5 | pHRR-25.9% THR-40.2% | [46] |
| PPDEG-EP | DDM | - | 203 | Y800 = 30.47 | V-0 | 32.1 | THR-21.0% | [48] |
| GPEP | DDM | 130.0 | 298.1 | Y800 = 31.0 | V-0 | 31.2 | pHRR-77.7% THR-65% | [49] |
| BEU-EP | DDM | 112.3 | 300.5 | Y700 = 23.4 | V-0 | 38.4 | pHRR-84.9% | [50] |
| TEUP-EP | DDM | 203.7 | 320.3 | Y800 = 43.8 | V-0 | 31.4 | pHRR-63.1% THR-57.4% | [51] |
| EP1 | DDM | 183 | 340 | Y700 = 53 | V-0 | 31.4 | - | [54] |
| EP2 | DDM | 214 | 353 | Y700 = 58 | V-0 | 32.8 | - | [54] |
| MDE | DDM | 178 | 361.4 | Y750 = 28.3 | V-0 | 44.9 | pHRR-64.5% THR-59.2% | [56] |
| DGEM | DDS | 279 | 402 | Y800 = 42.8 | V-0 | - | pHRR-70% THR-26% | [58] |
| MTEP | DDS | 326 | 377 | Y700 = 52.1 | V-0 | - | pHRR-56.7% | [59] |
| RESEP | DDM | 335 | 352.6 | Y800 = 38.6 | V-0 | 31.8 | - | [60] |
| DGEFA | DDM | 178.5 | 316.4 | Y700 = 40.6 | V-1 | 32.8 | pHRR-39% THR-43% | [61] |
| HCA-EP | DDM | 192.9 | 319.0 | Y700 = 31.6 | V-1 | 32.6 | pHRR-60% | [62] |
| DGEC | DDS | 300 | 324 | Y800 = 50.15 | V-0 | - | pHRR-74.8% THR-59.4% | [63] |
| GV-EP | DDM | 220 | 290.5 | Y800 = 44.9 | V-0 | 35.5 | pHRR-86.4% THR-48.1% | [64] |
| DGEBA | DDM | 187 | 368 | Y800 = 12.8 | no rating | 22.6 | pHRR = 646 W/g THR = 23.9 kJ/g | [64] |
| TVEP | difuran diamine | 162.7 | 275.4 | Y800 = 30.0 | V-0 | 28.5 | THR-52.6% | [65] |
| PH-ODA-EP | DDM | 204.9 | 280.1 | Y700 = 41.7 | V-0 | 40.5 | pHRR-92.9% THR-72.0% | [66] |
| DGED | DDM | 205 | 335 | Y800 = 42.9 | V-0 | 31.6 | - | [67] |
| DGEL | DDS | 314.4 | 379 | Y700 = 44.0 | V-0 | 32.5 | pHRR-74.5% THR-16.5% | [68] |
| DGEG | DDM | 223 | - | Y800 = 43.8 | V-0 | 33.1 | pHRR-36.9% THR-48.5% | [69] |
| DGEEA | DDM | 220 | 262 | Y800=38.5 | V-0 | 40.0 | pHRR-84.9% THR-70.1% | [70] |
| HMF-GU-EP | difuran diamine | 222 | 283 | Y800 = 55.8 | V-0 | 39.5 | pHRR-92.4% THR-73.1% | [71] |
| GSPZ-EP | DDM | 187 | 331 | Y700 = 42.3 | V-0 | - | pHRR-60.5% THR-54.4% | [72] |
| THMT-EP | DDS | 300 | 394 | Y800 = 44.8 | V-0 | 35.4 | pHRR-65.5% THR-29.3% | [73] |
| DGEBA | DDS | 180 | 391 | Y800 = 13.5 | no rating | 22.9 | pHRR = 423 W/g THR = 27.3 kJ/g | [73] |
| Triazole-VA-EP | DDM | 135 | - | Y800 = 34.7 | V-0 | 39.5 | pHRR-82.3% THR-52.8% | [74] |
| TDBE | DDM | 140 | 217 | Y700 = 29.8 | V-0 | 42.0 | pHRR-69.3% THR-53.0% | [38] |
| DGEBDB/ DGEBA = 1/9 | DDM | 176.4 | - | Y800 = 19.1 | V-1 | 30.2 | THR-11.9% | [39] |
| DGEBDB/ DGEBA = 2/8 | DDM | 169.2 | - | Y800 = 21.2 | V-0 | 32.4 | THR-18.3% | [39] |
| VDE | DDM | 129.6 | 248.8 | Y800 = 28 | V-0 | 34.5 | - | [40] |
| VSE | DDM | 176.1 | 254.9 | Y800 = 34.5 | V-0 | 38.7 | - | [40] |
| TEBA | DDM | 136 | 271 | Y700 = 29.9 | V-0 | 42.3 | pHRR-67% THR-27% | [41] |
| MEP/ DGEBA = 8/2 | DDM | 147.1 | 314.3 | - | V-0 | 27.5 | pHRR-34.7% THR-27.0% | [42] |
| VAD-EP/VDP-EP = 8/2 | D230 | 82.3 | 212.7 | Y800 = 32.8 | V-0 | 27.0 | - | [43] |
| VAD-EP/ VDP-EP = 7/3 | D230 | 80.3 | 205.3 | Y800 = 30.2 | V-0 | 28.7 | pHRR-47.9% THR-32.0% | [43] |
| EHEP | D230 | 122 | 270 | Y700 = 39.0 | V-0 | 31.0 | pHRR-66% THR-65% | [44] |
| HECarCP | DDM | - | - | Y800 = 14.3 | V-0 | 33.0 | pHRR-63% THR-24% | [45] |
| BEEP/DGEBA = 2/8 | DDM | 138.7 | 332.2 | Y800 = 23.9 | V-0 | 27.5 | pHRR-25.9% THR-40.2% | [46] |
| PPDEG-EP | DDM | - | 203 | Y800 = 30.47 | V-0 | 32.1 | THR-21.0% | [48] |
| GPEP | DDM | 130.0 | 298.1 | Y800 = 31.0 | V-0 | 31.2 | pHRR-77.7% THR-65% | [49] |
| BEU-EP | DDM | 112.3 | 300.5 | Y700 = 23.4 | V-0 | 38.4 | pHRR-84.9% | [50] |
| TEUP-EP | DDM | 203.7 | 320.3 | Y800 = 43.8 | V-0 | 31.4 | pHRR-63.1% THR-57.4% | [51] |
| EP1 | DDM | 183 | 340 | Y700 = 53 | V-0 | 31.4 | - | [54] |
| EP2 | DDM | 214 | 353 | Y700 = 58 | V-0 | 32.8 | - | [54] |
| MDE | DDM | 178 | 361.4 | Y750 = 28.3 | V-0 | 44.9 | pHRR-64.5% THR-59.2% | [56] |
| DGEM | DDS | 279 | 402 | Y800 = 42.8 | V-0 | - | pHRR-70% THR-26% | [58] |
| MTEP | DDS | 326 | 377 | Y700 = 52.1 | V-0 | - | pHRR-56.7% | [59] |
| RESEP | DDM | 335 | 352.6 | Y800 = 38.6 | V-0 | 31.8 | - | [60] |
| DGEFA | DDM | 178.5 | 316.4 | Y700 = 40.6 | V-1 | 32.8 | pHRR-39% THR-43% | [61] |
| HCA-EP | DDM | 192.9 | 319.0 | Y700 = 31.6 | V-1 | 32.6 | pHRR-60% | [62] |
| DGEC | DDS | 300 | 324 | Y800 = 50.15 | V-0 | - | pHRR-74.8% THR-59.4% | [63] |
| GV-EP | DDM | 220 | 290.5 | Y800 = 44.9 | V-0 | 35.5 | pHRR-86.4% THR-48.1% | [64] |
| DGEBA | DDM | 187 | 368 | Y800 = 12.8 | no rating | 22.6 | pHRR = 646 W/g THR = 23.9 kJ/g | [64] |
| TVEP | difuran diamine | 162.7 | 275.4 | Y800 = 30.0 | V-0 | 28.5 | THR-52.6% | [65] |
| PH-ODA-EP | DDM | 204.9 | 280.1 | Y700 = 41.7 | V-0 | 40.5 | pHRR-92.9% THR-72.0% | [66] |
| DGED | DDM | 205 | 335 | Y800 = 42.9 | V-0 | 31.6 | - | [67] |
| DGEL | DDS | 314.4 | 379 | Y700 = 44.0 | V-0 | 32.5 | pHRR-74.5% THR-16.5% | [68] |
| DGEG | DDM | 223 | - | Y800 = 43.8 | V-0 | 33.1 | pHRR-36.9% THR-48.5% | [69] |
| DGEEA | DDM | 220 | 262 | Y800 = 38.5 | V-0 | 40.0 | pHRR-84.9% THR-70.1% | [70] |
| HMF-GU-EP | difuran diamine | 222 | 283 | Y800 = 55.8 | V-0 | 39.5 | pHRR-92.4% THR-73.1% | [71] |
| GSPZ-EP | DDM | 187 | 331 | Y700 = 42.3 | V-0 | - | pHRR-60.5% THR-54.4% | [72] |
| THMT-EP | DDS | 300 | 394 | Y800 = 44.8 | V-0 | 35.4 | pHRR-65.5% THR-29.3% | [73] |
| DGEBA | DDS | 180 | 391 | Y800 = 13.5 | no rating | 22.9 | pHRR = 423 W/g THR = 27.3 kJ/g | [73] |
| Triazole-VA-EP | DDM | 135 | - | Y800 = 34.7 | V-0 | 39.5 | pHRR-82.3% THR-52.8% | [74] |
| Epoxy Resin | Curing Agent | Tg | Tx% (°C) | E′ (30 °C, MPa) | Flame Retardancy | Antibacterial Rate | Ref. | |
|---|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | |||||||
| SA-GA-EP | DIFFA | 204 | T10% = 300 | 2188.7 | V-0 | antibacterial | antibacterial | [155] |
| MGOL-EP-SC | - | 265 | Td5% = 360 T10% = 388 | 3789 | low flammability | 48.4% | - | [157] |
| MGOL-EP | DDM | 204 | Td5% = 347 T10% = 378 | 3884 | low flammability | 31.98% | - | [157] |
| DGEBA | DDM | 189 | Td5% = 383 T10% = 388 | 1727 | no rating, flammable | 13.1% | [157] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, X.; Wan, M.; Zhu, Y.; Zhang, K. Recent Development of Functional Bio-Based Epoxy Resins. Molecules 2024, 29, 4428. https://doi.org/10.3390/molecules29184428
Zhang Y, Liu X, Wan M, Zhu Y, Zhang K. Recent Development of Functional Bio-Based Epoxy Resins. Molecules. 2024; 29(18):4428. https://doi.org/10.3390/molecules29184428
Chicago/Turabian StyleZhang, Yuan, Xuemei Liu, Mengting Wan, Yanjie Zhu, and Kan Zhang. 2024. "Recent Development of Functional Bio-Based Epoxy Resins" Molecules 29, no. 18: 4428. https://doi.org/10.3390/molecules29184428
APA StyleZhang, Y., Liu, X., Wan, M., Zhu, Y., & Zhang, K. (2024). Recent Development of Functional Bio-Based Epoxy Resins. Molecules, 29(18), 4428. https://doi.org/10.3390/molecules29184428






