Electrospun Nanofiber-Based Biosensors for Foodborne Bacteria Detection
Abstract
1. Introduction
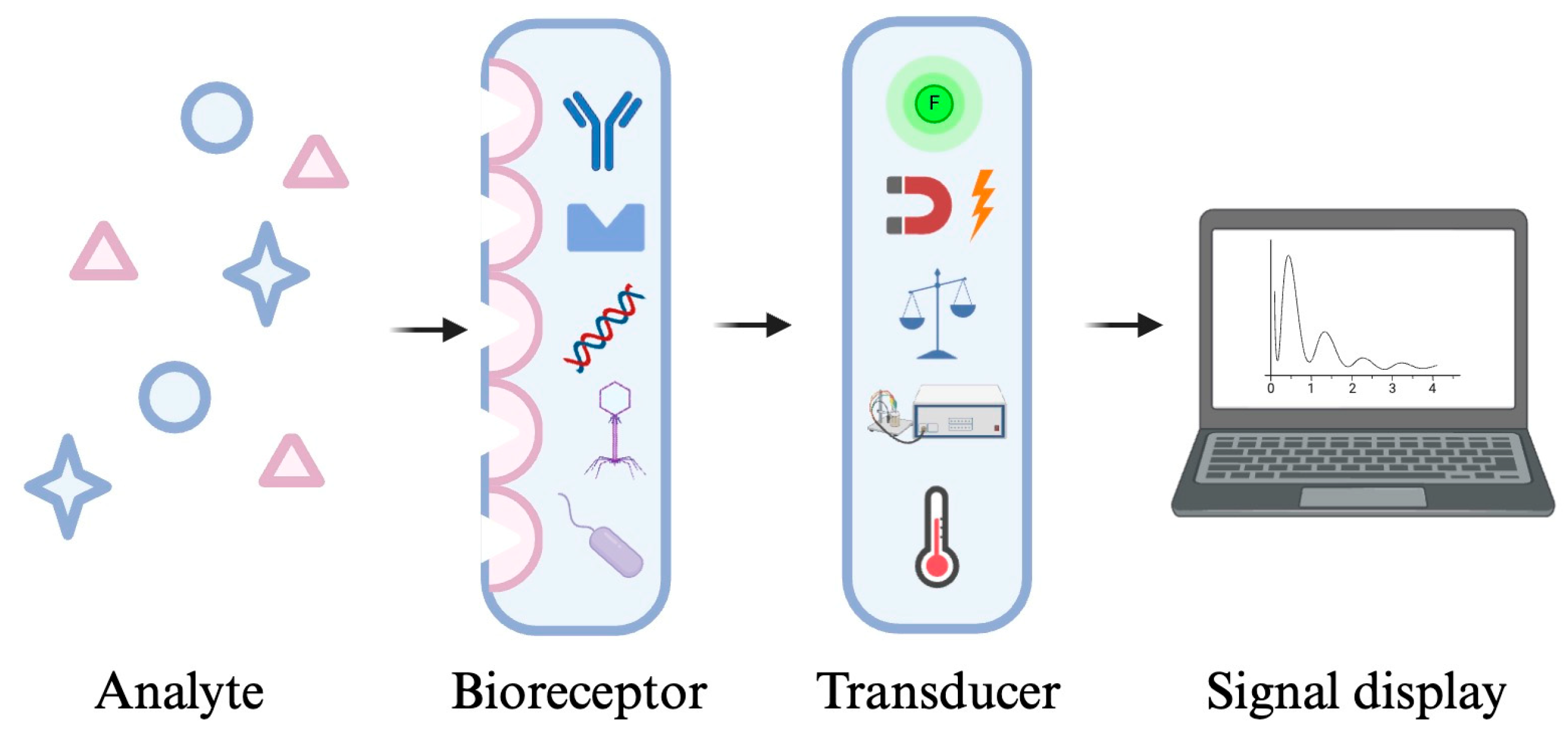
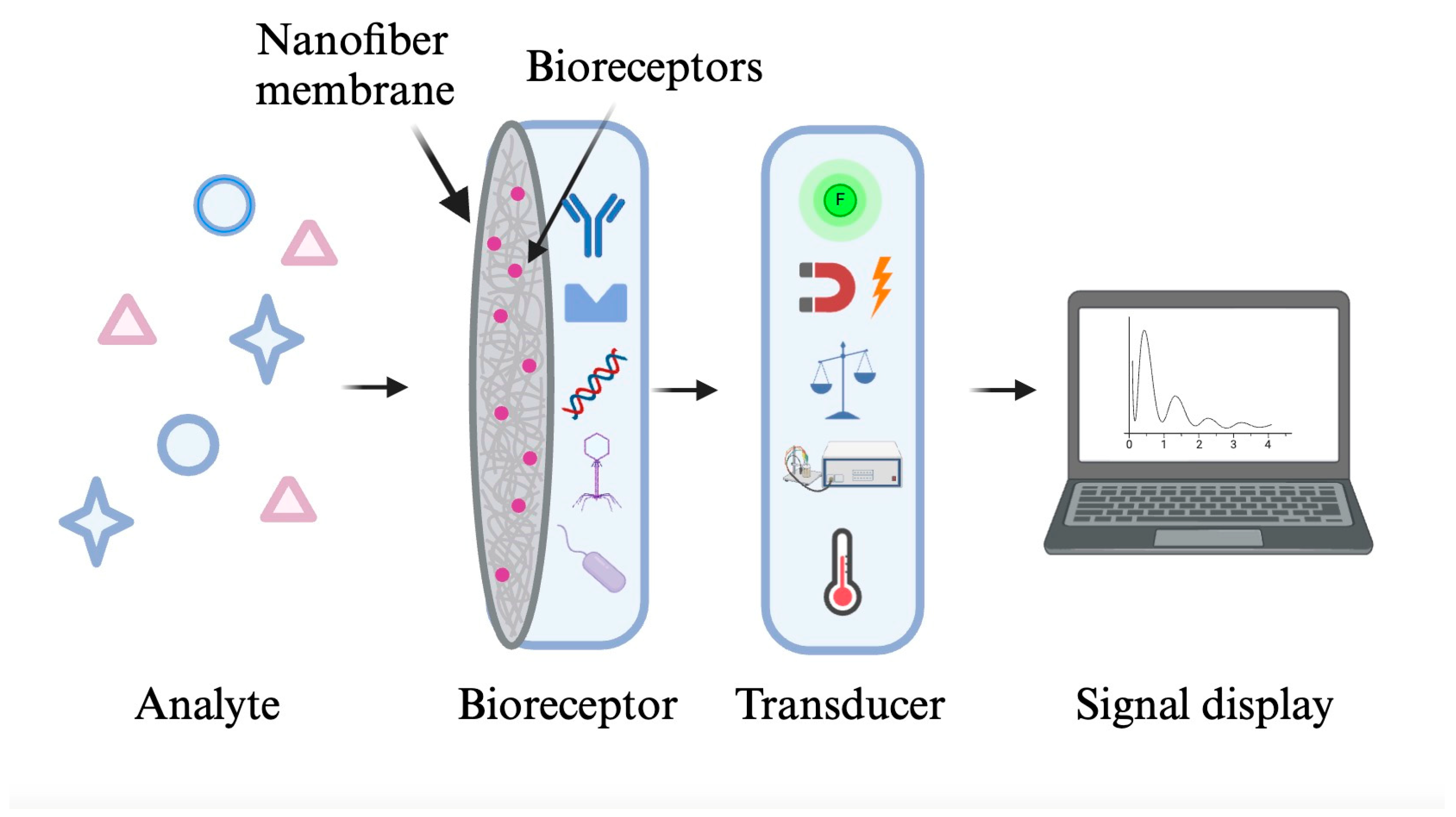
2. Biosensors
3. Electrospinning and Nanofibers
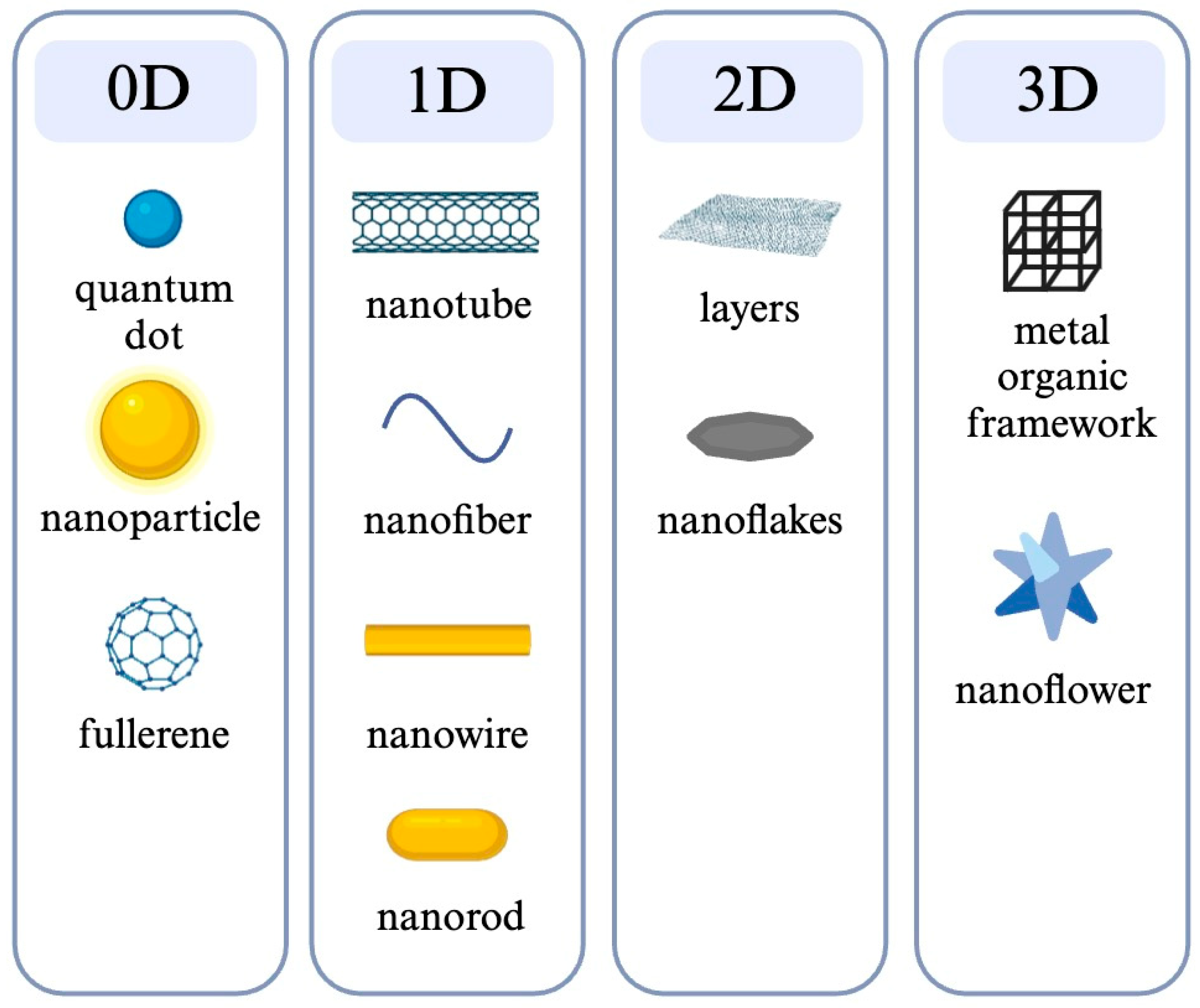
3.1. Nanofiber
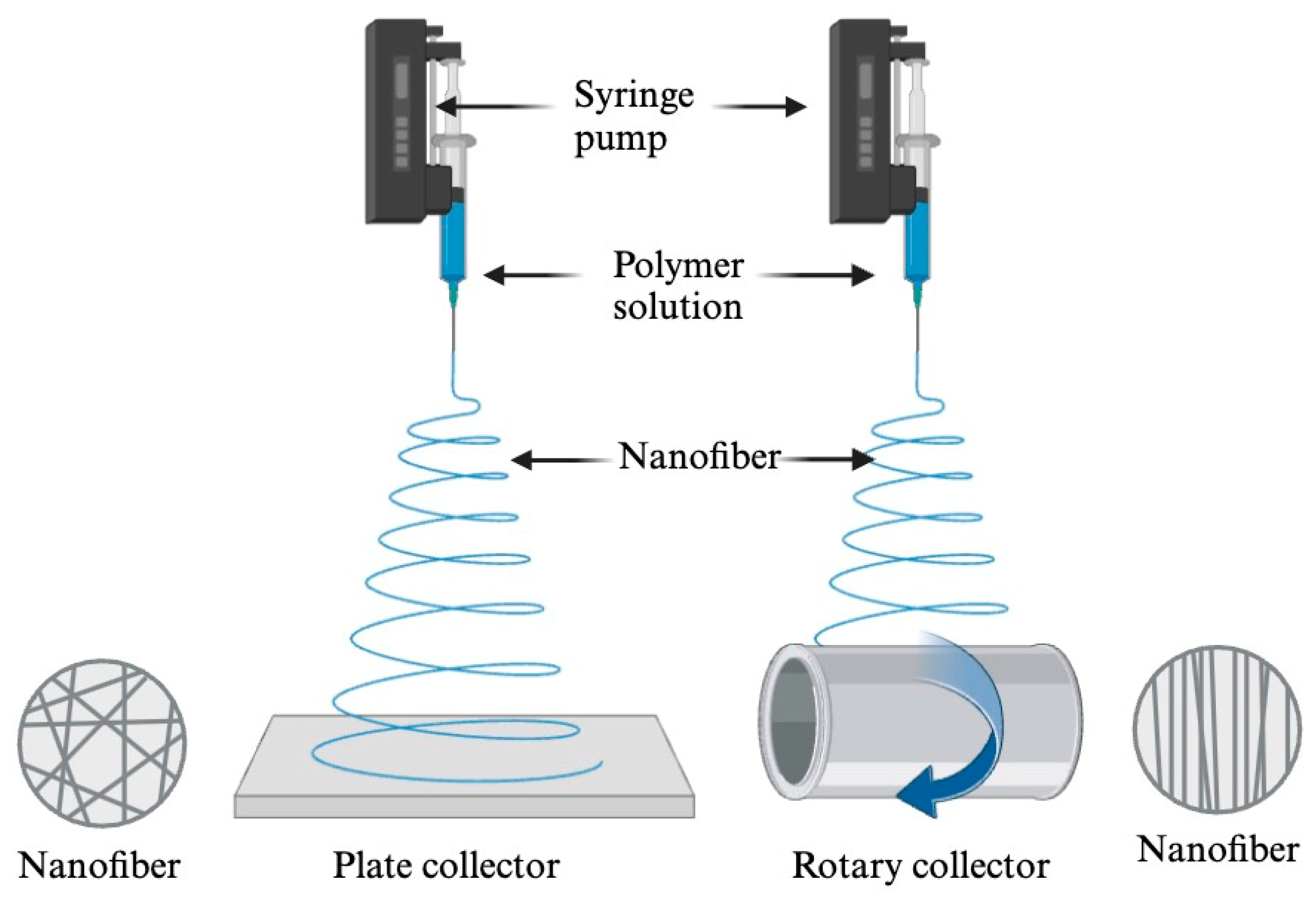
3.2. Electrospinning
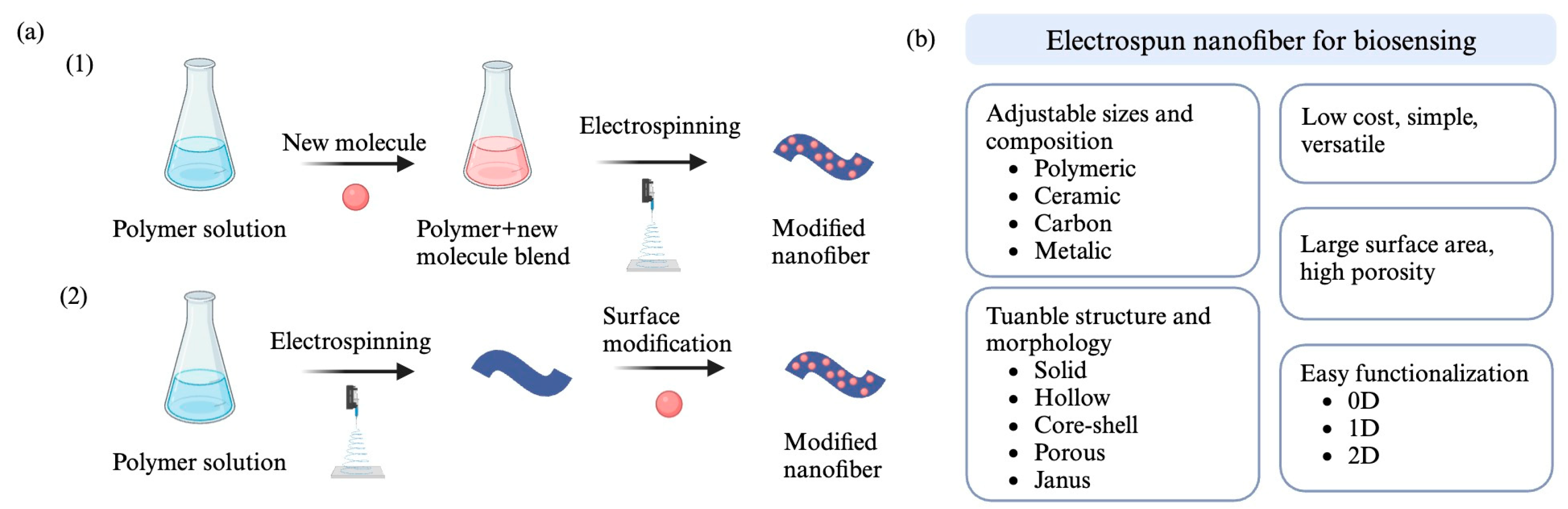
3.3. Electrospun Nanofibers in Biosensors
4. Application of Electrospun Nanofibers for Detecting Foodborne Bacteria
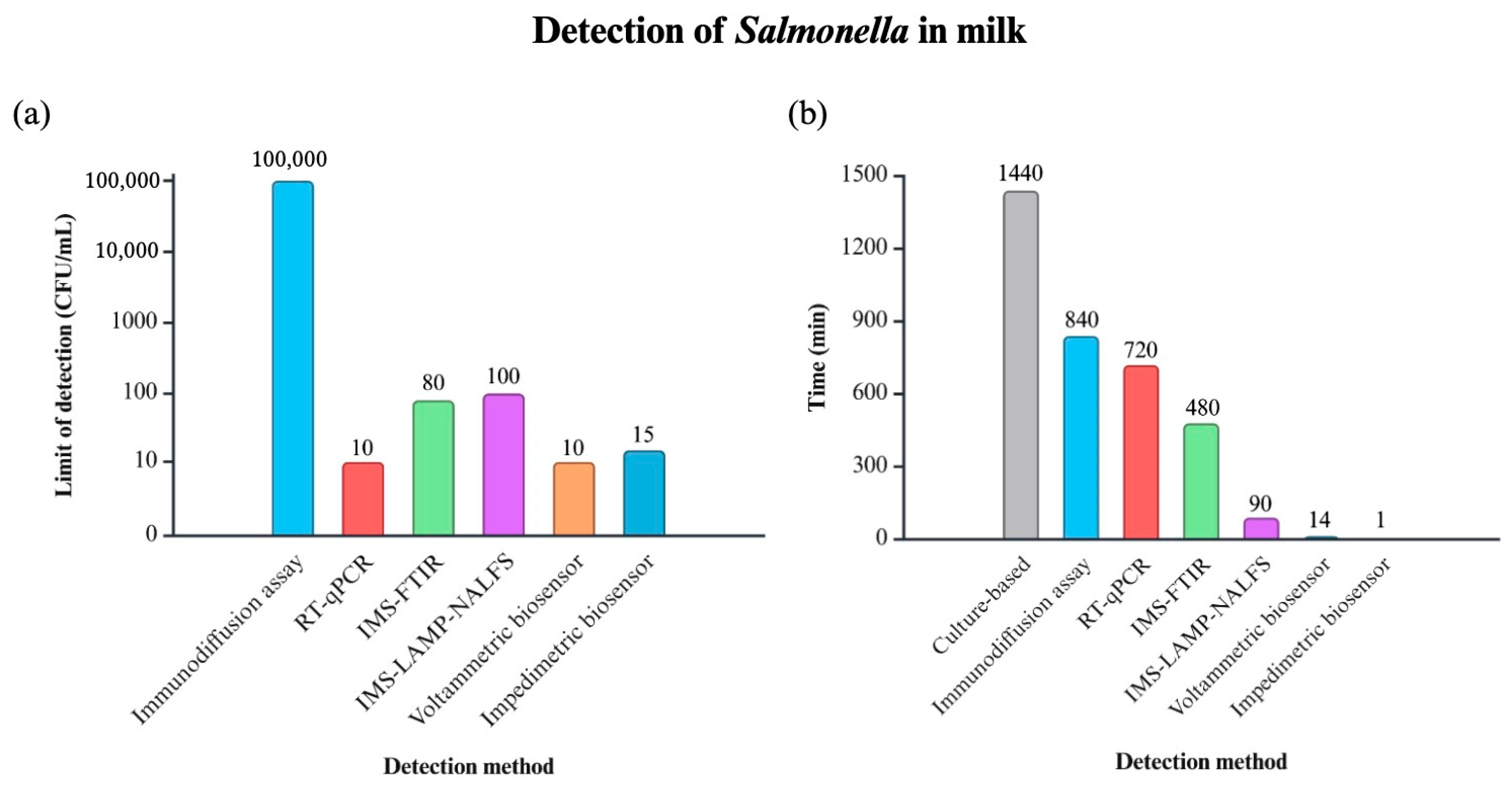
4.1. Salmonella
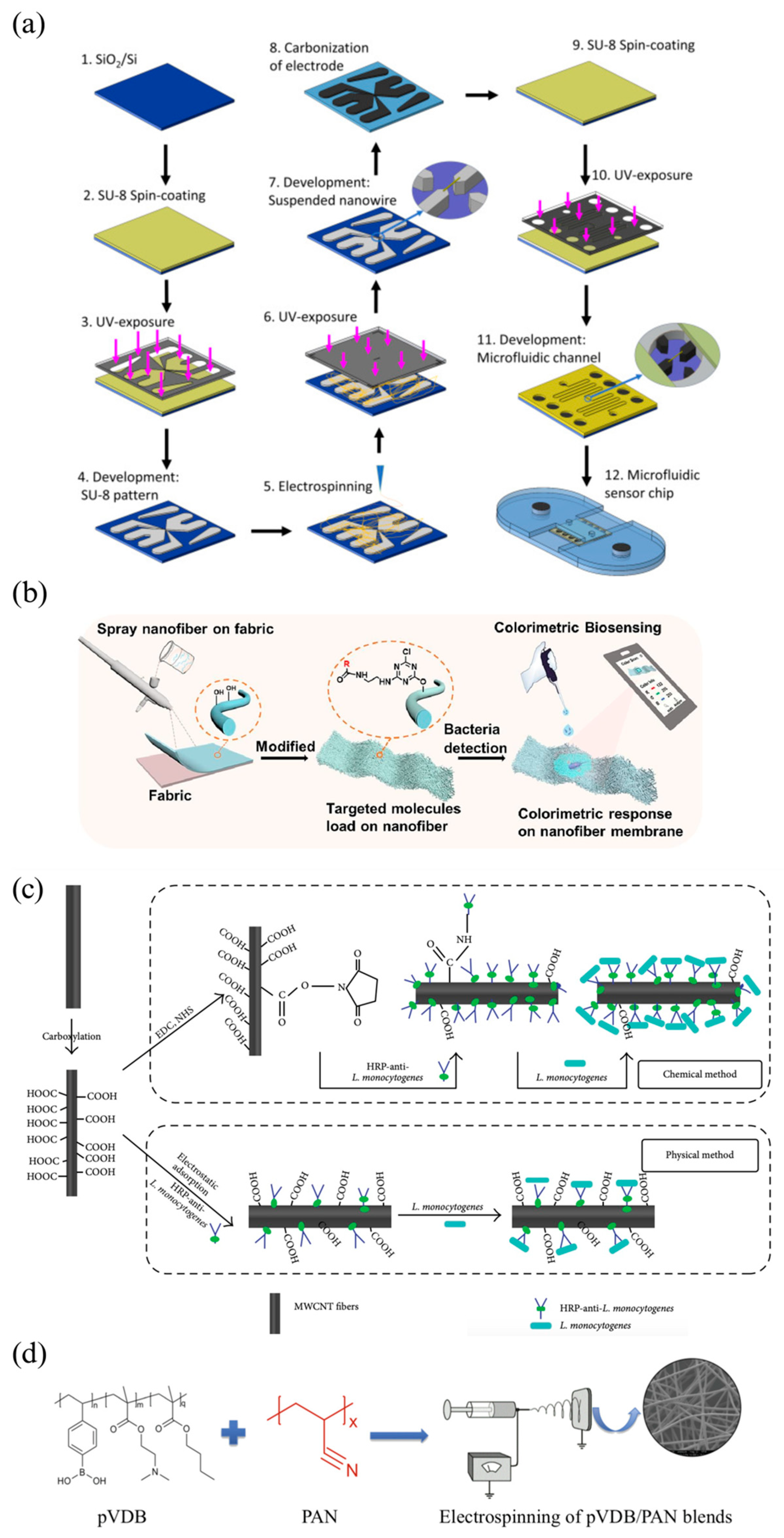
4.2. E. coli
4.3. L. monocytogenes
4.4. S. aureus and P. putida
5. Future Outlook
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Kamala, K.; Kumar, V.P. Chapter 1—Food Products and Food Contamination. In Microbial Contamination and Food Degradation; Holban, A.M., Grumezescu, A.M., Eds.; Handbook of Food Bioengineering; Academic Press: Cambridge, MA, USA, 2018; pp. 1–19. [Google Scholar] [CrossRef]
- WHO. Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 10 August 2024).
- Aladhadh, M. A Review of Modern Methods for the Detection of Foodborne Pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Detalle, L.; Vanheusden, K.; Sargentini-Maier, M.L.; Stöhr, T. 2.20—Translational Aspects in Drug Discovery. In Comprehensive Medicinal Chemistry III; Chackalamannil, S., Rotella, D., Ward, S.E., Eds.; Elsevier: Oxford, UK, 2017; pp. 495–529. [Google Scholar] [CrossRef]
- Espy, M.; Uhl, J.; Sloan, L.; Buckwalter, S.; Jones, M.; Vetter, E.; Yao, J.; Wengenack, N.; Rosenblatt, J.; Cockerill, F.; et al. Real-Time PCR in Clinical Microbiology: Applications for Routine Laboratory Testing. Clin. Microbiol. Rev. 2006, 19, 165–256. [Google Scholar] [CrossRef] [PubMed]
- Akkina, R.C.; Payala, V.; Maganti, S.S.; Akkina, R.C.; Payala, V.; Maganti, S.S. Tools for Rapid Detection and Control of Foodborne Microbial Pathogens. In Foodborne Pathogens—Recent Advances in Control and Detection; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Arduini, F.; Palleschi, G.; Rea, G. Biosensing Technology for Sustainable Food Safety. TrAC Trends Anal. Chem. 2014, 62, 1–10. [Google Scholar] [CrossRef]
- Kabiraz, M.P.; Majumdar, P.R.; Mahmud, M.M.C.; Bhowmik, S.; Ali, A. Conventional and Advanced Detection Techniques of Foodborne Pathogens: A Comprehensive Review. Heliyon 2023, 9, e15482. [Google Scholar] [CrossRef]
- Mercante, L.A.; Pavinatto, A.; Pereira, T.S.; Migliorini, F.L.; dos Santos, D.M.; Correa, D.S. Nanofibers Interfaces for Biosensing: Design and Applications. Sens. Actuators Rep. 2021, 3, 100048. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef]
- Pokropivny, V.V.; Skorokhod, V.V. Classification of Nanostructures by Dimensionality and Concept of Surface Forms Engineering in Nanomaterial Science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Park, S.-H.; Ryu, S.; Kang, D.-H. Development of an Improved Selective and Differential Medium for Isolation of Salmonella spp. J. Clin. Microbiol. 2020, 50, 3222–3226. [Google Scholar] [CrossRef]
- Lee, K.-M.; Runyon, M.; Herrman, T.J.; Phillips, R.; Hsieh, J. Review of Salmonella Detection and Identification Methods: Aspects of Rapid Emergency Response and Food Safety. Food Control 2015, 47, 264–276. [Google Scholar] [CrossRef]
- Bai, Y.; Cui, Y.; Suo, Y.; Shi, C.; Wang, D.; Shi, X. A Rapid Method for Detection of Salmonella in Milk Based on Extraction of mRNA Using Magnetic Capture Probes and RT-qPCR. Front. Microbiol. 2019, 10, 770. [Google Scholar] [CrossRef]
- Koluman, A.; Celik, G.; Unlu, T. Salmonella Identification from Foods in Eight Hours: A Prototype Study with Salmonella Typhimurium. Iran. J. Microbiol. 2012, 4, 15–24. [Google Scholar] [PubMed]
- Li, Q.; Zhang, J.; Chen, X.; Jiang, T.; Lin, L.; Zhao, L. Real-Time and Visual Detection of Viable Salmonella in Milk from Remote Pasture via IMS-LAMP-NALFS. Microchem. J. 2024, 197, 109732. [Google Scholar] [CrossRef]
- Hou, Y.; Tang, W.; Qi, W.; Guo, X.; Lin, J. An Ultrasensitive Biosensor for Fast Detection of Salmonella Using 3D Magnetic Grid Separation and Urease Catalysis. Biosens. Bioelectron. 2020, 157, 112160. [Google Scholar] [CrossRef]
- Malvano, F.; Pilloton, R.; Albanese, D. A Novel Impedimetric Biosensor Based on the Antimicrobial Activity of the Peptide Nisin for the Detection of Salmonella spp. Food Chem. 2020, 325, 126868. [Google Scholar] [CrossRef]
- Halica, K.; Cabaj, J. Electrospun Nanofibers for Sensing and Biosensing Applications-A Review. Int. J. Mol. Sci. 2021, 22, 6357. [Google Scholar] [CrossRef]
- Ji, G.; Chen, Z.; Li, H.; Awuye, D.E.; Guan, M.; Zhu, Y. Electrospinning-Based Biosensors for Health Monitoring. Biosensors 2022, 12, 876. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Luo, Z.; Lu, Y. Conventional and Emerging Techniques for Detection of Foodborne Pathogens in Horticulture Crops: A Leap to Food Safety. Food Bioprocess Technol. 2022, 15, 1248–1267. [Google Scholar] [CrossRef]
- Baldassarre, A.; Mucci, N.; Lecca, L.I.; Tomasini, E.; Parcias-do-Rosario, M.J.; Pereira, C.T.; Arcangeli, G.; Oliveira, P.A.B. Biosensors in Occupational Safety and Health Management: A Narrative Review. Int. J. Environ. Res. Public Health 2020, 17, 2461. [Google Scholar] [CrossRef]
- Samota, S.; Rani, R.; Chakraverty, S.; Kaushik, A. Biosensors for Simplistic Detection of Pathogenic Bacteria: A Review with Special Focus on Field-Effect Transistors. Mater. Sci. Semicond. Process. 2022, 141, 106404. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Lu, X.; Zheng, X.; Yang, Z. Recent Advances in Electrochemical Biosensors for the Detection of Foodborne Pathogens: Current Perspective and Challenges. Foods 2023, 12, 2795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, J.; Du, X. Electrochemical Biosensors for Detection of Foodborne Pathogens. Micromachines 2019, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Fan, C.; Chen, M.; Zhang, X.; Yan, Q.; Wang, Y.; Zhang, S.; Gong, Z.; Shi, L.; Li, X.; et al. Recent Advances of Fluorescent Biosensors Based on Cyclic Signal Amplification Technology in Biomedical Detection. J. Nanobiotechnol. 2021, 19, 403. [Google Scholar] [CrossRef]
- Zhao, V.X.T.; Wong, T.I.; Zheng, X.T.; Tan, Y.N.; Zhou, X. Colorimetric Biosensors for Point-of-Care Virus Detections. Mater. Sci. Energy Technol. 2020, 3, 237–249. [Google Scholar] [CrossRef]
- Nejadmansouri, M.; Majdinasab, M.; Nunes, G.S.; Marty, J.L. An Overview of Optical and Electrochemical Sensors and Biosensors for Analysis of Antioxidants in Food during the Last 5 Years. Sensors 2021, 21, 1176. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, M.; Chen, Z.; Liu, L.; Liu, Y.; Yang, W.; Ramakrishna, S. A Review on Recent Advances in Application of Electrospun Nanofiber Materials as Biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 174–189. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Issaabadi, Z.; Sajjadi, M.; Sajadi, S.M.; Atarod, M. Types of Nanostructures. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 29–80. [Google Scholar] [CrossRef]
- Gugulothu, D.; Barhoum, A.; Afzal, S.M.; Venkateshwarlu, B.; Uludag, H. Structural Multifunctional Nanofibers and Their Emerging Applications. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 693–732. [Google Scholar] [CrossRef]
- Terra, I.A.A.; Mercante, L.A.; Andre, R.S.; Correa, D.S. Fluorescent and Colorimetric Electrospun Nanofibers for Heavy-Metal Sensing. Biosensors 2017, 7, 61. [Google Scholar] [CrossRef]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005. [Google Scholar]
- Akhavan-Mahdavi, S.; Mirbagheri, M.S.; Assadpour, E.; Sani, M.A.; Zhang, F.; Jafari, S.M. Electrospun Nanofiber-Based Sensors for the Detection of Chemical and Biological Contaminants/Hazards in the Food Industries. Adv. Colloid Interface Sci. 2024, 325, 103111. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun Nanofibers: New Generation Materials for Advanced Applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Smoak, M.M.; Hogan, K.J.; Grande-Allen, K.J.; Mikos, A.G. Bioinspired Electrospun dECM Scaffolds Guide Cell Growth and Control the Formation of Myotubes. Sci. Adv. 2021, 7, eabg4123. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer Nanofibers Assembled by Electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Kenry; Lim, C.T. Nanofiber Technology: Current Status and Emerging Developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Nie, G.; Zhang, Z.; Wang, T.; Wang, C.; Kou, Z. Electrospun One-Dimensional Electrocatalysts for Oxygen Reduction Reaction: Insights into Structure–Activity Relationship. ACS Appl. Mater. Interfaces 2021, 13, 37961–37978. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J. Electrospinning for the Manufacture of Biosensor Components: A Mini-Review. Med. DEVICES Sens. 2021, 4, e10136. [Google Scholar] [CrossRef]
- Sundera Murthe, S.; Mohamed Saheed, M.S.; Perumal, V.; Mohamed Saheed, M.S.; Mohamed, N.M. 11—Electrospun Nanofibers for Biosensing Applications. In Nanobiosensors for Biomolecular Targeting; Gopinath, S.C.B., Lakshmipriya, T., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–267. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Recent Advancements in Nanobiosensors: Current Trends, Challenges, Applications, and Future Scope. Biosensors 2022, 12, 892. [Google Scholar] [CrossRef]
- Jiménez-Rodríguez, M.G.; Silva-Lance, F.; Parra-Arroyo, L.; Medina-Salazar, D.A.; Martínez-Ruiz, M.; Melchor-Martínez, E.M.; Martínez-Prado, M.A.; Iqbal, H.M.N.; Parra-Saldívar, R.; Barceló, D.; et al. Biosensors for the Detection of Disease Outbreaks through Wastewater-Based Epidemiology. Trends Anal. Chem. TRAC 2022, 155, 116585. [Google Scholar] [CrossRef]
- Chen, J.; Rong, F.; Xie, Y. Fabrication, Microstructures and Sensor Applications of Highly Ordered Electrospun Nanofibers: A Review. Materials 2023, 16, 3310. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Brenes-Acuña, M.; Castro-Rojas, A.; Cordero-Salmerón, R.; Lopretti-Correa, M.; Vega-Baudrit, J.R. Biosensors for the Detection of Bacterial and Viral Clinical Pathogens. Sensors 2020, 20, 6926. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Z.; Wang, X.; Hu, H.; Wu, M. MXene/Carbon Composites for Electrochemical Energy Storage and Conversion. Mater. Today Sustain. 2023, 22, 100350. [Google Scholar] [CrossRef]
- Wu, L.; Song, Y.; Xing, S.; Li, Y.; Xu, H.; Yang, Q.; Li, Y. Advances in Electrospun Nanofibrous Membrane Sensors for Ion Detection. RSC Adv. 2022, 12, 34866–34891. [Google Scholar] [CrossRef] [PubMed]
- Sinha Ray, S.; Chen, S.-S.; Li, C.-W.; Cong Nguyen, N.; Thi Nguyen, H. A Comprehensive Review: Electrospinning Technique for Fabrication and Surface Modification of Membranes for Water Treatment Application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Thiha, A.; Ibrahim, F.; Muniandy, S.; Dinshaw, I.J.; Teh, S.J.; Thong, K.L.; Leo, B.F.; Madou, M. All-Carbon Suspended Nanowire Sensors as a Rapid Highly-Sensitive Label-Free Chemiresistive Biosensing Platform. Biosens. Bioelectron. 2018, 107, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Fathi, S.; Saber, R.; Adabi, M.; Rasouli, R.; Douraghi, M.; Morshedi, M.; Farid-Majidi, R. Novel Competitive Voltammetric Aptasensor Based on Electrospun Carbon Nanofibers-Gold Nanoparticles Modified Graphite Electrode for Salmonella Enterica Serovar Detection. Biointerface Res. Appl. Chem. 2020, 11, 8702–8715. [Google Scholar] [CrossRef]
- Guler Gokce, Z.; Akalın, P.; Kok, F.N.; Sarac, A.S. Impedimetric DNA Biosensor Based on Polyurethane/Poly(m-Anthranilic Acid) Nanofibers. Sens. Actuators B Chem. 2018, 254, 719–726. [Google Scholar] [CrossRef]
- Nicolini, A.M.; Fronczek, C.F.; Yoon, J.-Y. Droplet-Based Immunoassay on a ‘Sticky’ Nanofibrous Surface for Multiplexed and Dual Detection of Bacteria Using Smartphones. Biosens. Bioelectron. 2015, 67, 560–569. [Google Scholar] [CrossRef]
- Li, W.; Sun, W.; Jia, L.; Dong, Y.; Wu, L.; Saldaña, M.D.A.; Sun, W. Poly-l-Lactic Acid (PLLA)/Anthocyanin Nanofiber Color Indicator Film for Headspace Detection of Low-Level Bacterial Concentration. Int. J. Biol. Macromol. 2022, 215, 123–131. [Google Scholar] [CrossRef]
- Zhang, J.; Hurren, C.; Lu, Z.; Wang, D. Nanofiber-Based Colorimetric Platform for Point-of-Care Detection of E. coli. Chem. Eng. J. 2023, 463, 142357. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Goodall, E.; Popat, K.C.; Zou, L.; Li, Y.V. Selective Detection of Gram-Negative Bacteria and Antibacterial Properties of Colorimetric Polydiacetylene Nanofibers. J. Mater. Sci. 2023, 58, 8261–8273. [Google Scholar] [CrossRef]
- Shaibani, P.M.; Jiang, K.; Haghighat, G.; Hassanpourfard, M.; Etayash, H.; Naicker, S.; Thundat, T. The Detection of Escherichia coli (E. coli) with the pH Sensitive Hydrogel Nanofiber-Light Addressable Potentiometric Sensor (NF-LAPS). Sens. Actuators B Chem. 2016, 226, 176–183. [Google Scholar] [CrossRef]
- Shaibani, P.M.; Etayash, H.; Jiang, K.; Sohrabi, A.; Hassanpourfard, M.; Naicker, S.; Sadrzadeh, M.; Thundat, T. Portable Nanofiber-Light Addressable Potentiometric Sensor for Rapid Escherichia coli Detection in Orange Juice. ACS Sens. 2018, 3, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Nartker, S.; Miller, H.; Hochhalter, D.; Wiederoder, M.; Wiederoder, S.; Setterington, E.; Drzal, L.T.; Alocilja, E.C. Surface Functionalization of Electrospun Nanofibers for Detecting E. coli O157:H7 and BVDV Cells in a Direct-Charge Transfer Biosensor. Biosens. Bioelectron. 2010, 26, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Nartker, S.; Wiederoder, M.; Miller, H.; Hochhalter, D.; Drzal, L.T.; Alocilja, E.C. Novel Biosensor Based on Electrospun Nanofiber and Magnetic Nanoparticles for the Detection of E. coli O157:H7. IEEE Trans. Nanotechnol. 2012, 11, 676–681. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Zhao, Y.; Li, W.; Qiu, L.; Li, L. A Novel and Disposable Enzyme-Labeled Amperometric Immunosensor Based on MWCNT Fibers for Listeria monocytogenes Detection. J. Nanomater. 2016, 2016, e3895920. [Google Scholar] [CrossRef]
- Wang, R.; Ruan, C.; Kanayeva, D.; Lassiter, K.; Li, Y. TiO2 Nanowire Bundle Microelectrode Based Impedance Immunosensor for Rapid and Sensitive Detection of Listeria Monocytogenes. Nano Lett. 2008, 8, 2625–2631. [Google Scholar] [CrossRef]
- Quirós, J.; Amaral, A.J.R.; Pasparakis, G.; Williams, G.R.; Rosal, R. Electrospun Boronic Acid-Containing Polymer Membranes as Fluorescent Sensors for Bacteria Detection. React. Funct. Polym. 2017, 121, 23–31. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa Oil/Chitosan Nanoparticles Embedded Gelatin Nanofibers for Food Packaging against Listeria monocytogenes and Staphylococcus aureus on Cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Kim, M.O.; Khan, M.Q.; Ullah, A.; Phan, D.-N.; Zhu, C.; Lee, J.-S.; Kim, I.S. Fabrication and Characterization of Colorimetric Polymer Based Novel Nanofibers for Sensing and Blocking of Bacterial. Mater. Res. Express 2020, 7, 085405. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Mustapha, A. Rapid and Simultaneous Quantitation of Escherichia coli O157:H7, Salmonella, and Shigella in Ground Beef by Multiplex Real-Time PCR and Immunomagnetic Separation. J. Food Prot. 2007, 70, 1366–1372. [Google Scholar] [CrossRef]
- Chen, S.; Wang, F.; Beaulieu, J.C.; Stein, R.E.; Ge, B. Rapid Detection of Viable Salmonellae in Produce by Coupling Propidium Monoazide with Loop-Mediated Isothermal Amplification. Appl. Environ. Microbiol. 2011, 77, 4008–4016. [Google Scholar] [CrossRef]
- Villamizar, R.A.; Maroto, A.; Rius, F.X.; Inza, I.; Figueras, M.J. Fast Detection of Salmonella Infantis with Carbon Nanotube Field Effect Transistors. Biosens. Bioelectron. 2008, 24, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Sayad, A.; Ibrahim, F.; Mukim Uddin, S.; Cho, J.; Madou, M.; Thong, K.L. A Microdevice for Rapid, Monoplex and Colorimetric Detection of Foodborne Pathogens Using a Centrifugal Microfluidic Platform. Biosens. Bioelectron. 2018, 100, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Etayash, H.; Azmi, S.; Naicker, S.; Hassanpourfard, M.; Shaibani, P.M.; Thakur, G.; Kaur, K.; Thundat, T. Rapid Label-Free Detection of E. coli Using Antimicrobial Peptide Assisted Impedance Spectroscopy. Anal. Methods 2015, 7, 9744–9748. [Google Scholar] [CrossRef][Green Version]
- Xue, C.; Yang, C.; Xu, T.; Zhan, J.; Li, X. A Wireless Bio-Sensing Microfluidic Chip Based on Resonating “μ-Divers”. Lab Chip 2015, 15, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, V.; Bhunia, A.K.; Tu, S.-I.; Paoli, G.C.; Brewster, J.D. SPR Biosensor for the Detection of L. monocytogenes Using Phage-Displayed Antibody. Biosens. Bioelectron. 2007, 23, 248–252. [Google Scholar] [CrossRef]
- Magliulo, M.; Simoni, P.; Guardigli, M.; Michelini, E.; Luciani, M.; Lelli, R.; Roda, A. A Rapid Multiplexed Chemiluminescent Immunoassay for the Detection of Escherichia coli O157:H7, Yersinia Enterocolitica, Salmonella Typhimurium, and Listeria Monocytogenes Pathogen Bacteria. J. Agric. Food Chem. 2007, 55, 4933–4939. [Google Scholar] [CrossRef]
- Beumer, R.R.; Brinkman, E. Detection of Listeria spp. with a Monoclonal Antibody-Based Enzyme-Linked Immunosorbent Assay (ELISA). Food Microbiol. 1989, 6, 171–177. [Google Scholar] [CrossRef]
- Khazaei, M.; Hosseini, M.S.; Haghighi, A.M.; Misaghi, M. Nanosensors and Their Applications in Early Diagnosis of Cancer. Sens. Bio-Sens. Res. 2023, 41, 100569. [Google Scholar] [CrossRef]
- Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Nanomaterial-Based Biosensors for Sensing Key Foodborne Pathogens: Advances from Recent Decades. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1465–1487. [Google Scholar] [CrossRef]
| Detection Method | Description | Types | Advantages | Disadvantages | |
|---|---|---|---|---|---|
| Conventional methods | Culture-based method | Traditional method where bacteria are grown on selective media to detect and quantify viable organisms. Colonies are counted after incubation to determine bacterial load. | Pre-enrichment, selective enrichment | Cost-effective, selective and distinctive | Time-consuming (18–24 h or several days), labor intensive |
| Biochemical test method | A growth-promoting method where specific compounds are used as indicators of pathogen presence. | Oxidase test, catalase test, indole production test, methyl red, blood agar plates, motility agar, etc. | Accurate, high specificity | Slow | |
| Immunological based method | Uses antibodies to detect specific bacterial antigens. | Enzyme-linked immunosorbent assay (ELISA), lateral flow immunoassay, immunofluorescence assay, immunomagnetic separation, latex agglutination, immunodiffusion assays, etc. | Highly specific and can be rapid | Expensive, requires pre-enrichment steps, false positive results | |
| Nucleic acid based method | Detects bacteria by amplifying or identifying their DNA or RNA | Polymerase chain reaction (PCR), multiplex PCR, real-time PCR, quantitative real-time PCR (qPCR), and reverse transcriptase PCR | Highly sensitive, specific, and faster than culture methods. | Expensive, requires specialized equipment, and may not distinguish between live and dead bacteria. | |
| Novel methods | Hybridization-based method | Uses complementary nucleic acid probes to bind to specific bacterial DNA or RNA sequences, enabling detection. | Fluorometric, colorimetric, electrochemical, and chemiluminescent | Rapid, stable, and sensitive | Requires instrumentation |
| Array-based method | Involves immobilizing multiple probes on a solid surface to detect several bacterial species or genes simultaneously. | DNA microarray, alternative array-based detection | Rapid, sensitive, high accuracy, and throughput | Confusion of first-time users, non-reproducible results | |
| Spectroscopy technique | Uses light absorption, scattering, or emission to detect bacterial components, such as lipids, proteins, or nucleic acids. | Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, Hyperspectral imaging techniques (HSI) | Sensitive, rapid | Time-consuming, and interference with fluorescence | |
| Loop-mediated isothermal amplification (LAMP) | A nucleic acid amplification method that uses four sets of primers to identify six distinct zones on the targeted gene. | LAMP | Rapid, sensitive, reliable, do not require trained personnel | Low throughput | |
| Biosensor | Combines biological recognition elements (e.g., antibodies, enzymes, or DNA) with a transducer to detect bacteria through electrical, optical, or chemical signals. | Electrochemical biosensor (Amperometric, Voltammetric, Potentiometric, Impedimetric), Optical biosensor (Colorimetric, Fluorescent, Surface plasmon resonance (SPR) biosensor, Surface-enhanced Raman scattering (SERS)), Mass-sensitive biosensor (Piezoelectric, Magnetostrictive) | Easy, low cost, rapid, and highly selective | Still under development for commercialization in foodborne pathogen detection, and may have limitations in complex food matrices |
| Target Foodborne Bacteria | Detection Method | Nanofiber Composition | Food Marix | LOD | Response Time |
|---|---|---|---|---|---|
| (CFU/mL) | |||||
| Salmonella Typhimurium [51] | Chemiresistive | SU-8 photoresist | Beef | 10 | 5 min |
| Salmonella [52] | Differential pulse voltammetry | GE-MB/Au NPs/CNFs/Chi | Full-fat milk | 1.223 | - |
| Salmonella [53] | Impedimetric | PU/P3ANA | - | - | - |
| Salmonella, E. coli [54] | Immunoassay | PCL | - | 10² | 12 min |
| E. coli, | Colorimetric | PLLA/anthocyanin | - | 10² | - |
| L. monocytogenes [55] | |||||
| E. coli [56] | Colorimetric | PVA-co-PE | - | 26 | 15 min |
| Cellulose acetate butyrate | 69 | 30 min | |||
| E. coli [57] | Colorimetric | PDA | - | - | 30 min−1 h |
| E. coli [58] | Potentiometric | PAA/PVA | Water | 20 | - |
| E. coli [59] | Potentiometric | PAA/PVA | Orange juice | 10² | <1 h |
| E. coli [60] | Conductometric | PVDC/NC | Water | 61 | 8 min |
| E. coli [61] | Magnetic immunoassay | CN NFs/MNPs | - | 67 | 8 min |
| L. monocytogenes [62] | Amperometric | MWCNT | Milk | 1.07 × 102 | - |
| L. monocytogenes [63] | Impedimetric | TiO2 | - | 4.7 × 102 | 50 min |
| S. aureus, P. putida [64] | Fluorescence | PAN/pVDB | - | - | - |
| S. aureus [65] | Colorimetric | MO@CNPs/Gelatin nanofibers | Cheese | - | - |
| S. aureus [66] | Colorimetric | PDA/PU | - | 45 × 102 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Yan, S.; Yang, T. Electrospun Nanofiber-Based Biosensors for Foodborne Bacteria Detection. Molecules 2024, 29, 4415. https://doi.org/10.3390/molecules29184415
Yang H, Yan S, Yang T. Electrospun Nanofiber-Based Biosensors for Foodborne Bacteria Detection. Molecules. 2024; 29(18):4415. https://doi.org/10.3390/molecules29184415
Chicago/Turabian StyleYang, Haoming, Song Yan, and Tianxi Yang. 2024. "Electrospun Nanofiber-Based Biosensors for Foodborne Bacteria Detection" Molecules 29, no. 18: 4415. https://doi.org/10.3390/molecules29184415
APA StyleYang, H., Yan, S., & Yang, T. (2024). Electrospun Nanofiber-Based Biosensors for Foodborne Bacteria Detection. Molecules, 29(18), 4415. https://doi.org/10.3390/molecules29184415








