Enzymatic Hydrolysis as an Effective Method for Obtaining Wheat Gluten Hydrolysates Combining Beneficial Functional Properties with Health-Promoting Potential
Abstract
1. Introduction
2. Results and Discussion
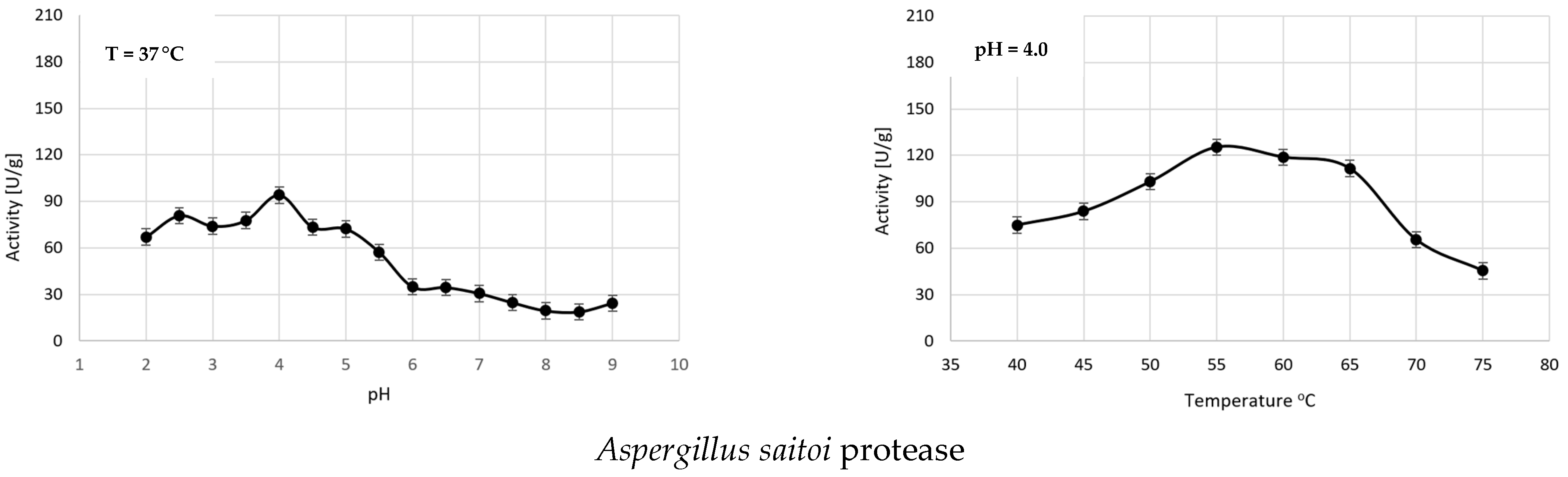
2.1. Optimization of Enzymatic Gluten Hydrolysis Conditions
2.2. Determination of the Maximum Activity and Optimal Dose of Proteases for WG Hydrolysis
2.3. The Effect of Protease Dose on the Gluten Hydrolysis Degree and Protein Recovery in WGHs
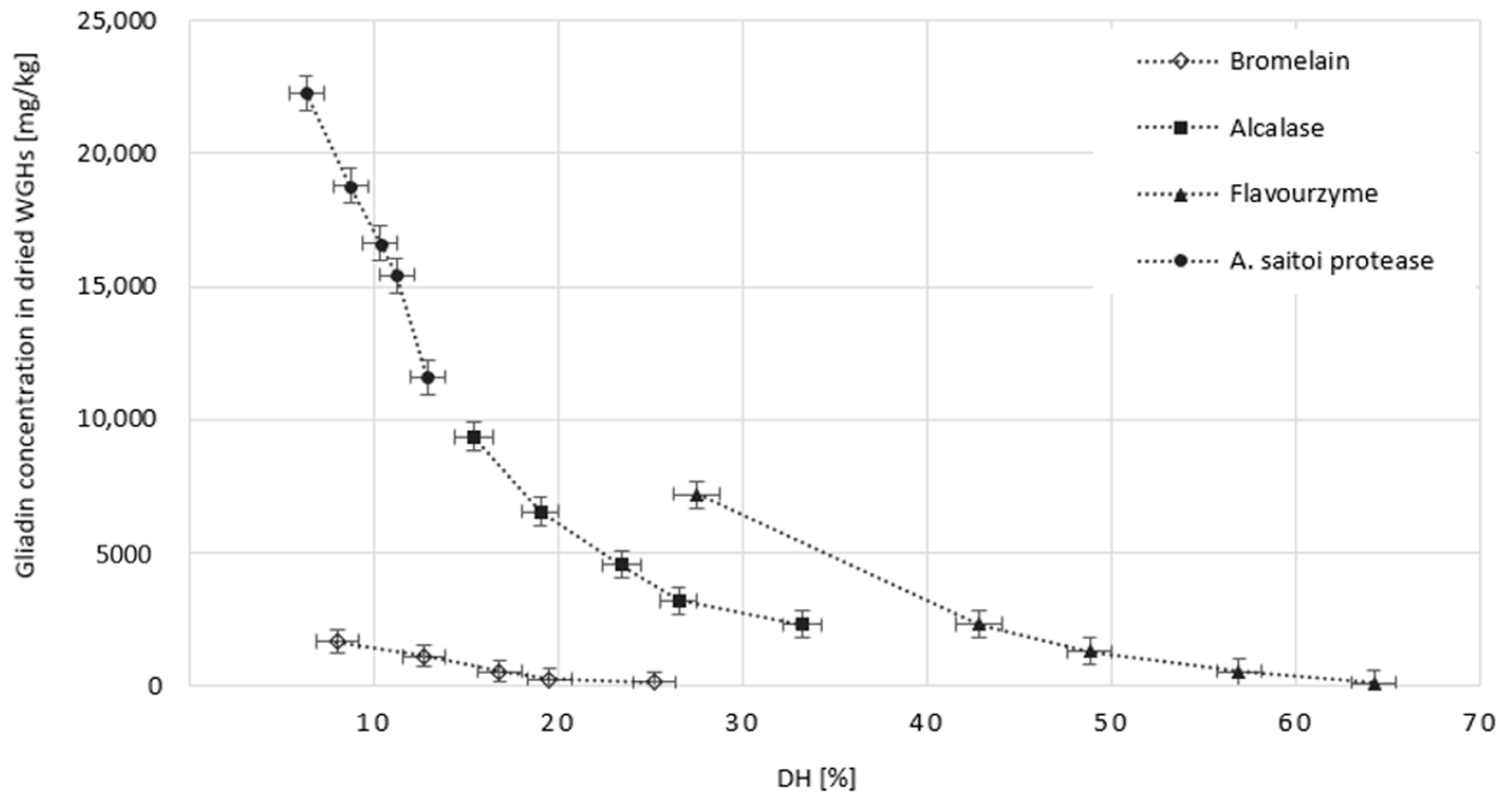
2.4. The Effect of Dose and Type of Protease on the Allergenic Potential of the Obtained WGHs
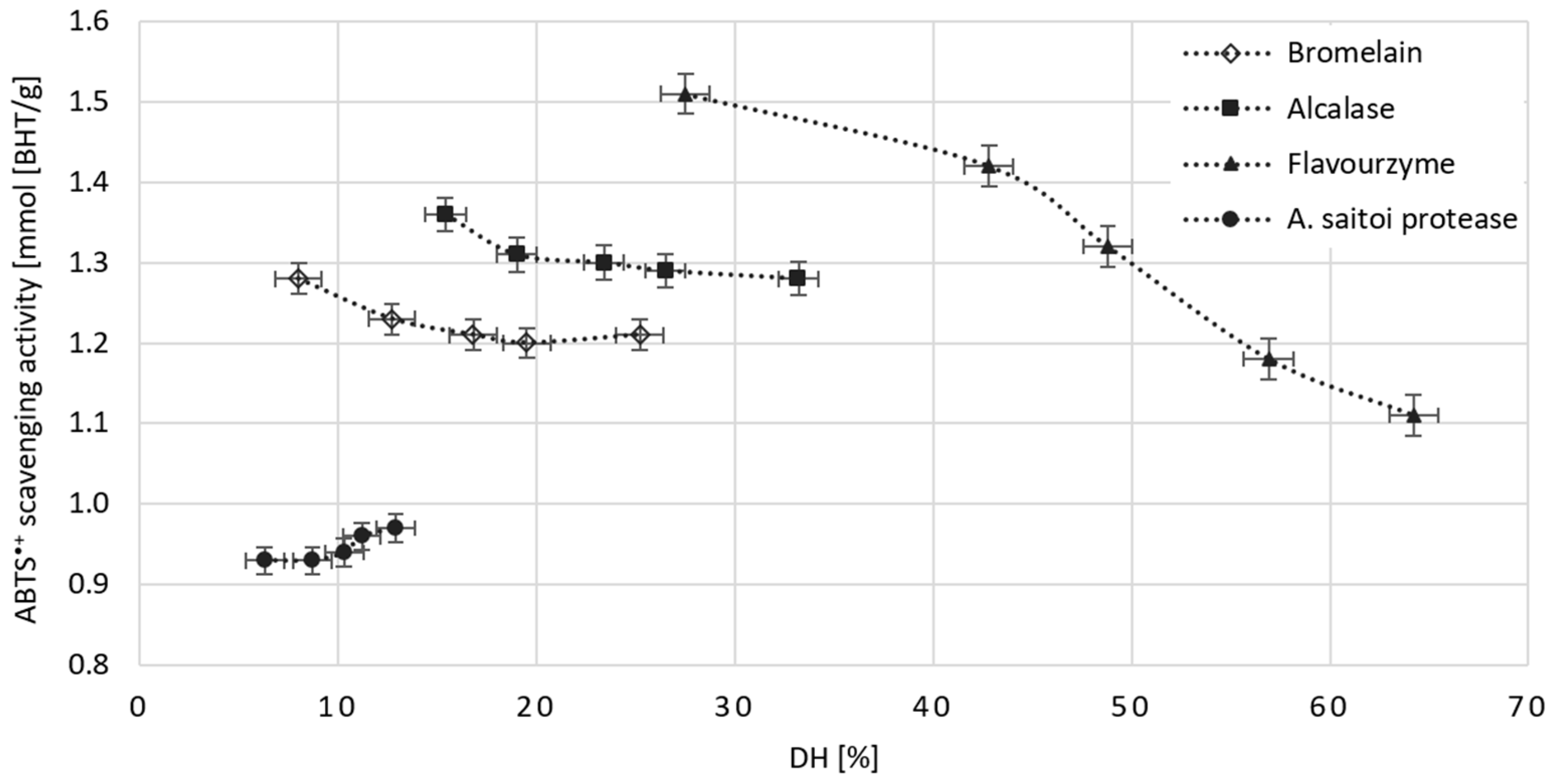
2.5. The Effect of Dose and Type of Protease on the Antioxidant Potential of Obtained WGHs
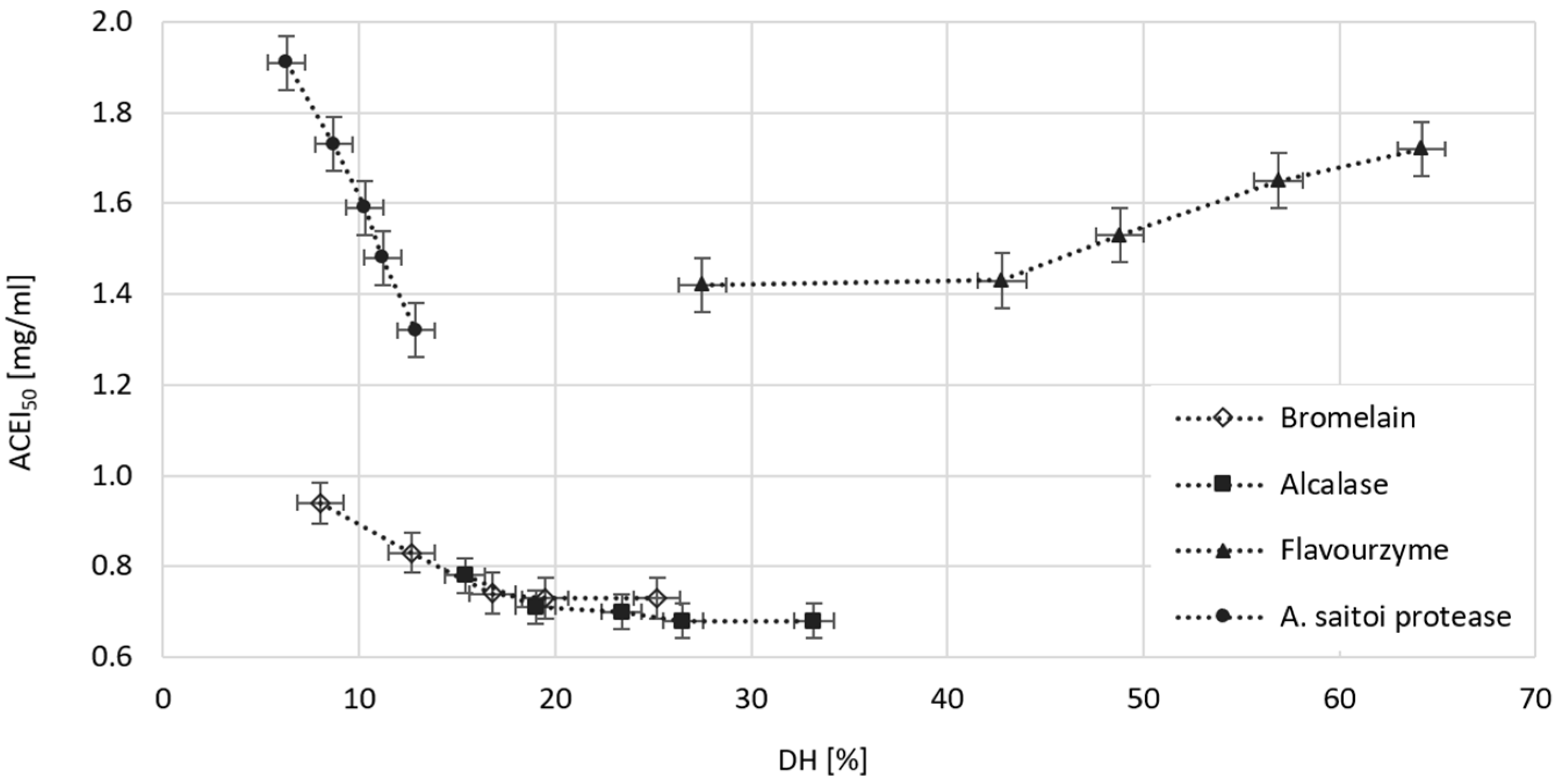
2.6. The Effect of Dose and Type of Protease on the Ability of Obtained WGHs to Inhibit ACE
2.7. The Effect of Dose and Type of Protease on the Functional Properties of Obtained WGHs
3. Materials and Methods
3.1. Materials
3.2. Determination of the Optimal pH for Gluten Hydrolysis by the Tested Proteases
3.3. Determination of the Optimal Temperature for Gluten Hydrolysis by the Tested Proteases
3.4. Preparation of Wheat Gluten Hydrolysates (WGHs)
3.5. Determination of the Gluten Hydrolysis Degree (DH%)
3.6. Determination of Protein Recovery (PR%)
3.7. Determination of Biological Properties of WGHs
3.7.1. Angiotensin Converting Enzyme Inhibitory (ACEI) Activity Assay
3.7.2. Determination of Allergenic Sequences in Gluten
3.7.3. ABTS Radical Scavenging Assay
3.8. Determination of Functional Properties of WGHs
3.8.1. Foaming Capacity and Foam Stability Assay
3.8.2. Determination of Emulsifying Activity and Emulsion Stability
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Day, L.; Augustin, M.A.; Batey, I.L.; Wrigley, C.W. Wheat-gluten uses and industry needs. Trends Food Sci. Technol. 2006, 17, 82–90. [Google Scholar] [CrossRef]
- Scherf, K.A.; Koehler, P.; Wieser, H. Gluten and wheat sensitivities—An overview. J. Cereal Sci. 2016, 67, 2–11. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of wheat gluten proteins: Qualitative composition. Cereal Chem. 2023, 100, 23–35. [Google Scholar] [CrossRef]
- Pourmohammadi, K.; Abedi, E. Hydrolytic enzymes and their directly and indirectly effects on gluten and dough properties: An extensive review. Food Sci. Nutr. 2021, 9, 3988–4006. [Google Scholar] [CrossRef] [PubMed]
- Bradauskiene, V.; Vaiciulyte-Funk, L.; Cernauskas, D.; Dzingeleviciene, R.; Lima, J.P.M.; Bradauskaite, A.; Tita, M.A. The efficacy of plant enzymes bromelain and papain as a tool for reducing gluten immunogenicity from wheat bran. Processes 2022, 10, 1948. [Google Scholar] [CrossRef]
- Merz, M.; Eisele, T.; Berends, P.; Appel, D.; Rabe, S.; Blank, I.; Stressler, T.; Fischer, L.J. Flavourzyme an enzyme preparation with industrial relevance: Automated nine-step purification and partial characterization of eight enzymes. J. Agric. Food Chem. 2015, 63, 5682–5693. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.-H.; Tavano, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of Alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef]
- Nishinoaki, M.; Asakura, T.; Watanabe, T.; Kunizaki, E.; Matsumoto, M.; Eto, W.; Tamura, T.; Minami, M.; Obata, A.; Abe, K.; et al. Application of an Aspergillus saitoi protease preparation to soybean curd to modify its functional and rheological properties. Biosci. Biotechnol. Biochem. 2008, 72, 587–590. [Google Scholar] [CrossRef]
- Jančič, U.; Gorgieva, S. Bromelain and nisin: The natural antimicrobials with high potential in biomedicine. Pharmaceutics 2022, 14, 76. [Google Scholar] [CrossRef]
- Okayama, A.; Cabral, H.; Orlando, B.R.G. Isolation and biochemical characterization of ananassains, cysteine peptidases from the fruits of ananas ananassoides. Curr. Chem. Biol. 2023, 17, 170–181. [Google Scholar] [CrossRef]
- Zhao, R.; Lu, S.; Li, S.; Shen, H.; Wang, Y.; Gao, Y.; Shen, X.; Wang, F.; Wu, J.; Liu, W.; et al. Enzymatic preparation and processing properties of DPP-IV inhibitory peptides derived from wheat gluten: Effects of pretreatment methods and protease types. Foods 2024, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Kim, N. Production of wheat gluten hydrolyzates by enzymatic process at high pressure. Food Sci. Biotechnol. 2017, 26, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Elmalimadi, M.B. Functional and Biological Properties of Enzymatically Modified Wheat Gluten. Ph.D. Dissertation, University of Belgrade, Beograd, Serbia, 2018. [Google Scholar]
- Kong, X.; Zhou, H.; Qian, H. Enzymatic hydrolysis of wheat gluten by proteases and properties of the resulting hydrolysates. Food Chem. 2007, 102, 759–763. [Google Scholar] [CrossRef]
- Hardt, N.A.; Goot, A.J.; Boom, R.M. Influence of high solid concentrations on enzymatic wheat gluten hydrolysis and resulting functional properties. J. Cereal Sci. 2013, 57, 531–536. [Google Scholar] [CrossRef]
- He, W.; Yang, R.; Zhao, W. Effect of acid deamidation-alcalase hydrolysis induced modification on functional and bitter-masking properties of wheat gluten hydrolysates. Food Chem. 2019, 277, 655–663. [Google Scholar] [CrossRef]
- Giesler, L.; Linke, D.; Berger, R.G. Factors limiting the enzymatic hydrolysis of wheat gluten. J. Agric. Food Chem. 2014, 62, 4762–4768. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1. [Google Scholar] [CrossRef]
- Thakur, D.; Pandit, S.B. Substrate promiscuity: A continuum feature of enzymes. Biophys. J. 2022, 121, 344A. [Google Scholar] [CrossRef]
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An overview of industrial application and purification strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef] [PubMed]
- Goettig, P. Reversed Proteolysis -Proteases as Peptide Ligases. Catalysts 2021, 11, 33. [Google Scholar] [CrossRef]
- Chompoorat, P.; Fasasi, A.; Lavine, B.K.; Rayas-Duarte, P. Gluten conformation at different temperatures and additive treatments. Foods 2022, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Y.; Zhang, J.-T.; Miyakawa, T.; Li, G.-M.; Gu, R.-Z.; Tanokura, M. Antioxidant properties and inhibition of angiotensin-converting enzyme by highly active peptides from wheat gluten. Sci. Rep. 2021, 11, 5206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Li, S.; Ma, H.; Zhang, H. Mechanism study of multimode ultrasound pretreatment on the enzymolysis of wheat gluten. J. Sci. Food Agric. 2018, 98, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.H.; Bae, I.Y.; Lee, S.; Lee, D.H.; Hur, B.S.; Lee, H.G. Evaluation of wheat gluten hydrolysates as taste-active compounds with antioxidant activity. J. Food Sci. Technol. 2014, 51, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Merz, M.; Kettner, L.; Langolf, E.; Appel, D.; Blank, I.; Stresslera, T.; Fischera, L. Production of wheat gluten hydrolysates with reduced antigenicity employing enzymatic hydrolysis combined with downstream unit operations. J. Sci. Food Agric. 2016, 96, 3358–3364. [Google Scholar] [CrossRef]
- Banerjee, S.; Ranganathan, V.; Arora, A.; Patti, A.F. Green approach towards hydrolysing wheat gluten using waste ingredients from pineapple processing industries. Int. J. Food Sci. Technol. 2021, 56, 1724–1733. [Google Scholar] [CrossRef]
- Song, M.K.; Guo, X.N.; Zhu, K.X. Insights into heat-induced molecular-level interactions between wheat and common buckwheat proteins. Food Chem. 2023, 404, 134730. [Google Scholar] [CrossRef]
- Richter, P.; Sebald, K.; Fischer, K.; Behrens, M.; Schnieke, A.; Somoza, V. Bitter peptides YFYPEL, VAPFPEVF, and YQEPVLGPVRGPFPIIV, released during gastric digestion of casein, stimulate mechanisms of gastric acid secretion via bitter taste receptors TAS2R16 and TAS2R38. J. Agric. Food Chem. 2022, 70, 11591–11602. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Goktepe, I.; Ahmedna, M. The potential of papain and alcalase enzymes and process optimizations to reduce allergenic gliadins in wheat flour. Food Chem. 2016, 196, 1338–1345. [Google Scholar] [CrossRef]
- Scherf, K.A.; Wieser, H.; Koehler, P. Novel approaches for enzymatic gluten degradation to create high-quality gluten-free products. Food Res. Int. 2018, 110, 62–72. [Google Scholar] [CrossRef]
- Newstead, S.; Drew, D.; Cameron, A.D.; Postis, V.L.G.; Xia, X.; Fowler, P.W.; Ingram, J.C.; Carpenter, E.P.; Sansom, M.S.P.; McPherson, M.J.; et al. Crystal structure of a prokaryotic homologue of the mammalian oligopeptide–proton symporters, PepT1 and PepT2. EMBO J. 2010, 30, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J. Research progress in structure-activity relationship of bioactive peptides. J. Med. Food 2015, 18, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wouters, A.G.B.; Fierens, E.; Rombouts, I.; Brijs, K.; Joey, I.J.; Delcour, J.A. Exploring the relationship between structural and air-water interfacial properties of wheat (Triticum aestivum L.) gluten hydrolysates in a food system. J. Agric. Food Chem. 2017, 65, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Joye, J.; McClements, D.J. Emulsifying and emulsion-stabilizing properties of gluten hydrolysates. J. Agric. Food Chem. 2014, 62, 2623–2630. [Google Scholar] [CrossRef]
- Pan, X.; Fang, Y.; Wang, L.; Xie, M.; Hu, B.; Zhu, Y.; Zhao, E.; Pei, F.; Shen, F.; Li, P.; et al. Effect of enzyme types on the stability of oil-in-water emulsions formed with rice protein hydrolysates. J. Sci. Food Agric. 2019, 99, 6731–6740. [Google Scholar] [CrossRef]
- Shumoy, H.; Pattyn, S.; Raes, K. Tef protein: Solubility characterization, in-vitro digestibility and its suitability as a gluten free ingredient. LWT 2018, 89, 697–703. [Google Scholar] [CrossRef]
- Asoodeh, A.; Haghighi, L.; Chamani, J.; Ansari-Ogholbeyk, M.A.; Mojallal-Tabatabaei, Z.; Lagzian, M. Potential angiotensin I converting enzyme inhibitory peptides from gluten hydrolysate: Biochemical characterization and molecular docking study. J. Cereal Sci. 2014, 60, 92–98. [Google Scholar] [CrossRef]
- Sadahira, M.; Lopes, F.; Rodrigues, M.; Netto, F. Influence of protein-pectin electrostatic interaction on the foam stability mechanism. Carbohydr. Polym. 2014, 103, 55–61. [Google Scholar] [CrossRef]

| Proteolytic Preparation | Optimal pH/T | Activity | |
|---|---|---|---|
| Bromelain | 6.0/60 °C | 188.7 ± 9.7 b U/g | 3145 ± 162 b nkat/g |
| Alcalase | 8.0/55 °C | 224.2 ± 4.8 c U /mL | 3737 ± 80 c nkat/mL |
| Flavourzyme | 5.5/50 °C | 137.2 ± 5.1 a U/mL | 2286 ± 85 a nkat/mL |
| A. saitoi protease | 4.0/55 °C | 125.3 ± 7.7 a U/g | 2088 ± 128 a nkat/g |
| Enzyme (th, pH, T) | Bromelain (4 h, 6.0, 60 °C) | Alcalase (4 h, 8.0, 55 °C) | ||||||||
| E/S [U/g] | 2.5 | 5 | 10 | 15 | 25 | 2.5 | 5 | 10 | 15 | 25 |
| DH [%] | 8.0 ± 0.6 a | 12.7 ± 2.1 b | 16.8 ± 1.3 c | 19.5 ± 0.2 d | 25.2 ± 1.2 e | 15.4 ± 0.4 a | 19.0 ± 0.4 b | 23.4 ± 0.6 c | 26.5 ± 0.4 d | 33.2 ± 0.6 e |
| PR [%] | 81.3 ± 3.4 a | 86.5 ± 1.5 ab | 86.5 ± 0.7 b | 89.6 ± 1.7 bc | 92.1 ± 1.6 c | 81.2 ± 1.9 a | 83.5 ± 1.9 ab | 85.5 ± 1.7 b | 89.5 ± 2.2 c | 91.8 ± 2.1 c |
| Enzyme (th, pH, T) | Flavourzyme (4 h, 5.5, 50 °C) | A. saitoi Protease (4 h, 4.0, 55 °C) | ||||||||
| E/S [U/g] | 2.5 | 5 | 10 | 15 | 25 | 2.5 | 5 | 10 | 15 | 25 |
| DH [%] | 27.5 ± 1.6 a | 40.8± 2.2 b | 48.8 ± 1.8 c | 56.9 ± 2.4 d | 64.2 ± 1.3 e | 6.3 ± 0.7 a | 8.7 ± 0.3 b | 10.3 ± 0.1 bc | 11.2 ± 0.2 c | 12.9 ± 0.6 d |
| PR [%] | 81.4 ± 0.7 a | 86.7 ± 2.3 b | 91.1 ± 1.4 c | 92.8 ± 0.8 c | 92.2 ± 1.4 c | 50.9 ± 1.4 a | 59.8 ± 1.3 b | 67.7 ± 2.4 c | 72.6 ± 2.4 d | 71.1 ± 1.3 d |
| Preparation | WGHs of Bromelain | WGHs of Alcalase | Gluten | ||||||||
| E/S [U/g] | 2.5 | 5 | 10 | 15 | 25 | 2.5 | 5 | 10 | 15 | 25 | |
| FC [%] | 440 ± 22 d | 300 ± 16 c | 260 ± 11 b | 200 ± 20 a | 198 ± 13 a | 300 ± 26 c | 268± 17 c | 200 ± 14 b | 112 ± 17 a | 85 ± 12 a | 197 ± 15 |
| FS [%] | 41 ± 2 c | 31 ± 3 b | 28 ± 2 b | 22 ± 2 a | 23 ± 2 a | 13 ± 1 b | 11± 2 ab | 9 ± 1 a | 8 ± 2 a | 8 ± 2 a | 31 ± 2 |
| EA [%] | 56 ± 2 c | 54 ± 2 bc | 51 ± 2 ab | 50 ± 2 a | 49 ± 2 a | 45 ± 2 d | 31± 2 c | 21 ± 1 b | 8 ± 2 a | 7 ± 3 a | 47 ± 2 |
| ESLT [%] | 102 ± 4 c | 90 ± 4 b | 89 ± 3 b | 92 ± 4 b | 81 ± 3 a | 51 ± 5 c | 24± 7 b | 10 ± 5 a | 9 ± 4 a | 7 ± 4 a | 102 ± 4 |
| ESHT [%] | 95 ± 7 c | 69 ± 7 b | 36 ± 5 a | 33 ± 6 a | 30 ± 5 a | 25 ± 4 b | 23± 7 b | 9 ± 6 a | 7 ± 4 a | 6 ± 4 a | 92 ± 4 |
| Preparation | WGHs of Flavourzyme | WGHs of A. saitoi protease | Ovalbumin | ||||||||
| E/S [U/g] | 2.5 | 5 | 10 | 15 | 25 | 2.5 | 5 | 10 | 15 | 25 | |
| FC [%] | 117 ± 26 c | 83 ± 17 bc | 65 ± 1 b | 70 ± 10 b | 23 ± 13 a | 325 ± 19 a | 402 ± 20 b | 445 ± 21 b | 342 ± 16 a | 340 ± 11 a | 400 ± 17 |
| FS [%] | 14 ± 2 b | 11 ± 2 ab | 11 ± 2 ab | 10 ± 2 a | 10 ± 1 a | 13 ± 1 a | 22 ± 2 b | 27 ± 2 c | 20 ± 2 b | 21 ± 1 b | 35 ± 2 |
| EA [%] | 43 ± 2 b | 8 ± 2 a | 7 ± 3 a | 5 ± 3 a | 5 ± 3 a | 56 ± 2 a | 60 ± 2 b | 60 ± 2 b | 65 ± 3 bc | 66 ± 2 c | 67 ± 2 |
| ESLT [%] | 65 ± 4 a | 24 ± 6 b | 8 ± 3 a | 7 ± 3 a | 5 ± 3 a | 99 ± 6 a | 101 ± 7 ab | 101 ± 5 ab | 100 ± 4 b | 100 ± 4 ab | 99 ± 3 |
| ESHT [%] | 66 ± 8 c | 34 ± 5 b | 7 ± 3 a | 6 ± 3 a | 5 ± 3 a | 58 ± 9 a | 93 ± 4 b | 94 ± 3 b | 94 ± 3 b | 95 ± 4 b | 102 ± 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mika, M.; Wikiera, A. Enzymatic Hydrolysis as an Effective Method for Obtaining Wheat Gluten Hydrolysates Combining Beneficial Functional Properties with Health-Promoting Potential. Molecules 2024, 29, 4407. https://doi.org/10.3390/molecules29184407
Mika M, Wikiera A. Enzymatic Hydrolysis as an Effective Method for Obtaining Wheat Gluten Hydrolysates Combining Beneficial Functional Properties with Health-Promoting Potential. Molecules. 2024; 29(18):4407. https://doi.org/10.3390/molecules29184407
Chicago/Turabian StyleMika, Magdalena, and Agnieszka Wikiera. 2024. "Enzymatic Hydrolysis as an Effective Method for Obtaining Wheat Gluten Hydrolysates Combining Beneficial Functional Properties with Health-Promoting Potential" Molecules 29, no. 18: 4407. https://doi.org/10.3390/molecules29184407
APA StyleMika, M., & Wikiera, A. (2024). Enzymatic Hydrolysis as an Effective Method for Obtaining Wheat Gluten Hydrolysates Combining Beneficial Functional Properties with Health-Promoting Potential. Molecules, 29(18), 4407. https://doi.org/10.3390/molecules29184407









