Anti-Inflammatory Effects of the Combined Treatment of Resveratrol- and Protopanaxadiol-Enriched Rice Seed Extract on Lipopolysaccharide-Stimulated RAW264.7 Cells
Abstract
1. Introduction
2. Results
2.1. Effect of Resveratrol- and PPD-Enriched Rice Extract on RAW264.7 Cell Viability
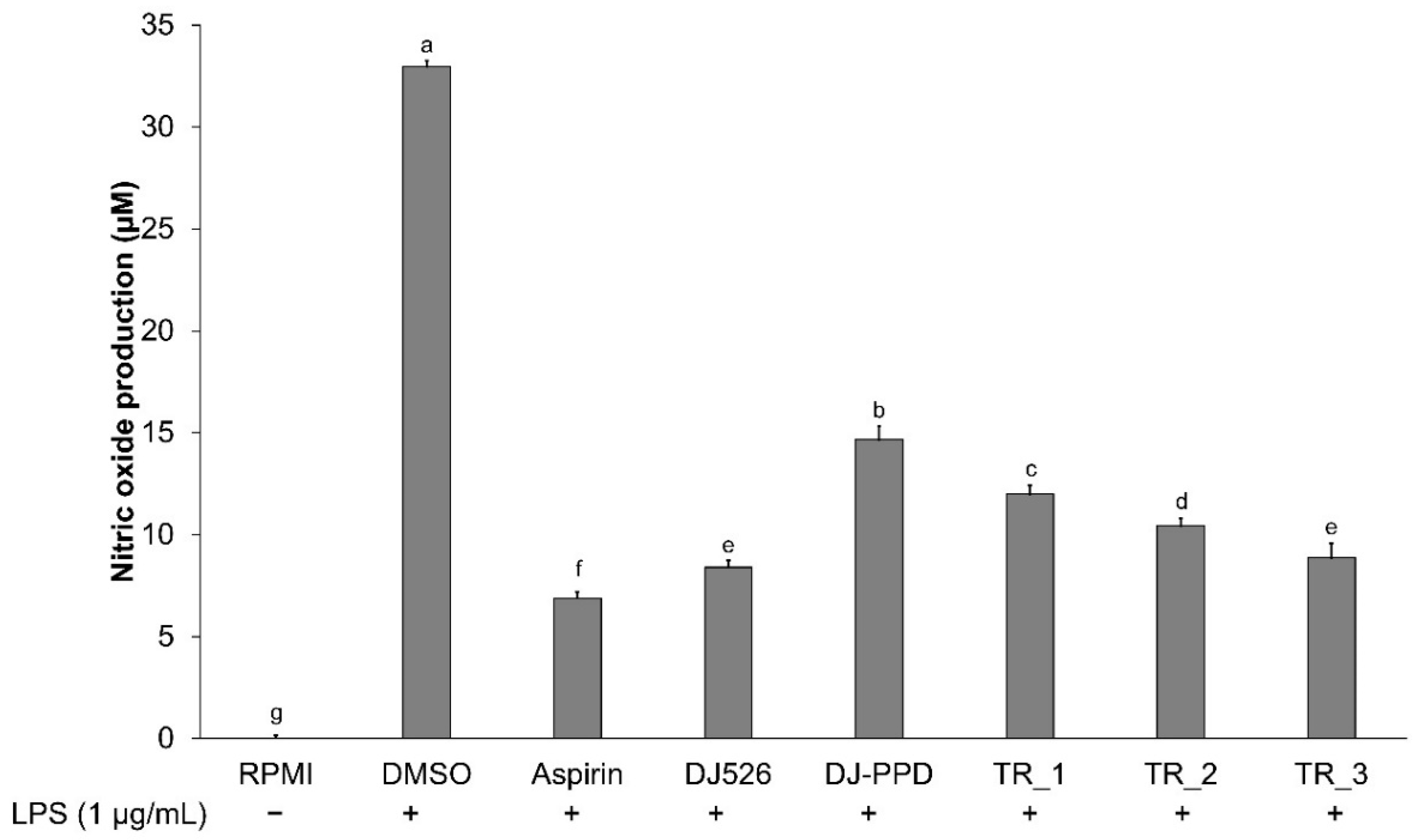
2.2. Effect of Resveratrol- and PPD-Enriched Rice Extract on NO Production
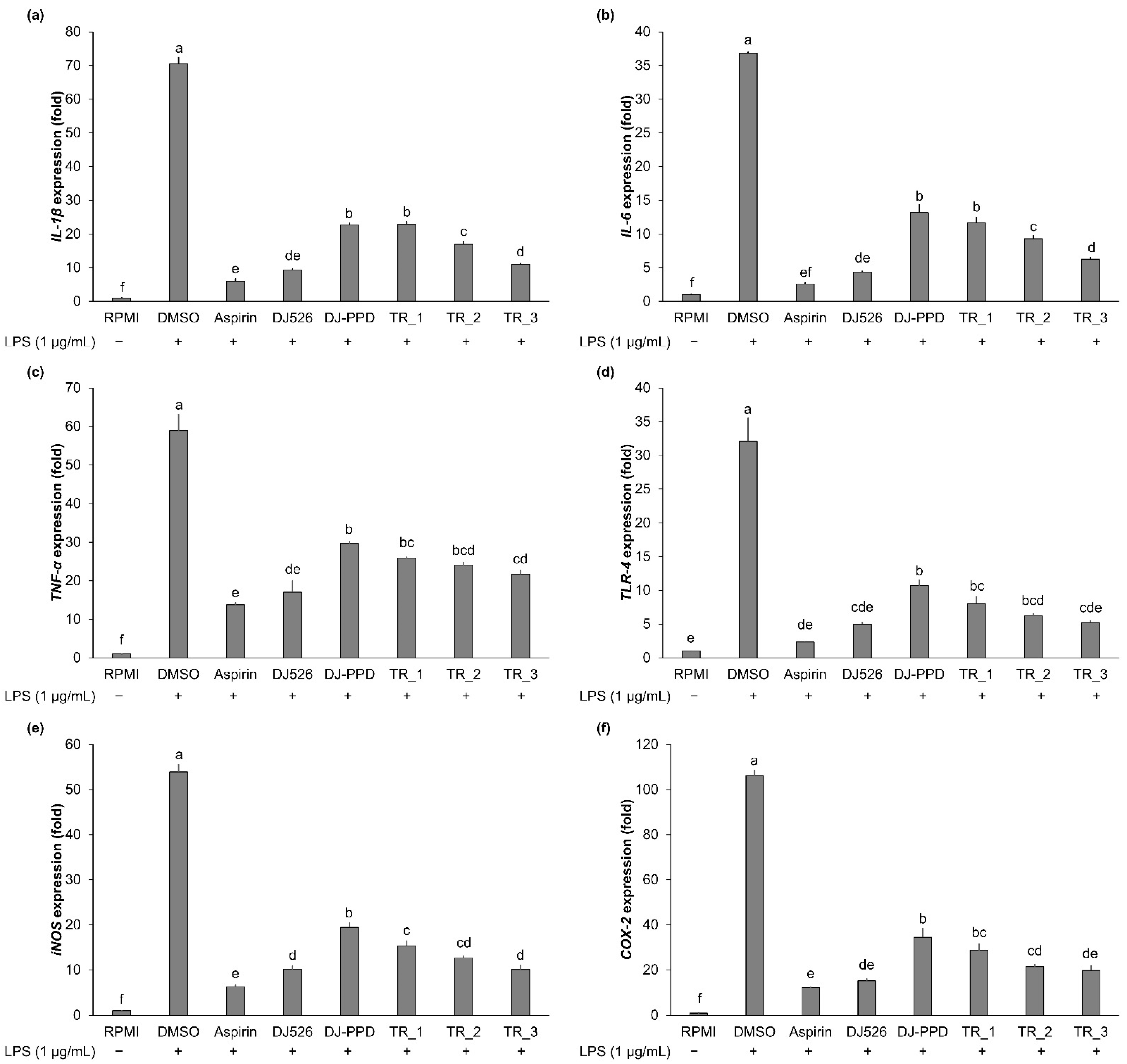
2.3. Effect of Resveratrol- and PPD-Enriched Rice Extract on Inflammatory Cytokine Expression
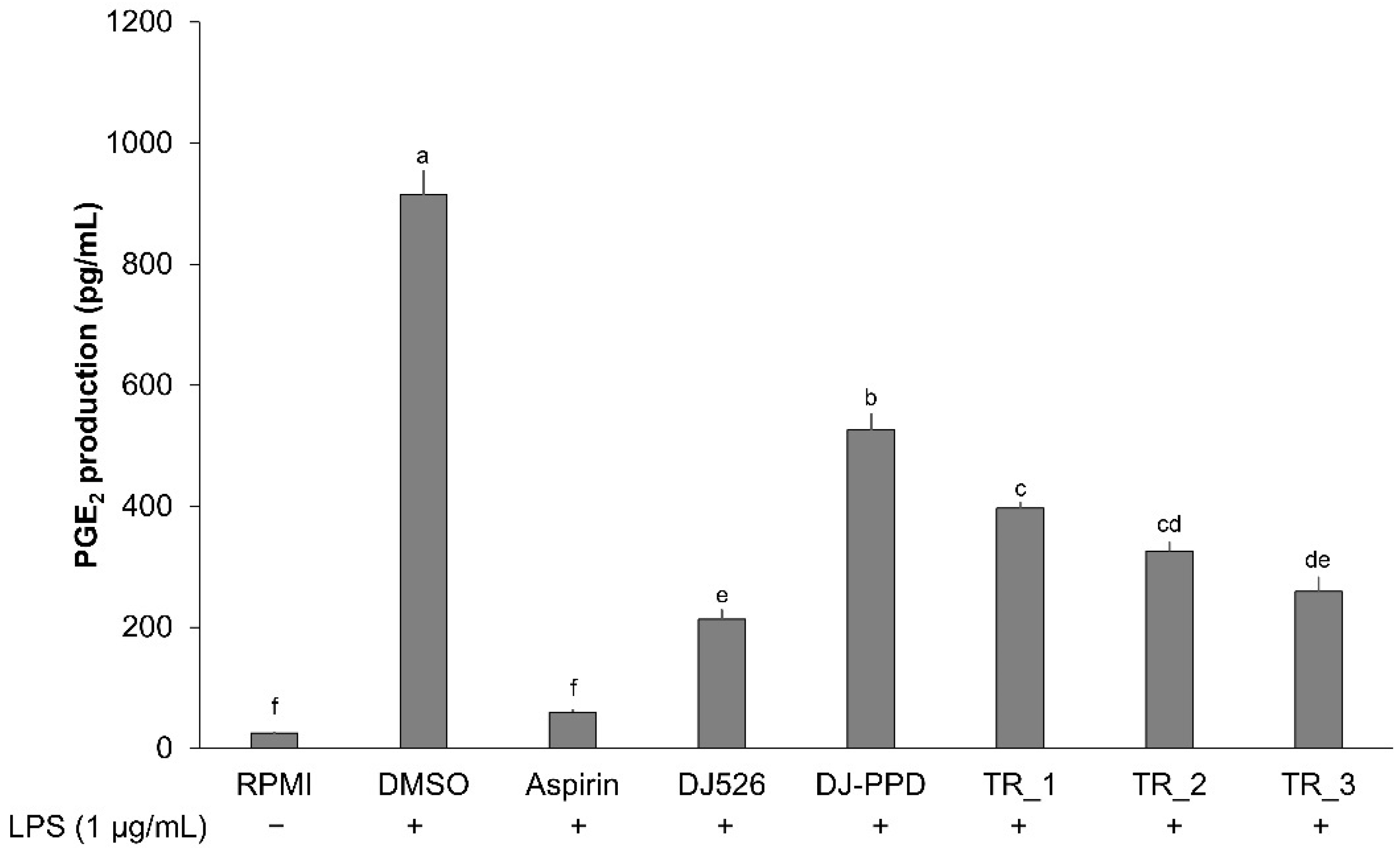
2.4. Effect of Resveratrol- and PPD-Enriched Rice Extract on PGE2 Production
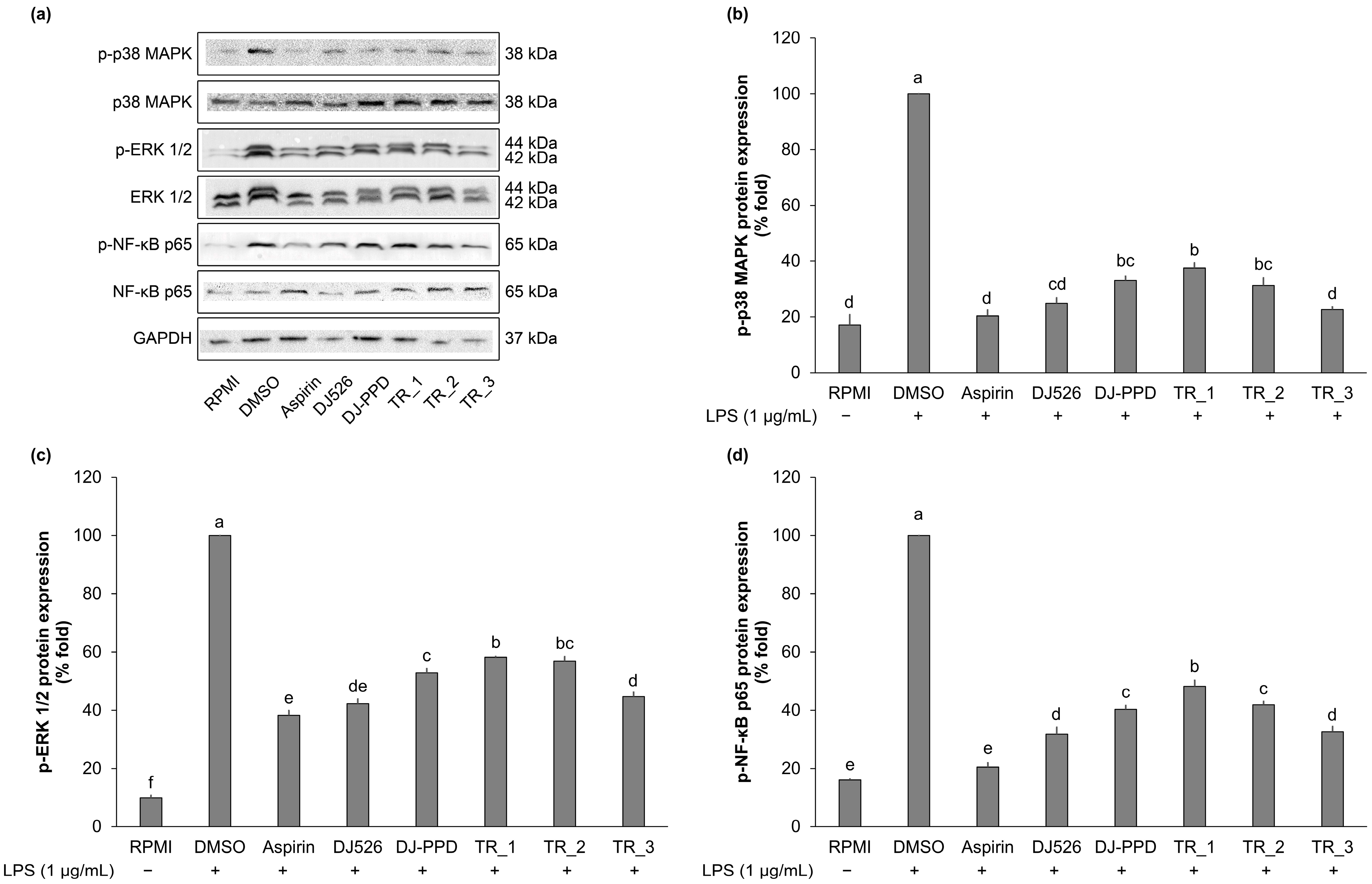
2.5. Effect of Resveratrol- and PPD-Enriched Rice Extract on Inflammatory Pathway Signaling
3. Discussion
4. Materials and Methods
4.1. Treatment Preparation
4.2. Cell Viability and NO Production
4.3. RNA Extraction
4.4. RNA Quantification and cDNA Synthesis
4.5. Inflammatory-Related Gene Expression Measurement
4.6. PGE2 Production Assay
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, E.-J.; Shin, S.-Y.; Kim, J.-K.; Woo, E.-R.; Kim, Y.-M. Anti-inflammatory effects of amentoflavone on modulation of signal pathways in LPS-stimulated RAW264.7 cells. Bull. Korean Chem. Soc. 2012, 33, 2878–2882. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, J.; Wu, L.; Yu, Y.; Ye, R.D.; Zhang, Y.; Liang, X. In vitro immunomodulatory effects of human milk oligosaccharides on murine macrophage RAW264.7 cells. Carbohydr. Polym. 2019, 207, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest 1997, 112 (Suppl. S6), 321s–329s. [Google Scholar] [CrossRef] [PubMed]
- Boniakowski, A.E.; Kimball, A.S.; Joshi, A.; Schaller, M.; Davis, F.M.; denDekker, A.; Obi, A.T.; Moore, B.B.; Kunkel, S.L.; Gallagher, K.A. Murine macrophage chemokine receptor CCR2 plays a crucial role in macrophage recruitment and regulated inflammation in wound healing. Eur. J. Immunol. 2018, 48, 1445–1455. [Google Scholar] [CrossRef]
- Chihara, N.; Aranami, T.; Sato, W.; Miyazaki, Y.; Miyake, S.; Okamoto, T.; Ogawa, M.; Toda, T.; Yamamura, T. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc. Natl. Acad. Sci. USA 2011, 108, 3701–3706. [Google Scholar] [CrossRef]
- Yencilek, F.; Yildirim, A.; Yilmaz, S.G.; Altinkilic, E.M.; Dalan, A.B.; Bastug, Y.; Isbir, T. Investigation of interleukin-1β polymorphisms in prostate cancer. Anticancer Res. 2015, 35, 6057–6061. [Google Scholar]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Conway, E.M.; Pikor, L.A.; Kung, S.H.; Hamilton, M.J.; Lam, S.; Lam, W.L.; Bennewith, K.L. Macrophages, Inflammation, and Lung Cancer. Am. J. Respir. Crit. Care Med. 2016, 193, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef]
- Kip, E.; Parr-Brownlie, L.C. Reducing neuroinflammation via therapeutic compounds and lifestyle to prevent or delay progression of Parkinson’s disease. Ageing Res. Rev. 2022, 78, 101618. [Google Scholar] [CrossRef]
- Fuggetta, M.P.; Bordignon, V.; Cottarelli, A.; Macchi, B.; Frezza, C.; Cordiali-Fei, P.; Ensoli, F.; Ciafrè, S.; Marino-Merlo, F.; Mastino, A.; et al. Downregulation of proinflammatory cytokines in HTLV-1-infected T cells by Resveratrol. J. Exp. Clin. Cancer Res. 2016, 35, 118. [Google Scholar] [CrossRef] [PubMed]
- Lopes Pinheiro, D.M.; Sales de Oliveira, A.H.; Coutinho, L.G.; Fontes, F.L.; de Medeiros Oliveira, R.K.; Oliveira, T.T.; Fonseca Faustino, A.L.; da Silva, V.L.; Araujo de Melo Campos, J.T.; Petta Lajus, T.B. Resveratrol decreases the expression of genes involved in inflammation through transcriptional regulation. Free Radic. Biol. Med. 2019, 130, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.-M.; Zong, Y.; Sun, L.; Guo, J.-Z.; Zhang, W.; He, Y.; Song, R.; Wang, W.-M.; Xiao, C.-J.; Lu, D. Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS ONE 2012, 7, e32195. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Carofiglio, V.; Acquafredda, A.; Cavallo, P.; Cianciulli, A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-κB activation in Caco-2 and SW480 human colon cancer cells. Br. J. Nutr. 2012, 108, 1623–1632. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Y.; Dong, L.; Li, M.; Cai, W. Anti-inflammatory effect of resveratrol through the suppression of NF-κB and JAK/STAT signaling pathways. Acta Biochim. Biophys. Sin. 2015, 47, 207–213. [Google Scholar] [CrossRef]
- Li, X.; Li, F.; Wang, F.; Li, J.; Lin, C.; Du, J. Resveratrol inhibits the proliferation of A549 cells by inhibiting the expression of COX-2. OncoTargets Ther. 2018, 11, 2981–2989. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent advances on the anti-inflammatory and antioxidant properties of red grape polyphenols: In vitro and in vivo studies. Antioxidants 2020, 9, 35. [Google Scholar] [CrossRef]
- Martín, A.R.; Villegas, I.; La Casa, C.; de la Lastra, C.A. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem. Pharmacol. 2004, 67, 1399–1410. [Google Scholar] [CrossRef]
- Zykova, T.A.; Zhu, F.; Zhai, X.; Ma, W.Y.; Ermakova, S.P.; Lee, K.W.; Bode, A.M.; Dong, Z. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol. Carcinog. 2008, 47, 797–805. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Alzahrani, M.Z.; Bakheet, S.A.; Attia, S.M. Resveratrol improves neuroimmune dysregulation through the inhibition of neuronal toll-like receptors and COX-2 signaling in BTBR T(+) Itpr3(tf)/J mice. Neuromol. Med. 2018, 20, 133–146. [Google Scholar] [CrossRef]
- Ruan, J.; Sun, F.; Zhang, Y.; Zheng, D.; Xiang, G.; Zhao, W.; Zhang, Y.; Wang, T. New 20(S)-protopanaxadiol type saponins from the leaves of Panax notoginseng and their potential anti-inflammatory activities. Steroids 2020, 162, 108696. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-M.; Kim, S.-D.; Kim, K.-S.; Kwak, Y.-S.; Park, H.-J.; Rhee, M.-H. Protopanaxadiol modulates LPS-induced inflammatory activity in murine macrophage RAW264.7 cells. J. Ginseng Res. 2006, 30, 181–187. [Google Scholar] [CrossRef]
- Lu, P.; Chen, J.; Chen, Y.; Quan, X.; Liu, J.; Han, Y.; Li, Y.; Yang, L.; Wan, J.-B.; Zhao, Y. 20(S)-Protopanaxadiol saponins isolated from Panax notoginseng target caveolin-1 against intestinal barrier dysfunction by alleviating inflammatory injury and oxidative stress in experimental murine colitis. Food Front. 2023, 4, 2081–2096. [Google Scholar] [CrossRef]
- Monmai, C.; Kim, J.-S.; Baek, S.-H. Use of germination to enhance resveratrol content and its anti-inflammatory activity in lipopolysaccharide-stimulated RAW264.7 cells. Molecules 2023, 28, 4898. [Google Scholar] [CrossRef] [PubMed]
- Monmai, C.; Kim, J.-S.; Baek, S.-H. Transgenic rice seed extracts exert immunomodulatory effects by modulating immune-related biomarkers in RAW264.7 macrophage cells. Nutrients 2022, 14, 4143. [Google Scholar] [CrossRef] [PubMed]
- Monmai, C.; Kim, J.-S.; Baek, S.-H. Effect of ginseng sapogenin protopanaxadiol-enriched rice (DJ-PPD) on immunomodulation. Plants 2023, 12, 767. [Google Scholar] [CrossRef]
- Baek, S.-H.; Shin, W.-C.; Ryu, H.-S.; Lee, D.-W.; Moon, E.; Seo, C.-S.; Hwang, E.; Lee, H.-S.; Ahn, M.-H.; Jeon, Y.; et al. Creation of resveratrol-enriched rice for the treatment of metabolic syndrome and related diseases. PLoS ONE 2013, 8, e57930. [Google Scholar] [CrossRef]
- Han, J.Y.; Baek, S.H.; Jo, H.J.; Yun, D.W.; Choi, Y.E. Genetically modified rice produces ginsenoside aglycone (protopanaxadiol). Planta 2019, 250, 1103–1110. [Google Scholar] [CrossRef]
- Cheng, D.; Zhu, C.; Liang, Y.; Xing, Y.; Shi, C. MiR-424 overexpression protects alveolar epithelial cells from LPS-induced apoptosis and inflammation by targeting FGF2 via the NF-κB pathway. Life Sci. 2020, 242, 117213. [Google Scholar] [CrossRef]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Bai, Y.; Qin, Y.; Li, J.; Feng, S.; et al. LPS-induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF-κB, STAT3 or AP-1 activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar] [CrossRef]
- Ren, J.; Su, D.; Li, L.; Cai, H.; Zhang, M.; Zhai, J.; Li, M.; Wu, X.; Hu, K. Anti-inflammatory effects of aureusidin in LPS-stimulated RAW264.7 macrophages via suppressing NF-κB and activating ROS- and MAPKs-dependent Nrf2/HO-1 signaling pathways. Toxicol. Appl. Pharmacol. 2020, 387, 114846. [Google Scholar] [CrossRef] [PubMed]
- Tucureanu, M.M.; Rebleanu, D.; Constantinescu, C.A.; Deleanu, M.; Voicu, G.; Butoi, E.; Calin, M.; Manduteanu, I. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of G(i)-protein inhibitor. Int. J. Nanomed. 2018, 13, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Zamyatina, A.; Heine, H. Lipopolysaccharide recognition in the crossroads of TLR4 and Caspase-4/11 mediated inflammatory pathways. Front. Immunol. 2020, 11, 585146. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Grylls, A.; Seidler, K.; Neil, J. Link between microbiota and hypertension: Focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed. Pharmacother. 2021, 137, 111334. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Beutler, B. Tlr4: Central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 2000, 12, 20–26. [Google Scholar] [CrossRef]
- Cho, J.W.; Lee, K.S.; Kim, C.W. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int. J. Mol. Med. 2007, 19, 469–474. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Choe, J. Prostaglandin E2 stimulates COX-2 expression via mitogen-activated protein kinase p38 but not ERK in human follicular dendritic cell-like cells. BMC Immunol. 2020, 21, 20. [Google Scholar] [CrossRef]
- Takai, E.; Tsukimoto, M.; Kojima, S. TGF-β1 downregulates COX-2 expression leading to decrease of PGE2 production in human lung cancer A549 cells, which is involved in fibrotic response to TGF-β1. PLoS ONE 2013, 8, e76346. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, H.; Du, S.; Zhang, H.; Wang, H.; Zhang, Y.; Yang, Z.; Zhang, X.; Ren, S.; Chen, S.; Wang, C.; et al. Facile engineering of resveratrol nanoparticles loaded with 20(S)-protopanaxadiol for the treatment of periodontitis by regulating the macrophage phenotype. Nanoscale 2023, 15, 7894–7908. [Google Scholar] [CrossRef]
- Lee, J.H.; Moon, S.H.; Kim, H.S.; Park, E.; Ahn, D.U.; Paik, H.D. Immune-enhancing activity of phosvitin by stimulating the production of pro-inflammatory mediator. Poult Sci. 2017, 96, 3872–3878. [Google Scholar] [CrossRef]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; Ruiz-Rosado Jde, D.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-de-Arellano, M. Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE 2015, 10, e0145342. [Google Scholar] [CrossRef]
- Wang, N.; Xu, C.; Li, N.; Wang, F.; Wang, F.; Li, Z.; Yu, Q.; Zhang, G. Synergistic anti-inflammatory effects of resveratrol and vitamin E in lipopolysaccharide-induced RAW264.7 cells. Food Sci. Technol. 2022, 42, e24122. [Google Scholar] [CrossRef]
- Ou, Y.; You, Z.; Yao, M.; Cao, Y.; Xue, X.; Chen, M.; Wu, R.; Gan, L.; Li, D.; Wu, P.; et al. Naproxen-derived new compound inhibits the NF-κB, MAPK and PI3K/Akt signaling pathways synergistically with resveratrol in RAW264.7 cells. Molecules 2023, 28, 3395. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zhang, Z.; Wan, J.Y.; Zhang, C.F.; Anderson, S.; He, X.; Yu, C.; He, T.C.; Qi, L.W.; Yuan, C.S. Protopanaxadiol, an active ginseng metabolite, significantly enhances the effects of fluorouracil on colon cancer. Nutrients 2015, 7, 799–814. [Google Scholar] [CrossRef]
- Ben-Eltriki, M.; Deb, S.; Shankar, G.; Meckling, G.; Hassona, M.; Yamazaki, T.; Fazli, L.; Chin, M.Y.; Tomlinson Guns, E.S. Anti-tumor effects of ginsenoside 20(S)-protopanaxadiol and 1,25-dihydroxyvitamin D3 combination in castration resistant prostate cancer. Medicines 2021, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, K.; Lu, L.; Li, M.; Han, M.; Guo, Y.; Wang, X. Improved therapeutic efficacy of CBD with good tolerance in the treatment of breast cancer through nanoencapsulation and in combination with 20(S)-protopanaxadiol (PPD). Pharmaceutics 2022, 14, 1533. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Zhang, S.; Han, S.; Qiu, B.; Lu, Y.; Huang, W.; Li, F.; Chen, D.; Berglund, B.; Xiao, H.; et al. The role of dihydroresveratrol in enhancing the synergistic effect of ligilactobacillus salivarius Li01 and resveratrol in ameliorating colitis in mice. Research 2022, 2022, 9863845. [Google Scholar] [CrossRef] [PubMed]
- Uzzaman, M.; Mahmud, T. Structural modification of aspirin to design a new potential cyclooxygenase (COX-2) inhibitors. Silico Pharmacol. 2020, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Serhan, C.N. Aspirin triggers formation of anti-inflammatory mediators: New mechanism for an old drug. Discov. Med. 2004, 4, 470–475. [Google Scholar]
- Liu, Y.; Fang, S.; Li, X.; Feng, J.; Du, J.; Guo, L.; Su, Y.; Zhou, J.; Ding, G.; Bai, Y.; et al. Aspirin inhibits LPS-induced macrophage activation via the NF-κB pathway. Sci. Rep. 2017, 7, 11549. [Google Scholar] [CrossRef]
- Vartiainen, N.; Goldsteins, G.; Keksa-Goldsteine, V.; Chan, P.H.; Koistinaho, J. Aspirin inhibits p44/42 mitogen-activated protein kinase and is protective against hypoxia/reoxygenation neuronal damage. Stroke 2003, 34, 752–757. [Google Scholar] [CrossRef]
- Zeng, Y.P.; Yang, C.; Li, Y.; Fan, Y.; Yang, H.J.; Liu, B.; Sang, H.X. Aspirin inhibits osteoclastogenesis by suppressing the activation of NF-κB and MAPKs in RANKL-induced RAW264.7 cells. Mol. Med. Rep. 2016, 14, 1957–1962. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Lee, K.-S.; Kim, J.-H.; Lee, D.-K.; Park, M.; Choi, S.; Park, W.; Kim, S.; Choi, Y.K.; Hwang, J.Y.; et al. Aspirin prevents TNF-α-induced endothelial cell dysfunction by regulating the NF-κB-dependent miR-155/eNOS pathway: Role of a miR-155/eNOS axis in preeclampsia. Free Radic. Biol. Med. 2017, 104, 185–198. [Google Scholar] [CrossRef]
- Darby, I.A.; Weller, C.D. Aspirin treatment for chronic wounds: Potential beneficial and inhibitory effects. Wound Repair Regen. 2017, 25, 7–12. [Google Scholar] [CrossRef]
- Müller, N. COX-2 inhibitors, aspirin, and other potential anti-inflammatory treatments for psychiatric disorders. Front. Psychiatry 2019, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Monmai, C.; Kim, J.-S.; Baek, S.-H. Anti-inflammatory efficacy of resveratrol-enriched rice callus extract on lipopolysaccharide-stimulated RAW264.7 macrophages. Immuno 2024, 4, 131–146. [Google Scholar] [CrossRef]



| Treatment | DJ526 (% w/w) | DJ-PPD (% w/w) | Piceid Content (×10−2 ng) | Resveratrol Content (×10−2 ng) | PPD Content (×10−2 ng) |
|---|---|---|---|---|---|
| DJ526 | 100 | 0 | 47.2 | 26.1 | 0.0 |
| DJ-PPD | 0 | 100 | 0.0 | 0.0 | 72.8 |
| TR_1 | 30 | 70 | 14.2 | 7.8 | 51.0 |
| TR_2 | 50 | 50 | 23.6 | 13.1 | 36.4 |
| TR_3 | 70 | 30 | 33.0 | 18.3 | 21.8 |
| Treatment | A260:A280 | A260:A230 | Concentration (ng/µL) | SD * | CV ** |
|---|---|---|---|---|---|
| Nontreatment (RPMI) | 1.974 | 1.851 | 351.49 | 8.12 | 2.31 |
| DMSO | 1.996 | 1.832 | 389.97 | 12.88 | 3.30 |
| Aspirin | 1.993 | 1.942 | 802.40 | 29.99 | 3.74 |
| DJ526 | 2.001 | 1.905 | 826.93 | 17.33 | 2.10 |
| DJ-PPD | 1.989 | 1.988 | 885.23 | 30.78 | 3.48 |
| TR_1 | 1.969 | 1.841 | 827.03 | 11.24 | 1.36 |
| TR_2 | 1.997 | 1.915 | 836.19 | 17.22 | 2.06 |
| TR_3 | 1.975 | 1.887 | 958.79 | 12.05 | 1.26 |
| Process | Temperature (°C) | Time | Number of Cycles |
|---|---|---|---|
| Predenaturation | 95 | 10 min | 1 cycle |
| Denaturation | 95 | 20 s | 40 cycles |
| Annealing | 60 | 20 s | |
| Elongation | 72 | 30 s | |
| Final elongation | 72 | 5 min | 1 cycle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monmai, C.; Baek, S.-H. Anti-Inflammatory Effects of the Combined Treatment of Resveratrol- and Protopanaxadiol-Enriched Rice Seed Extract on Lipopolysaccharide-Stimulated RAW264.7 Cells. Molecules 2024, 29, 4343. https://doi.org/10.3390/molecules29184343
Monmai C, Baek S-H. Anti-Inflammatory Effects of the Combined Treatment of Resveratrol- and Protopanaxadiol-Enriched Rice Seed Extract on Lipopolysaccharide-Stimulated RAW264.7 Cells. Molecules. 2024; 29(18):4343. https://doi.org/10.3390/molecules29184343
Chicago/Turabian StyleMonmai, Chaiwat, and So-Hyeon Baek. 2024. "Anti-Inflammatory Effects of the Combined Treatment of Resveratrol- and Protopanaxadiol-Enriched Rice Seed Extract on Lipopolysaccharide-Stimulated RAW264.7 Cells" Molecules 29, no. 18: 4343. https://doi.org/10.3390/molecules29184343
APA StyleMonmai, C., & Baek, S.-H. (2024). Anti-Inflammatory Effects of the Combined Treatment of Resveratrol- and Protopanaxadiol-Enriched Rice Seed Extract on Lipopolysaccharide-Stimulated RAW264.7 Cells. Molecules, 29(18), 4343. https://doi.org/10.3390/molecules29184343






