Estimation of the Vaporization Enthalpies and Vapor Pressures of α-Tocopherol and Δ9-Tetrahydrocannabinol via the Use of a Surrogate, Correlation Gas Chromatography, and Synthetic and Retrosynthetic Analysis
Abstract
1. Introduction
2. Vaporization Enthalpies via Synthetic Analysis
2.1. n-Alkanes
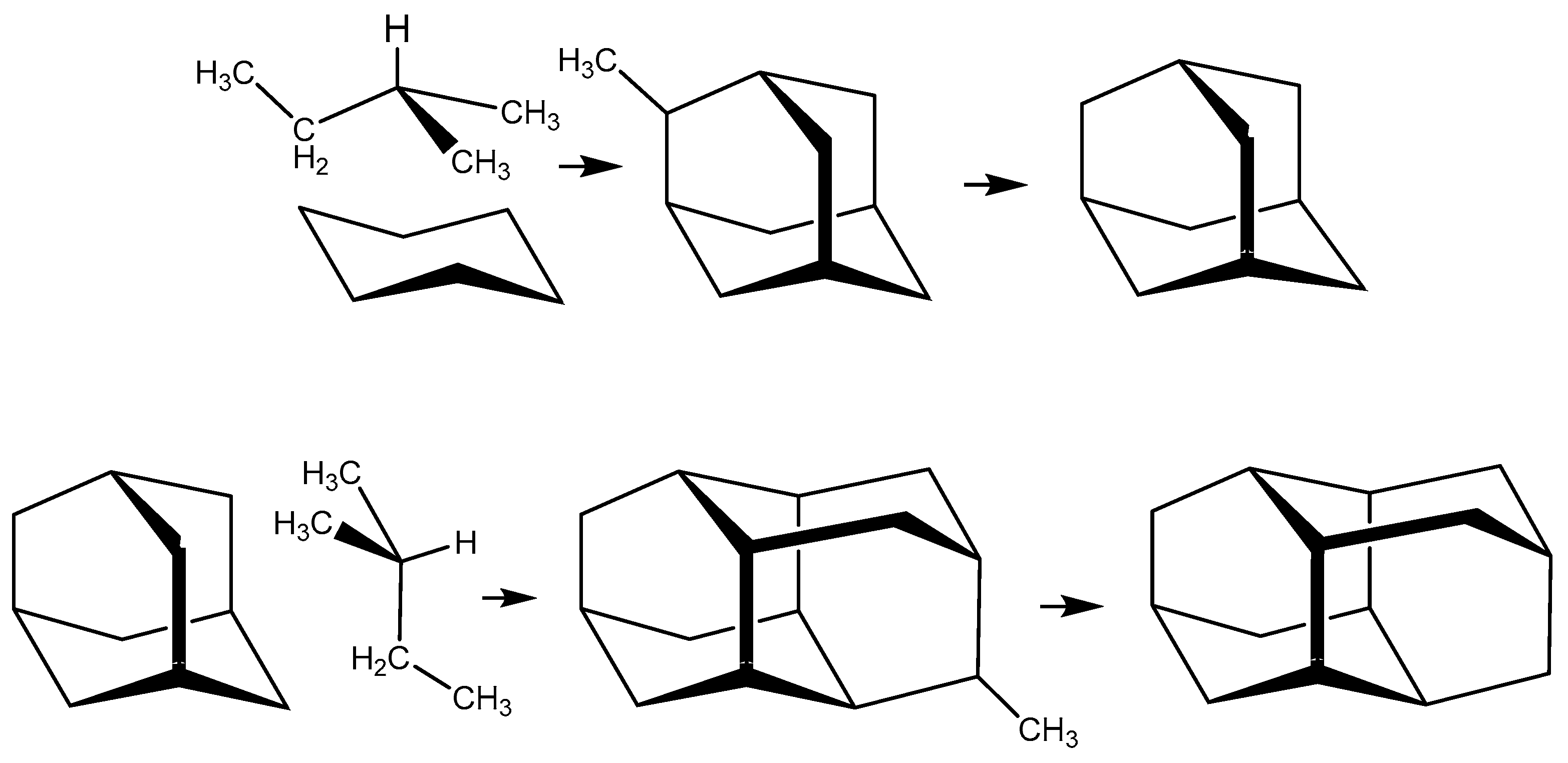
2.2. Synthetic Analysis of Adamantane and Diamantane at T = 298.15 K by Way of Their 2-Methyl Derivatives
2.3. Synthetic Analysis of Androstane and Cholestane
3. Evaluation of ΔlgH(298 K) of PMC at T = 298.15 K via Correlation Gas Chromatography
4. Experimental Methods
4.1. Methods
4.2. Evaluation of Vaporization Enthalpy
4.3. Evaluation of Vapor Pressure
4.4. Uncertainties
5. Experimental Results
5.1. Vaporization Enthalpies of PMC, Δ9-THC) and α-TOC at T = 298.15 via Correlation Gas Chromatography
5.2. Vapor Pressures of PMC, Δ9-THC, and α-TOC at T = 298.15 via Correlation Gas Chromatography Using n-Alkanes as Standards
6. Vaporization Enthalpies of α-TOC and Δ9-THC via Synthetic and Retrosynthetic Analysis
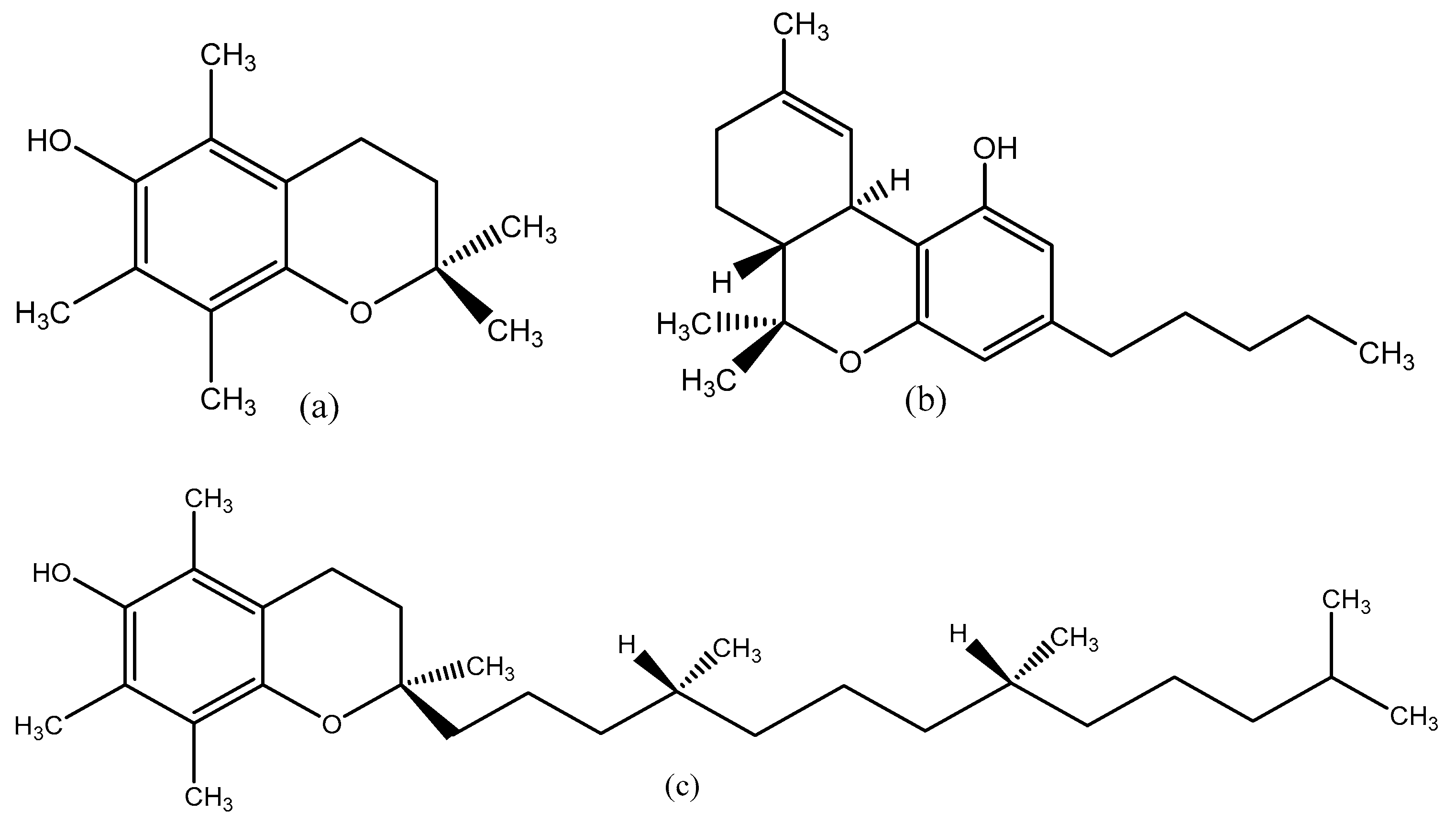
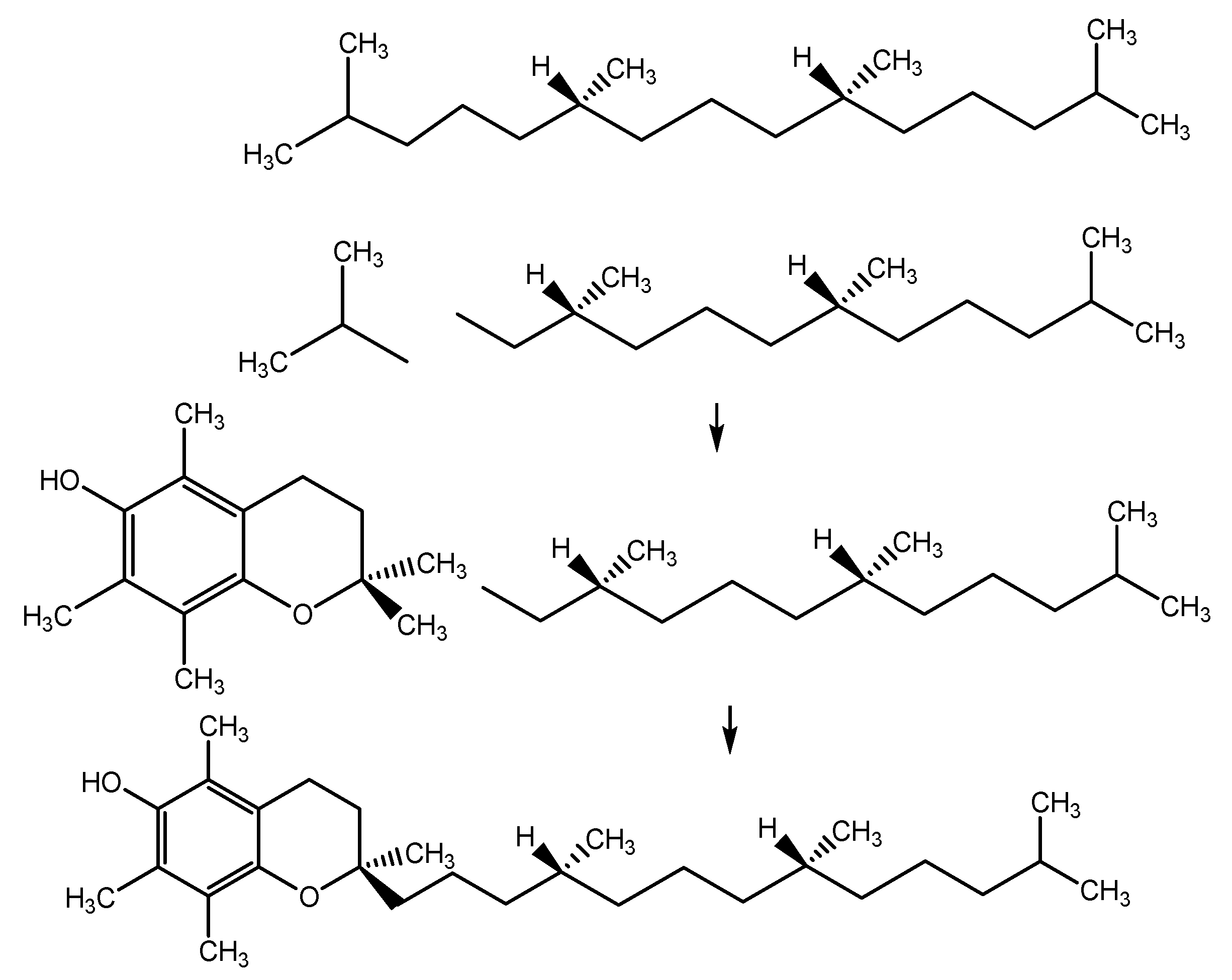
6.1. Estimation of α-TOC via Synthetic Analysis
6.2. Estimation of the Vaporization Enthalpy of Δ9-THC via Synthetic Analysis
7. Liquid Vapor Pressures of PMC, Δ9-THC, and α-TOC
8. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vitamin E. Available online: http://fnic.nal.usda.gov/food-composition/vitamins-and-minerals (accessed on 13 September 2016).
- Bernardes, C.E.S.; Simoes, R.G.; Diogo, H.P.; Minas da Piedade, M.E. Thermochemistry of 2,2,5,7,8-pentamethylchroman-6-ol (PMC) and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox). J. Chem. Thermodyn. 2014, 73, 140–147. [Google Scholar] [CrossRef]
- Russo, E. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effect. British J.Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Guy, G.W.; Robson, P.J. Cannabis, pain, and sleep: Lessons from therapeutic clinical trials of Sativex, a cannabis-based medicine. Chem. Biodivers. 2007, 4, 1729–1743. [Google Scholar] [CrossRef] [PubMed]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. The Absolute Configuration of Δ1-Tetrahydrocannabinol, the Major Active Constituent of Hashish. Tetrahedron Lett. 1967, 12, 1109–1111. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. A Total Synthesis of dl-Δ1-Tetrahydrocannabinol, the Active Constituent of Hashish. J. Am. Chem. Soc. 1965, 87, 3273–3274. [Google Scholar] [CrossRef]
- Desrosiers, N.A.; Himes, S.K.; Scheidweiler, K.B.; Concheiro-Guisan, M.; Gorelick, D.; Huestis, A.M.A. Phase I and II Cannabinoid Disposition in Blood and Plasma of Occasional and Frequent Smokers Following Controlled Smoked Cannabis. Clin. Chem. 2014, 60, 631–643. [Google Scholar] [CrossRef]
- Lovestead, T.M.; Bruno, T.J. Determination of cannabinoid vapor pressures to aid in vapor phase detection of intoxication. Forensic Chem. 2017, 5, 79–85. [Google Scholar] [CrossRef]
- Kolsk, Z.; Ruzicka, V.; Gani, R. Estimation of the Enthalpy of Vaporization and the Entropy of Vaporization for Pure Organic Compounds at 298.15 K and at Normal Boiling Temperature by a Group Contribution Method. Ind. Eng. Chem. Res. 2005, 44, 8436–8454. [Google Scholar] [CrossRef]
- Naef, R.; Acree, W.E., Jr. Calculation of the Vapour Pressure of Organic Molecules by Means of a Group-Additivity Method and Their Resultant Gibbs Free Energy and Entropy of Vaporization at 298.15 K. Molecules 2021, 26, 1045. [Google Scholar] [CrossRef]
- Chickos, J.S.; Acree, W.E., Jr. Enthalpies of Vaporization of Organic and Organometallic Compounds, 1880–2002. J. Phys. Chem. Ref. Data 2003, 32, 519–877. [Google Scholar] [CrossRef]
- Chickos, J.S.; Acree, W.E., Jr. Phase Transition Enthalpy Measurements of Organic Compounds. An Update of Sublimation, Vaporization, and Fusion Enthalpies from 1880 to 2010. J. Phys. Chem. Ref. Data 2022, 39, 043101. [Google Scholar]
- Acree, W.E., Jr.; Chickos, J.S. Phase Transition Enthalpy Measurements of Organic and Organometallic Compounds. Sublimation, Vaporization and Fusion Enthalpies From 1880 to 2015. Part 1. C1−C10. J. Phys. Chem. Ref. Data 2016, 45, 033101. [Google Scholar]
- Acree, W.E., Jr.; Chickos, J.S. Phase Transition Enthalpy Measurements of Organic and Organometallic Compounds. Sublimation, Vaporization and Fusion Enthalpies From 1880 to 2015. Part 2 C11–C192. J. Phys. Chem. Ref. Data 2017, 46, 013104. [Google Scholar]
- Acree, W.E., Jr.; Chickos, J.S. Phase Transition Enthalpy Measurements of Organic Compounds. An Update of Sublimation, Vaporization, and Fusion Enthalpies from 2016 to 2021. J. Phys. Chem. Ref. Data 2022, 51, 043101. [Google Scholar] [CrossRef]
- Chickos, J. An update on liquid heat capacity estimations of cyclic organic compounds by group additivity and their application in estimations of complex molecules by synthetic and retrosynthetic analysis. J. Chem. Thermodyn. 2023, 182, 107039. [Google Scholar] [CrossRef]
- Guthrie, J.P.; Taylor, K.F. Additivity methods for estimating heat of vaporization of organic molecules. J. Can. Chem. 1983, 61, 602–607. [Google Scholar] [CrossRef]
- Benson, S.W. Thermochemical Kinetics, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1976. [Google Scholar]
- Ruzicka, K.; Majer, V. Simultaneous Treatment of Vapor Pressures and Related Thermal Data Between the Triple and Normal Boiling Temperatures for n-Alkanes C5–C20. J. Phys. Chem. Ref. Data 1994, 23, 1–39. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hanshaw, W. Vapor Pressures and Vaporization Enthalpies of the n-Alkanes from C21 to C30 at T = 298.15 K by Correlation Gas Chromatography. J. Chem. Eng. Data 2004, 49, 77–85. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hanshaw, W. Vapor Pressures and Vaporization Enthalpies of the n-Alkanes from C31 to C38 at T = 298.15 K by Correlation Gas Chromatography. J. Chem. Eng. Data 2004, 49, 620–630. [Google Scholar] [CrossRef]
- Chickos, J.S.; Wang, T.; Sharma, E. Hypothetical Thermodynamic Properties: Vapor pressures and vaporization enthalpies of the even n-alkanes from C40 to C76 at T= 298.15 K by Correlation-gas chromatography. Are the vaporization enthalpies a linear function of carbon number. J. Chem. Eng. Data 2008, 53, 481–491. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hyman, A.S.; Ladon, L.H.; Liebman, J.F. Measurement of the Heats of vaporization of Hydrocarbons. J. Org. Chem. 1981, 4294–4296. [Google Scholar] [CrossRef]
- Diogo, H.P.; Santos, R.C.; Nunes, P.M.; Minas da Piedade, M.E. Ebulliometric apparatus for the measurement of enthalpies of vaporization. Thermochim. Acta 1995, 249, 113. [Google Scholar] [CrossRef]
- Wilhoit, R.C.; Zwolinski, B.J. Handbook of vapor pressures and heats of vaporization of hydrocarbons and related compounds. In API 44-TRC Publications in Science and Engineering; API: College Station, TX, USA, 1971. [Google Scholar]
- Nelson, C.; Chickos, J. The vaporization enthalpy and vapor pressures of liquid adamantane, diamantane and α- and β-cedrene by correlation gas chromatography. J. Chem. Thermodyn. 2018, 121, 175–186. [Google Scholar] [CrossRef]
- Osborne, N.S.; Ginnings, D.C. Measurements of Heat of Vaporization and Heat Capacity of a Number of Hydrocarbons. J. Res. Natl. Bur. Stand. 1947, 39, 453–477. [Google Scholar] [CrossRef]
- Camin, D.L.; Rossini, F.D. Physical Properties of 14 American Petroleum Institute Research Hydrocarbons, C9 to C15. J. Phys. Chem. 1955, 59, 1173–1179. [Google Scholar] [CrossRef]
- Fischer-Lodike, C.; Albinsaad, M.; Chickos, J.S. Vaporization Enthalpies and Vapor Pressures of 5α-Androstane and 5α-Cholestane by Correlation Gas Chromatography. Liquids 2024, 4, 456–469. [Google Scholar] [CrossRef]
- Mokbel, I.; Ruzicka, K.; Majer, V.; Ruzicka, V.; Ribeiro, M.; Jose, J.; Zabransky, M. Vapor pressures and thermal data for three high boiling compounds of petroleum interest: 1-phenyldodecane, (5α)-cholestane, adamantane. Fluid Phase Equil. 2000, 169, 191–207. [Google Scholar] [CrossRef]
- Damaceno, D.S.; Matricarde Falleiro, R.M.; Krahenbuhl, M.A.; Meirelles, A.J.A.; Ceriani, R. Boiling Points of Short-Chain Partial Acylglycerols and Tocopherols at Low Pressures by the Differential Scanning Calorimetry Technique. J. Chem. Eng. Data 2014, 59, 1515–1520. [Google Scholar] [CrossRef]
- Kerkache, H.; Bathily, A.; Chiriac, R.; Goutaudier, C.; Paricaud1, P.; Nicolle, A. Vapor-Liquid equilibria of α-tocopherol in transportation fuels surrogates: An experimental and modeling study. Fuel 2022, 319, 123866. [Google Scholar] [CrossRef]
- Available online: http://www.bipm.org/en/publications/guides/gum.html (accessed on 2 December 2019).
- Estimate. Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994–2024 ACD/Labs) through SciFinder, version 11.02, Chemical Abstracts Service: Columbus, OH, USA.
- Fischer-Lodike, C.; Zafar, A.; Chickos, J. The vapor pressure and vaporization of pristane and phytane by correlation gas chromatography. J. Chem. Thermodyn. 2000, 141, 105931. [Google Scholar] [CrossRef]
- Zabransky, M.; Ruzicka, V., Jr.; Majer, V.; Domalski, E.S. Heat Capacity of Liquids, Critical Review and Recommended Values. J. Phys. Chem. Ref. Data 1996, 1, R7. [Google Scholar] [CrossRef]
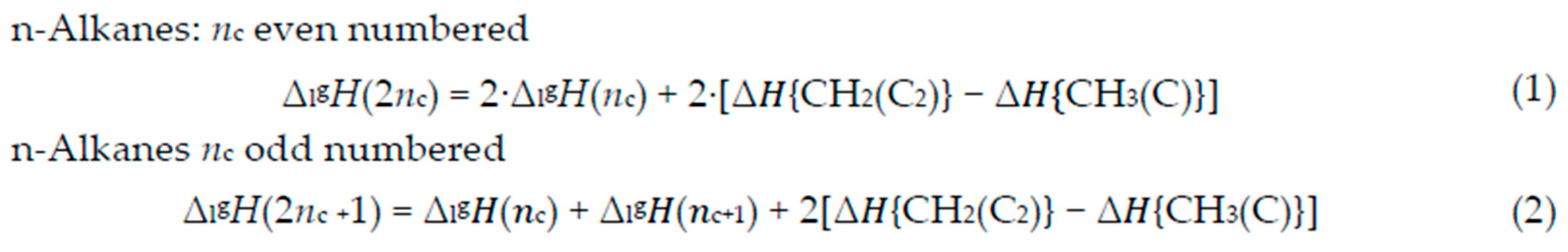





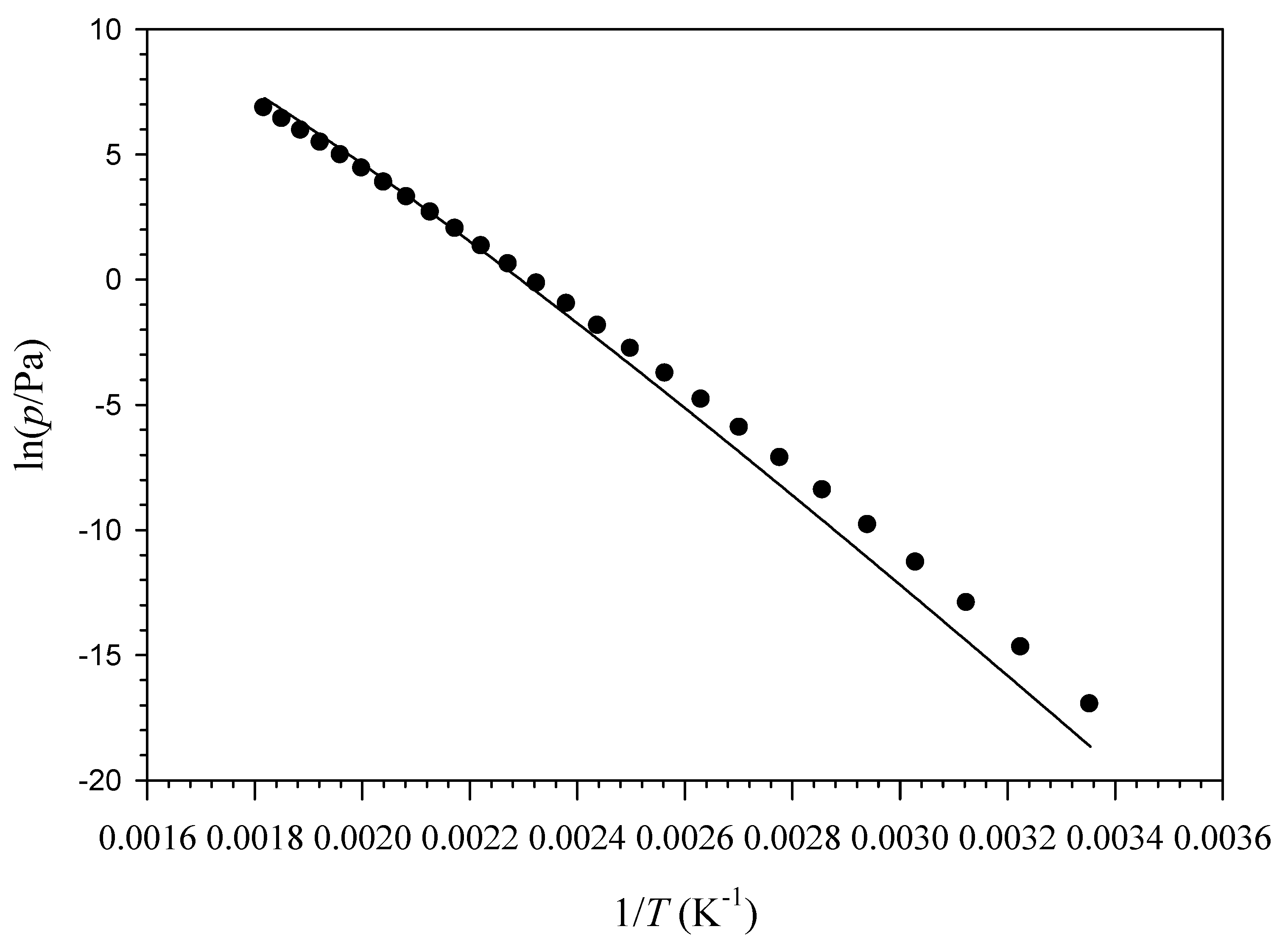
| Group Value | Uncertainty | Group | Group Value | Uncertainty | |
|---|---|---|---|---|---|
| CH3(C) | 5.69 | ±0.04 | CdH2 | 4.18 | ±1.5 |
| CH2(C2) | 5.06 | ±0.04 | CdH(C) | 5.15 | ±1.13 |
| CH(C3) | 3.05 | ±0.13 | Cd(C2) | 4.85 | ±1.17 |
| C(C4) | 0.335 | ±0.33 | CB(H) | 5.61 | ±0.08 |
| CH2(C)(Cd) | 5.61 | ±1.17 | CB(C) | 4.64 | ±0.21 |
| CH(C2)(Cd) | 4.77 | ±4.39 | CH2(C)(CB) | 3.31 | ±0.75 |
| C(C3)(Cd) | −1.21 | ±2.97 | CH(C2)(CB) | 1.00 | ±1.00 |
| n-Alkane | ΔlgH(298.15 K) Calcd. Lit. a | n-Alkane | ΔlgH(298.15 K) Calcd. Lit. a | ||
|---|---|---|---|---|---|
| n-Pentane | 26.42 ± 0.14 | n-Tricosane | 116.8 ± 0.8 | 117.0 ± 2.8 b | |
| n-Hexane | 31.52 ± 0.16 | n-Tetracosane | 121.8 ± 0.9 | 121.9 ± 2.8 b | |
| n-Heptane | 36.57 ± 0.18 | n-Pentacosane | 126.9 ± 0.9 | 126.8 ± 2.9 b | |
| n-Octane | 41.56 ± 0.21 | n-Hexacosane | 132.1 ± 1.0 | 131.7 ± 3.2 b | |
| n-Nonane | 46.55 ± 0.23 | n-Heptacosane | 137.2 ± 1.0 | 135.6 ± 3.3 b | |
| n-Decane | 51.6 ± 0.5 | 51.42 ± 0.26 | n-Octacosane | 142.2 ± 1.1 | 141.9 ± 4.9 b |
| n-Undecane | 56.7 ± 0.6 | 56.58 ± 0.57 | n-Nonacosane | 147.2 ± 1.1 | 147.1 ± 5.1 b |
| n-Dodecane | 61.8 ± 0.6 | 61.52 ± 0.62 | n-Tricontane | 152.3 ± 1.1 | 152.3 ± 5.3 b |
| n-Tridecane | 66.8 ± 0.7 | 66.68 ± 0.67 | n-Henetriacontane | 156.9 ± 1.9 | 157.2 ± 1.4 c |
| n-Tetradecane | 71.9 ± 0.7 | 71.73 ± 0.77 | n-Dotriacontane | 161.4 ± 2.4 | 162.5 ± 1.4 c |
| n-Pentadecane | 76.9 ± 0.8 | 76.77 ± 0.81 | n-Triatricontane | 166.6 ± 2.4 | 167.6 ± 1.4 c |
| n-Hexadecane | 81.9 ± 0.8 | 81.35 ± 0.87 | n-Tetratriacontane | 171.7 ± 2.5 | 172.7 ± 1.5 c |
| n-Heptadecane | 86.9 ± 0.3 | 86.47 ± 0.9 | n-Pentatriacontane | 176.7 ± 2.6 | 178.1 ± 5.4 c |
| n-Octadecane | 91.8 ± 0.3 | 91.44 ± 0.9 | n-Hexatriaconane | 181.6 ± 2.7 | 182.8 ± 5.5 c |
| n-Nonadecane | 96.7 ± 0.4 | 96.44 ± 1.0 | n-Heptatriacontane | 186.6 ± 2.7 | 187.5 ± 5.6 c |
| n-Eicosane | 101.6 ± 0.5 | 101.81 ± 1.0 | n-Octatriacontane | 191.6 ± 2.7 | 192.6 ± 5.7 c |
| n-Heneicosane | 106.7 ± 0.6 | 106.8 ± 2.5 b | n-Nonatriacontane | 197.0 ± 2.8 | NA d |
| n-Docosane | 111.9 ± 0.8 | 111.9 ± 2.7 b | n-Tetracontane | 202.4 ± 2.8 | 203.5 ± 2.9 e |
| Literature Values [2] | Estimated/Derived Values | ||
|---|---|---|---|
| Cp,m(cr)(298.15 K) | 315.3 ± 9.6 J.mol−1.K−1 | Cp,m(l)(298.15 K) | 429.1 kJ.mol−1.K−1 a |
| Tfus = | 365.3 K | ΔcrlH(298.15 K) | 22.0 ± 1.0 kJ·mol−1 |
| ΔcrlH(Tfus K) | 27.0 ± 0.2 kJ.mol−1 | ΔlgH(Tfus/K) | 77.8 ± 0.9 kJ·mol−1 |
| ΔcrgH(341.5 K) | 105.9 ± 1.3 kJ.mol−1 b | ΔlgH(298.15 K) | 85.8 ± 1.5 kJ·mol−1 b |
| ΔcrgH(298.15 K) | 107.4 ± 0.8 kJ.mol−1 | ptp= | 12.2 Pa c |
| ΔcrgH(298.15 K)/kJ·mol−1 | ΔcrlH(298.15 K)/kJ·mol−1 | ΔlgH(298.15 K)/kJ·mol−1 |
|---|---|---|
| 107.4 ± 0.8 [2] | 22.0 ± 1.0 a | 85.8 ± 1.5 b |
| Compound | CASRN’s | Supplier | Mass Fraction |
|---|---|---|---|
| n-Hexadecane | 544-76-3 | Sigma/Aldrich | 0.99 |
| n-Heptadecane | 629-78-7 | Sigma/Aldrich | 0.99 |
| n-Nonadecane | 629-92-5 | Sigma/Aldrich | 0.99 |
| n-Eicosane | 112-95-8 | Sigma/Aldrich | 0.99 |
| n-Heneicosane | 629-94-7 | Sigma/Aldrich | 0.98 |
| n-Docosane | 629-97-0 | Sigma/Aldrich | 0.99 |
| n-Tetracosane | 646-31-1 | Sigma/Aldrich | 0.99 |
| n-Pentacosane | 629-99-2 | Sigma/Aldrich | 0.99 |
| n-Hexacosane | 630-01-3 | Sigma/Aldrich | 0.99 |
| n-Octacosane | 630-02-4 | Sigma/Aldrich | 0.99 |
| n-Triacontane | 638-68-6 | Sigma/Aldrich | 0.98 |
| n-Dotriacontane | 544-85-4 | Sigma/Aldrich | 0.97 |
| 2,2,5,7,8-Pentamethylchroman-6-ol | 950-99-2 | Sigma/Aldrich | 0.97 |
| (-)-trans Δ9-Tetrahydrocannabinol | 1972-08-3 | Supelco/Aldrich | 0.90 a |
| (±) α-Tocopherol | 10,191-41-0 | Supelco/Aldrich | 0.96 |
| Compound | ΔlgH(298.15 K) a (kJ·mol−1) | Ao | 103·A1(K−1) | 106·A2(K−2) | To/K | ||
|---|---|---|---|---|---|---|---|
| n-Hexadecane | 81.35 ± 0.81 | 3.18271 | −2.002545 | 1.384476 | 559.978 | ||
| n-Heptadecane | 86.47 ± 1.7 | 3.21826 | −2.036553 | 1.383899 | 575.375 | ||
| n-Nonadecane | 96.44 ± 1.9 | 3.27626 | −2.062714 | 1.346737 | 603.989 | ||
| n-Eicosane a | 101.81 ± 2.0 | 3.31181 | −2.102218 | 1.348780 | 617.415 | ||
| ΔlgH(298.15 K) b | 10−6·A(K)3 | 10−4·B(K)2 | C(K) | D | |||
| n-Heneicosane | 106.8 ± 2.4 | 199.89 | −290.75 | −98.135 | 6.6591 | ||
| n-Docosane | 111.9 ± 2.7 | 217.13 | −311.76 | 110.72 | 6.5353 | ||
| n-Tricosane | 117.0 ± 2.8 | 233.86 | −332.2 | 310.77 | 6.4198 | ||
| n-Tetracosane | 121.9 ± 2.8 | 250.72 | −352.86 | 530.15 | 6.2817 | ||
| n-Pentacosane | 126.8 ± 2.9 | 267.38 | −373.07 | 741.19 | 6.1496 | ||
| n-Hexacosane | 131.7 ± 3.2 | 282.44 | −391.93 | 910.53 | 6.0704 | ||
| n-Octacosane | 141.9 ± 4.9 | 313.89 | −431.20 | 1279.4 | 5.8835 | ||
| n-Triacontane | 152.3 ± 5.3 | 334.04 | −469.98 | 1601.6 | 5.7696 | ||
| n-Dotriacontane | 162.5 ± 2.8 c | 375.24 | −509.21 | 1947.2 | 5.6303 | ||
| Equation (19) | ΔlgH(298.15 K) | A3 e | B3 d,e | C3 d,e | D3 d,e | E3 d,e | |
| TOC | 153.7 d | 50.449 | −20,228.9 | 0.9786 | − 0.0229 | 1 | |
| Equation (20) | ΔcrgH(298.15 K) | a | b | ||||
| PMC f | 107.4 ± 0.8 | 37.35 ± 0.24 | (12,728.8 ± 80.6) | ||||
| Run S1 | –Slope T/K | Intercept | ΔHtrn(468 K) kJ·mol−1 | ΔlgH(298 K) kJ·mol−1 (lit) b | ΔlgH(298 K) kJ·mol−1 (calc) | |
|---|---|---|---|---|---|---|
| n-Hexadecane | 6293.4 ± 22 | 12.933 ± 0.047 | 52.32 ± 0.18 | 81.35 ± 0.8 | 81.4 ± 1.1 | |
| n-Heptadecane | 6680.9 ± 22 | 13.385 ± 0.047 | 55.54 ± 0.18 | 86.47 ± 1.7 | 86.3 ± 1.1 | |
| PMC | 6405.4 ± 18 | 12.489 ± 0.039 | 53.25 ± 0.15 | 82.8 ± 1.1 | ||
| n-Nonadecane | 7484.9 ± 25 | 14.357 ± 0.054 | 62.23 ± 0.21 | 96.44 ± 1.9 | 96.7 ± 1.2 | |
| n-Eicosane | 7876.3 ± 25 | 14.828 ± 0.053 | 65.48 ± 0.20 | 101.81 ± 2.0 | 101.8 ± 1.2 | |
| n-Heneicosane | 8259.4 ± 0.27 | 15.28 ± 0.057 | 68.67 ± 0.22 | 106.8 ± 2.2 c | 106.7 ± 1.2 | |
| ΔlgH(298.15 K)/kJ·mol−1 = (1.55 ± 0.014)ΔHtrn(468 K) + 0.211 ± 0.0.83) r2 = 0.9998 | (21) | |||||
| Run S5 | –Slope T/K | Intercept | ΔHtrn(503 K) kJ·mol−1 | ΔlgH(298 K) kJ·mol−1 (lit) c | ΔlgH(298 K) kJ·mol−1 (calc) | |
| PMC | 6102.8 ± 41 | 11.858 ± 0.081 | 50.74 ± 0.34 | 84.1 ± 0.4 | ||
| n-Eicosane | 7426.9 ± 37 | 13.898 ± 0.074 | 61.74 ± 0.31 | 101.81 ± 2.0 b | 101.5 ± 0.4 | |
| n-Heneicosane | 7790.2 ± 36 | 14.314 ± 0.072 | 64.76 ± 0.30 | 106.8 ± 2.2 | 106.4 ± 0.4 | |
| n-Docosane | 8178.0 ± 35 | 14.781 ± 0.071 | 67.99 ± 0.30 | 111.9 ± 2.7 | 111.7 ± 0.4 | |
| n-Tetracosane | 8927.0 ± 37 | 15.667 ± 0.074 | 74.22 ± 0.31 | 121.9 ± 2.8 | 122.0 ± 0.4 | |
| Δ9-THC | 8620.8 ± 40 | 14.835 ± 0.080 | 71.67 ± 0.33 | 117.8 ± 0.4 | ||
| n-Pentacosane | 9292.8 ± 38 | 16.095 ± 0.075 | 77.26 ± 0.32 | 126.8 ± 2.9 | 127.0 ± 0.4 | |
| n-Hexacosane | 9649.0 ± 42 | 16.506 ± 0.83 | 80.22 ± 0.35 | 131.7 ± 3.2 | 131.9 ± 0.5 | |
| ΔlgH(298.15 K)/kJ·mol−1 = (1.612 ± 0.004)ΔHtrn(503 K) + 2.306 ± 0.33 r2 = 0.9997 | (22) | |||||
| Run S7 | –Slope T/K | Intercept | ΔHtrn(503 K) kJ·mol−1 | ΔlgH(298 K) kJ·mol−1 (lit) c | ΔlgH(298 K) kJ·mol−1 (calc) | |
| PMC | 5951.0 ± 28 | 11.557 ± 0.052 | 49.47 ± 0.23 | 82.1 ± 0.5 | ||
| n-Tetracosane | 8688.8 ± 29 | 15.191 ± 0.055 | 72.24 ± 0.24 | 121.7 ± 2.8 | 121.7 ± 0.6 | |
| n-Pentacosane | 9331.1 ± 30 | 16.180 ± 0.059 | 77.58 ± 0.25 | 126.8 ± 2.9 | 126.9 ± 1.3 | |
| n-Hexacosane | 9704.2 ± 24 | 16.625 ± 0.047 | 80.68 ± 0.20 | 131.7 ± 3.2 | 132.0 ± 1.3 | |
| n-Octacosane | 10,133.2 ± 32 | 16.875 ± 0.060 | 84.24 ± 0.27 | 141.9 ± 4.9 | 142.2 ± 0.7 | |
| (±) α-Tocopherol | 10,566.6 ± 42 | 16.938 ± 0.078 | 87.85 ± 0.35 | 148.3 ± 0.7 | ||
| Triacontane | 10,845.3 ± 31 | 17.708 ± 0.058 | 90.16 ± 0.26 | 152.3 ± 0.6 | 152.3 ± 0.7 | |
| n-Dotriacontane | 11,557.6 ± 36 | 18.544 ± 0.068 | 96.09 ± 0.30 | 162.5 ± 0.7 d | 162.4 ± 0.7 | |
| ΔlgH(298.15 K)/kJ·mol−1 = (1.705 ± 0.005)ΔHtrn(533 K) − (1.424 ± 0.456) r2 = 0.9999 | (23) | |||||
| Run | PMC | Δ9-THC | α-TOC |
|---|---|---|---|
| S1 | 82.8 ± 1.1 | ||
| S2 | 82.9 ± 1.1 | ||
| S3 | 117.9 ± 0.3 | ||
| S4 | 118.1 ± 0.4 | ||
| S5 | 84.1 ± 0.4 | 117.8 ± 0.4 | |
| S6 | 84.0 ± 0.3 | 117.6 ± 0.3 | |
| S7 | 82.7 ± 1.7 | 148.1 ± 2.0 | |
| S8 | 83.0 ± 1.4 | 148.3 ± 1.8 | |
| Average | 83.3 ± 1.1 | 117.9 ± 0.4 | 148.2 ± 1.9 |
| “Alkane Adjusted” | 85.7 ± 2.2 | 120.4 ± 1.8 | 150.7 ± 2.6 |
| 9A | ln(to/ta)avg | ln(p/po) | ln(p/po)calc | 104·p/Pa | 104·p/Pa(lit) a | |
|---|---|---|---|---|---|---|
| n-Hexadecane | −8.150 | −13.181 | −13.18 ± 0.10 | 1920 ± 190 | 1910 | |
| n-Heptadecane | −9.002 | −14.315 | −14.30 ± 0.10 | 620 ± 64 | 615 | |
| PMC | −8.977 | −14.27 ± 0.10 | 640 ± 66 | |||
| n-Nonadecane | −10.737 | −16.551 | −16.59 ± 0.11 | 63 ± 6.8 | 66 | |
| n-Eicosane | −11.579 | −17.696 | −17.70 ± 0.12 | 21 ± 2.4 | 21 | |
| n-Heneicosane | −12.425 | −18.836 | −18.81 ± 0.12 | 6.8 ± 0.82 | 6.7 | |
| ln(p/po) = (1.318 ± 0.007) ln(to/ta)avg − (2.43 ± 0.08) r2 = 0.9999 | (24) | |||||
| 9B | 104·p/Pa | 104·p/Pa | ||||
| PMC | −8.620 | −14.45 ± 0.08 | 530 | |||
| n-Eicosane | −11.027 | −17.696 | −17.72 ± 0.09 | 20 | 21 | |
| n-Heneicosane | −11.839 | −18.836 | −18.83 ± 0.09 | 6.8 | 6.7 b | |
| n-Docosane | −12.668 | −19.972 | −19.95 ± 0.10 | 2.2 | 2.1 b | |
| n-Tetracosane | −14.291 | −22.175 | −22.16 ± 0.10 | 0.24 | 0.24 b | |
| Δ9-THC | −14.089 | −21.88 ± 0.10 | 0.32 | 0.26 c | ||
| n-Pentacosane | −15.095 | −23.244 | −23.25 ± 0.10 | 0.08 | 0.08 b | |
| n-Hexacosane | −15.890 | −24.309 | −24.33 ± 0.11 | 0.028 | 0.028 b | |
| ln(p/po) = (1.358 ± 0.005) ln(to/ta)avg − (2.74 ± 0.07) r2 = 0.9999 | (25) | |||||
| 9C | 106·p/Pa | 106·p/Pa | ||||
| PMC | −8.407 | −14.34 ± 0.27 | 60,000 ± 18,000 | |||
| n-Tetracosane | −13.948 | −22.175 | −22.13 ± 0.43 | 25 ± 8.6 | 24 | |
| n-Octacosane | −17.101 | −26.490 | −26.57 ± 0.48 | 0.29 ± 0.11 | 32 | |
| n-Triacontane | −18.650 | −28.748 | −28.75 ± 0.51 | 0.033 ± 0.013 | 0.033 | |
| α-Tocopherol | −18.472 | −28.50 ± 0.27 | 0.042 ± 0.017 | 0.008 d | ||
| n-Dotriacontane | −20.195 | −30.964 | −30.92 ± 0.54 | 0.0038 ± 0.0016 | 0.0036 e | |
| ln(p/po) = (1.407 ± 0.015) ln(to/ta)avg − (2.51 ± 0.27) r2 = 0.9998 | (26) | |||||
| A | B | C | |||
|---|---|---|---|---|---|
| PMC | n-BP/K b | ||||
| Runs 1 and 2 | 9.555 ± 0.113 | −4119.6 ± 78 | −889,762 ± 13,295 | ||
| Runs 3 and 4 | 9.682 ± 0.101 | −4165.8 ± 70 | −903,771 ± 11,943 | ||
| Runs 5 and 6 | 9.632 ± 0.102 | −4185 ± 70.5 | −883,491 ± 12,076 | ||
| Runs 1−6 | 9.631 ± 0.003 | −4164.4 ± 0.2 | −890,573 ± 33.8 | 589 c | 617 ± 42 d |
| Δ9-THC | p298.15 K/Pa | ||||
| Runs 5 and 6 | 11.666 ± 0.128 | −5670.6 ± 122.6 | −1,296,183 ± 21,000 | 3.0·10−5 c | 2.57·10−5 e |
| (±) α-TOC | P549 K/Pa | ||||
| Runs 7 and 8 | 13.502 ± 0.241 | −7016.5 ± 166 | −7016.5 ± 28,452 | 900 c | 1100 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, C.; Fischer-Lodike, C.; Chickos, J.S. Estimation of the Vaporization Enthalpies and Vapor Pressures of α-Tocopherol and Δ9-Tetrahydrocannabinol via the Use of a Surrogate, Correlation Gas Chromatography, and Synthetic and Retrosynthetic Analysis. Molecules 2024, 29, 4332. https://doi.org/10.3390/molecules29184332
Nelson C, Fischer-Lodike C, Chickos JS. Estimation of the Vaporization Enthalpies and Vapor Pressures of α-Tocopherol and Δ9-Tetrahydrocannabinol via the Use of a Surrogate, Correlation Gas Chromatography, and Synthetic and Retrosynthetic Analysis. Molecules. 2024; 29(18):4332. https://doi.org/10.3390/molecules29184332
Chicago/Turabian StyleNelson, Carissa, Christian Fischer-Lodike, and James S. Chickos. 2024. "Estimation of the Vaporization Enthalpies and Vapor Pressures of α-Tocopherol and Δ9-Tetrahydrocannabinol via the Use of a Surrogate, Correlation Gas Chromatography, and Synthetic and Retrosynthetic Analysis" Molecules 29, no. 18: 4332. https://doi.org/10.3390/molecules29184332
APA StyleNelson, C., Fischer-Lodike, C., & Chickos, J. S. (2024). Estimation of the Vaporization Enthalpies and Vapor Pressures of α-Tocopherol and Δ9-Tetrahydrocannabinol via the Use of a Surrogate, Correlation Gas Chromatography, and Synthetic and Retrosynthetic Analysis. Molecules, 29(18), 4332. https://doi.org/10.3390/molecules29184332









