Promising Therapeutic Strategies for Hematologic Malignancies: Innovations and Potential
Abstract
1. Introduction
2. Therapeutic Strategies
2.1. Immune Checkpoint Inhibitors
2.2. Small Molecule Inhibitors
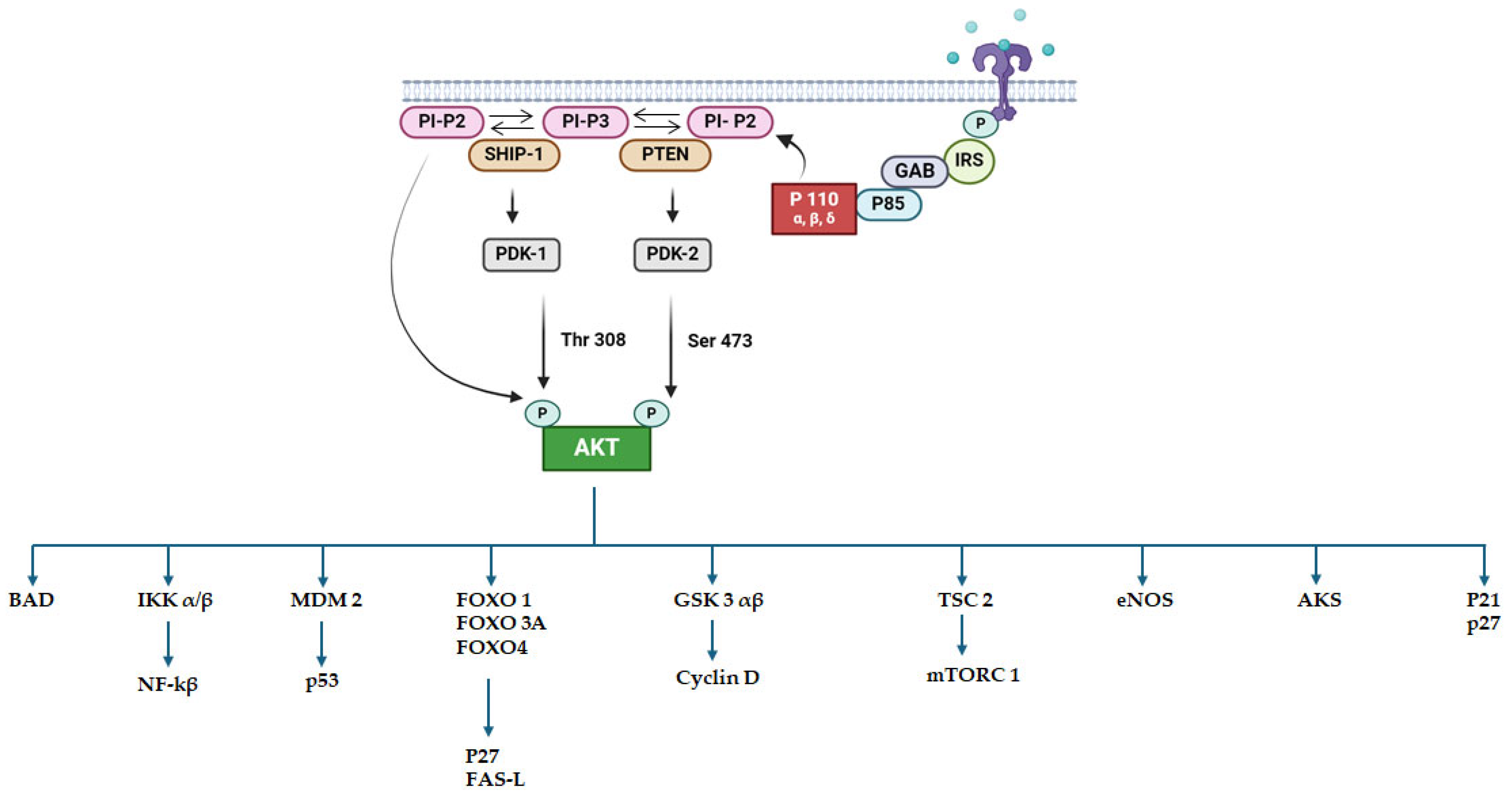
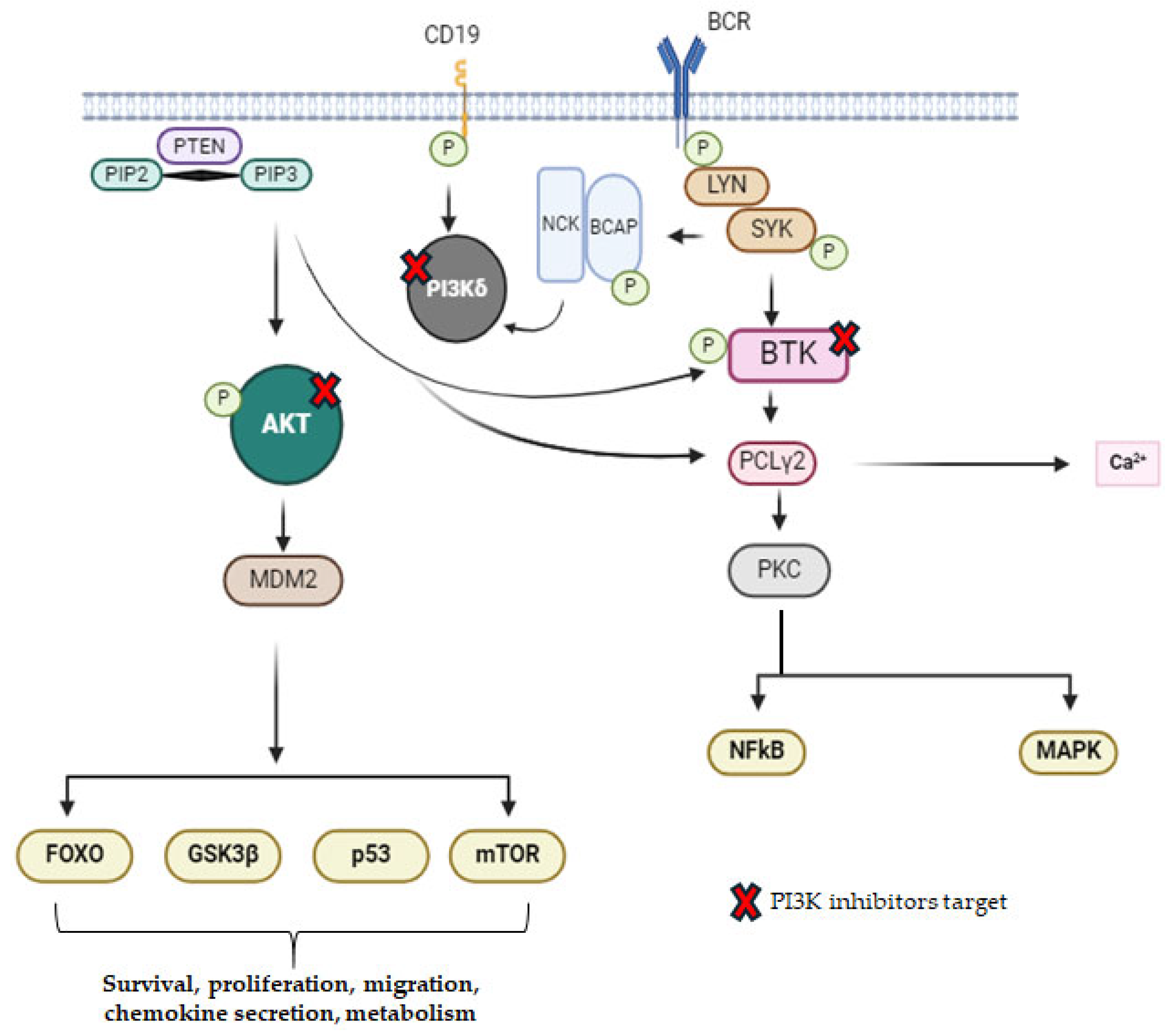
2.3. PI3K Inhibitors
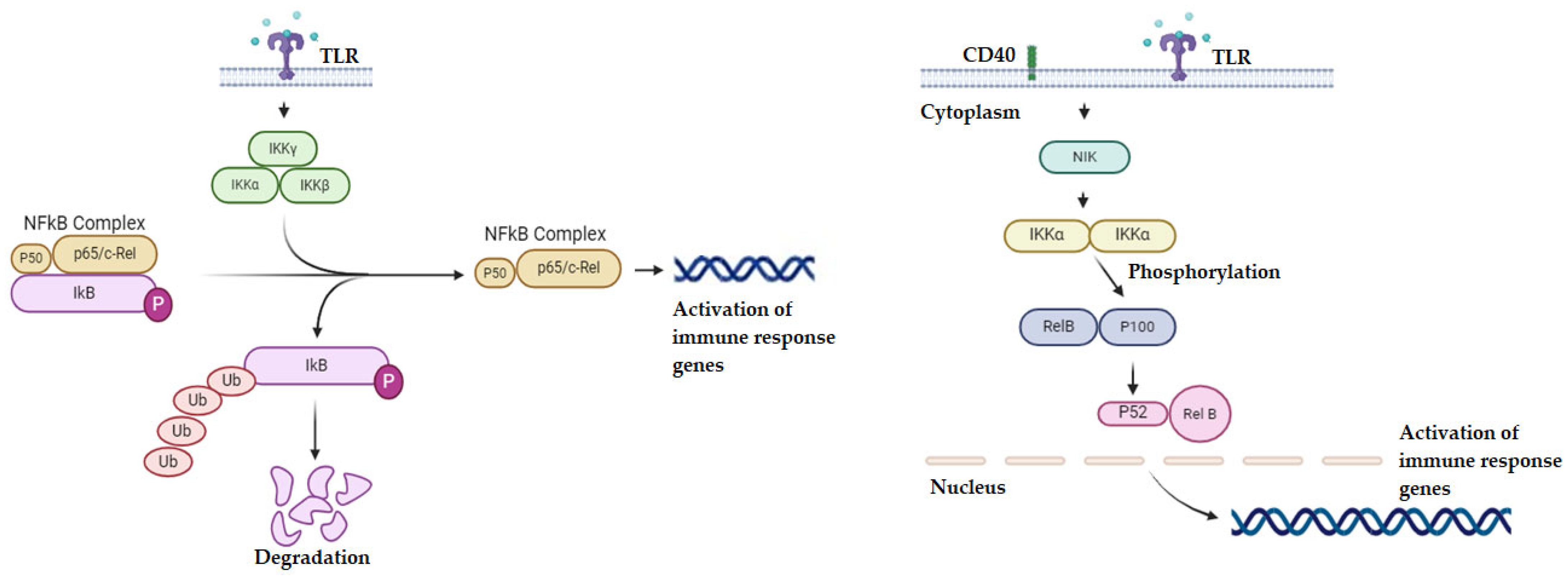
2.4. Targeting the NF-κB Pathway
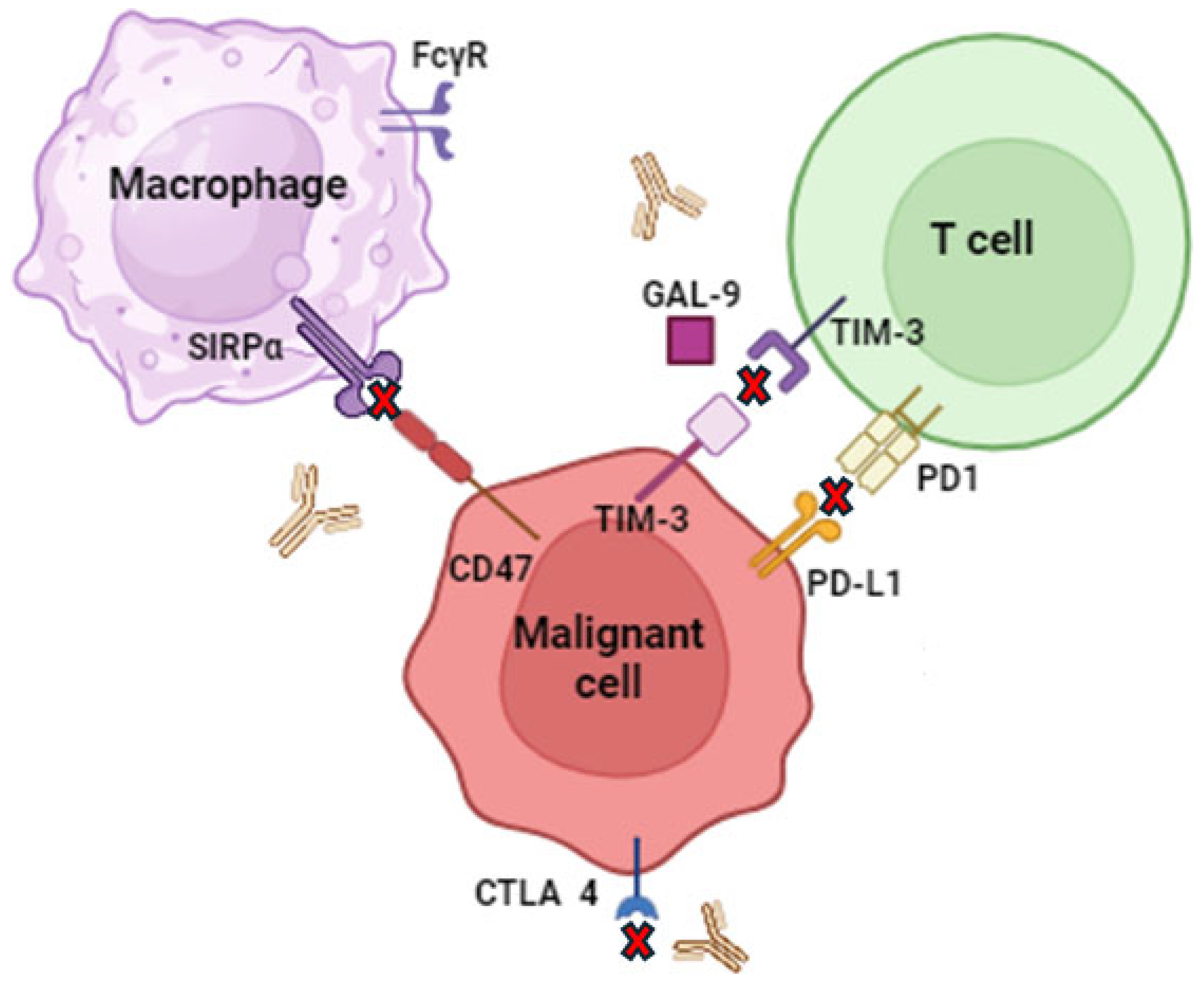
2.5. CD47 Inhibitors
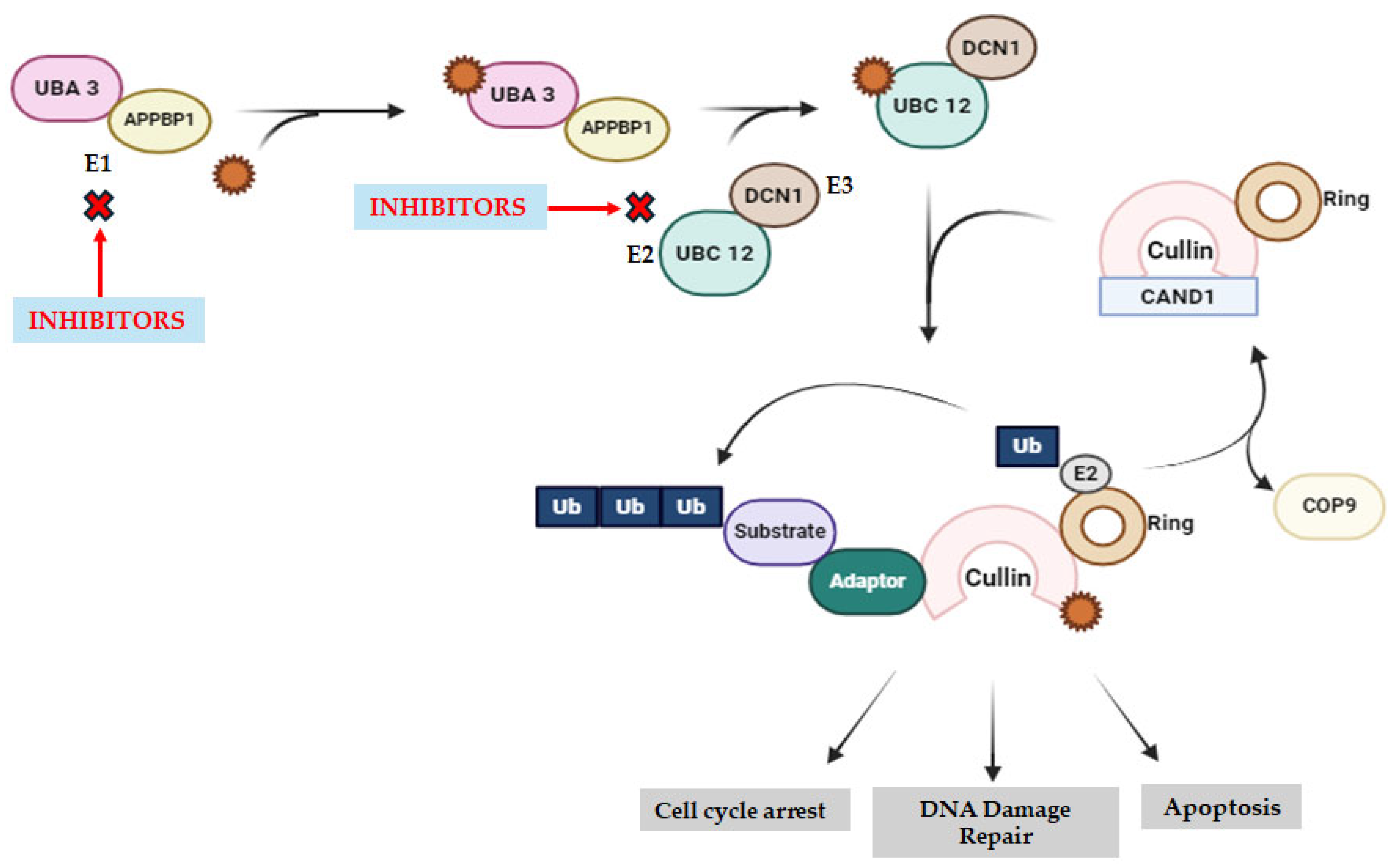
2.6. Neddylation Inhibitors
2.7. PD-1 Inhibitors
2.8. CTL-4 Inhibitors
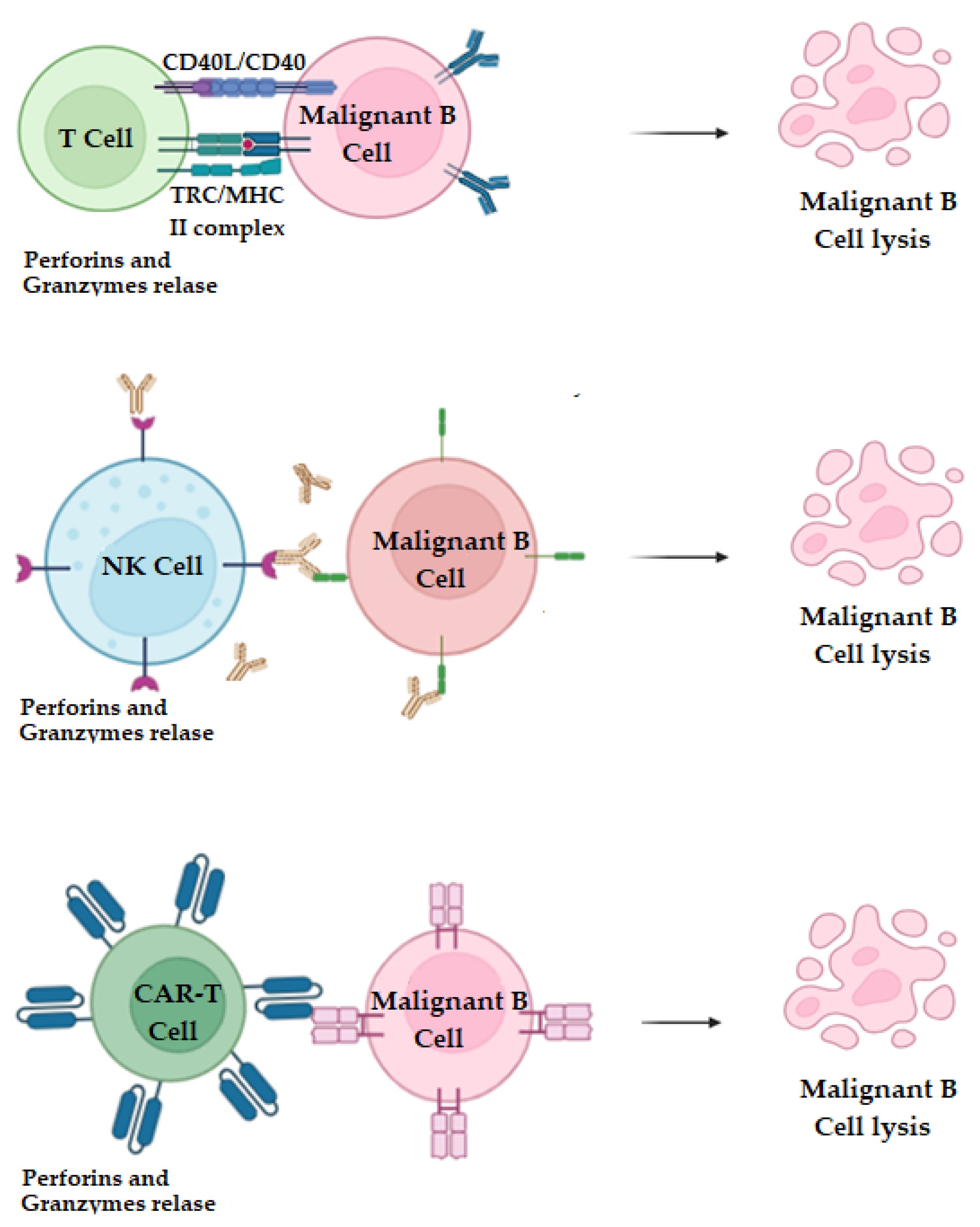
2.9. T Cell and NK-Cell Therapy
2.10. Macrophages
2.11. Summary the Proposed Therapies
3. Innovative Combination Therapies for Hematologic Malignancies: Enhancing Treatment Efficacy and Overcoming Resistance
3.1. PI3K Inhibitors in Combination Therapies
3.2. Immunological Checkpoint Inhibitors in Combination Therapies
3.3. NF-κB Inhibitors in Combination Therapies
3.4. Neddylation Inhibitors in Combination Therapies
3.5. Summary of Combination Therapies for Hematologic Malignancies
4. Other Innovative Combination Therapies for Hematologic Malignancies
Summary Table
5. Potentially Toxic Therapies
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, N.; Wu, J.; Wang, Q.; Liang, Y.; Li, X.; Chen, G.; Ma, L.; Liu, X.; Zhou, F. Global Burden of Hematologic Malignancies and Evolution Patterns over the Past 30 Years. Blood Cancer J. 2023, 13, 82. [Google Scholar] [CrossRef]
- Greim, H.; Kaden, D.A.; Larson, R.A.; Palermo, C.M.; Rice, J.M.; Ross, D.; Snyder, R. The Bone Marrow Niche, Stem Cells, and Leukemia: Impact of Drugs, Chemicals, and the Environment. Ann. N. Y. Acad. Sci. 2014, 1310, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, S.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.J.; Elcarte, E.; Withers, M.; et al. Disease Burden, Risk Factors, and Trends of Leukaemia: A Global Analysis. Front. Oncol. 2022, 12, 904292. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Liu, Y.; Fang, X.; Jiang, Y.; Ding, M.; Ge, X.; Yuan, D.; Lu, K.; Li, P.; Li, Y.; et al. The Epidemiological Patterns of Non-Hodgkin Lymphoma: Global Estimates of Disease Burden, Risk Factors, and Temporal Trends. Front. Oncol. 2023, 13, 1059914. [Google Scholar] [CrossRef]
- Sochacka-ćwikła, A.; Mączyński, M.; Regiec, A. FDA-Approved Drugs for Hematological Malignancies—The Last Decade Review. Cancers 2022, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Huang, Z.; Mei, H.; Hu, Y. Immunotherapy in Hematologic Malignancies: Achievements, Challenges and Future Prospects. Signal Transduct. Target. Ther. 2023, 8, 306. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef]
- Shalata, W.; Abu-salman, A.; Steckbeck, R.; Jacob, B.M.; Massalha, I.; Yakobson, A. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Cancers 2021, 13, 5218. [Google Scholar] [CrossRef]
- Skarbnik, A.P.; Faderl, S. The Role of Combined Fludarabine, Cyclophosphamide and Rituximab Chemoimmunotherapy in Chronic Lymphocytic Leukemia: Current Evidence and Controversies. Ther. Adv. Hematol. 2017, 8, 99. [Google Scholar] [CrossRef]
- Seipel, K.; Brügger, Y.; Mandhair, H.; Bacher, U.; Pabst, T. Rationale for Combining the BCL2 Inhibitor Venetoclax with the PI3K Inhibitor Bimiralisib in the Treatment of IDH2- and FLT3-Mutated Acute Myeloid Leukemia. Int. J. Mol. Sci. 2022, 23, 12587. [Google Scholar] [CrossRef]
- Muenzen, R.M.; Hughes, M.E.; Dwivedy Nasta, S.; Svoboda, J.; Landsburg, D.J.; Barta, S.K.; Tsai, D.; Schuster, S.J.; Chong, E.A. Rituximab, Cyclophosphamide, Etoposide and Prednisone for the Treatment of Post-Transplantation Lymphoproliferative Disorder. Blood 2022, 140, 6715–6716. [Google Scholar] [CrossRef]
- Nogami, A.; Sasaki, K. Therapeutic Advances in Immunotherapies for Hematological Malignancies. Int. J. Mol. Sci. 2022, 23, 11526. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Petrarca, C.; Di Gioacchino, M.; Casciaro, M.; Musolino, C.; Gangemi, S. Exosome-Mediated Therapeutic Strategies for Management of Solid and Hematological Malignancies. Cells 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, Z.; Liu, J.; Yan, W.; Xia, Y.; Yue, S.; Yu, J. Antibody-Based Immunotherapeutic Strategies for the Treatment of Hematological Malignancies. Biomed. Res. Int. 2020, 2020, 4956946. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.; Schnitter, J.M.; Gordon, L.I. Immune Checkpoint Blockade for the Treatment of Hodgkin Lymphoma. Immunotargets Ther. 2022, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of Immune Checkpoints—PD-1, PD-L1, CTLA-4—New Opportunities for Cancer Patients and a New Challenge for Internists and General Practitioners. Cancer Metastasis Rev. 2021, 40, 949. [Google Scholar] [CrossRef]
- Joller, N.; Anderson, A.C.; Kuchroo, V.K. LAG-3, TIM-3, and TIGIT: Distinct Functions in Immune Regulation. Immunity 2024, 57, 206–222. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 Comes of Age as an Inhibitory Receptor. Nat. Rev. Immunol. 2019, 20, 173–185. [Google Scholar] [CrossRef]
- Rowinsky, E.K.; Paner, A.; Berdeja, J.G.; Paba-Prada, C.; Venugopal, P.; Porkka, K.; Gullbo, J.; Linder, S.; Loskog, A.; Richardson, P.G.; et al. Phase 1 Study of the Protein Deubiquitinase Inhibitor VLX1570 in Patients with Relapsed and/or Refractory Multiple Myeloma. Investig. New Drugs 2020, 38, 1448–1453. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/MTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; Ribatti, D. Update in TIGIT Immune-Checkpoint Role in Cancer. Front. Oncol. 2022, 12, 871085. [Google Scholar] [CrossRef]
- Lang, T.J.L.; Damm, F.; Bullinger, L.; Frick, M. Mechanisms of Resistance to Small Molecules in Acute Myeloid Leukemia. Cancers 2023, 15, 4573. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Del Gaudio, N.; Conte, L.; Altucci, L. The Ubiquitin Proteasome System in Hematological Malignancies: New Insight into Its Functional Role and Therapeutic Options. Cancers 2020, 12, 1898. [Google Scholar] [CrossRef] [PubMed]
- Wiese, W.; Barczuk, J.; Racinska, O.; Siwecka, N.; Rozpedek-Kaminska, W.; Slupianek, A.; Sierpinski, R.; Majsterek, I. PI3K/Akt/MTOR Signaling Pathway in Blood Malignancies—New Therapeutic Possibilities. Cancers 2023, 15, 5297. [Google Scholar] [CrossRef] [PubMed]
- Hus, I.; Puła, B.; Robak, T. PI3K Inhibitors for the Treatment of Chronic Lymphocytic Leukemia: Current Status and Future Perspectives. Cancers 2022, 14, 1571. [Google Scholar] [CrossRef]
- Mattsson, A.; Sylvan, S.E.; Axelsson, P.; Ellin, F.; Kjellander, C.; Larsson, K.; Lauri, B.; Lewerin, C.; Scharenberg, C.; Tätting, L.; et al. Idelalisib (PI3Kδ Inhibitor) Therapy for Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia: A Swedish Nation-Wide Real-World Report on Consecutively Identified Patients. Eur. J. Haematol. 2023, 111, 715–721. [Google Scholar] [CrossRef] [PubMed]
- BASELINE HISTOLOGY Mehta-Shah, B.N.; Jacobsen, E.D.; Zinzani, P.L.; Zain, J.; Mead, M.; Casulo, C.; Gritti, G.; Pinter-Brown, L.; Izutsu, K.; Waters, S.; et al. Duvelisib in Patients with Relapsed/Refractory Peripheral T-Cell Lymphoma from the Phase 2 PRIMO Trial Expansion Phase: Outcomes by Baseline Histology. Hematol. Oncol. 2023, 41, 499–500. [Google Scholar] [CrossRef]
- Randall, J.; Evans, K.; Watts, B.; Smith, C.M.; Hughes, K.; Earley, E.J.; Erickson, S.W.; Pachter, J.A.; Teicher, B.A.; Smith, M.A.; et al. In Vivo Activity of the Dual PI3Kδ and PI3Kγ Inhibitor Duvelisib against Pediatric Acute Lymphoblastic Leukemia Xenografts. Pediatr. Blood Cancer 2023, 70, e30398. [Google Scholar] [CrossRef]
- Schweitzer, J.; Hoffman, M.; Graf, S.A. The Evidence to Date on Umbralisib for the Treatment of Refractory Marginal Zone Lymphoma and Follicular Lymphoma. Expert Opin. Pharmacother. 2022, 23, 535–541. [Google Scholar] [CrossRef]
- Dhillon, S.; Keam, S.J. Umbralisib: First Approval. Drugs 2021, 81, 857–866. [Google Scholar] [CrossRef]
- Davids, M.S.; O’Connor, O.A.; Jurczak, W.; Samaniego, F.; Fenske, T.S.; Zinzani, P.L.; Patel, M.R.; Ghosh, N.; Cheson, B.D.; Derenzini, E.; et al. Integrated Safety Analysis of Umbralisib, a Dual PI3Kδ/CK1ε Inhibitor, in Relapsed/Refractory Lymphoid Malignancies. Blood Adv. 2021, 5, 5332–5343. [Google Scholar] [CrossRef] [PubMed]
- Study Details|Phase 3 Study of Zandelisib (ME-401) in Combination With Rituximab in Patients With INHL-(COASTAL)|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04745832 (accessed on 1 July 2024).
- Belli, C.; Repetto, M.; Anand, S.; Porta, C.; Subbiah, V.; Curigliano, G. The Emerging Role of PI3K Inhibitors for Solid Tumour Treatment and Beyond. Br. J. Cancer 2023, 128, 2150. [Google Scholar] [CrossRef]
- Sauer, N.; Janicka, N.; Szlasa, W.; Skinderowicz, B.; Kołodzińska, K.; Dwernicka, W.; Oślizło, M.; Kulbacka, J.; Novickij, V.; Karłowicz-Bodalska, K. TIM-3 as a Promising Target for Cancer Immunotherapy in a Wide Range of Tumors. Cancer Immunol. Immunother. 2023, 72, 3405–3425. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Yao, J.; Li, H.; Yan, M.; Chang, W.C.; Hsu, J.M.; Cha, J.H.; et al. Galectin-9 Interacts with PD-1 and TIM-3 to Regulate T Cell Death and Is a Target for Cancer Immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, J.; Yoon, S.R.; Lee, H.G.; Jung, H. Regulation of Hematopoietic Stem Cell Fate and Malignancy. Int. J. Mol. Sci. 2020, 21, 4780. [Google Scholar] [CrossRef] [PubMed]
- Savinova, O.V.; Hoffmann, A.; Ghosh, G. The Nfkb1 and Nfkb2 Proteins P105 and P100 Function as the Core of High-Molecular-Weight Heterogeneous Complexes. Mol. Cell 2009, 34, 591–602. [Google Scholar] [CrossRef]
- Anilkumar, S.; Wright-Jin, E. NF-ΚB as an Inducible Regulator of Inflammation in the Central Nervous System. Cells 2024, 13, 485. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-ΚB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Prescott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory Feedback Control of NF-ΚB Signalling in Health and Disease. Biochem. J. 2021, 478, 2619–2664. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-ΚB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Cildir, G.; Low, K.C.; Tergaonkar, V. Noncanonical NF-ΚB Signaling in Health and Disease. Trends Mol. Med. 2016, 22, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Salvi, M.; Abdrabou, A.M. The Yin and Yang of IκB Kinases in Cancer. Kinases Phosphatases 2023, 2, 9–27. [Google Scholar] [CrossRef]
- Ghosh, G.; Wang, V.Y.F. Origin of the Functional Distinctiveness of NF-ΚB/P52. Front. Cell Dev. Biol. 2021, 9, 764164. [Google Scholar] [CrossRef]
- Ramadass, V.; Vaiyapuri, T.; Tergaonkar, V. Small Molecule NF-ΚB Pathway Inhibitors in Clinic. Int. J. Mol. Sci. 2020, 21, 5164. [Google Scholar] [CrossRef] [PubMed]
- BAY11-7082 Inhibits the Expression of Tissue Factor and Plasminogen Activator Inhibitor-1 in Type-II Alveolar Epithelial Cells Following TNF-α Stimulation via the NF-κB Pathway. Available online: https://www.spandidos-publications.com/10.3892/etm.2020.9608 (accessed on 1 July 2024).
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Xiao, A.; Akilov, O.E.; Xiao, A.; Akilov, O.E. Targeting the CD47-SIRPα Axis: Present Therapies and the Future for Cutaneous T-Cell Lymphoma. Cells 2022, 11, 3591. [Google Scholar] [CrossRef]
- Maute, R.; Xu, J.; Weissman, I.L. CD47–SIRPα-Targeted Therapeutics: Status and Prospects. Immuno-Oncol. Technol. 2022, 13, 100070. [Google Scholar] [CrossRef] [PubMed]
- Khalaji, A.; Yancheshmeh, F.B.; Farham, F.; Khorram, A.; Sheshbolouki, S.; Zokaei, M.; Vatankhah, F.; Soleymani-Goloujeh, M. Don’t Eat Me/Eat Me Signals as a Novel Strategy in Cancer Immunotherapy. Heliyon 2023, 9, e20507. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Lubben, B.; Adebayo, O.; Muz, B.; Azab, A.K. CD47-Targeting Antibodies as a Novel Therapeutic Strategy in Hematologic Malignancies. Leuk. Res. Rep. 2021, 16, 100268. [Google Scholar] [CrossRef]
- Ye, Z.H.; Yu, W.B.; Huang, M.Y.; Chen, J.; Lu, J.J. Building on the Backbone of CD47-Based Therapy in Cancer: Combination Strategies, Mechanisms, and Future Perspectives. Acta Pharm. Sin. B 2023, 13, 1467. [Google Scholar] [CrossRef]
- Jiang, Y.; Jia, L. Neddylation Pathway as a Novel Anti-Cancer Target: Mechanistic Investigation and Therapeutic Implication. Anticancer Agents Med. Chem. 2015, 15, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, L. Inhibition of NEDD8 NEDDylation Induced Apoptosis in Acute Myeloid Leukemia Cells via P53 Signaling Pathway. Biosci. Rep. 2022, 42, BSR20220994. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jiang, Y.; Luo, Q.; Li, L.; Jia, L. Neddylation: A Novel Modulator of the Tumor Microenvironment. Mol. Cancer 2019, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Che, X.; Wu, G. Deciphering the Role of Neddylation in Tumor Microenvironment Modulation: Common Outcome of Multiple Signaling Pathways. Biomark. Res. 2024, 12, 5. [Google Scholar] [CrossRef]
- Ochiiwa, H.; Ailiken, G.; Yokoyama, M.; Yamagata, K.; Nagano, H.; Yoshimura, C.; Muraoka, H.; Ishida, K.; Haruma, T.; Nakayama, A.; et al. TAS4464, a NEDD8-Activating Enzyme Inhibitor, Activates Both Intrinsic and Extrinsic Apoptotic Pathways via c-Myc-Mediated Regulation in Acute Myeloid Leukemia. Oncogene 2021, 40, 1217–1230. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, Y.; Sun, Y. Anticancer Drug Discovery by Targeting Cullin Neddylation. Acta Pharm. Sin. B 2020, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, Q.; Li, Z.; Zhao, Y.; Sun, Y. Protein Neddylation and Its Role in Health and Diseases. Signal Transduct. Target. Ther. 2024, 9, 85. [Google Scholar] [CrossRef]
- Solinas, C.; Aiello, M.; Rozali, E.; Lambertini, M.; Willard-Gallo, K.; Migliori, E. Programmed Cell Death-Ligand 2: A Neglected But Important Target in the Immune Response to Cancer? Transl. Oncol. 2020, 13, 100811. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination Strategies with PD-1/PD-L1 Blockade: Current Advances and Future Directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef]
- Lussier, D.M.; Johnson, J.L.; Hingorani, P.; Blattman, J.N. Combination Immunotherapy with α-CTLA-4 and α-PD-L1 Antibody Blockade Prevents Immune Escape and Leads to Complete Control of Metastatic Osteosarcoma. J. Immunother. Cancer 2015, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Lepage, E.; Brière, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N. Eng. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Weiss, B.M.; Usmani, S.Z.; Singhal, S.; Chari, A.; Bahlis, N.J.; Belch, A.; Krishnan, A.; Vescio, R.A.; Mateos, M.V.; et al. Daratumumab Monotherapy in Patients with Treatment-Refractory Multiple Myeloma (SIRIUS): An Open-Label, Randomised, Phase 2 Trial. Lancet 2016, 387, 1551–1560. [Google Scholar] [CrossRef]
- Younes, A.; Bartlett, N.L.; Leonard, J.P.; Kennedy, D.A.; Lynch, C.M.; Sievers, E.L.; Forero-Torres, A. Brentuximab Vedotin (SGN-35) for Relapsed CD30-Positive Lymphomas. N. Eng. J. Med. 2010, 363, 1812–1821. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Martinelli, G.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.; Wang, K.; Wang, T.; et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N. Eng. J. Med. 2016, 375, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.-V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Eng. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef]
- Fraser, G.; Smith, C.A.; Imrie, K.; Meyer, R. Alemtuzumab in Chronic Lymphocytic Leukemia. Curr. Oncol. 2007, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Babamohamadi, M.; Mohammadi, N.; Faryadi, E.; Haddadi, M.; Merati, A.; Ghobadinezhad, F.; Amirian, R.; Izadi, Z.; Hadjati, J. Anti-CTLA-4 Nanobody as a Promising Approach in Cancer Immunotherapy. Cell Death Dis. 2024, 15, 17. [Google Scholar] [CrossRef]
- Sobhani, N.; Tardiel-Cyril, D.R.; Davtyan, A.; Generali, D.; Roudi, R.; Li, Y. CTLA-4 in Regulatory T Cells for Cancer Immunotherapy. Cancers 2021, 13, 1440. [Google Scholar] [CrossRef]
- Rossi, F.; Fredericks, N.; Snowden, A.; Allegrezza, M.J.; Moreno-Nieves, U.Y. Next Generation Natural Killer Cells for Cancer Immunotherapy. Front. Immunol. 2022, 13, 886429. [Google Scholar] [CrossRef]
- Jogalekar, M.P.; Rajendran, R.L.; Khan, F.; Dmello, C.; Gangadaran, P.; Ahn, B.C. CAR T-Cell-Based Gene Therapy for Cancers: New Perspectives, Challenges, and Clinical Developments. Front. Immunol. 2022, 13, 925985. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Rayner, K.J. Macrophage Responses to Environmental Stimuli During Homeostasis and Disease. Endocr. Rev. 2021, 42, 407. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Guo, N.; Zhou, Y.; Chen, J.; Wei, Q.; Han, M. The Role of Tumor-Associated Macrophages (TAMs) in Tumor Progression and Relevant Advance in Targeted Therapy. Acta Pharm. Sin. B 2020, 10, 2156. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, H.; Yang, C.; He, L.; Zhang, X.; Peng, L.; Zhu, H.; Gao, L. Role and Mechanisms of Tumor-Associated Macrophages in Hematological Malignancies. Front. Oncol. 2022, 12, 933666. [Google Scholar] [CrossRef]
- Abdul-Rahman, T.; Ghosh, S.; Badar, S.M.; Nazir, A.; Bamigbade, G.B.; Aji, N.; Roy, P.; Kachani, H.; Garg, N.; Lawal, L.; et al. The Paradoxical Role of Cytokines and Chemokines at the Tumor Microenvironment: A Comprehensive Review. Eur. J. Med. Res. 2024, 29, 124. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, G. Macrophages in Leukemia Microenvironment. Blood Sci. 2019, 1, 29–33. [Google Scholar] [CrossRef]
- Sun, Q.; Hong, Z.; Zhang, C.; Wang, L.; Han, Z.; Ma, D. Immune Checkpoint Therapy for Solid Tumours: Clinical Dilemmas and Future Trends. Signal Transduct. Target. Ther. 2023, 8, 320. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nature Reviews Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and Wound Healing: The Role of the Macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, M.; Jia, S. Macrophage: Key Player in the Pathogenesis of Autoimmune Diseases. Front. Immunol. 2023, 14, 1080310. [Google Scholar] [CrossRef]
- Pan, K.; Farrukh, H.; Chittepu, V.C.S.R.; Xu, H.; Pan, C.X.; Zhu, Z. CAR Race to Cancer Immunotherapy: From CAR T, CAR NK to CAR Macrophage Therapy. J. Exp. Clin. Cancer Res. 2022, 41, 119. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, M.L.; Wang, J.C.; Fang, S. CAR-Macrophage versus CAR-T for Solid Tumors: The Race between a Rising Star and a Superstar. Biomol. Biomed. 2024, 24, 465–476. [Google Scholar] [CrossRef]
- Zhou, Q.; Munger, M.E.; Veenstra, R.G.; Weigel, B.J.; Hirashima, M.; Munn, D.H.; Murphy, W.J.; Azuma, M.; Anderson, A.C.; Kuchroo, V.K.; et al. Coexpression of Tim-3 and PD-1 Identifies a CD8+ T-Cell Exhaustion Phenotype in Mice with Disseminated Acute Myelogenous Leukemia. Blood 2011, 117, 4501–4510. [Google Scholar] [CrossRef]
- Fourcade, J.; Sun, Z.; Pagliano, O.; Chauvin, J.M.; Sander, C.; Janjic, B.; Tarhini, A.A.; Tawbi, H.A.; Kirkwood, J.M.; Moschos, S.; et al. PD-1 and Tim-3 Regulate the Expansion of Tumor Antigen-Specific CD8+ T Cells Induced by Melanoma Vaccines. Cancer Res. 2014, 74, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Kikushige, Y.; Miyamoto, T. TIM-3 as a Novel Therapeutic Target for Eradicating Acute Myelogenous Leukemia Stem Cells. Int. J. Hematol. 2013, 98, 627–633. [Google Scholar] [CrossRef]
- Kok, V.C. Current Understanding of the Mechanisms Underlying Immune Evasion From PD-1/PD-L1 Immune Checkpoint Blockade in Head and Neck Cancer. Front. Oncol. 2020, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Li, Z. Targeting Tim-3 in Cancer with Resistance to PD-1/PD-L1 Blockade. Front. Oncol. 2021, 11, 731175. [Google Scholar] [CrossRef]
- Tang, W.; Chen, J.; Ji, T.; Cong, X. TIGIT, a Novel Immune Checkpoint Therapy for Melanoma. Cell Death Dis. 2023, 14, 466. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Gu, Z.; Jiang, Z.; Zhao, S.; Song, Y.; Yu, J. Targeting TIGIT for Cancer Immunotherapy: Recent Advances and Future Directions. Biomark. Res. 2024, 12, 7. [Google Scholar] [CrossRef]
- Nininahazwe, L.; Liu, B.; He, C.; Zhang, H.; Chen, Z.S. The Emerging Nature of Ubiquitin-Specific Protease 7 (USP7): A New Target in Cancer Therapy. Drug Discov. Today 2021, 26, 490–502. [Google Scholar] [CrossRef]
- Yu, M.; Chen, J.; Xu, Z.; Yang, B.; He, Q.; Luo, P.; Yan, H.; Yang, X. Development and Safety of PI3K Inhibitors in Cancer. Arch. Toxicol. 2023, 97, 635. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Sun, P.; Bennett, S.; Charlesworth, O.; Tan, R.; Peng, X.; Gu, Q.; Kujan, O.; Xu, J. The Therapeutic Effect and Mechanism of Parthenolide in Skeletal Disease, Cancers, and Cytokine Storm. Front. Pharmacol. 2023, 14, 1111218. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, X.; Ma, W. Opportunities and Challenges of CD47-Targeted Therapy in Cancer Immunotherapy. Oncol. Res. 2024, 32, 49. [Google Scholar] [CrossRef] [PubMed]
- Gai, W.; Peng, Z.; Liu, C.H.; Zhang, L.; Jiang, H. Advances in Cancer Treatment by Targeting the Neddylation Pathway. Front. Cell Dev. Biol. 2021, 9, 653882. [Google Scholar] [CrossRef]
- Ojeda-Uribe, M.; Rimelen, V.; Marzullo, C. Good Profile of Efficacy/Tolerance of Bortezomib or Idelalisib in Waldenström Macroglobulinemia Associated with Acquired Von Willebrand Syndrome. J. Blood Med. 2020, 11, 67. [Google Scholar] [CrossRef]
- Castel, P.; Toska, E.; Engelman, J.A.; Scaltriti, M. The Present and Future of PI3K Inhibitors for Cancer Therapy. Nat. Cancer 2021, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Shayan, G.; Srivastava, R.; Li, J.; Schmitt, N.; Kane, L.P.; Ferris, R.L. Adaptive Resistance to Anti-PD1 Therapy by Tim-3 Upregulation Is Mediated by the PI3K-Akt Pathway in Head and Neck Cancer. Oncoimmunology 2017, 6, e1261779. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Jin, X.; Zhang, X.; Utsav, P.; Zhang, Y.; Guo, R.; Lu, W.; Zhao, M. Small-Molecule Compounds Boost CAR-T Cell Therapy in Hematological Malignancies. Cur.r Treat Options Oncol. 2023, 24, 184–211. [Google Scholar] [CrossRef]
- Varayathu, H.; Sarathy, V.; Thomas, B.E.; Mufti, S.S.; Naik, R. Combination Strategies to Augment Immune Check Point Inhibitors Efficacy-Implications for Translational Research. Front. Oncol. 2021, 11, 559161. [Google Scholar] [CrossRef]
- Sagiv-Barfi, I.; Kohrt, H.E.K.; Czerwinski, D.K.; Ng, P.P.; Chang, B.Y.; Levy, R. Therapeutic Antitumor Immunity by Checkpoint Blockade Is Enhanced by Ibrutinib, an Inhibitor of Both BTK and ITK. Proc. Natl. Acad. Sci. USA 2015, 112, E966–E972. [Google Scholar] [CrossRef]
- Hanna, B.S.; Yazdanparast, H.; Demerdash, Y.; Roessner, P.M.; Schulz, R.; Lichter, P.; Stilgenbauer, S.; Seiffert, M. Combining Ibrutinib and Checkpoint Blockade Improves CD8+ T-Cell Function and Control of Chronic Lymphocytic Leukemia in Em-TCL1 Mice. Haematologica 2021, 106, 968–977. [Google Scholar] [CrossRef]
- Petrazzuolo, A.; Maiuri, M.C.; Zitvogel, L.; Kroemer, G.; Kepp, O. Trial Watch: Combination of Tyrosine Kinase Inhibitors (TKIs) and Immunotherapy. Oncoimmunology 2022, 11, 2077898. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, N.; Abdulwahid, A.H.R.R.; Mansouri, A.; Karimi, N.; Bostani, R.J.; Beiranvand, S.; Adelian, S.; Khorram, R.; Vafadar, R.; Hamblin, M.R.; et al. Targeting the NF-ΚB Pathway as a Potential Regulator of Immune Checkpoints in Cancer Immunotherapy. Cell. Mol. Life Sci. 2024, 81, 106. [Google Scholar] [CrossRef] [PubMed]
- Sum, E.; Rapp, M.; Dürr, H.; Mazumdar, A.; Romero, P.J.; Trumpfheller, C.; Umaña, P. The Tumor-Targeted CD40 Agonist CEA-CD40 Promotes T Cell Priming via a Dual Mode of Action by Increasing Antigen Delivery to Dendritic Cells and Enhancing Their Activation. J. Immunother. Cancer 2022, 10, 3264. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, L.; Li, Y.; Liu, G.; Hoffman, R.M.; Jia, L. Neddylation Regulates Macrophages and Implications for Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 681186. [Google Scholar] [CrossRef]
- Pakjoo, M.; Ahmadi, S.E.; Zahedi, M.; Jaafari, N.; Khademi, R.; Amini, A.; Safa, M. Interplay between Proteasome Inhibitors and NF-ΚB Pathway in Leukemia and Lymphoma: A Comprehensive Review on Challenges Ahead of Proteasome Inhibitors. Cell Commun. Signal. 2024, 22, 105. [Google Scholar] [CrossRef]
- Logghe, T.; van Zwol, E.; Immordino, B.; Van den Cruys, K.; Peeters, M.; Giovannetti, E.; Bogers, J. Hyperthermia in Combination with Emerging Targeted and Immunotherapies as a New Approach in Cancer Treatment. Cancers 2024, 16, 505. [Google Scholar] [CrossRef]
- Shi, C.-S.; Kuo, K.-L.; Lin, W.-C.; Chen, M.-S.; Liu, S.-H.; Liao, S.-M.; Hsu, C.-H.; Chang, Y.-W.; Chang, H.-C.; Huang, K.-H. Neddylation Inhibitor, MLN4924 Suppresses Angiogenesis in Huvecs and Solid Cancers: In Vitro and in Vivo Study. Am. J. Cancer Res. 2020, 10, 953. [Google Scholar] [PubMed]
- Huntoon, K.; Jiang, W.; Kim, B.Y.S. Waking Immune-Resistant Tumors with Neddylation. J. Clin. Investig. 2023, 133, e167894. [Google Scholar] [CrossRef]
- Fu, D.J.; Wang, T. Targeting NEDD8-Activating Enzyme for Cancer Therapy: Developments, Clinical Trials, Challenges and Future Research Directions. J. Hematol. Oncol. 2023, 16, 87. [Google Scholar] [CrossRef]
- Sun, Y.; Baechler, S.A.; Zhang, X.; Kumar, S.; Factor, V.M.; Arakawa, Y.; Chau, C.H.; Okamoto, K.; Parikh, A.; Walker, B.; et al. Targeting Neddylation Sensitizes Colorectal Cancer to Topoisomerase I Inhibitors by Inactivating the DCAF13-CRL4 Ubiquitin Ligase Complex. Nat. Commun. 2023, 14, 3762. [Google Scholar] [CrossRef]
- Al-Haideri, M.; Tondok, S.B.; Safa, S.H.; Maleki, A.H.; Rostami, S.; Jalil, A.T.; Al-Gazally, M.E.; Alsaikhan, F.; Rizaev, J.A.; Mohammad, T.A.M.; et al. CAR-T Cell Combination Therapy: The next Revolution in Cancer Treatment. Cancer Cell Int. 2022, 22, 365. [Google Scholar] [CrossRef]
- Pérez-Moreno, M.A.; Ciudad-Gutiérrez, P.; Jaramillo-Ruiz, D.; Reguera-Ortega, J.L.; Abdel-kader Martín, L.; Flores-Moreno, S. Combined or Sequential Treatment with Immune Checkpoint Inhibitors and Car-T Cell Therapies for the Management of Haematological Malignancies: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 14780. [Google Scholar] [CrossRef] [PubMed]
- Kleinendorst, S.C.; Oosterwijk, E.; Bussink, J.; Westdorp, H.; Konijnenberg, M.W.; Heskamp, S. Combining Targeted Radionuclide Therapy and Immune Checkpoint Inhibition for Cancer Treatment. Clin. Cancer Res. 2022, 28, 3652–3657. [Google Scholar] [CrossRef]
- Lu, Q.; Li, H.; Wu, Z.; Zhu, Z.; Zhang, Z.; Yang, D.; Tong, A. BCMA/CD47-Directed Universal CAR-T Cells Exhibit Excellent Antitumor Activity in Multiple Myeloma. J. Nanobiotechnology 2024, 22, 279. [Google Scholar] [CrossRef]
- Van Duijn, A.; Van Der Burg, S.H.; Scheeren, F.A. CD47/SIRPα Axis: Bridging Innate and Adaptive Immunity. J. Immunother. Cancer 2022, 10, e004589. [Google Scholar] [CrossRef] [PubMed]
- Ahire, V.; Ahmadi Bidakhvidi, N.; Boterberg, T.; Chaudhary, P.; Chevalier, F.; Daems, N.; Delbart, W.; Baatout, S.; Deroose, C.M.; Fernandez-Palomo, C.; et al. Radiobiology of Combining Radiotherapy with Other Cancer Treatment Modalities. In Radiobiology Textbook; Springer: Cham, Switzerland, 2023; pp. 311–386. [Google Scholar]
- Vaz De Freitas, M.; Frâncio, L.; Haleva, L.; Da Silveira Matte, U. Protection Is Not Always a Good Thing: The Immune System’s Impact on Gene Therapy. Genet. Mol. Biol. 2022, 45, e20220046. [Google Scholar] [CrossRef]
- Jha, S.; Sharma, P.K.; Malviya, R. Hyperthermia: Role and Risk Factor for Cancer Treatment. Achiev. Life Sci. 2016, 10, 161–167. [Google Scholar] [CrossRef]
- Agarwal, N.; Rotz, S.; Hanna, R. Medical Emergencies in Pediatric Blood & Marrow Transplant and Cellular Therapies. Front. Pediatr. 2023, 11, 1075644. [Google Scholar] [CrossRef]
- Wang, E.S.; Baron, J. Management of Toxicities Associated with Targeted Therapies for Acute Myeloid Leukemia: When to Push through and When to Stop. Hematology 2020, 2020, 57–66. [Google Scholar] [CrossRef]
- Lu, D.; Wang, C.; Qu, L.; Yin, F.; Li, S.; Luo, H.; Zhang, Y.; Liu, X.; Chen, X.; Luo, Z.; et al. Histone Deacetylase and Enhancer of Zeste Homologue 2 Dual Inhibitors Presenting a Synergistic Effect for the Treatment of Hematological Malignancies. J. Med. Chem. 2022, 65, 12838–12859. [Google Scholar] [CrossRef]
- Xu, J.; Dong, X.; Huang, D.C.S.; Xu, P.; Zhao, Q.; Chen, B. Current Advances and Future Strategies for BCL-2 Inhibitors: Potent Weapons against Cancers. Cancers 2023, 15, 4957. [Google Scholar] [CrossRef] [PubMed]
- Charmsaz, S.; Scott, A.M.; Boyd, A.W. Targeted Therapies in Hematological Malignancies Using Therapeutic Monoclonal Antibodies against Eph Family Receptors. Exp. Hematol. 2017, 54, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Chandhok, N.S.; Prebet, T. Insights into Novel Emerging Epigenetic Drugs in Myeloid Malignancies. Ther. Adv. Hematol. 2019, 10, 2040620719866081. [Google Scholar] [CrossRef]
- Stuver, R.N.; Khan, N.; Schwartz, M.; Acosta, M.; Federico, M.; Gisselbrecht, C.; Horwitz, S.M.; Lansigan, F.; Pinter-Brown, L.C.; Pro, B.; et al. Single Agents vs Combination Chemotherapy in Relapsed and Refractory Peripheral T-Cell Lymphoma: Results from the Comprehensive Oncology Measures for Peripheral T-Cell Lymphoma Treatment (COMPLETE) Registry. Am. J. Hematol. 2019, 94, 641–649. [Google Scholar] [CrossRef]
- Uson Junior, P.L.S.; Santos, V.M.; Bugano, D.D.G.; Victor, E.D.S.; Rother, E.T.; Maluf, F.C. Systematic Review and Meta-Analysis of Docetaxel Perioperative Chemotherapy Regimens in Gastric and Esophagogastric Tumors. Sci. Rep. 2019, 9, 15806. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Syn, N.L.X.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.C.; Ho, J.; Unverzagt, S. Chemotherapy for Advanced Gastric Cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef] [PubMed]
- Gresham, G.K.; Wells, G.A.; Gill, S.; Cameron, C.; Jonker, D.J. Chemotherapy Regimens for Advanced Pancreatic Cancer: A Systematic Review and Network Meta-Analysis. BMC Cancer 2014, 14, 471. [Google Scholar] [CrossRef]
- Bertsimas, D.; O’Hair, A.; Relyea, S.; Silberholz, J. An Analytics Approach to Designing Combination Chemotherapy Regimens for Cancer. Manag. Sci. 2016, 62, 1511–1531. [Google Scholar] [CrossRef]
- Lonial, S.; Nooka, A.K. Novel Combination Approaches for Myeloma. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 286–293. [Google Scholar] [CrossRef][Green Version]
- Gupta, A.; Eisenhauer, E.A.; Booth, C.M. The Time Toxicity of Cancer Treatment. J. Clin. Oncol. 2022, 40, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Bottino, D.C.; Patel, M.; Kadakia, E.; Zhou, J.; Patel, C.; Neuwirth, R.; Iartchouk, N.; Brake, R.; Venkatakrishnan, K.; Chakravarty, A. Dose Optimization for Anticancer Drug Combinations: Maximizing Therapeutic Index via Clinical Exposure-Toxicity/Preclinical Exposure-Efficacy Modeling. Clin. Cancer Res. 2019, 25, 6633–6643. [Google Scholar] [CrossRef] [PubMed]
- Sergunova, V.; Leesment, S.; Kozlov, A.; Inozemtsev, V.; Platitsina, P.; Lyapunova, S.; Onufrievich, A.; Polyakov, V.; Sherstyukova, E. Investigation of Red Blood Cells by Atomic Force Microscopy. Sensors 2022, 22, 2055. [Google Scholar] [CrossRef] [PubMed]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef]
- Uscanga-Palomeque, A.C.; Chávez-Escamilla, A.K.; Alvizo-Báez, C.A.; Saavedra-Alonso, S.; Terrazas-Armendáriz, L.D.; Tamez-Guerra, R.S.; Rodríguez-Padilla, C.; Alcocer-González, J.M. CAR-T Cell Therapy: From the Shop to Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 15688. [Google Scholar] [CrossRef]
- Lekka, M. Discrimination Between Normal and Cancerous Cells Using AFM. Bionanoscience 2016, 6, 65–80. [Google Scholar] [CrossRef]
- Oehrlein, E.M.; Schoch, S.; Burcu, M.; McBeth, J.F.; Bright, J.; Pashos, C.L.; Willke, R.; Love, T.R.; Mattingly, T.J.; Perfetto, E.M. Developing Patient-Centered Real-World Evidence: Emerging Methods Recommendations from a Consensus Process. Value Health 2023, 26, 28–38. [Google Scholar] [CrossRef]
- Faulkner, S.D.; Somers, F.; Boudes, M.; Nafria, B.; Robinson, P. Using Patient Perspectives to Inform Better Clinical Trial Design and Conduct: Current Trends and Future Directions. Pharm. Med. 2023, 37, 129–138. [Google Scholar] [CrossRef]
- Yap, T.A.; Parkes, E.E.; Peng, W.; Moyers, J.T.; Curran, M.A.; Tawbi, H.A. Development of Immunotherapy Combination Strategies in Cancer. Cancer Discov. 2021, 11, 1368. [Google Scholar] [CrossRef]
- Vanneman, M.; Dranoff, G. Combining Immunotherapy and Targeted Therapies in Cancer Treatment. Nat. Rev. Cancer 2012, 12, 237. [Google Scholar] [CrossRef]
- Metzeler, K.H.; Platzbecker, U.; Winer, E.S.; Verma, A.; DeAngelo, D.J.; Severgnini, M.; Choudhary, G.S.; Zhao, W.; Lane, M.E.; Gallagher, C.; et al. Predictive Biomarkers of Response to the IRAK4/FLT3 Inhibitor Emavusertib in Hematological Malignancies. J. Clin. Oncol. 2024, 42, 6550. [Google Scholar] [CrossRef]
- Kiefer, J.D.; Neri, D. Immunocytokines and Bispecific Antibodies: Two Complementary Strategies for the Selective Activation of Immune Cells at the Tumor Site. Immunol. Rev. 2016, 270, 178–192. [Google Scholar] [CrossRef]
- Mun, J.Y.; Leem, S.H.; Lee, J.H.; Kim, H.S. Dual Relationship Between Stromal Cells and Immune Cells in the Tumor Microenvironment. Front. Immunol. 2022, 13, 864739. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, K.; He, Q.; Gu, X.; Jiang, C.; Wu, J. Hypoxia Signaling in Cancer: Implications for Therapeutic Interventions. MedComm 2023, 4, e203. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Shallis, R.; Stahl, M.; Zeidan, A.M. Epigenetic Therapy Combinations in Acute Myeloid Leukemia: What Are the Options? Ther. Adv Hematol 2019, 10, 2040620718816698. [Google Scholar] [CrossRef]
- Saito, M.; Momma, T.; Kono, K. Targeted Therapy According to next Generation Sequencing-Based Panel Sequencing. Fukushima J. Med. Sci. 2018, 64, 9. [Google Scholar] [CrossRef] [PubMed]
- Topatana, W.; Juengpanich, S.; Li, S.; Cao, J.; Hu, J.; Lee, J.; Suliyanto, K.; Ma, D.; Zhang, B.; Chen, M.; et al. Advances in Synthetic Lethality for Cancer Therapy: Cellular Mechanism and Clinical Translation. J. Hematol. Oncol. 2020, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- El Alaoui, Y.; Elomri, A.; Qaraqe, M.; Padmanabhan, R.; Taha, R.Y.; El Omri, H.; EL Omri, A.; Aboumarzouk, O. A Review of Artificial Intelligence Applications in Hematology Management: Current Practices and Future Prospects. J. Med. Internet Res. 2022, 24, e36490. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lin, W.; Zhu, L. Targeted Drug Delivery for the Treatment of Blood Cancers. Molecules 2022, 27, 1310. [Google Scholar] [CrossRef]
- Li, M.; Mei, S.; Yang, Y.; Shen, Y.; Chen, L. Strategies to Mitigate the On- and off-Target Toxicities of Recombinant Immunotoxins: An Antibody Engineering Perspective. Antib. Ther. 2022, 5, 164. [Google Scholar] [CrossRef]
| Drug | Mechanism of Action/Target/Type of Molecule | Type of Malignancy | Approval Status | Combination Therapies | Adverse Effects |
|---|---|---|---|---|---|
| MBG453 | TIM-3 inhibitor/Antibody | Lymphomas, advanced solid tumors | Clinical Trials II | Investigated with other immune checkpoint inhibitors | Fatigue, nausea |
| Sym023 | TIM-3 inhibitor/Monoclonal antibody | Advanced solid tumors, lymphomas | Clinical Trials I/II | Research ongoing for combinations | Fatigue, infusion reactions |
| LY3321367 | TIM-3 inhibitor/Antibody | Solid tumors, hematologic malignancies | Clinical Trials I/II | Being studied with other therapies | Under investigation |
| LY3415244 | Anti-TIM-3 antibody | Solid tumors and hematologic malignancies | Clinical Trials I/II | Combination immunotherapy | Under investigation |
| TSR-022 | Anti-TIM-3 antibody | Solid tumors and lymphomas | Clinical Trials I/II | Being tested with other checkpoint inhibitors | Under investigation |
| Lomvastomig: RO7121661 | TIM-3 inhibitor/Bispecific antibody | Advanced solid tumors and lymphomas | Clinical Trials I/II | Research ongoing for combinations | Under investigation |
| TSR-042 | Anti-PD-1 and TIM-3 antibody | Various cancers | Clinical Trials II | Being studied with other immunotherapies | Fatigue, immune-related adverse events |
| Agent Name | Mechanism of Action | Hematological Malignancies | Clinical Development Phase |
|---|---|---|---|
| Tiragolumab | Anti-TIGIT monoclonal antibody | NHL, MM | Phase II |
| Vibostolimab | Anti-TIGIT monoclonal antibody | HL | Phase II |
| Ociperlimab | Anti-TIGIT monoclonal antibody | AML | Phase I/II |
| AGEN1777 | Bispecific antibody targeting TIGIT and PD-1 | MM, NHL | Phase I/II |
| COM902 | Anti-TIGIT monoclonal antibody | MM | Phase I |
| AB154 | Anti-TIGIT monoclonal antibody | CLL | Phase I |
| Name of Molecule | Mechanism of Action | Type of Hematologic Malignancy | Approval Status | Clinical Trial Phase | Adverse Effects |
|---|---|---|---|---|---|
| Idelalisib (Zydelig) | Inhibits PI3Kδ, reducing cell proliferation and survival signals | CLL, FL, SLL | FDA Approved | N/A | Diarrhea, liver toxicity |
| Copanlisib (Aliqopa) | Inhibits PI3Kα and PI3Kδ, affecting cell growth and survival | FL | FDA Approved | N/A | Hyperglycemia, hypertension |
| Duvelisib (Copiktra) | Inhibits PI3Kδ and PI3Kγ, reducing cytokine synthesis and promoting apoptosis | CLL, SLL | FDA Approved | N/A | Diarrhea, colitis |
| TGR-1202 (Umbralisib) | Inhibits PI3Kδ and casein kinase-1ε (CK1ε), affecting cell adhesion and migration | CLL, MZL, FL | FDA Approved | N/A | Diarrhea, nausea |
| Zandelisib (ME-401) | Inhibits PI3Kδ, affecting cell proliferation and survival signals | FL, CLL, SLL, MZL, DLBCL | Clinical Trials | II/III | Diarrhea, liver toxicity |
| Linperlisib | Inhibits PI3Kδ, reducing cell proliferation and survival signals | FL | Clinical Trials | I/II | Fatigue, nausea |
| TQB3525 | Inhibits PI3Kα and PI3Kδ, affecting cell growth and survival | CLL, SLL | Clinical Trials | I/II | Under investigation |
| Acalisib | Inhibits PI3Kδ, reducing cell proliferation and survival signals | FL, DLBCL, MZL, MCL | Clinical Trials | I | Under investigation |
| SHC014748M | Inhibits PI3Kδ, reducing cell proliferation and survival signals | CLL | Preclinical | N/A | Under investigation |
| Venetoclax | Inhibits BCL-2, promoting apoptosis in cancer cells | CLL, AML | FDA Approved | N/A | Neutropenia, infections |
| Selinexor | Inhibits XPO1, blocking nuclear export and leading to apoptosis | MM, DLBCL | FDA Approved | N/A | Nausea, fatigue |
| Bortezomib | Inhibits proteasome activity, leading to the accumulation of pro-apoptotic proteins and triggering apoptosis | MM | FDA Approved | N/A | Peripheral neuropathy, fatigue |
| Melphalan | Binds at the N7 position of guanine, inducing inter-strand cross-links in DNA | MM | FDA Approved | N/A | Bone marrow suppression |
| P5091 | Inhibits USP7, blocking HDM2 and p21 signaling pathways | MM | Clinical Trials | I/II | Well tolerated in studies |
| Name of Molecule | Target | Type of Malignancy | Approval Status | Mechanism of Action | Combination Therapies | Adverse Effects |
|---|---|---|---|---|---|---|
| Idelalisib (Zydelig) | Inhibitor of PI3K-Delta | CLL, FL, SLL | FDA Approved | Induces caspase-dependent apoptosis | Being studied with anti-CD20 antibodies | Diarrhea, colitis, liver toxicity |
| Copanlisib (Aliqopa) | Inhibitor of PI3K-alpha and PI3K delta | FL | FDA Approved | Inhibits PI3K signaling, affecting cell proliferation and survival | Used with rituximab | Hyperglycemia, hypertension |
| Duvelisib (Copiktra) | Inhibitor of PI3K-delta and PI3K-gamma | CLL and SLL | FDA Approved | Reduces cytokine synthesis, direct cytotoxicity to leukemic cells | Investigated with BTK inhibitors | Diarrhea, colitis, pneumonitis |
| TGR-1202 (Umbralisib) | Dual PI3Kδ and CK1ε inhibitor | CLL, MZL, FL | FDA Approved | Inhibits PI3Kδ and CK1ε, reduces tumor cell adhesion and migration | Combined with BTK inhibitors | Diarrhea, nausea, fatigue |
| Zandelisib (ME-401) | Inhibitor of PI3K-Delta | FL, CLL, SLL, MZL, DLBCL | Phase II/III Clinical | Inhibits PI3K signaling, affecting cell proliferation and survival | Being tested with rituximab | Diarrhea, liver toxicity |
| Linperlisib | Inhibitor of PI3K-Delta | FL | Phase I/II Clinical | Inhibits PI3K signaling pathways | Combined with other chemotherapies | Fatigue, nausea |
| TQB3525 | Inhibitor of PI3K-alpha and PI3K delta | CLL, SLL | Phase I/II Clinical | Targets PI3K signaling, affects cell survival | Research ongoing for combinations | Under investigation |
| Acalisib | Inhibitor of PI3K-Delta | FL, DLBCL, MZL, MCL | Phase I Clinical | Inhibits PI3Kδ, impacting cell survival | Studied with chemotherapy agents | Under investigation |
| SHC014748M | Inhibitor of PI3K-Delta | CLL | Preclinical | Targets PI3K signaling pathways | Potential for combination therapy | Under investigation |
| Name of Molecule | Mechanism of Action | Type of Hematologic Malignancy | Type of Molecule | Approval Status | Combination Therapies | Adverse Effects |
|---|---|---|---|---|---|---|
| Curcumin | Inhibits NF-κB activation by suppressing various upstream signaling pathways | MM | Diarylheptanoid (curcuminoids group) | Preclinical | Research ongoing for combinations | Generally well tolerated |
| Bay 11-7082 | Inhibits NF-κB activation by targeting the IκB kinase complex, preventing phosphorylation of IκBα | MM, L | IκB Kinase (IKK) inhibitor | Preclinical | Investigated with other inhibitors | Under investigation |
| Parthenolide | Inhibits NF-κB activation by targeting the IκB kinase complex, preventing phosphorylation of IκBα | ALL, L | Germacranolide | Preclinical | Investigated with other NF-κB inhibitors | Cytotoxicity at high doses |
| IKK Inhibitor MLN120B | Targets IKK complex, preventing phosphorylation of IκBα | VL and L | Small molecule inhibitor | Preclinical | Investigated with chemotherapies | Under investigation |
| Resveratrol | Inhibits NF-κB activation by suppressing phosphorylation and degradation of IκBα, preventing NF-κB translocation | VL and L | Polyphenolic phytoalexin (Stilbene class) | Clinical Trials I/II | Combined with chemotherapies | Mild gastrointestinal symptoms |
| Name of Molecule | Target | Type of Malignancy | Type of Molecule | Approval Status | Combination Therapies | Adverse Effects |
|---|---|---|---|---|---|---|
| Hu5F9-G4 | Selectively binds to CD47 expressed on tumor cells and blocks the interaction with SIRPa | AML, MM, LBCL, and some solid tumors | Peptide (monoclonal antibody) | Clinical Trials I/II | Investigated with other chemotherapies | Anemia, fatigue |
| SIRPαFc (TTI-621) | Binds to CD47 on tumor cells, preventing inhibitory signals to macrophages, and engages FcγR to enhance phagocytosis | Relapsed/refractory hematologic malignancies and solid tumors | Peptide | Clinical Trials I/II | Combined with other immune checkpoint inhibitors | Thrombocytopenia, anemia |
| CC-90002 | Anti-CD47 antibody that inhibits CD47-SIRPα interaction, enabling macrophage-mediated killing of tumor cells | Relapsed/refractory hematologic malignancies and solid tumors | Peptide (antibody) | Clinical Trials I/II | Investigated with other mAbs | Cytokine release syndrome |
| ALX148 | Enhances macrophage phagocytosis of tumor cells and inhibits binding of wild-type SIRPα | Non-Hodgkin Lymphoma and solid tumors | Peptide (antibody) | Clinical Trials I/II | Combined with rituximab, pembrolizumab | Infusion reactions, anemia |
| Name of Molecule | Mechanism of Action | Type of Hematologic Malignancy | Type of Molecule | Approval Status | Combination Therapies | Adverse Effects |
|---|---|---|---|---|---|---|
| Pevonedistat (MLN4924) | Inhibits NEDD8-activating enzyme (NAE), disrupting neddylation, inducing apoptosis, senescence, and autophagy via p53 pathway activation | AML, MM, MDS | NEDD8-activating enzyme inhibitor | Clinical Trials II/III | Investigated with chemotherapies and immunotherapies | Nausea, fatigue, and hematologic toxicity |
| TAS4464 | Selectively inhibits NAE, leading to cullin neddylation inhibition and accumulation of CRL substrates, inducing antiproliferative activity | AML, MM | NEDD8-activating enzyme inhibitor | Clinical Trials I/II | Investigated with molecular and hormonal therapies | Under investigation |
| MLN4924 | Inhibits NAE, leading to the activation of the p53 signaling pathway and subsequent anti-leukemia effects | AML, MM | NEDD8-activating enzyme inhibitor | Clinical Trials II/III | Investigated with molecular, immunotherapy-based therapies | Nausea, fatigue, hematologic toxicity |
| TAS4464 | Highly potent NAE inhibitor, inducing cullin neddylation inhibition and CRL substrate accumulation, leading to widespread antiproliferative activity | AML, MM | NEDD8-activating enzyme inhibitor | Clinical Trials I/II | Combined with molecular therapies, CD47 receptor blockade | Under investigation |
| Name of Inhibitor | Clinical Trial Phase | Mechanism of Action | Type of Hematologic Malignancy | FDA Status | Combination Therapies | Adverse Effects |
|---|---|---|---|---|---|---|
| Nivolumab (Opdivo) | III/IV | Inhibits PD-1, preventing binding with PD-L1/PD-L2 and restoring T-cell activity | HL | Approved | Chemotherapy, targeted therapy, other immunotherapies | Fatigue, rash, diarrhea, hepatitis |
| Pembrolizumab (Keytruda) | III/IV | Inhibits PD-1, preventing binding with PD-L1/PD-L2 and restoring T-cell activity | HL, PMBCL | Approved | Chemotherapy, targeted therapy, other immunotherapies | Fatigue, pruritus, rash, pneumonitis |
| Cemiplimab (Libtayo) | III/IV | Inhibits PD-1, preventing binding with PD-L1/PD-L2 and restoring T-cell activity | HL | Clinical Trials | Chemotherapy, targeted therapy | Fatigue, rash, musculoskeletal pain |
| Sintilimab | III/IV | Inhibits PD-1, preventing binding with PD-L1/PD-L2 and restoring T-cell activity | HL | Approved (China) | Chemotherapy, targeted therapy, other immunotherapies | Pyrexia, hypothyroidism, pneumonia |
| Toripalimab | III/IV | Inhibits PD-1, preventing binding with PD-L1/PD-L2 and restoring T-cell activity | HL | Approved (China) | Chemotherapy, targeted therapy, other immunotherapies | Fatigue, fever, hypothyroidism |
| Camrelizumab | III/IV | Inhibits PD-1, preventing binding with PD-L1/PD-L2 and restoring T-cell activity | HL | Approved (China) | Chemotherapy, targeted therapy, other immunotherapies | Rash, pruritus, arthralgia |
| Target | Applications | Mechanism of Action | Clinical Impact |
|---|---|---|---|
| Rituximab (Rituxan) CD20 antigen on B cells [64]. | Rituximab is primarily used in the treatment of BNHL, including DLBCL and FL, as well as in CLL. | Rituximab binds to the CD20 antigen on B cells, leading to cell death through complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and direct induction of apoptosis. | The introduction of Rituximab has significantly improved survival rates in B-cell malignancies. It is often used in combination with chemotherapy (e.g., the R-CHOP regimen) and as maintenance therapy to prevent relapse. |
| Daratumumab (Darzalex) CD38 antigen on plasma cells [65]. | Daratumumab is widely used in the treatment of MM, both as a monotherapy and in combination with other agents like lenalidomide, bortezomib, and dexamethasone. | Daratumumab targets CD38, leading to cell death through CDC, ADCC, antibody-dependent cellular phagocytosis (ADCP), and apoptosis. | Daratumumab has transformed the treatment landscape for MM, offering significant improvements in progression-free survival and overall survival, particularly in relapsed and refractory settings. |
| Brentuximab Vedotin (Adcetris) CD30 antigen on Reed-Sternberg cells and some TCL [66]. | Brentuximab Vedotin is used in the treatment of HL and certain types of TCL, including ALCL. | This antibody-drug conjugate (ADC) consists of a CD30-directed monoclonal antibody linked to the cytotoxic agent monomethyl auristatin E (MMAE). Upon binding to CD30, the conjugate is internalized, and MMAE is released, leading to cell cycle arrest and apoptosis. | Brentuximab Vedotin has shown high efficacy in relapsed and refractory HL and ALCL, providing an important treatment option, especially for patients who have failed conventional chemotherapy. |
| Inotuzumab Ozogamicin (Besponsa) CD22 antigen on B cells [67]. | Inotuzumab Ozogamicin is used in the treatment of relapsed or refractory B-cell ALL. | This ADC targets CD22, delivering the cytotoxic antibiotic calicheamicin directly to the cancer cells, leading to DNA damage and cell death. | Inotuzumab Ozogamicin has improved outcomes in relapsed/refractory ALL, offering a targeted therapy option with high response rates in a difficult-to-treat patient population. |
| Elotuzumab (Empliciti) SLAMF7 (signaling lymphocytic activation molecule family member 7) on myeloma cells and natural killer (NK) cells [68]. | Elotuzumab is used in combination with lenalidomide and dexamethasone for the treatment of MM, particularly in relapsed/refractory cases. | Elotuzumab enhances NK cell-mediated ADCC against SLAMF7-expressing myeloma cells, while also activating NK cells to attack the cancer cells. | Elotuzumab has been shown to improve progression-free survival in patients with MM, especially when used in combination therapy. |
| Alemtuzumab (Campath) CD52 antigen on B and T cells [69]. | Alemtuzumab is used in the treatment of CLL and, in some cases, T-PL. | Alemtuzumab targets CD52, leading to cell death through CDC and ADCC. | Alemtuzumab has been effective in CLL, particularly in patients with 17p deletion who are typically resistant to other therapies. However, its use is limited due to significant immunosuppression and infection risks. |
| Name of Inhibitor | Mechanism of Action | Type of Hematologic Malignancy | Approval Status | Combination Therapies | Clinical Trial Phase | Adverse Effects |
|---|---|---|---|---|---|---|
| Ipilimumab (Yervoy) | Inhibits CTLA-4, leading to enhanced T cell activation and proliferation | RHL | FDA Approved | Combined with nivolumab (PD-1 inhibitor) | III/IV | Fatigue, diarrhea, rash, colitis |
| Tremelimumab | Inhibits CTLA-4, leading to enhanced T cell activation and proliferation | RHL | Clinical Trials | Combined with durvalumab (PD-L1 inhibitor) | III | Fatigue, nausea, rash, colitis |
| AGEN1884 | Inhibits CTLA-4, enhancing T cell activation and proliferation | VHM | Clinical Trials | Combined with other immunotherapies | I/II | Under investigation |
| RELA-067 | Inhibits CTLA-4, leading to enhanced T cell activation and proliferation | VHM | Clinical Trials | Combined with other immunotherapies | I/II | Under investigation |
| ONC-392 | Inhibits CTLA-4, reducing regulatory T cell suppression and enhancing effector T cell function | VHM | Clinical Trials | Combined with PD-1/PD-L1 inhibitors | I/II | Under investigation |
| XmAb20717 | Bispecific antibody targeting CTLA-4 and PD-1, enhancing T cell activation | VHM | Clinical Trials | Monotherapy and combination with other checkpoint inhibitors | I/II | Under investigation |
| Approach | Description | Therapeutic Application | Status |
|---|---|---|---|
| NK Cells + Immune Checkpoint Blockade | Combination of NK cells with checkpoint inhibitors to enhance immune response | VC | Clinical Trials |
| CAR-NK Cell Therapy | NK cells engineered to express chimeric antigen receptors for targeted cancer cell elimination | HM, ST | Clinical Trials |
| CAR-T Cell Therapy | T cells engineered to express chimeric antigen receptors for targeted cancer cell elimination | HM, ST | FDA Approved, Clinical Trials |
| Artificial Adjuvant Vector Cells | Artificial cells designed to enhance NK and T cell activation and targeting | VC | Preclinical/Clinical Trials |
| NK Cells + Monoclonal Antibodies | NK cells used in conjunction with monoclonal antibodies to target specific cancer cells | HM | Clinical Trials |
| TCR-Engineered T Cell Therapy | T cells engineered to express specific T cell receptors for precise targeting of cancer antigens | HM, ST | Clinical Trials |
| NK Cells + Cytokine Therapy | Combination of NK cells with cytokines to boost immune response against cancer cells | VC | Preclinical/Clinical Trials |
| Bispecific T-Cell Engagers (BiTEs) | Antibodies that simultaneously bind to T cells and cancer cells, bringing them into proximity | HM, ST | FDA Approved, Clinical Trials |
| NK Cell-Derived Exosomes | Exosomes derived from NK cells used for delivering therapeutic molecules | VC | Preclinical Trials |
| Trispecific Killer Engager (TriKE) | Molecules that engage NK cells with cancer cells and provide a co-stimulatory signal | HM | Preclinical/Clinical Trials |
| Dual-Affinity Re-Targeting (DART) molecules | Antibodies designed to bind two different antigens, enhancing immune cell targeting | VC | Preclinical/Clinical Trials |
| Macrophage Function | Action | Effect |
|---|---|---|
| Cytokines and Chemokines | Cytokines such as interferons (IFNs), tumor necrosis factor alpha (TNF-α), interleukins (e.g., IL-1, IL-6, IL-12), and chemokines released by other immune cells or produced by macrophages themselves can activate macrophages. | These small molecules bind to specific receptors on macrophages, initiating signaling pathways that induce their activation. |
| Opsonization | Opsonins, such as antibodies and complement proteins, coat pathogens and enhance their recognition and phagocytosis by macrophages. | Engagement of opsonin receptors on macrophages triggers signaling events that lead to their activation and phagocytic activity. |
| Phagocytic Receptors | Macrophages express various phagocytic receptors, including Fc receptors and complement receptors, which recognize opsonized pathogens and facilitate their internalization. | Successful binding of these receptors activates downstream signaling pathways that promote phagocytosis and microbial killing. |
| Inflammatory Mediators | Inflammatory mediators such as prostaglandins, leukotrienes, and reactive oxygen species (ROS) released during inflammation can activate macrophages. | These molecules contribute to the inflammatory response and induce macrophage activation, promoting enhanced anti-tumor activity in hematologic malignancies. |
| Toll-like receptor (TLR) agonists | TLRs are key molecular sensors that recognize the presence of pathogens and other danger signals. | Stimulation of TLRs on macrophages can lead to their activation and increased ability to eliminate hematologic cancer cells. |
| Interferons | When used in combination therapy, they can activate macrophages, stimulate the production of chemokines and pro-inflammatory cytokines, and increase the expression of MHC molecules on cancer cells. | This facilitates their recognition by the immune system, resulting in the activation of downstream signaling, deciding the fate of hematologic cancer cells. |
| CAR-M | Macrophages are engineered to express receptors on their surface, facilitating the recognition of surface antigens either with antibodies or specific ligands present on target cells. | CAR-Ms can phagocytose tumors directly after identifying specific antigens on hematologic cancer cells. Additionally, active CAR-Ms may secrete inflammatory molecules such as IFN-γ, IL-12, and TNF-α to encourage M1 polarization and activate antigen-presenting cells (APCs) in the tumor microenvironment (TME). |
| Name of Therapy | Potential Benefits | Disadvantages |
|---|---|---|
| TIM-3 (T cell immunoglobulin and mucin domain 3) | Combined PD-1/PD-L1 with TIM-3/Gal-9 blockade could prevent CD8+ T-cell exhaustion in advanced AML [85]. PD-1 combined with TIM-3 blockades could stimulate potential anti-tumor T cell responses in melanoma [86]. In xenograft models, anti-TIM-3 IgG2a antibody could improve cytotoxic activities and eradicate AML leukemic stem cells [87]. | Lack of valid biomarkers which can predict successful treatment with this combination [88]. Combinations will have to be patient-tailored since they are likely to be more toxic than single agents and more expensive. Cells usually have functionally redundant pathways which could override and compensate for each other [89]. |
| TIGIT | TIGIT suppresses both innate and adaptive immunity by a variety of mechanisms, such as initiating T/NK cell-intrinsic inhibition, producing immunosuppressive DCs, blocking CD226 signaling, boosting Treg immunosuppression, and encouraging Fap2-induced T/NK cell inhibition [90]. | There is currently no reliable biomarker for anti-TIGIT therapy. As a result, future studies should concentrate on identifying new biomarkers or targeting TIGIT using alternative strategies, such as CAR-T cells, antibody-drug conjugates, and bispecific antibodies [91]. |
| Small molecule inhibitors | Easier cellular entry, oral effectiveness, and comparatively cost-efficient synthesis [8]. In vivo studies indicate that P5091 is well tolerated, inhibits malignant cell growth, and extends survival [23]. | Pulmonary toxicity in preclinical studies [19]. Studies on biochemical and cellular characterization of lead compounds, in addition to extensive PK, pharmacodynamics, and toxicology studies, are required [92]. |
| PI3K inhibitors | Several inhibitors passed clinical trials and are approved by FDA. Demonstrated desired therapeutic effects on various cancers. Several inhibitor alternatives available in market [93]. | Adverse effects remain major concern for this therapy. On-target toxicities severely limit the development of PI3K inhibitors [93]. |
| NFkB inhibitors | Inhibits NF-κB activation by sup-pressing phosphorylation and degradation of IκBα, preventing NF-κB translocation [47]. Inhibits NF-κB activation by targeting the IκB kinase complex and preventing phosphorylation of IκBα [94]. | Mild gastro-intestinal symptoms [47]. Cytotoxicity at high doses [94]. |
| CD47 Inhibitors | This therapy aims to synergistically boost the immune system’s ability to target and eliminate cancer cells, while also overcoming resistance mechanisms that tumors may develop [52]. | These limitations include resistance mechanisms, toxicity, lack of predictive biomarkers, inadequate effectiveness as a monotherapy, and production difficulties [95]. |
| Neddylation Inhibitors | Widespread antiproliferative activity in cancer cell lines and patient-derived tumor cells, making it a promising agent for hematologic tumors [57]. Currently under phase II/III clinical trials for anti-tumor treatment and shows good safety and tolerability, indicating its good development prospects [96]. | Drug resistance is a major challenge [96]. |
| Combination Therapy | Components | Mechanism of Action | Potential Benefits | Clinical Status |
|---|---|---|---|---|
| PI3K Inhibitor + Proteasome Inhibitor | Idelalisib + Bortezomib | PI3K inhibitors impede signaling pathways regulating cell growth; proteasome inhibitors block protein degradation | Synergistic anti-cancer effects, reduced cell proliferation, enhanced apoptosis | Clinical Trials |
| PI3K Inhibitor + Immunological Checkpoint Inhibitor | Idelalisib + TIM-3/TIGIT inhibitors | PI3K inhibitors reduce immunosuppression; checkpoint inhibitors enhance T cell activation | Enhanced immune response, suppressed tumor cell proliferation | Preclinical/Clinical Trials |
| Immunological Checkpoint Inhibitor + Proteasome Inhibitor | PD-1/CTLA-4 inhibitors + Bortezomib | Checkpoint inhibitors boost immune response; proteasome inhibitors regulate apoptosis-controlling protein expression | Enhanced tumor cell apoptosis, boosted immune response | Clinical Trials |
| NF-κB Inhibitor + TIGIT Inhibitor | NF-κB inhibitors + TIGIT inhibitors | NF-κB inhibitors regulate signaling pathways; TIGIT inhibitors reduce Treg-mediated immunosuppression | Reduced tumor cell proliferation, heightened immune response | Preclinical |
| NF-κB Inhibitor + Monoclonal Antibody Therapy | NF-κB inhibitors + Monoclonal antibodies | NF-κB inhibitors block survival pathways; monoclonal antibodies target cancer cell receptors | Synergistic anti-tumor impact, enhanced immune-mediated tumor eradication | Preclinical/Clinical Trials |
| Neddylation Inhibitor + Tumorigenesis Inhibitor | MLN4924 + Tumorigenesis inhibitors | Neddylation inhibitors block protein degradation; tumorigenesis inhibitors impede growth and proliferation processes | Enhanced tumor cell apoptosis, restrained tumor growth and metastasis | Preclinical |
| Neddylation Inhibitor + Antigen Complex Therapy | MLN4924 + Antigen complex therapy | Neddylation inhibitors prevent protein degradation; antigen complex therapy elicits immune response | Increased tumor cell apoptosis, mounted immune response | Preclinical |
| CAR-T Cell Therapy + Immunological Checkpoint Inhibitor | CAR-T cells + Pembrolizumab | CAR-T cells target and eliminate cancer cells; checkpoint inhibitors enhance CAR-T cell persistence and function | Augmented CAR-T cell efficacy, enhanced immune response | Clinical Trials |
| CAR-T Cell Therapy + Radiotherapy | CAR-T cells + Radiotherapy | CAR-T cells target cancer cells; radiotherapy enhances tumor cell destruction | Improved anti-cancer immune response, enhanced tumor cell destruction | Clinical Trials |
| CAR-T Cell Therapy + Immunomodulatory Drugs | CAR-T cells + Immunomodulatory drugs | CAR-T cells target tumor cells; immunomodulatory drugs boost T cell proliferation and persistence | Effective tumor eradication, enhanced cytokine production | Clinical Trials |
| Signaling Cascade Inhibitors + Immune Checkpoint Inhibitors | Ibrutinib + Anti-PD-1/PD-L1 antibodies | Signaling inhibitors regulate growth pathways; checkpoint inhibitors boost immune response | Overcome resistance, enhanced anti-tumor immune response | Clinical Trials |
| CD47 Inhibitor + CAR-T Therapy | Hu5F9-G4 + CAR-T cells | CD47 inhibitors increase phagocytosis of cancer cells; CAR-T cells target and eliminate cancer cells | Enhanced phagocytosis, robust immune response | Clinical Trials |
| NF-κB Inhibitor + Hyperthermia Therapy | NF-κB inhibitors + Hyperthermia | NF-κB inhibitors block survival pathways; hyperthermia increases treatment sensitivity | Increased apoptosis, enhanced treatment efficacy | Preclinical |
| Combination Therapy | Components | Mechanism of Action | Potential Benefits | Clinical Status |
|---|---|---|---|---|
| CAR-T Therapy + Oncolytic Virus Therapy | CAR-T cells + Oncolytic viruses | Enhanced CAR-T cell infiltration and activity, direct oncolytic effects | Increased CAR-T cell efficacy, enhanced immune response | Preclinical/ Clinical Trials |
| Epigenetic Modifiers + Immunotherapy | Epigenetic drugs + CAR-T cells/checkpoint inhibitors | Improved tumor antigen expression, enhanced immune recognition and response | Improved immune response, enhanced tumor antigen presentation | Preclinical/ Clinical Trials |
| Metabolic Inhibitors + Immune Checkpoint Inhibitors | Metabolic inhibitors + Checkpoint inhibitors | Disrupted cancer cell metabolism, reduced tumor growth, enhanced immune response | Enhanced antitumor response, reduced tumor growth | Preclinical/ Clinical Trials |
| PARP Inhibitors + Immunotherapy | PARP inhibitors + CAR-T cells/checkpoint inhibitors | Increased DNA damage, improved immune recognition, and response | Enhanced tumor cell death, improved immune response | Preclinical/ Clinical Trials |
| Autophagy Inhibitors + Chemotherapy | Autophagy inhibitors + Chemotherapy | Increased chemotherapy efficacy, reduced cancer cell survival | Enhanced chemotherapy effects, reduced tumor cell survival | Preclinical/ Clinical Trials |
| Bcl-2 Inhibitors + Immunotherapy | Bcl-2 inhibitors + CAR-T cells/checkpoint inhibitors | Increased tumor cell apoptosis, enhanced immune response | Improved tumor cell death, enhanced immune response | Preclinical/ Clinical Trials |
| Proteasome Inhibitors + Histone Deacetylase Inhibitors | Proteasome inhibitors + Histone deacetylase inhibitors | Synergistic induction of apoptosis, improved tumor cell death | Enhanced apoptosis, improved tumor cell death | Preclinical/ Clinical Trials |
| Checkpoint Inhibitors + TLR Agonists | Checkpoint inhibitors + TLR agonists | Enhanced activation of innate and adaptive immune responses, improved antitumor activity | Improved immune response, enhanced tumor destruction | Preclinical/ Clinical Trials |
| Angiogenesis Inhibitors + Immune Checkpoint Inhibitors | Angiogenesis inhibitors + Checkpoint inhibitors | Reduced tumor vascularization, enhanced immune response | Reduced tumor growth, improved immune cell infiltration | Preclinical/ Clinical Trials |
| Anti-CD47 Therapy + Radiotherapy | Anti-CD47 antibodies + Radiotherapy | Enhanced phagocytosis, improved immune response, increased tumor cell death | Improved tumor clearance, enhanced immune response | Preclinical/ Clinical Trials |
| Therapy | Target Disease | Combination | Reason for Failure |
|---|---|---|---|
| Orelabrutinib | BCM | - | Significant safety concerns |
| Nemtabrutinib (formerly ARQ 531) | CLL and MCL | - | Efficacy and safety issues |
| TTI-621 | Relapsed or RHM | - | Significant safety issues |
| Hu8F4 | AML and CMML | - | Limited efficacy and significant toxicity |
| Ivosidenib and Venetoclax with or without Azacitidine | IDH1-mutated HM | Combination of Ivosidenib and Venetoclax, sometimes with Azacitidine | Safety challenges and unmet therapeutic outcomes |
| Afuresertib and Fulvestrant | HR+/HER2 BC (with implications for HM) | Combination of Afuresertib and Fulvestrant | Insufficient efficacy in Phase III trials |
| Rilotumumab | GC and HM | - | Safety concerns and lack of efficacy in Phase III trials |
| Bavituximab | NSCLC and HM | - | Lack of efficacy in Phase III trials |
| Selinexor | MM and other HM | - | Significant toxicity and limited efficacy in later-stage trials |
| Future Actions and Direction Lines for Combinational Therapies | |
|---|---|
| Targeted Combinations | As genomic profiling becomes more sophisticated, therapies will increasingly be tailored to the genetic and molecular characteristics of individual patients’ tumors. This approach will enable the selection of drug combinations that target specific mutations, pathways, or microenvironmental factors driving the malignancy [142,143]. |
| Predictive Biomarkers | The identification and validation of biomarkers that predict response to specific drug combinations will play a crucial role in personalizing therapy. For example, using biomarkers to guide the use of immunotherapy combinations with targeted therapies could optimize treatment efficacy and minimize toxicity [144]. |
| Immunotherapy Combinations | CAR-T cells with immune checkpoint inhibitors: CAR-T therapy has shown remarkable success in some hematologic malignancies, but resistance and relapse remain challenges. Combining CAR-T cells with immune checkpoint inhibitors (e.g., anti-PD-1/PD-L1 or anti-CTLA-4) could enhance the persistence and efficacy of CAR-T cells by overcoming the immunosuppressive tumor microenvironment [142,143]. |
| Bispecific Antibodies and Cytokines | The use of bispecific antibodies that target both the cancer cells and immune cells, combined with cytokine therapies to boost the immune response, is a promising strategy. These combinations aim to enhance the immune system’s ability to recognize and eliminate cancer cells more effectively [145]. |
| Stromal and Immune Modulators | The tumor microenvironment, including stromal cells, immune cells, and the extracellular matrix, plays a critical role in the progression and resistance of hematologic malignancies. Combinational therapies that target both the cancer cells and their supportive microenvironment could prevent resistance and improve outcomes [146]. |
| Hypoxia-Targeted Therapies | Targeting hypoxia-inducible factors (HIFs) in the tumor microenvironment, in combination with other therapies, could reduce the adaptation of cancer cells to hypoxic conditions, which is often associated with resistance to therapy [147]. |
| Epigenetic Modifiers with Chemotherapy | Combining epigenetic therapies, such as DNA methyltransferase inhibitors or histone deacetylase inhibitors with standard chemotherapy could enhance the sensitivity of cancer cells to treatment. Epigenetic modifications often drive resistance, so targeting these changes could overcome resistance mechanisms [148]. |
| Combining Epigenetic and Immunotherapies | There is growing interest in combining epigenetic drugs with immunotherapies to increase the immunogenicity of tumors. For example, epigenetic drugs could upregulate the expression of antigens or immune-related genes, making the cancer cells more susceptible to immune attack [148]. |
| Next-Generation Targeted Therapies | The development of next-generation small molecule inhibitors that target previously “undruggable” proteins or that have greater specificity and potency is a major focus. These could be used in combination with existing therapies to enhance efficacy and reduce side effects [149]. |
| Synthetic Lethality Approaches | Combining drugs that exploit synthetic lethality—where the simultaneous inhibition of two genes or pathways leads to cancer cell death, but inhibition of either alone does not—could provide a powerful strategy against hematologic malignancies with specific genetic alterations [150]. |
| Sequential and Adaptive Combinations | Instead of static combination regimens, future therapies might involve adaptive or sequential combinations, where treatments are adjusted based on the real-time monitoring of tumor evolution and resistance patterns. This dynamic approach could help prevent the emergence of drug-resistant clones. |
| Dual-Targeting Strategies | Combining two or more drugs that target different aspects of the same pathway or cellular process could prevent the cancer from developing resistance through alternative pathways [145]. |
| Big Data for Predictive Modeling | Integrating data from genomics, proteomics, and patient outcomes into predictive models can help forecast which combinations will be most effective for specific patient populations. This approach could lead to the rapid identification of novel combinations that might not have been considered through traditional research methods [151]. |
| Targeted Delivery Systems | Advances in drug delivery technologies, such as nanoparticles or conjugated antibodies, could allow for more precise targeting of drug combinations to cancer cells while sparing healthy tissues. This approach could minimize side effects and improve patients’ quality of life during treatment [152]. |
| Reducing Off-Target Effects | Combining therapies that have complementary mechanisms of action but non-overlapping toxicity profiles could reduce the cumulative side effects experienced by patients, making long-term treatment more tolerable [153]. |
| Master Protocols | Future clinical trials for combinational therapies are likely to involve master protocols where multiple therapies are tested simultaneously across different subtypes of hematologic malignancies. This approach can accelerate the identification of effective combinations [142,143,144]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lica, J.J.; Pradhan, B.; Safi, K.; Jakóbkiewicz-Banecka, J.; Hellmann, A. Promising Therapeutic Strategies for Hematologic Malignancies: Innovations and Potential. Molecules 2024, 29, 4280. https://doi.org/10.3390/molecules29174280
Lica JJ, Pradhan B, Safi K, Jakóbkiewicz-Banecka J, Hellmann A. Promising Therapeutic Strategies for Hematologic Malignancies: Innovations and Potential. Molecules. 2024; 29(17):4280. https://doi.org/10.3390/molecules29174280
Chicago/Turabian StyleLica, Jan Jakub, Bhaskar Pradhan, Kawthar Safi, Joanna Jakóbkiewicz-Banecka, and Andrzej Hellmann. 2024. "Promising Therapeutic Strategies for Hematologic Malignancies: Innovations and Potential" Molecules 29, no. 17: 4280. https://doi.org/10.3390/molecules29174280
APA StyleLica, J. J., Pradhan, B., Safi, K., Jakóbkiewicz-Banecka, J., & Hellmann, A. (2024). Promising Therapeutic Strategies for Hematologic Malignancies: Innovations and Potential. Molecules, 29(17), 4280. https://doi.org/10.3390/molecules29174280





