The Effect of the Lysine Acetylation Modification of ClpP on the Virulence of Vibrio alginolyticus
Abstract
1. Introduction
2. Results
2.1. Characteristics of clpP
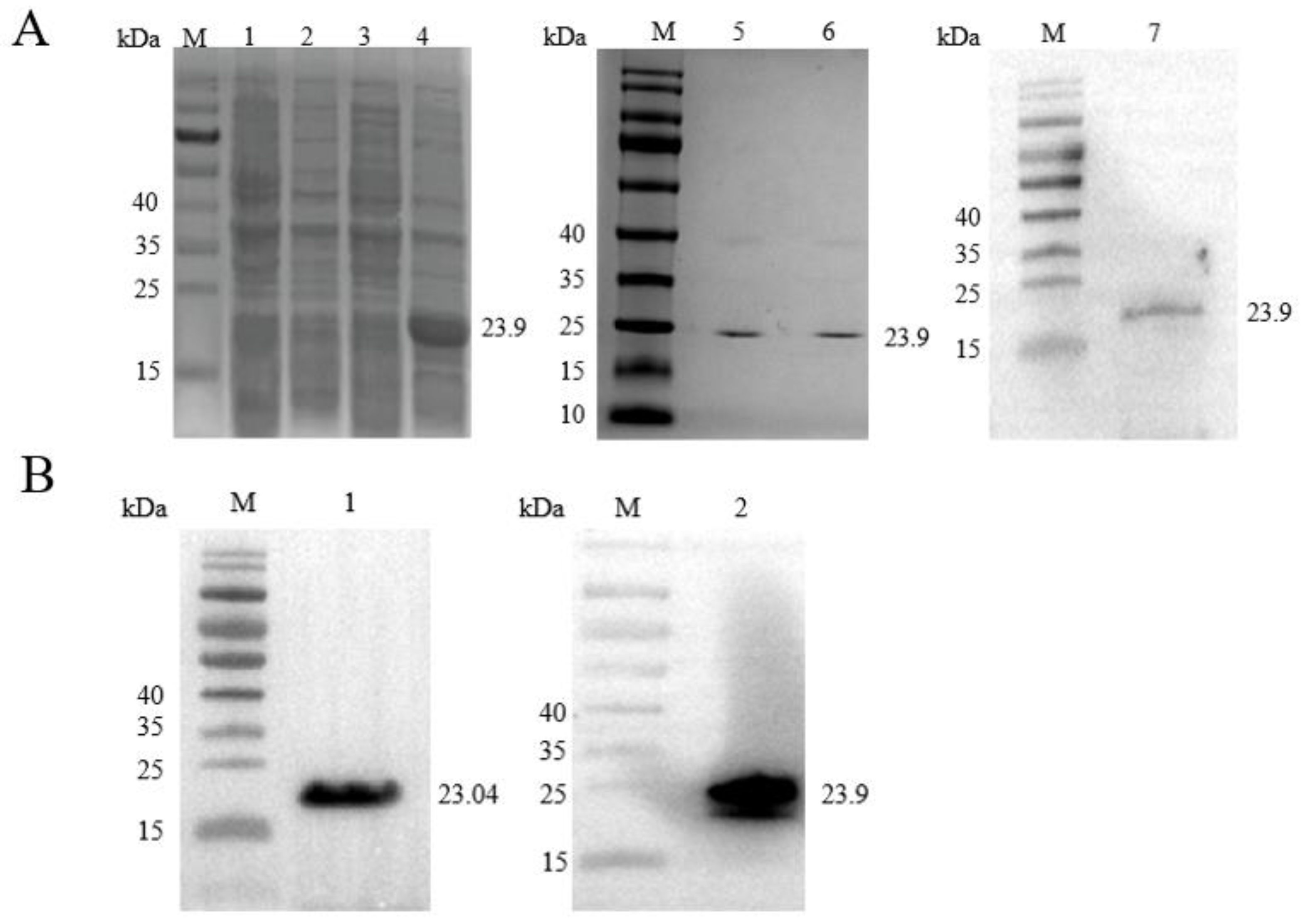
2.2. Recombinant ClpP Expression, Purification and Antibody Validation
2.3. Validation of ClpP Lysine-Acetylated Proteins Using Immunoprecipitation and Western Blot
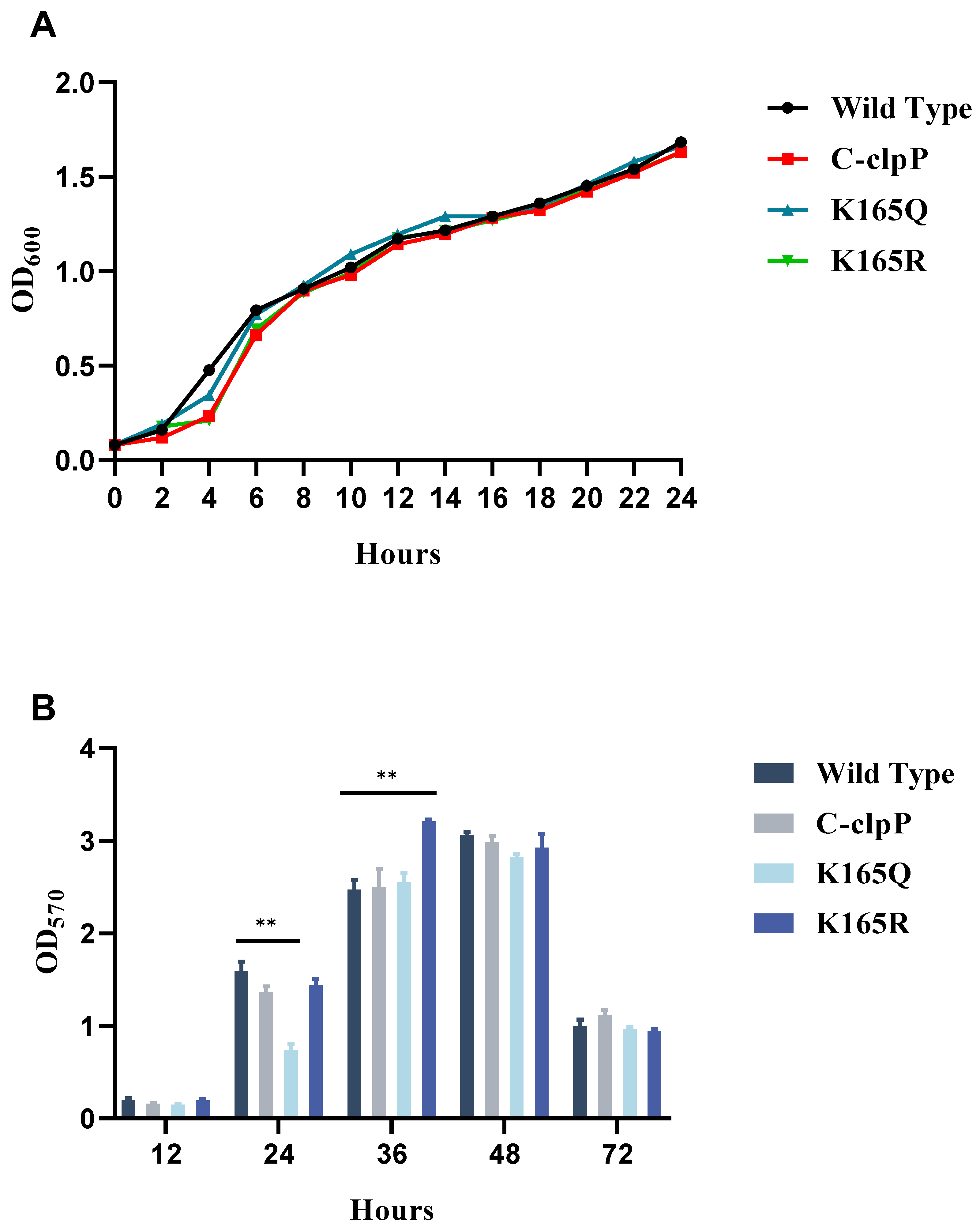
2.4. Growth Curve Measurement
2.5. Effect of Lysine Acetylation on Biofilm Formation
2.6. Effect of Site-Directed Mutagenesis Strains on LD50 of Zebrafish
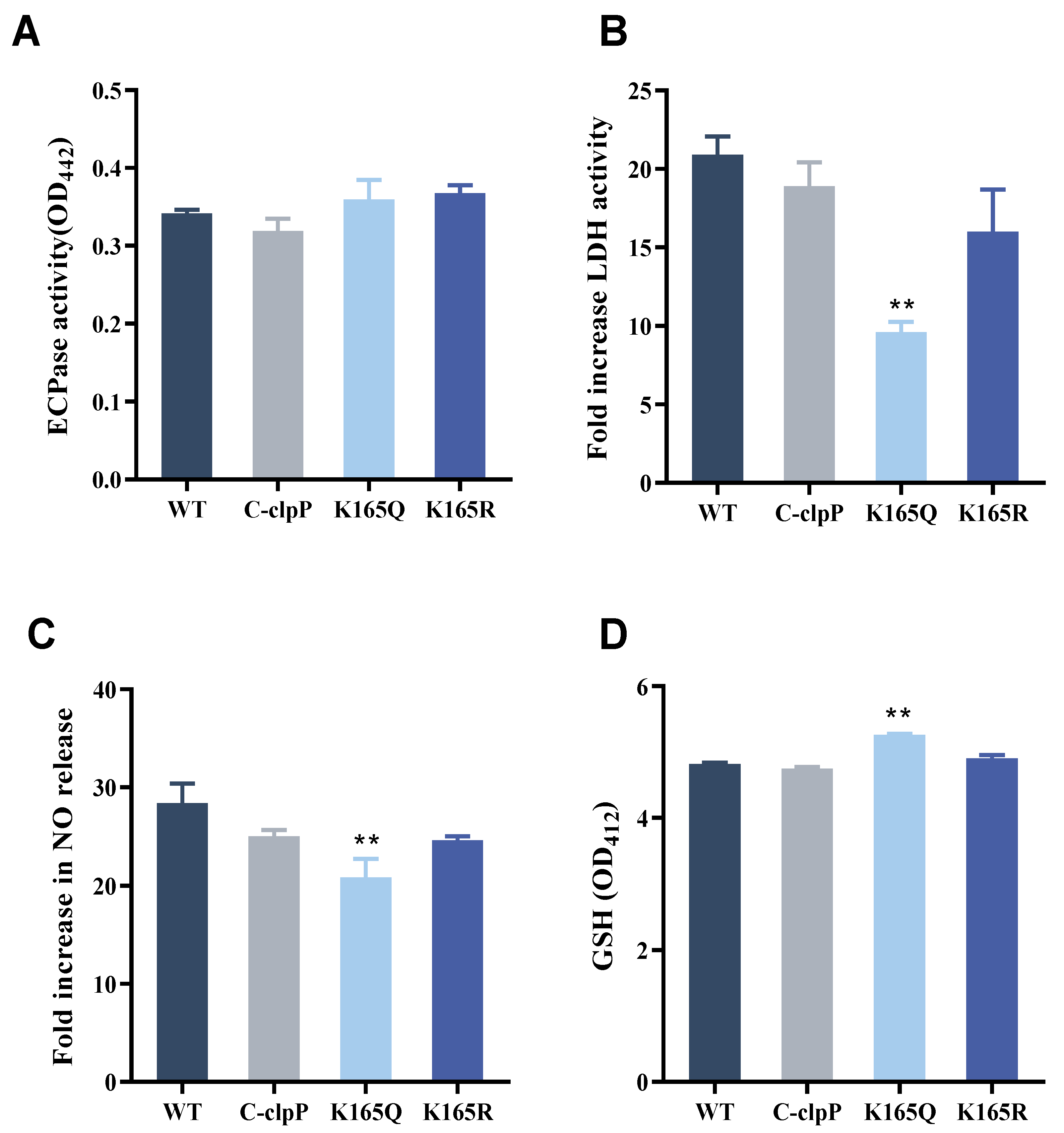
2.7. Effect of Site-Directed Mutagenesis Strains on ECPase Activity
2.8. Effects of Site-Directed Mutagenesis Strains on GS Cells
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Fish
4.2. Cloning and Bioinformatics Analysis of clpP Gene from V. alginolyticus HY9901
4.3. Prokaryotic Expression of clpP and Antibody Preparation
4.4. Immunoprecipitation and Western Blot
4.5. Construction of clpP Complementation Strain (C-clpP) and Acetylation Site-Directed Mutagenesis Strains
4.6. Growth Curve of Bacteria
4.7. Quantification of Biofilm Biomass
4.8. Fifty Percent Lethal Dose (LD50)
4.9. Detection of Extracellular Protease (ECPase) Activity
4.10. GS Cell Cytotoxicity Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernal, V.; Castaño-Cerezo, S.; Gallego-Jara, J.; Écija-Conesa, A.; de Diego, T.; Iborra, J.L.; Cánovas, M. Regulation of Bacterial Physiology by Lysine Acetylation of Proteins. N. Biotechnol. 2014, 31, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine Acetylation Targets Protein Complexes and Co-Regulates Major Cellular Functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Minguez, P.; Parca, L.; Diella, F.; Mende, D.R.; Kumar, R.; Helmer-Citterich, M.; Gavin, A.; van Noort, V.; Bork, P. Deciphering a Global Network of Functionally Associated Post-translational Modifications. Mol. Syst. Biol. 2012, 8, 599. [Google Scholar] [CrossRef]
- Deutscher, J.; Aké, F.M.D.; Derkaoui, M.; Zébré, A.C.; Cao, T.N.; Bouraoui, H.; Kentache, T.; Mokhtari, A.; Milohanic, E.; Joyet, P. The Bacterial Phosphoenolpyruvate: Carbohydrate Phosphotransferase System: Regulation by Protein Phosphorylation and Phosphorylation-Dependent Protein-Protein Interactions. Microbiol. Mol. Biol. Rev. 2014, 78, 231–256. [Google Scholar] [CrossRef]
- Reverdy, A.; Chen, Y.; Hunter, E.; Gozzi, K.; Chai, Y. Protein Lysine Acetylation Plays a Regulatory Role in Bacillus subtilis Multicellularity. PLoS ONE 2018, 13, e0204687. [Google Scholar] [CrossRef]
- Fang, Z.; Lai, F.; Cao, K.; Zhang, Z.; Cao, L.; Liu, S.; Duan, Y.; Yin, X.; Ge, R.; He, Q.-Y.; et al. Potential Role of Lysine Acetylation in Antibiotic Resistance of Escherichia coli. mSystems 2022, 7, e00649-22. [Google Scholar] [CrossRef] [PubMed]
- Gaviard, C.; Broutin, I.; Cosette, P.; Dé, E.; Jouenne, T.; Hardouin, J. Lysine Succinylation and Acetylation in Pseudomonas aeruginosa. J. Proteome Res. 2018, 17, 2449–2459. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sang, Y.; Tan, Y.; Tao, J.; Ni, J.; Liu, S.; Fan, X.; Zhao, W.; Lu, J.; Wu, W.; et al. Acetylation of Lysine 201 Inhibits the DNA-Binding Ability of PhoP to Regulate Salmonella Virulence. PLoS Pathog. 2016, 12, e1005458. [Google Scholar] [CrossRef]
- Singh, K.K.; Bhardwaj, N.; Sankhe, G.D.; Udaykumar, N.; Singh, R.; Malhotra, V.; Saini, D.K. Acetylation of Response Regulator Proteins, TcrX and MtrA in M. tuberculosis Tunes Their Phosphotransfer Ability and Modulates Two-Component Signaling Crosstalk. J. Mol. Biol. 2019, 431, 777–793. [Google Scholar] [CrossRef]
- Sang, Y.; Ren, J.; Qin, R.; Liu, S.; Cui, Z.; Cheng, S.; Liu, X.; Lu, J.; Tao, J.; Yao, Y.-F. Acetylation Regulating Protein Stability and DNA-Binding Ability of HilD, Thus Modulating Salmonella typhimurium Virulence. J. Infect. Dis. 2017, 216, 1018–1026. [Google Scholar] [CrossRef]
- Li, D.; Ramanathan, S.; Wang, G.; Wu, Y.; Tang, Q.; Li, G. Acetylation of Lysine 7 of AhyI Affects the Biological Function in Aeromonas hydrophila. Microb. Pathog. 2020, 140, 103952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, Z.; Tang, H.; Song, Q.; Song, H.; Yao, J.; Li, Z.; Xie, X.; Lin, Y.; Lin, X. The Lysine Acetylation Modification in the Porin Aha1 of Aeromonas hydrophila Regulates the Uptake of Multidrug Antibiotics. Mol. Cell Proteom. 2022, 21, 100248. [Google Scholar] [CrossRef]
- Pang, H.; Li, W.; Zhang, W.; Zhou, S.; Hoare, R.; Monaghan, S.J.; Jian, J.; Lin, X. Acetylome Profiling of Vibrio alginolyticus Reveals Its Role in Bacterial Virulence. J. Proteom. 2020, 211, 103543. [Google Scholar] [CrossRef]
- Norfolk, W.A.; Shue, C.; Henderson, W.M.; Glinski, D.A.; Lipp, E.K. Vibrio alginolyticus Growth Kinetics and the Metabolic Effects of Iron. Microbiol. Spectr. 2023, 11, e02680-23. [Google Scholar] [CrossRef]
- Austin, B. Vibrios as Causal Agents of Zoonoses. Vet. Microbiol. 2010, 140, 310–331. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Q.; Yang, Q.; Fan, H.; Yu, G.; Liu, F.; Bello, B.K.; Zhang, X.; Zhang, T.; Dong, J.; et al. Vibrio alginolyticus Triggers Inflammatory Response in Mouse Peritoneal Macrophages via Activation of NLRP3 Inflammasome. Front. Cell. Infect. Microbiol. 2021, 11, 769777. [Google Scholar] [CrossRef]
- Lee, K.-K.; Yu, S.-R.; Liu, P.-C. Alkaline Serine Protease Is an Exotoxin of Vibrio alginolyticus in Kuruma Prawn, Penaeus Japonicus. Curr. Microbiol. 1997, 34, 110–117. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Cao, X.; Yang, M.; Zhang, Y. Characterization of Two TonB Systems in Marine Fish Pathogen Vibrio alginolyticus: Their Roles in Iron Utilization and Virulence. Arch. Microbiol. 2008, 190, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Liu, J.; Liu, K.; Zhang, Z.; Ma, Z.; Dan, S.F.; Lan, Z.; Lu, M.; Fang, H.; Zhang, Y.; et al. Cloning and Expression of Two Anti-Lipopolysaccharide Factors in Eriocheir Hepuensis under Vibrio alginolyticus-Induced Stress. J. Fish Biol. 2023, 102, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Aljghami, M.E.; Barghash, M.M.; Majaesic, E.; Bhandari, V.; Houry, W.A. Cellular Functions of the ClpP Protease Impacting Bacterial Virulence. Front. Mol. Biosci. 2022, 9, 1054408. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, F.; Wang, Z.; Tang, J.; Cai, S.; Jian, J. Construction and Evaluation of Vibrio alginolyticus ΔclpP Mutant, as a Safe Live Attenuated Vibriosis Vaccine. Fish Shellfish. Immunol. 2020, 98, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Diwan, G.D.; Gonzalez-Sanchez, J.C.; Apic, G.; Russell, R.B. Next Generation Protein Structure Predictions and Genetic Variant Interpretation. J. Mol. Biol. 2021, 433, 167180. [Google Scholar] [CrossRef]
- Gu, K.; Ouyang, P.; Hong, Y.; Dai, Y.; Tang, T.; He, C.; Shu, G.; Liang, X.; Tang, H.; Zhu, L.; et al. Geraniol Inhibits Biofilm Formation of Methicillin-Resistant Staphylococcus aureus and Increase the Therapeutic Effect of Vancomycin in Vivo. Front. Microbiol. 2022, 13, 960728. [Google Scholar] [CrossRef] [PubMed]
- Arslan, E.; Coşkun, M.K.; Çobanoğlu, Ş.; Aslan, M.H.; Yazıcı, A. Effects of Four Antibiotics on Pseudomonas aeruginosa Motility, Biofilm Formation, and Biofilm-Specific Antibiotic Resistance Genes Expression. Diagn. Microbiol. Infect. Dis. 2023, 106, 115931. [Google Scholar] [CrossRef]
- Dong, Y.; Geng, J.; Liu, J.; Pang, M.; Awan, F.; Lu, C.; Liu, Y. Roles of Three TonB Systems in the Iron Utilization and Virulence of the Aeromonas hydrophila Chinese Epidemic Strain NJ-35. Appl. Microbiol. Biotechnol. 2019, 103, 4203–4215. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Nero, T.; Mukherjee, S.; Olson, R.; Yan, J. Searching for the Secret of Stickiness: How Biofilms Adhere to Surfaces. Front. Microbiol. 2021, 1, 686793. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ma, Q.; Yan, J.; Gong, T.; Huang, J.; Chen, J.; Li, J.; Qiu, Y.; Wang, X.; Lei, Z.; et al. Inhibition of Streptococcus mutans Growth and Biofilm Formation through Protein Acetylation. Mol. Oral Microbiol. 2024, 39, 334–343. [Google Scholar] [CrossRef]
- Osei-Adjei, G.; Huang, X.; Zhang, Y. The Extracellular Proteases Produced by Vibrio parahaemolyticus. World J. Microbiol. Biotechnol. 2018, 34, 68. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L.; Wang, X.; Wan, M.; Tang, M.; Ding, Y. Characterizing the Effect of the Lysine Deacetylation Modification on Enzyme Activity of Pyruvate Kinase I and Pathogenicity of Vibrio alginolyticus. Front. Vet. Sci. 2022, 9, 877067. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Qin, J.; Cheng, S.; Yeo, W.-S.; He, L.; Ma, X.; Liu, X.; Li, M.; Bae, T. The ATP-Dependent Protease ClpP Inhibits Biofilm Formation by Regulating Agr and Cell Wall Hydrolase Sle1 in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2017, 7, 18. [Google Scholar] [CrossRef]
- Korzeniewski, C.; Callewaert, D.M. An Enzyme-Release Assay for Natural Cytotoxicity. J. Immunol. Methods 1983, 64, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Crocetta, V.; Confalone, P.; Nicoletti, M.; Petrucca, A.; Guarnieri, S.; Fiscarelli, E.; Savini, V.; Piccolomini, R.; Di Bonaventura, G. Adhesion to and Biofilm Formation on IB3-1 Bronchial Cells by Stenotrophomonas maltophilia Isolates from Cystic Fibrosis Patients. BMC Microbiol. 2010, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Crabbé, A.; Liu, Y.; Matthijs, N.; Rigole, P.; De La Fuente-Nùñez, C.; Davis, R.; Ledesma, M.A.; Sarker, S.; Van Houdt, R.; Hancock, R.E.W.; et al. Antimicrobial Efficacy against Pseudomonas aeruginosa Biofilm Formation in a Three-Dimensional Lung Epithelial Model and the Influence of Fetal Bovine Serum. Sci. Rep. 2017, 7, 4332. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bossche, S.; Vandeplassche, E.; Ostyn, L.; Coenye, T.; Crabbé, A. Bacterial Interference with Lactate Dehydrogenase Assay Leads to an Underestimation of Cytotoxicity. Front. Cell. Infect. Microbiol. 2020, 10, 494. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione Depletion Induces Ferroptosis, Autophagy, and Premature Cell Senescence in Retinal Pigment Epithelial Cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef]
- Gao, Z.; Zhu, H.; Chen, J.; Liu, W.; Huo, J.; He, C.; Chen, J. Clinical Value of BRE-AS1 in Myocardial Infarction and Its Role in Myocardial Infarction-Induced Cardiac Muscle Cell Apoptosis. Scand. Cardiovasc. J. 2024, 58, 2347290. [Google Scholar] [CrossRef]
- Khademvatan, S.; Yousefi, E.; Asadi, N.; Abasi, E. Evaluation of In Vitro Cytotoxic and Apoptotic Effects of Miltefosine on the Toxoplasma Gondii RH Strain. Iran J. Parasitol. 2024, 19, 52–60. [Google Scholar]
- Lind, M.; Hayes, A.; Caprnda, M.; Petrovic, D.; Rodrigo, L.; Kruzliak, P.; Zulli, A. Inducible Nitric Oxide Synthase: Good or Bad? Biomed. Pharmacother. 2017, 93, 370–375. [Google Scholar] [CrossRef]
- Forbes, S.P.; Alferiev, I.S.; Chorny, M.; Adamo, R.F.; Levy, R.J.; Fishbein, I. Modulation of NO and ROS Production by AdiNOS Transduced Vascular Cells through Supplementation with L-Arg and BH4: Implications for Gene Therapy of Restenosis. Atherosclerosis 2013, 230, 23–32. [Google Scholar] [CrossRef]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. The Key Role of GSH in Keeping the Redox Balance in Mammalian Cells: Mechanisms and Significance of GSH in Detoxification via Formation of Conjugates. Antioxidants 2023, 12, 1953. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Diotallevi, M.; Checconi, P.; Palamara, A.T.; Celestino, I.; Coppo, L.; Holmgren, A.; Abbas, K.; Peyrot, F.; Mengozzi, M.; Ghezzi, P. Glutathione Fine-Tunes the Innate Immune Response toward Antiviral Pathways in a Macrophage Cell Line Independently of Its Antioxidant Properties. Front. Immunol. 2017, 8, 1239. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and Sequential Cell Death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef]
- Cai, S.H.; Wu, Z.H.; Jian, J.C.; Lu, Y.S. Cloning and Expression of the Gene Encoding an Extracellular Alkaline Serine Protease from Vibrio alginolyticus Strain HY9901, the Causative Agent of Vibriosis in Lutjanus Erythopterus (Bloch). J. Fish Dis. 2007, 30, 493–500. [Google Scholar] [CrossRef]
- Pang, H.; Qiu, M.; Zhao, J.; Hoare, R.; Monaghan, S.J.; Song, D.; Chang, Y.; Jian, J. Construction of a Vibrio alginolyticus hopPmaJ (Hop) Mutant and Evaluation of Its Potential as a Live Attenuated Vaccine in Orange-Spotted Grouper (Epinephelus Coioides). Fish Shellfish. Immunol. 2018, 76, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yu, X.; Abdullahi, A.Y.; Hu, W.; Pan, W.; Shi, X.; Tan, L.; Song, M.; Li, G. Sequence Analysis and Prokaryotic Expression of Giardia Lamblia α-18 Giardin Gene. Infect. Genet. Evol. 2016, 38, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Pang, H.; Chen, Y.; Zheng, H.; Li, W.; Ramanathan, S.; Hoare, R.; Monaghan, S.J.; Lin, X.; Jian, J. First Succinylome Profiling of Vibrio alginolyticus Reveals Key Role of Lysine Succinylation in Cellular Metabolism and Virulence. Front. Cell. Infect. Microbiol. 2021, 10, 626574. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sang, Y.; Ni, J.; Tao, J.; Lu, J.; Zhao, M.; Yao, Y.-F. Acetylation Regulates Survival of Salmonella Enterica Serovar typhimurium under Acid Stress. Appl. Environ. Microbiol. 2015, 81, 5675–5682. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Huang, Y.; Jiang, B.; Li, X.; Huang, M.; Huang, Y.; Jian, J. Molecular Characterization of a Novel C-Type Lectin Receptors (CD302) in Nile Tilapia (Oreochromis Niloticus) and Its Functional Analysis in Host Defense against Bacterial Infection. Aquac. Rep. 2022, 27, 10140. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, W.; Xie, J.; Zhu, Y.; Pan, Y.; Ou, J.; Zhao, Y.; Liu, H. Comparison on the Growth Heterogeneity of Vibrio parahaemolyticus Coupled with Strain Source and Genotype Analyses in Different Oligotrophic Conditions. J. Food Prot. 2021, 84, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, S.; Sun, E.; Chen, Y.; Hua, L.; Wang, X.; Zhou, R.; Chen, H.; Peng, Z.; Wu, B. A Temperate Siphoviridae bacteriophage Isolate from Siberian Tiger Enhances the Virulence of Methicillin-Resistant Staphylococcus aureus through Distinct Mechanisms. Virulence 2022, 13, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Percent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, H.; Yang, S.; Huang, Y.; Cai, S.; Jian, J.; Cai, J.; Qin, Q. The Role of dctP Gene in Regulating Colonization, Adhesion and Pathogenicity of Vibrio alginolyticus Strain HY9901. J. Fish Dis. 2022, 45, 421–434. [Google Scholar] [CrossRef]
- Qin, Q.W.; Wu, T.H.; Jia, T.L.; Hegde, A.; Zhang, R.Q. Development and Characterization of a New Tropical Marine Fish Cell Line from Grouper, Epinephelus Coioides Susceptible to Iridovirus and Nodavirus. J. Virol. Methods 2006, 131, 58–64. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Y.; Sun, J.; Han, X.; Qin, Q. Characterization of Two Grouper epinephelus Akaara Cell Lines: Application to Studies of Singapore Grouper Iridovirus (SGIV) Propagation and Virus–Host Interaction. Aquaculture 2009, 292, 172–179. [Google Scholar] [CrossRef]

| Strain | QMEAN | Cβ | All Atom | Solvation | Torsion |
|---|---|---|---|---|---|
| ClpP | 1.14 | 1.03 | 1.03 | 1.88 | 0.32 |
| K165Q | 1.26 | 0.98 | 1.10 | 1.88 | 0.44 |
| K165R | 1.14 | 1.03 | 1.01 | 1.93 | 0.31 |
| WT | C-clpP | K165Q | K165R | |
|---|---|---|---|---|
| LD50 (cfu/mL) | 5.82 × 106 | 5.89 × 106 | 9.75 × 107 ** | 7.34 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Jiang, Y.; Zhang, W.; Wei, Y.; Xiao, X.; Wei, Z.; Wen, X.; Dong, Y.; Jian, J.; Wang, N.; et al. The Effect of the Lysine Acetylation Modification of ClpP on the Virulence of Vibrio alginolyticus. Molecules 2024, 29, 4278. https://doi.org/10.3390/molecules29174278
Wang S, Jiang Y, Zhang W, Wei Y, Xiao X, Wei Z, Wen X, Dong Y, Jian J, Wang N, et al. The Effect of the Lysine Acetylation Modification of ClpP on the Virulence of Vibrio alginolyticus. Molecules. 2024; 29(17):4278. https://doi.org/10.3390/molecules29174278
Chicago/Turabian StyleWang, Shi, Yingying Jiang, Weijie Zhang, Yingzhu Wei, Xing Xiao, Zhiqing Wei, Xiaoxin Wen, Yuhang Dong, Jichang Jian, Na Wang, and et al. 2024. "The Effect of the Lysine Acetylation Modification of ClpP on the Virulence of Vibrio alginolyticus" Molecules 29, no. 17: 4278. https://doi.org/10.3390/molecules29174278
APA StyleWang, S., Jiang, Y., Zhang, W., Wei, Y., Xiao, X., Wei, Z., Wen, X., Dong, Y., Jian, J., Wang, N., & Pang, H. (2024). The Effect of the Lysine Acetylation Modification of ClpP on the Virulence of Vibrio alginolyticus. Molecules, 29(17), 4278. https://doi.org/10.3390/molecules29174278






