Polyamine Derived Photosensitizer: A Novel Approach for Photodynamic Therapy of Cancer
Abstract
1. Introduction
2. Results
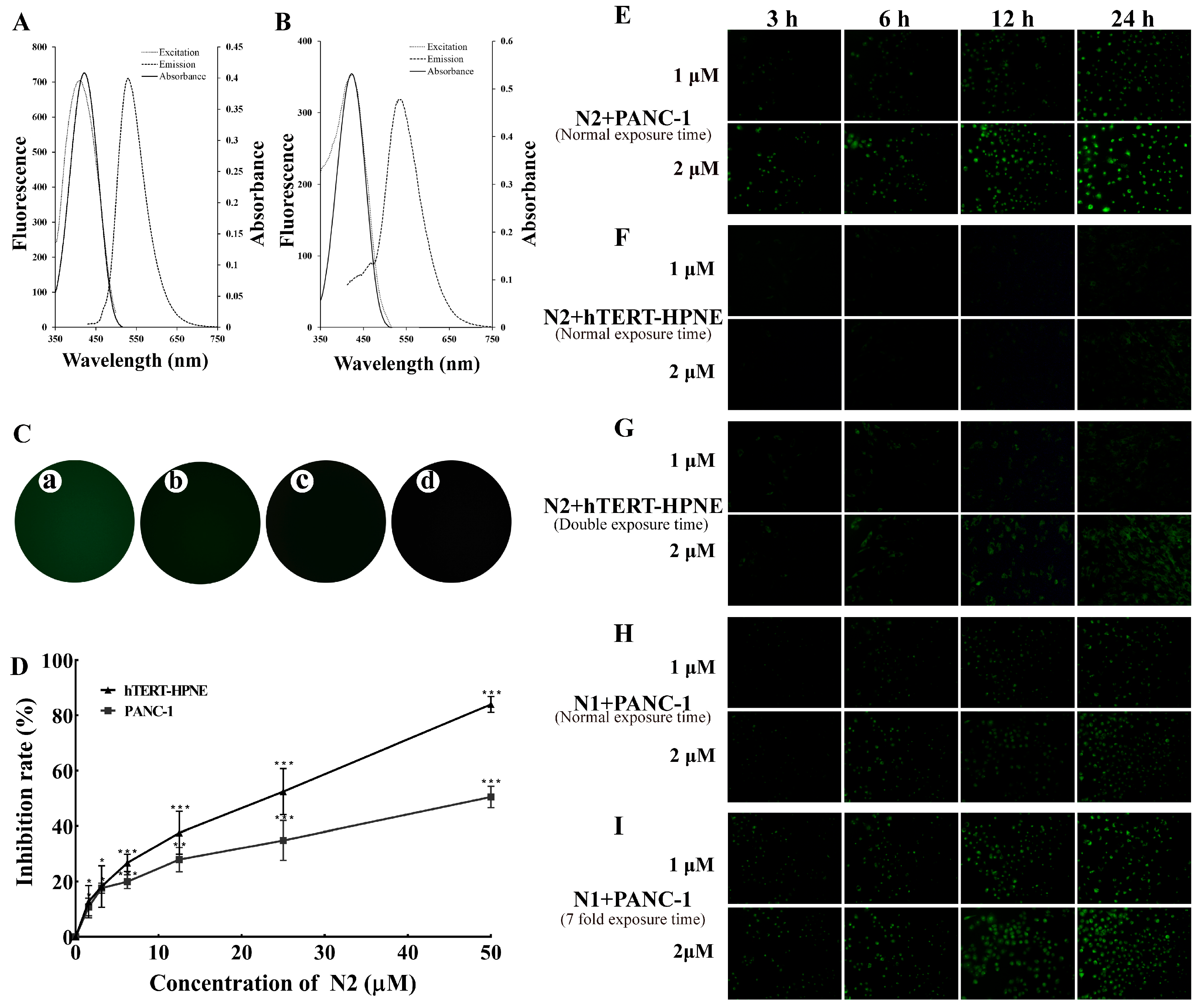
2.1. N2 Was Specifically Taken into PANC-1 Cells
2.2. N2 Was Taken into PANC-1 Cells via PTS
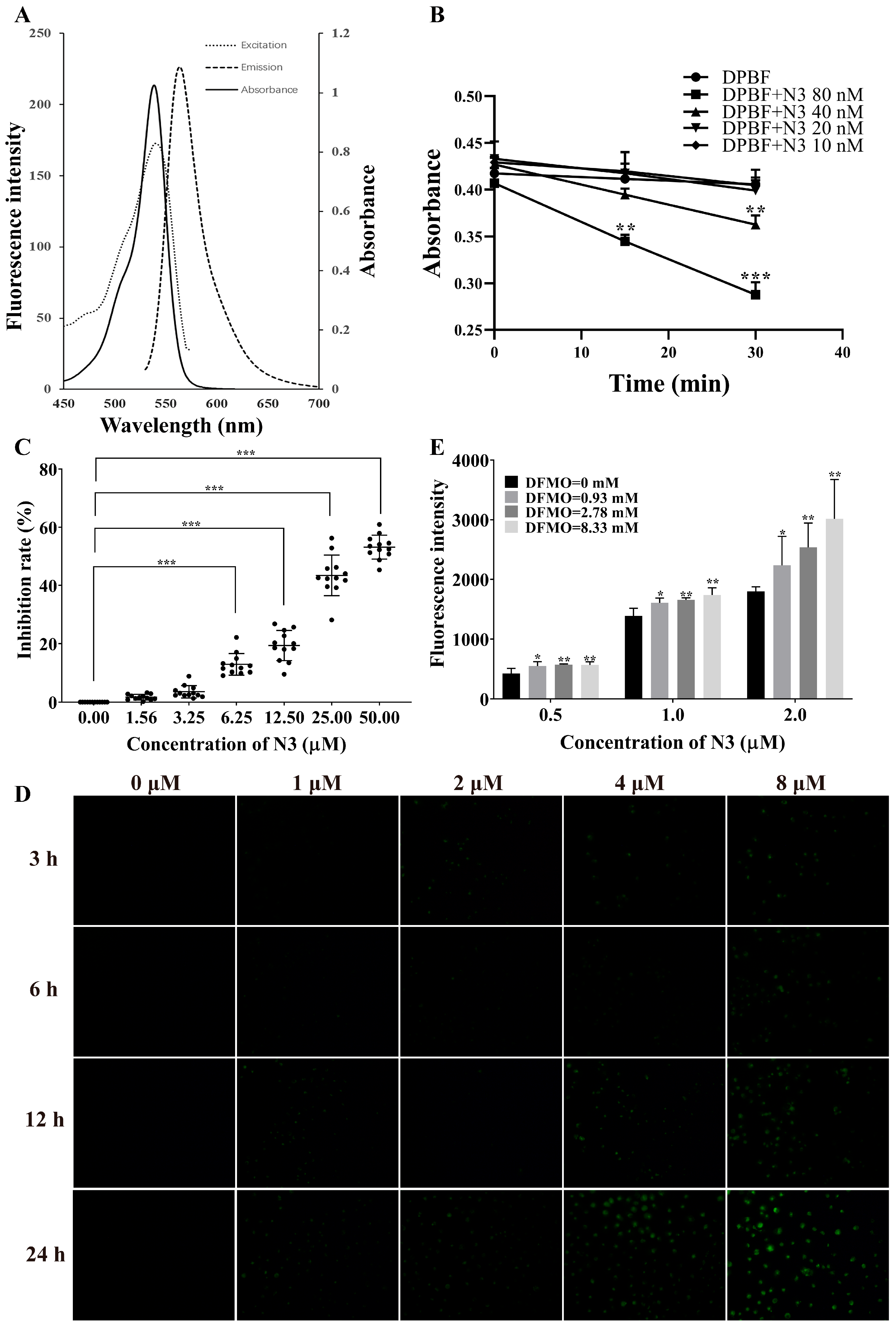
2.3. Low Concentration of DFMO Promoted Uptake of N2 into PANC-1 Cells
2.4. Evaluation of Production of Singlet Oxygen of N3 and the Uptake of N3 into PANC-1 Cells
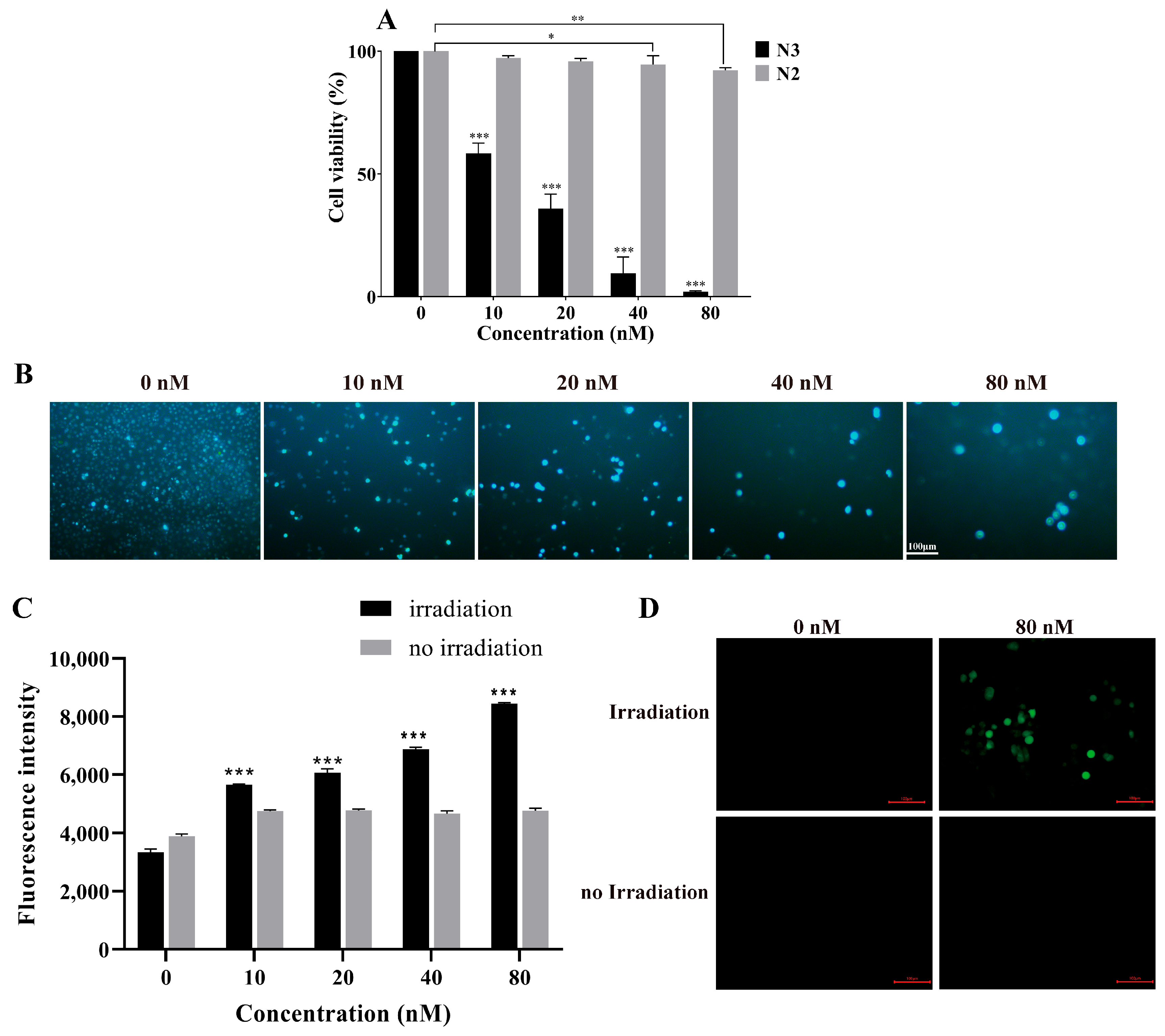
2.5. N3 Induced PDT in Pancreatic Cancer Cells
N3-PDT Inhibited the Tumor Growth on the CDX Mouse Model
3. Discussion
4. Materials and Methods
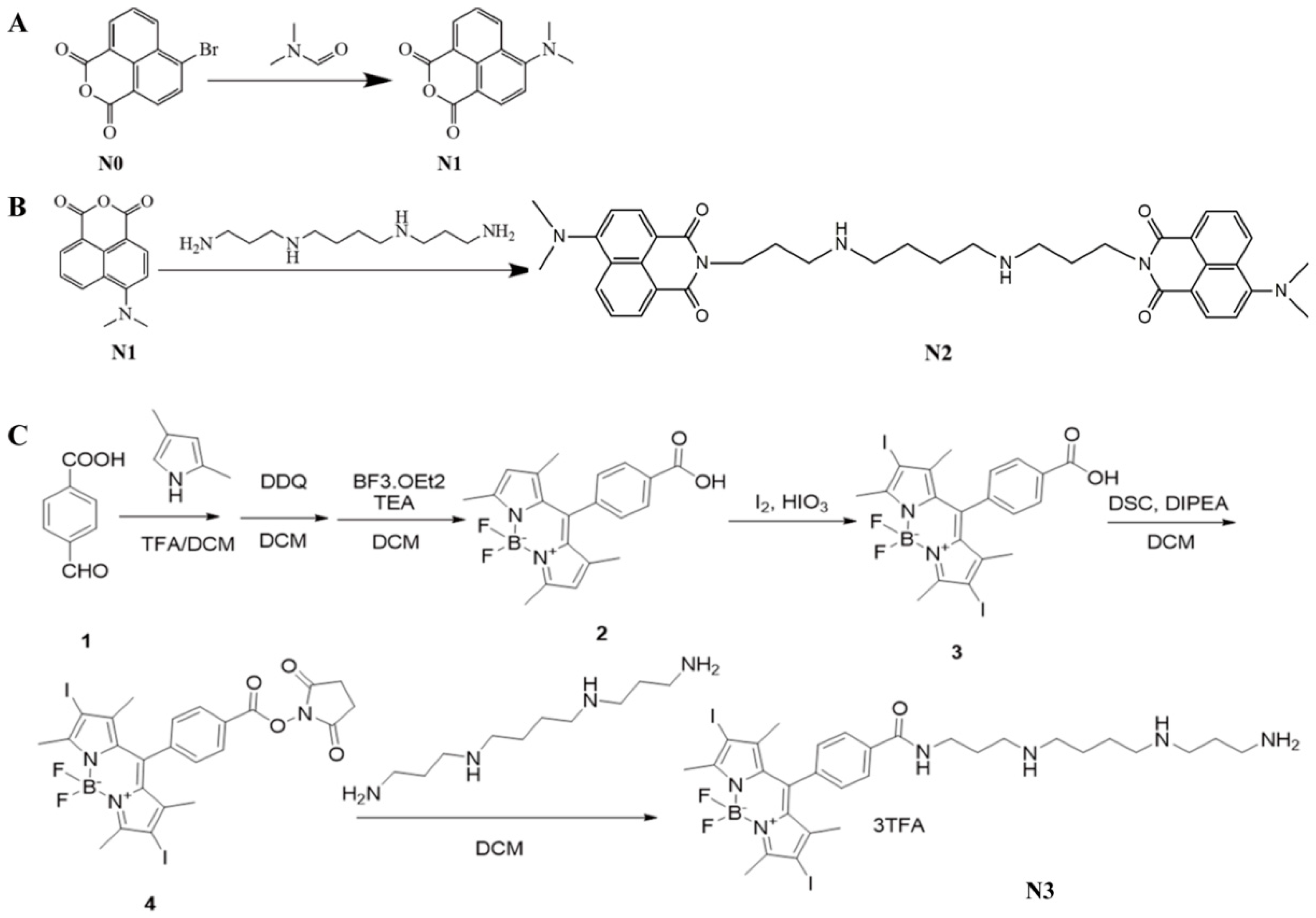
4.1. Synthesis of N1, N2, and N3
- ①
- Preparation of compound 2
- ②
- Preparation of compound 3
- ③
- Preparation of N3
4.1.1. Determination of Photophysical Properties of Compounds
4.1.2. Cell Cultures
4.1.3. Cytotoxicity Detected with Cell Counting Kit 8
4.1.4. The Uptake of N1, N2, and N3 into Cells Observed Using Fluorescence Microscope
4.1.5. The Uptake of N2 into Cells Evaluated Using Flow Cytometry
4.1.6. The Uptake of N2 and N3 into Cells Evaluated Using Spectrofluorometry
4.1.7. Competition Assay Evaluated Using Spectrofluorometry
4.1.8. Productivity of Singlet Oxygen Evaluated Using Spectrofluorometry
4.1.9. Phototoxicity in Cells
4.1.10. Evaluation of ROS in Cells
4.1.11. Animals
4.1.12. Acute and Chronic Toxicity of N3 In Vivo
4.1.13. Cell-Derived Xenograft Mouse Model of Pancreatic Cancer
4.1.14. The Dark Toxicity of N3 on CDX Mouse Model
4.1.15. The Phototoxicity of N3 on CDX Mouse Model
4.1.16. Molecular Structure Analysis of N2
4.1.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Liu, L.X. Use of photodynamic therapy in malignant lesions of stomach, bile duct, pancreas, colon and rectum. Hepatogastroenterology 2007, 54, 718–724. [Google Scholar]
- Gitto, S.B.; Pandey, V.; Oyer, J.L.; Copik, A.J.; Hogan, F.C.; Phanstiel, O.; Altomare, D.A. Difluoromethylornithine Combined with a Polyamine Transport Inhibitor Is Effective against Gemcitabine Resistant Pancreatic Cancer. Mol. Pharm. 2018, 15, 369–376. [Google Scholar] [CrossRef]
- Karimnia, V.; Slack, F.J.; Celli, J.P. Photodynamic Therapy for Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 4354. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.B.; Friedberg, J.S.; Glatstein, E.; Stevenson, J.P.; Sterman, D.H.; Hahn, S.M.; Cengel, K.A. Photodynamic therapy for the treatment of non-small cell lung cancer. J. Thorac. Dis. 2011, 4, 63–75. [Google Scholar] [CrossRef]
- Qumseya, B.J.; David, W.; Wolfsen, H.C. Photodynamic Therapy for Barrett’s Esophagus and Esophageal Carcinoma. Clin. Endosc. 2013, 46, 30. [Google Scholar] [CrossRef]
- Kobza-Mroczkowska, D.; Khot, M.I. The current situation of 5-aminolevulinic acid mediated photodynamic therapy in bladder cancer. Photodiagnosis Photodyn. Ther. 2020, 31, 101794. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. 5-Aminolevulinic acid-mediated photodynamic therapy for bladder cancer. Int. J. Urol. 2017, 24, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, 1900132. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef]
- Quilbe, A.; Moralès, O.; Baydoun, M.; Kumar, A.; Mustapha, R.; Murakami, T.; Leroux, B.; de Schutter, C.; Thecua, E.; Ziane, L.; et al. An Efficient Photodynamic Therapy Treatment for Human Pancreatic Adenocarcinoma. J. Clin. Med. 2020, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Douglas Holyoke, E. New surgical approaches to pancreatic cancer. Cancer 1981, 47, 1719–1723. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Huggett, M.T.; Jermyn, M.; Gillams, A.; Illing, R.; Mosse, S.; Novelli, M.; Kent, E.; Bown, S.G.; Hasan, T.; Pogue, B.W.; et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. Cancer 2014, 110, 1698–1704. [Google Scholar] [CrossRef]
- Hanada, Y.; Pereira, S.P.; Pogue, B.; Maytin, E.V.; Hasan, T.; Linn, B.; Mangels-Dick, T.; Wang, K.K. EUS-guided verteporfin photodynamic therapy for pancreatic cancer. Gastrointest. Endosc. 2021, 94, 179–186. [Google Scholar] [CrossRef]
- DeWitt, J.M.; Sandrasegaran, K.; O’Neil, B.; House, M.G.; Zyromski, N.J.; Sehdev, A.; Perkins, S.M.; Flynn, J.; McCranor, L.; Shahda, S. Phase 1 study of EUS-guided photodynamic therapy for locally advanced pancreatic cancer. Gastrointest. Endosc. 2019, 89, 390–398. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhou, L.; Lu, J.; Jiang, B.; Liu, C.; Guo, J. Photodynamic therapy of pancreatic cancer: Where have we come from and where are we going? Photodiagnosis Photodyn. Ther. 2020, 31, 101876. [Google Scholar] [CrossRef]
- Poulin, R.; Casero, R.A.; Soulet, D. Recent advances in the molecular biology of metazoan polyamine transport. Amino Acids 2011, 42, 711–723. [Google Scholar] [CrossRef]
- Houdou, M.; Jacobs, N.; Coene, J.; Azfar, M.; Vanhoutte, R.; Van den Haute, C.; Eggermont, J.; Daniëls, V.; Verhelst, S.H.L.; Vangheluwe, P. Novel Green Fluorescent Polyamines to Analyze ATP13A2 and ATP13A3 Activity in the Mammalian Polyamine Transport System. Biomolecules 2023, 13, 337. [Google Scholar] [CrossRef]
- Palmer, A.J.; Wallace, H.M. The polyamine transport system as a target for anticancer drug development. Amino Acids 2009, 38, 415–422. [Google Scholar] [CrossRef]
- Holbert, C.E.; Cullen, M.T.; Casero, R.A.; Stewart, T.M. Polyamines in cancer: Integrating organismal metabolism and antitumour immunity. Nat. Rev. Cancer 2022, 22, 467–480. [Google Scholar] [CrossRef]
- Casero, R.A.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Delcros, J.-G.; Tomasi, S.; Duhieu, S.; Foucault, M.; Martin, B.; Le Roch, M.; Eifler-Lima, V.; Renault, J.; Uriac, P. Effect of Polyamine Homologation on the Transport and Biological Properties of Heterocyclic Amidines. J. Med. Chem. 2005, 49, 232–245. [Google Scholar] [CrossRef]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, H.; Chen, Z.; Dong, X.; Zhao, W.; Shi, Y.; Zhu, Q. Discovery of an Amino Acid-Modified Near-Infrared Aza-BODIPY Photosensitizer as an Immune Initiator for Potent Photodynamic Therapy in Melanoma. J. Med. Chem. 2022, 65, 3616–3631. [Google Scholar] [CrossRef]
- Yin, J.; Jiang, X.; Sui, G.; Du, Y.; Xing, E.; Shi, R.; Gu, C.; Wen, X.; Feng, Y.; Shan, Z.; et al. The tumor phototherapeutic application of nanoparticles constructed by the relationship between PTT/PDT efficiency and 2,6- and 3,5-substituted BODIPY derivatives. J. Mater. Chem. B 2021, 9, 7461–7471. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Sola-Llano, R.; Agarrabeitia, A.R.; García-Fresnadillo, D.; López-Arbeloa, I.; Villanueva, A.; Ortiz, M.J.; Moya, S.; Martínez-Martínez, V. Exploring BODIPY Derivatives as Singlet Oxygen Photosensitizers for PDT. Photochem. Photobiol. 2020, 96, 458–477. [Google Scholar] [CrossRef]
- Yogo, T.; Urano, Y.; Ishitsuka, Y.; Maniwa, F.; Nagano, T. Highly Efficient and Photostable Photosensitizer Based on BODIPY Chromophore. J. Am. Chem. Soc. 2005, 127, 12162–12163. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, N.N.; Van den Haute, C.; Vanhoutte, R.; Sannerud, R.; Azfar, M.; Mayer, R.; Cortés Calabuig, Á.; Swinnen, J.V.; Agostinis, P.; Baekelandt, V.; et al. ATP13A3 is a major component of the enigmatic mammalian polyamine transport system. J. Biol. Chem. 2021, 296, 100182. [Google Scholar] [CrossRef]
- van Veen, S.; Martin, S.; Van den Haute, C.; Benoy, V.; Lyons, J.; Vanhoutte, R.; Kahler, J.P.; Decuypere, J.-P.; Gelders, G.; Lambie, E.; et al. ATP13A2 deficiency disrupts lysosomal polyamine export. Nature 2020, 578, 419–424. [Google Scholar] [CrossRef]
- Vanhoutte, R.; Kahler, J.P.; Martin, S.; van Veen, S.; Verhelst, S.H.L. Clickable Polyamine Derivatives as Chemical Probes for the Polyamine Transport System. Chem. Eur. J. Chem. Bio. 2018, 19, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Minchin, R.F.; Martin, R.L.; Summers, L.A.; Ilett, K.F. Inhibition of putrescine uptake by polypyridinium quaternary salts in B16 melanoma cells treated with difluoromethylornithine. Biochem. J. 1989, 262, 391–395. [Google Scholar] [CrossRef]
- Barret, J.-M.; Kruczynski, A.; Vispé, S.; Annereau, J.-P.; Brel, V.; Guminski, Y.; Delcros, J.-G.; Lansiaux, A.; Guilbaud, N.; Imbert, T.; et al. F14512, a Potent Antitumor Agent Targeting Topoisomerase II Vectored into Cancer Cells via the Polyamine Transport System. Cancer Res. 2008, 68, 9845–9853. [Google Scholar] [CrossRef] [PubMed]
- Jeter, J.M.; Alberts, D.S. Difluoromethylornithine: The Proof Is in the Polyamines. Cancer Prev. Res. 2012, 5, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Gamble, L.D.; Upton, D.H.; Ung, C.; Yu, D.M.T.; Ehteda, A.; Pandher, R.; Mayoh, C.; Hébert, S.; Jabado, N.; et al. Dual targeting of polyamine synthesis and uptake in diffuse intrinsic pontine gliomas. Nat. Commun. 2021, 12, 971. [Google Scholar] [CrossRef]
- Meyskens, F.L.; Gerner, E.W. Development of Difluoromethylornithine (DFMO) as a Chemoprevention Agent. Clin. Cancer Res. 1999, 5, 945–951. [Google Scholar]
- Vlastos, A.; West, L.; Atkinson, E.; Boiko, I.; Malpica, A.; Hong, W.; Follen, M. Results of a phase II double-blinded randomized clinical trial of difluoromethylornithine for cervical intraepithelial neoplasia grades 2 to 3. Clin. Cancer Res. 2005, 11, 390–396. [Google Scholar] [CrossRef]
- Meyskens, F.; Kingsley, E.; Glattke, T.; Loescher, L.; Booth, A. A phase II study of alpha-difluoromethylornithine (DFMO) for the treatment of metastatic melanoma. Investig. New Drugs 1986, 4, 257–262. [Google Scholar] [CrossRef]
- Kreul, S.M.; Havighurst, T.; Kim, K.; Mendonça, E.A.; Wood, G.S.; Snow, S.; Borich, A.; Verma, A.; Bailey, H.H. A Phase III Skin Cancer Chemoprevention Study of DFMO: Long-term Follow-up of Skin Cancer Events and Toxicity. Cancer Prev. Res. 2012, 5, 1368–1374. [Google Scholar] [CrossRef]
- Ozfiliz, P.; Kizilboga, T.; Demir, S.; Alkurt, G.; Palavan-Unsal, N.; Arisan, E.D.; Dinler-Doganay, G. Bag-1 promotes cell survival through c-Myc-mediated ODC upregulation that is not preferred under apoptotic stimuli in MCF-7 cells. Cell Biochem. Funct. 2015, 33, 293–307. [Google Scholar] [CrossRef]
- Massaro, C.; Thomas, J.; Phanstiel, O. Investigation of Polyamine Metabolism and Homeostasis in Pancreatic Cancers. Med. Sci. 2017, 5, 32. [Google Scholar] [CrossRef]
- Hayes, C.S.; Shicora, A.C.; Keough, M.P.; Snook, A.E.; Burns, M.R.; Gilmour, S.K. Polyamine-Blocking Therapy Reverses Immunosuppression in the Tumor Microenvironment. Cancer Immunol. Res. 2014, 2, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi-Tago, M.; Sumi, K.; Kasahara, T.; Tago, K. Critical Roles of Myc-ODC Axis in the Cellular Transformation Induced by Myeloproliferative Neoplasm-Associated JAK2 V617F Mutant. PLoS ONE 2013, 8, e52844. [Google Scholar] [CrossRef]
- Samal, K.; Zhao, P.; Kendzicky, A.; Yco, L.P.; McClung, H.; Gerner, E.; Burns, M.; Bachmann, A.S.; Sholler, G. AMXT-1501, a novel polyamine transport inhibitor, synergizes with DFMO in inhibiting neuroblastoma cell proliferation by targeting both ornithine decarboxylase and polyamine transport. Int. J. Cancer 2013, 133, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Koomoa, D.-L.T.; Geerts, D.; Lange, I.; Koster, J.; Pegg, A.E.; Feith, D.J.; Bachmann, A.S. DFMO/eflornithine inhibits migration and invasion downstream of MYCN and involves p27Kip1 activity in neuroblastoma. Int. J. Oncol. 2013, 42, 1219–1228. [Google Scholar] [CrossRef]
- Hogarty, M.D.; Norris, M.D.; Davis, K.; Liu, X.; Evageliou, N.F.; Hayes, C.S.; Pawel, B.; Guo, R.; Zhao, H.; Sekyere, E.; et al. ODC1 Is a Critical Determinant of MYCN Oncogenesis and a Therapeutic Target in Neuroblastoma. Cancer Res. 2008, 68, 9735–9745. [Google Scholar] [CrossRef]
- van Veen, S.; Kourti, A.; Ausloos, E.; Van Asselberghs, J.; Van den Haute, C.; Baekelandt, V.; Eggermont, J.; Vangheluwe, P. ATP13A4 Upregulation Drives the Elevated Polyamine Transport System in the Breast Cancer Cell Line MCF7. Biomolecules 2023, 13, 918. [Google Scholar] [CrossRef] [PubMed]
- Rangaraju, L.P.; Kunapuli, G.; Every, D.; Ayala, O.D.; Ganapathy, P.; Mahadevan-Jansen, A. Classification of burn injury using Raman spectroscopy and optical coherence tomography: An ex-vivo study on porcine skin. Burns 2019, 45, 659–670. [Google Scholar] [CrossRef]
- Osborne, C.L.; Petersson, C.; Graham, J.E.; Meyer, W.J.; Simeonsson, R.J.; Suman, O.E.; Ottenbacher, K.J. The multicenter benchmarking study of burn injury: A content analysis of the outcome measures using the international classification of functioning, disability and health. Burns 2016, 42, 1396–1403. [Google Scholar] [CrossRef]
- Klein, M.B.; Gibran, N.S.; Emerson, D.; Sullivan, S.R.; Honari, S.; Engrav, L.H.; Heimbach, D.M. Patterns of grease burn injury: Development of a classification system. Burns 2005, 31, 765–767. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. Management of Burns. N. Engl. J. Med. 2019, 380, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Cram, R.H. The Management Of Burns. Lancet 1951, 257, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.F.; Deitch, E.A. The Management of Burns. N. Engl. J. Med. 1990, 323, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Matiatou, M.; Giannios, P.; Koutsoumpos, S.; Toutouzas, K.G.; Zografos, G.C.; Moutzouris, K. Data on the refractive index of freshly-excised human tissues in the visible and near-infrared spectral range. Result Phys. 2021, 22, 103833. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Friebel, M.; Helfmann, J.; Netz, U.; Meinke, M. Influence of oxygen saturation on the optical scattering properties of human red blood cells in the spectral range 250 to 2000 nm. J. Biomed. Opt. 2009, 14, 034001. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, H.; Meyers, A.D.; Musani, A.I.; Wang, L.; Tagg, R.; Barqawi, A.B.; Chen, Y.K. Photodynamic Therapy for Treatment of Solid Tumors—Potential and Technical Challenges. Technol. Cancer Res. Treat. 2008, 7, 309–320. [Google Scholar] [CrossRef]
- Hafiz, S.S.; Xavierselvan, M.; Gokalp, S.; Labadini, D.; Barros, S.; Duong, J.; Foster, M.; Mallidi, S. Eutectic Gallium–Indium Nanoparticles for Photodynamic Therapy of Pancreatic Cancer. ACS Appl. Nano Mater. 2022, 5, 6125–6139. [Google Scholar] [CrossRef]
- Deng, K.; Li, C.; Huang, S.; Xing, B.; Jin, D.; Zeng, Q.; Hou, Z.; Lin, J. Recent Progress in Near Infrared Light Triggered Photodynamic Therapy. Small 2017, 13, 1702299. [Google Scholar] [CrossRef] [PubMed]


| Group | Drug | Tumor | N3-PDT 1st (%) | N3-PDT 2nd (%) | N3-PDT 3rd (%) | |||
|---|---|---|---|---|---|---|---|---|
| III | II | III | II | III | II | |||
| N3 | N3 | + | 70 | 30 | 20 | 80 | 11.1 | 88.9 |
| N3 con | N3 | − | 100 | 0 | 100 | 0 | 33.3 | 66.7 |
| DMSO | DMSO | + | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Xie, K.; Hu, L.; Liu, X.; Li, Q.; Xie, D.; Xiang, F.; Liu, W.; Zheng, W.; Xiao, S.; et al. Polyamine Derived Photosensitizer: A Novel Approach for Photodynamic Therapy of Cancer. Molecules 2024, 29, 4277. https://doi.org/10.3390/molecules29174277
Deng H, Xie K, Hu L, Liu X, Li Q, Xie D, Xiang F, Liu W, Zheng W, Xiao S, et al. Polyamine Derived Photosensitizer: A Novel Approach for Photodynamic Therapy of Cancer. Molecules. 2024; 29(17):4277. https://doi.org/10.3390/molecules29174277
Chicago/Turabian StyleDeng, Hao, Ke Xie, Liling Hu, Xiaowen Liu, Qingyun Li, Donghui Xie, Fengyi Xiang, Wei Liu, Weihong Zheng, Shuzhang Xiao, and et al. 2024. "Polyamine Derived Photosensitizer: A Novel Approach for Photodynamic Therapy of Cancer" Molecules 29, no. 17: 4277. https://doi.org/10.3390/molecules29174277
APA StyleDeng, H., Xie, K., Hu, L., Liu, X., Li, Q., Xie, D., Xiang, F., Liu, W., Zheng, W., Xiao, S., Zheng, J., & Tan, X. (2024). Polyamine Derived Photosensitizer: A Novel Approach for Photodynamic Therapy of Cancer. Molecules, 29(17), 4277. https://doi.org/10.3390/molecules29174277






