Utilizing Morphological and Physiological Parameters of Lemna minor for Assessing Tetracyclines’ Removal
Abstract
1. Introduction
2. Results
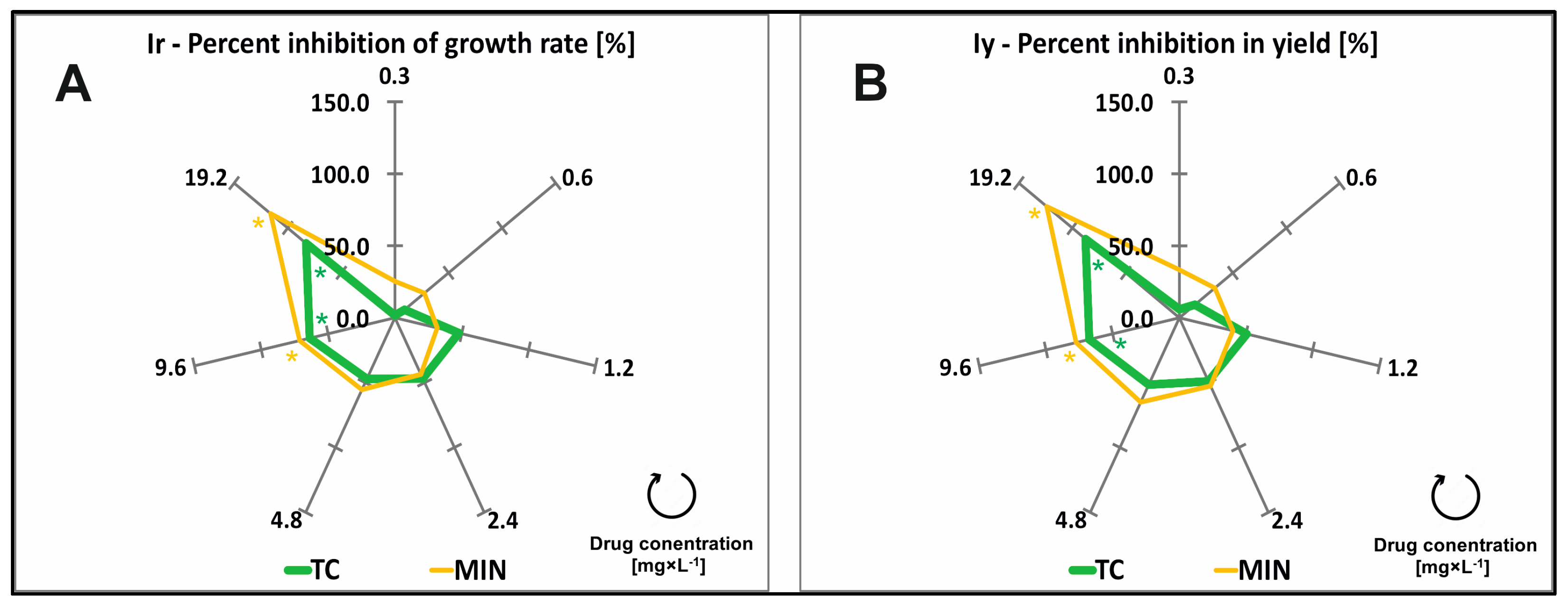
2.1. Effect of TCs on the Ir and Iy of Common Duckweed
2.2. Fresh Mass and Dry Matter Content
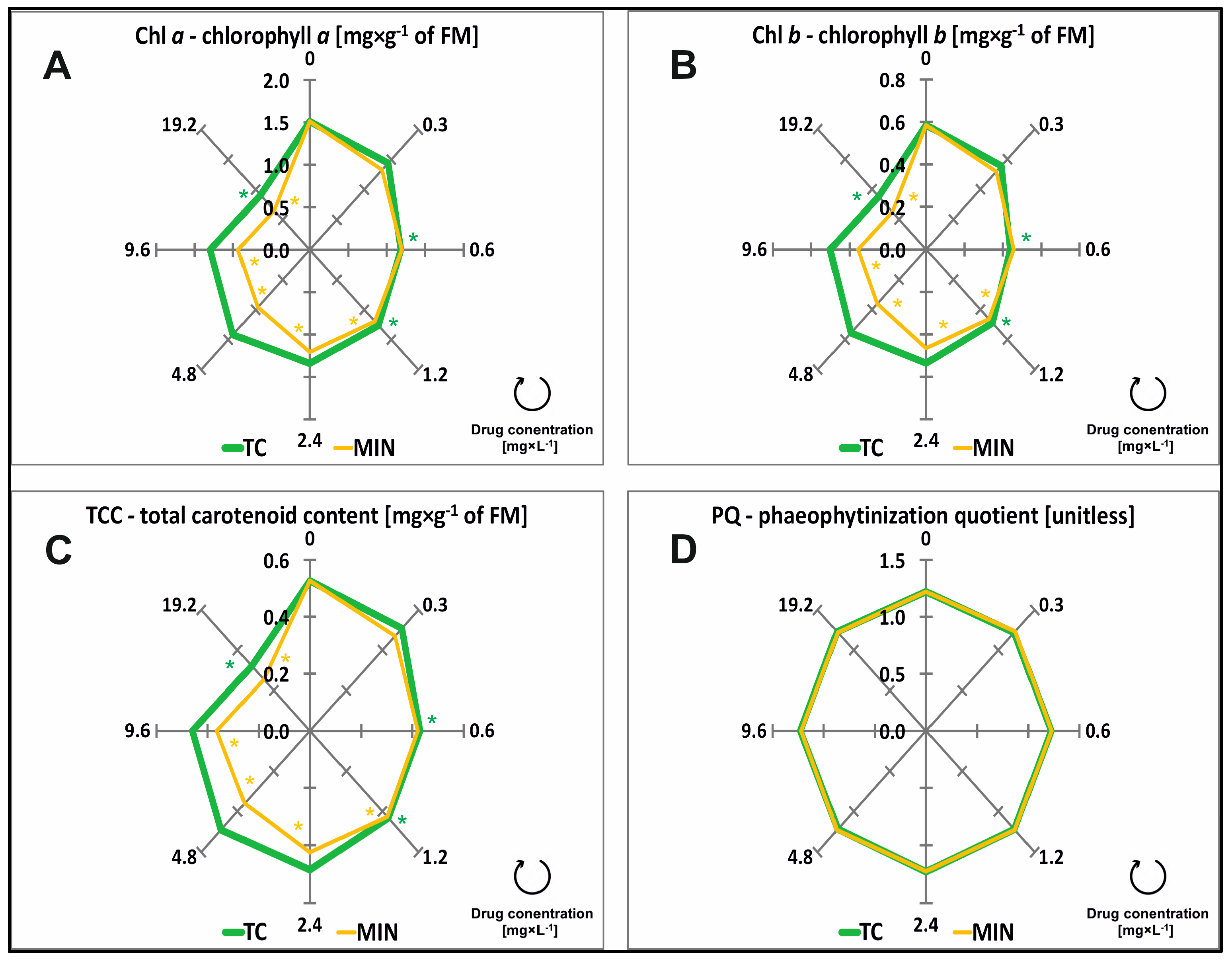
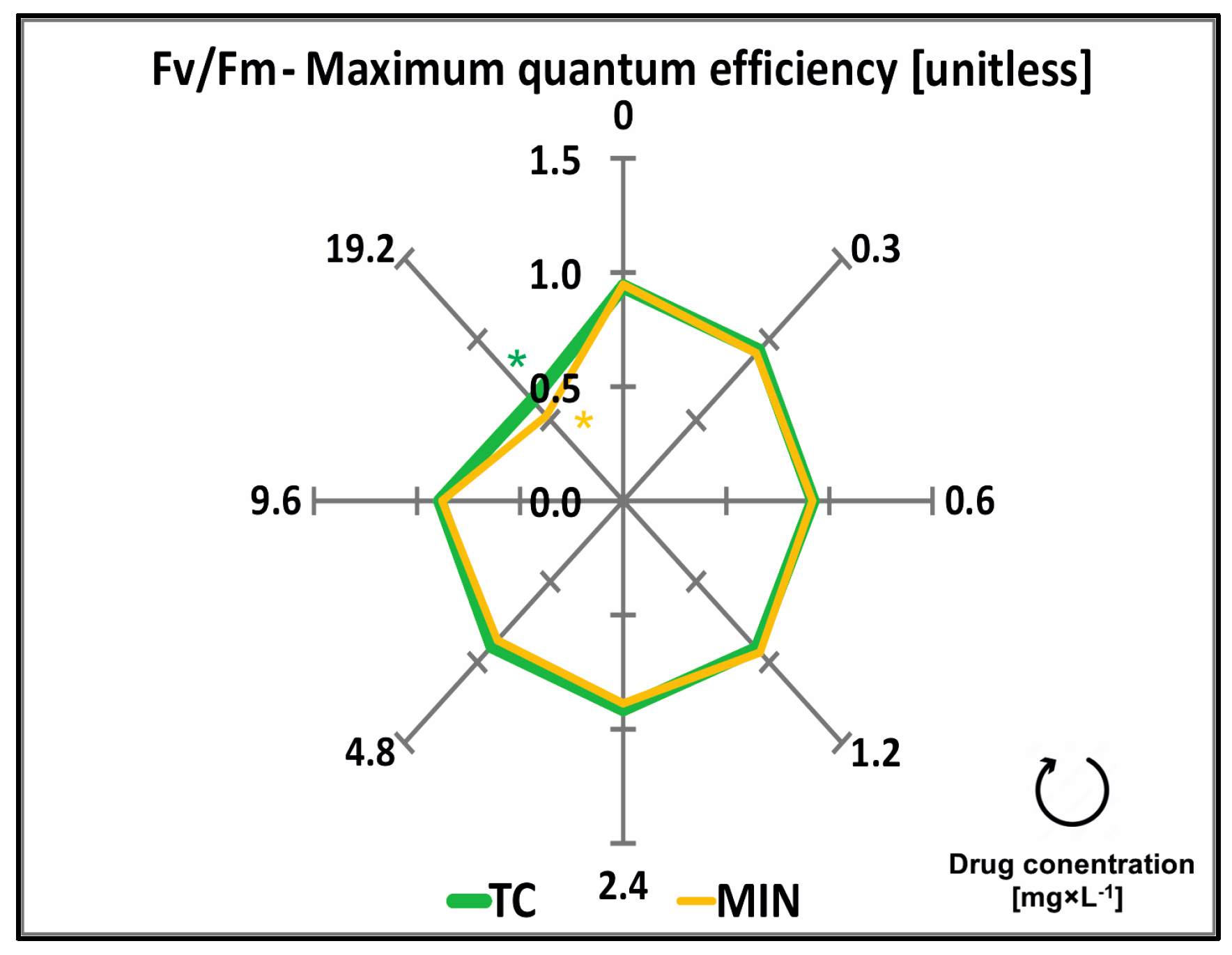
2.3. Chlorophylls, Carotenoids, Phaeophytinization, and Fluorescence
2.4. Antibiotic Biosorption—Results
3. Discussion
4. Materials and Methods
4.1. Plant Biosorbent
4.2. Chemical Adsorbates
4.3. Lemna Test
4.4. Chlorophyll, Total Carotenoid Content, and Phaeophytinization Quotient
4.5. Chlorophyll Fluorescence
4.6. Antibiotic Biosorption
4.6.1. Spectrophotometric Measurements
4.6.2. Biosorption Measurements
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TCs | tetracyclines |
| TC | tetracycline |
| MIN | minocycline |
| FM | fresh mass |
| DM | dry matter content |
| DW | dry weight |
| Ir | percent inhibition of growth rate |
| Iy | percent reduction in yield |
| Chl a | chlorophyll a |
| Chl b | chlorophyll b |
| TCC | total carotenoid content |
| PQ | phaeophytinization quotient |
| Fv/Fm | maximum quantum efficiency |
| ECx | effective concentration associated with x% response (20% and 50%) |
| Bs | antibiotic biosorption |
Appendix A
| Feature | Tetracycline Hydrochloride, mg × L−1 | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.30 | 0.60 | 1.20 | 2.40 | 4.80 | 9.66 | 19.20 | |
| ±SD for Percent inhibition of growth rate (Ir) | - | 7.07 | 14.35 | 20.26 | 17.68 | 20.52 | 11.68 | 15.18 |
| ±SD for Percent inhibition in yield (Iy) | - | 12.42 | 14.81 | 20.32 | 11.56 | 18.81 | 20.66 | 26.86 |
| ±SD for Fresh mass (FM) | 4.74 | 18.52 | 1.20 | 11.32 | 4.41 | 6.15 | 6.71 | 8.72 |
| ±SD for Dry matter content (DM) | 1.48 | 1.76 | 1.75 | 1.85 | 2.27 | 2.12 | 1.40 | 1.82 |
| ±SD for Chlorophyll a content (Chl a) | 0.03 | 0.06 | 0.06 | 0.02 | 0.00 | 0.01 | 0.02 | 0.02 |
| ±SD for Chlorophyll b content (Chl b) | 0.02 | 0.03 | 0.02 | 0.01 | 0.04 | 0.01 | 0.01 | 0.01 |
| ±SD for Total carotenoid content (TCC) | 0.01 | 0.02 | 0.02 | 0.01 | 0.03 | 0.01 | 0.01 | 0.01 |
| ±SD for Phaeophytinization quotient (PQ) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| ±SD for Maximum quantum efficiency (Fv/Fm) | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.04 |
| ±SD for Antibiotic biosorption (Bs) | - | 0.05 | 0.03 | 0.04 | 0.10 | 0.19 | 0.66 | 0.85 |
| Feature | Minocycline hydrochloride, mg × L−1 | |||||||
| 0.00 | 0.30 | 0.60 | 1.20 | 2.40 | 4.80 | 9.66 | 19.20 | |
| ±SD for Percent inhibition of growth rate (Ir) | - | 12.63 | 8.45 | 17.77 | 6.91 | 11.42 | 10.31 | 20.62 |
| ±SD for Percent inhibition in yield (Iy) | - | 12.06 | 9.14 | 16.12 | 11.56 | 21.43 | 20.66 | 41.32 |
| ±SD for Fresh mass (FM) | 4.74 | 19.78 | 4.41 | 16.00 | 4.74 | 30.30 | 6.86 | 8.91 |
| ±SD for Dry matter content (DM) | 6.35 | 7.20 | 7.23 | 7.36 | 7.55 | 7.76 | 7.65 | 9.94 |
| ±SD for Chlorophyll a content (Chl a) | 0.13 | 0.17 | 0.06 | 0.06 | 0.05 | 0.04 | 0.03 | 0.04 |
| ±SD for Chlorophyll b content (Chl b) | 0.05 | 0.07 | 0.02 | 0.03 | 0.02 | 0.01 | 0.01 | 0.01 |
| ±SD for Total carotenoid content (TCC) | 0.04 | 0.06 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 |
| ±SD for Phaeophytinization quotient (PQ) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| ±SD for Maximum quantum efficiency (Fv/Fm) | 0.01 | 0.04 | 0.02 | 0.02 | 0.08 | 0.06 | 0.03 | 0.04 |
| ±SD for Antibiotic biosorption (Bs) | - | 0.00 | 0.06 | 0.25 | 1.51 | 0.76 | 0.98 | 0.00 |
References
- Jamee, R.; Siddique, R. Biodegradation of Synthetic Dyes of Textile Effluent by Microorganisms: An Environmentally and Economically Sustainable Approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy Metal Water Pollution: A Fresh Look about Hazards, Novel and Conventional Remediation Methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment; Springer: Berlin/Heidelberg, Germany, 2012; pp. 133–164. [Google Scholar]
- Siddiqui, E.; Pandey, J. Assessment of Heavy Metal Pollution in Water and Surface Sediment and Evaluation of Ecological Risks Associated with Sediment Contamination in the Ganga River: A Basin-Scale Study. Environ. Sci. Pollut. Res. 2019, 26, 10926–10940. [Google Scholar] [CrossRef]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of Pesticides on Environment. In Plant, Soil and Microbes; Springer International Publishing: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar]
- Adomas, B.; Sikorski; Bęś, A.; Warmiński, K. Exposure of Lemna minor L. to Gentian Violet or Congo Red Is Associated with Changes in the Biosynthesis Pathway of Biogenic Amines. Chemosphere 2020, 254, 126752. [Google Scholar] [CrossRef]
- Baciak, M.; Sikorski, Ł.; Piotrowicz-Cieślak, A.I.; Adomas, B. Content of Biogenic Amines in Lemna minor (Common Duckweed) Growing in Medium Contaminated with Tetracycline. Aquat. Toxicol. 2016, 180, 95–102. [Google Scholar] [CrossRef]
- Alduina, R. Antibiotics and Environment. Antibiotics 2020, 9, 202. [Google Scholar] [CrossRef]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public. Health 2021, 18, 4909. [Google Scholar] [CrossRef]
- Danner, M.-C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic Pollution in Surface Fresh Waters: Occurrence and Effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Jjemba, P.K. The Potential Impact of Veterinary and Human Therapeutic Agents in Manure and Biosolids on Plants Grown on Arable Land: A Review. Agric. Ecosyst. Environ. 2002, 93, 267–278. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Sengeløv, G.; Tjørnelund, J. Toxicity of Tetracyclines and Tetracycline Degradation Products to Environmentally Relevant Bacteria, Including Selected Tetracycline-Resistant Bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Fritz, J.; Zuo, Y. Simultaneous Determination of Tetracycline, Oxytetracycline, and 4-Epitetracycline in Milk by High-Performance Liquid Chromatography. Food Chem. 2007, 105, 1297–1301. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant Activity of Taxifolin: An Activity–Structure Relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an Antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- DrugBank. DB00759, Tetracycline. Available online: https://go.drugbank.com/drugs/DB00759 (accessed on 30 April 2024).
- Thiele-Bruhn, S. Pharmaceutical Antibiotic Compounds in Soils—A Review. J. Plant Nutr. Soil. Sci. 2003, 166, 145–167. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary Antibiotics in the Aquatic and Terrestrial Environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Addamo, M.; Augugliaro, V.; Di Paola, A.; García-López, E.; Loddo, V.; Marcì, G.; Palmisano, L. Removal of Drugs in Aqueous Systems by Photoassisted Degradation. J. Appl. Electrochem. 2005, 35, 765–774. [Google Scholar] [CrossRef]
- Martínez-Carballo, E.; González-Barreiro, C.; Scharf, S.; Gans, O. Environmental Monitoring Study of Selected Veterinary Antibiotics in Animal Manure and Soils in Austria. Environ. Pollut. 2007, 148, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Vallé, Q.; Roques, B.B.; Bousquet-Mélou, A.; Dahlhaus, D.; Ramon-Portugal, F.; Dupouy, V.; Bibbal, D.; Ferran, A.A. Prediction of Minocycline Activity in the Gut From a Pig Preclinical Model Using a Pharmacokinetic -Pharmacodynamic Approach. Front. Microbiol. 2021, 12, 671376. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chang, P.-H.; Jean, J.-S.; Jiang, W.-T.; Wang, C.-J. Interaction between Tetracycline and Smectite in Aqueous Solution. J. Colloid. Interface Sci. 2010, 341, 311–319. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, S.; Li, J. Fast and Highly Efficient Tetracyclines Removal from Environmental Waters by Graphene Oxide Functionalized Magnetic Particles. Chem. Eng. J. 2013, 225, 679–685. [Google Scholar] [CrossRef]
- Sikorski, Ł.; Bęś, A.; Warmiński, K. The Effect of Quinolones on Common Duckweed Lemna minor L., a Hydrophyte Bioindicator of Environmental Pollution. Int. J. Environ. Res. Public. Health 2023, 20, 5089. [Google Scholar] [CrossRef]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.; Jiang, J.; Shen, Y.-M.; Pang, S.-Y.; Wang, Z.; Duan, J.; Guo, Q.; Guan, C.; Ma, J. Transformation of Tetracycline Antibiotics during Water Treatment with Unactivated Peroxymonosulfate. Chem. Eng. J. 2020, 379, 122378. [Google Scholar] [CrossRef]
- Kümmerer, K.; Henninger, A. Promoting Resistance by the Emission of Antibiotics from Hospitals and Households into Effluent. Clin. Microbiol. Infect. 2003, 9, 1203–1214. [Google Scholar] [CrossRef]
- Sellier, A.; Khaska, S.; Le Gal La Salle, C. Assessment of the Occurrence of 455 Pharmaceutical Compounds in Sludge According to Their Physical and Chemical Properties: A Review. J. Hazard. Mater. 2022, 426, 128104. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive Removal of Antibiotics from Water and Wastewater: Progress and Challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Huang, S.-T.; Chen, X.; Zou, M.-Y.; Liu, H.; Guo, P.-R.; Kong, L.-J.; Chu, W. Peroxymonosulfate-Assisted Photocatalytic Degradation of Antibiotic Norfloxacin by a Calcium-Based Ag3PO4 Composite in Water: Reactivity, Products and Mechanism. J. Clean. Prod. 2022, 330, 129806. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Jin, J.-C.; Zou, M.-Y.; Liu, H.; Qin, J.-Q.; Zhou, X.-H.; Qian, W.; Guo, P.-R.; Kong, L.-J.; Chu, W. Simultaneous Degradation of Amoxicillin and Norfloxacin by TiO2@nZVI Composites Coupling with Persulfate: Synergistic Effect, Products and Mechanism. Sep. Purif. Technol. 2021, 278, 119620. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Sathishkumar, K.; AlSalhi, M.S.; Sanganyado, E.; Devanesan, S.; Arulprakash, A.; Rajasekar, A. Sequential Electrochemical Oxidation and Bio-Treatment of the Azo Dye Congo Red and Textile Effluent. J. Photochem. Photobiol. B 2019, 200, 111655. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.; Hauxwell, J.; Haber, E.A. Distribution and Abundance of Aquatic Plants—Human Impacts. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Liu, L.; Liu, Y.; Liu, C.; Wang, Z.; Dong, J.; Zhu, G.; Huang, X. Potential Effect and Accumulation of Veterinary Antibiotics in Phragmites Australis under Hydroponic Conditions. Ecol. Eng. 2013, 53, 138–143. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current Perspectives on Concept, Definition and Application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- ISO 20079: 2005; Water Quality—Determination of Toxic. Effect of Water Constituents and Wastewater on Duckweed (Lemma Minor)—Duckweed Growth Inhibition Test. The International Organization for Standardization: Geneva, Switzerland, 2005.
- Test No. 221: Lemna sp. Growth Inhibition Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2006; ISBN 1-280-60670-3.
- Nunes, B.; Veiga, V.; Frankenbach, S.; Serôdio, J.; Pinto, G. Evaluation of Physiological Changes Induced by the Fluoroquinolone Antibiotic Ciprofloxacin in the Freshwater Macrophyte Species Lemna minor and Lemna gibba. Environ. Toxicol. Pharmacol. 2019, 72, 103242. [Google Scholar] [CrossRef]
- OECD 221. OECD Guidelines for the Testing of Chemicals Revised Proposal for A New Guideline 221; OECD Publishing: Paris, France, 2002. [Google Scholar]
- Akhter, S.; Bhat, M.A.; Hashem, A.; Abd_Allah, E.F.; Ahmed, S.; Siddiqi, W.A.; Kulsoom, I.; Un Nisa, F. Profiling of Antibiotic Residues in Soil and Vegetables Irrigated Using Pharmaceutical-Contaminated Water in the Delhi Stretch of the Yamuna River, India. Water 2023, 15, 4197. [Google Scholar] [CrossRef]
- Baciak, M.; Sikorski, Ł.; Piotrowicz-Cieślak, A.I.; Adomas, B. Role of Decarboxylases in the Biosynthesis of Biogenic Amines of Pea Growing in Soil Contaminated with Lomefloxacin. Appl. Ecol. Environ. Res. 2017, 15, 1131–1148. [Google Scholar] [CrossRef]
- Sikorski, Ł.; Adomas, B.; Dobiesz, M.; Baciak, M.; Piotrowicz-Cieślak, A.I. Morphological and Biochemical Responses of Lemna minor L. (Common Duckweed) to Ciprofloxacin. Fresenius Environ. Bull. 2014, 23, 363–371. [Google Scholar]
- Sikorski, Ł.; Baciak, M.; Piotrowicz-Cieślak, A.I.; Michalczyk, D.J.; Bęś, A.; Adomas, B. The Bioaccumulation and Metabolic Effects of Ciprofloxacin-HCl and Ciprofloxacin Free Base in Yellow Lupin (Lupinus luteus L.) Seedlings. Appl. Ecol. Environ. Res. 2017, 15, 1287–1300. [Google Scholar] [CrossRef]
- Migliore, L.; Cozzolino, S.; Fiori, M. Phytotoxicity to and Uptake of Enrofloxacin in Crop Plants. Chemosphere 2003, 52, 1233–1244. [Google Scholar] [CrossRef]
- Brain, R.A.; Hanson, M.L.; Solomon, K.R.; Brooks, B.W. Aquatic Plants Exposed to Pharmaceuticals: Effects and Risks; Springer: Berlin/Heidelberg, Germany, 2008; pp. 67–115. [Google Scholar]
- Huang, W.; Kong, R.; Chen, L.; An, Y. Physiological Responses and Antibiotic-Degradation Capacity of Duckweed (Lemna Aequinoctialis) Exposed to Streptomycin. Front. Plant Sci. 2022, 13, 1065199. [Google Scholar] [CrossRef]
- Krupka, M.; Michalczyk, D.J.; Žaltauskaitė, J.; Sujetovienė, G.; Głowacka, K.; Grajek, H.; Wierzbicka, M.; Piotrowicz-Cieślak, A.I. Physiological and Biochemical Parameters of Common Duckweed Lemna Minor after the Exposure to Tetracycline and the Recovery from This Stress. Molecules 2021, 26, 6765. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, L.; Piotrowicz-Cieślak, A.I.; Adomas, B. Phytotoxicity of Sodium Chloride towards Common Duckweed (Lemna minor L.) and Yellow Lupin (Lupinus luteus L.). Arch. Environ. Prot. 2013, 39, 117–128. [Google Scholar] [CrossRef][Green Version]
- Sikorski, Ł.; Baciak, M.; Bęś, A.; Adomas, B. The Effects of Glyphosate-Based Herbicide Formulations on Lemna minor, a Non-Target Species. Aquat. Toxicol. 2019, 209, 70–80. [Google Scholar] [CrossRef]
- Kalčíková, G.; Žgajnar Gotvajn, A.; Kladnik, A.; Jemec, A. Impact of Polyethylene Microbeads on the Floating Freshwater Plant Duckweed Lemna minor. Environ. Pollut. 2017, 230, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Moullan, N.; Mouchiroud, L.; Wang, X.; Ryu, D.; Williams, E.G.; Mottis, A.; Jovaisaite, V.; Frochaux, M.V.; Quiros, P.M.; Deplancke, B.; et al. Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research. Cell Rep. 2015, 10, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Bęś, A.; Warmiński, K.; Adomas, B. Long-Term Responses of Scots Pine (Pinus sylvestris L.) and European Beech (Fagus sylvatica L.) to the Contamination of Light Soils with Diesel Oil. Environ. Sci. Pollut. Res. 2019, 26, 10587–10608. [Google Scholar] [CrossRef]
- Zhang, H.B.; Dong, C.C.; Yang, Y.J.; Fu, J.K.; Liu, L.; He, X.Y.; Shi, J.-Q.; Wu, Z.-X. The Toxic Effect of Streptomycin on the Growth and Photosynthesis of Nostoc Using the Chlorophyll Fluorescence Analysis. Acta Hydrobiol. Sin. 2019, 43, 664–669. [Google Scholar]
- Yang, T.; Jiang, Z.; Lin, D.; Guo, L.; Wu, T.; Li, Z.; He, Z.; Zhou, X. Effects of Tetracycline and Oxytetracycline on Growth and Chlorophyll Fluorescence in Lettuce Seedlings. IOP Conf. Ser. Earth Environ. Sci. 2020, 474, 022013. [Google Scholar] [CrossRef]
- Iannelli, M.A.; Bellini, A.; Venditti, I.; Casentini, B.; Battocchio, C.; Scalici, M.; Ceschin, S. Differential Phytotoxic Effect of Silver Nitrate (AgNO3) and Bifunctionalized Silver Nanoparticles (AgNPs-Cit-L-Cys) on Lemna Plants (Duckweeds). Aquat. Toxicol. 2022, 250, 106260. [Google Scholar] [CrossRef]
- Huang, W.; Sun, D.; Wang, R.; An, Y. Integration of Transcriptomics and Metabolomics Reveals the Responses of Sugar Beet to Continuous Cropping Obstacle. Front. Plant Sci. 2021, 12, 711333. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Biosorption: Critical Review of Scientific Rationale, Environmental Importance and Significance for Pollution Treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Maldonado, I.; Moreno Terrazas, E.G.; Vilca, F.Z. Application of Duckweed (Lemna Sp.) and Water Fern (Azolla Sp.) in the Removal of Pharmaceutical Residues in Water: State of Art Focus on Antibiotics. Sci. Total Environ. 2022, 838, 156565. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, Y.-S.; Park, J.M. The Past, Present, and Future Trends of Biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A Reappraisal of the Use of DMSO for the Extraction and Determination of Chlorophylls a and b in Lichens and Higher Plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]


| SoV | Ir | Iy | FM | DM | Chl a | Chl b | TCC | PQ | Fv/Fm | Bs |
|---|---|---|---|---|---|---|---|---|---|---|
| F-value | ||||||||||
| Intercept | 107.78 * | 13,485 * | 62,396.31 * | 12,574.18 * | 33,592.27 * | 37,794.55 * | 39,283.75 * | 12,559,803 * | 108,103.88 * | 1856.19 * |
| Antibiotic (A) | 1.98 | 2.89 | 44.16 * | 10.39 * | 32.09 * | 35.02 * | 28.87 * | 7.16 * | 19.38 * | 164.58 * |
| Concentration (C) | 12.87 * | 15.49 * | 29.14 * | 3.07 * | 58.01 * | 56.98 * | 52.69 * | 8.04 * | 11.51 * | 279.28 * |
| A × C | 0.56 | 0.42 | 3.04 * | 0.81 | 6.13 * | 8.85 * | 5.65 * | 7.17 * | 1.23 | 54.77 * |
| Antibiotic | Parameter | Effective Concentration, mg × L−1 | |
|---|---|---|---|
| EC20 | EC50 | ||
| TC | Ir | 0.73 | 5.69 |
| Iy | 0.65 | 1.22 | |
| Mean Ir and Iy | 0.69 | 3.46 | |
| FM | 2.41 | - | |
| DM | 15.43 | - | |
| Chl a | 0.67 | - | |
| Chl b | 0.63 | - | |
| TCC | 15.78 | - | |
| PQ | - | - | |
| Fv/Fm | 16.18 | - | |
| MIN | Ir | 0.26 | 3.45 |
| Iy | 0.17 | 2.14 | |
| Mean Ir and Iy | 0.22 | 2.78 | |
| FM | 11.43 | - | |
| DM | 4.18 | - | |
| Chl a | 0.60 | 12.83 | |
| Chl b | 0.57 | 14.10 | |
| TCC | 2.43 | 18.15 | |
| PQ | - | - | |
| Fv/Fm | 13.89 | - | |
| Chemical Compound | Structural Formula | Empirical Formula | CAS Number | Molecular Weight [g × mol−1] | Form/Color |
|---|---|---|---|---|---|
| Tetracycline hydrochloride (TC) | 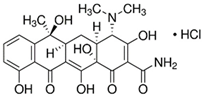 | C22H24N2O8 · HCl | 64-75-5 | 480.90 | powder/ yellow |
| Minocycline hydrochloride (MIN) | 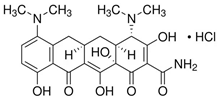 | C23H27N3O7 · HCl | 13614-98-7 | 493.94 | powder/ yellow |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorski, Ł.; Bęś, A.; Warmiński, K.; Truszkowski, W.; Kowal, P. Utilizing Morphological and Physiological Parameters of Lemna minor for Assessing Tetracyclines’ Removal. Molecules 2024, 29, 3971. https://doi.org/10.3390/molecules29163971
Sikorski Ł, Bęś A, Warmiński K, Truszkowski W, Kowal P. Utilizing Morphological and Physiological Parameters of Lemna minor for Assessing Tetracyclines’ Removal. Molecules. 2024; 29(16):3971. https://doi.org/10.3390/molecules29163971
Chicago/Turabian StyleSikorski, Łukasz, Agnieszka Bęś, Kazimierz Warmiński, Wojciech Truszkowski, and Przemysław Kowal. 2024. "Utilizing Morphological and Physiological Parameters of Lemna minor for Assessing Tetracyclines’ Removal" Molecules 29, no. 16: 3971. https://doi.org/10.3390/molecules29163971
APA StyleSikorski, Ł., Bęś, A., Warmiński, K., Truszkowski, W., & Kowal, P. (2024). Utilizing Morphological and Physiological Parameters of Lemna minor for Assessing Tetracyclines’ Removal. Molecules, 29(16), 3971. https://doi.org/10.3390/molecules29163971







