Role of Functional Groups in Tuning Luminescence Signature of Solution-Processed Graphene Quantum Dots: Experimental and Theoretical Insights
Abstract
1. Introduction
2. Results
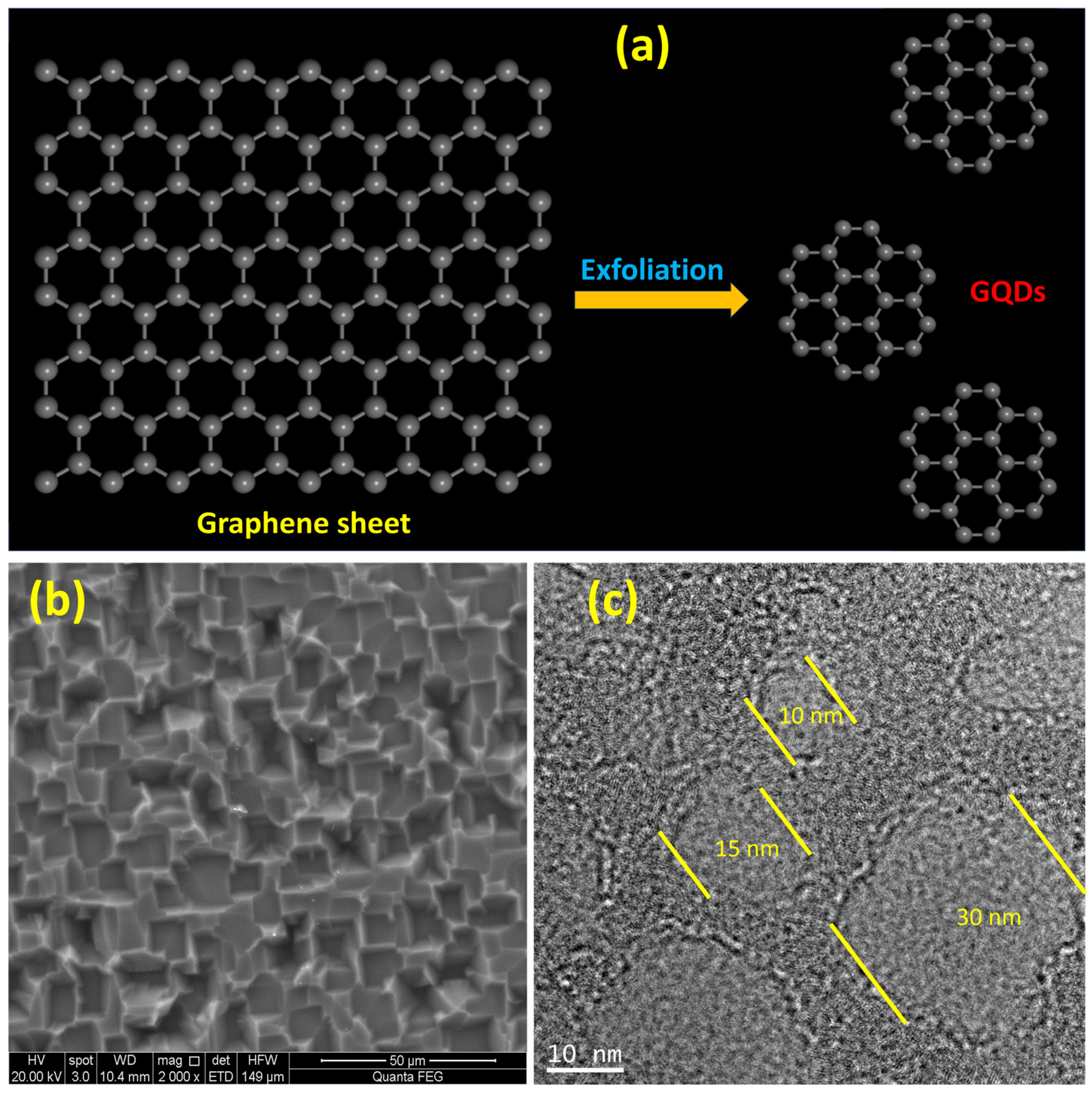
2.1. Morphological Characterization
2.2. Fourier-Transform Infrared Spectroscopy
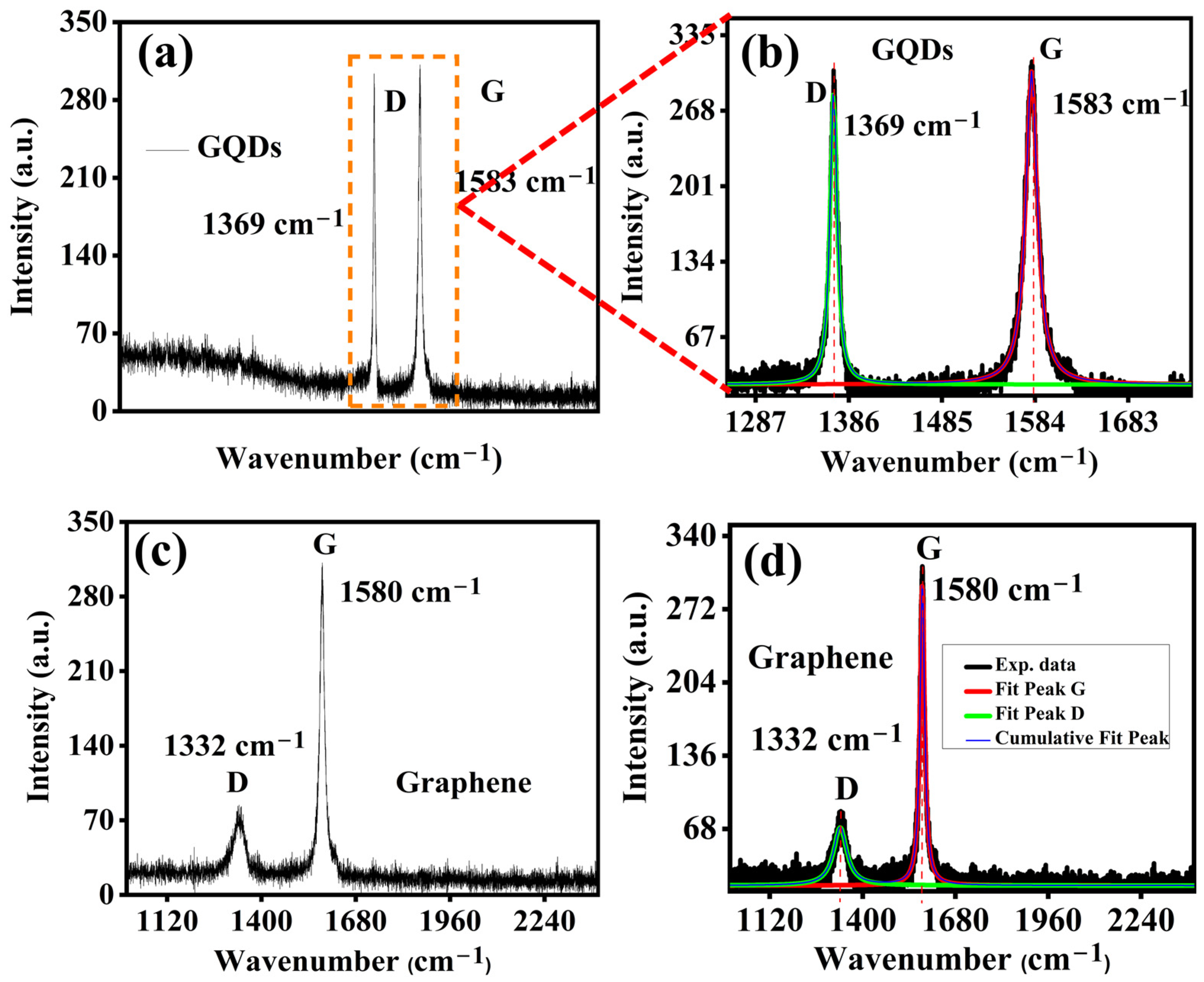
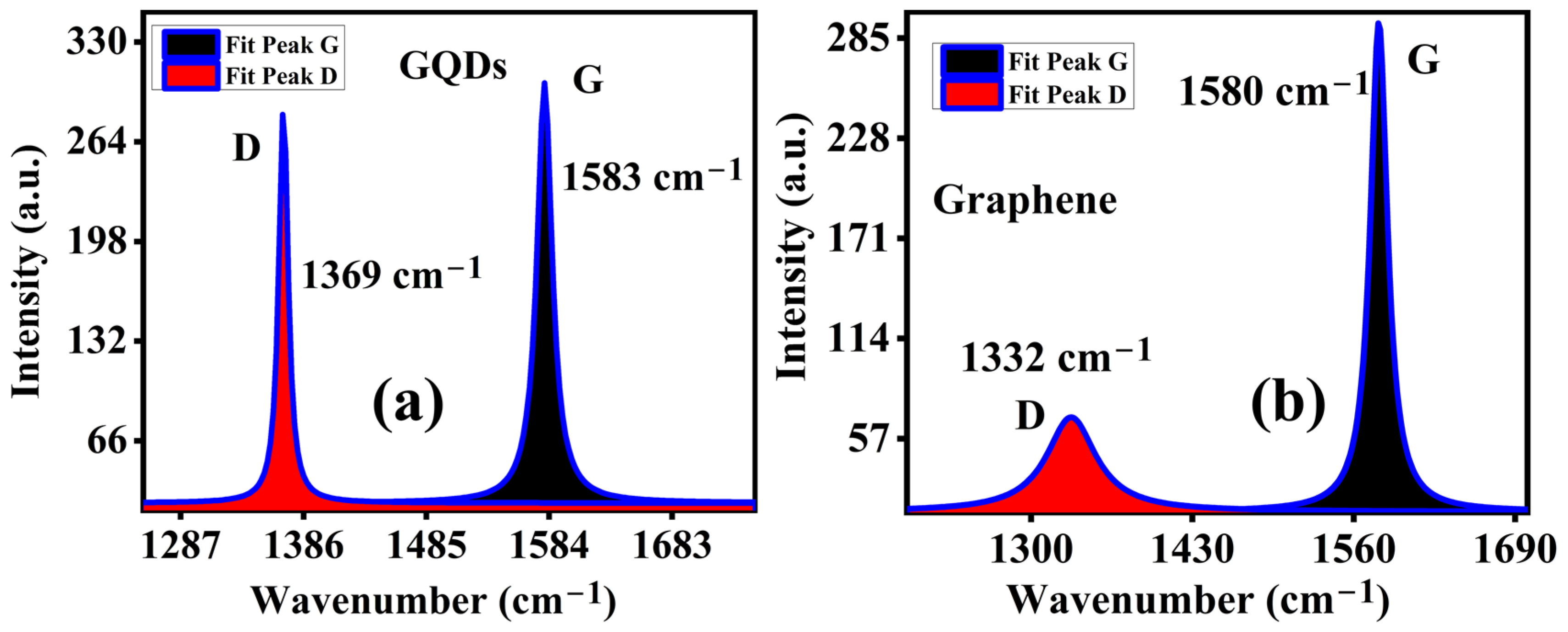
2.3. Raman Spectroscopy and Component Analysis
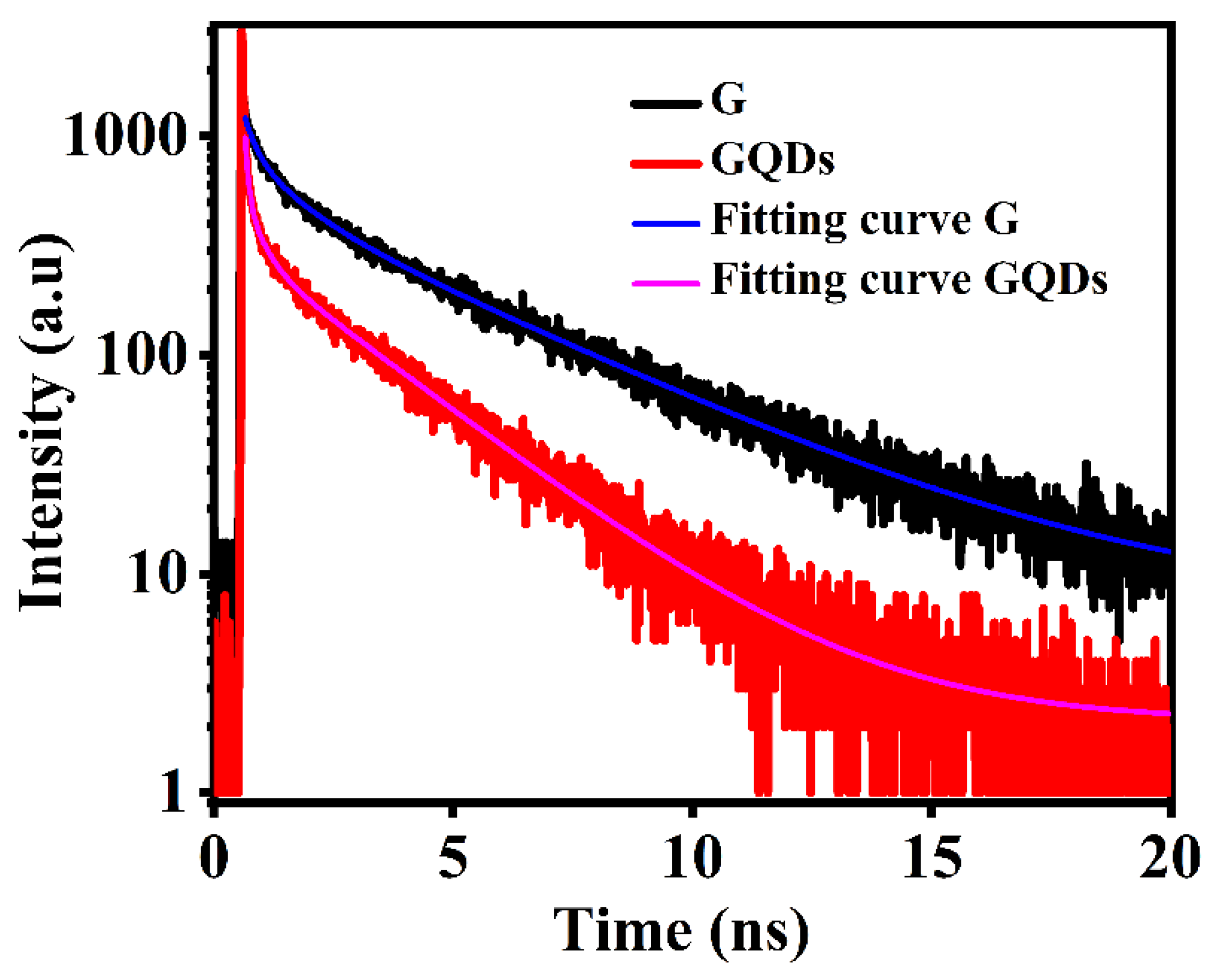
2.4. Photoluminescence Investigations
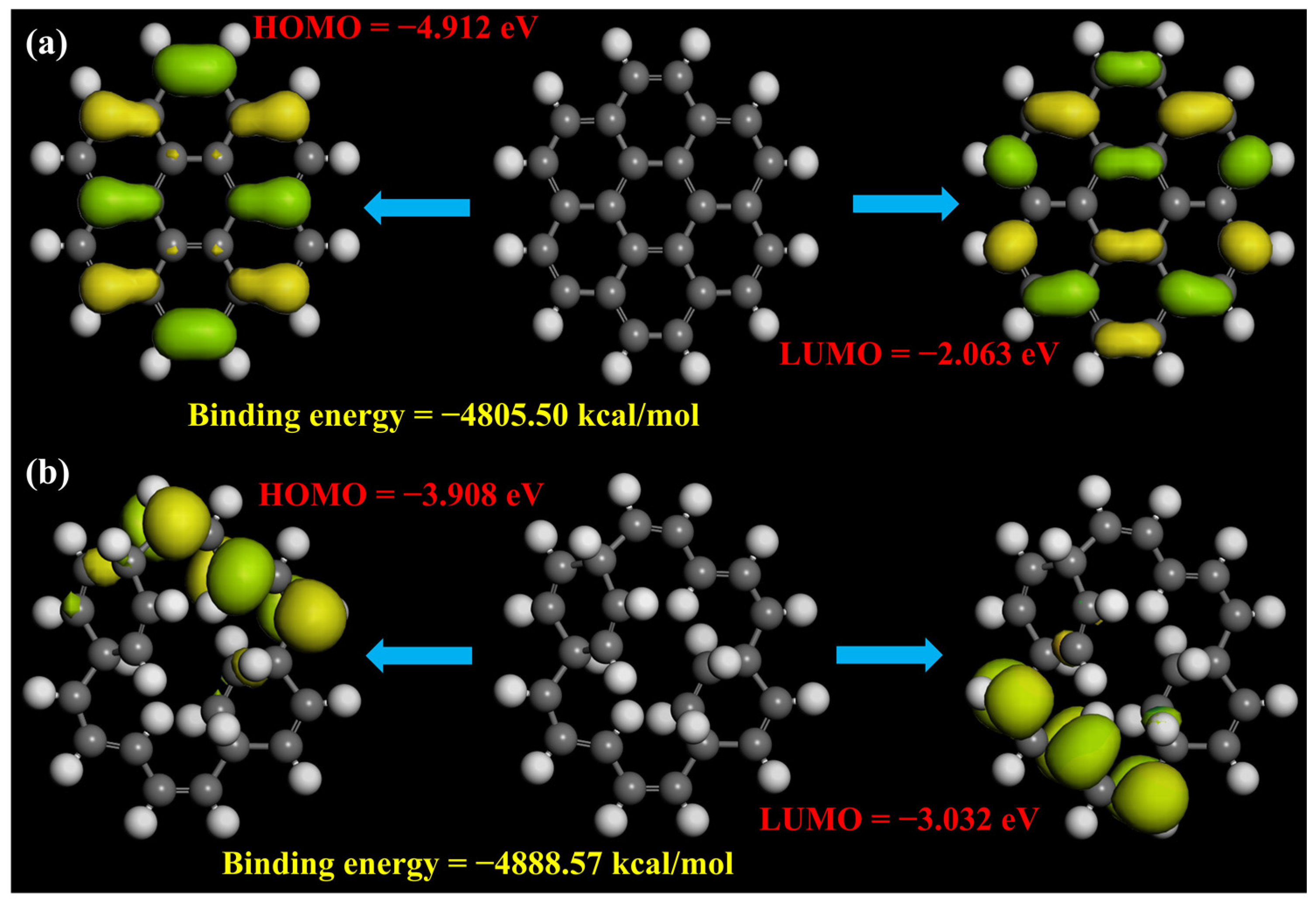
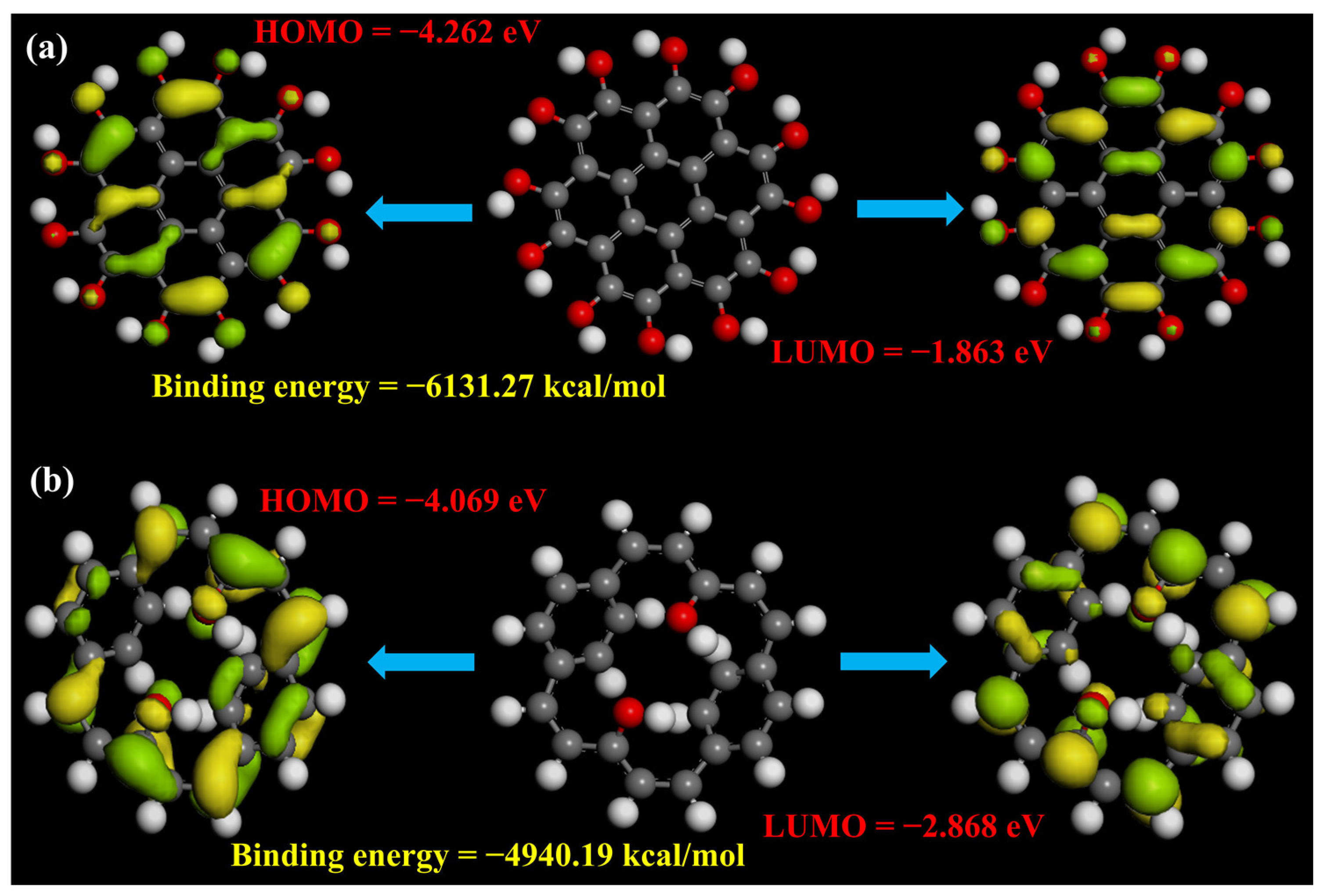
2.5. DFT Calculations
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, S.; Wang, L.; Li, B.; Song, Y.; Zhao, X.; Zhang, G.; Zhang, S.; Lu, S.; Zhang, J.; Wang, H.; et al. Investigation of photoluminescence mechanism of graphene quantum dots and evaluation of their assembly into polymer dots. Carbon 2014, 77, 462–472. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Wu, B.; Li, Z.; Pan, D.; Wu, M. Room-temperature synthesis of graphene quantum dots via electron-beam irradiation and their application in cell imaging. Chem. Eng. J. 2017, 309, 374–380. [Google Scholar] [CrossRef]
- Kim, J.K.; Bae, S.; Yi, Y.; Park, M.J.; Kim, S.J.; Myoung, N.; Lee, C.-L.; Hong, B.H.; Hyeok Park, J. Origin of White Electroluminescence in Graphene Quantum Dots Embedded Host/Guest Polymer Light Emitting Diodes. Sci. Rep. 2015, 5, 11032. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarkhah, A.; Hosseini, E.; Kamkar, M.; Sehat, A.A.; Dordanihaghighi, S.; Allahbakhsh, A.; van der Kuur, C.; Synthesis, M.A. Applications, and Prospects of Graphene Quantum Dots: A Comprehensive Review. Small 2022, 18, 2102683. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Zhao, Y.; Shi, G.; Deng, L.; Hou, Y.; Qu, L. An Electrochemical Avenue to Green-Luminescent Graphene Quantum Dots as Potential Electron-Acceptors for Photovoltaics. Adv. Mater. 2011, 23, 776–780. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, F.; Xue, Y.; Xiao, D.; Guo, Y. A Time-Dependent DFT Study of the Absorption and Fluorescence Properties of Graphene Quantum Dots. ChemPhysChem 2014, 15, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Lingam, K.; Podila, R.; Qian, H.; Serkiz, S.; Rao, A.M. Evidence for Edge-State Photoluminescence in Graphene Quantum Dots. Adv. Funct. Mater. 2013, 23, 5062–5065. [Google Scholar] [CrossRef]
- Sreeprasad, T.S.; Nguyen, P.; Alshogeathri, A.; Hibbeler, L.; Martinez, F.; McNeil, N.; Berry, V. Graphene Quantum Dots Interfaced with Single Bacterial Spore for Bio-Electromechanical Devices: A Graphene Cytobot. Sci. Rep. 2015, 5, 9138. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chapman, R.; Stevens, M.M. Integrative Self-Assembly of Graphene Quantum Dots and Biopolymers into a Versatile Biosensing Toolkit. Adv. Funct. Mater. 2015, 25, 3183–3192. [Google Scholar] [CrossRef]
- Chakraborti, H.; Sinha, S.; Ghosh, S.; Pal, S.K. Interfacing water soluble nanomaterials with fluorescence chemosensing: Graphene quantum dot to detect Hg2+ in 100% aqueous solution. Mater. Lett. 2013, 97, 78–80. [Google Scholar] [CrossRef]
- Sudolská, M.; Otyepka, M. Exact roles of individual chemical forms of nitrogen in the photoluminescent properties of nitrogen-doped carbon dots. Appl. Mater. Today 2017, 7, 190–200. [Google Scholar] [CrossRef]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Synthesis and Characterization of Monodisperse Nanocrystals and Close-Packed Nanocrystal Assemblies. Annu. Rev. Mater. Sci. 2000, 30, 545–610. [Google Scholar] [CrossRef]
- Efros, A.L.; Rosen, M. The Electronic Structure of Semiconductor Nanocrystals. Annu. Rev. Mater. Sci. 2000, 30, 475–521. [Google Scholar] [CrossRef]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene Quantum Dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Lin, L.; Rong, M.; Luo, F.; Chen, D.; Wang, Y.; Chen, X. Luminescent graphene quantum dots as new fluorescent materials for environmental and biological applications. TrAC Trends Anal. Chem. 2014, 54, 83–102. [Google Scholar] [CrossRef]
- Teymourinia, H.; Salavati-Niasari, M.; Amiri, O.; Safardoust-Hojaghan, H. Synthesis of graphene quantum dots from corn powder and their application in reduce charge recombination and increase free charge carriers. J. Mol. Liq. 2017, 242, 447–455. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.; Huang, C.; Su, W.; Xiao, Q. Amino-functionalized graphene quantum dots based ratiometric fluorescent nanosensor for ultrasensitive and highly selective recognition of horseradish peroxidase. Sens. Actuators B Chem. 2016, 234, 255–263. [Google Scholar] [CrossRef]
- Feng, J.; Dong, H.; Yu, L.; Dong, L. The optical and electronic properties of graphene quantum dots with oxygen-containing groups: A density functional theory study. J. Mater. Chem. C 2017, 5, 5984–5993. [Google Scholar] [CrossRef]
- Li, S.-Y.; He, L. Recent progresses of quantum confinement in graphene quantum dots. Front. Phys. 2021, 17, 33201. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Li, X.; Teng, K.S.; Lau, S.P. Size-Dependent Structural and Optical Characteristics of Glucose-Derived Graphene Quantum Dots. Part. Part. Syst. Charact. 2013, 30, 523–531. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Zhao, D.; Li, Q. The dual roles of functional groups in the photoluminescence of graphene quantum dots. Nanoscale 2016, 8, 7449–7458. [Google Scholar] [CrossRef]
- Zou, F.; Zhou, H.; Tan, T.V.; Kim, J.; Koh, K.; Lee, J. Dual-Mode SERS-Fluorescence Immunoassay Using Graphene Quantum Dot Labeling on One-Dimensional Aligned Magnetoplasmonic Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 12168–12175. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, S.-J.; Wang, H.-Y.; Qu, S.-N.; Zhang, Y.-L.; Zhang, J.-H.; Chen, Q.-D.; Xu, H.-L.; Han, W.; Yang, B.; et al. Common Origin of Green Luminescence in Carbon Nanodots and Graphene Quantum Dots. ACS Nano 2014, 8, 2541–2547. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Voitsihovska, O.O.; Rudenko, R.M.; Povarchuk, V.Y.; Abakumov, A.A.; Bychko, I.B.; Stetsenko, M.O.; Rudenko, M.P. The effect of electron irradiation on the electrical properties of reduced graphene oxide paper. Mater. Lett. 2019, 236, 334–336. [Google Scholar] [CrossRef]
- Rajender, G.; Giri, P.K. Formation mechanism of graphene quantum dots and their edge state conversion probed by photoluminescence and Raman spectroscopy. J. Mater. Chem. C 2016, 4, 10852–10865. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman spectroscopy of bottom-up synthesized graphene quantum dots: Size and structure dependence. Nanoscale 2019, 11, 16571–16581. [Google Scholar] [CrossRef]
- Herziger, F.; Vierck, A.; Laudenbach, J.; Maultzsch, J. Understanding double-resonant Raman scattering in chiral carbon nanotubes: Diameter and energy dependence of the $D$ mode. Phys. Rev. B 2015, 92, 235409. [Google Scholar] [CrossRef]
- Lyding, J. Influence of Edge Structure, Substrate Structure and Grain Boundaries on the Electronic Properties of Graphene Quantum Dots and Transferred Graphene; Bulletin of the American Physical Society: Dallas, TX, USA, 2011; p. T2.005. [Google Scholar]
- Eda, G.; Lin, Y.-Y.; Mattevi, C.; Yamaguchi, H.; Chen, H.-A.; Chen, I.S.; Chen, C.-W.; Chhowalla, M. Blue Photoluminescence from Chemically Derived Graphene Oxide. Adv. Mater. 2010, 22, 505–509. [Google Scholar] [CrossRef]
- Mei, Q.; Zhang, K.; Guan, G.; Liu, B.; Wang, S.; Zhang, Z. Highly efficient photoluminescent graphene oxide with tunable surface properties. Chem. Commun. 2010, 46, 7319–7321. [Google Scholar] [CrossRef]
- Pan, D.; Guo, L.; Zhang, J.; Xi, C.; Xue, Q.; Huang, H.; Li, J.; Zhang, Z.; Yu, W.; Chen, Z.; et al. Cutting sp2clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J. Mater. Chem. 2012, 22, 3314–3318. [Google Scholar] [CrossRef]
- Cao, L.; Meziani, M.J.; Sahu, S.; Sun, Y.-P. Photoluminescence Properties of Graphene versus Other Carbon Nanomaterials. Acc. Chem. Res. 2013, 46, 171–180. [Google Scholar] [CrossRef]
- Song, L.; Shi, J.; Lu, J.; Lu, C. Structure observation of graphene quantum dots by single-layered formation in layered confinement space. Chem. Sci. 2015, 6, 4846–4850. [Google Scholar] [CrossRef]
- Koyama, T.; Shimizu, S.; Miyata, Y.; Shinohara, H.; Nakamura, A. Ultrafast formation and decay dynamics of trions in $p$-doped single-walled carbon nanotubes. Phys. Rev. B 2013, 87, 165430. [Google Scholar] [CrossRef]
- Park, J.S.; Hirana, Y.; Mouri, S.; Miyauchi, Y.; Nakashima, N.; Matsuda, K. Observation of Negative and Positive Trions in the Electrochemically Carrier-Doped Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2012, 134, 14461–14466. [Google Scholar] [CrossRef]
- Hao, Y.; Gan, Z.; Zhu, X.; Li, T.; Wu, X.; Chu, P.K. Emission from Trions in Carbon Quantum Dots. J. Phys. Chem. C 2015, 119, 2956–2962. [Google Scholar] [CrossRef]
- Shen, R.; Song, K.; Liu, H.; Li, Y.; Liu, H. Fluorescence Enhancement and Radiolysis of Carbon Dots through Aqueous γ Radiation Chemistry. J. Phys. Chem. C 2012, 116, 15826–15832. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Lu, D.; Wang, G.-G.; Huangfu, J.; Wu, Q.-B.; Wang, X.-F.; Liu, L.-F.; Ye, D.-M.; Yan, B.; Han, J. Highly Efficient Orange Emissive Graphene Quantum Dots Prepared by Acid-Free Method for White LEDs. ACS Sustain. Chem. Eng. 2020, 8, 6657–6666. [Google Scholar] [CrossRef]
- Park, J.; Moon, J.; Kim, C.; Kang, J.H.; Lim, E.; Park, J.; Lee, K.J.; Yu, S.-H.; Seo, J.-H.; Lee, J.; et al. Graphene quantum dots: Structural integrity and oxygen functional groups for high sulfur/sulfide utilization in lithium sulfur batteries. NPG Asia Mater. 2016, 8, e272. [Google Scholar] [CrossRef]
- Stetsenko, M.; Pullano, S.A.; Margitych, T.; Maksimenko, L.; Hassan, A.; Kryvyi, S.; Hu, R.; Huang, C.; Ziniuk, R.; Golovynskyi, S.; et al. Antireflection Enhancement by Composite Nanoporous Zeolite 3A–Carbon Thin Film. Nanomaterials 2019, 9, 1641. [Google Scholar] [CrossRef]
- Hassan, A.; Iqbal, M.F.; Maksym, S.; Tetiana, M.; Azam, M.; Kanwal, Z.; Irfan, I.; Li, B.; Jiang, Y. Near-band-edge emission enhancement and suppression of the deep levels in Ga-doped ZnO via surface plasmon-exciton coupling without a dielectric spacer. J. Mater. Sci. Mater. Electron. 2019, 30, 20544–20550. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Elhaes, H.; Ibrahim, M.A. First principles study of edge carboxylated graphene quantum dots. Phys. B Condens. Matter 2018, 537, 77–86. [Google Scholar] [CrossRef]
- Feng, J.; Guo, Q.; Song, N.; Liu, H.; Dong, H.; Chen, Y.; Yu, L.; Dong, L. Density functional theory study on optical and electronic properties of co-doped graphene quantum dots based on different nitrogen doping patterns. Diam. Relat. Mater. 2021, 113, 108264. [Google Scholar] [CrossRef]
- Feng, J.; Dong, H.; Pang, B.; Shao, F.; Zhang, C.; Yu, L.; Dong, L. Theoretical study on the optical and electronic properties of graphene quantum dots doped with heteroatoms. Phys. Chem. Chem. Phys. 2018, 20, 15244–15252. [Google Scholar] [CrossRef]
- Ojeda-Martínez, M.; Martínez, A.N.P.; El Hamdaoui, J.; Piedrahita, M.C.; Feddi, E.M.; Thirumuruganandham, S.P.; Martínez, M.L.O.; Figueroa, J.L.C.; Ordoñez, C.V.; Mowbray, D.J. Tuning the energy gap of graphene quantum dots functionalized by OH and COOH radicals: First principle study. Mater. Chem. Phys. 2024, 311, 128543. [Google Scholar] [CrossRef]
- Abozeed, A.A.; Younis, O.; Al-Hossainy, A.F.; El-Mawla, N.A.; Sayed, M.; El-dean, A.M.K.; Tolba, M.S. Combined experimental and TD-DFT/DMOl3 investigations, optical properties, and photoluminescence behavior of a thiazolopyrimidine derivative. Sci. Rep. 2022, 12, 15674. [Google Scholar] [CrossRef]
- Kumar, A.; Sayyed, M.I.; Punina, D.; Naranjo, E.; Jácome, E.; Abdulameer, M.K.; Albazoni, H.J.; Shariatinia, Z. Graphene quantum dots (GQD) and edge-functionalized GQDs as hole transport materials in perovskite solar cells for producing renewable energy: A DFT and TD-DFT study. RSC Adv. 2023, 13, 29163–29173. [Google Scholar] [CrossRef]
- Becke, A.D. A multicenter numerical integration scheme for polyatomic molecules. J. Chem. Phys. 1988, 88, 2547–2553. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]





| GQDs | Graphene | ||||
|---|---|---|---|---|---|
| Bands | A | B | C | A | D |
| Peak Position (nm) | 544.52 ± 1.22 | 515.79 ± 0.90 | 455.34 ± 0.82 | 513.18 ± 0.16 | 752.62 ± 1.04 |
| Peak Intensity (a.u.) | 1.105 × 106 | 436,141.01 | 496,059.13 | 1.293 × 106 | 78,384.74 |
| FWHM (nm) | 165.41 ± 1.42 | 69.96 ± 1.30 | 70.18 ± 1.25 | 121.61 ± 0.71 | 65.28 ± 4.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, Z.; Azam, M.; Ali, S.; Zubair, M.; Cao, Y.; Khan, A.A.; Hassan, A.; Xue, W. Role of Functional Groups in Tuning Luminescence Signature of Solution-Processed Graphene Quantum Dots: Experimental and Theoretical Insights. Molecules 2024, 29, 2790. https://doi.org/10.3390/molecules29122790
Ke Z, Azam M, Ali S, Zubair M, Cao Y, Khan AA, Hassan A, Xue W. Role of Functional Groups in Tuning Luminescence Signature of Solution-Processed Graphene Quantum Dots: Experimental and Theoretical Insights. Molecules. 2024; 29(12):2790. https://doi.org/10.3390/molecules29122790
Chicago/Turabian StyleKe, Zhicheng, Muhammad Azam, Shujat Ali, Muhammad Zubair, Yu Cao, Abbas Ahmad Khan, Ali Hassan, and Wei Xue. 2024. "Role of Functional Groups in Tuning Luminescence Signature of Solution-Processed Graphene Quantum Dots: Experimental and Theoretical Insights" Molecules 29, no. 12: 2790. https://doi.org/10.3390/molecules29122790
APA StyleKe, Z., Azam, M., Ali, S., Zubair, M., Cao, Y., Khan, A. A., Hassan, A., & Xue, W. (2024). Role of Functional Groups in Tuning Luminescence Signature of Solution-Processed Graphene Quantum Dots: Experimental and Theoretical Insights. Molecules, 29(12), 2790. https://doi.org/10.3390/molecules29122790







