Abstract
The objective of this research was to investigate natural products for their potential against pathogenic microorganisms. Sabinene hydrate (SH), a monoterpenoid, is synthesised by numerous different plants as a secondary metabolite. At present, there is a lack of definite investigations regarding the antimicrobial activity of SH itself and its different isomers. The antimicrobial effects of commercially available SH (composed mainly of trans-isomer) were evaluated within a range of concentrations in three types of contact tests: solid and vapor diffusion and the macro-broth dilution method. Moreover, the effects of SH on the rate of linear growth and spore germination were also examined. Ethanolic SH solutions were tested against an array of microorganisms, including blue-stain fungi (Ceratocystis polonica, Ophiostoma bicolor, O. penicillatum), frequently originating from bark beetle galleries; three fungal strains (Musicillium theobromae, Plectosphaerella cucumerina, and Trichoderma sp.) isolated from a sapwood underneath bark beetle galleries (Ips typographus) on spruce (Picea abies) stems; Verticillium fungicola, isolated from diseased I. typographus larvae; two Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus), two Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa); five yeasts (Candida albicans, C. krusei, C. parapsilosis, Saccharomyces cerevisiae, and Rhodotorula muscilaginosa), and two saprophytic fungi (Aspergillus niger and Penicillium notatum). In solid agar disc diffusion tests, Gram-positive bacteria exhibited greater susceptibility to SH than Gram-negative bacteria, followed by yeasts and fungi. The most resistant to SH in both the disc diffusion and broth macro-dilution methods were P. aeruginosa, A. niger, and Trichoderma sp. strains. Blue-stain fungi and fungi isolated from the Picea sapwood were the most resistant among the fungal strains tested. The minimum inhibition concentrations (MICs) generated by SH and determined using a disc volatilization method were dependent on the fungal species and played an important role in the development of microorganism inhibition. The two Gram-positive bacteria, B. subtilis and S. aureus (whose MICs were 0.0312 and 0.0625 mg/mL, respectively), were the organisms most susceptible to SH, followed by the Gram-negative bacterium, E. coli (MIC = 0.125 mg/mL) and two yeasts, C. albicans and C. kruei (MIC was 0.125 mg/mL and 0.25 mg/mL, respectively). C. parapsilosis (MIC = 0.75 mg/mL) was the yeast most resistant to SH. The investigation of antimicrobial properties of plant secondary metabolites is important for the development of a new generation of fungicides.
1. Introduction
Sabinene hydrate (SH, also known as 4-thujanol) is a volatile organic compound and belongs to the class of bicyclic monoterpene alcohols. Biosynthesis of monoterpenes occurs mainly by the 2-methyl-erythritol-4-phosphate pathway in plant cell plastids, and geranyl/neryl diphosphate is a key intermediary in the formation of cyclic monoterpenoids [1]. It is important to note that in many cases, the enzymatic synthesis of SH isomers occurs without the formation of free intermediates, e.g., via sabinene or α-thujene [2]. The role of metal ions (such as Mn2+ or Mg2+) as catalysts is very important for the biosynthesis of SH in plants [2,3]. SH could be in cis or trans form, it has optical isomers [2,4], and different enzymes are responsible for the biosynthesis of (1S,2R,4S)-(Z)-sabinene hydrate and (1S,2S,4R)-(E)-sabinene hydrate [4]. This secondary metabolite occurs naturally in a large number of plants and their essential oils (EOs), including Citrus [5,6], Salvia [7], Myristica [8,9,10], Origanum [11,12,13,14,15,16,17,18,19,20,21,22], Thymus [3,23,24,25,26,27,28], Mentha [22,29], Juniperus [30,31] and other species. It has been observed that the presence of terpinen-4-ol in the mature leaves and in the steam distillates of Melaleuca argentea, M. dissitiflora, and M. linariifolia is related to high levels of cis- and trans-sabinene hydrate in the young leaves. Isomers of SH serve as the precursors of terpinen-4-ol, which is formed through non-enzymatic chemical conversion [32]. A significant number of EOs containing appreciable quantities of SH isomers have demonstrated bioactive properties, including strong antifungal and antibacterial activities [12,18,20,28,29,33,34,35,36,37,38,39,40]. A valuable study was conducted to investigate the cultivable bacterial communities of Origanum species, from which EOs containing high amounts of SH were obtained [41]. EOs were found to be rich in trans-sabinene hydrate (24.0% and 30.8% for O. vulgare ssp. vulgare and O. vulgare ssp. hirtum, respectively), and a high degree of biodiversity was found in the bacterial endophytic microbiome. A hypothesis has been proposed that the composition of the EOs may be involved in the formation of microbial communities and that bacteria may be able to colonize the plants by resisting the antimicrobial activity exhibited by the EOs themselves and/or by using some compounds as carbon and energy sources. The composition of microbial communities of plants can impact or modify the EOs’ composition [41].
Plants produce volatile organic compounds for many reasons, and one of them is as a defensive activity against pests and their associated fungi. Terpenoids can be involved in the systemic resistance mechanisms of plants [42,43,44]. It has been reported how the profile of main terpenoids and enantiomeric ratio of major monoterpenes changed in the stem bark of Norway spruce (Picea abies) trees inoculated with blue-stain fungus Ceratocystis polonica [45]. However, it has also been found that various fungal strains can degrade tree defence metabolites (phenolic compounds) and use them as a carbon source [46,47,48]. Furthermore, the different strains of Endoconidiophora, Ophiostoma or Grosmannia associated with the bark beetle Ips typographus Linnaeus (Coleoptera, Curculionidae: Scolytinae) can metabolize monoterpenes in Norway spruce resin, producing volatiles with attractant properties [49]. Bornyl acetate can be metabolized into camphor and α- and β-pinene to trans-4-thujanol (SH); both monoterpenoids at specific doses attracted the I. typographus beetles in the above study. Additionally, it has been discussed how the volatile emissions of conifers may provide clues regarding tree vitality and suitability for I. typographus attacks, i.e., to find suitable host trees for the beetles [50]. On other hand, the repellent effects of SH on both sexes of bark beetle I. typographus have been evaluated [51,52,53]. I. typographus is a common pest of conifers that attacks Norway spruce, a dominant tree species in the European boreal, montane, and sub-alpine forests, as well as Yezo spruce (Picea jezoensis) and Sakhalin spruce (Picea glehnii (Fr. Schm.) Masters), dominant trees in eastern Asia [54,55,56,57,58]. During periods when the beetles are not active, they breed in felled, fallen, or windthrown trees, i.e., stumps and logs. During outbreaks, they kill healthy trees, which can have a considerable impact on the forest ecosystem and global environment [59,60].
The interaction between plants and herbivores is often not a simple two-way process; rather, it is mediated by microorganisms, resulting in a complex three-way interaction between plants, herbivores, and microorganisms [55]. The bark beetle I. typographus carries spores of several phytopathogenic fungi, including Ceratocystiopsis minuta, Ceratocystis polonica (Siem.) C. Moreau, Ophiostoma bicolor Davidson & Wells, O. penicillatum (Grosmann.) Siemaszko (syn., Grosmannia penicillata), O. piceae and O. japonicum [55,56,57,58]. A well-studied example of a three-way, plant–herbivore–microbe interaction is that observed between conifer trees and tree-killing bark beetles, in which fungal pathogens, developed in the phloem and cambium, are thought to facilitate the beetles’ ability to kill trees [57,58,61].
The main objectives of the investigation were to evaluate the activity of SH (composed mainly of trans-isomer), a volatile secondary metabolite of plants against pests’ associated microorganisms:
- (i)
- blue-stain fungi: Ceratocystis polonica, Ophiostoma bicolor and O. penicillatum (syn. Grosmannia penicillata)), frequently originating from bark beetle galleries;
- (ii)
- other fungi associated with Ips. typographus, i.e., three fungal strains: Musicillium theobromae, Plectosphaerella cucumerina and Trichoderma sp., isolated from sapwood underneath bark beetle galleries (Ips typographus) on spruce (Picea abies) stems;
- (iii)
- Verticillium fungicola, isolated from diseased I. typographus larvae;
- (iv)
- and to compare SH activity against:
- (v)
- two Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus),
- (vi)
- two Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa);
- (vii)
- five yeasts (Candida albicans, C. krusei, C. parapsilosis, Saccharomyces cerevisiae, and Rhodotorula muscilaginosa),
- (viii)
- two saprophytic fungi (Aspergillus niger and Penicillium notatum).
As we mentioned above, a significant number of studies have been conducted on the antimicrobial activity of plant EOs containing appreciable amounts of SH. However, to the best of our knowledge, at present there is a definite lack of publications on the antimicrobial properties of SH itself and its different isomers [12].
It must be emphasised that there are no previous reports related to SH’s effects on most of the microorganisms tested in the present research.
2. Results
2.1. Agar Disc Diffusion Method
The diameters of inhibition zones (IZs), including paper disc diameter (6 mm), are presented in Table 1.

Table 1.
Mean diameters of inhibition zones (mm) of microorganisms using sabinene hydrate in an agar disc diffusion test *.
Microbial strains belonging to different groups (bacteria, yeasts, blue-stain, and other fungi) were differently susceptible to SH in the disc diffusion method. SH was more active against Gram-positive bacteria, followed by Gram-negative bacteria and yeasts. Fungi were more resistant than yeasts and bacteria. Blue-stain fungi as well as other fungi showed low susceptibility to SH in the disc diffusion method. The most resistant blue-stain fungus was O. penicillatum, with an IZ of only 13.1 ± 0.7 mm even at the highest SH concentration (1000 μg/disc corresponding 100 mg/mL SH concentration). At the lowest SH concentrations (25 μg/disc and 250 μg/disc), O. penicillatum (syn. Grosmannia penicillata) showed the full resistance to this monoterpene alcohol. Moreover, a stimulation of melanin synthesis by SH was noticeable in fungus cultures. A similar SH resistance level was detected for C. polonica. O. bicolor was the most sensitive to SH among the blue-stain fungi as well as among all tested fungi. Very low, albeit detectable, growth inhibition was detected even at the lowest SH concentration (125 μg/disc); IZ diameter increased with SH concentration on the paper disc and exceeded 17.2 ± 3.8 mm at 1000 μg/disc SH concentration.
Surprisingly, in this investigation, the saprotrophic fungi and fungi used (whose trophic attribution is not yet determined), isolated from bark beetle galleries, were more resistant to SH than blue-stain fungi were (Table 1). A. niger was chosen for our treatments due to its versatility and ability to grow well in a variety of media with different parameters. None of the SH concentrations inhibited A. niger or Trichoderma sp. strains. All these strains were developed on the paper discs impregnated with 125–250 μg/disc SH content. A. niger tolerated also at 500 μg/disc SH concentration. Additionally, A. niger was found as a fungus whose sporulation and colony pigmentation were stimulated by SH. P. notatum was inhibited only at an SH concentration of 1000 μg/disc, and its IZ was very low (7.9 ± 0.6 mm). P. notatum was selected for our studies as a fungus that is ubiquitous in its environment and is able to produce biologically active secondary metabolites. M. theobromae showed the largest IZ (average 15.4 mm) as well as P. cucumerina, which was susceptible to the lower SH concentration (750 μg/disc).
Gram-positive B. subtilis was the most sensitive to SH; its average IZ ranged from 7.0 to 33.5 mm with increased SH concentration, followed by S. aureus (Gram-positive), whose average IZ values were 28.8 mm at the highest SH concentration (100 mg/mL) (Table 1). None of the SH concentrations tested inhibited Gram-negative bacterium P. aeruginosa. This strain as well as the saprotrophic fungi A. niger and Trichoderma sp. strains were the microorganisms most resistant to SH in the agar disc diffusion test.
2.2. Broth Macro-Dilution Method
The minimum inhibitory concentrations (MICs) of SH were determined against four bacterial and five yeast strains (Table 2).

Table 2.
Minimum inhibition concentrations (mg/mL) of SH for microbial strains by the macro-broth dilution method.
Gram-positive bacteria, B. subtilis and S. aureus, with MIC values of 0.0312 and 0.0625 mg/mL, respectively, were the organisms most susceptible to SH, followed by the Gram-negative bacterium E. coli (MIC = 0.125 mg/mL) and two yeasts, C. albicans and C. kruei (MIC values were determined to be 0.125 mg/mL and 0.25 mg/mL, respectively). C. parapsilosis was the yeast most resistant to SH (MIC = 0.75 mg/mL), while S. cerevisiae and R. muscilaginosa showed a medium resistance to this monoterpenoid. None of the concentrations of SH inhibited P. aeruginosa (Gram-negative) in this investigation.
The MICs induced in the microbial cultures by SH were from 10 to 100 times lower than those induced by the standard antibiotics (Table 2). Strains of B. subtilis and P. aeruginosa were the most resistant to chloramphenicol (MICs = 62.50 μg/mL). E. coli (MIC = 31.25 μg/mL) was less sensitive, followed by S. aureus, as the most susceptible to chloramphenicol.
All filamentous fungi tested were more resistant to SH than yeasts and bacteria; however, none of the SH concentrations induced 100% inhibition of the fungi in their early development stages (germination or germ-tube formation rate). The most susceptible blue-stain fungus was O. bicolour (Table 3).

Table 3.
Germination effects (%) of sabinene hydrate on fungi development with the macro-broth dilution method *.
Inhibition of spores started from the lowest tested SH concentration (0.0625 mg/mL) and gradually increased with the increased concentration of the monoterpenoid. Spore germination and inhibition of the ophiostomatoid fungi C. polonica and O. penicillatum also started from the same SH concentration (2.25 μg/mL). However, O. penicillatum showed higher susceptibility (higher inhibition percentages) than C. polonica at all SH concentrations tested. Conidia of A. niger germinated and were resistant even to very high SH concentrations. An average 0.82% and 1.45% inhibition were caused at 7.5 and 10.0 μg/mL SH concentrations, respectively. Surprisingly, very high resistance to SH was shown by the M. theobromae and P. cucumerina strains isolated from partially decomposed spruce sapwood underneath I. typographus galleries.
As in the case of the broth macro-dilution method used for bacteria and yeasts, nystatin-dihydrate (N-D) activity against the fungi tested was significantly higher than SH activity (Table 3). In the broth macro-dilution method, all fungal strains were fully inhibited by N-D at a concentration of 300 μg/mL, and only a small portion of the propagules of four fungi, including C. polonica and fungi associated with beetle galleries (P. cucumerina, A. niger, and M. theobromae) survived at an N-D concentration of 150 μg/mL. C. polonica was the strain most resistant to N-D among the all fungi tested. The lowest concentration of N-D, causing 26.71 ± 11.14% inhibition, was 37.5 μg/mL. The lowest N-D concentration inhibited spore germination of both Ophiostoma species, and the conidium germination of other fungi was 9.375 μg/mL. However, inhibition percentages were low and insignificant due to the high dispersal of the data.
2.3. Sabinene Hydrate Volatilization from Paper Disc (Vapour Phase Activity Test)
In Petri dishes, the atmosphere created from paper discs containing 1 mg, 2 mg, or 3 mg of SH induced inhibition of the radial growth rate, which was fungus species-dependent (Figure 1). Inhibition of the radial growth (%) of the blue-stain fungi by SH supplied during the vapor phase was contradictory. Rate of radial growth of C. polonica and other fungi tested were inhibited by SH vapor, while O. penicillatum was stimulated up to 47.57% compared to the control by the vapor phase obtained from 1 mg SH in the 9 cm3 Petri dish. Less stimulation was detected with increasing SH content. C. polonica was the least susceptible to SH supplied in the vapor phase, followed by O. bicolor.
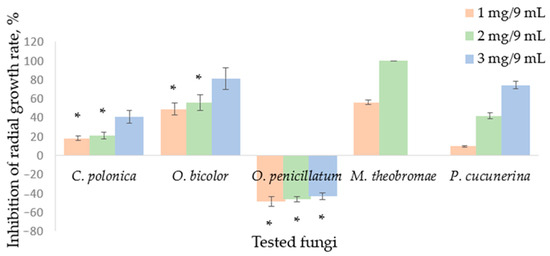
Figure 1.
Inhibition of the radial growth rate of fungi by sabinene hydrate supplied in the vapor phase: Ceratocystis polonica, Ophiostoma bicolour, Ophiostoma penicillatum (syn. Grosmannia penicillata), Musicillium theobromae, and Plectosphaerella cucunerina. Mean values represent data from three replicate experiments, bar—SD (standard deviation). Unmarked values are significantly different (p < 0.05). A lack of statistically significant difference between the experimental data is marked with asterisks (*).
Two yeasts investigated were also affected by SH supplied in the vapor phase. Neither of the two strains was stimulated by SH in the disc volatilization method (Figure 2). Yeast cell budding as well as pseudo-mycelium formation rate was influenced in the atmosphere containing SH vapor evaporated from 3 mg SH (Figure 2). Greater damage from SH vapor, evaluated as cell budding inhibition percentage (Figure 2), and determined for C. albicans. C. parapsilosis was more resistant in both cell budding (Figure 2) and pseudo-hypha development (Figure 3).
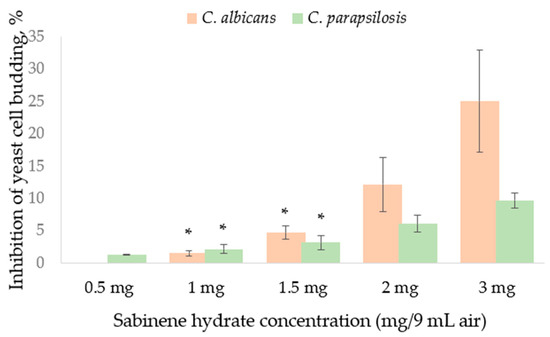
Figure 2.
Inhibition of yeast cell budding by the sabinene hydrate supplied in the vapor phase in the disc volatilization method. Mean from three experiments (n = 3), bar—SD (standard deviation). Unmarked values are significantly different (p < 0.05). A lack of statistically significant difference between the experimental data is marked with asterisks (*).
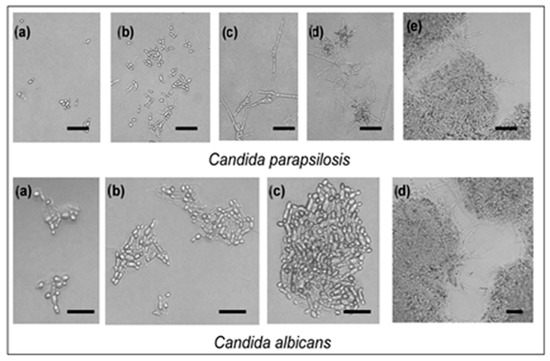
Figure 3.
Effect of sabinene hydrate supplied in the vapor phase (volatile fraction evaporating into 9 cm3 Petri dish atmosphere from paper disc impregnated with 1 g of this compound) on yeast cell budding and pseudo-mycelium formation. Scale is 10 μm; (a–c) conidia and conidiophores; (d,e) conidiophores and conidial masses in preparations. The most representative images were selected from each of the three replicates (n = 3).
2.4. Results of Dual Culture Tests
Results obtained from dual culture tests differed significantly depending on the culture medium used (malt extract medium (MEA) or malt extract medium amended with spruce phloem extract (MEA + SPE)). Significant differences between the inter-inhibition levels were detected for the blue-stain fungi (Table 4).

Table 4.
Inhibition (%) of radial growth in dual culture test *.
The most active antagonist on the MEA agar medium was O. bicolor, whose colonies developed very rapidly and suppressed the growth other ophiostomatoid fungi, especially C. polonica. On MEA medium, O. bicolor inhibited radial growth of C. polonica and O. penicillatum by about 99% and 43%, respectively, while C. polonica radial growth of both O. bicolor and O. penicillatum was inhibited by an average of 12.08 and 78.91%, respectively (Table 4). On MEA medium, other fungi studied, including fungicolous L. fungicola, entomopathogenic B. bassiana and M. anissopliae, were resistant to the blue-stain fungi tested and showed high concurrence peculiarities when grown on MEA medium in dual cultures. In this investigation, L. fungicola was the most active against the blue-stain fungi. This fungus completely (100%) inhibited growth of the O. penicillata and suppressed colony development of C. polonica and O. bicolor by approximately 82.42% and 38.34%, respectively (Table 4). O. penicillatum was the strain most susceptible to L. fungicola as well as to fungal entomopathogens, while O. bicolor was the most resistant to these fungi on MEA in the dual culture tests. Very strong inhibition of the radial growth rate of the blue-stain fungi was caused by B. bassiana and M. anisopliae. B. bassiana inhibited growth of C. polonica, O. bicolor, and O. penicillatum by an average of 50.77, 22.16, and 94.11%, respectively (Table 4). Sterile zones (3–5 mm wide) were found on MEA in dual M. anisopliae and C. polonica cultures (Figure 4). The growth inhibition percentage induced by M. anissopliae ranged from 17.89 to 62.19% for O. bicolor and O. penicillatum, respectively (Table 4). Results also confirmed high P. cucumerina antagonistic peculiarities against the blue-stain fungi, especially against O. penicillatum (~90%). M. theobromae was significantly less active; however, its activity markedly increased after two to three weeks of incubation, the when mycelium and sporulation structures of the impacted fungi were intensively degraded (Figure 4). Additional illustrations of the growth of tested fungal colonies are shown in Figures S1–S5 in Supplementary Materials.
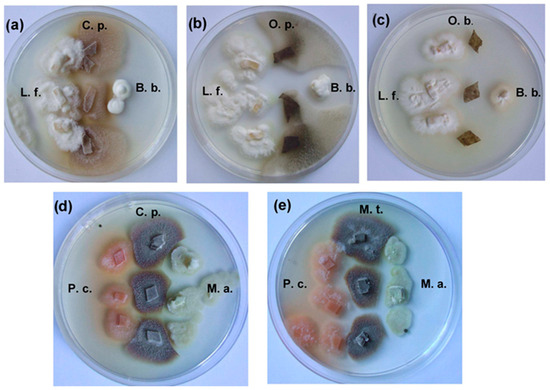
Figure 4.
Antagonistic effects of fungi after 10 days’ growth on 2% MEA from (a–e): C. p.—Ceratocystis polonica, O. b.—Ophiostoma bicolor, O. p.—Ophiostoma penicillatum (syn. Grosmannia penicillata), B. b.—Beauveria bassiana, L. f.—Lecanicillium fungicola, P. c.—Plectosphaerella cucumerina, M. t.—Musicillium theobromae, and M. a.—Metarhizium anissopliae. The most representative images were selected from each of at least three replicates (n ≥ 3).
In dual culture tests, a different situation was detected when fungal cultures were grown on malt extract medium amended with spruce phloem extract (MEA + SPE). The antagonistic abilities of the C. polonica strain changed controversially: O. bicolor inhibition increased 4-fold (from 12.08% to 49.31% inhibition), while C. polonica partially lost activity against O. penicillatum (inhibition activity decreased from 78.91% to 46.77%) (Table 4). Proportionally, the antagonistic activity of O. bicolor against C. polonica decreased from an average of 98.89 to 24.68% on MEA and MEA + SPE media, respectively. The ability of O. bicolor to suppress the radial growth of O. penicillatum was almost similar on both culture media. On the MEA + SPE medium, inhibition of C. polonica growth rate induced by O. penicillatum markedly increased (from 12.43% to 49.27%) (Table 4).
Only P. cucumerina kept its antagonistic peculiarities against blue-stain fungi in the dual cultures on MEA + SPE medium. The fungus was stimulated by spruce phloem extracts in MEA medium in control plates. Conversely, M. theobromae was inhibited by spruce phloem extracts in the control, and thus its antagonistic peculiarities against blue-stain fungi on this medium decreased by 10–25% as compared with those on MEA medium.
The fungal entomopathogens B. bassiana and M. anissopliae grew more slowly on the malt extract medium with spruce phloem extract amendments; however, their activity almost did not change in dual culture tests against blue-stain fungi. However, the radial growth rate of the L. fungicola strain was stimulated by SH extracts, and its antagonistic abilities against blue-stain fungi increased, with the exception of O. penicillatum (100%—inhibition did not change).
3. Discussion
Some recent publications concerning bacterial and fungal communities associated with the Ips typographus insect have considerably enhanced our understanding of these microbial ecosystems [62,63,64,65]. The bark beetle, its associated fungi, and fungal pathogen invasion are all actually related to the host’s defensive chemistry [66,67,68]. After infection of the host, fungi use a variety of strategies to gain access to the host’s nutrients [69]. Biotrophic fungi acquire nutrients directly from living cells by penetrating them and causing little visible damage [70]. Necrotrophic fungi, in contrast, produce enzymes and toxins to kill plant cells, and then the fungi feed on the nutrients from the dead tissue [71]. Additionally, potential antifungal defense compounds of the plant may contact with necrotrophs [47]. In the case of Norway spruce infestation with C. polonica, the plants act by increasing their total terpene content and accumulating monoterpenes with antifungal properties [45,47,61,72,73]. The correlation between terpene content and fungal resistance is strengthened by the data showing that trees that survive C. polonica inoculation have much higher terpene concentrations than trees killed by the fungus [73]. This can be explained by several reasons. If microorganisms can improve the fitness of pests by facilitating their nutrient uptake, they must be resistant to terpenes; if the microorganisms reduce the toxicity of host defence compounds (that is, they might be able to transform compounds to be less toxic), they must be resistant to chemical defence.
Our study is the first report concerning the antimicrobial activity of SH (composed mainly of trans-isomer) regarding blue-stain fungus and other microorganisms associated with bark beetle I. typographus and its development habitat (galleries created in the phloem). Antimicrobial activity of SH against some strains of microorganisms was detected; however, high SH concentrations (up to 500–1000 μg/disc or mL−1) were necessary in the broth agar disc diffusion and broth macro-dilution methods. SH was less effective than antibiotics, with a narrow spectrum (affecting only few microorganisms used in the present study as indicators for antimicrobial activity) and with small inhibition zones in the agar diffusion method. SH was found to have low toxicity in the agar diffusion method, whereas it showed high toxicity in the broth macro-dilution method. The reason for this may be that in the broth macro-dilution method, the fungal spores are in direct contact with dissolved SH molecules, which are able to exhibit a greater effect than in the agar diffusion method. As SH is insoluble in water, some re-crystallisation may occur in the medium prepared in water. It is well known that the agar diffusion method has its accuracy impaired as the hydrophobic nature of most EOs or their components prevents the uniform diffusion of these substances through agar medium, which may account for differences in the obtained results.
From the data obtained by the broth macro-dilution method in this study, it can be seen that SH has a significant influence on growth of microbial strains, which is in accordance with the published data regarding EOs containing appreciable amounts of SH [12,20,28,29,34,37,38,39,40].
B. subtilis was the most susceptible to all tested SH concentrations, while P. aeruginosa was the least susceptible—no SH concentration inhibited its growth. Our results indicated greater susceptibility on the part of the Gram-positive bacteria (B. subtilis and S. aureus), which has been also reported by other authors regarding EOs containing SH [12,20,36,38].
The weak antibacterial activity against Gram-negative bacteria (E. coli and P. aeruginosa) can be explained by the presence of an outer membrane. Hydrophilic polysaccharide chains act as a barrier to hydrophobic essential oils and do not allow them to enter and have antibacterial activity. In addition, the resistance of P. aeruginosa may be due to its ability to utilise terpenes for its growth [74], or it may be related to the possible resistance genes in plasmids, which can inactivate SH, having antimicrobial potential. High resistance of P. aeruginosa against essential oils is well documented [75,76].
An EO from Origanum majorana, containing appreciable amounts of both isomers of SH (25.18 and 5.44% of trans- and cis-, respectively) was assessed against S. aureus, and E. coli strains; MIC values of 0.125–0.250% and 30–61 µM were obtained, respectively [12]. In the efflux pump inhibitory assay, this EO exhibited substantial activity, especially for the E. coli strain. In the case of S. aureus strains, the EO and SH exhibited moderate potency on the drug-resistant phenotype. SH was found to be an effective inhibitor of biofilm formation (inhibition 36–86%) in E. coli and S. aureus, while the EO was ineffective on these strains. In contrast to this, biofilms formed by E. coli and S. aureus were significantly inhibited by the EO [12]. However, research on the antimicrobial properties of EOs commonly used as condiments in Brazil against Clostridium perfringens revealed an MIC = 5.0 mg/mL for marjoram EO with appreciable SH content [38].
Additionally, an antimicrobial activity of EOs with the most characteristic components trans-sabinene hydrate and terpinen-4-ol, obtained from several Origanum species cultivated in Poland, was evaluated against human respiratory pathogens, such as S. aureus, Haemophilus influenzae, H. parainfluenzae, and Pseudomonas aeruginosa [20]. O. majorana EO was the most active in the MIC assay and had the highest inhibitory rate in the anti-biofilm assay against all strains in the above study.
In another study [36], a well diffusion assay revealed that EOs from O. majorana and O. vulgare (containing 10.26 and 27.48% of cis-sabinene hydrate, respectively) were active against both the tested Gram-positive, viz., B. subtilis, Micrococcus luteus, and S. aureus; and Gram-negative, viz., E. coli, Klebsiella pneumoniae, and P. aeruginosa, bacteria. MIC values (v/v) indicated the highest efficacy of O. majorana EO against B. subtilis (0.5%), M. luteus (1%), and S. aureus (1%), while O. vulgare was most efficient against E. coli (2%) and K. pneumoniae (2%).
EOs from Tunisian A. absinthium L. were tested for their antibacterial and antifungal activity against 10 indicator microorganisms including seven pathogenic bacterial references (E. coli, S. typhimurium, S. aureus, P. aeruginosa, A. hydrophila, L. monocytogenes, and B. cereus), and against three fungus species (A. flavus, A. niger and C. albicans); and displayed antimicrobial activity against all tested strains with variable degrees. For Gram-negative bacterial strains, comparable levels of antibacterial activities were observed (MIC range from 12.5 to 25% v/v) for the oil containing of 11.83% (Z)-sabinene hydrate; the highest activity was recorded against the bacteria P. aeruginosa [39].
Fungi, especially filamentous fungi, were more resistant to SH than bacteria were. C. albicans was the most sensitive yeast, and A. niger as well as Trichoderma sp. were the most resistant fungi. Blue-stain fungus O. bicolor was the most susceptible fungus in both the disc diffusion and broth macro-dilution methods as compare to other blue-stain fungi. The pioneer Picea tree tissue invader C. polonica was more resistant to SH than O. bicolor and O. penicillatum. It was determined that spores from the blue-stain fungi were the most susceptible to SH. No inhibition was found in the growing mycelium. Conversely, some stimulatory effects such as increased radial growth and melanin synthesis were observed, especially in the case of O. penicillatum cultures (Figure 1).
SH induced changes in the morphology of blue-stain fungi: dominance of one stage with elimination of the other. It must be noted that blue-stain fungi and saprotrophic fungi were in a strong antagonistic relationship in this investigation. Moreover, saprotrophic fungi were also resistant to SH.
Fungal entomopathogens (Beauveria bassiana, (Bals.-Criv) Vuill. and Metarhizium anissopliae (Metschn.) Sorokin) were among the fungi distributed in I. typographus galleries; they were found on the larva cadavers. Thus, it was interesting to determine their relationship with blue-stain fungi in an in situ experiment. As was mentioned above, phytopathogens are well known to alter plant chemistry. However, even though several studies have considered the effects of plant chemistry on insect pathogens, knowledge on how phytopathogenic fungi may influence herbivores’ vulnerability to entomopathogens is almost completely lacking. Contradictory results in studies with fungal entomopathogens have been found [77,78,79]. It has been suggested that this might reflect differences in their dependence on host insects [80,81]. Entomopathogens should be considered important natural enemies of bark beetles, and their importance has been discussed regarding other herbivores [82].
In our study, two fungi, M. theobromae and P. cucumerina, isolated from the I. typographus galleries and their phloem and one strain from the genus Lecanicillium W. Gams & Zare [L. fungicola (Preaus) Zare & W. Gams], isolated from I. typographus cadavers, were resistant to SH and very active antagonists to blue-stain fungi when grown on standard MEA medium (Figure 4). Their antagonistic properties weakened when phloem extract medium was used for the investigation of the dual cultures, suggesting that they are not pioneer sapwood invaders and develop using partially degraded phloem and/or secondary metabolites from other fungi. Despite this, M. theobromae and P. cucumerina inhibited development of the blue-stain fungi and overgrew their mycelium and sporulation structures (Figure 4). Only a few studies have addressed the question of how a decrease in host plant suitability might affect the efficiency of entomopathogens. O. majorana EOs containing SH exhibited strong antifungal properties against rice seed-borne fungi, such as Bipolaris orzyae, Curvularia lunata, Fusarium verticilliodies, and F. graminearum [18]. Additionally, Mentha spicata EO (content of SH: 7.04%) showed strong antifungal activity to plant-pathogenic fungi, such as Fusarium oxysporum f. sp. radicis-lycopersici (Sacc.) W.C. Synder & H.N. Hans, Rhizoctonia solani J.G. Kuhn. Alternaria solani, and Verticillium dahliae Kleb; and against selected bacterial strains of Xanthomonas spp. [29].
In our investigation, the results of the paired cultures indicated the possibility that they are associated with bark beetle–plant–fungal pathogen systems. If they inhibit the growth of blue-stain fungi, which are hypothesized as its feeding source, they thus influence the development of insects indirectly through feeding. Some explanation could be found in an investigation conducted by Rostás, M. et al., in which the duration of larval development from hatching to pupation increases by about 9% when Phaedon cochleariae (Chrysomelidae) larvae feed upon fungus-infected Chinese cabbage leaves. Higher larva mortality under prolonged development was induced by the entomopathogenic fungus Metarhizium anisopliae (Metsch.) Sorok. [83]. I. typographus larvae feeding on healthy tree tissues could also survive entomopathogen attacks; while feeding on diseased tree tissues, this ability can be lost. Other factors must be also considered, such as, e.g., the induction of defensive plant compounds by the phytopathogen. The effects of plant allelochemicals influencing insect susceptibility to entomopathogens are known [83]. Artificial diets containing plant secondary metabolites may enhance insect mortality caused by entomopathogens. Thus, knowledge about I. typographus fungal pathogens is valuable too, and investigations could be continued in the future.
4. Materials and Methods
4.1. Sabinene Hydrate (Dissolution and Preparation for Bioassays)
A commercial synthetic SH (analytical standard, ≥97.0% (GC), composed of isomers, containing a predominant quantity of trans-sabinene hydrate, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) was used for the investigations. SH, being insoluble in water, was dissolved in ethanol (96%). Stock solutions of sabinene hydrate [10% (100 mg/mL) or 20% (200 mg/mL)] were prepared, from which half-and-half dilutions in a range of sabinene hydrate concentrations (6.25, 12.50, 25.00, 50.00, 100.00, and 200.0 mg/mL) were used for in vitro treatments against test organisms.
4.2. Test Organisms
The selected test organisms used to evaluate the antifungal and antibacterial activity of the sabinene hydrate were as follows:
- (a)
- blue-stain fungi: Ceratocystis polonica (Siem.) C. Moreau 1994-169/113, C. polonica BIGTC-2133, Ophiostoma bicolor Davidson & Wells BIGTC-2133, and O. penicillatum (Grosmann.) Siemaszko (syn. Grosmannia penicillata (Grosmann) Goid) 2006-209/44/2;
- (b)
- other mycelial fungi, which were isolated from Ips typographus Linnaeous (Coleoptera, Curculionidae, Scolytinae) galleries in Picea abies L. stemps and from its discolorated sapwood, such as Musicillium theobromae (Turconi) Zare & W. Gams (syn. Stachylidium theobromae Turconi, Verticillium theobromae E.W. Mason & S. Hughes BIGTC-20132 and Plectosphaerella cucumerina (Lindt.) W. Gams BIGTC-136, Aspergillus niger Tiegh. BIGTC-9823, Penicillium notatum Wehmer BIGTC-8914, and Trichoderma sp. BIGTC-2131;
- (c)
- yeasts Candida albicans BIGTC-MK2, C. parapsilosis BIGTC-MK9, C. krusei BIGTC-MK3 (Blastomycetes, Cryptococcales, Cryptococcaceae) and Saccharomyces cerevisiae BIGTC-MK11 (Saccharomycetes, Saccharomycetales, Saccharomycetaceae) phylogenetically related to the Ascomycota, as well as Rhodotorula muscilaginosa BIGTC-056 (Urediomycetes, Sporidiales) phylogenetically related to Basidiomycota;
- (d)
- Gram-positive bacteria Staphylococcus aureus BIGTC-BK06 and Bacillus subtilis BIGTC-BK09;
- (e)
- Gram-negative bacteria Escherichia coli BIGTC-BK08 and Pseudomonas aeruginosa BIGTC-BK19.
All cultures were obtained from the Microorganism Culture Collection of Nature Research Centre (Vilnius, Lithuania), except three strains, C. polonica 1994-169/113, O. bicolor, and G. penicillata 2006-209/44/2, which were received from the KTH Royal Institute of Technology, Stockholm, Sweden.
As reference compounds, chloramphenicol and nystatin-dihydrate were used for treatments with bacteria and fungi, respectively.
4.3. Preparation of Test Organisms
The cultures of test organisms were maintained in agar slants at 4 °C (bacteria in nutrient agar (NA), fungi in 2% malt extract agar (MEA), and yeasts in Sabourad agar (SA); all media were purchased from Liofilchem SRL, Roseto degli Abruzzi (TE), Italy). To obtain stock cultures, fungi were pre-cultured on 2% MEA at 25 °C for four weeks (Ophiostomatoid fungi) and 7 days (other fungi), yeasts—on SA for 3 days, and bacteria—on NA for 2 days. Fungal conidia were taken from the slants using sterile saline (0.9% NaCl, w/v) containing 0.05% Tween 80. Mycelia were removed by filtration through sterile gauze, and the filtrate was adjusted up to 1–2 × 105 conidia mL−1. Bacterial and yeast cells were picked from freshly grown slant cultures using 10 mL of sterile saline, and decimal cell solutions were prepared in the sterile saline up to 107 and 106 colony-forming units (CFU) mL−1 (bacteria and yeasts, respectively).
4.4. In Vitro Antifungal and Antibacterial Activity Testing
The CLSI (Clinical and Laboratory Standards Institute) method for antimicrobial susceptibility testing was modified for sabinene hydrate testing. We used two preliminary methods: the agar diffusion method for all strains of microorganisms and the disc volatilization method (vapor phase activity) for microbial strains selected as the most sensitive in the agar diffusion method. Additionally, direct SH activities (minimal inhibition concentrations, MICs) against bacteria and yeasts tested were detected by the micro-broth dilution method; additionally, SH direct activity against the fungi was determined. Activity of SH against spore (or conidium) germination was evaluated for bacterial and yeast strains. A standard antibiotics, nystatine-dihydrate (ROTH, Carl Roth GmbH + Co., Karlsruhe, Germany), was used to control the sensitivity of the tested fungi; chloramphenicol (ROTH, Carl Roth GmbH + Co., Germany) was applied to control the sensitivity of the tested bacteria. Concentrations were explored depending on the method used. Turbidity measurements of the medium were not performed.
4.4.1. Agar Disk Diffusion Method
The evaluation of SH activity against test strains of microorganisms was carried out by the disc diffusion method, which is normally used as a preliminary check and to select between different oils and their constituents. Solidified 2% MEA, SA, and NA (for fungi, yeasts, and bacteria, respectively) media were used for the SH activity treatments. Media were inoculated with 100 μL inoculums of appropriate microorganisms (107, 106, and 105 CFU mL−1 suspension concentrations of bacteria, yeasts, and fungi respectively) and were spread over the plates using a sterile rod display in order to obtain a uniform microbial growth on both control and test plates. After the inoculums’ absorption by agar, sterile filter discs (Whatman no 1, 5 mm diameter) were impregnated with 10 μL solutions of sabinene hydrate (at 6.25, 12.50, 25.00, 50.00, 100.00, and 200.00 mg/mL concentration in 96% ethanol). These preparations corresponded to SH concentrations of 0.0625, 0.125, 0.25, 0.50, 1.0, and 2.0 mg per disc, respectively. Filter discs moistened with ethanol alcohol were placed on the seeded Petri dish as a negative control. Filter discs impregnated with nystatine-dihydrate (30 mg/mL corresponding 30 μg/disc) for fungi and chloramphenicol (30 mg/mL, corresponding 30 μg/disc) for bacteria were used as a reference control.
All Petri dishes were sealed with sterile laboratory parafilm to avoid the eventual evaporation of SH. The dishes were left for 30 min at room temperature to allow the diffusion of oil, and then were incubated for 24 h for bacteria at 30 °C, for 48 h for yeasts, and 48–98 h for fungi at 25 °C. After the incubation period, the mean diameter of the inhibition halo where the test microorganisms did not grow (clearly visible inhibition zone) or the growth was partly inhibited was measured in millimeters.
4.4.2. Broth Macro-Dilution Tests
The minimal inhibitory concentration (MIC) values were determined for bacterial and yeast strains using the macro-well dilution method as described [84]. Bacterial strains were cultured for 18 h at 30 °C in Müller-Hinton broth (MHB, Liofilchim, Roseto Degli Abruzzi (TE), Italy); yeasts were cultured for 48 h at 25 °C in 2% malt extract broth (MEB, Liofilchem, Italy). Then, test cultures were suspended in sterile saline (0.9% NaCl) and diluted to produce a final density of 2.5 × 106 cfu mL−1 for the bacteria and 1.5 × 105 cfu mL−1 for the yeasts.
The 24-well plates were prepared by dispensing into each well 490 μL of appropriate nutrient broth. An aliquot (10 μL) of SH, initially prepared at a 10% concentration (100 mg/mL; w/v ethanol, was added to each of the first wells, followed by 2-fold serial dilution to obtain concentrations in a range from 1.0 to 0.0625 mg/mL w/v (corresponding from 0.1% to 0.000625% w/v concentrations). The prepared inoculums of the test cultures were separately added to the wells (500 μL each). The final volume in each well was 1 mL. The last well, containing 500 μL of MHB (or MEB) without test substance and 500 μL of the inoculum, was used as a negative control. Standard antibiotics, tested at concentrations ranging (chloramphenicol) from 50.0 μg/mL to 1.5625 μg/mL and (nystatine-dihydrate) from 30.0 μg/mL to 0.9375 μg/mL, were used as positive controls. Ethanol was not tested because the even highest concentration used in the plate well was equal 0.096 % (10 μL ethanol (96%) per 1 mL well content = 0.096%). Culture medium + test sample of the sabinene hydrate was used as the sterility control. The plates were covered with a sterile plate sealer and incubated at 30 °C (bacteria) and 25 °C (yeasts) for 18–24 h and 48 h, respectively. Growth of the bacteria and yeasts was indicated by the presence of turbidity and/or white fur at the bottom of the well. The minimum inhibition concentrations (MICs) were determined as the lowest concentration preventing visible growth. Additionally, viability was proven by making subcultures from 50 μL from each well showing no turbidity and negative control on nutrient agar (NA; Liofilchem, Italy) or SA medium. For each strain, the growth conditions were checked and plates were incubated as described above.
Fungal strains in the macro-broth dilution method were treated by some modifications of the method. Fungi spore inoculums, corresponding approximately 1.0–1.5 × 104 conidia mL−1, were prepared as stocks, from which 0.1 mL aliquots were transferred into each plate well containing either 2% MB medium or 2% MB amended with an appropriate sabinene hydrate concentration. Plates were incubated for 4–8 h, and then 100 mL−1 of each well content was sub-cultured for 24–48 h on 2% MEA medium (three replicate plates were used per well). After incubation, the number of growing colonies in the control and SH treated variants was counted. Growth inhibition percentage was calculated by comparing the number of CFUs in the SH-treated culture with that of the control. Spores which did not form colonies were considered as having lost their viability. Microscopic observations were made to determine growth (germination and germ tube length) retardation under SH impact.
4.4.3. Disc Volatilization Method (Vapor Phase Activity)
This method was used to evaluate the activity of SH vapor from impregnated paper discs against the blue-stain fungal strains isolated from P. abies sapwood and two fungal strains associated with I. typographus galleries, the same strains which were used in the disc diffusion method. Agar plugs of actively growing fungi (blue-stain fungi after 2 weeks and other fungi after 7 days’ growth on the 2% MEA) were placed in the center of the Petri dish (covered with 2% MEA). Different aliquots (10 μL, 20 μL, and 30 μL) of the SH solution were added to 6 mm sterile blank paper discs placed in the center of the cover of the glass Petri dish (6 cm diameter). The dishes were sealed with laboratory parafilm to avoid eventual evaporation of SH followed by incubation. The volume of the dish in which SH vapor was distributed was 9 cm3. Blanks were prepared by adding 10 μL of ethanol alcohol to the paper discs. Practically, vapor from either of the SH contents (1 g, 2 g, or 3 g per dish) evaporated into the dish atmosphere above the disc. The effectiveness of SH was calculated as a percentage inhibition of the linear fungus growth (in mm) compare to the control (disc with appropriate content of water). Each assay in these experiments was repeated three times and the results (inhibition %) were expressed as average values ± SD (standard deviation).
4.4.4. Dual Culture Tests
Agar plugs (5 mm diameter) from two actively growing test fungi (blue-stain fungi after 2 weeks and other fungi after 7 days growth on the MEA medium were placed at the different ends of the Petri dish dishes, covered with either of MEA medium or MEA amended with spruce phloem-extract (MEA + SPE)). Dishes were incubated at 25 °C in the dark. The radial growth of mycelium (in mm) was measured daily and compared with that in the MEA and MEA + SPE plates simultaneously inoculated with each fungus only as control.
Percent of test fungus inhibition by the other fungi was evaluated and expressed as calculated percent inhibition (PI):
where C is the growth of the test fungus (mm) in the absence of the other fungi; T is the growth of the test fungus (mm) in the presence of the opposite fungus.
4.5. Statistical Analysis
The obtained data were statistically processed and expressed as means and standard deviation (SD) values, using the XLSTAT program (trial version, Addinsoft 2014, Paris, France). Convergence of results was based on at least three independent measurements. Evaluation of statistically significant differences between tested parameters was performed by one-way ANOVA statistical analysis. Differences between obtained values were compared using Student’s t-test, at a significance level of α = 0.05. IBM SPSS Statistics software (Version 28.0.1.1(15), New York, NY, USA) was applied to calculate the p-values. Statistically significant differences were set at p values lower than 0.05.
5. Conclusions
The current study has made a contribution to knowledge regarding the in situ antimicrobial properties of sabinene hydrate (SH), which is a quite common secondary metabolite in plants. For the first time, the effects of SH (composed mainly of trans-isomer) were revealed against the following microorganisms: blue-stain fungi (Ceratocystis polonica, Ophiostoma bicolor, O. penicillatum), frequently originating from bark beetle galleries; three fungal strains (Musicillium theobromae, Plectosphaerella cucumerina, and Trichoderma sp.), isolated from sapwood under bark beetle galleries (Ips typographus) on spruce (Picea abies) stems; and Verticillium fungicola, isolated from diseased I. typographus larvae. Additionally, monoterpenoid activity was tested and compared against two saprophytic fungi (Aspergillus niger and Penicillium notatum), two Gram-positive (Bacillus subtilis and Staphylococcus aureus), and two Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa); and five yeasts (Candida albicans, C. krusei, C. parapsilosis, Saccharomyces cerevisiae, and Rhodotorula muscilaginosa). The antimicrobial effects were evaluated over a range of SH concentrations in three types of contact tests, namely solid and vapor diffusion and the macro-broth dilution method. SH was less effective than antibiotics, such as chloramphenicol and nystatin-dihydrate, in the agar diffusion method. SH was found to have low toxicity in the agar diffusion method against the micro-organisms tested, whereas it showed a high toxicity in the broth macro-dilution method. In solid agar disc diffusion tests, Gram-positive bacteria were more susceptible to SH than Gram-negative bacteria, followed by yeasts and fungi. The most resistant to SH in both the disc diffusion and broth macro-dilution methods were P. aeruginosa, A. niger, and Trichoderma sp. strains. Blue-stain fungi and fungi isolated from the Picea sapwood were the most resistant among the fungal strains tested. The values of the minimum inhibition concentrations generated by SH and determined using the disc volatilization method depended on the tested fungus species. SH showed a more or less important role in the development of microbial inhibition. The Gram-positive bacteria B. subtilis and S. aureus were the organisms most susceptible to SH, followed by Gram-negative bacterium, E. coli and two yeasts, C. albicans and C. kruei. The strains of C. parapsilosis were the yeasts most resistant to SH. Further research may be focused on evaluating the bioactivity of SH against other pathogenic microorganisms and/or investigation of antimicrobial properties of another isomer (cis-) of this monoterpenoid.
The investigation of antimicrobial properties of plant secondary metabolites is important for the development of a new generation of fungicides. In general, this study has provided new comprehension regarding the application of SH as a natural product derived from plants. The use of renewable products offers great prospects for more economical, environmentally friendly, and effective development of industry and agriculture.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29174252/s1, Figure S1: Ceratocystis polonica: (a) colonies after 14 days’ growth on 2% MEA medium; (b,d) synnemata in culture; (c,e,f) synnemata and conidia in preparates; (g) micro ascus. Scales are indicated in pictures. Figure S2: Ophiostoma penicillatum (syn. Grosmannia penicillata): (a–c) colonies after 14 days’ growth on 2% MEA, PDA and SA media, respectively; (d) synnemata in culture; (e) conidiogenesis in culture; (f) synnemata in culture; (g,h) Leptographium state; (a–c) scales are 10 μm; other scales are 100 μm. Figure S3: Musicillium theobromae: (a) colonies after 10 days’ growth on 2% MEA; (b–d) conidiophores and conidial masses in culture; (e,f) conidiophores and conidial masses (heads) in preparates; (g) aggregates from mycelium in culture; (h,i) conidiophores and conidia. Scales are indicated in pictures. Figure S4: Plectosphaerella cucumerina: (a) colonies after 10 days’ growth on 2% MEA; (b–d) hyphae, conidiophores, and conidia in culture; (e,f) conidiophores and conidia in preparates. Scales are indicated in pictures. Figure S5: Ceratocystis polonica colonies after 6 days’ growth on: (a) MEA medium, (b) mineral medium without glucose and with phloem extract medium, and (c) mineral medium with phloem. Ophiostoma penicillatum (syn. Grosmannia penicillata) colonies after 6 days’ growth on: (d) MEA medium, (e) mineral medium without glucose and with phloem extract medium, and (f) mineral medium with phloem.
Author Contributions
Conceptualization, A.J. and I.N.; methodology, D.P.; investigation, D.P.; resources, A.J.; data curation, A.J. and I.N.; writing—original draft preparation, A.J.; writing—review and editing, A.J.; supervision, A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Biosynthesis of essential oils in aromatic plants: A review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- Hallachan, T.W.; Croteau, R. Monoterpene biosynthesis: Demonstration of a geranyl pyrophosphate: Sabinene hydrate cyclase in soluble enzyme preparations from sweet marjoram (Majorana hortensis). Arch. Biochem. Biophys. 1988, 264, 618–631. [Google Scholar] [CrossRef]
- Arsenijević, J.; Marković, J.; Šoštarić, I.; Ražić, S. A chemometrics as a powerful tool in the elucidation of the role of metals in the biosynthesis of volatile organic compounds in Hungarian thyme samples. Plant Physiol. Biochem. 2013, 71, 298–306. [Google Scholar] [CrossRef]
- Krause, S.T.; Köllner, T.G.; Asbach, J.; Degenhardt, J. Stereochemical mechanism of two sabinene hydrate synthases forming antipodal monoterpenes in thyme (Thymus vulgaris). Arch. Biochem. Biophys. 2013, 529, 112–121. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile compounds in Citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Hernández, M.G.; Sánchez-Bravo, P.; Hernández, F.; Carbonell-Barrachina, Á.A.; Pastor-Pérez, J.J.; Legua, P. Determination of the volatile profile of lemon peel oils as affected by rootstock. Foods 2020, 9, 241. [Google Scholar] [CrossRef]
- Flamini, G.; Najar, B.; Leonardi, M.; Ambryszewska, K.E.; Cioni, P.L.; Parri, F.; Melai, B.; Pistelli, L. Essential oil composition of Salvia rosmarinus Spenn. wild samples collected from six sites and different seasonal periods in Elba Island (Tuscan Archipelago, Italy). Nat. Prod. Res. 2020, 36, 1919–1925. [Google Scholar] [CrossRef]
- Matulyte, I.; Marksa, M.; Ivanauskas, L.; Kalvėnienė, Z.; Lazauskas, R.; Bernatonienė, J. GC-MS analysis of the composition of the extracts and essential oil from Myristica fragrans seeds using magnesium aluminometasilicate as excipient. Molecules 2019, 24, 1062. [Google Scholar] [CrossRef]
- Adiani, V.; Gupta, S.; Chatterjee, S.; Variyar, P.S.; Sharma, A. Activity guided characterization of antioxidant components from essential oil of Nutmeg (Myristica fragrans). J. Food Sci. Technol. 2013, 52, 221–230. [Google Scholar] [CrossRef]
- Kapoor, I.P.S.; Singh, B.; Singh, G.; De Heluani, C.S.; De Lampasona, M.P.; Catalan, C.A.N. Chemical composition and antioxidant activity of essential oil and oleoresins of nutmeg (Myristica fragrans Houtt.) fruits. Int. J. Food Prop. 2013, 16, 1059–1070. [Google Scholar] [CrossRef]
- Danin, A.; Ravid, U.; Umano, K.; Shibamoto, T. Essential oil composition of Origanum ramonense Danin leaves from Israel. J. Essent. Oil Res. 1997, 9, 411–417. [Google Scholar] [CrossRef]
- Ghazal, T.S.A.; Schelz, Z.; Vidács, L.; Szemerédi, N.; Veres, K.; Spengler, G.; Hohmann, J. Antimicrobial, multidrug resistance reversal and biofilm formation inhibitory effect of Origanum majorana extracts, essential oil and monoterpenes. Plants 2022, 11, 1432. [Google Scholar] [CrossRef] [PubMed]
- Arafat, K.; Al-Azawi, A.M.; Sulaiman, S.; Attoub, S. Exploring the anticancer potential of Origanum majorana essential oil monoterpenes alone and in combination against non-small cell lung cancer. Nutrients 2023, 15, 5010. [Google Scholar] [CrossRef] [PubMed]
- Dudai, N.; Larkov, O.; Chaimovitsh, D.; Lewinsohn, E.; Freiman, L.; Ravid, U. Essential oil compounds of Origanum dayi Post. Flavour. Fragr. J. 2003, 18, 334–337. [Google Scholar] [CrossRef]
- Tabanca, N.; Özek, T.; Baser, K.H.C. Comparison of the essential oils of Origanum majorana L. and Origanum x majoricum Cambess. J. Essent. Oil Res. 2004, 16, 248–252. [Google Scholar] [CrossRef]
- Raina, A.P.; Negi, K.S. Essential oil composition of Origanum majorana and Origanum vulgare ssp. hirtum growing in India. Chem. Nat. Compd. 2012, 47, 1015–1017. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef]
- Mohamed, A.A.; El-Hefny, M.; El-Shanhorey, N.A.; Ali, H.M. Foliar application of bio-stimulants enhancing the production and the toxicity of Origanum majorana essential oils against four rice seed-borne fungi. Molecules 2020, 25, 2363. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and antibacterial capacities of Origanum vulgare L. essential oil from the Arid Andean region of Chile and its chemical characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Piasecki, B.; Balázs, V.L.; Kieltyka-Dadasiewicz, A.; Szabó, P.; Kocsis, B.; Horváth, G.; Ludwiczuk, A. Microbiological studies on the influence of essential oils from several Origanum species on respiratory pathogens. Molecules 2023, 28, 3044. [Google Scholar] [CrossRef]
- Kandoudi, W.; Radácsi, P.; Gosztola, B.; Zámboriné Németh, É. Elicitation of medicinal plants in vivo—Is it a realistic tool? The effect of methyl jasmonate and salicylic acid on Lamiaceae species. Horticulturae 2022, 8, 5. [Google Scholar] [CrossRef]
- Farouk, A.; Mohsen, M.; Ali, H.; Shaaban, H.; Albaridi, N. Antioxidant activity and molecular docking study of volatile constituents from different aromatic Lamiaceous plants cultivated in Madinah Monawara, Saudi Arabia. Molecules 2021, 26, 4145. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential oil characterization of Thymus vulgaris from various geographical locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Bumbulytė, G.; Būdienė, J.; Būda, V. Essential oils and their components control behaviour of yellow mealworm (Tenebrio molitor) larvae. Insects 2023, 14, 636. [Google Scholar] [CrossRef]
- Groendahl, E.; Ehlers, B.K.; Keefover-Ring, E. A new cis-sabinene hydrate chemotype detected in large thyme Thymus pulegioides L. growing wild in Denmark. J. Essent. Oil Res. 2008, 20, 40–41. [Google Scholar] [CrossRef]
- Kim, S.-W.; Lee, H.-R.; Jang, M.-J.; Jung, C.-S.; Park, I.-K. Fumigant toxicity of Lamiaceae plant essential oils and blends of their constituents against adult rice weevil Sitophilus oryzae. Molecules 2016, 21, 361. [Google Scholar] [CrossRef]
- Trendafilova, A.; Todorova, M.; Ivanova, V.; Zhelev, P.; Aneva, I. Essential oil composition of ten species from sect. Serpyllum of genus Thymus growing in Bulgaria. Diversity 2023, 15, 759. [Google Scholar] [CrossRef]
- Wiese, N.; Fischer, J.; Heidler, J.; Lewkowski, O.; Degenhardt, J.; Erler, S. The terpenes of leaves, pollen, and nectar of thyme (Thymus vulgaris) inhibit growth of bee disease-associated microbes. Sci. Rep. 2018, 8, 14634. [Google Scholar] [CrossRef]
- Bayan, Y.; Küsek, M. Chemical composition and antifungal and antibacterial activity of Mentha spicata L. volatile oil. Cien. Inv. Agr. 2018, 45, 64–69. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, L.B.S.; Ahmad, J.; Dubey, N.; Puri, S. Chemical composition of commercial Juniperus communis L. leaf oil. J. Essent. Oil Bear. Plants 2013, 10, 310–313. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Jeliazkova, E.A.; Tatman, A.O.; Schlegel, V. Distillation time alters essential oil yield, composition and antioxidant activity of female Juniperus scopulorum trees. J. Essent. Oil Res. 2013, 25, 62–69. [Google Scholar] [CrossRef]
- Cornwell, C.P.; Leach, D.N.; Wyllie, S.W. The origin of terpinen-4-ol in the steam distillates of Melaleuca argentea, M. dissitiflora and M. linariifolia. J. Essent. Oil Res. 1999, 11, 49–53. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Rathore, S.; Mukhia, S.; Kumar, R.; Kumar, R. Essential oil composition and antimicrobial potential of aromatic plants grown in the mid-hill conditions of the Western Himalayas. Sci. Rep. 2023, 13, 4878. [Google Scholar] [CrossRef]
- Santoyo, S.; Cavero, S.; Jaime, L.; Ibañez, E.; Señoráns, F.J.; Reglero, G. Supercritical carbon dioxide extraction of compounds with antimicrobial activity from Origanum vulgare L.: Determination of optimal extraction parameters. J. Food Prot. 2006, 69, 369–375. [Google Scholar] [CrossRef]
- Radaelli, M.; da Silvaa, B.P.; Weidlich, L.; Hoehne, L.; Flach, A.; Mendonc, L.A.; da Costa, A.; Ethur, E.M. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens. Braz. J. Microbiol. 2016, 47, 424–430. [Google Scholar] [CrossRef]
- Riahi, L.; Ghazghazi, H.; Ayari, B.; Aouadhi, C.; Klay, I.; Chograni, H.; Cherif, A.; Zoghlami, N. Effect of environmental conditions on chemical polymorphism and biological activities among Artemisia absinthium L. essential oil provenances grown in Tunisia. Ind. Crops Prod. 2015, 66, 96–102. [Google Scholar] [CrossRef]
- Paudel, P.N.; Satyal, P.; Satyal, R.; Setzer, W.N.; Gyawali, R. Chemical composition, enantiomeric distribution, antimicrobial and antioxidant activities of Origanum majorana L. essential oil from Nepal. Molecules 2022, 27, 6136. [Google Scholar] [CrossRef]
- Semenzato, G.; Del Duca, S.; Vassallo, A.; Zaccaroni, M.; Mucci, N.; Greco, C.; Padula, A.; Castronovo, L.M.; Chioccioli, S.; Pistelli, L.; et al. Exploring the nexus between the composition of essential oil and the bacterial phytobiome associated with different compartments of the medicinal plants Origanum vulgare ssp. vulgare, O. vulgare ssp. hirtum, and O. heracleot. Ind. Crops Prod. 2023, 191, 115997. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Ishangulyyeva, G.; Erbilgin, N.; Bonello, P. Terpenoids are involved in the expression of systemic-induced resistance in Austrian pine. Plant Cell Environ. 2024, 47, 2206–2227. [Google Scholar] [CrossRef]
- Rahmani, R.; Hedenström, E.; Schroeder, M. SPME collection and GC-MS analysis of volatiles emitted during the attack of male Polygraphus poligraphus (Coleoptera, Curcolionidae) on Norway spruce. Z. Für Naturforschung C 2015, 70, 265–273. [Google Scholar] [CrossRef]
- Toffolatti, L.; Maddalena, G.; Passera, A.; Casati, P.; Bianco, P.A.; Quaglino, F. 16-Role of terpenes in plant defense to biotic stress. In Biocontrol Agents and Secondary Metabolites; Applications and Immunization for Plant Growth and Protection; Woodhead publishing: Sawston, UK, 2021; pp. 401–417. [Google Scholar] [CrossRef]
- Zhao, T.; Krokene, P.; Björklund, N.; Långström, B.O.; Solheim, H.; Christiansen, E.; Borg-Karlson, A.-K. The influence of Ceratocystis polonica inoculation and methyl jasmonate application on terpene chemistry of Norway spruce, Picea abies. Phytochemistry 2010, 71, 1332–1341. [Google Scholar] [CrossRef]
- Wadke, N.; Kandasamy, D.; Vogel, H.; Lah, L.; Wingfield, B.D.; Paetz, C.; Wright, L.P.; Gershenzon, J.; Hammerbacher, A. The bark-beetle-associated fungus, Endoconidiophora polonica, utilizes the phenolic defence compounds of its host as a carbon source. Plant Physiol. 2016, 171, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Hammerbacher, A.; Schmidt, A.; Wadke, N.; Wright, L.P.; Schneider, B.; Joerg Bohlmann, J.; Brand, W.A.; Fenning, T.M.; Gershenzon, J.; Paetz, C. A common fungal associate of the spruce bark beetle metabolizes the stilbene defenses of Norway spruce. Plant Physiol. 2013, 162, 1324–1336. [Google Scholar] [CrossRef]
- Zhao, T.; Kandasamy, D.; Krokene, P.; Chen, J.; Gershenzon, J.; Hammerbacher, A. Fungal associates of the tree-killing bark beetle, Ips typographus, vary in virulence, ability to degrade conifer phenolics and influence bark beetle tunneling behavior. Fungal Ecol. 2019, 38, 71–79. [Google Scholar] [CrossRef]
- Kandasamy, D.; Zaman, R.; Nakamura, Y.; Zhao, T.; Hartmann, H.; Andersson, M.N.; Hammerbacher, A.; Gershenzon, J. Conifer-killing bark beetles locate fungal symbionts by detecting volatile fungal metabolites of host tree resin monoterpenes. PLoS Biol. 2023, 21, e3001887. [Google Scholar] [CrossRef]
- Lehmanski, L.M.A.; Kandasamy, D.; Andersson, M.N.; Netherer, S.; Alves, E.G.; Huang, J.; Hartmann, H. Addressing a century-old hypothesis–do pioneer beetles of Ips typographus use volatile cues to find suitable host trees? New Phytol. 2023, 238, 1762–1770. [Google Scholar] [CrossRef]
- Blažytė-Čereškienė, L.; Apšegaitė, V.; Radžiutė, S.; Mozūraitis, R.; Būda, V.; Pečiulytė, D. Electrophysiological and behavioural responses of Ips typographus (L.) to trans-4-thujanol—A host tree volatile compound. Ann. For. Sci. 2016, 73, 247–256. [Google Scholar] [CrossRef]
- Schiebe, C.; Unelius, C.R.; Ganji, S.; Binyameen, M.; Birgersson, G.; Schlyter, F. Styrene, (+)-trans-(1R,4S,5S)-4-thujanol and oxygenated monoterpenes related to host stress elicit strong electrophysiological responses in the bark beetle Ips typographus. J. Chem. Ecol. 2019, 45, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Jirošová, A.; Kalinová, B.; Modlinger, R.; Jakuš, R.; Unelius, C.R.; Blaženec, M.; Schlyter, F. Anti-attractant activity of (+)-trans-4-thujanol for Eurasian spruce bark beetle typographus: Novel potency for females. Pest Manag. Sci. 2022, 78, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Trubin, A.; Mezei, P.; Afratakhti, K.Z.; Surovy, P.; Jakuš, R. Northernmost European spruce bark beetle Ips typographus outbreak: Modelling tree mortality using remote sensing and climate data. For. Ecol. Manag. 2022, 505, 119829. [Google Scholar] [CrossRef]
- Baños-Quintana, A.P.; Gershenzon, J.; Kaltenpoth, M. The Eurasian spruce bark beetle Ips typographus shapes the microbial communities of its offspring and the gallery environment. Front. Microbiol. 2024, 15, 1367127. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Wingfield, M.J.; Takahashi, I.; Solheim, H. Ophiostomatoid fungi associated with the spruce bark beetle Ips typographus f. aponicus in Japan. Mycol. Res. 1997, 1001, 1215–1227. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Takahashi, I.; Iguchi, K. Virulence of ophiostomatoid fungi associated with the spruce bark beetle Ips typographus f. japonicus in Yezo spruce. J. For. Res. 2000, 5, 87–94. [Google Scholar] [CrossRef]
- Kirisits, T. Fungal associates of European bark beetles with special emphasis on the opiostomatoid fungi. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Gregoire, J.-C., Evans, H.F., Eds.; Kluwer, Acad: Dordrecht, The Netherlands, 2004; pp. 181–236. [Google Scholar] [CrossRef]
- Bidart-Bouzat, M.G.; Imeh-Nathaniel, A. Global change effects on plant chemical dfenses against insect herbivores. J. Integr. Plant Biol. 2008, 50, 1339–1354. [Google Scholar] [CrossRef]
- Kurz, W.A.; Dymond, C.C.; Stinson, G.; Rampley, G.J.; Neilson, E.T.; Carroll, A.L.; Ebata, T.; Safranyik, L. Mountain pine beetle and forest carbon feedback to climate change. Nature 2008, 452, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Krokene, P. Chapter 5—Conifer defense and resistance to bark beetles. In Bark Beetles; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015; pp. 177–207. [Google Scholar] [CrossRef]
- Fang, J.X.; Zhang, S.F.; Liu, F.; Zhang, X.; Zhang, F.B.; Guo, X.B.; Zhang, Z.; Zhang, Q.H.; Kong, X.B. Differences in gut bacterial communities of Ips typographus (Coleoptera: Curculionidae) induced by enantiomer-specific alpha-pinene. Environ. Entomol. 2020, 49, 1198–1205. [Google Scholar] [CrossRef]
- Chakraborty, A.; Purohit, A.; Khara, A.; Modlinger, R.; Roy, A. Life-stage and geographic location determine the microbial assemblage in Eurasian spruce bark beetle, Ips typographus L. (Coleoptera: Curculionidae). Front. Forest. Glob. Chang. 2023, 6, 1176160. [Google Scholar] [CrossRef]
- Moussa, A.; Nones, S.; Vannucchi, P.E.; Shahzad, G.I.R.; Dittmer, J.; Corretto, E.; Schebeck, M.; Faccoli, M.; Battisti, A.; Stauffer, C.; et al. The bacterial community of the European spruce bark beetle in space and time. bioRxiv 2023. [Google Scholar] [CrossRef]
- Veselská, T.; Švec, K.; Kostovčík, M.; Peral-Aranega, E.; Garcia-Fraile, P.; Křížková, B.; Havlíček, V.; Saati-Santamaría, Z.; Kolařík, M. Proportions of taxa belonging to the gut core microbiome change throughout the life cycle and season of the bark beetle Ips typographus. FEMS Microbiol. Ecol. 2023, 99, fiad072. [Google Scholar] [CrossRef] [PubMed]
- Wermelinger, B. Ecology and management of the spruce bark beetle Ips typographus–a review of recent research. For. Ecol. Manag. 2004, 2002, 67–82. [Google Scholar] [CrossRef]
- Netherer, S.; Kandasamy, D.; Jirosová, A.; Kalinová, B.; Schebeck, M.; Schlyter, F. Interactions among Norway spruce, the bark beetle Ips typographus and its fungal symbionts in times of drought. J. Pest Sci. 2021, 94, 591–614. [Google Scholar] [CrossRef]
- Tanin, S.M.; Kandasamy, D.; Krokene, P. Fungal interactions and host tree preferences in the spruce bark beetle Ips typographus. Front. Microbiol. 2021, 12, 695167. [Google Scholar] [CrossRef]
- Johns, L.E.; Goldman, G.H.; Ries, L.N.A.; Brown, N.A. Nutrient sensing and acquisition in fungi: Mechanisms promoting pathogenesis in plant and human hosts. Fung. Biol. Rev. 2021, 36, 1–14. [Google Scholar] [CrossRef]
- Fei, W.; Liu, Y. Biotrophic fungal pathogens: A critical overview. Appl. Biochem. Biotechnol. 2023, 195, 1–16. [Google Scholar] [CrossRef]
- Liao, C.-J.; Hailemariam, S.; Sharon, A.; Mengiste, T. Pathogenic strategies and immune mechanisms to necrotrophs: Differences and similarities to biotrophs and hemibiotrophs. Curr. Opin. Plant Biol. 2022, 69, 102291. [Google Scholar] [CrossRef]
- Zeneli, G.; Krokene, P.; Chistiansen, E.; Krekling, T.; Gershenzon, J. Methyl jasmonate treatment of mature Norway spruce (Picea abies) trees increases the accumulation of terpenoid resin components and protects against infection by Ceratocystis polonica, a bark beetle-associated fungus. Tree Physiol. 2006, 26, 977–988. [Google Scholar] [CrossRef]
- Novak, M.; Urbanek Krajnc, A.; Lah, L.; Zupanec, N.; Kraševec, N.; Križman, M.; Bohlmann, J.; Komel, R. Low-density Ceratocystis polonica inoculation of Norway spruce (Picea abies) triggers accumulation of monoterpenes with antifungal properties. Eur. J. Forest Res. 2014, 133, 573–583. [Google Scholar] [CrossRef]
- Förster-Fromme, K.; Höschle, B.; Mack, C.; Bott, M.; Armbruster, W.; Jendrossek, D. Identification of genes and proteins necessary for catabolism of acyclic terpenes and leucine/isovalerate in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2006, 72, 4819–4828. [Google Scholar] [CrossRef] [PubMed]
- Van, L.T.; Hagiu, I.; Popovici, A.; Marinescu, F.; Gheorghe, I.; Curutiu, C.; Ditu, L.M.; Holban, A.-M.; Sesan, T.E.; Lazar, V. Antimicrobial efficiency of some essential oils in antibiotic-resistant Pseudomonas aeruginosa isolates. Plants 2022, 11, 2003. [Google Scholar] [CrossRef]
- Iseppi, R.; Mariani, M.; Condò, C.; Sabia, C.; Messi, P. Essential Oils: A natural weapon against antibiotic-resistant bacteria responsible for nosocomial infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef]
- Deka, B.; Baruah, C.; Babu, A. Entomopathogenic microorganisms: Their role in insect pest management. Egypt. J. Biol. Pest Control 2021, 31, 121. [Google Scholar] [CrossRef]
- Altinok, H.H.; Altinok, M.A.; Koca, A.S. Modes of action of entomopathogenic fungi. Curr. Trends Nat. Sci. 2019, 8, 117–124. Available online: http://natsci.upit.ro (accessed on 18 June 2024).
- Bugti, G.A.; Bin, W.; Memon, S.A.; Khaliq, G.; Jaffar, M.A. Entomopathogenic fungi: Factors involved in successful microbial control of insect pests. J. Entomol. 2020, 17, 74–83. Available online: https://scialert.net/abstract/?doi=je.2020.74.83 (accessed on 8 July 2024). [CrossRef]
- Qu, S.; Wang, S. Interaction of entomopathogenic fungi with the host immune system. Dev. Comp. Immunol. 2018, 83, 96–103. [Google Scholar] [CrossRef]
- Davis, T.S.; Stewart, J.E.; Mann, A.; Bradley, C.; Hofstetter, R.W. Evidence for multiple ecological roles of Leptographium abietinum, a symbiotic fungus associated with the north American spruce beetle. Fungal Ecol. 2019, 38, 62–70. [Google Scholar] [CrossRef]
- Elliot, S.L.; Sabelis, M.W.; Janssen, A.; Van Der Geest, L.P.S.; Beerling, E.A.M.; Fransen, J. Can plants use entomopathogens as bodyguards? Ecol. Lett. 2000, 3, 228–235. [Google Scholar] [CrossRef]
- Rostás, M.; Simon, M.; Hilker, M. Ecological cross-effects of induced plant responses towards herbivores and phytopathogenic fungi. Basic Appl. Ecol. 2003, 4, 43–62. [Google Scholar] [CrossRef]
- Zgoda, J.P.; Porter, J.R. A convenient microdilution method for screening natural products against bacteria and fungi. Pharm. Biol. 2001, 39, 221–225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).