Abstract
The reaction of indigo with two equivalents of the electrophile ethyl bromoacetate with caesium carbonate as a base result in the formation of structurally complex polyheterocyclics, including a fused spiroimidazole and a spiro[1,3]oxazino derivative, together with a biindigoid-type derivative, through a convenient one-pot reaction. Further assessment of the reaction using five equivalents of the electrophile gave rise to other molecules incorporating the 2-(7,13,14-trioxo-6,7,13,14-tetrahydropyrazino[1,2-a:4,3-a′]diindol-6-yl) scaffold. The reaction of ethyl bromoacetate with the less reactive indirubin resulted in the synthesis of three derivatives of a new class of polyheterocyclic system via a cascade process, although yields were low. These compounds were derived from the parent indolo[1,2-b]pyrrolo[4,3,2-de]isoquinoline skeleton. Despite the modest yields of the reactions, they represent quick cascade routes to a variety of heterocycles from cheap starting materials, with these structures otherwise being difficult to synthesise in a traditional stepwise manner. These outcomes also contribute significantly to the detailed understanding of the indigo/indirubin cascade reaction pathways initiated by base-catalysed N-alkylation.
1. Introduction
Indigo 1 (Figure 1) is a natural dye with a rich history dating back thousands of years [1]. Significant research interest continues in indigo and derivatives not only as colourants or dyes [2] but increasingly in fundamental photophysics [3,4,5] as well as chemical aspects [1,6,7]. Indigo has recently been the subject of great interest in synthetic chemistry due to its aromatic core, which possesses a number of functional groups in close proximity, thus offering a versatile platform for designing cascade reactions. This understanding arises from previous studies that have shown indigo 1 participating in cascade processes with a variety of functionalised electrophiles, yielding unprecedented polyheterocyclic systems [8,9]. For example, the reaction of indigo 1 with allyl halides yielded the 8′H-spiro[indoline-2,9′-pyrido[1,2-a]indol]-3-one 2 and its Claisen rearranged derivative 8′H-spiro[indoline-2,9′-pyrido[1,2-a]indole]-3,10′-(9a′H)dione 3. Alternatively, the reaction with bioxirane gave rise to the spiro compounds 4 and 5 (Figure 1) [10].
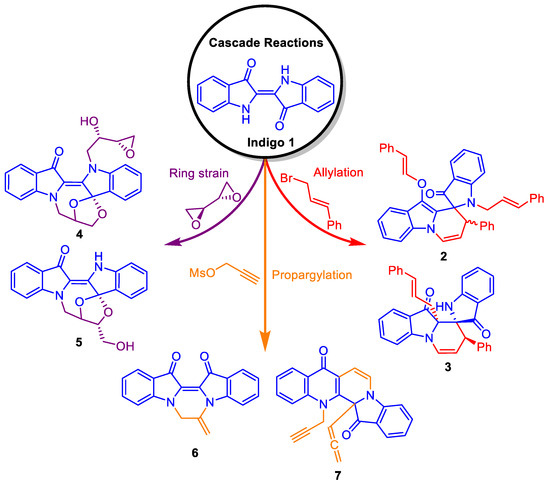
Figure 1.
Examples of polyheterocyclic systems arising from cascade reactions of indigo 1.
Reactions of indigo 1 with different propargyl derivatives yielded a variety of products, including straightforward addition-cyclisation products, e.g., 6, as well as cascade-induced products such as the ring-expanded heterocycle 7 (Figure 1) [8]. Further, by altering the leaving group and terminal substitution in the propargyl electrophiles, a variety of diverse outcomes were achieved, with the formation of naphthyridiones (e.g., 9), pyrazinodiindolodiones (e.g., 10 and 12), azepinodiindoles (e.g., 11), and other intriguing heterocyclic systems (Scheme 1) [11]. Further, it was revealed that the reaction of indigo 1 with bromoacetonitrile 13 produced the mono-N-alkylated indigo 14 along with the cyclised compound 15, both in low yield (Scheme 1). This last outcome was interesting as the strongly electron-withdrawing cyano moiety was expected to facilitate anion formation at the adjacent methylene carbon in alkylated intermediates, with subsequent cascade cyclisation reactions producing new polycyclic derivatives.
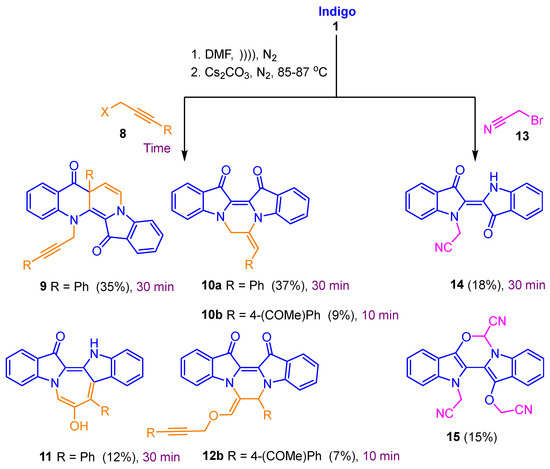
Scheme 1.
Cascade reaction products from the reaction of indigo with X-CH2C≡C-R (X = Br, Cl, OMs or OTs) compared with those from Br-CH2CN. Reaction times are presented in purple.
The outcomes of these reactions have demonstrated an ability to swiftly access structurally diverse heterocycles, yielding a plethora of new chemical compounds with a possible wide range of applications. They also show that the use of an electrophile containing an electron-withdrawing substituent had a significant effect on the outcome of cascade reactions of indigo 1. One of the major aspirations of this line of research was to investigate the cascade chemistry of indigo 1 to the point of predictability, thus enabling access to unprecedented polyheterocyclic compounds while exploring diverse molecular space. Therefore, the aim of this current study was to further investigate the N-alkylation-initiated chemistry of indigo 1 using electrophiles that contained an electron-withdrawing substituent. Ethyl bromoacetate 16 was chosen as it contained a leaving group to allow for the initial N-alkylation of indigo. The presence of the ester-activated methylene group was also anticipated to enable carbanion formation under the basic conditions of the typical cascade reactions, while the ester carbonyl also presents a further electrophilic site (Figure 2), a combination primed to allow cascade reactions to occur. Some potential reactive electrophilic and nucleophilic sites in indigo 1 are highlighted in Figure 2. Given that the reaction of indigo 1 with bromoacetonitrile 13 gave poor yields, we anticipated that the less strongly electron withdrawing effects of the ester functionality versus the cyano functionality may promote better yields of cascade products due to less activated intermediates allowing more controlled cascade processes, although nucleophilic addition-elimination processes at the ester carbonyl might still proceed. Further, we planned to investigate analogous reactions using indirubin 1a (Figure 2), which is known to undergo cascade reactions [12] and is less reactive than indigo 1.
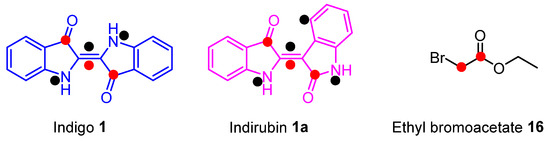
Figure 2.
The structures of indigo 1, indirubin 1a, and ethyl bromoacetate 16 show electrophilic (●) and nucleophilic sites (●).
2. Results
2.1. Reaction of Indigo with Ethyl Bromoacetate
In a typical reaction, a suspension of indigo 1 in anhydrous DMF was sonicated under a static nitrogen environment to facilitate maximum dissolution. The resulting suspension was cannulated into a reaction flask containing pre-dried caesium carbonate and 4 Å molecular sieves and was stirred under a nitrogen environment at 85–88 °C for 90 min. Ethyl bromoacetate 16 was added, and after 15 min the reaction was quenched, and the new heterocyclic systems 17, 18, and 20 were recovered, together with the N,Nʹ-disubstituted indigo derivative 19, in low yields after silica gel column chromatography (Scheme 2).
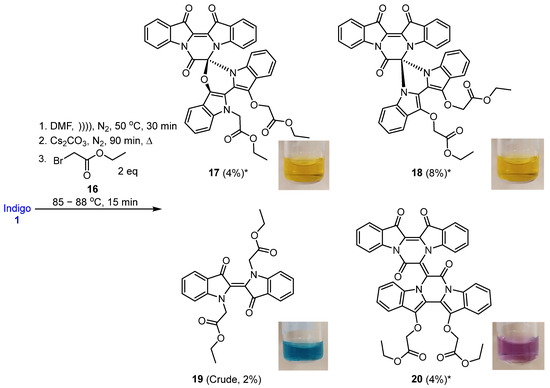
Scheme 2.
Base-promoted cascade reaction of indigo 1 with ethyl bromoacetate 16 to produce compounds 17–20. Note: Relative configurations are shown for 17 and 18. * X-ray crystal structures are available (see Figure 3).
Analysis of the 1H NMR spectrum of compound 19 revealed only four aromatic resonances at 7.67, 7.63, 7.38, and 7.13, assigned to H4 and H4′, H6 and H6′, H7 and H7′ and H5 and H5′, respectively, which indicated a symmetrical structure. The 1H NMR resonances at 1.15, 4.08, and 4.92 were assigned to the N-ethoxycarbonylmethyl (CH3CH2OCOCH2) moieties as H5′′ and H5′′′, H4′′ and H4′′′ and H1′′ and H1′′′, respectively. The 1H NMR spectrum also indicated the presence of some minor impurities, and therefore a 2% crude yield is reported. The HRMS-ESI analysis of 19 revealed an ion peak at m/z 435.1572, assigned as the [M + H]+ ion for the molecular formula C24H23N2O6. In contrast, structures of the diindigoids 17, 18, and 20 structures were solved by X-ray crystallographic analysis (Figure 3). All three molecules consisted of a pyrazinodiindolinone bonded to a biindole through either the spirocyclic compounds 17 and 18 or a twisted unsaturated core, 20.
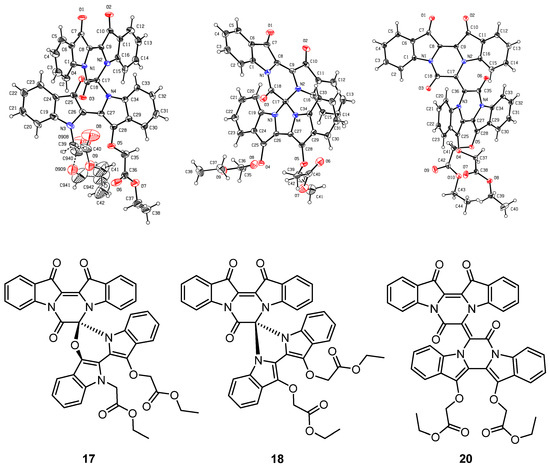
Figure 3.
X-ray crystallographic structures of compounds 17, 18, and 20. Note: Atom numbering in the X-ray structures is different from those used in the systematic numbering.
Diindigoid 17 was isolated as a yellow solid from which crystals were obtained by slow evaporation from CH2Cl2/hexane. HRMS-ESI spectrum analysis of the solids showed an ion peak at m/z 735.2086, assigned [M + H]+ for a molecular formula of C42H31N4O9. This suggested the presence of two moieties derived from indigo with three additional ethyl bromoacetate-derived units, with one of the units also involving loss of the EtO group. Analysis of the COSY spectrum indicated 4 distinct ortho-coupled proton networks, which suggested the presence of two non-symmetrical indigo cores (Figure 4 and Figure S3). Analysis of the 1H NMR spectrum revealed resonances at 1.29, 1.31, 4.25, 4.31, 4.97, and 5.54/5.75, which corresponded with two ethoxycarbonylmethyl substituents and were assigned as H2′′′′′′, H2′′′′, H1′′′′′′, H1′′′′, H2′′′, and H2′′′′a/H2′′′′b, respectively. Analysis of the HMBC spectrum (Figure S4) revealed correlations of 1H NMR resonances 7.99 (H12) and 7.91 (H1) with the 13C NMR resonances 178.6 (C13) and 180.4 (C14), respectively. The ester carbonyls were observed at 169.5 (C1′′′′′) and 168.6 (C1′′′), which correlated with a diastereotopic doublet 5.65 (H2′′′′′) and a singlet 4.97 (H2′′′), respectively. This assignment was supported by HMBC correlations of resonances 4.25 (H1′′′′′′) and 4.31 (H1′′′′) with 169.5 (C1′′′′′) and 168.6 (C1′′′), respectively. Analysis of the DEPTq135 spectrum revealed an additional 13C resonance downfield at 155.4 ppm, which corresponded to a quaternary carbon. The HMBC analysis indicated this resonance correlated with no 1H NMR resonances and it was therefore assigned to the carbonyl C7. Analysis of the NOESY spectrum indicated the diastereotopic pair of doublets at 5.65 correlated with the aromatic 7.26 resonance. Given the diastereotopic splitting is typically observed with sterically hindered CH2 N-substitutions, the 5.65 and 7.26 ppm resonances were assigned to H2′′′′′ and H7′′, respectively. The other CH2 resonance at 4.97 ppm was a singlet and therefore possesses a greater degree of freedom, which is typical of an O-substitution. By HMBC spectral analysis, the 1H resonance at 4.97 ppm correlated with a 13C NMR resonance at 133.3 ppm, which in turn correlated with the 1H NMR resonance at 7.59 and these were assigned to H2′′′, C3′, and H4′, respectively. The indolinones of the pyrazinoindolotrione half of the molecule were distinguished based on differences in chemical shifts. It was proposed the C7-N8 amide would withdraw the free electron pair on N8, reducing its capacity to resonate with the aromatic ring, i.e., resulting in upfield chemical shifts. Subsequently, analysis of the DEPTq135 spectrum indicated resonances at 144.4 and 148.0, which were assigned to C8a and C4a, respectively.
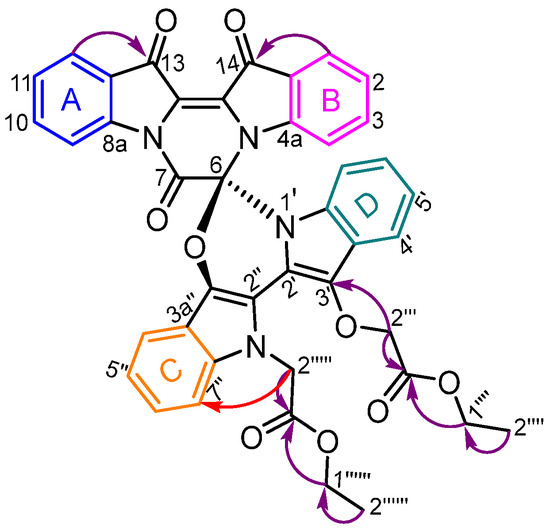
Figure 4.
HMBC correlations in 17 annotated as purple and NOESY as red. COSY correlations with the four ortho-coupled aromatic systems annotated, as follows: (A, blue) 8.27 (H9)—7.61 (H10)—7.40 (H11)—7.99 (H12), (B, magenta) 7.91 (H1)—7.14 (H2)—7.31 (H3)—6.87 (H4), (C, orange) 7.49 (H4′′)—7.08 (H5′′)—7.25 (H6′′)—7.26 (H7′′) and (D, teal) 6.96 (H7′)—7.02 (H6′)—7.11 (H5′)—7.59 (H4′).
Diindigoid 18 was isolated as a yellow amorphous solid and eluted with a similar HPLC retention time to 17. A molecular formula of C42H30N4O9Na was assigned to the [M + Na]+ ion peak at m/z 757.1927+ by analysis of the HRMS-ESI spectrum. Analysis of the 1H NMR spectrum revealed 16 aromatic protons with 4 resonances that integrated to 2, which indicated one indigo-derived unit was symmetrical. Only one set of CH2CO2C2H5 group resonances were observed with the quartet at 4.31 (H1′′′′) and triplet at 1.31 (H2′′′′), which integrated for 10 H in agreement with a symmetrical indigo moiety being present. Analysis of the DEPTq135 spectrum indicated a 13C NMR resonance at 155.1 ppm, which had no HMBC correlations, and this was assigned to C7, similar to the assignment of diindigoid 17 (Figure S7). The presence of the diastereotopic pair of doublets at 5.20 (H2′′′) suggested the ethyl acetate moieties were N-linked to the indigo core; however, the HMBC spectrum analysis indicated a single correlation with the 13C NMR resonance at 132.9 (C3′), which correlated with the 1H NMR resonance 7.72 (H4′). Therefore, the ethyl acetate moieties were proposed to be attached via O-alkylation. This was confirmed by observation of the correlation between 7.72 and 5.20 in the NOESY spectrum, which were assigned H4′ and H2′′, respectively. Therefore, structure 18 was proposed as a structural isomer of 17 with the indigoids linked via an imidazole-derived ring.
The purple colour of diindigoid 20 suggested extended conjugation was present in the molecule. The HRMS-ESI analysis indicated an ion peak at m/z 797.1859+ assigned to [M + Na]+ for a molecular formula of C44H30N4O10Na, which corresponded to the presence of two indigo units with two CH2CO2C2H5 moieties plus an additional C4O2 unit. Analysis of the 1H NMR spectrum revealed resonances of two ethyl moieties at 1.29, 1.32 and 4.24, which were assigned to H2′′′′′, H2′′′ and H1′′′′′/H1′′′, respectively. Two diastereotopic doublets were observed at 4.93 and 4.83 and were assigned H2′′ and H2′′′′, respectively. Analysis of the HMBC spectrum revealed the ester carbonyls at 169.1 (C1′′ and C1′′′′) correlated with the diastereotopic doublets at 4.93 and 4.83 (H2′′/H2′′′′), which themselves correlated with 13C resonances at 139.4 and 139.2, respectively. These 13C resonances also correlated with the 1H resonances at 7.89 and 7.76 and were therefore assigned to C14′, C13′, H1′ and H12′, respectively. Analysis of the DEPTq135 indicated two quaternary carbons at 153.6 and 154.8, which were assigned to C7′ and C7, respectively. The presence of these two carbonyls and no additional protons suggested the formation of two pyrazinone rings, which were fused via a tetrasubstituted double bond. No heteronuclear correlations were observed to enable confirmation of the stereochemistry of the double bond in view of the nature of the substituents present. The E-stereochemistry was established from X-ray crystallography (Figure 3) in which unfavourable interactions between the two carbonyl groups would be obviated.
2.2. Investigation of the Effect of Reaction Conditions on Product Outcomes for the Indigo and Ethyl Bromoacetate Reaction
The reaction of indigo 1 with ethyl bromoacetate 16 resulted in the formation of the structurally notable compounds 17, 18, and 20, plus 19, albeit in low yield (Scheme 3, entry 1). This outcome strongly indicated that ethyl bromoacetate 16 is highly reactive, resulting in cascade pathways incorporating alkylation, cyclization, and coupling reactions. It was thought that the poor yield was mainly due to the low amount of electrophile (2 eq) used in the initial reaction studies, which activated the reactive -CH2 group quickly, leading to the diverse array of pathways in the reaction. Therefore, the reaction of indigo 1 with ethyl bromoacetate 16 was re-investigated, changing the number of equivalents of electrophile 16 used, the reaction temperature, and the reaction time (Scheme 3, entries 2–4). Thus, to a stirred mixture of indigo 1, anhydrous caesium carbonate, and 4 Å molecular sieves in DMF under a nitrogen atmosphere, ethyl bromoacetate 16 (either 5 or 20 equivalents) was added to the reaction mixture for 15 or near 12 min of reaction time. Subsequent workup and multiple rounds of silica gel column chromatography afforded a number of products, including the N,N′-dialkylated product 19 and the coupled product 20, although not compounds 17 or 18. (Scheme 3, entries 2–4). The addition of 5 equivalents of 16 for 15 min of reaction time yielded N,N-dialkylated indigo 19 (6%), biindigoid 20 (2%), cyclised compounds 22 (2%), and 23 (3%) along with the known product tryptanthrin 24 [13,14] (2%) and a non-separable mixture of other compounds (Scheme 3, entry 2). The fused pyrazino derivatives 22 and 23 had not been isolated previously. Compound 19 was also isolated pure (6%), in contrast to previous attempts. Analysis of the 1H NMR spectrum of compound 22 revealed eight aromatic resonances 8.49, 7.94, 7.85, 7.69, 7.60, 7.38, 7.15, and 7.04, assigned to H4, H1, H12, H3, H10, H2, H11, and H9, respectively. This indicated the presence of the indigo core within the molecule. Additionally, in the upfield region of the spectrum, four resonances at 5.17, 4.01, 3.31, and 1.06 were assigned to H6, H1′′, H2′, and H2′′′. HMBC spectral analysis showed a resonance at 5.17 ppm correlated to 160.6, 168.8, 126.1, and was assigned to C7, C1′ and C13b, respectively (Figure S29). 13C NMR analysis further supported these findings by revealing four characteristic resonances at 180.3, 178.4, 168.8, and 160.6 ppm, which were assigned to C13, C14, C1′, and C7, respectively. HRMS-ESI spectral analysis of compound 22 revealed an ion peak at m/z 411.0959+, which was assigned to the [M + Na]+ ion for the molecular formula C22H16N2O5Na. The HRMS-ESI spectrum of compound 23 gave an ion peak at m/z 475.1505, which was assigned to [M + H]+ ion for the molecular formula of C26H23N2O7, consistent with the presence of the indigo core and two additional ethyl acetate-derived moieties.
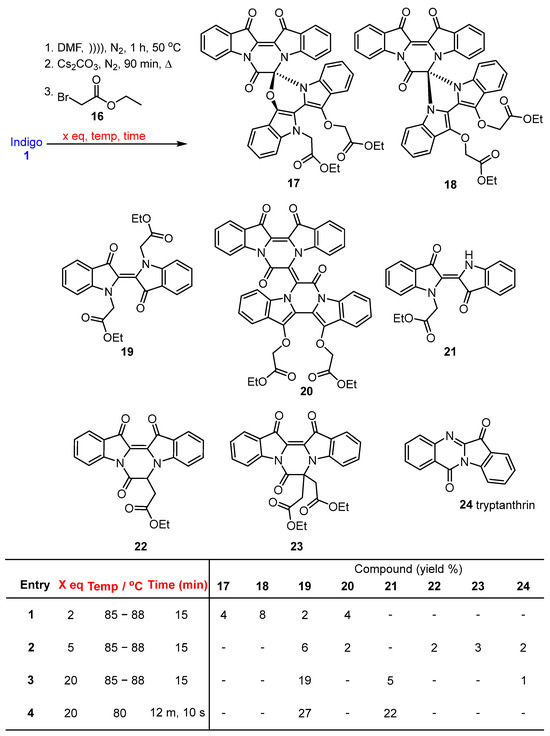
Scheme 3.
Optimisation of the reaction of indigo 1 with ethyl bromoacetate 16.
Analysis of the 1H NMR spectrum of compound 23 showed eight aromatic resonances and two sets of ethyl acetate resonances with cumulative integrations of 14 protons. Among these, a quartet at 3.92 in 1H NMR spectrum was assigned as H1′′ and H1′′′′, which correlated to the resonances at 167.9 (C1′ and C1′′′), and 13.8 (C2′′ and C2′′′′), respectively, in the HMBC spectrum. A doublet of doublets at 3.57 in the 1H NMR spectrum was assigned to H2′ and H2′′′, correlating to the resonances at 163.8 (C7), 167.9 (C1′ and C1′′′), 61.5 (C1′′ and C1′′′′). and 40.8 (C2′ and C2′′′), respectively, in the HMBC spectrum. A triplet 0.97 assigned to H2′′ and H2′′′′, correlated to a resonance at 13.8 and 13C were attributed as C2′′ and C2′′′′.
The biindigoid 20 was isolated in 2% yield, and trypanthrin 24 (2%) was identified as a reaction disintegration product, with NMR data in agreement with that previously reported [13].
Scheme 3, entry 3, with the addition of 20 equivalents of electrophile 16 in the reaction mixture for 15 min at 85–88 °C, gave N,N′-dialkylated indigo 19 (19%), N-alkylated indigo 21 (5%), and tryptanthrin 24 (1%) along with a mixture of minor products and baseline material. With 20 equivalents of electrophile 16, a reaction temperature slightly reduced (from 88 to 80 °C) and a reaction time shortened to just over 12 min, two major products were isolated, two with improved yields: N,N′-dialkylated indigo 19 (22%), and N-alkylated indigo 21 (27%) (Scheme 3, entry 4). Furthermore, there was evidence of baseline polymeric material, likely arising from the formation of undesired minor products or disintegration of the starting materials. It is of note here that previous work by others on the reaction of indigo with tert-butyl bromoacetate (in DMF with Cs2CO3 as a base in a sealed vial) at 23 °C over 24 h gave a moderately good yield (66%) of the corresponding (E)-N,N′-dialkylation product, N,N′-bis(tert-butyloxycarbonylmethyl)indigo, and no other reaction products were reported [15]. The same low-temperature method was used again more recently to make this dialkylation product [16].
2.3. Reaction of Indirubin with Ethyl Bromoacetate
Indirubin 1a (Figure 2), a naturally occurring regioisomer of indigo 1, is renowned for its range of biological activities and has been employed in traditional Chinese medicine for anticancer treatment [17,18,19,20,21]. Many derivatives of indirubin have been made in connection with probing their anti-tumour properties, including the development of the first hybrid dual inhibitors [22] of glutathione peroxidase 4 (GPX4) and cyclin-dependent kinase (CDK), as well as molecules inducing damage to DNA and targeting the poly (ADP-ribose) polymerases (PARPs) involved in DNA repair [23]. Beyond its significance in the realm of biology and medicine, indirubin 1a possesses interesting chemical properties [12,24,25]. Notably, in our previous work [12], it was found that indirubim is less reactive than indigo, and it was reasoned that this might impart greater control in reactions with ethyl bromoacetate. Therefore, indirubin 1a was selected for further investigation with ethyl bromoacetate 16 in an attempt to invoke polyheterocycle formation via cascade reactions in a controlled manner.
In this context, indirubin 1a was reacted with 16 using conditions that were previously optimised when allyl bromide was used as the electrophile [12]. Therefore, indirubin 1a was dissolved in DMF and subjected to sonication for 10 min under a static nitrogen environment. The resulting suspension was cannulated into a reaction flask containing pre-dried caesium carbonate and stirred vigorously for 90 min at 90 °C. Subsequently, the temperature was reduced to 80 °C, and ethyl bromoacetate 16 was added over 30 min at this reaction temperature. Upon workup and subsequent purification by silica gel column chromatography, the monosubstituted N-alkylated indirubin 25 (48%), and the disubstituted N,N′-dialkylated indirubin 26 (20%) were isolated as dark pink and dark purple fluffy solids, respectively, along with minor products. In an attempt to invoke the cyclisation of these molecules, subsequent reactions were performed at a higher temperature (110 °C) and the addition of the electrophile 16 over an extended reaction time of 1 h. This afforded sets of polyheterocyles: N-alkylated indirubin 25 (<1%), N,N′-dialkylated indirubin 26 (25%), compound 27 (4%), compound 28 (2%), compound 29 (3%), and the degradation product 30 (3%) (Scheme 4). Compounds 27, 28, and 29 are derivatives of a previously undescribed fused indirubin skeleton, the indolo[1,2-b]pyrrolo[4,3,2-de]isoqinoline system.
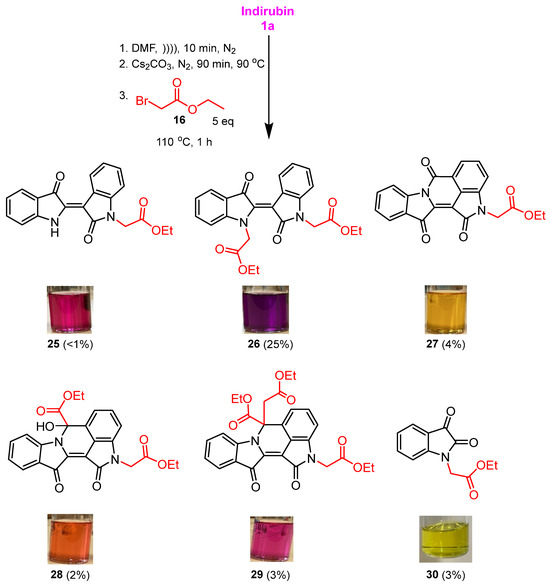
Scheme 4.
Base-promoted cascade reaction of indirubin 1a with 16 to produce polyheterocyles 25–29, plus the N-substituted isatin 30.
Analysis of the HRMS-ESI spectrum of compound 27 revealed an ion peak at m/z 397.0815, which was assigned as the [M + Na]+ ion for the molecular formula of C22H10N6O5Na and corresponded to the presence of the indirubin core and one CH2CO2C2H5 moiety. Analysis of the 1H NMR spectrum showed seven resonances at 8.81, 7.92, 7.87, 7.75, 7.57, 7.40, and 6.99 integrating to seven protons and assigned as H8, H11, H5, H9, H4, H10, and H13, respectively. The N-ethyl acetate moiety resonances were observed at 4.62, 4.25, and 1.29 and assigned to H1′, H4′, and H5′, respectively. Analysis of the 13C NMR spectrum of 27 showed significant resonances at 180.8, and 162.2, assigned C12, and C1 carbonyls, whereas the 13C resonances at 159.0, and 167.4, were assigned to C6, and C2′, corresponding to the amide and ester carbonyls, respectively. Analysis of the HMBC spectrum further supported these correlations by showing the resonances of H11 at 180.8 (C12), H1′ at 162.2, and 167.4 (C1, C2′), and H5 at 159.0 (C6), respectively.
Analysis of the HRMS-ESI spectrum of compound 28 revealed an ion peak at m/z 471.1180, which was assigned as [M + Na]+ ion for the molecular formula of C25H16N6O3Na, and indicated the presence of the indirubin core and two ethyl acetate moieties with loss of a CH2 group but with the addition of an OH group. Analysis of the 1H NMR spectrum of compound 28 displayed seven aromatic resonances at 7.67, 7.52, 7.33, 7.22, 7.11, 7.08, and 6.99, assigned to H11, H9, H4, H8, H5, H10, and H3, respectively. The N-ethyl acetate moiety resonances were observed at 4.31, 4.28–4.15, and 1.08 in the 1H NMR spectrum were assigned as H2′, H1′′, and H2′′. Analysis of the 1H NMR spectrum further showed other resonances which could be assigned as follows: at 5.39 (OH), 4.43 (H1′′′) and 1.26 (H2′′′). Analysis of the 13C NMR spectrum revealed resonances at δ 181.8, and 162.9, assigned to carbonyls C12, and C1, and two 13C resonances at δ 169.2, and 167.8, assigned to the ester carbonyls as C1′′′, and C1′, respectively. Analysis of the HMBC spectrum displayed the correlations of the OH to a resonance at 169.2 (C1′′′), H11 to 181.8 (C12), H2′ to 162.8 (C1) and 167.8 (C1′), and H5 to 66.2 (C6). In contrast to compound 28, analysis of the 1H NMR spectrum of compound 29 displayed a similar trend with the exception of an additional ethyl ester moiety in the upfield region of the spectrum. Interestingly, the absence of a resonance for the OH moiety in the 1H NMR spectrum of 29 suggested that the additional ethoxycarbonylmethyl moiety was replaced with an OH group at the C-6 position. This conclusion was supported by the HMBC analysis, which showed the correlations between the additional moiety’s diastereotopic proton H2′′′′′ab to a 13C resonances at 169.0, 168.0 and 126.0 ppm, corresponding to the carbons as C1′′′, C1′′′′′, and C5a respectively. Furthermore, the HMBC analysis indicated that the quartet at 3.81, assigned to H1′′′′′′ correlated to the resonances at 168.0 and 13.6 ppm, leading to the assignments C1′′′′′, and C2′′′′′′, respectively (Figure S59). The HRMS-ESI spectrum of compound 29 revealed an ion peak at m/z 519.1767, which was assigned as [M + Na]+ ion for the molecular formula of C28H26N2O8Na (Figures S56–S60).
Based on the cascade reaction of indirubin 1a, the product outcomes indicated that N,N′-dialkylated indirubin 26 could potentially serve as the starting material for the formation of cascade products 27–29. To understand the reaction mechanism and improve the product yields, the study was extended to include some control mechanism experiments. Therefore, N,N′-dialkylated indirubin 26 was synthesised according to previously optimised reaction conditions. This compound 26 was then dissolved in DMF with ultrasonication, two equivalents of anhydrous caesium carbonate were added, and the mixture was stirred vigorously at 110 °C under a nitrogen environment. Notably, no ester 16 was added to the reaction mixture. After a reaction time of 3.5 h, crushed ice was added, and subsequent work-up, including multiple rounds of preparative TLC, yielded the compounds 27–29 with improved yields (Scheme 5).
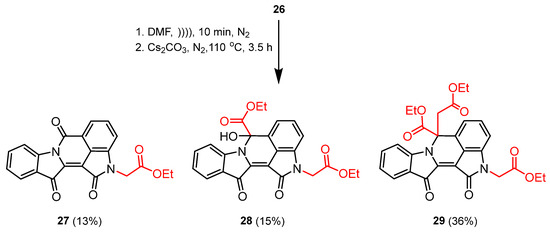
Scheme 5.
Reaction of N,N′-dialkylated indirubin 26 in the presence of a base to yield compounds 27–29.
2.4. Mechanistic Discussion of the Indigo-Ethyl Bromoacetate Reactions
The proposed reaction mechanisms for the formation of the cascade products 17, 18, and 20–23 are summarised in Scheme 6, Scheme 7, Scheme 8 and Scheme 9. For the synthesis of 17, the first step is the N,N′-dialkylation of indigo 1 leading to the formation of intermediate 19 (Scheme 6). Subsequent O-alkylation could then give rise to intermediate 30. Base removal of the acidic methylene hydrogen would then give rise to iminium-based intermediate 31. In the presence of bicarbonate and water, intermediate 31 could be converted to 32. The base-induced removal of glyoxylate 33 would then furnish O,O′-dialkylindigo 34 after C-C bond rotation. The condensation reaction of O,O′-dialkylindigo 34 with oxalylindigo 35 produced in situ (see Scheme 10 for a proposed route to this known [26] compound under the reaction conditions) would then afford access to intermediate 36. The subsequent 6-endo-trig cyclisation of intermediate 36 in the presence of base would afford compound 17 (Scheme 6).
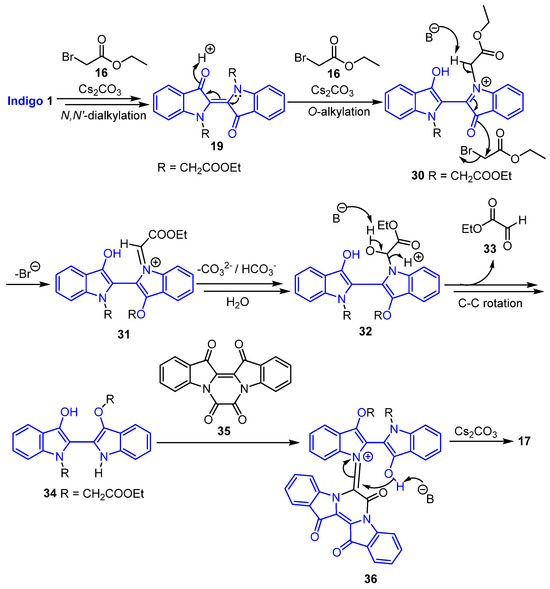
Scheme 6.
Proposed mechanism for the formation of the spirocycle 17.
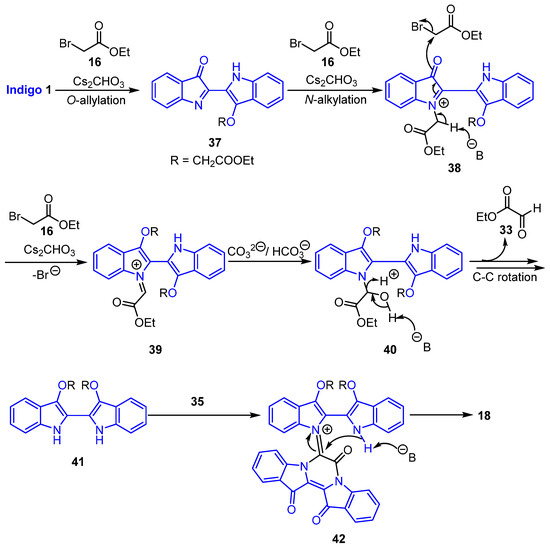
Scheme 7.
Proposed mechanism for the formation of the spirocyclic aminal 18.
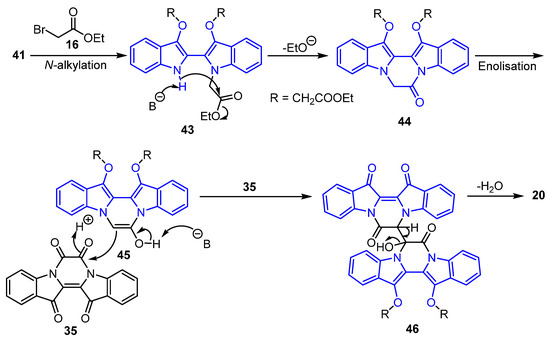
Scheme 8.
Proposed reaction mechanism for the formation of the double bond-linked compound 20.
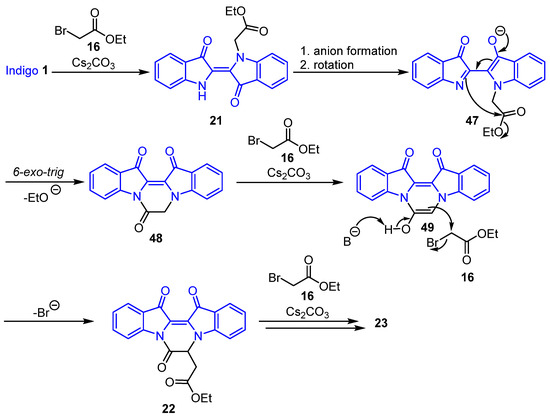
Scheme 9.
Proposed reaction mechanism for the formation of compounds 22–23 via N-alkylation of indigo 1.
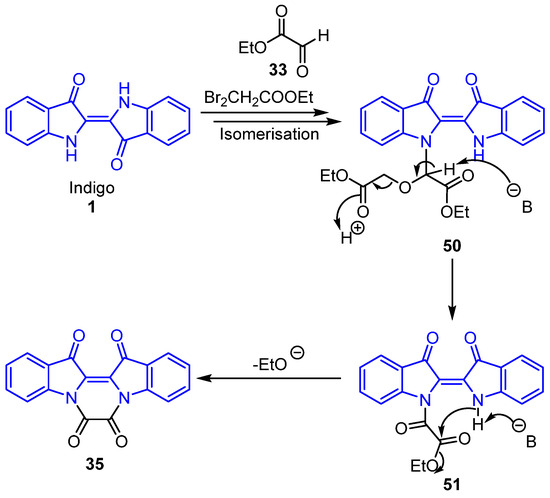
Scheme 10.
Formation of proposed intermediate 35. Ethyl glyoxylate 33 may be generated in situ as noted in Scheme 6 and Scheme 7, or possibly from a carbonate ester intermediate (see Supplementary Materials, Scheme S1).
The proposed mechanism of formation of compound 18 is described in Scheme 7 and begins with the reaction of indigo 1 with 16 in the presence of a base to generate O-alkylindigo 37. Subsequent N-alkylation could then lead to intermediate 38 with subsequent base removal of an acidic methylene hydrogen giving rise to the iminium-based intermediate 39. In the presence of bicarbonate, intermediate 39 could be converted to 40, followed by base-initiated removal of glyoxylate 33 and C-C bond rotation to access O,O′-dialkylindigo 41 with bicarbonate also acting as a proton source. The reaction of 41 with 35 could give an intermediate 42. Base removal of the acidic methylene proton of 42 and subsequent cyclisation would then result in compound 18 (Scheme 7).
The proposed mechanism for the formation of compound 20 is suggested, to begin with the N-alkylation of branch point intermediate 41 in the presence of base and ethyl bromoacetate 16 to produce 43, which undergoes cyclisation in the presence of a base to generate lactam intermediate 44. The lactam 44 could undergo enolization, and a subsequent reaction with intermediate 35 in the presence of base would furnish intermediate 46. Finally, dehydration of intermediate 46 could give compound 20 (Scheme 8).
The proposed mechanism of formation for the cascade products 22 and 23 arises from the N-alkylation of indigo 1 producing 21, with subsequent anion formation and rotation giving rise to the intermediate 47. After a 6-exo-trig cyclisation step followed by the elimination of ethoxide, the reaction would furnish lactam 48. Alkylation of the carbonyl α-position with 16 as the electrophile produces the product 22. Further C-alkylation would afford 23 (Scheme 9).
The reaction between indigo 1 and ethyl bromoacetate 16 resulted in the formation of a wide range of polyheterocycles 17–23. Notable key intermediates, e.g., oxalylindigo 35 and lactam 48, are critical for the construction of the more complex systems and derivatives and could be formed in situ during the reaction but were not isolated during the optimisation process. The suggested reaction mechanism for the formation of 48 is detailed in Scheme 9, while the proposed mechanism for the formation of intermediate 35 is illustrated in Scheme 10. This involves the reaction of indigo 1 with ethyl bromoacetate 16 in the presence of ethyl glyoxylate 33 and base to produce intermediate 50 after isomerisation about the C=C bond (Scheme 10). Subsequent base removal of the acidic proton at the ester carbonyl α-position may follow, which would then allow for the elimination of ethyl acetate and the formation of intermediate 51. Base removal of the proton of the -NH group of 51 and subsequent cyclisation at the adjacent carbonyl with ethoxide ion loss would result in oxalylindigo 35 (Scheme 10).
2.5. Mechanistic Discussion of the Indirubin-Ethyl Bromoacetate Reaction
The proposed mechanism for the formation of compounds 25–29 is illustrated in Scheme 11. The initial steps involve indirubin N,N′-dialkylation in the presence of Cs2CO3 with ethyl bromoacetate 16, resulting in the production of N,N′-dialkylated indirubin 26, probably via the N-alkylated compound 25. Nucleophilic attack by the carbonate ion at the electron-deficient α-position to the ester carbonyl with loss of the carbonate 53 (an in situ precursor of bis(ethoxycarbonylmethyl)carbonate 59; see Scheme S1) would then provide access to the key imine intermediate 54. Some 54 might also result more directly from the N-monosubstituted indirubin 25. The imine 54 could then serve as a point of divergence in pathways. In Path A, intermediate 54 could react with the bis-carbonate 59 to generate 55. Subsequent aromatic electrophilic substitution of 55 to give intermediate 56, followed by elimination of ethyl glycolate 57, would ultimately yield the isolated derivative 27.
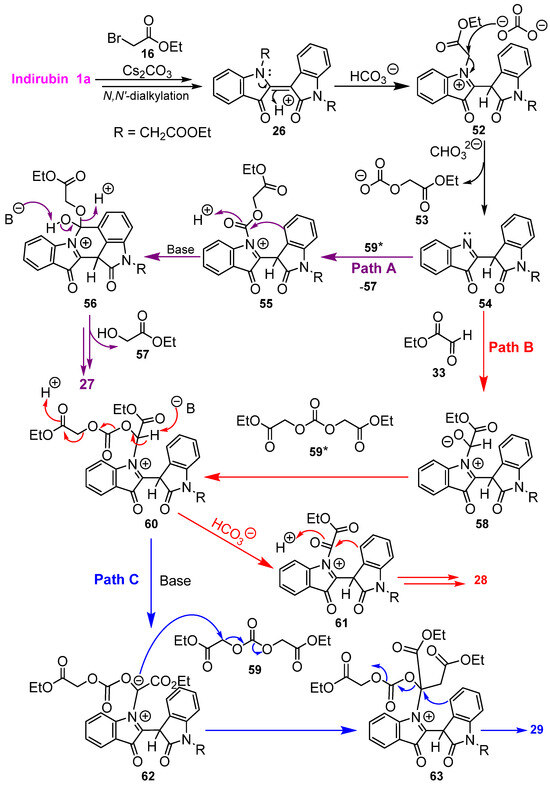
Scheme 11.
Proposed reaction mechanisms for the formation of compounds 26–29 from cascade reaction of indirubin 1a with ethyl bromoacetate 16. * For the proposed synthesis in situ synthesis of 59, see the Supplementary Materials (Scheme S1).
In Path B, the imine intermediate 54 could add to the aldehyde group in ethyl glyoxylate 33 to produce intermediate 58, which in turn could participate in a nucleophilic substitution reaction with the bis-carbonate 59 giving rise to 60. Intermediate 60 could then proceed to the isolated cyclised compound 28 by subsequent steps, first involving conversion to 61 (with the elimination of ethyl acetate and carbon dioxide) and then intramolecular electrophilic aromatic substitution. The formation of the unsymmetrically gem-disubstituted derivative 29 may also possibly arise from the intermediate 60 (Path C) in an alternative pathway via deprotonation to produce 62, which could react with the carbonate 59 or possibly with ethyl bromoacetate 16, leading to the formation of 63. Finally, intramolecular aromatic electrophilic substitution and proton loss steps in the presence of base would afford compound 29. The fact that the disubstituted compound 26 could be converted independently to the cyclized products 27–29 on heating at a higher temperature with Cs2CO3 in DMF is consistent with the proposed mechanistic pathways A–C with the plausible presumption that both the other reactants 59 and 33 could still be generated in situ. The carbonate ion 53 could possibly compete as a nucleophile in the step from 52 to 54 in Scheme 11, thus giving rise also to 59, which in turn could act as a precursor of glyoxylic ester 33 (Scheme S1).
3. Materials and Methods
3.1. Chemistry
Anhydrous dimethylformamide (DMF) was purchased from Merck (Sigma-Aldrich, St. Louis, MO, USA), and used without further purification. High-performance liquid chromatography (HPLC) grade dichloromethane (CH2Cl2) was used, and all other solvents were purchased reagent grade and used without further purification unless otherwise stated. Indigo was purchased from Merck (St. Louis, MO, USA) and AK Scientific (Union City, CA, USA) and used without further purification. Indirubin was synthesised as reported [27]. Ethyl bromoacetate was purchased from Sigma-Aldrich. Deionised (reverse osmosis; RO) water was used for extractions and preparation of aqueous solutions and was obtained from a Millipore purification system. Salt solutions such as brine or NaHCO3 were prepared from the commercially available salt and are saturated aqueous solutions unless specified otherwise. Nitrogen used in reactions was passed through a 20 cm tube filled with a silica-based drying agent (blue→yellow indicator) or anhydrous calcium chloride. Cs2CO3 was stored in a desiccator and pre-dried by heating at 85–87 °C for an hour under a high vacuum. Molecular sieves (4 Å) were activated in a furnace at 300 °C overnight before use. Melting point temperatures are expressed in degrees Celsius (°C) and are uncorrected. 1H and 13C NMR spectra (CDCl3) were recorded either at 500 and 125 MHz (Bruker Avance 500), or 400 and 100 MHz (Bruker Avance 400), respectively, with chemical shifts (δ) reported as parts per million relative to TMS (δ = 0.00 ppm) or CDCl3 (δ = 7.26; 77.0 ppm); the numbering used for resonance assignments may be different from the systematic numbering. Coupling constants (J) are reported in Hertz (Hz). Multiplicities are reported as singlets (s), doublets (d), triplets (t), doublet of doublets (dd), quartets (q), sextets (s), heptets (hept), or multiplets (m). Electrospray (ESI single quadrupole, Shimadzu LCMS-2020) mass spectra are reported as ion mass to charge values (m/z), with the relative abundances as a percentage in parentheses. Infrared (IR) spectra were recorded neatly on a Bruker Vertex 70 FTIR (Bruker Optik GmbH, Ettlingen, Germany). UV−visible absorption spectra were recorded in dichloromethane solutions at room temperature using a double-beam spectrophotometer. Thin-layer chromatography (TLC) was performed using silica gel F254 coated with aluminium sheets. Preparative thin-layer chromatography (PTLC) was performed. Column chromatography was performed using silica gel 60 (0.063−0.200 mm). Eluents are reported in volume-to-volume (v/v) ratios. Solvent extracts and chromatographic fractions were concentrated by rotary evaporation in vacuo. PS 40–60 refers to petroleum spirit, bp range 40–60 °C.
3.2. Reactions of Indigo with Ethyl Bromoacetate
3.2.1. Method 1
Anhydrous DMF (40 mL) was added via cannula to a flask containing indigo (263.1 mg, 1.003 mmol) under nitrogen flow. The resulting suspension was sonicated for 30 min at 50 °C under a static N2 atmosphere. The solution was transferred via cannula to a flask containing pre-dried Cs2CO3 (1.321 g, 4.055 mmol) and stirred at 85–88 °C under a static N2 atmosphere for 90 min. Ethyl bromoacetate 16 (0.22 mL, 2.0 mmol) was added via syringe, and the reaction was left to stir for 15 min at 85–88 °C and then quenched in ice water. The organic material was extracted with EtOAc (1 × 40 mL, 3 × 20 mL) and then washed with water (3 × 50 mL) and brine (1 × 50 mL). The organic layer was dried (MgSO4), concentrated in vacuo, and the residue was subjected to silica gel column chromatography (40 g, 20% P.S/CH2Cl2 to 2% MeOH/EtOAc), which afforded a major yellow band (98.1 mg), a major purple band (32.5 mg), and significant decomposition (195.8 mg). The yellow band was subjected to further silica gel column chromatography (16 g silica, 30% EtOAc/PS 40–60 to 40% EtOAc/PS 40–60), which yielded a mixture of two yellow compounds (48.9 mg) that could be separated using preparative HPLC (15 mL.min−1, 70% MeCN/H2O with 0.1% TFA) to afford the compounds 17–18. The major purple band was purified by silica gel column chromatography (13 g silica, 30% to 40% EtOAc/PS 40–60) to yield compound 19 (9.6 mg, crude yield 2%) and a major purple fraction that was further purified by recrystallization (slow evaporation from 1:1 CH2Cl2/hexane) to yield compound 20 (18.9 mg, 0.024 mmol, 4%).
3.2.2. Method 2
Indigo (262.27 mg, 1.00 mmol) was dissolved in anhydrous DMF (40 mL), and the resulting suspension was sonicated for 1 h at 50 °C under a static N2 atmosphere. The solution was transferred to a flask containing pre-dried Cs2CO3 (1.303 g, 4.00 mmol) with activated 4 Å molecular sieves and stirred at 85–88 °C under a static N2 atmosphere for 90 min. Ethyl bromoacetate 16 (0.55 mL, 5 mmol) was added via syringe. The reaction mixture was stirred vigorously for 15 min at 85–88 °C under a static N2 atmosphere. The reaction mixture was then quenched by pouring into crushed ice (200 mL) and transferred to a separatory funnel. The aqueous layer was extracted with ethyl acetate (3 × 40 mL). The combined organic layers were washed with H2O (1 × 30 mL), brine (1 × 30 mL), dried (MgSO4) and concentrated in vacuo. The mixture was adsorbed on silica and loaded to a silica gel (100 g) column and elution with (100% hexane → 10% EtOAc/hexane → 2% MeOH/DCM) resulted in three major fractions (F1, F2, and F3), including minor fractions (F4 and F5), and baseline decomposition. Fraction F1 was identified as a disintegration product tryptanthrine 24 as a yellow waxy solid. Fraction F2 was subjected to silica gel (30 g) column chromatography, and the mixture was eluted with (100% hexane → EtOAc 10% to 20%) to give a partially pure compound 19 as a dark blue waxy solid. Further purification by multiple rounds of PTLC (1:9 EtOAc:hexane) gave a pure form of compound 19 as a dark blue waxy solid. Compound 22 was isolated as dark pink powder by fractional recrystallization (1:5 EtOAc:hexane) of fraction F3. The remaining aliquot of the recrystallisation was dried and adsorbed on PTLC using 3% MeCN in DCM, yielding compound 23 as a red, pink waxy solid. Fractions F4 and F5 were attempted to be purified either with silica gel column chromatography or multiple rounds of PTLC, resulting in the collection of six minor fractions, from which compound 20 was isolated as a dark purple waxy solid as a partially pure compound.
3.2.3. Method 3
Indigo (262.27 mg, 1.00 mmol) was dissolved in anhydrous DMF (40 mL), and the resulting suspension was sonicated for 1 h at 50 °C under a static N2 atmosphere. The solution was transferred to a flask containing pre-dried Cs2CO3 (1.303 g, 4.00 mmol) with activated 4 Å molecular sieves and stirred at 90 °C under a static N2 atmosphere for 90 min. Ethyl bromoacetate 16 (2.22 mL, 20 mmol) was added via syringe. The reaction mixture was stirred vigorously for 12 min 10 s at 80 °C under a static N2 atmosphere. The reaction mixture was then quenched by pouring into crushed ice (200 mL) and transferred to a separatory funnel. The aqueous layer was extracted with ethyl acetate (3 × 40 mL). The combined organic layers were washed with H2O (1 × 30 mL), brine (1 × 30 mL), dried (MgSO4) and concentrated in vacuo. The mixture was adsorbed on silica and loaded to a silica gel (100 g) column, and elution with (100% hexane → 10% EtOAc/hexane → 2% MeOH/DCM) resulted in three major fractions (F1, F2, and F3) including baseline decomposition. Fraction F1 contained an intense blue papery solid 19 and fraction F2 was subjected to silica gel (30 g) column chromatography, and the mixture was eluted with (100% hexane → EtOAc 10% to 20%) to afford a partially pure compound. Further purification by recrystallisation in 1:9 EtOAc:hexane gave compound 21 in pure form as a dark blue waxy solid.
3.3. Reactions of Indirubin with Ethyl Bromoacetate
3.3.1. Alkylation Reaction of Indirubin 1a with Ethyl Bromoacetate 16
Indirubin 1a (131.16 mg, 0.50 mmol) was dissolved in DMF (20 mL) and sonicated for 10 min under a nitrogen atmosphere for further dissolution. The resulting suspension was transferred via syringe to a flask containing pre-dried Cs2CO3 (390.98 mg, 1.20 mmol) and continued stirring at 90 °C. After 90 min, the reaction temperature was reduced to 70 °C, and ethyl bromoacetate 16 (0.27 mL, 2.50 mmol) was added via syringe and continued stirring vigorously under a nitrogen environment. After 1 h of reaction time, the mixture was poured into crushed ice (100 mL). When the ice melted, the resulting suspension was transferred to the separating funnel and the aqueous layer was partitioned with ethyl acetate (3 × 30 mL). The combined organic layers were washed with H2O (1 × 30 mL), with brine (1 × 20 mL), dried (MgSO4), and concentrated in vacuo. The residue was purified by silica gel column chromatography and eluted with 5% EtOAc/hexane to 100% EtOAc yielding N-alkylated indirubin 25 (82.97 mg, 48%) and N,N′-dialkylated indirubin 26 (44.50 mg, 20%) as a dark pink and dark purple fluffy solids.
3.3.2. Cascade Reaction of Indirubin 1a with Ethyl Bromoacetate 16
Anhydrous DMF (20 mL) was added via syringe to a flask containing indirubin 1a (131.13 mg, 0.50 mmol) under a nitrogen atmosphere. The resulting mixture was sonicated for 10 min at 50 °C under a static N2 atmosphere. The solution was transferred via cannula to a flask containing pre-dried Cs2CO3 (684.22 mg, 4.1 mmol), and the mixture was heated at 90 °C for 90 min. Ethyl bromoacetate 16 (0.27 mL, 2.5 mmol, 5 eq) was then added via syringe, the nitrogen inlet was removed, and the reaction was stirred vigorously for 1 h at 110 °C. The reaction mixture was quenched in ice water (150 mL), and the organic layer was extracted with ethyl acetate (2 × 40 mL), brine (1 × 20 mL), washed with H2O (1 × 30 mL), dried (MgSO4), and concentrated in vacuo. The residue was subjected to silica gel (70 g) column chromatography eluting with 10% EtOAc/hexane → 100% EtOAc, which afforded four major fractions (F1, F2, F3, and F4). Fraction F1 was identified as the disintegration product N-alkylated isatin 30 obtained as a yellow waxy solid. The fractions F2 and F3 were recrystallized (1:5 EtOAc/Hex) by slow evaporation to afford a pink solid 25 and a purple solid 26. Fraction F4 was purified by multiple rounds of PTLC using (2% MeCN/CHCl3) developing solvent mixture and gave compounds 27–29 as orange-yellow, red-pink, and dark purple solids, respectively.
3.3.3. Mechanistic Investigation of N,N′-Dialkylated Indirubin 26
A 50 mL round-bottomed flask containing a stirring bar was charged with anhydrous caesium carbonate (65.16 mg, 0.2 mmol). The solution of N,N′-dialkylated indirubin 26 (43.41 mg, 0.1 mmol) in anhydrous DMF (15 mL) was added, and the mixture was vigorously stirred at 110 °C under a nitrogen environment. After 3.5 h, the resulting mixture was poured into crushed ice (50 mL), and the organic layer was extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with H2O (1 × 40 mL), brine (1 × 30 mL), dried (MgSO4), and concentrated in vacuo. The crude mixture was purified by multiple rounds of preparative TLC using 1–2% MeCN/CHCl3 and afforded compounds 27 (13%), 28 (15%), and 29 (36%) as orange-yellow, red-pink, and dark-purple solids, respectively.
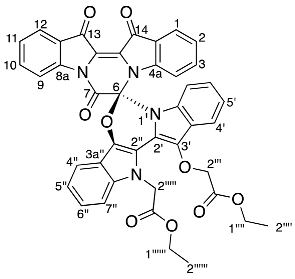
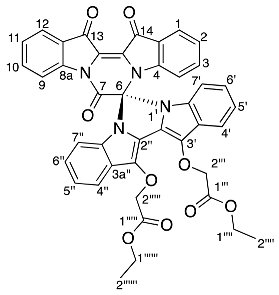
- Ethyl (R)-2-(13′-(2-ethoxy-2-oxoethoxy)-7,13,14-trioxo-13,14-dihydro-7H,12′H-spiro[pyrazino[1,2-a:4,3-a′]diindole-6,6′-[1,3]oxazino[3,4-a:5,6-b′]diindol]-12′-yl)acetate 17
Yield (18.7 mg, 4%), as an amorphous yellow solid. 1H NMR (500 MHz, CDCl3) δ 8.27 (1H, d, J = 8.2 Hz, H9), 7.99 (1H, d, J = 7.2 Hz, H12), 7.91 (1H, d, J = 7.1 Hz, H1), 7.61 (1H, td, J = 7.8, 1.2 Hz, H10), 7.59 (1H, d, J = 8.0 Hz, H4′), 7.49 (1H, d, J = 8.0 Hz, H4′′), 7.40 (1H, t, J = 7.3 Hz, H11), 7.31 (1H, td, J = 7.8, 1.3 Hz, H3), 7.26 (1H, m, H7′′), 7.25 (1H, m, H6′′), 7.14 (1H, t, J = 7.4 Hz, H2), 7.11 (1H, td, J = 7.6, 0.8 Hz, H5′), 7.08 (1H, td, J = 7.1, 1.6 Hz H5′′), 7.02 (1H, td, J = 7.1, 1.0 Hz, H6′), 6.96 (1H, d, J = 8.4 Hz, H7′), 6.87 (1H, d, J = 8.2 Hz, H4), 5.75 (1H, d, J = 18.0 Hz, H2′′′′a), 5.54 (1H, d, J = 18.0 Hz, H2′′′′b), 4.97 (2H, s, H2′′′), 4.31 (2H, qd, J = 7.2, 1.7 Hz, H1′′′′), 4.25 (2H, q, J = 7.2 Hz, H1′′′′′′), 1.31 (3H, t, J = 7.2 Hz, H2′′′′), 1.29 (3H, t, J = 7.2 Hz, H2′′′′′′). 13C NMR (126 MHz, CDCl3) 180.4 (C14), 178.6 (C13), 169.5 (C1′′′′′), 168.6 (C1′′′), 155.4 (C7), 148.0 (C4a), 144.4 (C8a), 136.9 (C3), 136.8 (C7a′′), 136.2 (C10), 133.5 (C7a′), 133.3 (C3′), 132.4 (C3′′), 126.8 (C11), 125.6 (C1), 125.5 (C12a), 124.8 (C6′), 124.7 (C12), 124.4 (C2), 124.2 (C7′′), 123.5 (C14a), 122.8 (C3a′), 122.2 (C5′), 120.5 (C5′′), 118.2 (C4′), 117.8 (C4′′), 117.8 (C9), 117.1 (C3a′′), 114.0 (C4), 113.9 (C2′′), 110.3 (C7′), 109.5 (C6′′), 92.6 (C6), 70.3 (C2′′′), 61.5 (C1′′′′′′), 61.4 (C1′′′′), 47.7 (C2′′′′′), 14.2 (C2′′′′′′), 14.1 (C2′′′′). FTIR (neat) max 2919 (s), 2850 (m), 1732 (s), 1595 (s), 1462 (s), 1372 (s), 1157 (s), 1095 (s), 902 (s), 738 (s), 685 (m), 425 (m) cm−1. LRMS-ESI (100%) 757 [M + Na]+, HRMS-ESI C42H31N4O9 calculated 735.2091, found 735.2086.
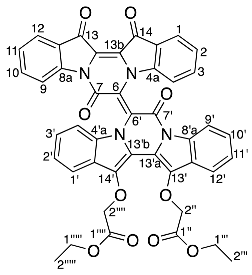
- Diethyl 2,2′-((7′,13′,14′-trioxo-13′,14′-dihydro-7′H-spiro[imidazo[1,5-a:3,4-a′]diindole-6,6′-pyrazino[1,2-a:4,3-a′]diindole]-12,13-diyl)bis(oxy))diacetate 18
Yield (30.2 mg, 8%), as an amorphous yellow solid, 1H NMR (500 MHz, CDCl3) 8.30 (1H, d, J = 8.2 Hz, H9), 8.06 (1H, d, J = 7.2 Hz, H12), 7.82 (1H, d, J = 7.0 Hz, H1), 7.72 (2H, d, J = 7.4 Hz, H4′),7.66 (1H, td, J = 7.8, 1.3 Hz, H10), 7.47 (1H, t, J = 7.1 Hz, H11), 7.24 (1H, td, J = 7.8, 1.3 Hz, H3), 7.07 (5H, m, H2, H5′ and H6′), 6.79 (2H, d, J = 7.8 Hz, H7′), 6.38 (1H, d, J = 8.3 Hz, H4), 5.25 (2H, d, J = 16.0 Hz, H2′′′a and H2′′′′′a), 5.15 (2H, d, J = 16.0 Hz, H2′′′b and H2′′′′′b), 4.31 (4H, q, J = 7.2 Hz, H1′′′′ and H1′′′′′′), 1.31 (6H, t, J = 7.2 Hz, H2′′′′ and H2′′′′′′). 13C NMR (126 MHz, CDCl3) 180.1 (C14), 178.5 (C13), 168.9 (C1′′′), 155.1 (C7), 147.4 (C4a), 143.9 (C8a), 137.2 (C3), 136.4 (C10), 132.0 (C3′), 130.2 (C7a′), 127.4 (C11), 126.2 (C3a′), 125.8 (C1), 125.5 (C12a), 125.0 (C6′), 124.8 (C12), 124.6 (C2), 124.4 (C13b), 123.0 (C14a), 121.6 (C5′), 120.2 (C4′), 117.8 (C9), 117.6 (C2′), 116.5 (C13a), 113.0 (C4), 108.4 (C7′), 82.6 (C6), 70.5 (C2′′′), 61.4 (C1′′′), 14.0 (C2′′′′). FTIR (neat) max 2919 (s), 2850 (m), 1703 (s), 1645 (s), 1462 (s), 1303 (s), 1192 (s), 1120 (s), 1014 (s), 937 (m), 738 (s), 685 (m), 538 (m) cm−1. LRMS-ESI (100%) 757 [M + Na]+. HRMS-ESI for C42H30N4O9Na calculated 757.1910, found 757.1927.
- Diethyl (E)-2,2′-(3,3′-dioxo-[2,2′-biindolinylidene]-1,1′-diyl) diacetate 19
Yield (98.2 mg, 22%), as a dark blue waxy solid, Rf (20% EtOAc/hexane) 0.53; mp. 244–245 °C, 1H NMR (400 MHz, CDCl3) δ 7.74 (1H, d, J = 7.7 Hz, H4 and H4′), 7.53 (1H, ddd, J = 8.2, 7.3, 1.3 Hz, H6 and H6′), 7.07 (1H, td, J = 7.4, 0.8 Hz, H5 and H5′), 7.02 (1H, d, J = 8.2, Hz, H7, and H7′), 4.82 (4H, s, H2′′ and H2′′′′), 4.21 (4H, q, J = 7.1 Hz, H1′′′ and H1′′′′′), 1.25 (6H, t, J = 7.1 Hz, H2′′′ and H2′′′′′). 13C NMR (101 MHz, CDCl3) δ 186.3 (C3 and C3′), 168.9 (C1′′ and C1′′′′), 153.7 (C7a and C7a′), 135.6 (C6 and C6′), 127.0 (C2 and C2′), 124.4 (C4 and C4′), 122.0 (C3a and C3a′), 121.9 (C5 and C5′), 111.0 (C7 and C7′), 61.4 (C1′′′ and C1′′′′′), 51.0 (C2′′ and C2′′′′), 14.1 (C2′′′ and C2′′′′′). FTIR (neat) max 2982 (s), 2935 (s), 1739 (m), 1650 (s), 1607 (s), 1470 (s), 1373 (s), 1202 (s), 1083 (s), 753 (s) cm−1. UV-vis (CH2Cl2) λmax/nm (ε M−1cm−1) 624.00 (2709096). LRMS-ESI (100%) 435 [M + H]+, HRMS-ESI for C24H22N2O6Na calculated 457.1363, found 457.1376.
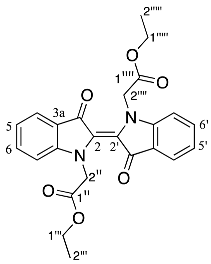
- Diethyl (E)-2,2′-((7,7′,13′,14′-tetraoxo-13′,14′-dihydro-7H,7′H-[6,6′-bipyrazino[1,2-a:4,3-a′]diindolylidene]-13,14-diyl)bis(oxy)) diacetate 20
Yield (18.9 mg, 4%), as a purple powder. Rf (30% EtOAc/P.S) 0.33; mp 214–220 °C. 1H NMR (500 MHz, CDCl3) 8.38 (1H, d, J = 8.4 Hz, H9), 8.35 (1H, d, J = 7.4 Hz, H9′), 8.04–7.96 (1H, dd, J = 0.9, 7.7 Hz, H1), 7.92 (1H, dd, J = 0.7, 7.6 Hz, H12), 7.90–7.88 (1H, m, H12′), 7.78–7.71 (1H, m, H1′), 7.59–7.54 (2H, m, H3 and H10), 7.53–7.45 (1H, m, H4′), 7.44–7.38 (1H, m, H4), 7.37–7.27 (6H, m, H2, H11, H2′, H3′, H10′ and H11′), 5.04 (1H, d, J = 16.1 Hz, H2′′b) 4.93 (1H, d, J = 15.8 Hz, H2′′′′b), 4.82 (1H, d, J = 16.1 Hz, H2′′a), 4.74 (1H, d, J = 15.8 Hz, H2′′′′a), 4.36–4.12 (4H, m, H1′′′ and H1′′′′′), 1.32 (3H, t, J = 7.2 Hz, H2′′′), 1.29 (3H, t, J = 7.2 Hz, H1′′′′′). 13C NMR (126 MHz, CDCl3) 178.6 (C14), 178.4 (C13), 169.1 (C1′′′′), 169.1 (C1′′), 154.8 (C7), 153.6 (C7′), 150.8 (C4a), 145.6 (C8a), 139.4 (C14′), 139.2 (C13′), 135.9 (C10), 135.8 (C3, C8a′), 132.9 (C4a′), 126.6 (C11′), 126.5 (C10′), 125.6 (C12a′), 125.5 (C3′), 125.4 (C1, C11), 125.1 (C2′), 124.9 (C12), 124.4 (C12a), 124.2 (C14a′), 123.4 (C14a), 123.2 (C2), 122.8 (C6), 120.8 (C13b), 119.6 (C12′), 119.0 (C13a), 118.8 (C1′), 118.2 (C9), 116.8 (C9′), 116.6 (C6′), 114.0 (C13b′), 113.4 (C13a′), 113.1 (C4), 112.5 (C4′), 71.4 (C2′′), 71.3 (C2′′′′), 61.3 (C1′′′), 61.2 (C1′′′′′′), 14.2 (C2′′′), 14.1 (C2′′′′′). FTIR (neat) max 1766 (m), 1716 (m), 1462 (m), 1299 (s), 1191 (s), 1063 (s), 755 (s) cm−1. LRMS-ESI (100%) 798 [M + Na]+, HRMS-ESI for calculated C44H30N4O10Na 797.1860, found 797.1859.
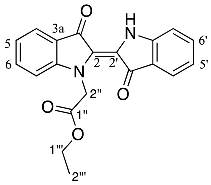
- Ethyl (E)-2-(3,3′-dioxo-[2,2′-biindolinylidene]-1-yl)acetate 21
Yield (95.8 mg, 27%), as an intense blue papery solid, Rf (20% EtOAc/hexane) 0.75; mp. 217–220 °C, 1H NMR (400 MHz, CDCl3) δ 10.58 (1H, s, NH), 7.77 (1H, d, J = 7.7 Hz, H4), 7.65 (1H, d, J = 7.6 Hz, H4′), 7.54 (1H, ddd, J = 8.4, 7.2, 1.3 Hz, H6), 7.46 (1H, ddd, J = 8.1, 7.2, 1.3 Hz, H6′), 7.06 (1H, ddd, J = 7.8, 7.2, 0.7 Hz, H5), 6.99 (1H, d, J = 8.0 Hz, H7′), 6.97 (1H, d, J = 8.1 Hz, H7), 6.93 (1H, ddd, J = 7.9, 7.3, 0.8 Hz, H5′), 5.34 (2H, s, H2′′), 4.21 (2H, q, J = 7.1 Hz, H1′′′), 1.26 (3H, t, J = 7.1 Hz, H2′′′). 13C NMR (101 MHz, CDCl3) δ 189.3 (C3), 187.6 (C3′), 169.0 (C1′′), 152.7 (C7a), 151.8 (C7a′), 136.4 (C6′), 135.9 (C6), 125.7 (C2′), 124.9 (C4′), 124.3 (C4), 123.4 (C2), 121.3 (C5), 121.1 (C3a), 120.7 (C5′), 120.1 (Ca′), 111.9 (C7′), 110.1 (C7), 61.5 (C1′′′), 49.0 (C2′′), 14.2 (C2′′′). FTIR (neat) max 3273 (s), 2926 (s), 2855 (s), 1739 (s), 1692 (s), 1640 (s), 1464 (s), 1430(s), 1209 (s), 1046 (s), 747 (s) cm−1. UV-vis (CH2Cl2) λmax/nm (ε M−1cm−1) 617.30 (164854), LRMS-ESI (100%) 349 [M + H]+, HRMS-ESI for C20H16N2O4Na calculated 371.1003, found 371.1008.
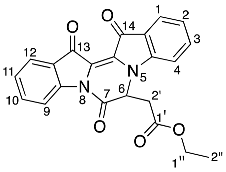
- Ethyl 2-(7,13,14-trioxo-6,7,13,14-tetrahydropyrazino[1,2-a:4,3-a′]diindol-6-yl) acetate 22
Yield (8.2 mg, 2%), as a dark pink powder, Rf (40% EtOAc/hexane) 0.61; mp. 210–216 °C, 1H NMR (400 MHz, CDCl3) δ 8.49 (1H, d, J = 8.2 Hz, H4), 7.94 (1H, d, J = 7.9 Hz, H1), 7.85 (1H, d, J = 8.1 Hz, H12), 7.69 (1H, ddd, J = 8.4, 7.3, 1.4 Hz, H3), 7.60 (1H, ddd, J = 7.7, 1.3 Hz, H10), 7.38 (1H, td, J = 7.6, 0.9 Hz, H2), 7.15 (1H, t, J = 7.5 Hz, H11), 7.04 (1H, d, J = 8.0 Hz, H9), 5.17 (1H, dd, J = 5.2, 4.0 Hz, H6), 4.01 (2H, q, J = 7.2, Hz, H1′′), 3.31–3.12 (2H, m, H2′), 1.06 (3H, t, J = 7.2 Hz, H2′′). 13C NMR (101 MHz, CDCl3) δ 180.3 (C13), 178.4 (C14), 168.8 (C1′), 160.6 (C7), 149.1 (C8a), 144.3 (C4a), 137.0 (C13a), 136.3 (C10), 135.6 (C3), 126.2 (C12), 128.0 (C13b), 126.0 (C2), 125.4 (C14a), 124.3 (C1), 122.9 (C11), 122.6 (C12a), 117.2 (C4), 110.0 (C9), 61.7 (C1′′), 54.1 (C6), 35.7 (C2′) 13.9 (C2′′). FTIR (neat) max 2927 (s), 2854 (s), 1728 (s), 1700 (s), 1640 (s), 1604 (s), 1467 (s), 1300 (s), 1208 (s), 1030 (s), 754 (s) cm−1. UV-vis (CH2Cl2) λmax/nm (ε M−1cm−1) 526.00 (1134143). LRMS-ESI (100%) 389 [M + H]+, HRMS-ESI for C22H16N2O5Na calculated 411.0957, found 411.0959.
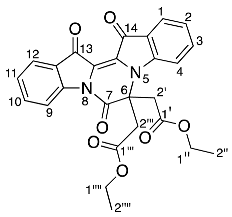
- Diethyl 2,2′-(7,13,14-trioxo-6,7,13,14-tetrahydropyrazino[1,2-a:4,3-a′]diindole-6,6-diyl) diacetate 23
Yield (15.4 mg, 3%), as a red waxy solid, Rf (40% EtOAc/hexane) 0.57; 1H NMR (400 MHz, CDCl3) δ 8.51 (1H, d, J = 8.2 Hz, H4), 7.96 (1H, d, J = 7.6 Hz, H1), 7.89 (1H, dd, J = 7.4, 1.7 Hz, H12), 7.70 (1H, ddd, J = 8.3, 7.4, 1.4 Hz, H3), 7.58 (1H, ddd, J = 8.4, 7.4, 1.5 Hz, H10), 7.38 (1H, td, J = 7.5, 0.9 Hz, H2), 7.17 (1H, d, J = 8.2 Hz, H9), 7.15 (1H, t, J = 7.5 Hz, H11), 3.92 (4H, q, J = 14.21 Hz, H1′′ and H1′′′′), 3.63–3.47 (4H, m, H2′ and H2′′′), 0.93 (6H, t, J = 14.26 Hz, H2′′, and H2′′′′). 13C NMR (101 MHz, CDCl3) δ 180.3 (C13), 178.7 (C14), 167.9 (C1′), 163.8 (C7), 148.3 (C8a), 144.2 (C4a), 135.9 (C10), 135.5 (C3), 131.3 (C13a), 126.3 (C12), 125.8 (C2), 125.4 (C14a), 124.3 (C1), 123.2 (C12a), 122.5 (C11), 117.8 (C13b), 117.1 (C4), 111.6 (C9), 63.5 (C6), 61.5 (C1′′ and C1′′′), 40.8 (C2′ and C2′′′), 13.8 (C2′′ and C2′′′′). FTIR (neat) max 2954 (s), 2925 (s), 2870 (s), 1735 (m), 1638 (s), 1604 (s), 1466 (s), 1197 (m), 1098 (s), 758 (s) cm−1. UV-vis (CH2Cl2) λmax/nm (ε M−1cm−1) 523.00 (4959190). LRMS-ESI (100%) 475 [M + H]+, HRMS-ESI for C26H22N2O7H calculated 475.1504, found 475.1505.
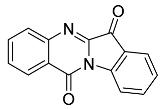
- Tryptanthrin 24
Yield 5.4 mg, 2%), yellow waxy solid. 1H NMR (400 MHz, CDCl3) δ 8.51 (d, J = 8.12 Hz, 1H), 8.39 (d, J = 1.22 Hz, 1H), 7.99 (d, J = 7.64 Hz, 1H), 7.86–7.72 (m, 3H), 7.60 (t, J = 7.1 Hz, 1H), 7.36 (t, J = 7.0, 0.56 Hz, 1H), LRMS-ESI 249 [M + H]+. This was consistent with the data in [13].
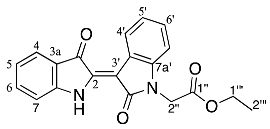
- Ethyl (Z)-2-(2′,3-dioxo-[2,3′-biindolinylidene]-1′-yl)acetate 25
Yield (1.8 mg, <1%), as a dark pink fluffy solid, Rf (20% EtOAc/hexane) 0.68; mp. 272–273 °C, 1H NMR (400 MHz, CDCl3) δ 10.46 (1H, s, NH), 8.92 (1H, d, J = 7.3 Hz, H4′), 7.74 (1H, d, J = 7.7 Hz, H4), 7.51 (1H, t, J = 8.0 Hz, H6), 7.30 (1H, td, J = 7.7, 1.2 Hz, H6′), 7.16 (1H, td, J = 7.7, 1.1 Hz, H5′), 7.02 (1H, td, J = 7.5 Hz, H5), 6.98 (1H, d, J = 7.9 Hz, H7), 6.78 (1H, d, J = 7.7 Hz, H7′), 4.58 (2H, s, H2′′), 4.24 (2H, q, J = 7.1 Hz, H1′′′), 1.27 (3H, t, J = 7.1 Hz, H2′′′). 13C NMR (101 MHz, CDCl3) δ 188.2 (C3), 170.7 (C2′), 167.6 (C1′′), 151.6 (C7a), 140.7 (C7a′), 139.7 (C3a′), 137.0 (C6), 129.2 (C6′), 125.7 (C4′), 125.3 (C4), 123.1 (C5′), 121.8 (C5), 121.2 (C2), 120.1 (C3a), 111.9 (C7), 107.8 (C7′), 106.0 (C3′), 61.7 (C1′′′), 41.3 (C2′′), 14.2 (C2′′′). FTIR (neat) max 3307 (s), 2920 (s), 1739 (s), 1654 (s), 1629 (s), 1469 (s), 1220 (s), 747 (s) cm−1. UV-vis (CH2Cl2) λmax/nm (ε M−1cm−1) 534.00 (50950), LRMS-ESI (100%) 349 [M + H]+, HRMS-ESI for C20H16N2O4Na calculated 371.1011, found 371.1008.

- Diethyl (Z)-2,2′-(2′,3-dioxo-[2,3′-biindolinylidene]-1,1′-diyl) diacetate 26
Yield (54.2 mg, 25%), as a dark purple fluffy solid, Rf (20% EtOAc/hexane) 0.48; mp. 151–152 °C, 1H NMR (500 MHz, CDCl3) δ 8.72 (1H, ddd, J = 8.0, 1.2, 0.6 Hz, H4′), 7.75 (1H, ddd, J = 7.6, 1.4, 0.6 Hz, H4), 7.56 (1H, ddd, J = 8.2, 7.4, 1.3 Hz, H6), 7.27 (1H, td, J = 7.7, 1.2 Hz, H6′), 7.12 (1H, td, J = 7.5, 0.8 Hz, H5), 7.08 (1H, td, J = 7.7, 1.1 Hz, H5′), 6.99 (1H, d, J = 8.1 Hz, H7), 6.70 (1H, dt, J = 7.8, 0.9 Hz, 7′), 4.86 (2H, s, H2′′′′), 4.53 (2H, s, H2′′), 4.26 (2H, q, J = 7.1 Hz, H1′′′), 4.22 (2H, q, J = 7.1 Hz, H1′′′′′), 1.27 (3H, t, J = 7.1 Hz, H2′′′′′). 1.25 (3H, t, J = 7.1 Hz, H2′′′). 13C NMR (126 MHz, CDCl3) δ 187.8 (C3), 168.9 (C1′′′′), 167.8 (C1′′), 166.9 (C2′), 154.3 (C7a), 143.2 (C2), 141.5 (C7a′), 136.7 (C6), 129.9 (C6′), 126.1 (C4′), 124.9 (C4), 122.9 (C5), 122.3 (C5′), 121.8 (C3a′), 121.4 (C3a), 111.4 (C7), 110.4 (C3′), 107.7 (C7′), 61.7 (C1′′′), 61.5 (C1′′′′′), 51.8 (C2′′′′), 41.5 (C2′′), 14.1 (C2′′′ and C2′′′′). FTIR (neat) max 2957 (s), 2850 (s), 1741 (s), 1680 (s), 1610 (s), 1470 (s), 1337 (s), 1205 (s), 753 (s) cm−1. UV-vis (CH2Cl2) λmax/nm (ε M−1cm−1) 555.50 (67336), LRMS-ESI (100%) 435 [M + H]+, HRMS-ESI for C24H22N2O6Na calculated 457.1377, found 457.1376.
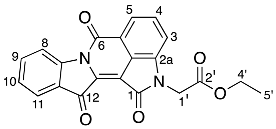
- Ethyl 2-(1,6,12-trioxo-6,12-dihydroindolo[1,2-b]pyrrolo[4,3,2-de]isoquinolin-2(1H)-yl) acetate 27
Yield (8.2 mg, 4%), as an orange yellow amorphous solid, Rf (3% MeCN/CHCl3) 0.86; 1H NMR (400 MHz, CDCl3) δ 8.81 (1H, d, J = 8.2 Hz, H8), 7.92 (1H, dd, J = 7.5, 2.1 Hz, H11), 7.87 (1H, d, J = 8.1 Hz, H5), 7.75 (1H, ddd, J = 8.3, 7.5, 1.4 Hz, H9), 7.57 (1H, t, J = 7.8 Hz, H4), 7.40 (1H, td, J = 7.5, 0.9 Hz, H10), 6.99 (1H, d, J = 7.6 Hz, H3), 4.62 (2H, s, H1′), 4.25 (2H, q, J = 7.1 Hz, H4′), 1.29 (3H, t, J = 7.1 Hz, H5′). 13C NMR (126 MHz, CDCl3) δ 180.8 (C12), 167.4 (C2′), 162.2 (C1), 159.0 (C6), 148.6 (C7a), 141.4 (C2a), 137.3 (C9), 134.9 (C12a), 132.4 (C4), 126.9 (C10), 126.5 (C2b), 125.6 (C5a), 125.3 (C11), 123.4 (C11a), 120.0 (C5), 118.5 (C8), 111.3 (C3), 109.9 (C12b), 62.0 (C4′), 41.8 (C1′), 14.2 (C5). FTIR (neat) max 2953 (s), 2917 (s), 2850 (s), 1731 (m), 1710 (m), 1624 (m), 1461 (s), 1337 (s), 1205 (s), 760 (s) cm−1. UV-vis (CH2Cl2) λmax/nm (ε M−1cm−1) 457.50 (2104), LRMS-ESI 375 [M + H]+, HRMS-ESI for C22H10N6O5Na calculated 397.0814, found 397.0815.
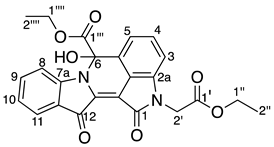
- Ethyl 2-(2-ethoxy-2-oxoethyl)-6-hydroxy-1,12-dioxo-1,2,6,12-tetrahydroindolo[1,2-b]pyrrolo[4,3,2-de]isoquinoline-6-carboxylate 28
Yield (5.4 mg, 2%), as a red, pink, waxy solid, Rf (3% MeCN/CHCl3) 0.28; mp. 280–281 °C, 1H NMR (500 MHz, CDCl3) δ 7.67 (1H, dd, J = 7.6, 1.3 Hz, H11), 7.52 (1H, ddd, J = 8.2, 7.4, 1.4 Hz, H9), 7.33 (1H, t, J = 7.8 Hz, H4), 7.22 (1H, d, J = 8.2, Hz, H8), 7.11 (1H, dd, J = 8.0, 0.5 Hz, H5), 7.08 (1H, td, J = 7.5, 0.8 Hz, H10), 6.69 (1H, d, J = 7.8 Hz, H3), 5.39 (1H, s, OH), 4.43 (1H, d, J = 17.7 Hz, H2′a), 4.31 (1H, d, J = 17.1 Hz, H2′b), 4.28–4.15 (4H, m, H1′′′′ and H1′′), 1.26 (3H, t, J = 7.1 Hz, H2′′′′), 1.08 (3H, t, J = 7.1 Hz, H2′′). 13C NMR (126 MHz, CDCl3) δ 181.8 (C12), 169.2 (C1′′′), 167.8 (C1′), 162.9 (C1), 150.3 (C7a), 139.6 (C2a), 136.4 (C9), 134.7 (C12a), 131.3 (C4), 126.7 (C5a), 125.4 (C11), 123.1 (C10), 121.8 (C11a), 119.2 (C2b), 117.7 (C5), 112.8 (C8), 108.5 (C3), 104.1 (C12b), 84.5 (C6), 63.9 (C1′′), 61.8 (C1′′′′), 41.5 (C2′), 14.1 (C2′′′′), 13.8 (C2′′). FTIR (neat) max 3376 (s), 2980 (s), 1744 (s), 1712 (s), 1592 (s), 1469 (s), 1303 (s), 1200 (s), 756 (s) cm−1. UV-vis (CH2Cl2) λmax/nm (ε M−1cm−1) 512.50 (3921), LRMS-ESI (100%) 449 [M + H]+, HRMS-ESI for C25H16N6O3Na for calculated 471.1182, found 471.1180.
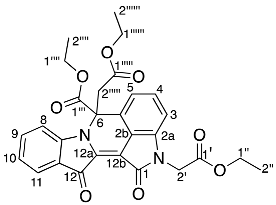
- Diethyl 2,2′-(6-(ethoxycarbonyl)-1,12-dioxo-6,12-dihydroindolo[1,2-b]pyrrolo[4,3,2-de]isoquinoline-2,6(1H)-diyl) diacetate 29
Yield (7.8 mg, 3%), as a dark purple waxy solid, Rf (3% MeCN/CHCl3) 0.75; 1H NMR (500 MHz, CDCl3) δ 7.86 (1H, dd, J = 7.6, 1.5 Hz, H11), 7.52 (1H, ddd, J = 8.2, 7.4, 1.4 Hz, H9), 7.24 (1H, t, J = 7.9 Hz, H4), 7.12 (1H, td, J = 7.5, 0.7 Hz, H10), 6.86 (1H, d, J = 7.8 Hz, H5), 6.84 (1H, d, J = 8.2 Hz, H8), 6.63 (1H, d, J = 7.6 Hz, H3), 4.57 (1H, d, J = 17.7 Hz, H2′a), 4.48 (1H, d, J = 17.7 Hz, H2′b), 4.27 (2H, q, J = 7.1 Hz, H1′′′′), 4.22 (2H, q, J = 7.1 Hz, H1′′), 3.81 (2H, q, J = 7.1 Hz, H1′′′′′′), 3.63 (1H, d, J = 15.6 Hz, H2′′′′′a), 3.46 (1H, d, J = 15.5 Hz, H2′′′′′b), 1.28 (3H, t, J = 7.1 Hz, H2′′), 1.10 (3H, t, J = 7.1 Hz, H2′′′′), 0.86 (3H, t, J = 7.1 Hz, H2′′′′′′). 13C NMR (126 MHz, CDCl3) δ 181.5 (C12), 169.0 (C1′′′), 168.0 (C1′′′′′), 167.8 (C1′), 162.8 (C1), 150.3 (C7a), 140.0 (C2a), 136.3 (C12a), 136.0 (C9), 131.3 (C4), 126.0 (C5a), 125.9 (C11), 122.4 (C10), 121.9 (C11a), 119.2 (C2b), 116.5 (C5), 110.8 (C8), 108.0 (C3), 105.0 (C12b), 66.2 (C6), 63.4 (C1′′′′), 61.7 (C1′′), 61.0 (C1′′′′′′), 41.6 (C2′), 41.5 (C2′′′′′), 14.1 (C2′′), 13.8 (C2′′′′), 13.6 (C2′′′′′′). FTIR (neat) max 2956 (s), 2920 (s), 2851 (s), 1735 (s), 1720 (s), 1629 (s), 1604 (s), 1469 (s), 1302 (s), 1198 (s), 757 (s) cm−1. UV-vis (CH2Cl2) λmax/nm (ε M−1cm−1) 558.00 (3907), LRMS-ESI 519 [M + H]+, HRMS-ESI for C28H26N2O8H calculated 519.1781, found 519.1767.
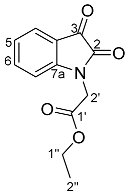
- Ethyl 2-(2,3-dioxo-1H-indol-1-yl)acetate 30
(Yield 3.5 mg, 3%), yellow waxy solid. 1H NMR (400 MHz, CDCl3) δ 8.63 (d, J = 8.4 Hz, 1H), 7.91 (d, J = 7.9 Hz, 1H), 7.63–7.55 (m, 1H), 7.40 (t, J = 7.5 Hz, 1H), 5.36 (s, 2H), 4.24 (q, J = 7.1 Hz, 2H), 1.27 (t, J = 7.2 Hz, 3H). LRMS-ESI 234 [M + H]+. This data were consistent with that reported in reference [28].
4. Conclusions
This study explored the reaction of indigo 1 with ethyl bromoacetate 16 in the presence of caesium carbonate as a base, revealing the new polyheterocyclic compounds 17, 18 and 20, albeit in low yield. Further optimisation of the reaction allowed enhanced control over the reaction to furnish key compounds 19, and 21–23, highlighting the influence of the dual electrophilic sites in ethyl bromoacetate 16 in these cascade processes. The acidic methylene hydrogen involvement led to numerous side reactions, resulting in high reactivity with less control of the reaction. Despite this, the optimised reactions yielded a diverse range of new polyheterocyclic compounds that could be challenging to prepare from a step-by-step synthesis. The cascade reaction of ethyl bromoacetate 16 with the more stable (and less reactive) indirubin 1a under basic conditions yielded representatives of a new heterocyclic class, in particular dihydroindolopyrroloisoquinoline-2-accetate 27, the tetrahydroindolopyrroloisoquinoline-6-carboxylate 28, and the dihydroindolopyrroloisoquinoline-2,6-diacetate 29, as well as N- and N,N′-alkylation products 25 and 26 respectively. Detailed mapping of the proposed cascade mechanistic pathways to these compounds is presented which should be useful in informing any future synthetic studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29174242/s1, Reports all NMR data for synthesised compounds, the X-ray crystallographic data for compounds 17, 18 and 20, and the proposed in situ synthesis of compounds 33 and 59.
Author Contributions
Conception and design, S.A., P.M.M., P.A.K., J.B.B. and S.G.P.; development of methodology, S.A., P.M.M., P.A.K. and J.B.B.; acquisition of data S.A., P.M.M. and A.C.W.; analysis and interpretation of data S.A., P.M.M., P.A.K., J.B.B. and C.R.; writing—review and/or revision of the manuscript, S.A., P.A.K. and J.B.B.; study supervision, P.A.K., S.G.P. and J.B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
SA acknowledges the UOW for the provision of a scholarship. PMM acknowledges the UOW and the Australian Department of Education for financial assistance through the Research Training Program and Postgraduate Endeavour Scholarship, respectively. This research was conducted with assistance and resources from the School of Chemistry and Molecular Bioscience (UOW), the Centre for Molecular and Medical Bioscience, Molecular Horizons (UOW), and the Research School of Chemistry (ANU). We also thank Michael Gardner (ANU) for guidance in the X-ray data presentation.
Conflicts of Interest
The authors declare that they have no competing financial interests.
References
- Choi, K.-Y. Pigments, A review of recent progress in the synthesis of bio-indigoids and their biologically assisted end-use applications. Dye. Pigment. 2020, 181, 108570. [Google Scholar] [CrossRef]
- Ma, L.; Sun, T.; Liu, Y.; Zhao, Y.; Liu, X.; Li, Y.; Chen, X.; Cao, L.; Kang, Q.; Guo, J. Enzymatic synthesis of indigo derivatives by tuning P450 BM3 peroxygenases. Synth. Syst. Biotechnol. 2023, 8, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Hecht, S. A blueprint for transforming indigos to photoresponsive molecular tools. Chem. Eur. J. 2023, 29, e202300981. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Bora, S.R.; Bishen, S.M.; Mishra, H.; Kalita, D.J.; Wahab, A. Photophysics of a monoannulated indigo: Intra-and intermolecular charge transfer. J. Phys. Chem. A 2024, 128, 2565–2573. [Google Scholar] [CrossRef]
- Kaplan, G.; Seferoğlu, Z.; Berdnikova, D.V. Photochromic derivatives of indigo: Historical overview of development, challenges and applications. Beilstein J. Org. Chem. 2024, 20, 228–242. [Google Scholar] [CrossRef]
- Reva, I.; Lapinski, L.; Nowak, M.J. Stability of monomeric indigo in the electronic ground state: An experimental matrix isolation infrared and theoretical study. Dyes. Pigment. 2024, 222, 111827. [Google Scholar] [CrossRef]
- Xu, H.; Chakraborty, R.; Adak, A.K.; Das, A.; Yang, B.; Meier, D.; Riss, A.; Reichert, J.; Narasimhan, S.; Barth, J.V.; et al. On-surface isomerization of indigo within 1D coordination polymers. Angew. Chem. Int. Ed. 2024, 136, e202319162. [Google Scholar] [CrossRef]
- Shakoori, A.; Bremner, J.B.; Willis, A.C.; Haritakun, R.; Keller, P.A. Rapid cascade synthesis of poly-heterocyclic architectures from indigo. J. Org. Chem. 2013, 78, 7639–7647. [Google Scholar] [CrossRef]
- Shakoori, A.; Bremner, J.B.; Abdel-Hamid, M.K.; Willis, A.C.; Haritakun, R.; Keller, P.A. Further exploration of the heterocyclic diversity accessible from the allylation chemistry of indigo. Beilstein J. Org. Chem. 2015, 11, 481–492. [Google Scholar] [CrossRef]
- Butler, N.M.; Hendra, R.; Bremner, J.B.; Willis, A.C.; Lucantoni, L.; Avery, V.M.; Keller, P.A. Cascade reactions of indigo with oxiranes and aziridines: Efficient access to dihydropyrazinodiindoles and spiro-oxazocinodiindoles. Org. Biomol. Chem. 2018, 16, 6006–6016. [Google Scholar] [CrossRef]
- McCosker, P.M.; Butler, N.M.; Shakoori, A.; Perry, M.J.; Mullen, J.W.; Willis, A.C.; Clark, T.; Bremner, J.B.; Guldi, D.M.; Keller, P.A.; et al. The cascade reactions of indigo with propargyl substrates for heterocyclic and photophysical diversity. Chem. Eur. J. 2021, 27, 3708–3721. [Google Scholar] [CrossRef]
- Sele, A.M.; Bremner, J.B.; Willis, A.C.; Haritakun, R.; Griffith, R.; Keller, P.A. A cascade synthetic route to new bioactive spiroindolinepyrido [1, 2-a] indolediones from indirubin. Tetrahedron 2015, 71, 8357–8367. [Google Scholar] [CrossRef]
- Kaur, R.; Manjal, S.K.; Rawal, R.K.; Kumar, K.J. B. Recent synthetic and medicinal perspectives of tryptanthrin. Bioorg. Med. Chem. 2017, 25, 4533–4552. [Google Scholar] [CrossRef]
- Pinheiro, D.; Pineiro, M.; Pina, J.; Brandão, P.; Galvão, A.M.; Seixas de Melo, J.S. Tryptanthrin from indigo: Synthesis, excited state deactivation routes and efficient singlet oxygen sensitization. Dye. Pigment. 2020, 175, 108125. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Bonasera, A.; Hristov, L.; Garmshausen, Y.; Schmidt, B.M.; Jacquemin, D.; Hecht, S. N,N′-disubstituted indigos as readily available red-light photoswitches with tunable thermal half-lives. J. Am. Chem. Soc. 2017, 139, 15205–15211. [Google Scholar] [CrossRef]
- Kihara, Y.; Tani, S.; Higashi, Y.; Teramoto, T.; Nagasawa, Y. Ultrafast excited state dynamics of forward and reverse trans-cis photoisomerization of red-light-absorbing indigo derivatives. J. Phys. Chem. B 2022, 126, 3539–3550. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Wei, C.; Wang, J.; Xu, Y.; Bai, G.; Yao, Q.; Zhang, L.; Chen, Y. Anticancer potential of indirubins in medicinal chemistry: Biological activity, structural modification, and structure-activity relationship. J. Med. Chem. 2021, 223, 113652. [Google Scholar] [CrossRef]
- Jautelat, R.; Brumby, T.; Schäfer, M.; Briem, H.; Eisenbrand, G.; Schwahn, S.; Krüger, M.; Lücking, U.; Prien, O.; Siemeister, G. From the insoluble dye indirubin towards highly active, soluble CDK2-inhibitors. ChemBioChem 2005, 6, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Eisenbrand, G.; Hippe, F.; Jakobs, S.; Muehlbeyer, S. Molecular mechanisms of indirubin and its derivatives: Novel anticancer molecules with their origin in traditional Chinese phytomedicine. J. Cancer Res. Clin. Oncol. 2004, 130, 627–635. [Google Scholar] [CrossRef]
- Alex, D.; Lam, I.K.; Lin, Z.; Lee, S.M. Y. Indirubin shows anti-angiogenic activity in an in vivo zebrafish model and an in vitro HUVEC model. J. Ethnopharmacol. 2010, 131, 242–247. [Google Scholar] [CrossRef]
- Meijer, L.; Shearer, J.; Bettayeb, K.; Ferandin, Y. Diversity of the Intracellular Mechanisms Underlying the Anti-Tumor Properties of Indirubins. Int. Congr. Ser. 2007, 1304, 60–74. [Google Scholar]
- Zhu, J.; Cai, Y.; Kong, M.; Li, Y.; Zhu, L.; Zhang, J.; Yu, Z.; Xu, S.; Hong, L.; Chen, C.; et al. Design, Synthesis, and Biological Evaluation for First GPX4 and CDK Dual Inhibitors. J. Med. Chem. 2024, 67, 2758–2776. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Chen, X.; Yin, F.; Li, S.; Zhang, Y.; Luo, H.; Luo, Z.; Cui, N.; Chen, Y.; Li, X.; et al. Indirubin derivatives as bifunctional molecules inducing DNA damage and targeting PARP for the treatment of cancer. Eur. J. Med. Chem. 2023, 261, 115843. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-W.; He, J.-B.; You, Y.-H.; Zhang, Y.-M.; Zhang, S.-L. Spectroelectrochemistry of solid indirubin and its sulfonated form. Electrochim. Acta 2011, 56, 1219–1226. [Google Scholar] [CrossRef]
- Nobre, D.C.; Delgado-Pinar, E.; Cunha, C.; Galvao, A.M.; Seixas de Melo, J.S. Indirubin: Nature finding efficient light-activated protective molecular mechanisms. Dye. Pigment. 2023, 212, 111116. [Google Scholar] [CrossRef]
- Görner, H.; Pouliquen, J.; Kossanyi, J. Trans to cis photoisomerization of N,N′-disubstituted indigo dyes via excited singlet states; a laser flash photolysis and steady state irradiation study. Can. J. Chem. 1987, 65, 708–717. [Google Scholar] [CrossRef]
- Wang, C.; Yan, J.; Du, M.; Burlison, J.A.; Li, C.; Sun, Y.; Zhao, D.; Liu, J. One step synthesis of indirubins by reductive coupling of isatins with KBH4. Tetrahedron 2017, 73, 2780–2785. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; Ramadan, E.S.; Hamid, H.A.; Hagar, M. Microwave irradiation for enhancing the regioselective synthesis of 6H-indolo [2,3-b] quinoxalines. J. Chem. Res. 2005, 2005, 229–232. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).