Effect of Ionic Liquids with Different Structures on Rheological Properties of Water-Based Drilling Fluids and Mechanism Research at Ultra-High Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
- (1)
- Preparation of drilling fluid
- (2)
- Performance testing
- (3)
- Mechanism analysis
3. Results and Discussion
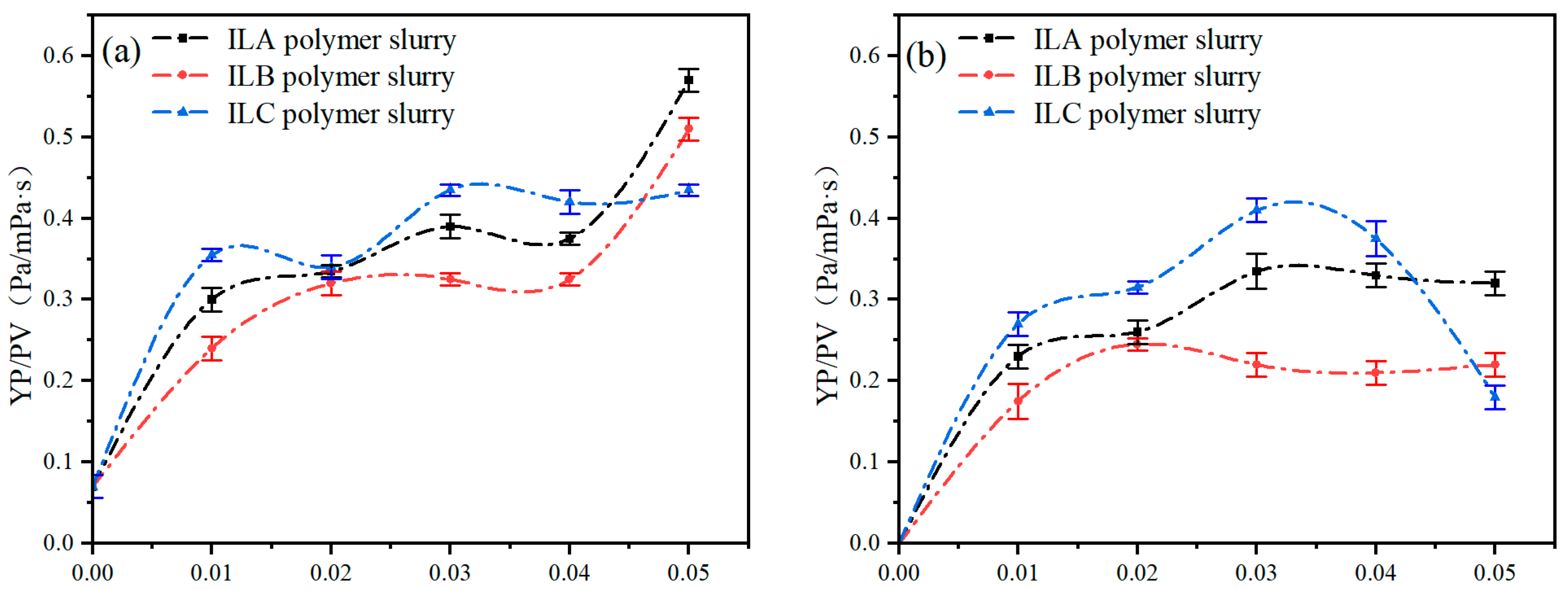
3.1. Effect of ILs with Different Anionic Structures on the Rheological Properties of Polymer Slurries
3.2. Effect of ILs with Different Cationic Structures on the Rheological Properties of Polymer Slurries
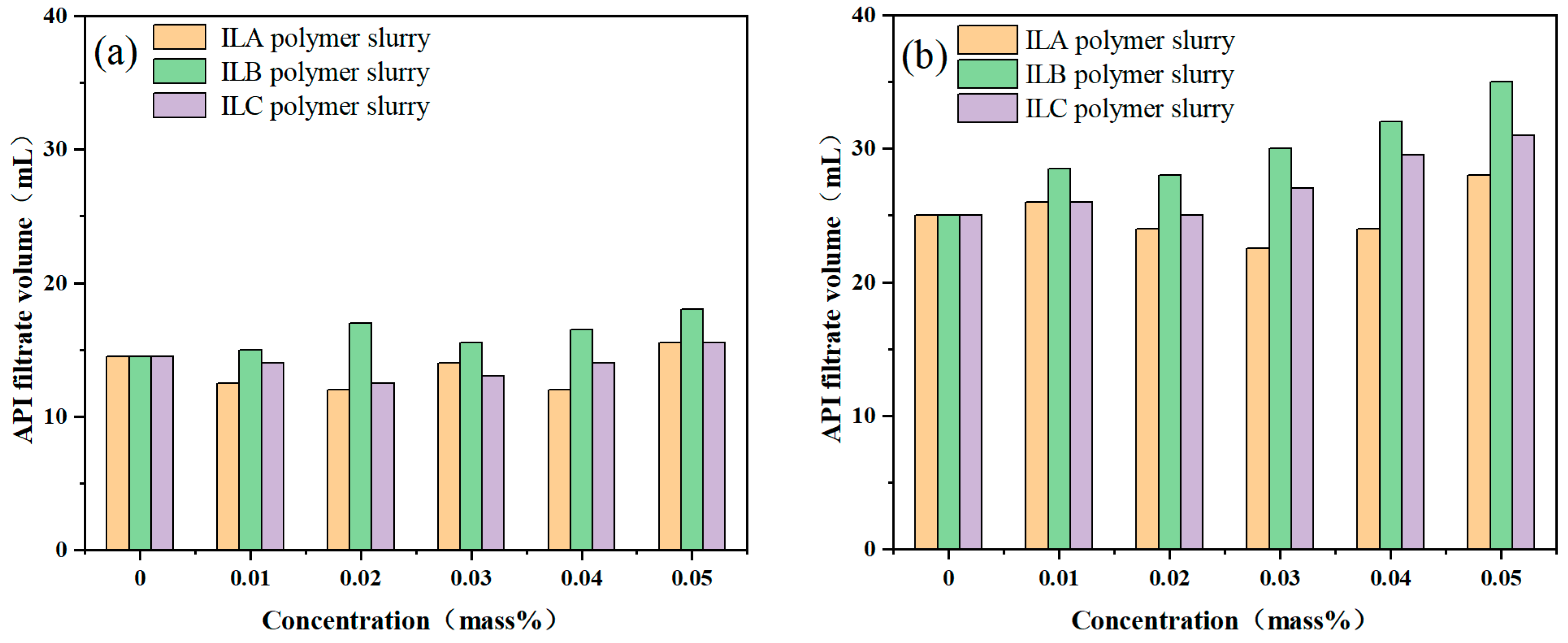
3.3. Influence of ILs on the Filtration Loss of Polymer Slurries
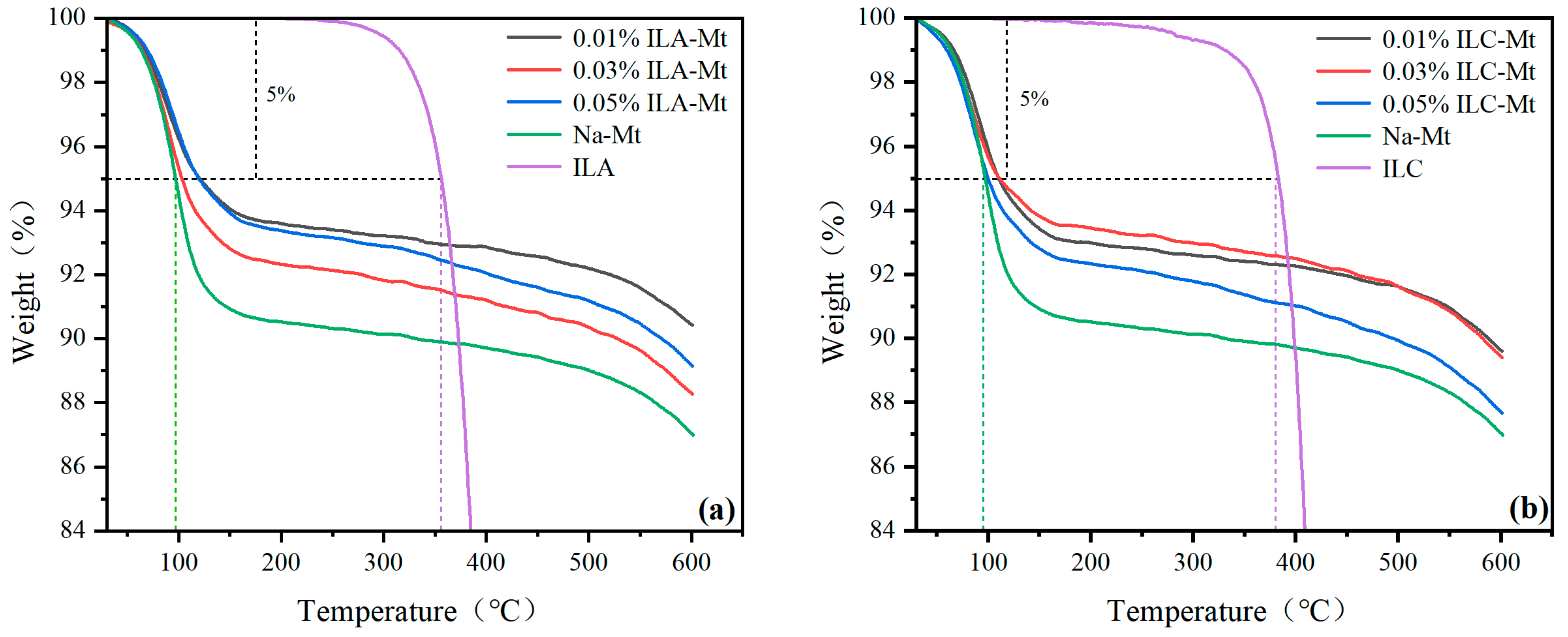
3.4. TGA
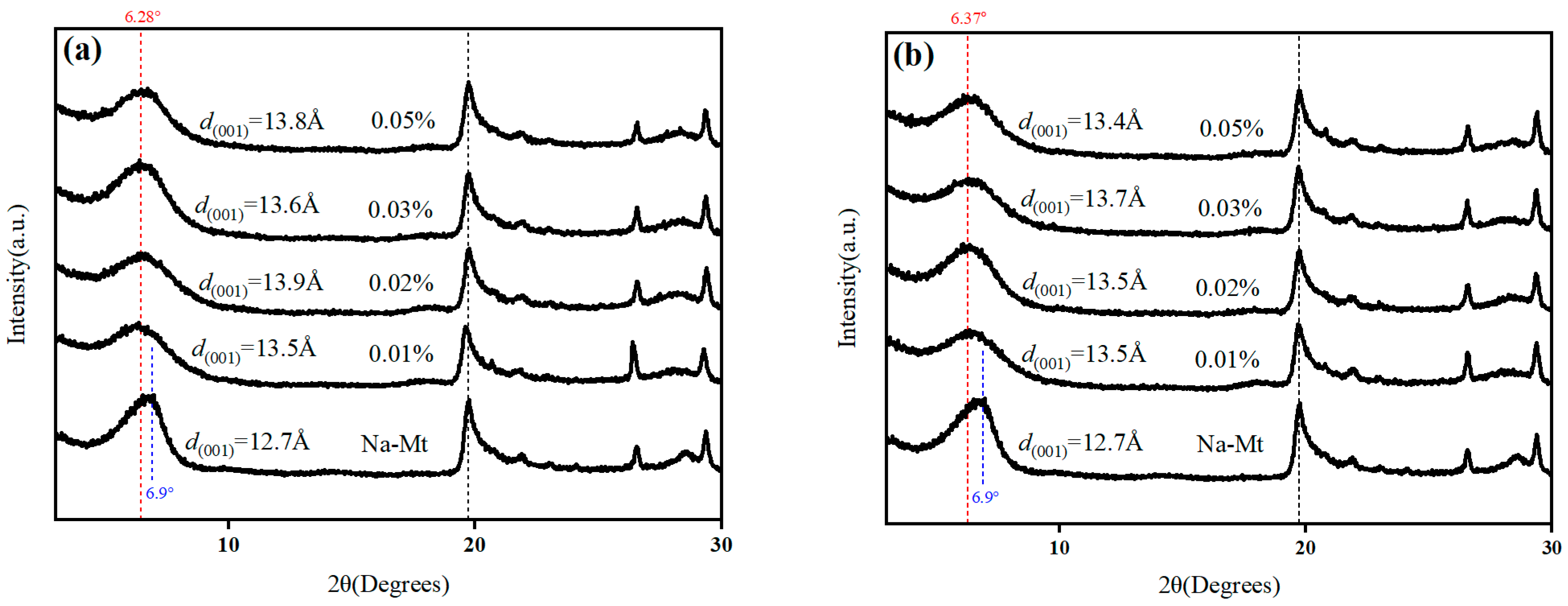
3.5. XRD
3.6. Contact Angle
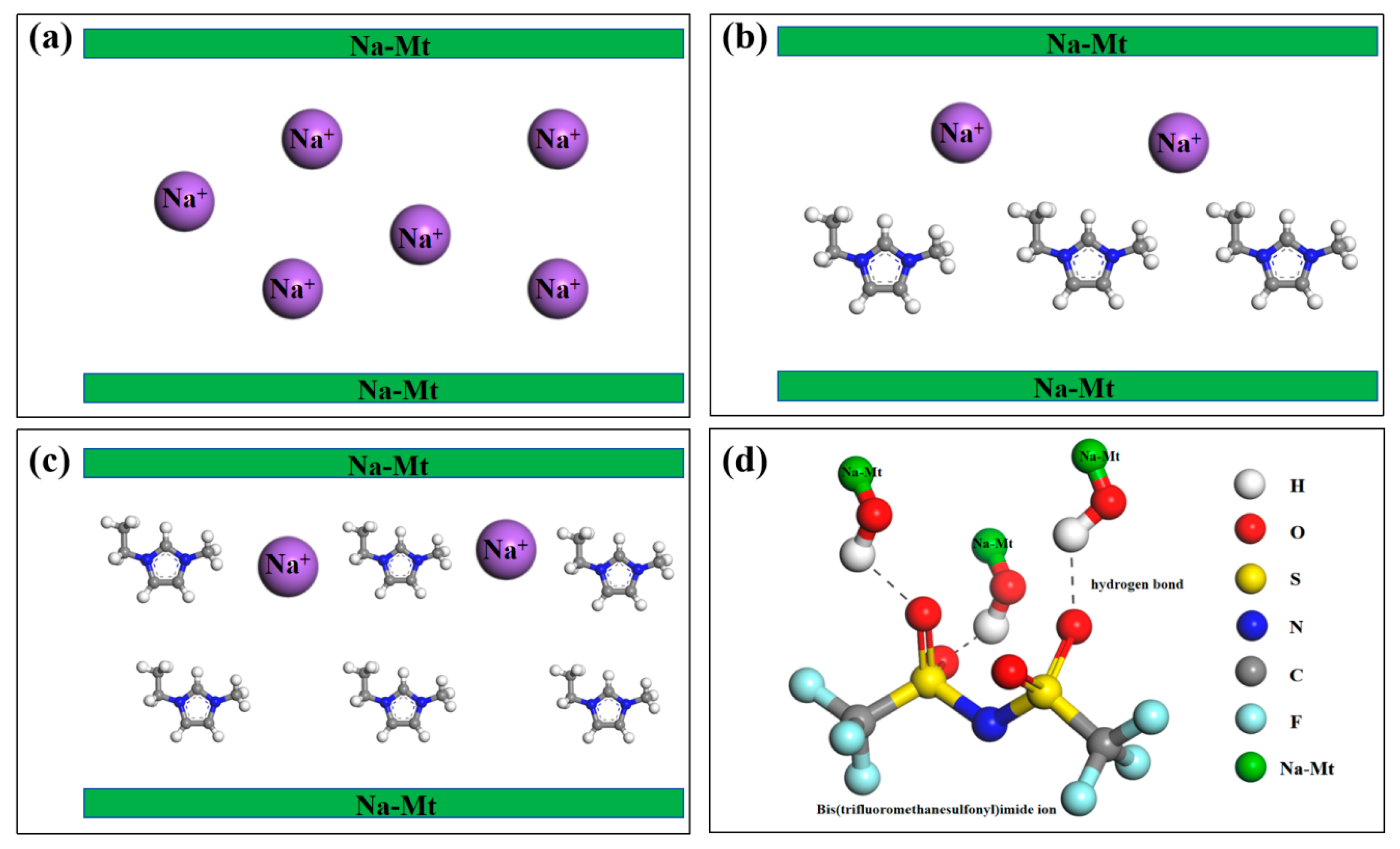
4. Mechanism Analysis
5. Conclusions
- (1)
- Compared with the PAM slurry without ionic liquids, the YP/PV ratio of PAM slurry formulated with the three ionic liquids at low concentrations increased significantly after aging at ultra-high temperatures of 200 °C and 240 °C. ILA and ILB with different anion structures can improve the ultra-high temperature rheological properties of aqueous drilling fluids, and ILA and ILC with different cation structures can also improve the ultra-high temperature rheological properties of aqueous drilling fluids.
- (2)
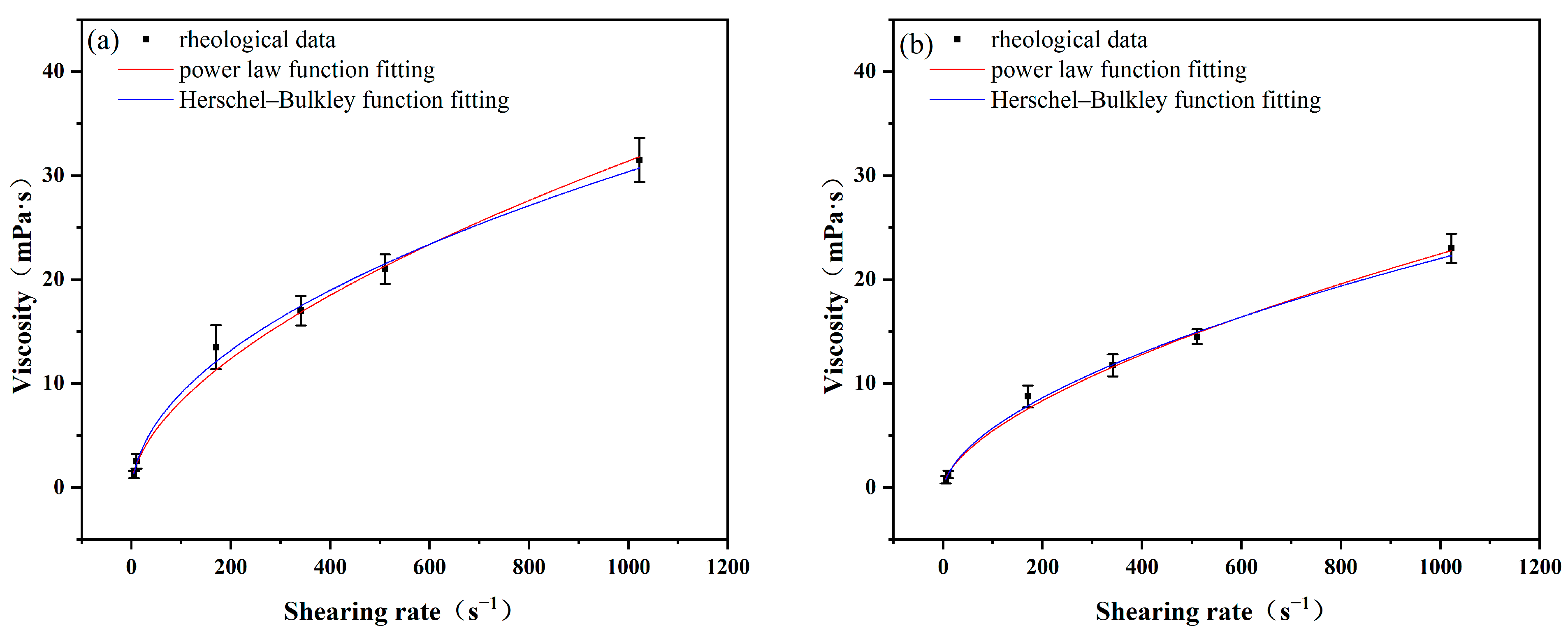
- The TG thermal stability of N-methyl, butylpyrrolidinium bis(trifluoromethanesulfonimide) (ILC) is higher than that of 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonimide) (ILA), and that of 1-ethyl-3-methylimidazolium tetrafluoroborate (ILA) is higher than that of 1-ethyl-3-methylimidazolium tetrafluoroborate (ILB), which is consistent with the fact that the three ionic liquids improve the YP/PV ratio of drilling fluids under ultra-high temperatures. Both the Herschel–Bulkley model and the Power law model can be fitted with the rheological model of PAM slurries formulated with ILA and ILC.
- (3)
- The cationic interlayer exchange between the organic cations of ionic liquids and sodium montmorillonite can enhance the ultra-high temperature stability of sodium montmorillonite and improve the rheological properties of aqueous drilling fluids; the s=o bond in bis(trifluorosulfonyl)imide ions and the hydroxyl group in sodium montmorillonite may form hydrogen bonds to improve the rheology of water-based drilling fluids.
- (4)
- None of the three ionic liquids—ILA, ILB and ILC—can reduce the ultra-high temperature filtration loss of PAM-based drilling fluids.
- (5)
- ILA, ILB and ILC may be used to improve the rheological properties of drilling fluids at ultra-high temperatures in deep or ultra-deep drilling because of the higher thermal stability they provide.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IEO. International Energy Outlook; U.S. Energy Information Administration (EIA): Washington, DC, USA, 2013. [Google Scholar]
- Yang, E.; Fang, Y.; Liu, Y.; Li, Z.; Wu, J. Research and application of microfoam selective water plugging agent in shallow low-temperature reservoirs. J. Petrol. Sci. Eng. 2020, 193, 107354. [Google Scholar] [CrossRef]
- Pang, X.-Q.; Jia, C.-Z.; Wang, W.-Y. Petroleum geology features and research developments of hydrocarbon accumulation in deep petroliferous basins. Petrol. Sci. 2015, 12, 1–53. [Google Scholar] [CrossRef]
- Si, N.; Ye, H.; Niu, X.; Wang, L.; Feng, J.; Jin, R. Analysis on the Adaptability of Oil and Gas Drilling Technologies in Development for Hot Dry Rocks. Pet. Drill. Tech. 2019, 47, 35–40. [Google Scholar]
- Shadravan, A.; Amani, M. HPHT 101-what every engineer or geoscientist should know about high pressure high temperature wells. In Proceedings of the SPE Kuwait International Petroleum Conference and Exhibition, Kuwait City, Kuwait, 12–14 December 2012; p. SPE-163376-MS. [Google Scholar]
- Sun, J.; Huang, X.; Lv, K.; Shao, Z.; Meng, X.; Wang, J.; Li, W. Methods, technical progress and research advance of improving high-temperature stability of water based drilling fluids. J. China Univ. Pet. 2019, 43, 73–81. [Google Scholar]
- Luo, Z.; Pei, J.; Wang, L.; Yu, P.; Chen, Z. Influence of an ionic liquid on rheological and filtration properties of water-based drilling fluids at high temperatures. Appl. Clay Sci. 2017, 136, 96–102. [Google Scholar] [CrossRef]
- Caenn, R.; Darley, H.C.H.; Gray, G.R. Composition and Properties of Drilling and Completion Fluids; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Elward-Berry, J.; Darby, J.B. Rheologically stable, nontoxic, high-temperature water-base drilling fluid. SPE Drill. Complet. 1997, 12, 158–162. [Google Scholar] [CrossRef]
- Liu, J.; Dai, Z.; Xu, K.; Yang, Y.; Lv, K.; Huang, X.; Sun, J. Water-based drilling fluid containing bentonite/poly (sodium 4-styrenesulfonate) composite for ultrahigh-temperature ultra-deep drilling and its field performance. SPE J. 2020, 25, 1193–1203. [Google Scholar] [CrossRef]
- Lin, L.; Luo, P. Amphoteric hydrolyzed poly (acrylamide/dimethyl diallyl ammonium chloride) as a filtration reducer under high temperatures and high salinities. J. Appl. Polym. Sci. 2015, 132, 41581. [Google Scholar] [CrossRef]
- Huo, J.; Peng, Z.; Ye, Z.; Feng, Q.; Zheng, Y.; Zhang, J.; Liu, X. Investigation of synthesized polymer on the rheological and filtration performance of water-based drilling fluid system. J. Pet. Sci. Eng. 2018, 165, 655–663. [Google Scholar] [CrossRef]
- Su, J.; Chu, Q.; Ren, M. Properties of high temperature resistance and salt tolerance drilling fluids incorporating acrylamide/2-acrylamido-2-methyl-1-propane sulfonic acid/N-vinylpyrrolidone/dimethyl diallyl ammonium chloride quadripolymer as fluid loss additives. J. Polym. Eng. 2014, 34, 153–159. [Google Scholar] [CrossRef]
- Tehrani, M.A.; Popplestone, A.; Guarneri, A.; Carminati, S. Water-Based Drilling Fluid for HT/HP Applications. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007. [Google Scholar]
- Li, Z.; Pu, X.; Tao, H.; Liu, L.; Su, J. Synthesis and properties of acrylamide 2-acrylamido-2-methypropane sulfonic acid sodium styrene sulfonate N-vinyl pyrrolidone quadripolymer and its reduction of drilling fluid filtration at high temperature and high salinity. J. Polym. Eng. 2014, 34, 125–131. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, C.; Qiu, Z.; Leong, Y.K.; Zhong, H.; Cao, J. Synthesis, characterization and evaluation of a quadripolymer with low molecular weight as a water based drilling fluid viscosity reducer at high temperature (245 °C). Polym. Int. 2015, 64, 1352–1360. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Zheng, T.; Liu, H.; Zhang, M.; Zhang, R.; Si, C. Recent advances in cellulose and its derivatives for oilfield applications. Carbohydr. Polym. 2021, 259, 117740. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Qiu, Z.; Shen, Z.; Huang, W.; Zhong, H.; Dai, W. Novel hydrophobic associated polymer based nano-silica composite with core–shell structure for intelligent drilling fluid under ultra-high temperature and ultra-high pressure. Prog. Nat. Sci. Mater. Int. 2015, 25, 90–93. [Google Scholar] [CrossRef]
- Ofei, T.N.; Bavoh, C.B.; Rashidi, A.B. Insight into ionic liquid as potential drilling mud additive for high temperature wells. J. Mol. Liq. 2017, 242, 931–939. [Google Scholar] [CrossRef]
- Huang, X.; Lv, K.; Sun, J.; Lu, Z.; Bai, Y.; Shen, H.; Wang, J. Enhancement of thermal stability of drilling fluid using laponite nanoparticles under extreme temperature conditions. Mater. Lett. 2019, 248, 146–149. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, L.; Pei, J.; Yu, P.; Xia, B. A novel star-shaped copolymer as a rheology modifier in water-based drilling fluids. J. Pet. Sci. Eng. 2018, 168, 98–106. [Google Scholar] [CrossRef]
- Mao, H.; Yang, Y.; Zhang, H.; Huang, Y. A critical review of the possible effects of physical and chemical properties of subcritical water on the performance of water-based drilling fluids designed for ultra-high temperature and ultra-high pressure drilling applications. J. Pet. Sci. Eng. 2020, 187, 106795. [Google Scholar] [CrossRef]
- Amanullah, M.; Alarfaj, M.K.; Al-Abdullatif, Z.A. Preliminary test results of nano-based drilling fluids for oil and gas field application. In Proceedings of the SPE/IADC Drilling Conference and Exhibition, Amsterdam, The Netherlands, 1–3 March 2011; pp. 1–9. [Google Scholar]
- Anwar, A.; Erum, P.; Tayyaba, N. Applications of Emerging Nanomaterials in Drilling Fluids. ChemistrySelect 2022, 7, e202202383. [Google Scholar]
- Aftab, A.; Ismail, A.R.; Ibupoto, Z.H. Enhancing the rheological properties and shale inhibition behavior of water-based mud using nanosilica, multi-walled carbon nanotube, and graphene nanoplatelet. Egypt. J. Pet. 2017, 26, 291–299. [Google Scholar] [CrossRef]
- Kosynkin, D.V.; Ceriotti, G.; Wilson, K.C.; Lomeda, J.R.; Scorsone, J.T.; Patel, A.D.; Tour, J.M. Graphene oxide as a high-performance fluid-loss-control additive in water-based drilling fluids. ACS Appl. Mater. Interfaces 2012, 4, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Alsaba, M.; Al Marshad, A.; Abbas, A.; Abdulkareem, T.; Al-Shammary, A.; Al-Ajmi, M.; Kebeish, E. Laboratory evaluation to assess the effectiveness of inhibitive nano-water-based drilling fluids for Zubair shale formation. J. Pet. Explor. Prod. Technol. 2020, 10, 419–428. [Google Scholar] [CrossRef]
- Mady, A.; Mahmoud, O.; Dahab, A.S. Nanoparticle-based drilling fluids as promising solutions to enhance drilling performance in Egyptian oil and gas fields. Int. J. Ind. Sustain. Dev. 2020, 1, 24–38. [Google Scholar] [CrossRef][Green Version]
- Mahmoud, O.; Nasr-El-Din, H.A.; Vryzas, Z.; Kelessidis, V.C. Nanoparticle-based drilling fluids for minimizing formation damage in HP/HT applications. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, LO, USA, 24–26 February 2016; pp. 1–26. [Google Scholar]
- Ahmed Khan, R.; Murtaza, M.; Abdulraheem, A.; Kamal, M.S.; Mahmoud, M. Imidazolium-based ionic liquids as clay swelling inhibitors: Mechanism, performance evaluation, and effect of different anions. ACS Omega 2020, 5, 26682–26696. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, G.; Shi, Y.; Yang, X. Application of ionic liquid and polymeric ionic liquid as shale hydration inhibitors. Energy Fuels 2017, 31, 4308–4317. [Google Scholar] [CrossRef]
- Wróbel, P.; Kubisiak, P.; Eilmes, A. Hydrogen Bonding and Infrared Spectra of Ethyl-3-methylimidazolium Bis (trifluoromethylsulfonyl) imide/Water Mixtures: A View from Molecular Dynamics Simulations. J. Phys. Chem. B 2022, 126, 10922–10932. [Google Scholar] [CrossRef]
- Huang, Z.; Choudhury, S.; Gong, H.; Cui, Y.; Bao, Z. A cation-tethered flowable polymeric interface for enabling stable deposition of metallic lithium. J. Am. Chem. Soc. 2020, 142, 21393–21403. [Google Scholar] [CrossRef] [PubMed]
- API Recommendation 13B-1; Recommended Practice for Field Testing Water-Based Drilling Fluids. ISO: Geneva, Switzerland, 2009.
- Standard API RP 13I; Recommended Practice for Laboratory Testing of Drilling Fluids. American Petroleum Institute: Washington, DC, USA, 2009.
- Jańczuk, B.; Białopiotrowicz, T. Components of surface free energy of some clay minerals. Clays Clay Miner. 1988, 36, 243–248. [Google Scholar] [CrossRef]
- Zhong, H.; Qiu, Z.; Huang, W.; Cao, J. Shale inhibitive properties of polyether diamine in water-based drilling fluid. J. Pet. Sci. Eng. 2011, 78, 510–515. [Google Scholar] [CrossRef]
- Wiśniowski, R.; Skrzypaszek, K.; Małachowski, T. Selection of a Suitable Rheological Model for Drilling Fluid Using Applied Numerical Methods. Energies 2020, 13, 3192. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, H.; Jiang, X.; Lu, F.; Zhou, Y.; Yin, W.; Ji, X. Modification of montmorillonite surfaces using a novel class of cationic gemini surfactants. J. Colloid Interface Sci. 2009, 332, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Chapeaux, A.; Simoni, L.D.; Stadtherr, M.A.; Brennecke, J.F. Liquid phase behavior of ionic liquids with water and 1-octanol and modeling of 1-octanol/water partition coefficients. J. Chem. Eng. Data 2007, 52, 2462–2467. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, S.; Zha, M.; Wang, Z. Physicochemical adsorption and aggregative structures of the organic cation [C18mim]+ in the interlayer of montmorillonite. Acta Phys.-Chim. Sin. 2006, 22, 548–551. [Google Scholar] [CrossRef]
- Pei, J.; Xing, X.; Xia, B.; Wang, Z.; Luo, Z. Study on the adsorption behavior between an imidazolium ionic liquid and Na-montmorillonite. Molecules 2019, 24, 1396. [Google Scholar] [CrossRef]




| Concentration of ILA | Experimental Conditions | AV (mPa·s) | PV (mPa·s) | YP (Pa) | YP/PV (Pa/mPa·s) |
|---|---|---|---|---|---|
| 0 | room temperature | 22.5 | 12.5 | 10 | 0.8 |
| 200 °C | 6.5 | 6 | 0.5 | 0.08 | |
| 240 °C | 6.5 | 6.5 | 0 | 0 | |
| 0.01% | room temperature | 18.75 | 10.5 | 8.25 | 0.79 |
| 200 °C | 5.25 | 4 | 1.25 | 0.31 | |
| 240 °C | 5.5 | 4.5 | 1.0 | 0.22 | |
| 0.02% | room temperature | 19 | 10 | 9.0 | 0.90 |
| 200 °C | 6.0 | 4.0 | 1.5 | 0.33 | |
| 240 °C | 8.25 | 6.5 | 1.75 | 0.27 | |
| 0.03% | room temperature | 20 | 12 | 8 | 0.67 |
| 200 °C | 7.0 | 5.0 | 2.0 | 0.40 | |
| 240 °C | 13.5 | 10.0 | 3.5 | 0.35 | |
| 0.04% | room temperature | 18 | 11 | 7 | 0.64 |
| 200 °C | 11.0 | 8.0 | 3.0 | 0.38 | |
| 240 °C | 12.5 | 9.5 | 3.0 | 0.32 | |
| 0.05% | room temperature | 19 | 11 | 8 | 0.73 |
| 200 °C | 19 | 12 | 7 | 0.58 | |
| 240 °C | 12 | 9 | 3 | 0.33 |
| Concentration of ILB | Experimental Conditions | AV (mPa·s) | PV (mPa·s) | YP (Pa) | YP/PV (Pa/mPa·s) |
|---|---|---|---|---|---|
| 0 | room temperature | 22.5 | 12.5 | 10 | 0.80 |
| 200 °C | 6.5 | 6 | 0.5 | 0.08 | |
| 240 °C | 6.5 | 6.5 | 0 | 0 | |
| 0.01% | room temperature | 23.5 | 14 | 9.5 | 0.68 |
| 200 °C | 5 | 4 | 1 | 0.25 | |
| 240 °C | 4.75 | 4.0 | 0.75 | 0.19 | |
| 0.02% | room temperature | 20.5 | 11.5 | 9 | 0.78 |
| 200 °C | 8.5 | 6.5 | 2.0 | 0.31 | |
| 240 °C | 5 | 4 | 1 | 0.25 | |
| 0.03% | room temperature | 22 | 14.5 | 7.5 | 0.52 |
| 200 °C | 8 | 6 | 2 | 0.33 | |
| 240 °C | 6.75 | 5.5 | 1.25 | 0.23 | |
| 0.04% | room temperature | 20 | 10.5 | 9.5 | 0.90 |
| 200 °C | 4 | 3 | 1 | 0.33 | |
| 240 °C | 6 | 5 | 1 | 0.2 | |
| 0.05% | room temperature | 20.0 | 10 | 10 | 1.0 |
| 200 °C | 3 | 2 | 1 | 0.5 | |
| 240 °C | 7.25 | 6 | 1.25 | 0.21 |
| Concentration of ILC | Experimental Conditions | AV (mPa·s) | PV (mPa·s) | YP (Pa) | YP/PV (Pa/mPa·s) |
|---|---|---|---|---|---|
| 0 | room temperature | 22.5 | 12.5 | 10 | 0.80 |
| 200 °C | 6.5 | 6 | 0.5 | 0.08 | |
| 240 °C | 6.5 | 6.5 | 0 | 0 | |
| 0.01% | room temperature | 21 | 12 | 9 | 0.75 |
| 200 °C | 4.75 | 3.5 | 1.25 | 0.36 | |
| 240 °C | 5.75 | 4.5 | 1.25 | 0.28 | |
| 0.02% | room temperature | 18 | 11 | 7 | 0.64 |
| 200 °C | 6.75 | 5.0 | 1.75 | 0.35 | |
| 240 °C | 13.75 | 10.5 | 3.25 | 0.31 | |
| 0.03% | room temperature | 19 | 13 | 6 | 0.46 |
| 200 °C | 14.25 | 10 | 4.25 | 0.43 | |
| 240 °C | 8.5 | 6 | 2.5 | 0.42 | |
| 0.04% | room temperature | 17 | 11.5 | 5.5 | 0.48 |
| 200 °C | 14.25 | 10 | 4.25 | 0.43 | |
| 240 °C | 12.5 | 9 | 3.5 | 0.39 | |
| 0.05% | room temperature | 19.5 | 12 | 7.5 | 0.63 |
| 200 °C | 23 | 16 | 7 | 0.44 | |
| 240 °C | 10.5 | 9 | 1.5 | 0.17 |
| Temperature | Rheology Model | Ratio | ILA-PAM | ILC-PAM |
|---|---|---|---|---|
| 200 °C | Power law | 1.3640 | 0.7929 | |
| 0.5184 | 0.5428 | |||
| R2 | 0.9961 | 0.9958 | ||
| Herschel–Bulkley | 1.2630 | 0.5361 | ||
| 0.5294 | 0.5994 | |||
| 0.3168 | 0.9840 | |||
| R2 | 0.9961 | 0.9975 | ||
| 240 °C | Power law | 0.6542 | 0.2419 | |
| 0.5602 | 0.7468 | |||
| R2 | 0.9945 | 0.9916 | ||
| Herschel–Bulkley | 0.6039 | 0.2414 | ||
| 0.5717 | 0.7471 | |||
| 0.1918 | 0.0054 | |||
| R2 | 0.9945 | 0.9916 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Yu, Y.; Wang, Y.; Ning, Z.; Luo, Z. Effect of Ionic Liquids with Different Structures on Rheological Properties of Water-Based Drilling Fluids and Mechanism Research at Ultra-High Temperatures. Molecules 2024, 29, 4206. https://doi.org/10.3390/molecules29174206
Shi H, Yu Y, Wang Y, Ning Z, Luo Z. Effect of Ionic Liquids with Different Structures on Rheological Properties of Water-Based Drilling Fluids and Mechanism Research at Ultra-High Temperatures. Molecules. 2024; 29(17):4206. https://doi.org/10.3390/molecules29174206
Chicago/Turabian StyleShi, Haoxian, Yanjiang Yu, Yingsheng Wang, Zijie Ning, and Zhihua Luo. 2024. "Effect of Ionic Liquids with Different Structures on Rheological Properties of Water-Based Drilling Fluids and Mechanism Research at Ultra-High Temperatures" Molecules 29, no. 17: 4206. https://doi.org/10.3390/molecules29174206
APA StyleShi, H., Yu, Y., Wang, Y., Ning, Z., & Luo, Z. (2024). Effect of Ionic Liquids with Different Structures on Rheological Properties of Water-Based Drilling Fluids and Mechanism Research at Ultra-High Temperatures. Molecules, 29(17), 4206. https://doi.org/10.3390/molecules29174206




