Simultaneous Determination of Tobacco Smoke Exposure and Stress Biomarkers in Saliva Using In-Tube SPME and LC-MS/MS for the Analysis of the Association between Passive Smoking and Stress
Abstract
1. Introduction
| Biomarker 1 | Analytical Method 2 | Linearity Range (ng mL–1) | LOD (pg mL–1) | LOQ (pg mL–1) | Precision RSD (%) | Recovery (%) | Remarks | Ref. |
|---|---|---|---|---|---|---|---|---|
| Nic, Cot, OH-Cot | SPE GC–MS/MS | 0.5–1000 | 500 | 500 | 1.56–9.62 | 89–92 | Oral fluid 0.2 mL, derivatization | [48] |
| Nic, Cot | HPLC–UV | Saliva 5 mL | [49] | |||||
| Nic, Cot, alkaloids | IT-SPME LC–MS | 0.5–20 | 15–40 | - | 0.53–4.73 | 83–98 | Saliva 0.1–0.2 mL | [50] |
| Nic, Cot, alkaloids | SPE LC–MS/MS | 1–100 | 250–1000 | 1000 | ≤10 | 80–119 | Oral fluid 0.5 mL | [51] |
| CRT | Immuno FET sensor | 0.01–15 | 5 | ≤10 | 104 | Saliva 0.05 mL | [55] | |
| CRT, CRN | SPE HPLC–UV | 2.0–40 | 36–72 | 2.7–7.0 | 88–99 | Saliva 0.05 mL | [57] | |
| CRT, CRN | IL-DDDME LC–UV/Vis | 5–500 | 110–160 | 370–540 | 2.8–5.5 | 83–116 | Saliva 1–1.5 mL | [58] |
| CRT, CRN, corticosterone | MEPS-HPLC–DAD | 5–100 | 1500 | 5000 | 2.6–4.9 | 82–86 | Saliva 0.4 mL | [59] |
| CRT, CRN, MEL | TF LC–MS/MS | 0.2–10 0.001–0.1 (MEL) | - | 200–1900 1.4 (MEL) | ≤5 | 95–106 | Saliva 0.05 mL | [60] |
| CRT, TES, DHEA | IT-SPME LC–MS | 0.002–100 | 0.3–8.9 | 10–290 | 1.0–4.9 | 94–106 | Saliva 0.1 mL | [67] |
| CRT, DHEA-S | LC–MS/MS | 1.0–25.0 | 20–30 | 30–60 | 4.6–17.9 | 95–110 | Saliva 0.4 mL | [61] |
| CRT, CRN | SPE UPLC–MS/MS | 0.005–10 | 5 | 10–50 | 2–4 | 95–103 | Saliva 0.2 mL | [62] |
| CRT, other steroids | SPE UPLC–MS/MS | 0.005–5.0 | - | 50 (CRT) | 7.2 (CRT) | - | Saliva 0.2 mL | [63] |
| CRT, TES, DHEA, DHEA-S | IT-SPME LC–MS | 0.01–20 | 0.40–8.5 | 36–768 | 0.9–6.1 | 95–106 | Saliva 0.05 mL | [20] |
| CRT, CRN, MEL | LLE UPLC–MS/MS | 0.2–10 0.001–0.1 (MEL) | 36–54 0.7 (MEL) | 181–360 2.3(MEL) | 7–14 | 86–99 | Saliva 0.25 mL | [64] |
| Nic, Cot, CRT, DA, 5-HT, MEL, OXT | IT-SPME LC–MS | 0.01–25 | 0.12–3.7 | 4–124 | 1.3–7.9 | 82–107 | Saliva 0.05 mL | This study |
2. Results and Discussion
2.1. Optimization of IT-SPME and Desorption of Biomarkers
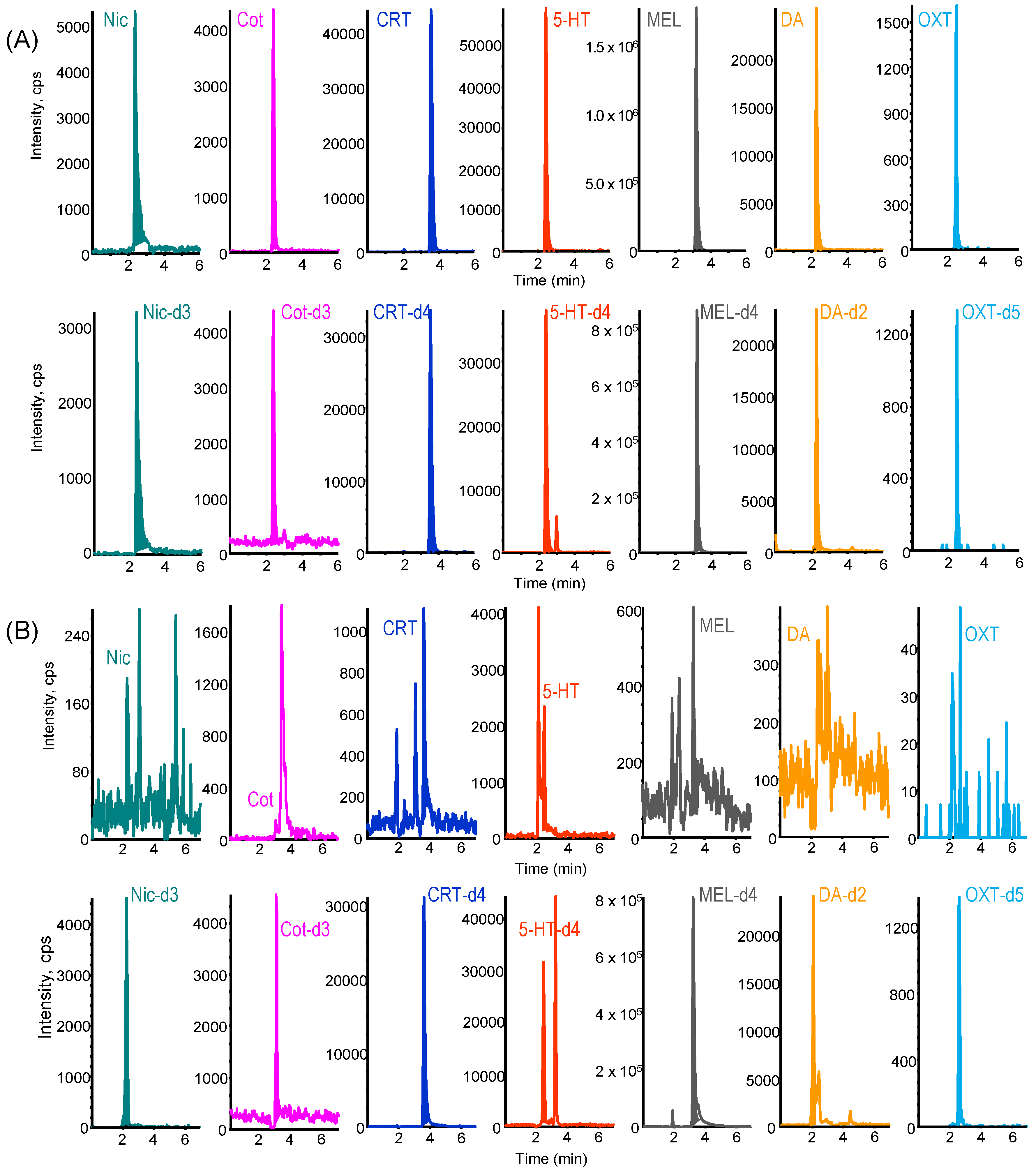
2.2. LC–MS/MS Analysis of Biomarkers
2.3. Validation of the Developed IT-SPME LC–MS/MS Method
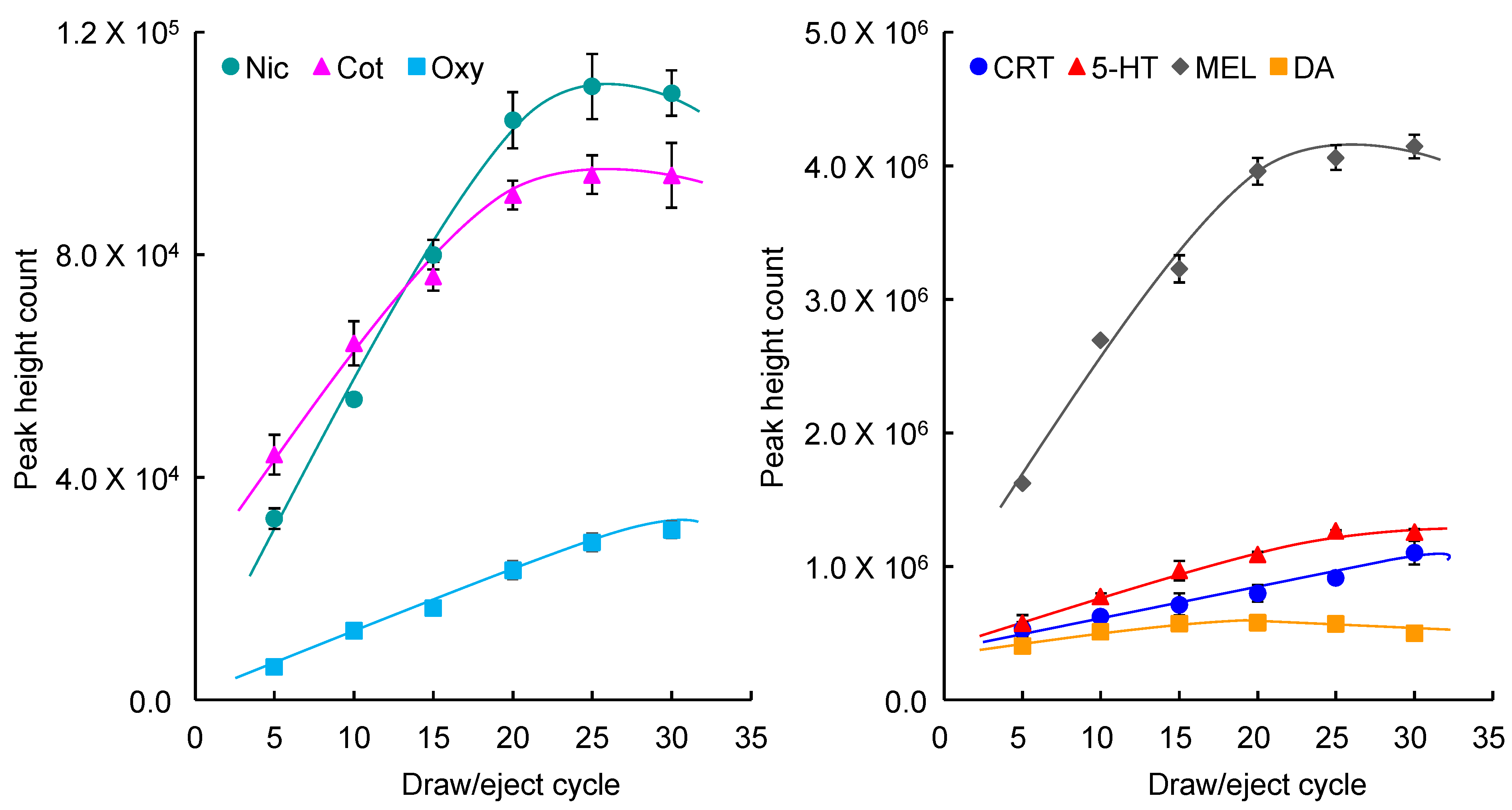
2.4. Analysis of Saliva Samples
2.5. Analysis of the Relationship between Stress and Tobacco Smoke Exposure by Measurements of Salivary Biomarkers and HRV
3. Materials and Methods
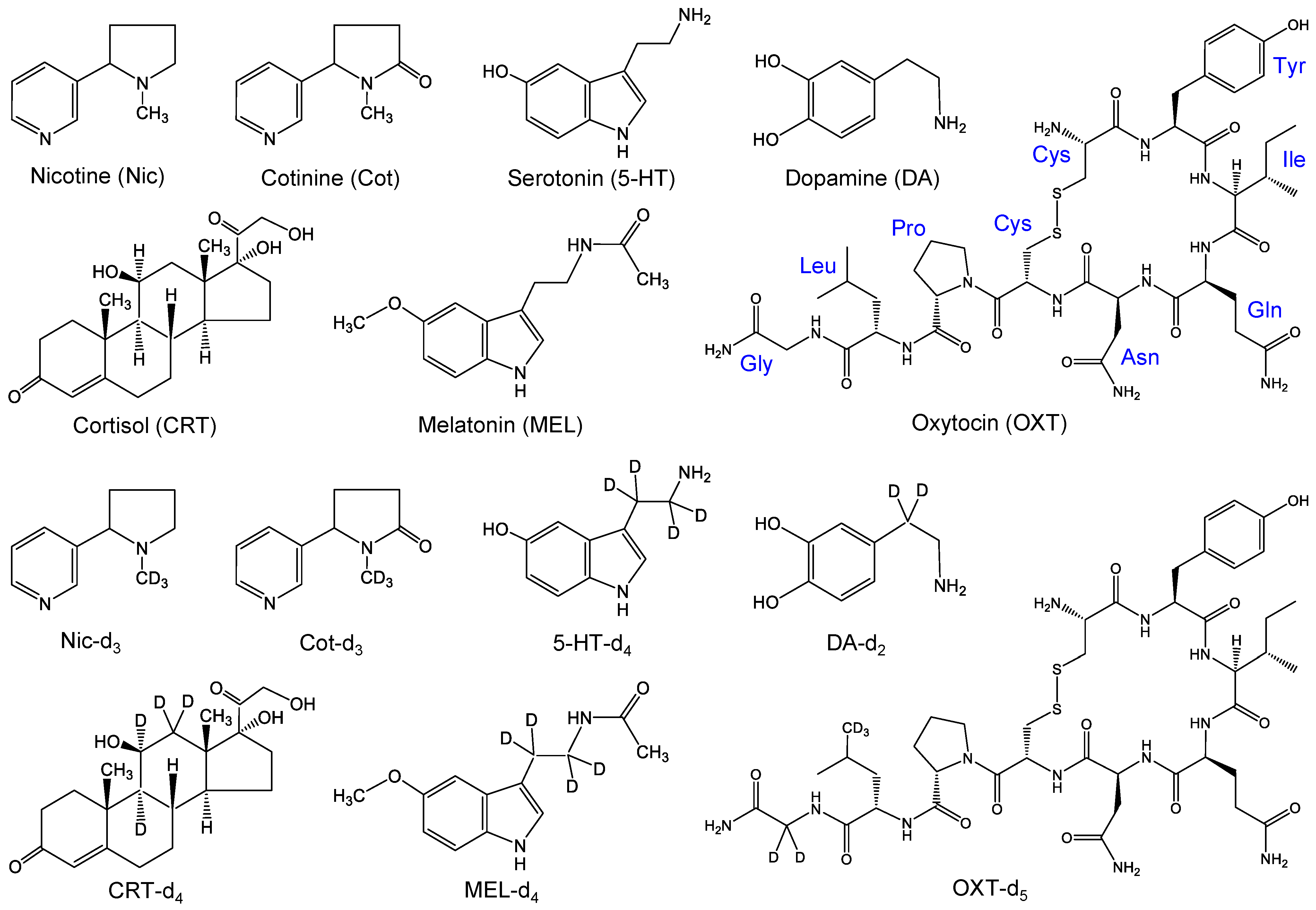
3.1. Reagents and Materials
3.2. Sampling, Preparation and Analysis of Saliva Samples
3.3. LC–MS/MS Conditions
3.4. IT-SPME Procedure and On-Line Coupling with LC–MS/MS
3.5. Method Validation Study
3.6. HRV Analysis by Fingertip Pulse Wave Measurement
3.7. Tobacco Smoke Exposure Assessment by Biomarker and HRV Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Q.; Hou, H. (Eds.) Tobacco Smoke Exposure Biomarkers; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Sikorska-Jaroszyńska, M.H.; Mielnik-Błaszczak, M.; Krawczyk, D.; Nasiłowska-Barud, A.; Błaszczak, J. Passive smoking as an environmental health risk factor. Ann. Agric. Environ. Med. 2012, 19, 547–550. [Google Scholar] [PubMed]
- Mattes, W.; Yang, X.; Orr, M.S.; Richter, P.; Mendrick, D.L. Biomarkers of tobacco smoke exposure. Adv. Clin. Chem. 2014, 67, 1–45. [Google Scholar] [CrossRef]
- Ni, X.; Xu, N.; Wang, Q. Meta-analysis and systematic review in environmental tobacco smoke risk of female lung cancer by research type. Int. J. Environ. Res. Public Health 2018, 15, 1348. [Google Scholar] [CrossRef]
- Torres, S.; Merino, C.; Paton, B.; Correig, X.; Ramírez, N. Biomarkers of exposure to secondhand and thirdhand tobacco smoke: Recent advances and future perspectives. Int. J. Environ. Res. Public Health 2018, 15, 2693. [Google Scholar] [CrossRef]
- Hori, M.; Tanaka, H.; Wakai, K.; Sasazuki, S.; Katanoda, K. Secondhand smoke exposure and risk of lung cancer in Japan: A systematic review and meta-analysis of epidemiologic studies. Jpn. J. Clin. Oncol. 2016, 46, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, N.; Inoue, M.; Liu, Y.; Iwasaki, M.; Sasazuki, S.; Sobue, T.; Tsugane, S. Passive smoking and lung cancer in Japanese non-smoking women: A prospective study. Int. J. Cancer 2008, 122, 653–657. [Google Scholar] [CrossRef]
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values. Front. Mol. Biosci. 2019, 6, 91. [Google Scholar] [CrossRef]
- Vinkers, C.H.; Kuzminskaite, E.; Lamers, F.; Giltay, E.J.; Penninx, B.W. An integrated approach to understand biological stress system dysregulation across depressive and anxiety disorders. J. Affect. Disord. 2021, 283, 139–146. [Google Scholar] [CrossRef]
- Chojnowska, S.; Ptaszyńska-Sarosiek, I.; Kępka, A.; Knaś, M.; Waszkiewicz, N. Salivary Biomarkers of Stress, Anxiety and Depression. J. Clin. Med. 2021, 10, 517. [Google Scholar] [CrossRef]
- Wekenborg, M.K.; von Dawans, B.; Hill, L.K.; Thayer, J.F.; Penz, M.; Kirschbaum, C. Examining reactivity patterns in burnout and other indicators of chronic stress. Psychoneuroendocrinology 2019, 106, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Strain, J.J. The psychobiology of stress, depression, adjustment disorders and resilience. World J. Biol. Psychiatry 2018, 19 (Suppl. S1), S14–S20. [Google Scholar] [CrossRef]
- Kivimaki, M.; Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 2018, 15, 215–229. [Google Scholar] [CrossRef]
- Chami, R.; Monteleone, A.M.; Treasure, J.; Monteleone, P. Stress hormones and eating disorders. Mol. Cell. Endocrinol. 2019, 497, 110349. [Google Scholar] [CrossRef]
- Tomiyama, A.J. Stress and Obesity. Annu. Rev. Psychol. 2019, 70, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.; Kreek, M.J. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry 2007, 164, 1149–1159. [Google Scholar] [CrossRef]
- Gould, G.S.; Havard, A.; Lim, L.L.; Kumar, R. Exposure to tobacco, environmental tobacco smoke and nicotine in pregnancy: A pragmatic overview of reviews of maternal and child outcomes, effectiveness of interventions and barriers and facilitators to quitting. Int. J. Environ. Res. Public Health 2020, 17, 2034. [Google Scholar] [CrossRef]
- Kataoka, H.; Kaji, S.; Moai, M. Risk Assessment of Passive Smoking Based on Analysis of Hair Nicotine and Cotinine as Exposure Biomarkers by In-Tube Solid-Phase Microextraction Coupled On-Line to LC-MS/MS. Molecules 2021, 26, 7356. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Ohshima, H.; Ohkawa, T. Simultaneous analysis of multiple steroidal biomarkers in saliva for objective stress assessment by on-line coupling of automated in-tube solid-phase microextraction and polarity-switching LC-MS/MS. Talanta Open 2023, 7, 100177. [Google Scholar] [CrossRef]
- Wadsworth, M.E.; Broderick, A.V.; Loughlin-Presnal, J.E.; Bendezu, J.J.; Joos, C.M.; Ahlkvist, J.A.; Perzow, S.E.D.; McDonald, A. Co-activation of SAM and HPA responses to acute stress: A review of the literature and test of differential associations with preadolescents’ internalizing and externalizing. Dev. Psychobiol. 2019, 61, 1079–1093. [Google Scholar] [CrossRef]
- Bleker, L.S.; van Dammen, L.; Leeflang, M.M.G.; Limpens, J.; Roseboom, T.J.; de Rooij, S.R. Hypothalamic-pituitary-adrenal axis and autonomic nervous system reactivity in children prenatally exposed to maternal depression: A systematic review of prospective studies. Neurosci. Biobehav. Rev. 2020, 117, 243–252. [Google Scholar] [CrossRef]
- Mueller, B.; Figueroa, A.; Robinson-Papp, J. Structural and functional connections between the autonomic nervous system, hypothalamic-pituitary-adrenal axis, and the immune system: A context and time dependent stress response network. Neurol. Sci. 2022, 43, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, A.; Scherer, E.; Eckhoff, R.; Furberg, R. Measurement of Human Stress: A Multidimensional Approach; RTI Press: Research Triangle Park, NC, USA, 2022. [Google Scholar] [CrossRef]
- Stefano, G.B.; Fricchione, G.L.; Esch, T. Relaxation: Molecular and physiological significance. Med. Sci. Monit. 2006, 12, HY21–HY31. [Google Scholar] [PubMed]
- Moberg, K.U.; Handlin, L.; Petersson, M. Neuroendocrine mechanisms involved in the physiological effects caused by skin-to-skin contact—With a particular focus on the oxytocinergic system. Infant Behav. Dev. 2020, 61, 101482. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, G.; Scholten, A.; Parr, M.K. Current methods for stress marker detection in saliva. J. Pharm. Biomed. Anal. 2020, 191, 113604. [Google Scholar] [CrossRef]
- Łoś, K.; Waszkiewicz, N. Biological Markers in Anxiety Disorders. J. Clin. Med. 2021, 10, 1744. [Google Scholar] [CrossRef]
- Noushad, S.; Ahmed, S.; Ansari, B.; Mustafa, U.H.; Saleem, Y.; Hazrat, H. Physiological biomarkers of chronic stress: A systematic review. Int. J. Health Sci. 2021, 15, 46–59. [Google Scholar]
- Kataoka, H. Application of In-Tube SPME to Analysis of Stress-Related Biomarkers. In Evolution of SPME Technology; Pawliszyn, J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2023; Chapter 14; pp. 419–440. ISBN 978-1-83916-680-8. [Google Scholar]
- Ali, N.; Nater, U.M. Salivary Alpha-Amylase as a Biomarker of Stress in Behavioral Medicine. Int. J. Behav. Med. 2020, 27, 337–342. [Google Scholar] [CrossRef]
- Obayashi, K. Salivary mental stress proteins. Clin. Chim. Acta 2013, 425, 196–201. [Google Scholar] [CrossRef]
- Carter, C.S.; Pournajafi-Nazarloo, H.; Kramer, K.M.; Ziegler, T.E.; White-Traut, R.; Bello, D.; Schwertz, D. Oxytocin: Behavioral associations and potential as a salivary biomarker. Ann. N. Y. Acad. Sci. 2007, 1098, 312–322. [Google Scholar] [CrossRef]
- Steckl, A.; Ray, P. Stress Biomarkers in Biological Fluids and Their Point-of-Use Detection. ACS Sens. 2018, 3, 2025–2044. [Google Scholar] [CrossRef]
- Zamkah, A.; Hui, T.; Andrews, S.; Dey, N.; Shi, F.; Sherratt, R.S. Identification of Suitable Biomarkers for Stress and Emotion Detection for Future Personal Affective Wearable Sensors. Biosensors 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Chung, S.; Chang, A.-Y.; Wang, J.; Hall, D.A. A non-invasive wearable stress patch for real-time cortisol monitoring using a pseudoknot-assisted aptamer. Biosens. Bioelectron. 2023, 227, 115097. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Sieciński, S.; Kostka, P.S.; Tkacz, E.J. Heart Rate Variability Analysis on Electrocardiograms, Seismocardiograms and Gyrocardiograms on Healthy Volunteers. Sensors 2020, 20, 4522. [Google Scholar] [CrossRef]
- Natarajan, A.; Pantelopoulos, A.; Emir-Farinas, H.; Natarajan, P. Heart rate variability with photoplethysmography in 8 million individuals: A cross-sectional study. Lancet Digit. Health 2020, 2, e650–e657. [Google Scholar] [CrossRef]
- Nayak, S.K.; Pradhan, B.; Mohanty, B.; Sivaraman, J.; Ray, S.S.; Wawrzyniak, J.; Jarzębski, M.; Pal, K. A Review of Methods and Applications for a Heart Rate Variability Analysis. Algorithms 2023, 16, 433. [Google Scholar] [CrossRef]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Cao, R.; Rahmani, A.M.; Lindsay, K.L. Prenatal stress assessment using heart rate variability and salivary cortisol: A machine learning-based approach. PLoS ONE 2022, 17, e0274298. [Google Scholar] [CrossRef]
- Immanuel, S.; Teferra, M.N.; Baumert, M.; Bidargaddi, N. Heart Rate Variability for Evaluating Psychological Stress Changes in Healthy Adults: A Scoping Review. Neuropsychobiology 2023, 82, 187–202. [Google Scholar] [CrossRef]
- Elgendi, M. On the Analysis of Fingertip Photoplethysmogram Signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-K.; Hu, Y.-F.; Lin, S.-F. Methodological considerations in calculating heart rate variability based on wearable device heart rate samples. Comput. Biol. Med. 2018, 102, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.; Ni, S.; Peng, K. Happiness at Your Fingertips: Assessing Mental Health with Smartphone Photoplethysmogram-Based Heart Rate Variability Analysis. Telemed. J. e-Health 2020, 26, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Ino, T.; Ohta, M.; Otani, T.; Hanada, S.; Sakuraoka, A.; Matsumoto, A.; Ichiba, M.; Hara, M. Enzyme-linked immunosorbent assay of nicotine metabolites. Environ. Health Prev. Med. 2010, 15, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, B.M.; Moreno, I.E.; Magalhães, A.R.; Barroso, M.; Queiroz, J.A.; Ravara, S.; Calheiros, J.; Gallardo, E. Determination of biomarkers of tobacco smoke exposure in oral fluid using solid-phase extraction and gas chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 889–890, 116–122. [Google Scholar] [CrossRef]
- Shaik, F.B.; Nagajothi, G.; Swarnalatha, K.; Kumar, C.S.; Maddu, N. Quantification of Nicotine and Cotinine in Plasma, Saliva, and Urine by HPLC Method in Chewing Tobacco Users. Asian Pac. J. Cancer Prev. 2019, 20, 3617–3623. [Google Scholar] [CrossRef]
- Kataoka, H.; Inoue, R.; Yagi, K.; Saito, K. Determination of nicotine, cotinine, and related alkaloids in human urine and saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 108–114. [Google Scholar] [CrossRef]
- Miller, E.I.; Norris, H.R.; Rollins, D.E.; Tiffany, S.T.; Moore, C.M.; Vincent, M.J.; Agrawal, A.; Wilkins, D.G. Identification and quantification of nicotine biomarkers in human oral fluid from individuals receiving low-dose transdermal nicotine: A preliminary study. J. Anal. Toxicol. 2010, 34, 357–366. [Google Scholar] [CrossRef]
- Chang, Y.J.; Muthukumaran, R.B.; Chen, J.L.; Chang, H.Y.; Hung, Y.C.; Hu, C.W.; Chao, M.R. Simultaneous determination of areca nut- and tobacco-specific alkaloids in saliva by LC-MS/MS: Distribution and transformation of alkaloids in oral cavity. J. Hazard. Mater. 2022, 426, 128116. [Google Scholar] [CrossRef]
- Tahara, Y.; Huang, Z.; Kiritoshi, T.; Onodera, T.; Toko, K. Development of Indirect Competitive Immuno-Assay Method Using SPR Detection for Rapid and Highly Sensitive Measurement of Salivary Cortisol Levels. Front. Bioeng. Biotechnol. 2014, 2, 15. [Google Scholar] [CrossRef]
- Pritchard, B.T.; Stanton, W.; Lord, R.; Petocz, P.; Pepping, G.J. Factors Affecting Measurement of Salivary Cortisol and Secretory Immunoglobulin A in Field Studies of Athletes. Front. Endocrinol. 2017, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Ben Halima, H.; Bellagambi, F.G.; Brunon, F.; Alcacer, A.; Pfeiffer, N.; Heuberger, A.; Hangouët, M.; Zine, N.; Bausells, J.; Errachid, A. Immuno field-effect transistor (ImmunoFET) for detection of salivary cortisol using potentiometric and impedance spectroscopy for monitoring heart failure. Talanta 2023, 257, 123802. [Google Scholar] [CrossRef]
- Casals, G.; Ballesteros, M.A.; Zamora, A.; Martínez, I.; Fernández-Varo, G.; Mora, M.; Hanzu, F.A.; Morales-Ruiz, M. LC-HRMS and GC-MS Profiling of Urine Free Cortisol, Cortisone, 6beta-, and 18-Hydroxycortisol for the Evaluation of Glucocorticoid and Mineralocorticoid Disorders. Biomolecules 2024, 14, 558. [Google Scholar] [CrossRef] [PubMed]
- De Palo, E.F.; Antonelli, G.; Benetazzo, A.; Prearo, M.; Gatti, R. Human saliva cortisone and cortisol simultaneous analysis using reverse phase HPLC technique. Clin. Chim. Acta 2009, 405, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Abujaber, F.; Corps Ricardo, A.I.; Ríos, Á.; Guzmán Bernardo, F.J.; Rodríguez Martín-Doimeadios, R.C. Ionic liquid dispersive liquid-liquid microextraction combined with LC-UV-Vis for the fast and simultaneous determination of cortisone and cortisol in human saliva samples. J. Pharm. Biomed. Anal. 2019, 165, 141–146. [Google Scholar] [CrossRef]
- Saracino, M.A.; Iacono, C.; Somaini, L.; Gerra, G.; Ghedini, N.; Raggi, M.A. Multimatrix assay of cortisol, cortisone and corticosterone using a combined MEPS-HPLC procedure. J. Pharm. Biomed. Anal. 2014, 88, 643–648. [Google Scholar] [CrossRef]
- Fustinoni, S.; Polledri, E.; Mercadante, R. High-throughput determination of cortisol, cortisone, and melatonin in oral fluid by on-line turbulent flow liquid chromatography interfaced with liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 1450–1460. [Google Scholar] [CrossRef]
- Cao, Z.T.; Wemm, S.E.; Han, L.; Spink, D.C.; Wulfert, E. Noninvasive determination of human cortisol and dehydroepiandrosterone sulfate using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 1203–1210. [Google Scholar] [CrossRef]
- Bakusicm, J.; De Nys, S.; Creta, M.; Godderis, L.; Duca, R.C. Study of temporal variability of salivary cortisol and cortisone by LC-MS/MS using a new atmospheric pressure ionization source. Sci. Rep. 2019, 9, 19313. [Google Scholar] [CrossRef]
- Gregory, S.; Denham, S.G.; Lee, P.; Simpson, J.P.; Homer, N.Z.M. Using LC-MS/MS to determine salivary steroid reference intervals in a European older adult population. Metabolites 2023, 13, 265. [Google Scholar] [CrossRef]
- Lanfermeijer, M.; van Winden, L.J.; Starreveld, D.E.J.; Razab-Sekh, S.; van Faassen, M.; Bleiker, E.M.A.; van Rossum, H.H. An LC-MS/MS-based method for the simultaneous quantification of melatonin, cortisol and cortisone in saliva. Anal. Biochem. 2024, 689, 115496. [Google Scholar] [CrossRef]
- Kataoka, H. In-tube solid-phase microextraction: Current trends and future perspectives. J. Chromatogr. A 2021, 1636, 461787. [Google Scholar] [CrossRef]
- Inukai, T.; Kaji, S.; Kataoka, H. Analysis of nicotine and cotinine in hair by on-line in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry as biomarkers of exposure to tobacco smoke. J. Pharm. Biomed. Anal. 2018, 156, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Ehara, K.; Yasuhara, R.; Saito, K. Simultaneous determination of testosterone, cortisol and dehydroepiandrosterone in saliva by stable isotope dilution on-line in-tube solid-phase microextraction coupled with liquid chromatography−tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, E.; Kataoka, H. Automated analysis of oxytocin by on-line in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. Chromatography 2015, 2, 382–391. [Google Scholar] [CrossRef]
- Ishizaki, A.; Uemura, A.; Kataoka, H. A sensitive method to determine melatonin in saliva by automated online in-tube solid-phase microextraction coupled with stable isotope-dilution liquid chromatography-tandem mass spectrometry. Anal. Methods 2017, 9, 3134–3140. [Google Scholar] [CrossRef]
- Kataoka, H.; Nakayama, D. Online in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry for automated analysis of four sulfated steroid metabolites in saliva samples. Molecules 2022, 27, 3225. [Google Scholar] [CrossRef]
- Hitomi, T.; Kataoka, H. Development of Noninvasive Method for the Automated Analysis of Nine Steroid Hormones in Saliva by Online Coupling of In-Tube Solid-Phase Microextraction with Liquid Chromatography–Tandem Mass Spectrometry. Analytica 2024, 5, 233–249. [Google Scholar] [CrossRef]




| Biomarker | Linearity | LOD 2 (pg mL−1) | LOQ 3 (pg mL−1) | ||
|---|---|---|---|---|---|
| Range (ng mL−1) | CC 1 | Direct Injection | IT-SPME | IT-SPME | |
| Nic | 0.05–2.5 | 0.9977 | 7.0 | 0.22 | 7.7 |
| Cot | 0.01–0.5 | 0.9999 | 2.6 | 0.12 | 4.0 |
| CRT | 0.2–10 | 0.9997 | 23 | 0.78 | 27 |
| 5-HT | 0.02–1.0 | 1.0000 | 18 | 0.39 | 13 |
| MEL | 0.01–0.5 | 0.9994 | 24 | 0.45 | 16 |
| DA | 0.2–10 | 0.9993 | 31 | 1.4 | 48 |
| OXT | 0.5–25 | 0.9998 | 138 | 3.7 | 124 |
| Compound | Concentration (ng mL−1) | Precision (RSD 1 %), (n = 5) | |
|---|---|---|---|
| Intra–Day | Inter–Day | ||
| Nic | 0.25 | 5.7 | 9.5 |
| 1 | 5.9 | 9.9 | |
| 2.5 | 5.8 | 9.4 | |
| Cot | 0.05 | 3.4 | 7.3 |
| 0.2 | 6.8 | 13.5 | |
| 0.5 | 3.2 | 5.0 | |
| CRT | 1 | 6.1 | 6.7 |
| 4 | 2.6 | 6.8 | |
| 10 | 1.6 | 3.8 | |
| 5-HT | 0.5 | 6.7 | 14.6 |
| 2 | 3.0 | 5.7 | |
| 5 | 1.9 | 2.8 | |
| MEL | 0.05 | 4.8 | 5.5 |
| 0.2 | 3.0 | 2.3 | |
| 0.5 | 1.3 | 2.2 | |
| DA | 1 | 5.0 | 7.8 |
| 4 | 3.9 | 4.7 | |
| 10 | 3.4 | 2.9 | |
| OXT | 2.5 | 7.9 | 8.0 |
| 10 | 3.7 | 4.0 | |
| 25 | 3.6 | 3.7 | |
| Compound | Spiked (ng mL−1 Saliva) (ng mL−1 Saliva) | Recovery ± SD (%), (n = 3) (n = 3) |
|---|---|---|
| Nic | 2.5 | 105.4 ± 2.5 |
| 10 | 96.8 ± 0.6 | |
| 25 | 98.2 ± 0.9 | |
| Cot | 0.5 | 105.4 ± 4.0 |
| 2 | 99.1 ± 0.7 | |
| 5 | 100.4 ± 0.4 | |
| CRT | 10 | 99.1 ± 1.4 |
| 40 | 97.9 ± 4.4 | |
| 100 | 101.0 ± 1.2 | |
| 5-HT | 5 | 98.2 ± 2.2 |
| 20 | 98.9 ± 1.5 | |
| 50 | 99.2 ± 2.6 | |
| MEL | 0.5 | 96.9 ± 0.5 |
| 2 | 96.2 ± 0.7 | |
| 5 | 102.9 ± 5.9 | |
| DA | 10 | 82.3 ± 2.6 |
| 40 | 96.7 ± 3.9 | |
| 100 | 82.1 ± 1.2 | |
| OXT | 25 | 93.1 ± 3.7 |
| 100 | 106.6 ± 1.1 | |
| 250 | 93.8 ± 2.5 |
| Content 1 (pg mL−1 Saliva) | HRV Indicator | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nic | Cot | CRT | 5-HT | MEL | DA | OXT | SDNN 3 | Ln LF/Ln HF 4 | Ln TP 5 | |
| Max. | 807 | 54 | 732 | 3706 | <LOQ 2 | 224 | 691 | 88 | 1.7 | 9.1 |
| Med. | 377 | 12 | 482 | 164 | <LOQ | 44 | 114 | 47 | 0.9 | 6.6 |
| Min. | 207 | 4 | 220 | 23 | <LOQ | <LOQ | <LOQ | 19 | 0.7 | 3.8 |
| Ave. | 395 | 18 | 497 | 457 | <LOQ | 64 | 178 | 47 | 1.0 | 6.4 |
| SD | 153 | 12 | 134 | 792 | – | 46 | 162 | 15 | 0.2 | 1.2 |
| Compound | Mass Transition (m/z) | DP 1 (V) | EP 2 (V) | CE 3 (V) | CXP 4 (V) |
|---|---|---|---|---|---|
| Nicotine (Nic) | 163.1 → 132.1 | 70 | 10 | 20 | 10 |
| Cotinine (Cot) | 177.1 → 80.2 | 75 | 10 | 30 | 15 |
| Cortisol (CRT) | 363.0 → 120.9 | 70 | 10 | 30 | 10 |
| Serotonin (5-HT) | 177.2 → 160.2 | 25 | 5 | 15 | 3 |
| Melatonin (MEL) | 233.1 → 174.1 | 20 | 9 | 20 | 12 |
| Dopamine (DA) | 154.2 → 91.1 | 50 | 4 | 30 | 8 |
| Oxytocin (OXT) | 1008.3 → 724.5 | 60 | 9 | 40 | 12 |
| Nic-d3 | 166.1 → 132.1 | 70 | 10 | 20 | 10 |
| Cot-d3 | 180.1 → 80.2 | 75 | 10 | 30 | 15 |
| CRT-d4 | 367.1 → 121.4 | 70 | 10 | 30 | 10 |
| 5-HT-d4 | 181.2 → 164.3 | 25 | 5 | 15 | 3 |
| MEL-d4 | 237.1 → 178.1 | 20 | 9 | 20 | 12 |
| DA-d2 | 156.2 → 93.1 | 50 | 4 | 30 | 8 |
| OXT-d5 | 1013.3 → 724.5 | 60 | 9 | 40 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataoka, H.; Miyata, S.; Ehara, K. Simultaneous Determination of Tobacco Smoke Exposure and Stress Biomarkers in Saliva Using In-Tube SPME and LC-MS/MS for the Analysis of the Association between Passive Smoking and Stress. Molecules 2024, 29, 4157. https://doi.org/10.3390/molecules29174157
Kataoka H, Miyata S, Ehara K. Simultaneous Determination of Tobacco Smoke Exposure and Stress Biomarkers in Saliva Using In-Tube SPME and LC-MS/MS for the Analysis of the Association between Passive Smoking and Stress. Molecules. 2024; 29(17):4157. https://doi.org/10.3390/molecules29174157
Chicago/Turabian StyleKataoka, Hiroyuki, Saori Miyata, and Kentaro Ehara. 2024. "Simultaneous Determination of Tobacco Smoke Exposure and Stress Biomarkers in Saliva Using In-Tube SPME and LC-MS/MS for the Analysis of the Association between Passive Smoking and Stress" Molecules 29, no. 17: 4157. https://doi.org/10.3390/molecules29174157
APA StyleKataoka, H., Miyata, S., & Ehara, K. (2024). Simultaneous Determination of Tobacco Smoke Exposure and Stress Biomarkers in Saliva Using In-Tube SPME and LC-MS/MS for the Analysis of the Association between Passive Smoking and Stress. Molecules, 29(17), 4157. https://doi.org/10.3390/molecules29174157







