Phytochemicals, Two New Sulphur Glycosides and Two New Natural Products, from Shepherd’s Purse Seed and Their Activities
Abstract
1. Introduction
2. Results
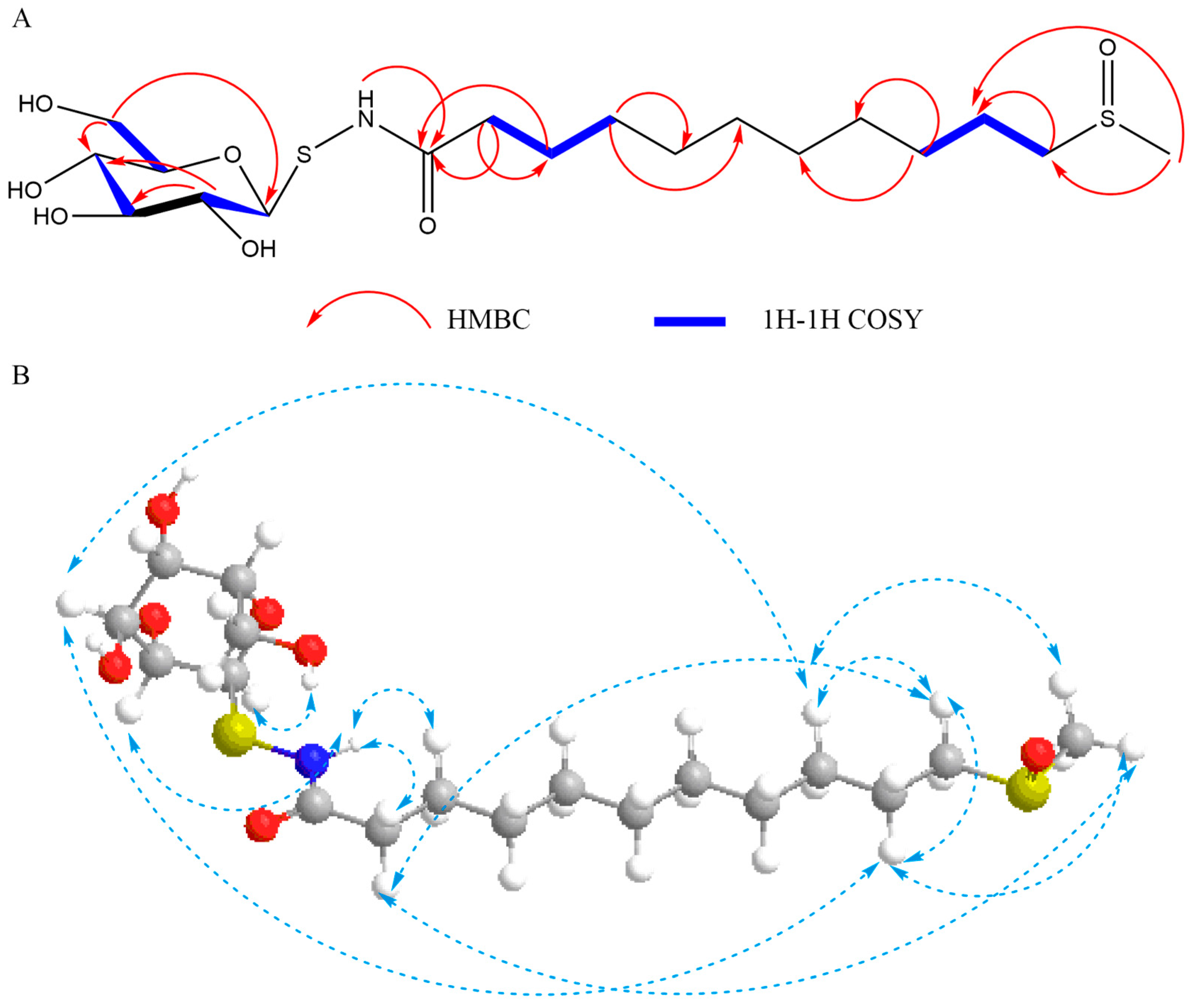
2.1. Structure Elucidation
2.2. Structural Information of 1–2
2.3. Proposed Biosynthetic Pathway
2.4. Anti-Radiation Activity
2.5. Molecular Docking Analysis
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Isolations of Compounds
3.4. Cells and Cell Culture
3.5. Anti-Radiation Assay
3.6. Molecular Docking Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, J.; Hu, T.; Li, J.; Du, J.; Zhu, K.; Cheng, B.; Li, K. Shepherd’s Purse Polyphenols Exert Its Anti-Inflammatory and Antioxidative Effects Associated with Suppressing MAPK and NF-κB Pathways and Heme Oxygenase-1 Activation. Oxid. Med. Cell Longev. 2019, 2019, 7202695. [Google Scholar] [CrossRef] [PubMed]
- Apaydin Yildirim, B.; Aydin, T.; Kordali, S.; Yildirim, S.; Cakir, A.; Yildirim, F. Antihemorrhoidal activity of organic acids of Capsella bursa-pastoris on croton oil-induced hemorrhoid in rats. J. Food Biochem. 2020, 9, e13343. [Google Scholar]
- Cha, J.M.; Suh, W.S.; Lee, T.H.; Subedi, L.; Kim, S.Y.; Lee, K.R. Phenolic Glycosides from Capsella bursa-pastoris (L.) Medik and Their Anti-Inflammatory Activity. Molecules 2017, 6, 1023. [Google Scholar] [CrossRef] [PubMed]
- Wątły, J.; Szarszoń, K.; Mikołajczyk, A.; Grelich-Mucha, M.; Matera-Witkiewicz, A.; Olesiak-Bańska, J.; Rowińska-Żyrek, M. Zn(II) Induces Fibril Formation and Antifungal Activity in Shepherin I, An Antimicrobial Peptide from Capsella bursa-pastoris. Inorg. Chem. 2023, 48, 19786–19794. [Google Scholar] [CrossRef]
- Öztürk, O.U.; Ugur, M.; Güzel, Y.; Öztürk, M.A.; Gürsoy, D.; Doğan, S.; Temiz, M. Hemostatic effects of traditional Inula viscosa and Capsella bursa-pastoris plant mixture extract on rat liver parenchymal bleeding model. Ulus. Travma. Acil. Cerrahi. Derg. 2022, 8, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Naafe, M.; Kariman, N.; Keshavarz, Z.; Khademi, N.; Mojab, F.; Mohammadbeigi, A. Effect of Hydroalcoholic Extracts of Capsella bursa-pastoris on Heavy Menstrual Bleeding: A Randomized Clinical Trial. J. Altern. Complement Med. 2018, 7, 694–700. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Balasubramanian, N.; Quinn, M.T. Neutrophil Immunomodulatory Activity of Natural Organosulfur Compounds. Molecules 2019, 9, 1809. [Google Scholar] [CrossRef]
- Torres Palazzolo, C.; Martín Giménez, V.M.; Mazzei, L.; De Paola, M.; Quesada, I.; Cuello Carrión, F.D.; Fornés, M.W.; Camargo, A.B.; Castro, C.; Manucha, W. Consumption of oil macerated with garlic produces renovascular protective effects in adult apolipoprotein E-deficient mice. Food Funct. 2022, 15, 8131–8142. [Google Scholar] [CrossRef]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Gil-Martinez, L.; Ariza-Romero, J.J.; Maroto-Tello, A.; Baños-Arjona, A.; Gutierrez-Fernandez, J. Antibacterial and Antifungal Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate, Two Organosulfur Compounds from Allium cepa: In Vitro Antimicrobial Effect via the Gas Phase. Pharmaceuticals 2020, 1, 21. [Google Scholar] [CrossRef]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- Ahmed, T.; Wang, C.K. Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules 2021, 16, 5028. [Google Scholar] [CrossRef] [PubMed]
- Egbujor, M.C.; Petrosino, M.; Zuhra, K.; Saso, L. The Role of Organosulfur Compounds as Nrf2 Activators and Their Antioxidant Effects. Antioxidants 2022, 11, 1255. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, M.; Huang, D. Dietary Organosulfur-Containing Compounds and Their Health-Promotion Mechanisms. Annu Rev. Food Sci. Technol. 2022, 13, 287–313. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.Z.; Zhou, T.Q.; Xia, Z.M.; Liu, S.F.; Li, M.; Zhang, G.J.; Tian, Y.; Li, B.; Wang, L. Four organosulfur compounds from the seeds of Capsella bursa-pastoris and their anti-inflammatory activities. Nat. Prod. Res. 2022, 37, 2688–2696. [Google Scholar] [CrossRef]
- Zhou, T.Q.; Wei, Z.Z.; Zhang, J.R.; Dong, J.H.; Liu, C.Y.; Jiang, C.Z.; Xia, Z.M.; Liu, S.F.; Li, M.; Zhang, G.J.; et al. Phytochemical Constituents from the Seeds of Capsella bursa-pastoris and Their Antioxidant Activities. Plant Foods Hum. Nutr. 2023, 78, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.S.; Li, C.G.; Zheng, X.K.; Li, L.L.; Chen, W.J.; Zhang, Y.L.; Cao, Y.G.; Gong, J.H.; Kuang, H.X. Three new sulphur glycosides from the seeds of Descurainia sophia. Nat. Prod. Res. 2016, 30, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, P.; Qu, L.; Ruan, J.; Yang, S.; Yu, H.; Zhang, Y.; Wang, T. Bioactive Constituents Obtained from the Seeds of Lepidium apetalum Willd. Molecules 2017, 22, 540. [Google Scholar] [CrossRef] [PubMed]
- Petkowski, J.J.; Bains, W.; Seager, S. Natural Products Containing a Nitrogen-Sulfur Bond. J. Nat. Prod. 2018, 81, 423–446. [Google Scholar] [CrossRef]
- Katoch, O.; Khan, G.A.; Dwarakanath, B.S.; Agrawala, P.K. Mitigation of hematopoietic radiation injury by diallyl sulphide. J. Environ. Pathol. Toxicol. Oncol. 2012, 4, 357–365. [Google Scholar] [CrossRef]
- Brucer, M.; Mewissen, D.J. Late effects of gamma radiation on mice protected with cysteamine or cystamine. Nature 1957, 4552, 201–202. [Google Scholar]
- Ji, L.; Cui, P.; Zhou, S.; Qiu, L.; Huang, H.; Wang, C.; Wang, J. Advances of Amifostine in Radiation Protection: Administration and Delivery. Mol. Pharm. 2023, 11, 5383–5395. [Google Scholar] [CrossRef] [PubMed]
- Kouloulias, V.E.; Kouvaris, J.R. Cytoprotective efficacy of amifostine against radiation-induced rectal toxicity: Objective and subjective grading scales for radiomucositis. Molecules 2008, 4, 892–903. [Google Scholar] [CrossRef]
- Poma, P. NF-κB and Disease. Int. J. Mol. Sci. 2020, 21, 9181. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.Z.; Zhou, T.Q.; Zhang, J.R.; Xia, Z.M.; Liu, S.F.; Liu, C.Y.; Li, M.; Zhang, G.J.; Tian, Y.; Li, B.; et al. Discovery of pentacyclic triterpenoid glycosides with anti-proliferative activities from Ardisialindleyana. Carbohydr. Res. 2023, 524, 108761. [Google Scholar] [CrossRef]
- Zhou, T.Q.; Wei, Z.Z.; Fu, Q.Y.; Rui, Q.; Zhang, G.J.; Li, B.; Dong, J.X.; Zeng, C.C. A new oleanane-type triterpene from Ardisia lindleyana D.Dietr and its cytotoxic activity. Nat. Prod. Res. 2023, 37, 2517–2524. [Google Scholar]
- Yang, J.; Zhou, Y.; Liu, H.; Wang, J.; Hu, J. MCI extraction from Turkish galls played protective roles against X-ray-induced damage in AHH-1 cells. Int. J. Clin. Exp. Pathol. 2015, 8, 8122–8128. [Google Scholar]
- El-Hachem, N.; Haibe-Kains, B.; Khalil, A.; Kobeissy, F.H.; Nemer, G. AutoDock and AutoDockTools for Protein-Ligand Docking: Beta-Site Amyloid Precursor Protein Cleaving Enzyme 1(BACE1) as a Case Study. Methods Mol. Biol. 2017, 1598, 391–403. [Google Scholar]





| No | Compond 3 (CD3OD) | Compond 4 (DMSO-d6) | ||
|---|---|---|---|---|
| δH (J in Hz) | δc | δH (J in Hz) | δc | |
| 1 | - | 179.2 | - | 177.7 |
| 2 | 2.29 (2H, t, 7.3) | 37.3 | 2.27 (2H, m) | 35.2 |
| 3 | 1.61 (2H, m) | 26.9 | 1.51 (2H, m) | 25.2 |
| 4 | 1.32–1.38 (2H, m) | 29.7 | 1.24–1.30 (2H, m) | 28.4 |
| 5 | 1.32–1.38 (2H, m) | 30.2 | 1.24–1.30 (2H, m) | 28.6 |
| 6 | 1.32–1.38 (2H, m) | 30.3 | 1.24–1.30 (2H, m) | 28.8 |
| 7 | 1.32–1.38 (2H, m) | 30.4 | 1.24–1.30 (2H, m) | 28.7 |
| 8 | 1.32–1.38 (2H, m) | 30.4 | 1.24–1.30 (2H, m) | 28.4 |
| 9 | 1.48 (2H, m) | 30.2 | 1.37 (2H, m) | 28.1 |
| 10 | 1.75 (2H, m) | 23.6 | 1.61 (2H, m) | 21.9 |
| 11 | 2.80 (2H, m) | 54.9 | 2.73 (1H, m) 2.62 (1H, m) | 53.2 |
| CH3SO | 2.63 (3H, s) | 38.1 | 2.50 (3H, s) | 38.0 |
| 1′ | 5.43 (1H, d, 5.8) | 90.3 | 4.12 (1H, d, 9.4) | 88.9 |
| 2′ | 3.75 (1H, dd, 5.8, 3.9) | 73.1 | 2.99 (1H, m) | 70.2 |
| 3′ | 4.07 (1H, m) | 74.6 | 2.86 (1H, m) | 70.1 |
| 4′ | 3.37 (1H, t, 9.9) | 71.2 | 3.19 (1H, m) | 76.6 |
| 5′ | 3.49 (1H, t, 9.3) | 75.8 | 3.24 (1H, m) | 81.5 |
| 6′ | 3.78 (1H, dd, 11.8, 2.5) 3.74 (1H, dd, 11.0, 5.1) | 62.4 | 3.69 (1H, dd, 11.8, 5.4) 3.41 (1H, m) | 61.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.-Z.; Ge, C.-B.; Wang, Y.-J.; Li, B.; Tian, Y.; Zhou, T.-Q.; Liu, S.-C.; Yi, J.-F. Phytochemicals, Two New Sulphur Glycosides and Two New Natural Products, from Shepherd’s Purse Seed and Their Activities. Molecules 2024, 29, 4145. https://doi.org/10.3390/molecules29174145
Wei Z-Z, Ge C-B, Wang Y-J, Li B, Tian Y, Zhou T-Q, Liu S-C, Yi J-F. Phytochemicals, Two New Sulphur Glycosides and Two New Natural Products, from Shepherd’s Purse Seed and Their Activities. Molecules. 2024; 29(17):4145. https://doi.org/10.3390/molecules29174145
Chicago/Turabian StyleWei, Zhen-Zhen, Chun-Bo Ge, Yu-Jie Wang, Bin Li, Ying Tian, Ti-Qiang Zhou, Shu-Chen Liu, and Jian-Feng Yi. 2024. "Phytochemicals, Two New Sulphur Glycosides and Two New Natural Products, from Shepherd’s Purse Seed and Their Activities" Molecules 29, no. 17: 4145. https://doi.org/10.3390/molecules29174145
APA StyleWei, Z.-Z., Ge, C.-B., Wang, Y.-J., Li, B., Tian, Y., Zhou, T.-Q., Liu, S.-C., & Yi, J.-F. (2024). Phytochemicals, Two New Sulphur Glycosides and Two New Natural Products, from Shepherd’s Purse Seed and Their Activities. Molecules, 29(17), 4145. https://doi.org/10.3390/molecules29174145







