Abstract
The increasingly severe antibiotic pollution has become one of the most critical issues. In this study, a zinc peroxide/peroxymonosulfate (ZnO2/PMS) double-oxidation system was developed for tetracycline (TC) degradation. A small amount of ZnO2 (10 mg) and PMS (30 mg) could effectively degrade 82.8% of TC (100 mL, 50 mg/L), and the degradation process could be well described by the pseudo-second-order kinetic model. Meanwhile, the ZnO2/PMS double-oxidation system showed high adaptability in terms of reaction temperature (2–40 °C), initial pH value (4–12), common inorganic anions (Cl−, NO3−, SO42− and HCO3−), natural water source and organic pollutant type. The quenching experiment and electron paramagnetic resonance (EPR) characterization results confirmed that the main reactive oxygen species (ROS) was singlet oxygen (1O2). Moreover, three possible pathways of TC degradation were deduced according to the analyses of intermediates. On the basis of comparative characterization and experiment results, a synergistic activation mechanism was further proposed for the ZnO2/PMS double-oxidation system, accounting for the superior degradation performance. The released OH− and H2O2 from ZnO2 could activate PMS to produce major 1O2 and minor superoxide radicals (•O2−), respectively.
1. Introduction
Antibiotics have been widely applied in the fields of clinical treatment, animal husbandry and crop growth [1,2,3,4,5,6]. However, the reckless use of antibiotics results in their frequent detection in water bodies [7,8,9] since the majority of antibiotics are poorly absorbed by organisms or degraded in nature [10]. Residual antibiotics in the environment easily trigger serious environmental problems, like fostering antibiotic resistance [11], inducing physiological toxicity [12] and threatening human health [13]. It has been reported that the concentration of antibiotics in some pig farm wastewater could even reach up to 25 mg/L [14], posing a great need for the efficient treatment of antibiotic-polluted wastewater.
Advanced oxidation processes (AOPs), like photocatalysis [15], ozonation [16], Fenton/Fenton-like oxidation [17] and electrocatalysis [18], have received widespread attention due to the fact that the generated reactive oxygen species (ROS) can degrade and mineralize organic pollutants with high efficiency and environmental friendliness [19,20,21]. Recently, peroxymonosulfate (PMS)-based AOPs have been considered promising and have become one of the research hotspots. This is because a variety of methods, including carbon materials [22], transition metals [23], heat [24], ultraviolet light [25], ultrasound [26] and alkali [27], can facilely activate PMS to degrade organic contaminants. Moreover, the generated ROS contain not only free radicals (like superoxide (•O2–), hydroxyl (•OH) and sulfate (SO4•–)) but also non-free radical singlet oxygen (1O2), which greatly expands the applications of PMS-based AOPs [28].
Among PMS-based AOPs, a hydrogen peroxide (H2O2)/PMS dual-oxidant system possesses superior performance towards antibiotics degradation. Sun et al. claimed that more than 80% of tetracycline (TC) could be degraded in a biochar/nZVI/MoS2-activated H2O2/PMS double-oxidation system [29]. Under the catalysis of pipe deposits, the H2O2/PMS double-oxidation system could remove around 80% of chloramphenicol within 120 min [30]. A MnO2/UIO-66-activated H2O2/PMS system could efficiently degrade 79.5% of oxytetracycline with high adaptability towards common inorganic anions [31]. Despite the excellent performance of H2O2/PMS double-oxidation systems, H2O2 suffers from the defects of being workable only under acidic conditions and its poor stability [32], severely restricting the practical applications of H2O2/PMS double-oxidation systems.
Accordingly, some metal peroxides, with relatively high stability and adaptability in a wide pH range, have been developed as an excellent alternative to H2O2, which can slowly produce H2O2 by reacting with H2O. In addition, the hydrolysis processes of metal peroxides can produce alkaline metal hydroxides, contributing to the activation of PMS. So far, calcium peroxide (CaO2) [33,34] has been reported in persulfate-based double-oxidation systems and has exhibited outstanding results in antibiotic degradation. Compared with CaO2, zinc peroxide (ZnO2) has a lower hydrolysis rate and can release H2O2 more slowly [35], which is certainly conducive to the effective utilization of H2O2. This means that ZnO2 possesses broad potential in environmental remediation fields. However, ZnO2/PMS double-oxidation systems have never been reported in the field of organic pollutant degradation.
In this manuscript, a ZnO2/PMS double-oxidation system was developed for the effective degradation of TC. The degradation performance of the ZnO2/PMS double-oxidation system was investigated in terms of ZnO2 dosage, PMS dosage, reaction temperature and initial solution pH value. Moreover, common inorganic matrices, different natural water sources and various types of organic pollutants were used to assess the adaptability of the ZnO2/PMS double-oxidation system. Quenching experiments and electron paramagnetic resonance (EPR) tests were conducted to reveal the main ROS responsible for TC degradation. Furthermore, the possible TC degradation pathways were clarified by an analysis of the intermediate products, and the synergistic effect of the ZnO2/PMS double-oxidation system was revealed on the basis of comparative experimental results.
2. Experimental Section
2.1. Chemicals
Zinc nitrate hexahydrate (Zn(NO3)2·6H2O), hydrogen peroxide (H2O2), ammonium hydroxide (NH3·H2O), sodium hydroxide (NaOH), sulfuric acid (H2SO4), anhydrous ethanol (C2H5OH), sodium chloride (NaCl), sodium nitrate (NaNO3), sodium sulfate (Na2SO4) and sodium hydrogen carbonate (NaHCO3) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Ningbo, China). Tetracycline (C22H24N2O8, TC), doxycycline hydrochloride (C22H25N2O8Cl, DOXH), Congo red (C32H22N6Na2O6S2, CR), Chloramphenicol hydrochloride (C22H22Cl2N2O7, CTCH), 5,5-dimethyl-1-pyrroline N-oxide (DMPO), 2,2,6,6-tetramethylpiperidine (TEMP) and 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL) were provided by Aladdin Chemistry Reagent Chemistry Co., Ltd. (Shanghai, China). Methanol (CH3OH, MeOH), isopropanol (C3H8O, IPA) and furfuryl alcohol (C5H6O2, FFA) were supplied by Shanghai Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals were of analytical grade and used without further purification.
2.2. Synthesis of ZnO2 Particles
Briefly, 1.5 mL of H2O2 was added to 20 mL of Zn(NO3)2·6H2O solution (0.5 mol/L). Then, 1.6 mL of NH3·H2O was slowly dropped into the above solution under vigorous agitation. In order to investigate the effect of agitation time on the product, a series of preparation experiments were conducted under different agitation times (10 min, 30 min, 60 min and 240 min). After the reaction, the generated white precipitates were separated by centrifugation and washed several times. Finally, the products were reserved after being dried in an oven at 60 °C for 12 h.
2.3. Characterizations
The phase structure was determined using an X-ray diffractometer (XRD, x’pert3 powder). The functional group was clarified via a Fourier transform infrared spectrometer (FTIR, Spectrum 3, PerkinElmer, Waltham, MA, USA). The thermal behavior was investigated using a thermogravimetry analyzer (TGA 5500, Milford, MA, USA). The microstructure was characterized by scanning electron microscopy (SEM, Nova NanoSEM 450, FEI, Eindhoven, Netherlands) and transmission electron microscopy (TEM, Tecnai G2 20, FEI, Eindhoven, The Netherlands). The chemical oxygen demand (COD) was tested using a multi-parameter water quality analyzer (LH-T640, Lianhua Technology, Beijing, China), and total organic carbon (TOC) concentration was measured via a TOC analyzer (TOC-L CPH, Shimadzu, Kyoto, Japan). The generated ROS were characterized using an electron paramagnetic resonance (EPR) spectrometer (EMXmicro-6/1, Bruker, Karlsruhe, Germany). The intermediates generated during the TC degradation process were determined using high-resolution liquid chromatography–mass spectrometry (HR-LC-MS, Thermo Fisher Orbitrap Q Exactive, Thermo Fisher Waltham, MA, USA). The specific analysis conditions and parameters are described in Text S1 and Table S1, which are provided in the Supplementary Materials.
2.4. Catalytic Degradation Experiment
At the preset temperature (2–40 °C), a certain amount of ZnO2 (0–15 mg) and PMS (0–40 mg) were sequentially added to the TC solution (100 mL, 50 mg/L) with different initial pH values (2–12) to start the degradation reaction under continuous agitation. Each group of degradation experiments was performed in triplicate. At specific time intervals, 3 mL of the mixture was extracted and filtered through a 0.22 μm filter. The residual TC concentration was measured by a UV-Vis spectrometer (UV-8000S), and the degradation rate was calculated using Equation (1).
where Co and Ct (mg/L) were TC concentrations at initial and certain times (t), respectively.
Degradation rate (%) = (1 − Ct/Co) × 100%
3. Results and Discussion
3.1. Characterizations of ZnO2
The XRD patterns of as-synthesized ZnO2 particles under different agitation times are displayed in Figure 1a. Evidently, these four characteristic diffraction peaks centered at 31.9°, 36.9°, 53.4° and 63.3° could be well assigned to the (111), (200), (220) and (311) planes of ZnO2 with the standard cubic phase (JCPDS 76−1364), respectively. Furthermore, the intensity of the diffraction peaks was enhanced with the agitation time, which indicated that extending the agitation time could be well conducive to the crystallinity. According to the Scherrer equation, the crystallite size of ZnO2 under agitation for 240 min was calculated to be 10.72 nm (Table S2), which was consistent with an earlier reported study [36]. As for the FTIR spectra of ZnO2 (Figure 1b), the absorption peaks around 3450 and 1572 cm−1 were attributed to -OH vibration [37]. In addition, the two adsorption peaks located at 1388 and 849 cm−1 were assigned to the characteristic O-O vibration [9]. It could be clearly observed that the intensity of the adsorption peak at 1388 improved with the agitation time. This point confirmed that extending the agitation time contributed to the formation of the O-O bond, which is consistent with the XRD results.
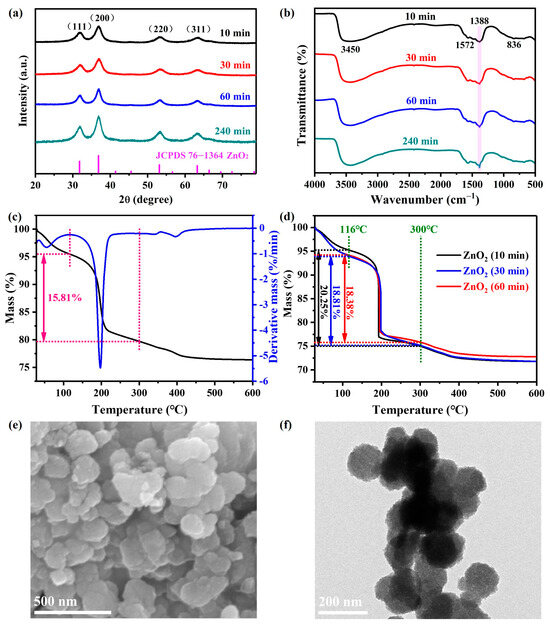
Figure 1.
XRD spectra (a) and FTIR patterns (b) of the products under different conditions; TG plots of ZnO2 samples under agitation for 240 min (c) and other agitation times (d); SEM (e) and TEM (f) pictures of as-synthesized ZnO2 under agitation for 240 min.
Figure 1c displays the thermal behavior of ZnO2 under agitation for 240 min. It could be observed that around 4.5% of the mass was lost from 30 to 116 °C, which is attributed to the desorption of adsorbed water. The main mass loss (15.81%) rapidly occurred between 116 and 300 °C, which was attributed to the thermal decomposition of ZnO2. Thus, the purity could be calculated to be around 96.4% in view of the theoretical value (16.4%). Meanwhile, the mass losses of ZnO2 samples under agitation for 10 min, 30 min and 60 min at this stage were 20.25, 18.81 and 18.38%, respectively (Figure 1d). They were all higher than the theoretical value, which indicated the generation of amorphous impurities due to incomplete oxidation. Accordingly, the ZnO2 sample prepared after agitation for 240 min was chosen for subsequent characterizations and degradation experiments.
The microscopic morphology of as-prepared ZnO2 was characterized by SEM and TEM techniques. As shown in Figure 1e, the as-prepared ZnO2 was mainly composed of multiple dispersive spherules with a particle size of around 100 nm. As seen from the TEM image (Figure 1f), the spherules were indeed assembled from much smaller primary particles, which was verified by the crystallite size calculation results. Undoubtedly, the highly dispersed particles would tend to expose active sites, thus contributing to the catalytic activity.
3.2. Degradation Performance of ZnO2/PMS Double-Oxidation System
3.2.1. Effects of PMS and ZnO2 Dosages
In order to investigate the effect of PMS dosage on the performance of the ZnO2/PMS double-oxidation system, the ZnO2 dosage was fixed at 10 mg. As shown in Figure 2a, only around 5% of TC was removed without PMS addition, which can be attributed to the physical adsorption of ZnO2. After PMS addition, the degradation rate of TC increased with the PMS dosage. After 60 min, the degradation rate of TC rose significantly from 49.3% to 80.2% when the dosage of PMS was increased from 10 mg to 30 mg. However, the degradation rate was slightly enhanced as the PMS dosage was further increased to 40 mg. In consideration of the reagent cost and degradation efficiency, 30 mg of PMS was selected for the ZnO2/PMS double-oxidation system in subsequent degradation experiments.
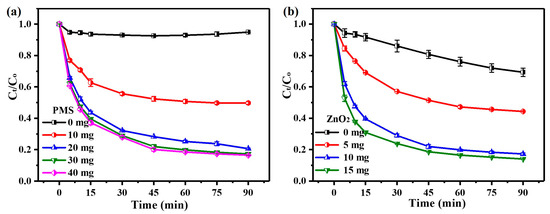
Figure 2.
Effects of PMS dosage (a) and ZnO2 dosage (b) on TC degradation.
The influence of the ZnO2 dosage is displayed in Figure 2b. It can be clearly observed that nearly 31% of TC was degraded within 90 min by PMS alone. After the addition of a small dosage of ZnO2 (5 mg), the degradation rate improved remarkably to 55.7% after 90 min, indicating the significant role of ZnO2 in the degradation system. It was noted that a slight enhancement of the degradation rate (only 3.2%) was realized after increasing the ZnO2 dosage from 10 to 15 mg. Hence, 10 mg of ZnO2 was deemed suitable for the ZnO2/PMS double-oxidation system in subsequent degradation experiments. Meanwhile, pseudo-zero-order, pseudo-first-order and pseudo-second-order kinetic models (Equations (S1)–(S3)) were employed to explore the kinetic characteristics of TC degradation (Text S2). The results (Figure S1) indicate that the pseudo-second-order kinetic model was more suitable to describe TC degradation on the basis of linear correlation coefficients, as listed in Table S3. This point illustrated that the degradation rate of TC was positively correlated with the square of dominant ROS concentrations generated in the ZnO2/PMS double-oxidation system.
3.2.2. Effects of Temperature and Initial Solution pH
The performance of the ZnO2/PMS double-oxidation system for TC degradation was examined under different temperatures (2–40 °C). The results (Figure 3a) exhibit that over 75% of TC could be degraded even at a low temperature (2 °C), revealing the terrific degradation performance of the ZnO2/PMS double-oxidation system. The degradation rate was continuously enhanced with the rise in temperature, and the degradation rate reached up to 80% within 60 min at 20 °C. Considering the extent of improvement became quite weak as the temperature exceeded 20 °C, the subsequent degradation experiments were conducted under 20 °C.

Figure 3.
Effects of temperature (a) and initial solution pH (b) on TC degradation; COD (c) and TOC (d) removal in the ZnO2/PMS double-oxidation system.
To explore the effect of initial pH, the degradation experiments were performed with different initial pH values (2–12). As seen from Figure 3b, under strong acid conditions (pH = 2), only 7.8% of TC was degraded after 90 min. This was due to the fact that ZnO2 had been completely decomposed by the large numbers of hydrogen ions (H+) in the solution. The generation of ROS via the self-decomposition of PMS was greatly inhibited under strong acid conditions [38], resulting in the low degradation rate of TC. Notably, the degradation rate was significantly improved with the increase in the initial solution pH, and around 77% of TC could be effectively degraded when the initial solution pH was adjusted to 4. It could be found that the ZnO2/PMS double-oxidation system showed excellent performance for TC degradation in a wide initial pH range (4–12).
Moreover, the Arrhenius equation (Equation (S4)) was applied to calculate the reaction activation energy (Text S3). As illustrated in Figure S2, the positive activation energy (32.3 KJ/mol) indicated that the TC degradation process was endothermic and in agreement with the contribution of increasing temperature to the degradation performance of the ZnO2/PMS double-oxidation system. Figure 3c exhibits the change in COD during the TC degradation process. It can be observed that the COD value significantly dropped from an initial 82.16 to 39.03 mg/L after 90 min, and the COD removal efficiency was over 52%. The TOC removal results (Figure 3d) show that the TOC concentration declined from 25.29 to 10.60 mg/L. Over 58% of TOC was removed after 180 min, suggesting a high mineralization efficiency of the ZnO2/PMS double-oxidation system in TC degradation.
3.2.3. Adaptability of ZnO2/PMS Double-Oxidation System
Firstly, the adaptability of the ZnO2/PMS double-oxidation system was evaluated by four common matrix species (NaCl, NaNO3, Na2SO4 and NaHCO3) with different concentrations (10–100 mM). As shown in Figure 4a–c, NaCl, NaNO3 and Na2SO4 could rarely affect TC degradation. Surprisingly, the existence of NaHCO3 remarkably enhanced TC degradation (Figure 4d), and this promotion effect could be attributed to the improvement of the solution pH value after NaHCO3’s addition (Figure S3a), which was consistent with the effect of the initial solution pH on TC degradation (Figure 3b).
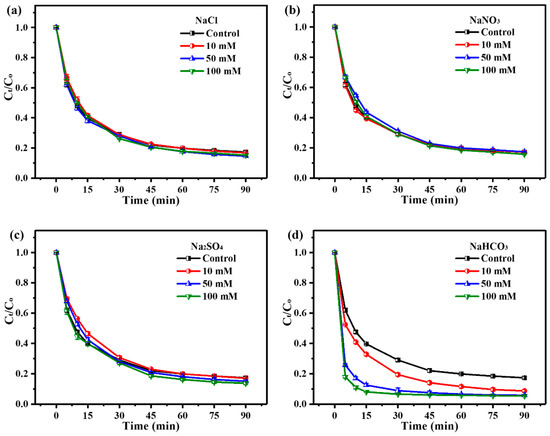
Figure 4.
Adaptability experiments of ZnO2 + PMS system to Cl− (a), NO3− (b), SO42− (c) and HCO3− (d).
Then, several natural water resources (Yangtze River water, Han River water, East Lake water, Yujia Lake water and tap water) were studied to explore the adaptability of the ZnO2/PMS double-oxidation system. The specific water quality parameters of these natural water resources are listed in Table S4. Unexpectedly, the results (Figure 5a) show that TC degradation was enhanced in these natural water resources. This could be due to the higher initial pH values of the TC solution when prepared using natural water resources (Figure S3b).
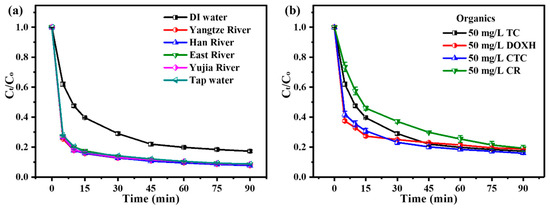
Figure 5.
Adaptability experiments of ZnO2 + PMS system to natural water resources (a) and organic pollutant types (b).
Additionally, diverse kinds of organic pollutants (DOXH, CR and CTCH) were used to investigate the adaptability of the ZnO2/PMS double-oxidation system. As displayed in Figure 5b, the degradation rates of DOXH, CR and CTCH could reach 81.2%, 80.1% and 84.1% after 90 min, respectively. On the basis of the above results, it was confirmed that the ZnO2/PMS double-oxidation system possessed high adaptability to common matrix species, water resources and organic pollutant types.
3.3. Mechanism Analysis
3.3.1. Quenching Experiments
Quenching experiments were conducted to explore the generated ROS in the ZnO2/PMS double-oxidation system. Specifically, MeOH, IPA and FFA were selected to quench the possible SO4•−, •OH and 1O2 generated in the system, respectively. Due to the fact that p-BQ can activate PMS to produce non-radical 1O2 [39], TEMPOL was utilized to quench generated •O2− radicals as a substitute for p-BQ [40]. As displayed in Figure 6a,b, the addition of MeOH and IPA with different concentrations (10–100 mM) had little effect on TC degradation, which indicated that the ZnO2/PMS double-oxidation system could hardly produce SO4•− and •OH radicals.

Figure 6.
Quenching experiments with different concentrations of MeOH (a), IPA (b), TEMPOL (c) and FFA (d).
As seen from Figure 6c, there was a slight inhibition effect, and the degradation rate only decreased by 5% after 30 mM TEMPOL was added into the system, suggesting the existence of a small amount of •O2− radicals. As for FFA (Figure 6d), it could significantly inhibit TC degradation, and the degradation rate dropped from 82.8% to 55.1% after the addition of 50 mM FFA. As the concentration of FFA reached 500 mM, TC degradation was completely inhibited in the system. Accordingly, the above results indicated that the main ROS was 1O2, accompanied by a small amount of •O2−.
Further, EPR tests were carried out to identify the ROS generated in the ZnO2/PMS double-oxidation system. It was noted that SO4•−, •OH and •O2− radicals could react with DMPO to form characteristic signals. As shown in Figure 7a, no obvious signals could be detected in the ZnO2 + PMS system. This point demonstrated that the ZnO2 + PMS system could not produce SO4•− and •OH radicals, which is consistent with the quenching experiment results (Figure 6a,b). The characteristic signals related to the DMPO-•O2− adduct were not detected either, which might be attributed to the low number of •O2− radicals. The EPR results using TEMP as a capturing agent are exhibited in Figure 7b. Clearly, the characteristic triplet signal of the TEMP-1O2 adduct with an intensity ratio of 1:1:1 could be observed in the PMS system because PMS could generate 1O2 through a self-decomposition reaction [41]. As for the ZnO2 + PMS system, the signal intensity of TEMP-1O2 was much stronger than that in the sole PMS system. This point confirmed that ZnO2 can activate PMS to produce more 1O2, thereby contributing to the superior performance for TC degradation compared with other PMS-based systems (Table 1).
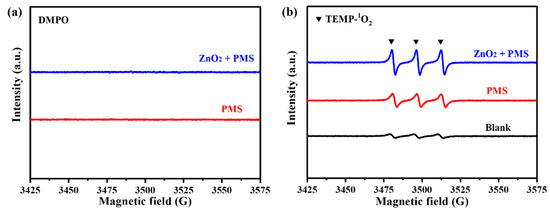
Figure 7.
EPR tests of ZnO2 + PMS system using DMPO (a) and TEMP (b).

Table 1.
Comparison of performances over the reported PMS-based systems.
3.3.2. Intermediate Identification and TC Degradation Pathway Investigation
To deduce the possible pathways of TC degradation in the ZnO2/PMS double-oxidation system, the HR-LC-MS technique was employed to determine the generated intermediates. As shown in the ESI-MS spectra (Figure S4), TC (P445) and nine main intermediates (P493, P449, P461, P477, P431, P417, P267, P163 and P114) were found at different retention times and the detailed information on this is supplied in Table S5. Based on the detected intermediates, three possible degradation pathways are proposed in Figure 8. In pathway A, a TC molecule first lost a methyl group around C4 to generate P431 [48] and formed P417 through further demethylation. In pathway B, the product of P461 was first generated through dipolar cycloaddition and the rearrangement of the hydroxyl group at C11a-C12 in the TC molecule. P461 progressively transformed into P477 via hydroxylation [49]. As for pathway C, the TC molecule opened the ring at C7 to produce P493 [50]. Then, P447 was produced via the decarboxylation of P493 at C8. Subsequently, the smaller intermediates, including P163, P267 and P114, were produced by further oxidation [51]. Finally, these intermediates were degraded and mineralized into CO2, H2O and NH4+.
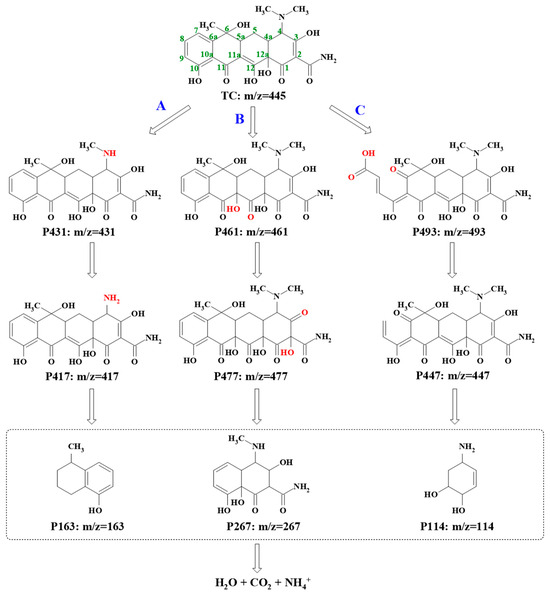
Figure 8.
Possible pathways for TC degradation in ZnO2/PMS double-oxidation system.
3.3.3. Activation Pathway Study
It was well known that hydroxide ions (OH−) could activate PMS to generate 1O2 [52,53] and that Zn(OH)2 released from ZnO2 would spontaneously produce OH− in solution via the ionization effect. Thus, it is of great significance to monitor the pH value of the ZnO2/PMS double-oxidation system during TC degradation in order to deeply understand the generation mechanism of ROS in the ZnO2/PMS double-oxidation system. As illustrated in Figure 9a, the pH value of the ZnO2/PMS double-oxidation degradation system (ZnO2 + PMS) increased from the initial 5.6 to 6.4 and gradually stabilized at around 6.6.

Figure 9.
Comparison of degradation performance of NaOH + PMS, NaOH + H2O2 + PMS and ZnO2 + PMS systems under the same pH conditions (a), and using equimolar amount of ZnO, Zn(OH)2 and ZnO2 to activate PMS for TC degradation (b).
Then, two comparative degradation experiments (NaOH + PMS and NaOH + H2O2 + PMS) were conducted under the same pH condition as the ZnO2 + PMS system, and the molar amount of H2O2 equaled the molar amount of ZnO2. The results showed that the degradation rate of TC in the NaOH + PMS system reached 67% within 90 min, proving that OH− could indeed activate PMS to produce 1O2. As for the NaOH + H2O2 + PMS system, on one hand, TC degradation was apparently enhanced in comparison with the NaOH + PMS system, demonstrating that PMS might be activated by H2O2. It was reported that H2O2 could activate PMS to produce •O2− radicals [54], which was consistent with the TEMPOL quenching experiment results (Figure 6c). On the other hand, the degradation performance of the NaOH + H2O2 + PMS system was still inferior to the ZnO2 + PMS system, revealing the contribution of ZnO2 release to the TC degradation in the ZnO2/PMS double-oxidation system.
Additionally, equimolar ZnO and Zn(OH)2 were selected to replace ZnO2 for PMS activation for TC degradation under the same conditions. The results (Figure 9b) showed that both the ZnO + PMS system and the Zn(OH)2 + PMS system had an inferior performance in TC degradation. This point confirmed that the released H2O2 from ZnO2 was indeed able to activate PMS, thereby promoting the degradation performance of the ZnO2/PMS double-oxidation degradation system.
Based on the above results and analyses, the generation mechanism of ROS in the ZnO2/PMS double-oxidation system could be deduced by the following equations (Equations (2)–(8)). It was well known that PMS could directly produce 1O2 through the self-decomposition reaction (Equation (2)), according to previous research [55]. In addition, as illustrated in Equation (3), ZnO2 would transform into Zn(OH)2 and H2O2 via hydrolysis in solution [36]. On the one hand, the generated Zn(OH)2 could spontaneously release OH− ions (Equation (4)). It was reported that OH− could activate PMS to generate 1O2 via Equation (5) [27], thus contributing to TC degradation. On the other hand, the ionization reaction of the produced H2O2 (Equation (6)) was greatly promoted under alkaline conditions, which was revealed in the base-activated PMS system [56]. Further, the ionization product HO2− would react with PMS to form •O2− radicals [33] through Equation (7). Therefore, TC was effectively degraded by the 1O2 and •O2− produced in the ZnO2/PMS double-oxidation system (Equation (8)).
HSO5− + SO52− → HSO4− + SO42− + 1O2
ZnO2 + 2H2O → Zn(OH)2 + H2O2
Zn(OH)2 → Zn2+ + 2OH−
HSO5− + SO52− + OH− → 2SO42− + H2O + 1O2
H2O2 → HO2− + H+
2SO52− + HO2− → 2SO42− + 2•O2− + H+
1O2 + •O2− + TC → Intermediate products + H2O + CO2 + NH4+
4. Conclusions
In summary, a ZnO2/PMS double-oxidation system was fabricated and applied to the degradation of organic pollutants. Over 80% of 50 mg/L TC could be effectively degraded by 0.1 g/L ZnO2 and 0.3 g/L PMS within 60 min. Surprisingly, the ZnO2/PMS double-oxidation system possessed a relatively strong anti-interference ability towards co-existing inorganic ions, natural water sources and organic pollutant types, exhibiting a great application potential for practical wastewater treatment. 1O2 was considered the dominant ROS responsible for TC degradation. According to a series of characterization analyses and comparative experiment results, OH− and H2O2 released from ZnO2 were able to activate PMS to generate 1O2 and minor •O2−, respectively. It was the existence of a synergistic activation mechanism that endowed the ZnO2/PMS double-oxidation system with a superior degradation performance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29174120/s1, Text S1. HR-LC-MS analysis conditions and parameters; Table S1. Gradient elution program; Table S2. Crystallite sizes of ZnO2 samples prepared under different agitation time; Text S2. Degradation kinetics analysis; Figure S1. Linear curves fitted by pseudo-zero order (a), pseudo-first order (b) and pseudo-second order (c) kinetic models; Table S3. Kinetic parameters of different kinetics models; Text S3. Degradation thermodynamics analysis; Figure S2. Linear curves fitted by Arrhenius equation; Figure S3. Initial pH values of TC solutions containing common inorganic anions (a) and prepared using different water sources (b); Table S4. Water quality parameters of natural water resources; Figure S4. The detected mass spectra of TC and intermediates; Table S5. Characteristics of intermediate products during TC degradation.
Author Contributions
Methodology, S.L., X.L. and N.D.; Software, S.D. and M.N.; Validation, W.W. and P.C.; Investigation, Y.Z.; Data curation, M.N.; Writing–original draft, S.L.; Writing–review & editing, Y.Z., S.D., X.L., W.W., N.D., M.N. and P.C.; Visualization, S.L. and Y.Z.; Supervision, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research and Development Program of Hubei Province (No. 2023BCB108), the Supporting Enterprise Technological Innovation and Development in Hubei Province (No. 2021BAB112), the 2022 Central Guidance Local Science and Technology Development Fund (No. 2022BGE005), and the Natural Science Foundation of Hubei Province (No. 2023AFB447).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials, further inquiries can be directed to the corresponding author.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Liu, J.; Dong, Y.; Liu, Q.; Liu, W.; Lin, H. MoS2-based nanocomposites and aerogels for antibiotic pollutants removal from wastewater by photocatalytic degradation process: A review. Chemosphere 2024, 354, 141582. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Liu, Y.; Feng, L.; Yang, S.; Tan, X.; Liu, Z. Magnetic Fe3O4-C@MoS2 composites coordinated with peroxymonosulfate catalysis for enhanced tetracycline degradation. J. Alloys Compd. 2024, 989, 174318. [Google Scholar] [CrossRef]
- Zhao, H.; He, T.; Zhang, Q.; Feng, H.; Mi, H.; Liang, Z.; Zhou, D.; Dong, W.; Xue, X. Interfacial bonded K-doped-C3N4@Bi2WO6 heterostructure for efficient photocatalytic degradation of tetracycline. J. Alloys Compd. 2024, 972, 172822. [Google Scholar] [CrossRef]
- Xu, X.; Shao, W.; Tai, G.; Yu, M.; Han, X.; Han, J.; Wu, G.; Xing, W. Single-atomic Co-N site modulated exciton dissociation and charge transfer on covalent organic frameworks for efficient antibiotics degradation via peroxymonosulfate activation. Sep. Purif. Technol. 2024, 333, 125890. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, M.; Huo, B.; Wang, J.; Yang, L.; Ma, W.; Qi, J.; Wang, Y.; Zhu, Z.; Meng, F. A novel ZnO/CQDs/PVDF piezoelectric system for efficiently degradation of antibiotics by using water flow energy in pipeline: Performance and mechanism. Nano Energy 2023, 107, 108162. [Google Scholar] [CrossRef]
- Goudarzi, M.; Abdulhusain, Z.H.; Salavati-Niasari, M. Low-cost and eco-friendly synthesis of Mn-doped Tl2WO4 nanostructures for efficient visible light photocatalytic degradation of antibiotics in water. Sol. Energy 2023, 262, 111912. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Zang, J.; Zhao, X.; Jiang, F.; Jiang, L.; Xiong, C.; Wang, N.; Fu, C. Antibiotic residues of drinking-water and its human exposure risk assessment in rural Eastern China. Water Res. 2023, 236, 119940. [Google Scholar] [CrossRef]
- Yu, X.; Yu, F.; Li, Z.; Zhan, J. Occurrence, distribution, and ecological risk assessment of pharmaceuticals and personal care products in the surface water of the middle and lower reaches of the Yellow River (Henan section). J. Hazard. Mater. 2023, 443, 130369. [Google Scholar] [CrossRef]
- Chen, P.; Dong, N.; Zhang, J.; Wang, W.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. Investigation on visible-light photocatalytic performance and mechanism of zinc peroxide for tetracycline degradation and Escherichia coli inactivation. J. Colloid Interface Sci. 2022, 624, 137–149. [Google Scholar] [CrossRef]
- Li, S.; Zhu, L. Copper regulates degradation of typical antibiotics by microalgal-fungal consortium in simulated swine wastewater: Insights into metabolic routes and dissolved organic matters. Water Res. 2023, 245, 120654. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Huang, Y.; Xiang, Z.; Zeng, R.; Zeng, S.; Liu, S. Polystyrene nanoplastics foster Escherichia coli O157, H7 growth and antibiotic resistance with a stimulating effect on metabolism. Environ. Sci. Nano 2023, 10, 1341–1351. [Google Scholar] [CrossRef]
- Zhang, S.; Han, W.; Liu, T.; Feng, C.; Jiang, Q.; Zhang, B.; Chen, Y.; Zhang, Y. Tetracycline inhibits the nitrogen fixation ability of soybean (Glycine max (L.) Merr.) nodules in black soil by altering the root and rhizosphere bacterial communities. Sci. Total Environ. 2024, 908, 168047. [Google Scholar] [CrossRef] [PubMed]
- Míguez-González, A.; Cela-Dablanca, R.; Barreiro, A.; Rodríguez-López, L.; Rodríguez-Seijo, A.; Arias-Estévez, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Castillo-Ramos, V.; Álvarez-Rodríguez, E. Adsorption of antibiotics on bio-adsorbents derived from the forestry and agro-food industries. Environ. Res. 2023, 233, 116360. [Google Scholar] [CrossRef]
- Zhi, S.; Shen, S.; Zhou, J.; Ding, G.; Zhang, K. Systematic analysis of occurrence, density and ecological risks of 45 veterinary antibiotics: Focused on family livestock farms in Erhai Lake basin, Yunnan, China. Environ. Pollut. 2020, 267, 115539. [Google Scholar] [CrossRef]
- Ali, H.; Masar, M.; Yasir, M.; Machovsky, M.; Monteiro, O.C.; Kuritka, I. Current trends in environmental and energy photocatalysis and ISO standardization. J. Environ. Chem. Eng. 2023, 11, 111541. [Google Scholar] [CrossRef]
- Mutke, X.A.M.; Swiderski, P.; Drees, F.; Akin, O.; Lutze, H.V.; Schmidt, T.C. Efficiency of ozonation and sulfate radical-AOP for removal of pharmaceuticals, corrosion inhibitors, X-ray contrast media and perfluorinated compounds from reverse osmosis concentrates. Water Res. 2024, 255, 121346. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; You, X.; Li, Y.; Lin, H.; Chen, J.P. A novel bimetallic Fe-Cu-CNT catalyst for effective catalytic wet peroxide oxidation: Reaction optimization and mechanism investigation. Chem. Eng. J. 2024, 479, 147320. [Google Scholar] [CrossRef]
- Zeng, J.; Xie, W.; Guo, Y.; Zhao, T.; Zhou, H.; Wang, Q.; Li, H.; Guo, Z.; Xu, B.B.; Gu, H. Magnetic field facilitated electrocatalytic degradation of tetracycline in wastewater by magnetic porous carbonized phthalonitrile resin. Appl. Catal. B Environ. 2024, 340, 123225. [Google Scholar] [CrossRef]
- Xie, Z.H.; He, C.S.; Zhou, H.Y.; Li, L.L.; Liu, Y.; Du, Y.; Liu, W.; Mu, Y.; Lai, B. Effects of molecular structure on organic contaminants’ degradation efficiency and dominant ROS in the advanced oxidation process with multiple ROS. Environ. Sci. Technol. 2022, 56, 8784–8795. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Meng, C.; Zhang, Z. Nanoconfined catalytic membranes assembled by cobalt-functionalized graphitic carbon nitride nanosheets for rapid degradation of pollutants. Appl. Catal. B Environ. 2023, 322, 122098. [Google Scholar] [CrossRef]
- Tang, R.; Zeng, H.; Deng, Y.; Xiong, S.; Li, L.; Zhou, Z.; Wang, J.; Tang, L. Dual modulation on peroxymonosulfate activation site and photocarrier separation in carbon nitride for efficient photocatalytic organics degradation: Efficacy and mechanism evaluation. Appl. Catal. B Environ. 2023, 336, 122918. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal-and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, H.; Yao, B.; Gao, X.; Yang, X.; Zhou, Y. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: A Review. Chem. Eng. J. 2021, 414, 128800. [Google Scholar] [CrossRef]
- Ahn, Y.Y.; Choi, J.; Kim, M.; Kim, M.S.; Lee, D.; Bang, W.H.; Yun, E.T.; Lee, H.; Lee, J.H.; Lee, C.; et al. Chloride-mediated enhancement in heat-induced activation of peroxymonosulfate: New reaction pathways for oxidizing radical production. Environ. Sci. Technol. 2021, 55, 5382–5392. [Google Scholar] [CrossRef] [PubMed]
- Alayande, A.B.; Hong, S. Ultraviolet light-activated peroxymonosulfate (UV/PMS) system for humic acid mineralization: Effects of ionic matrix and feasible application in seawater reverse osmosis desalination. Environ. Pollut. 2022, 307, 119513. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.; Cui, M.; Ren, Y.; Park, B.; Ma, J.; Han, Z.; Khim, J. Activation of peroxodisulfate and peroxymonosulfate by ultrasound with different frequencies: Impact on ibuprofen removal efficient, cost estimation and energy analysis. Chem. Eng. J. 2021, 413, 127487. [Google Scholar] [CrossRef]
- Wang, W.; Chen, M.; Wang, D.; Yan, M.; Liu, Z. Different activation methods in sulfate radical-based oxidation for organic pollutants degradation: Catalytic mechanism and toxicity assessment of degradation intermediates. Sci. Total Environ. 2021, 772, 145522. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, T.; Yang, C.; Ma, C.; Zhao, Z.; Wu, Z.; Cao, S.; Geng, W.; Wang, Y.; Yao, Y.; et al. Activity trends and mechanisms in peroxymonosulfate-assisted catalytic production of singlet oxygen over atomic metal-N-C catalysts. Angew. Chem. Int. Ed. 2021, 60, 22513–22521. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, B.; Wang, N.; Zhang, N.; Ma, Y.; Zang, L.; Li, Z.; Xue, R. Refractory organics removal in PMS and H2O2/PMS oxidation system activated by biochar/nZVI/MoS2 composite: Synthesis, performance, mechanism and dosing methods. J. Environ. Chem. Eng. 2023, 11, 109134. [Google Scholar] [CrossRef]
- Zhong, D.; Zhou, Z.; Ma, W.; Ma, J.; Lv, W.; Feng, W.; Du, X.; He, F. Study on degradation of chloramphenicol by H2O2/PMS double-oxidation system catalyzed by pipe deposits from water networks. J. Environ. Chem. Eng. 2022, 10, 107529. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, S.; Duan, X.; Zheng, W.; Shao, C.; Wu, W.; Jiang, Z.; Lai, W. MnO2/UIO-66 improves the catalysed degradation of oxytetracycline under UV/H2O2/PMS system. J. Solid State Chem. 2021, 300, 122231. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Liu, C.; Xia, D.; Ou, X.; Cai, Y.; Zhou, Y.; Jiang, J.; Han, B. Sulfone-Modified Covalent Organic Frameworks Enabling Efficient Photocatalytic Hydrogen Peroxide Generation via One-Step Two-Electron O2 Reduction. Angew. Chem. Int. Ed. 2023, 62, e202305355. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, W.; Dong, N.; Chen, P.; Ge, L.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. A dual-oxidant advanced oxidation process system containing CaO2 and peroxymonosulfate for organic pollutant degradation: High adaptability and synergistic effect. Sep. Purif. Technol. 2023, 308, 122909. [Google Scholar] [CrossRef]
- Abbas, Q.; Shakoor, A.; Naushad, M.; Naushad, M.; Yousaf, B. In-situ oxidative degradation of sulfamethoxazole by calcium peroxide/persulfate dual oxidant system in water and soil. Process Saf. Environ. Prot. 2022, 164, 696–705. [Google Scholar]
- Wolanov, Y.; Prikhodchenko, P.V.; Medvedev, A.V.; Pedahzur, R.; Lev, O. Zinc dioxide nanoparticulates: A hydrogen peroxide source at moderate pH. Environ. Sci. Technol. 2013, 47, 8769–8774. [Google Scholar] [CrossRef]
- Chen, P.; Wang, W.; Dong, N.; Zhang, J.; Yang, T.; Tan, F.; Tan, S.; Wang, X.; Qiao, X.; Wong, P.K. Facile in-situ fabrication of ZnO2/CQD composites with promoted visible-light photocatalytic activities for organic degradation and bacterial inactivation. Appl. Surf. Sci. 2022, 604, 154629. [Google Scholar] [CrossRef]
- Wang, B.; Hu, J.; Liu, K.; Zhang, L.; Jiang, H.; Li, C. Reinforcement mechanism of silica surface hydroxyl: The opposite effect. Appl. Surf. Sci. 2023, 13, 157000. [Google Scholar] [CrossRef]
- Nie, Y.; Zhou, H.; Tian, S.; Tian, X.; Yang, C.; Li, Y.; Tian, Y.; Wang, Y. Anionic ligands driven efficient ofloxacin degradation over LaMnO3 suspended particles in water due to the enhanced peroxymonosulfate activation. Chem. Eng. J. 2022, 427, 130998. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, K.; He, J.; Li, N.; You, H.; Jiang, J. Droplet spray ionization mass spectrometry for real-time monitoring of activation of peroxymonosulfate by 1,4-benzoquinone. Microchem. J. 2018, 139, 437–442. [Google Scholar] [CrossRef]
- Liang, S.; Zheng, W.; Zhu, L.; Duan, W.; Wei, C.; Feng, C. One-Step treatment of phosphite-laden wastewater: A single electrochemical reactor integrating superoxide radical-induced oxidation and electrocoagulation. Environ. Sci. Technol. 2019, 53, 5328–5336. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Li, W.; Zhong, R.; Lan, Y.; Guo, J. The efficient degradation of sulfisoxazole by singlet oxygen (1O2) derived from activated peroxymonosulfate (PMS) with Co3O4–SnO2/RSBC. Environ. Res. 2020, 187, 109665. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yan, L.; Li, X.; Yan, T.; Song, W.; Hou, T.; Tong, C.; Mu, J.; Xu, M. Goethite/biochar-activated peroxymonosulfate enhances tetracycline degradation: Inherent roles of radical and non-radical processes. Sci. Total Environ. 2021, 783, 147102. [Google Scholar] [CrossRef]
- Su, X.; Guo, Y.; Yan, L.; Wang, Q.; Zhang, W.; Li, X.; Song, W.; Li, Y.; Liu, G. MoS2 nanosheets vertically aligned on biochar as a robust peroxymonosulfate activator for removal of tetracycline. Sep. Purif. Technol. 2022, 282, 120118. [Google Scholar] [CrossRef]
- Luo, X.; Shen, M.; Liu, J.; Ma, Y.; Gong, B.; Liu, H.; Huang, Z. Resource utilization of piggery sludge to prepare recyclable magnetic biochar for highly efficient degradation of tetracycline through peroxymonosulfate activation. J. Clean. Prod. 2021, 294, 126372. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, X.; Yan, Y.; Sun, C.; Wu, H.; He, J.; Wang, D. Heterogeneous activation of peroxymonosulfate by different ferromanganese oxides for tetracycline degradation: Structure dependence and catalytic mechanism. Chem. Eng. J. 2018, 348, 263–270. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Wang, Y.; Jia, F.; Song, S. Construction of 3D-sized Mn (II)-doped MoS2@activated alumina beads as PMS activator for tetracycline degradation under light irradiation. Chem. Phys. Lett. 2022, 806, 139996. [Google Scholar] [CrossRef]
- Yan, X.; Yao, Y.; Zhang, H.; Xie, J.; Xiao, C.; Zhang, S.; Qi, J.; Sun, X.; Li, J. Zeolitic imidazolate framework (ZIF-8)/polyacrylonitrile derived millimeter-sized hierarchical porous carbon beads for peroxymonosulfate catalysis. Environ. Res. 2022, 206, 112618. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Lin, Q.; Huang, R.; Fu, H.; Zhang, X.; Luo, H.; Xiao, R. Oxidative degradation of tetracycline using persulfate activated by N and Cu codoped biochar. Chem. Eng. J. 2020, 380, 122608. [Google Scholar] [CrossRef]
- Wang, H.; Chen, T.; Chen, D.; Zou, X.; Li, M.; Huang, F.; Sun, F.; Wang, C.; Shu, D.; Liu, H. Sulfurized oolitic hematite as a heterogeneous Fenton-like catalyst for tetracycline antibiotic degradation. Appl. Catal. B Environ. 2020, 260, 118203. [Google Scholar] [CrossRef]
- Ge, L.; Yue, Y.; Wang, W.; Tan, F.; Zhang, S.; Wang, X.; Qiao, X.; Wong, P.K. Efficient degradation of tetracycline in wide pH range using MgNCN/MgO nanocomposites as novel H2O2 activator. Water Res. 2021, 198, 117149. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Zhang, R.; Ding, Y.; Ren, Z.; Fu, M.; Cao, X.; Zeng, G. Singlet oxygen-dominated activation of peroxymonosulfate by passion fruit shell derived biochar for catalytic degradation of tetracycline through a non-radical oxidation pathway. J. Hazard. Mater. 2021, 419, 126495. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Xu, T.; Faheem, M.; Xi, Y.; Zhou, T.; Moryani, H.T.; Bao, J.; Du, J. Evolution of singlet oxygen by activating peroxydisulfate and peroxymonosulfate: A review. Int. J. Environ. Res. 2021, 18, 3344. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Deng, Y.; Nie, S.; Yan, C.; Ding, M.; Dong, W.; Dai, Y.; Zhang, Y. Simultaneous removal of bisphenol A and phosphate from water by peroxymonosulfate combined with calcium hydroxide. Chem. Eng. J. 2019, 369, 35–45. [Google Scholar] [CrossRef]
- Cai, H.; Zou, J.; Lin, J.; Li, J.; Huang, Y.; Zhang, S.; Yuan, B.; Ma, J. Sodium hydroxide-enhanced acetaminophen elimination in heat/peroxymonosulfate system: Production of singlet oxygen and hydroxyl radical. Chem. Eng. J. 2022, 429, 132438. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Ma, J.; Lin, C.; Li, X.; Zhang, H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere 2016, 151, 280–288. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).